PURPOSE
Hypofractionated breast radiotherapy has been found to be equivalent to conventional fractionation in many clinical trials. Using data from the European Society for Radiotherapy and Oncology Global Impact of Radiotherapy in Oncology survey, we identified preferences for hypofractionation in breast cancer across World Bank income groups and the perceived facilitators and barriers to its use.
MATERIALS AND METHODS
An international, electronic survey was administered to radiation oncologists from 2018 to 2019. Demographics, practice characteristics, preferred hypofractionation regimen for specific breast cancer scenarios, and facilitators and barriers to hypofractionation were reported and stratified by World Bank income groups. Variables associated with hypofractionation were assessed using multivariate logistic regression models.
RESULTS
One thousand four hundred thirty-four physicians responded: 890 (62%) from high-income countries (HICs), 361 (25%) from upper-middle–income countries (UMICs), 183 (13%) from low- and lower-middle–income countries (LLMICs). Hypofractionation was preferred most frequently in node-negative disease after breast-conserving surgery, with the strongest preference reported in HICs (78% from HICs, 54% from UMICs, and 51% from LLMICs, P < .001). Hypofractionation for node-positive disease postmastectomy was more frequently preferred in LLMICs (28% from HICs, 15% from UMICs, and 35% from LLMICs, P < .001). Curative doses of 2.1 to < 2.5 Gy in 15-16 fractions were most frequently reported, with limited preference for ultra-hypofractionation, but significant variability in palliative dosing. In adjusted analyses, UMICs were significantly less likely than LLMICs to prefer hypofractionation across all curative clinical scenarios, whereas respondents with > 1 million population catchments and with intensity-modulated radiotherapy were more likely to prefer hypofractionation. The most frequently cited facilitators and barriers were published evidence and fear of late toxicity, respectively.
CONCLUSION
Preference for hypofractionation varied for curative indications, with greater acceptance in earlier-stage disease in HICs and in later-stage disease in LLMICs. Targeted educational interventions and greater inclusivity in radiation oncology clinical trials may support greater uptake.
INTRODUCTION
Breast cancer is the most common cancer worldwide, with the most rapid increases in incidence occurring in low- and middle-income countries (LMICs).1 Radiotherapy is an essential component of breast cancer treatment, but large global disparities in access have limited the extent to which women can benefit.2,3 Although these disparities are most pronounced in LMICs, they are also present in more well-resourced regions such as Europe.4 Hypofractionation, in which larger doses per day are delivered over shorter periods of time, has been identified as an important mechanism for improving access and reducing the cost of treatment without compromising outcomes,3 yet there is limited understanding of its adoption in the treatment of breast cancer across different resource settings and the factors affecting its use.
CONTEXT
Key Objective
To what extent do radiation oncologists prefer hypofractionated radiotherapy for breast cancer and what are the barriers and facilitators to its use across different resource settings?
Knowledge Generated
Hypofractionation was widely accepted for palliation, but there was significant variability for adjuvant curative indications, with concerns of worse toxicity and insufficient data limiting adoption. Stronger preference for hypofractionation was observed in early-stage disease among high-income respondents and in postmastectomy or node-positive disease among low- and middle-income country respondents.
Relevance
Addressing barriers to hypofractionation, including ultra-hypofractionated regimens, is an important mechanism for increasing access to radiotherapy around the world and improving the quality of care delivered.
The use of moderate hypofractionation for breast cancer radiotherapy has been well established in the adjuvant setting on the basis of several large randomized trials.5-8 More recently, the FAST and Fast-Forward trials have established the noninferiority of ultra-hypofractionation regimens involving five fractions for early-stage disease after breast-conserving surgery (BCS)9,10 and openly accruing trials are evaluating this regimen for node-positive disease.11 In the palliative setting, hypofractionation has been used to provide both effective symptom control and maintain quality of life.12 Economic analyses in different settings have also demonstrated the cost-effectiveness of these shortened fractionation schedules.13
Despite the strong evidence base for hypofractionation in breast cancer, evidence on its real-world use has been mixed. In 2020, the European Society for Radiotherapy and Oncology's Global Impact of Radiotherapy in Oncology (ESTRO-GIRO) initiative published an international survey of hypofractionation across several disease sites.14 Findings from the survey identified significant variation in the preference for hypofractionation across indications and regions, but the extent to which practice patterns differed between low- and lower–middle-income countries (LLMICs), upper-middle–income countries (UMICs), and high-income countries (HICs) and the factors affecting its use in breast cancer were not explored. Understanding the perspectives of practitioners on hypofractionation in the treatment of breast cancer in different resource settings is essential to promote the translation of evidence into practice. In the present study, we report on findings from the ESTRO-GIRO survey to describe the preferences for breast cancer hypofractionation among radiation oncologists across World Bank income group regions and the facilitators and barriers to its use.
