Abstract
Background
Recycling tenofovir and lamivudine/emtricitabine with dolutegravir (TLD) after failure of non-nucleoside transcriptase inhibitor (NNRTI) first-line antiretroviral therapy (ART) is more tolerable and scalable than dolutegravir plus optimized nucleoside reverse transcriptase inhibitors. Studies have demonstrated TLD’s efficacy as second-line, but long term follow-up is limited.
Methods
ARTIST is a single arm, prospective, interventional study conducted in Khayelitsha, South Africa, which switched 62 adults with two viral loads (VL) >1000 copies/mL from tenofovir, lamivudine/emtricitabine and an NNRTI to TLD. We report efficacy to 72 weeks and, in a post hoc analysis, evaluated VL trajectories of individuals with viraemic episodes.
Results
Virologic suppression was 86% (95% Confidence Interval (CI) 74-93), 74% (95% CI 61-84) and 75% (95% CI 63-86) <50 copies/mL, and 95%, 84% and 77% <400 copies/mL at week 24, 48 and 72 respectively, with 89% (50/56) resistant (Stanford score ≥15) to tenofovir and/or lamivudine pre-switch. No participants developed integrase-inhibitor resistance. Of the 20 participants not suppressed at week 24 and/or 48, two developed virologic failure, one switched regimen (adverse event), two were lost to follow-up, one missed the visit, one transferred out, nine resuppressed <50 copies/mL with enhanced adherence counselling and four remained viraemic (three with <200 copies/mL) at week 72.
Conclusions
Recycling NRTIs with dolutegravir was effective for most participants to 72 weeks. Most with viraemia did not develop virologic failure and subsequently suppressed with enhanced adherence counselling or continued to have low-level viraemia. No integrase-inhibitor resistance was detected despite low-level viraemia in a minority of participants.
Keywords: TLD, dolutegravir, recycling-NRTIs, low-level viraemia, second-line antiretroviral therapy
Introduction
The DAWNING study showed that dolutegravir was superior to ritonavir-boosted lopinavir after failing first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy (ART)1. In both arms, the backbone was dual nucleoside reverse transcriptase inhibitors (NRTI) with at least one NRTI fully active on resistance testing1, which led the World Health Organization to recommend a second-line regimen of zidovudine, lamivudine and dolutegravir (ALD) after failing a first-line NNRTI-based regimen with tenofovir plus lamivudine or emtricitabine (XTC) in settings with limited access to antiretroviral resistance testing2. This recommendation for the zidovudine and lamivudine NRTI backbone is based on the rationale that the resistance mutation selected for by tenofovir (K65R), does not compromise the activity of zidovudine3.
Recent evidence has emerged that recycling tenofovir and XTC with dolutegravir (TLD) in second-line ART, after failure of first-line NNRTI-based ART, is efficacious despite high rates of resistance to both tenofovir and XTC4,5. Such a strategy would be beneficial to both patients and the health system as tenofovir is better tolerated than zidovudine, and TLD is available as a single fixed dose tablet taken once daily while ALD requires twice daily dosing of zidovudine and lamivudine 6.
We have previously reported 24-week results of a single-arm interventional study of TLD as second-line ART (AntiRetroviral Therapy In Second-line: investigating Tenofovir-lamivudine-dolutegravir [ARTIST] trial), with 85% (51/60) achieving the primary end point of VL <50 copies/mL despite substantial baseline resistance to one or both of tenofovir and XTC (88.9%, 48/54). No participants developed study-defined virologic failure and no integrase resistance was detected in the one participant meeting criteria for resistance testing4.
The NADIA trial found similar results after randomising participants failing first-line ART consisting of an NNRTI, tenofovir, and XTC, first to darunavir or dolutegravir, and second to tenofovir or zidovudine with lamivudine. Dolutegravir was non-inferior to darunavir at 48 and 96 weeks5,7, and recycling tenofovir was non-inferior at 48 weeks5 and superior at 96 weeks7 to switching to zidovudine. This was achieved despite more than half of the participants having no fully active NRTI on resistance testing in the tenofovir arm5. In the VISEND trial, TLD or a regimen of dolutegravir with tenofovir alafenamide and emtricitabine, were both superior to boosted protease inhibitor regimens with zidovudine and lamivudine at week 48, with virologic suppression of 82%, 87% and 76% respectively8.
The efficacy of second-line TLD in the absence of a fully active NRTI is likely due to dolutegravir’s high genetic barrier to resistance coupled with a cost in fitness from NRTI resistance mutations9. However, the development of integrase inhibitor resistance has been described in clinical trials1,7,10–12 and post-market programmatic settings13,14. In the NADIA trial, three of the nine cases of treatment-emergent dolutegravir resistance occurred in the TLD group7. In a Malawian observational study following patients transitioning to TLD with a raised VL (n=101), dolutegravir resistance developed in two patients who had virologic failure on second-line TLD14.
Emergence of dolutegravir resistance in patients on second-line TLD could jeopardise the public health benefits that the combination potentially offers in lower and middle income country programmes. It is unclear whether the efficacy of TLD in those who have failed first-line NNRTI-based therapy is durable, particularly in those who develop viraemia, making longer follow-up crucial to inform treatment policy decisions15. Here we report follow-up results of the ARTIST trial participants to 72 weeks to evaluate the maintenance of the virologic suppression on second-line TLD. Additionally, we conducted a post hoc descriptive analysis of the VL trajectories of those who had episodes of viraemia.
Methods
Study design
We conducted a single arm, prospective, interventional study in two primary care clinics in Khayelitsha, South Africa. This study was designed to evaluate the virologic efficacy of TLD in second-line ART.
A full description of the study design was published with the week 24 primary endpoint results4. The protocol was approved by the University of Cape Town’s Human Research Ethics Committee (039/2019) and is available with the statistical analysis plan on ClinicalTrials.gov (NCT03991013). Adults who had virologic failure (defined as two consecutive VL >1000 copies/mL, 2-24 months apart) on a first-line regimen consisting of tenofovir, XTC and efavirenz or nevirapine were invited to participate. Exclusion criteria were CD4 cell count less than 100 cells/ml, active or suspected tuberculosis, active AIDS-defining conditions, an estimated glomerular filtration rate less than 50 ml/min per 1.73m2, haemoglobin less than 7.5 g/dl, alanine aminotransferase greater than 100 IU/l, a previous or current diagnosis of malignancy or any condition judged to put the patient at increased risk if participating, a condition judged likely to impact adherence (active psychiatric disease or substance abuse), pregnancy, breastfeeding, intention to fall pregnant or unable to take the study medication (allergy, intolerance or contraindicated drug interaction). Women of child-bearing potential were required to be on effective contraception.
Participants were enrolled and switched to TLD with additional 50 mg dolutegravir for two weeks to overcome efavirenz induction effects on dolutegravir metabolism and transport. Study visits with clinicians occurred every four weeks to week 24, then at week 36, 48 and 72. VL was assessed at baseline and every subsequent visit, with a repeat VL after two weeks of enhanced adherence counselling if VL was >50 copies/mL after week 12. If the repeat VL was >500 copies/mL, a genotypic resistance test was performed per protocol. Baseline genotypic resistance testing was performed retrospectively for all participants and was not available to inform treatment decisions.
Outcomes and analysis
The primary outcome was VL suppression (defined as VL <50 copies/mL), evaluated according to the FDA snapshot algorithm16. We used a ± two-week visit window for the first 20 weeks, then a ± six-week visit window from week 24 onwards. The extended window was introduced to accommodate COVID-19 restrictions in accessing health facilities. We conducted a modified intention-to-treat analysis (mITT), excluding those switching study drug due to contraception cessation, wishing to become pregnant, pregnancy, non-clinical transfer out or death from non-HIV and non-drug causes (assessed by the study doctor). Failure included: VL ≥50 copies/mL, missing VL within the visit window or loss to follow up, intolerance or adverse event due to any drug in the regimen requiring switch.