MATERIALS AND METHODS
The ESTRO-GIRO initiative administered an anonymous, electronic survey using the SurveyMonkey platform to radiation oncologists from January 2018 to January 2019 through the ESTRO membership database and liaisons of several national and regional professional radiation oncology societies globally. Before administration, the survey was pilot tested by radiation oncologists representing four different countries, with representation from HICs and LMICs. This ensured that the questions and response options were comprehensive and clearly articulated and that respondent burden was minimized. The survey was iteratively revised and considered validated when no further revisions were suggested and subsequently translated into English, Spanish, Japanese, and Mandarin. Complete details on the survey design have been described previously.14
Data on physician demographics and clinical practice characteristics were collected. Demographic characteristics of respondents included age, sex, country of current practice, and country of training. Clinical practice characteristics included scope of practice (university affiliation, public, private, and public-private partnership), population size of practice catchment area (< 10,000, 10,000-50,000, 50,000-100,000, 100,000-500,000, 500,000-1,000,000, and > 1,000,000), and available radiotherapy technology (intensity-modulated radiotherapy [IMRT], three-dimensional [3D] conformal radiotherapy, computed tomography–based 3D planning, and two-dimensional [2D planning]).
Respondents who treated at least one breast cancer case per month were invited to respond to a series of questions on hypofractionation in breast cancer management (Data Supplement). Respondents were presented with five different clinical scenarios on radiotherapy for breast cancer and asked to specify whether they preferred hypofractionation (≥ 2.1 Gy per fraction), conventional fractionation (1.8-2.0 Gy per fraction), or both. Those who selected both were asked to specify the proportion of patients for whom hypofractionation was preferred. Those who selected hypofractionation were asked to specify the dose per fraction and the number of fractions used. The clinical scenarios included the following: (1) node-negative disease after BCS, (2) node-negative disease after mastectomy, (3) node-positive disease after BCS, (4) node-positive disease after mastectomy, and (5) palliative symptom control. Respondents were asked to indicate the justifications and barriers that supported their decision.
Descriptive statistics were reported as counts and proportions. Analyses were reported for the full sample and were stratified by World Bank income groups on the basis of respondents' country of practice. The 2019 World Bank income group and regional classification system were used for this analysis.15 The association between justifications and barriers to hypofractionation and income group was assessed using the Fisher's exact test. Univariate and multivariate logistic regression analyses were performed to determine the factors predictive of hypofractionation by clinical scenario. All results from these models are presented as odds ratios with 95% CIs. The covariates included all demographic and practice characteristics of respondents collated in the survey. For this analysis, a hypofractionation user was defined as a respondent who preferred hypofractionation or both in ≥ 75% of their patients.14
A P value of < .05 was considered statistically significant, and all statistical tests were two-sided. All analyses were conducted using the R statistical environment, version 3.5.2 (R Foundation for Statistical Computing). The study received institutional review board exemption.
RESULTS
A total of 2,316 radiation oncologists completed the survey, of which 1,928 respondents treated at least one breast cancer case per month. Of the respondents who treated breast cancer, 494 did not respond to the breast-specific survey questions. The final sample size was 1,434 (breast practitioner survey response rate 74%), of which 890 respondents (62%) were from HICs, 361 (25%) from UMICs, and 183 (13%) from LLMICs. Because of the distribution method, an overall survey response rate could not be estimated. Most respondents reported access to linear accelerators (94%) and advanced radiotherapy planning techniques such as IMRT (87%). Full sample characteristics are shown in Table 1; characteristics of the excluded respondents are shown in the Data Supplement.
TABLE 1.
Characteristics of Respondents

Utilization of hypofractionation for each clinical scenario by income group region is shown in Figure 1. After BCS for node-negative disease, respondents in HICs were significantly more likely to report hypofractionation as their preferred fractionation compared with UMICs and LLMICs (75% from HICs, 52% from UMICs, and 48% from LLMIC; P < .001). There was greater preference for hypofractionation by physicians in LLMICs after mastectomy for node-positive disease than in HICs or UMICs (26% in HICs, 14% in UMICs, and 31% in LLMICs; P < .001). There was high overall utilization of hypofractionation for palliation (92% in HICs, 83% in UMICs, and 82% in LLMIC; Fig 1).
FIG 1.
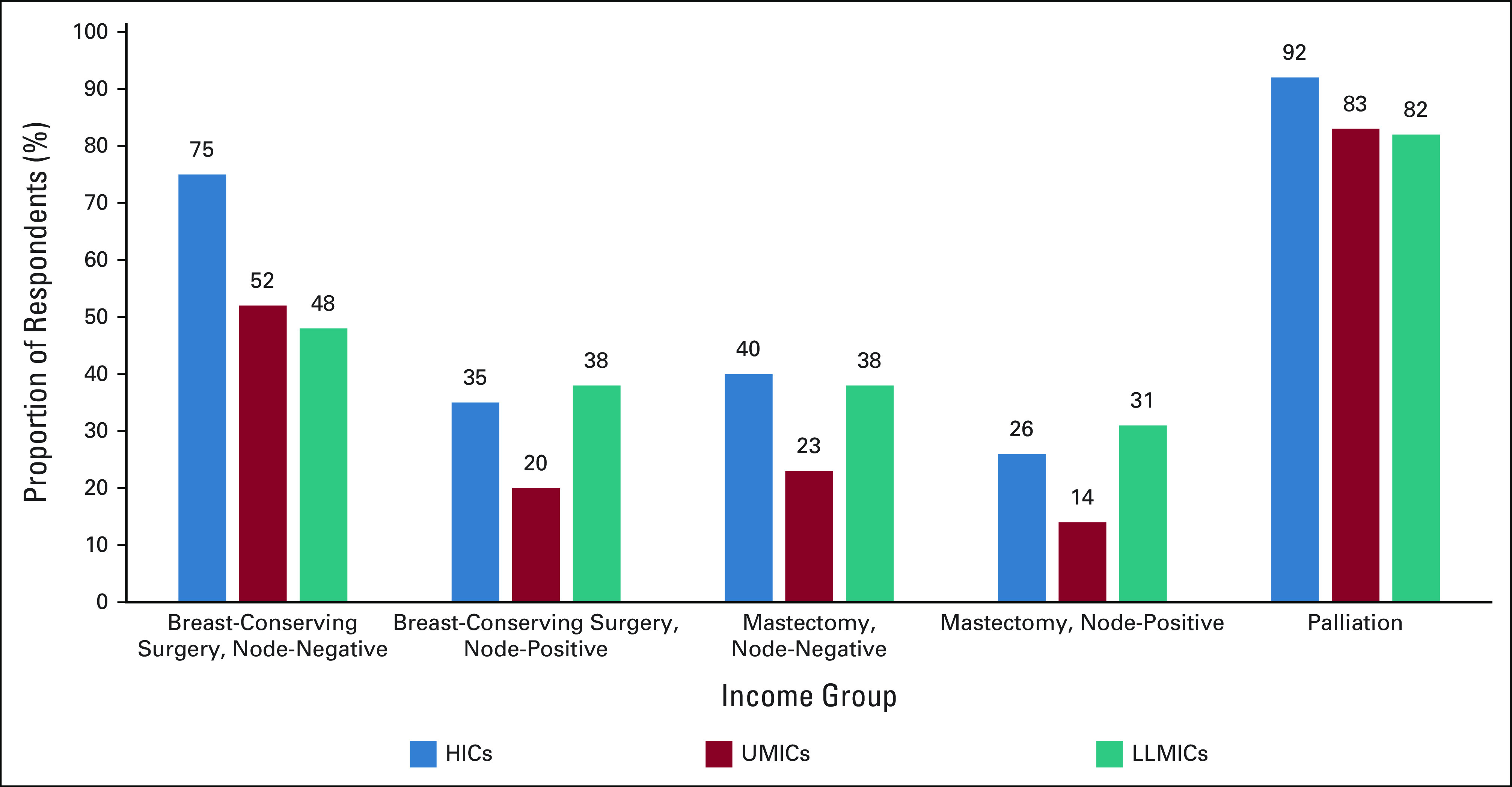
Hypofractionation refers to respondents who selected hypofractionation as their preferred fractionation scheme overall or in >75% of patients. All comparisons are statistically significant with P < .001. HICs, high-income countries; LLMICs, low- and lower-middle income countries; UMICs, upper middle-income countries.