Virological failure was defined as having two consecutive VL >1000 copies/mL after week 12. Genotypic resistance was classified using the Stanford algorithm (version 8.9-1), with a score ≥15 indicating at least low-level resistance. Results were categorised as two fully active NRTIs (both with a Stanford score <15), resistance to one NRTI (one with a Stanford score <15 and one ≥15) and resistance to both NRTIs (both with a Stanford score ≥15)17.
Virologic outcomes at week 24, 48 and 72 are presented as proportions with the corresponding exact binomial 95% confidence interval (CI). A pre-specified secondary analysis described VL suppression defined as <400 copies/mL. A sensitivity analysis of VL suppression excluded certain participants included in the mITT analysis: those lost to follow-up, missing a VL in the window and those who were changed from the study drug for reasons other than treatment failure. The pre-specified sensitivity analysis for the primary end-point of 24 weeks also excluded those with evidence of poor adherence at the visit (TFV-DP <350 fmol/punch), however these results were not available for the 48 and 72 week visits.
In a post hoc descriptive analysis, the VL trajectories of participants who were on TLD and had a VL ≥50 copies/mL at weeks 24 and/or 48 were graphed along with a narrative description of their baseline and follow-up resistance test results, reported adherence (taken from clinician notes) and missed visits (no visit within the window).
Results
Baseline characteristics
The baseline characteristics of the 62 participants enrolled between August 2019 and July 2020, are described in Table 1. Low, intermediate or high-level resistance to tenofovir, XTC or both was found in 89.3% (50/56 participants with results). Of these, 35.7% (20/56) had K65R mutations and 82.1% (46/56) had M184V/I mutations.
Table 1. Baseline characteristics of all participants.
| Baseline characteristics, n=62 | |
|---|---|
| Participant characteristics | |
| Female sex [n(%)] | 43 (69%) |
| Age (years) [median(IQR)] | 36.4 (30.9-46.0) |
| BMI (kg/m2) [median(IQR)] | 27.8 (22.6-32.4) |
| Weight (kg) [median(IQR)] | 71.2 (62.1-81.4) |
| HIV characteristics | |
| CD4 count (cells/μL) [median(IQR)] | 259 (175-380) |
| VL (copies/mL) [median(IQR)] | 9586 (2827-37243) |
| ART history | |
| ART duration (years) [median(IQR)] | 5.2 (2.8-8.2) |
| Prior exposure to stavudine or zidovudine [n(%)] | 10 (16%) |
| On efavirenz at enrolment [n(%)] | 61 (98%) |
| Baseline genotypic resistance* (n=56), [n(%)] | |
| NRTI genotypic resistance | |
| Two fully active NRTIs | 6/56 (11%) |
| Resistance to one NRTI | |
| - Tenofovir, not XTC | 0/56 (0%) |
| - XTC, not tenofovir | 14/56 (25%) |
| Resistance to both NRTIs | 36/56 (64%) |
| Efavirenz and/or nevirapine genotypic resistance | 54/56 (96%) |
ART (antiretroviral therapy); IQR (inter-quartile range); NRTI (nucleoside reverse transcriptase inhibitor); VL (viral load); XTC (lamivudine or emtricitabine).
Resistance classified using Stanford interpretation, where a score <15 indicates susceptible or potential low-level resistance to a drug, and ≥15 indicate low-level, intermediate, or high-level resistance to a drug. For this analysis resistance was defined as a score ≥15 17
Virologic outcomes at week 24, 48 and 72
The proportion of participants with virologic suppression (VL <50 copies/mL) decreased from week 24 to week 72 but was maintained above 70% across the mITT and sensitivity analyses. In the mITT analysis 75.4% of participants had a VL <50 copies/mL at 72 weeks. A sensitivity analysis examined virologic suppression in those with a VL result who remained on TLD throughout the study; at 72 weeks 83.6% had a VL <50 copies/mL in this analysis (Table 2).