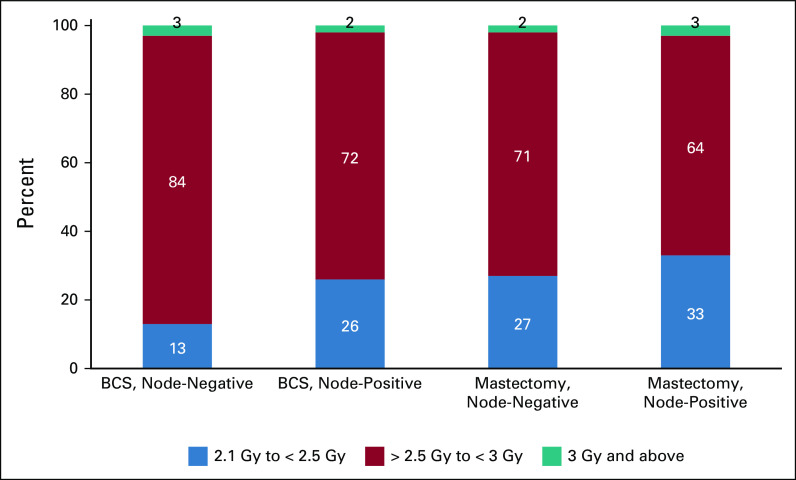
In the curative clinical scenarios, the most frequently preferred fraction dose was between 2.1 and < 2.5 Gy, which corresponded with 15 or 16 fractions (Fig 2). No respondents reported use of ultra-hypofractionation schedules (eg, five fractions). More heterogeneity was observed in the palliative dose regimens, with doses between 3 and < 4.0 Gy being the most frequently preferred by 46% of respondents.
FIG 2.
Preferred hypofractionation dose per fraction by clinical scenario. BCS, breast-conserving surgery.
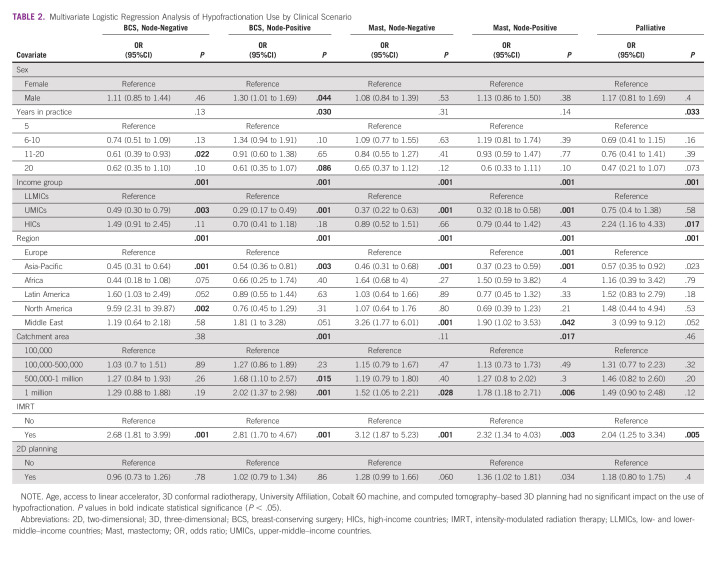
The results of the multivariable logistic regression analyses are presented in Table 2; univariable regression analyses are shown in the Data Supplement. Compared with respondents in LLMICs, those in UMICs were significantly less likely to prefer hypofractionation across all curative scenarios, with no significant differences in the palliative setting. Regionally, respondents in Asia-Pacific were significantly less likely to hypofractionate across curative scenarios, compared with respondents in Europe, with no differences in palliation. Respondents from North America were significantly more likely to hypofractionate for scenario 1, with no significant differences across other clinical scenarios. By contrast, those in the Middle East were significantly more likely to hypofractionate after mastectomy (scenarios 3 and 4) and for palliation. Respondents in large catchment areas and those with IMRT availability were significantly more likely to hypofractionate, with no significant differences among those using 2D planning.
TABLE 2.
Multivariate Logistic Regression Analysis of Hypofractionation Use by Clinical Scenario
Respondents were asked to report their perceived justifications and barriers to hypofractionation, which was stratified by the World Bank income group (Table 3). Published evidence was the most cited facilitator for hypofractionation overall but was cited more frequently in HICs (94%) compared with UMICs (81%) and LLMICs (79%; P < .001). Reimbursement was reported by only 5%, with no significant differences between income groups. Patient and provider preference, acceptance among peers, and convenience were more prominent in HICs than in other income groups (P < .001). Lack of long-term data and concerns about both acute and late toxicities were commonly reported barriers to hypofractionation and were reported more frequently in UMICs compared with other income groups (Table 3).
TABLE 3.
Facilitators and Barriers to Hypofractionation
DISCUSSION
In this large, international survey of hypofractionation, with a specific focus on breast cancer radiotherapy, hypofractionation was widely accepted for palliation, but significant differences were observed in its acceptability across adjuvant curative indications and across resource settings, with significantly lower reported use in UMICs. Preference for hypofractionation was lower in the setting of chest wall or node-positive radiation, where concerns about worse acute and late toxicities and perceptions of insufficient long-term data limited adoption.
The highest rates of hypofractionation were observed in early-stage disease after BCS, but 25% of respondents from HICs and half of the respondents from UMICs and LLMICs still preferred conventional fractionation. Respondents with IMRT had 2.68 greater odds of using hypofractionation (95% CI, 1.81 to 3.99; P < .001), with similar findings observed across all scenarios. In the postmastectomy setting, respondents from UMICs were significantly less likely to prefer hypofractionation, compared with respondents from LLMICs or HICs. These findings are consistent with another European survey, which found that overall rates of postmastectomy hypofractionated radiation were approximately 30% and that its use was less common in Eastern Europe,16 where the majority of European UMICs are located.
In our adjusted analyses, respondents from Asia-Pacific were significantly less likely than European respondents to hypofractionate overall, but significantly higher reported preferences were observed in the Middle East postmastectomy, with no significant differences between Africa and Latin America. This may reflect the heterogeneity of the regions, which include countries across a wide range of gross national incomes with differing resource constraints and practice patterns. Although variation in worldwide radiotherapy availability has been found to correspond to regional income level, previous work has demonstrated large variations in gross national incomes per capita for different countries in each of the geographic regions.17
Notably, respondents from LLMICs were the most likely to prefer hypofractionation in the node-positive setting. Because of advanced stage at presentation and the high rates of mastectomy in LLMICs, these scenarios are most commonly encountered by those respondents in routine practice.1,18-21 The moderate hypofractionation study by Wang et al8 included a small proportion of node-positive patients, and the results of currently open trials for node-positive patients may improve overall acceptability of hypofractionation for node-positive disease over time.11 Furthermore, in the DCBG HYPO trial, 42% of patients received chemotherapy, with no increase in toxicity, providing further support for hypofractionation in high-risk patients.22
Published evidence was the most cited facilitator of hypofractionation adoption across all income groups. Many of the practice changing trials for hypofractionation5-7 were conducted in patients after BCS, and many had node-negative disease. The well-established evidence base in low-risk disease is reflected in the higher overall preference for hypofractionation reported by survey respondents in this scenario. However, the differences between income groups and regions, as observed in BCS node-negative, where there was a 50% higher preference for hypofractionation in HICs compared with UMICs and LLMICs, may also reflect the fact that most trials for this clinical scenario were conducted in Europe and North America in HICs. A randomized controlled trial of moderate hypofractionation published by Wang et al8 in China, where uptake of hypofractionation was reported to be as low as 12%, may help to improve evidence diffusion in that region. Radiotherapy research seldom originates from LLMICs,23 and there are limited opportunities for clinicians from these regions to conduct studies in their practice settings with patients who are more representative of those they encounter in routine practice. The Cervical Cancer Research Network (Gynecologic Cancer Intergroup) has been making great advances in this area by promoting clinical trial participation and initiation in low-resource settings. This model could also be applied in breast cancer care.24
Peer acceptance was cited as a facilitator for hypofractionation by 35% of respondents although it was reported by twice as many respondents from HICs as LLMICs and UMICs. In many HICs, peer acceptance and the building of consensus around hypofractionation have been promoted within practice groups through the implementation of clinical protocols that indicate a preference for hypofractionation25 and by implementing utilization management strategies such as peer review or payer restrictions.26 A US study on payer restriction, in which claims for conventional radiotherapy were not reimbursed for patients eligible for hypofractionation, was associated with both direct and spillover increases in the use of hypofractionated radiotherapy.26 In Europe, a study by Prades et al25 in the Public Health Service in Spain found that utilization of hypofractionation for breast cancer ranged between 9% and 75% in 2015 and that factors such as endorsement of hypofractionation by department heads and the inclusion of hypofractionation in clinical protocols as a preferred regimen strongly influenced uptake. In LLMICs, and particularly in Africa, there are often fewer radiation oncologists in the country with reduced opportunities for peer audit and review.27 Initiatives such as the International Atomic Energy Agency's regional virtual tumor board projects through their Regional Cooperative Agreements have attempted to address this barrier.28 Patient preference was also more commonly cited in HICs than in UMICs or LLMICs, which may reflect cultural differences in the nature of the patient-provider relationship and the lack of decisional aids to support patients undergoing adjuvant radiotherapy and can help to facilitate shared decision making.29,30