Table 2. Viral load outcomes at week 24, 48 and 72.
| VL suppression <50copies/mL (n; percentage, 95% CI) |
VL suppression <400copies/mL (n; percentage, 95% CI) |
|||
|---|---|---|---|---|
| mITT analysis* | Sensitivity analysis** |
mITT analysis* | Sensitivity analysis** |
|
| Week 24 | 53/62; 85 (74-93) |
53/60; 88 (77-95) |
59/62; 95 (87-99) |
59/60; 98 (91-100) |
| Week 48 | 45/61; 74 (61-84) |
45/57; 79 (66-89) |
51/61; 84 (72-92) |
51/57; 89 (78-96) |
| Week 72 | 46/61; 75 (63-86) |
46/55 84 (71-92) |
47/61; 77 (65-87) |
47/55 85 (73-94) |
Modified intention-to-treat analysis (mITT) excludes those switching study drug for reasons of stopping contraception or wish to become pregnant, or becoming pregnant, transfer out for non-clinical reasons and death from non-HIV and non-drug causes.
Sensitivity analysis excludes those excluded from mITT analysis, as well as those lost to follow up, those missing a VL within the window and participants who stopped or were changed from the study drug for reasons other than failure of the regimen
The outcomes of those not suppressed at weeks 24, 48 and 72 are described in Table 3. At week 48, of the 16 participants not suppressed, 6/16 (38%), and 6/12 on TLD with VL results, had low level viraemia <200 copies/mL. At week 72, 5/15 (33%), and 5/9 of those on TLD with VL results, had VL <200 copies/mL.
Table 3. Outcomes of those not suppressed at the week 24, 48 and 72 visits.
| N(%) | N(%) | N(%) | |
|---|---|---|---|
| Not suppressed | 9/62 (15%) | 16/61 (26%) | 15/61 (25%) |
| Virologic failure (two consecutive VL >1000 copies/mL after 12 weeks) | 0/62 (0%) | 1/61 (2%): failed at week 36 |
2/61 (3%): failed at week 36 and week 52 |
| VL > 1000 copies/mL but did not meet the definition of failure | 0/62 (0%) | 3/61 (5%) | 0/61 (0%) |
| VL 400-999 copies/mL | 1/62 (2%) | 0/61 (0%) | 2/61 (3%) |
| VL 200-399 copies/mL | 0/62 (0%) | 2/61 (3%) | 0/61 (0%) |
| VL 100-199 copies/mL | 0/62 (0%) | 4/61 (7%) | 1/61 (2%) |
| VL 50-99 copies/mL | 6/62 (10%) | 2/61 (3%) | 4/61 (7%) |
| No VL data | 1/62 (2%): lost to follow-up at week 16 |
3/61 (5%): 1 missed a visit in the study window but still in the study, 2 lost to follow-up |
5/61 (8%): 3 missed a visit in the study window but still in the study, 2 lost to follow-up |
| Other | 1/62 (2%): switched due to an adverse event before 24 weeks |
1/61 (2%): switched due to an adverse event before 24 weeks |
|
Virologic failure
Two participants met criteria for virologic failure (two consecutive VL >1000 copies/mL after week 12) during the follow up period: one at 36 weeks and one at 52 weeks. Three participants were eligible for resistance testing and all three had resistance testing conducted (including the two participants with virological failure), but none were found to have integrase resistance mutations.
Suppression by baseline NRTI resistance
The relative risk of virologic suppression for those with any baseline NRTI resistance (Stanford score ≥15 for tenofovir, XTC or both3) compared to those with two active NRTIs at baseline was 0.84 (95% CI 0.74-0.95) for week 24, 0.91 (95% CI 0.61-1.34) for week 48 and 0.91 (85% CI 0.61-1.34) for week 72 (see Supplementary Figure 1 for proportion suppressed at each time point by baseline resistance to tenofovir, XTC or no resistance).