Reimbursement was cited as a barrier to hypofractionation by only a minority of respondents although no in-depth evaluation of the health system reimbursement methods was conducted in this survey. In Europe, for example, most radiotherapy reimbursement systems are still fee-for-service–based and fractionation-driven, which may pose an important hurdle on the adoption of hypofractionated treatment schemes.31 A survey of an international group of radiation oncologists from 13 countries reported that adoption of moderately hypofractionated radiotherapy for breast cancer would result in a financial loss of up to 40%, depending on the provider and setting.32 Recognizing the important recent trend toward ultra-hypofractionated radiotherapy in breast cancer, as in other common tumor types such as prostate, recommendations have been made to develop reimbursement systems supporting this evolution, such as episode-based payment models.33 A large proportion of respondents in the present survey identified hypofractionation as a mechanism for promoting more efficient use of resources. Whether hypofractionation ultimately lowers health system costs depends on whether excess capacity created by hypofractionation can be repurposed toward other patients.34
Less than 1% of physicians used ultra-hypofractionation in the curative setting although the survey was distributed before COVID-19 pandemic and the publication of the Fast-Forward Trial, which demonstrated the noninferiority at 5 years of 40 Gy in 15 fractions and 26 Gy in five fractions in early-stage breast cancer after BCS.9 The Fast-Forward publication coincided with the first wave of the COVID-19 pandemic, which catalyzed adoption of this regimen. Several international guidelines published by professional societies, experts, and institutions further promoted the adoption of hypofractionation, including one-week regimens, to minimize viral exposure through reduced hospital visits.35-41 In that regard, ESTRO recently published recommendations for the adoption of ultra-hypofractionation (five fractions) for non-nodal breast or chest wall (without reconstruction) radiotherapy either as standard of care or within a randomized trial or prospective cohort.42
Limitations of this study include possible selection bias as the survey was administered by convenience sampling through professional society membership databases, which may lead to over- or under-representation of groups from specific regions or income levels. Furthermore, 25% of the respondents who indicated that they treated breast cancer did not respond to the breast scenario questions and were therefore excluded from the analysis. Since a higher proportion of these excluded respondents were from LMICs, this may also bias the results. This survey-based study also measured reported preferences, which was not correlated with utilization. To encourage a high completion rate, it was not possible to address all nuances of hypofractionation use in the clinical scenarios surveyed. Although provider comfort with hypofractionation might have evolved during the COVID-19 pandemic and with the publication of new trials such as FAST-Forward,9 these results provide important global benchmark data. These data will facilitate evaluation of the impact of recent consensus statements published by ESTRO and the American Society of Radiation Oncology and African expert groups on breast hypofractionation.42-44 Similarly, the use of conventional fractionation for early-stage breast cancer was included in the recent publications of Indian and African Choosing Wisely guidelines, which reflect consensus statements on low-value clinical practices that should be avoided.44-47
In conclusion, the study demonstrated significant variability in the preference of hypofractionation in breast cancer across World Bank income groups for curative indications, with a lack of long-term evidence cited as the most common barrier to uptake. Targeted interventions tailored to different resource settings, such as through ESTRO, the Federation of Asian Organisations of Radiation Oncologists, Southeast Asia Radiation Oncology group, and African Organisation of Research and Training in Cancer, may be necessary to increase evidence-based adoption of hypofractionation for breast cancer. Inclusivity in multi-institutional radiation oncology clinical trials by supporting the accreditation of centers from diverse income groups may further promote knowledge diffusion and guideline implementation.
ACKNOWLEDGMENT
The authors would like to thank Chiara Gasparotto and Gabriella Axelsson (ESTRO) for their support in administering the survey. CG and GA were employees of ESTRO, but ESTRO did not participate in the study design, data analysis, data interpretation, or writing of the report. The authors also gratefully acknowledge the fellowship support received by DR from the Canadian Association of Radiation Oncology, the Royal College of Physicians and Surgeons of Canada, and The Commonwealth and the support received by SG from the Mentored Patient Oriented Career Research Development Award (1-K08CA230170-01A1) during the conduct of this study.
Osama Mohamad
Research Funding: Salesforce
Fabio Y. Moraes
Honoraria: AstraZeneca Canada
Consulting or Advisory Role: Elekta
Surbhi Grover
Honoraria: Varian Medical Systems
Consulting or Advisory Role: GenesisCare
Research Funding: Varian Medical Systems
Danielle Rodin
Consulting or Advisory Role: Need Inc
No other potential conflicts of interest were reported.
DISCLAIMER
None of these organizations had a role in study design, data collection, data analysis, data interpretation, or writing of the report. There was no funding source for this study. ESTRO provided logistical support to perform the survey. The corresponding author had full access to all the data and final responsibility for the decision to submit fo publication.
PRIOR PRESENTATION
Presented at the Canadian Association of Radiation Oncology (CARO) Annual Scientific Meeting, virtual, September 28-October 1, 2021; the European Society of Radiotherapy & Oncology (ESTRO) Congress, Madrid, Spain, Sept 27-31, 2021; and the American Society for Radiation Oncology (ASTRO) Annual Meeting, Chicago, IL, October 24-27, 2021.
AUTHOR CONTRIBUTIONS
Conception and design: Melinda Mushonga, Anna-Mary Nyakabau, Osama Mohamad, Bouchra Tawk, Fabio Y. Moraes, Mei Ling Yap, Eduardo Zubizarreta, Yolande Lievens, Danielle Rodin
Collection and assembly of data: Melinda Mushonga, Osama Mohamad, Bouchra Tawk, Surbhi Grover, Eduardo Zubizarreta, Danielle Rodin
Data analysis and interpretation: Melinda Mushonga, Jessica Weiss, Zhihui Amy Liu, Osama Mohamad, Bouchra Tawk, Fabio Y. Moraes, Surbhi Grover, Mei Ling Yap, Eduardo Zubizarreta, Danielle Rodin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Osama Mohamad
Research Funding: Salesforce
Fabio Y. Moraes
Honoraria: AstraZeneca Canada
Consulting or Advisory Role: Elekta
Surbhi Grover
Honoraria: Varian Medical Systems
Consulting or Advisory Role: GenesisCare
Research Funding: Varian Medical Systems
Danielle Rodin
Consulting or Advisory Role: Need Inc
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wahab M, Gondhowiardjo SS, Rosa AA, et al. : Global radiotherapy: Current status and future directions-white paper. JCO Glob Oncol 7:827-842, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atun R, Jaffray DA, Barton MB, et al. : Expanding global access to radiotherapy. Lancet Oncol 16:1153-1186, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Borras JM, Lievens Y, Dunscombe P, et al. : The optimal utilization proportion of external beam radiotherapy in European countries: An ESTRO-HERO analysis. Radiother Oncol 116:38-44, 2015 [DOI] [PubMed] [Google Scholar]
- 5.START Trialists' Group; Bentzen SM, Agrawal RK: The UK Standardisation of Breast Radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol 9:331-341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentzen SM, Agrawal RK, Aird EGA, et al. : The UK Standardisation of Breast Radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 371:1098-1107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffty BG: Long-term results of hypofractionated radiation therapy for breast cancer. Breast Dis 21:267-268, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Wang SL, Fang H, Song YW, et al. : Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 20:352-360, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Murray Brunt A, Haviland JS, Wheatley DA, et al. : Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 395:1613-1626, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunt AM, Haviland JS, Sydenham M, et al. : Ten-year results of fast: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol 38:3261-3272, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ontario Clinical Oncology Group (OCOG) . Hypofractionated LocoRegional Radiotherapy in Breast Cancer [Internet]. OCOG-2019-RHEAL. 2020. https://clinicaltrials.gov/ct2/show/NCT04228991
- 12.Jacobson G, Galper SL, Shahadi ID, et al. : Palliative breast radiation—Effectiveness, fractionation, and toxicity. Int J Radiat Oncol 99:S6-S7, 2017 [Google Scholar]
- 13.Monten C, Lievens Y: Adjuvant breast radiotherapy: How to trade-off cost and effectiveness? Radiother Oncol 126:132-138, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Rodin D, Tawk B, Mohamad O, et al. : Hypofractionated radiotherapy in the real-world setting: An international ESTRO-GIRO survey. Radiother Oncol 157:32-39, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Bank : World Bank list of economies (June 2018). World Bank Country and Lending Groups. 2018. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 16.Ratosa I, Chirilă ME, Steinacher M, et al. : Hypofractionated radiation therapy for breast cancer: Preferences amongst radiation oncologists in Europe—Results from an international survey. Radiother Oncol 155:17-26, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Zubizarreta E, Van Dyk J, Lievens Y: Analysis of global radiotherapy needs and costs by geographic region and income level. Clin Oncol 29:84-92, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Fadelu T, Damuse R, Lormil J, et al. : Patient characteristics and outcomes of nonmetastatic breast cancer in Haiti: Results from a retrospective cohort. Oncologist 25:1372-1381, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadzatsa W, Ndarukwa-Jambwa S: Breast cancer treatment in resource constrained countries: A Zimbabwean perspective. Curr Breast Cancer Rep 11:170-174, 2019 [Google Scholar]
- 20.Chipidza FE, Mushonga M, Kanda C, et al. : Utilization and predictors of postmastectomy radiation receipt in an Oncology Center in Zimbabwe. Breast Cancer Res Treat 189:701-709, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Elmore SNC, Mushonga M, Iyer HS, et al. : Breast cancer in Zimbabwe: Patterns of care and correlates of adherence in a national referral hospital radiotherapy center cohort from 2014 to 2018. Cancer Med 10:3489-3498, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offersen BV, Alsner J, Nielsen HM, et al. : Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: The DBCG HYPO trial. J Clin Oncol 38:3615-3625, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal A, Lewison G, Rodin D, et al. : Radiation therapy research: A global analysis 2001-2015. Int J Radiat Oncol Biol Phys 101:767-778, 2018 [DOI] [PubMed] [Google Scholar]
- 24.McCormack M, Gaffney D, Tan D, et al. : The Cervical Cancer Research Network (Gynecologic Cancer InterGroup) roadmap to expand research in low- and middle-income countries. Int J Gynecol Cancer 31:775-778, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prades J, Algara M, Espinàs JA, et al. : Understanding variations in the use of hypofractionated radiotherapy and its specific indications for breast cancer: A mixed-methods study. Radiother Oncol 123:22-28, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Parikh RB, Fishman E, Chi W, et al. : Association of utilization management policy with uptake of hypofractionated radiotherapy among patients with early-stage breast cancer. JAMA Oncol 6:839-846, 2020. doi: 10.1001/jamaoncol.2020.0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderpuye V, Hammad N, Martei Y, et al. : Cancer care workforce in Africa: Perspectives from a global survey. Infect Agent Cancer 14:11-18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Atomic Energy Agency : International Conference on Advances in Radiation Oncology ICARO2 (RDHR-2020). 2020. http://www-pub.iaea.org/iaeameetings/50815/International-Conference-on-Advances-in- Radiation-Oncology-ICARO2
- 29.Stacey D, Samant R, Bennett C: Decision making in oncology: A review of patient decision aids to support patient participation. CA Cancer J Clin 58:293-304, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Steffensen KD, Vinter M, Crüger D, et al. : Lessons in integrating shared decision-making into cancer care. JCO Oncol Pract 14:229-235, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Lievens Y, Defourny N, Corral J, et al. : How public health services pay for radiotherapy in Europe: An ESTRO-HERO analysis of reimbursement. Lancet Oncol 21:e42-e54, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Marta GN, Ramiah D, Kaidar-Person O, et al. : The financial impact on reimbursement of moderately hypofractionated postoperative radiation therapy for breast cancer: An international consortium report. Clin Oncol 33:322-330, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Borras JM, Corral J, Aggarwal A, et al. : Innovation, value and reimbursement in radiation and complex surgical oncology: Time to rethink. Radiother Oncol 169:114-123, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Spencer K, Defourny N, Tunstall D, et al. : Variable and fixed costs in NHS radiotherapy; consequences for increasing hypo fractionation. Radiother Oncol 166:180-188, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Braunstein LZ, Gillespie EF, Hong L, et al. : Breast radiation therapy under COVID-19 pandemic resource constraints—Approaches to defer or shorten treatment from a comprehensive cancer center in the United States. Adv Radiat Oncol 5:582-588, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson DJ, Yom SS, Saeed H, et al. : Radiation fractionation schedules published during the COVID-19 pandemic: A systematic review of the quality of evidence and recommendations for future development. Int J Radiat Oncol Biol Phys 108:379-389, 2020. doi: 10.1016/j.ijrobp.2020.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson W, Kamwa F, Samba R, et al. : Hypofractionated radiotherapy in African cancer centers. Front Oncol 10:618641, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Rashdan A, Roumeliotis M, Quirk S, et al. : Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: Hypo-fractionation and accelerated partial breast irradiation to address World Health Organization recommendations. Adv Radiat Oncol 5:575-576, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coles CE, Aristei C, Bliss J, et al. : International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol 32:279-281, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch CA, Lee G, Liu ZA, et al. : Rapid adaptation of breast radiation therapy use during the coronavirus disease 2019 pandemic at a large academic cancer center in Canada. Adv Radiat Oncol 5:749-756, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meattini I, Becherini C, Boersma L, et al. : European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol 23:e21-e31, 2022 [DOI] [PubMed] [Google Scholar]
- 42.Smith BD, Bellon JR, Blitzblau R, et al. : Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 8:145-152, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Kochbati L, Vanderpuye V, Moujahed R, et al. : Cancer care and COVID-19: Tailoring recommendations for the African radiation oncology context. Ecancermedicalscience 14:1144-1148, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loblaw DA, Prestrud AA, Somerfield MR, et al. : ASTRO choosing wisely list. Int J Radiat Oncol Biol Phys 14:1086-1094, 2018 [Google Scholar]
- 45.American Geriatrics Society : Ten things physicians and patients should question. Am Geriatr Soc 2013:21-24, 2015 [Google Scholar]
- 46.Rubagumya F, Mitera G, Ka S, et al. : Choosing wisely Africa: Ten low-value or harmful practices that should be avoided in cancer care. JCO Glob Oncol 6:1192-1199, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pramesh CS, Chaturvedi H, Reddy VA, et al. : Choosing wisely India: Ten low-value or harmful practices that should be avoided in cancer care. Lancet Oncol 20:e218-e223, 2019 [DOI] [PubMed] [Google Scholar]