Follow up of participants not suppressed at week 24 and/or week 48
Nine participants were not suppressed (VL <50 copies/mL) at week 24 (Table 3). Following these participants forward over time, one participant developed virologic failure, four re-suppressed, two had a VL <200 copies/mL and one was lost to follow-up by week 48. One had already switched from TLD before week 24 due to an adverse event (renal impairment). By week 72, three of those who had re-suppressed remained suppressed and one had a VL 500-999 copies/mL, one of the two with a VL <200 copies/mL remained viraemic (VL of 70 copies/mL) and one re-suppressed (Supplementary Figure 2).
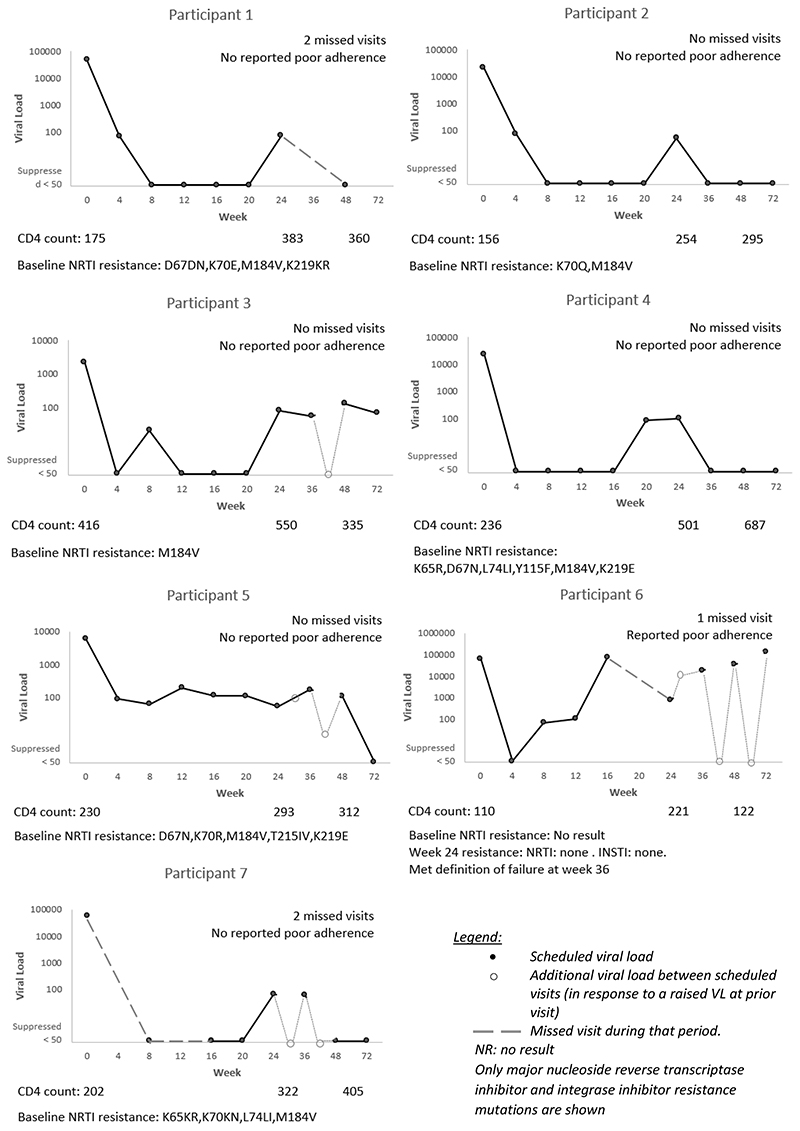
We plotted the VL trajectories for participants who were on TLD, had a VL conducted and had a result ≥50 copies/mL at week 24 and/or week 48. Figure 1 describes the VL trajectories for the seven participants who had a VL ≥50 copies/mL at week 24, excluding the participant who was not on TLD because they had switched due to an adverse event, and the participant who was classified as not suppressed due to a missing VL result at week 24.
Figure 1.
Individual trajectories of the seven participants on tenofovir, lamivudine and dolutegravir with a viral load result but not suppressed (VL ≥50 copies/mL) at week 24
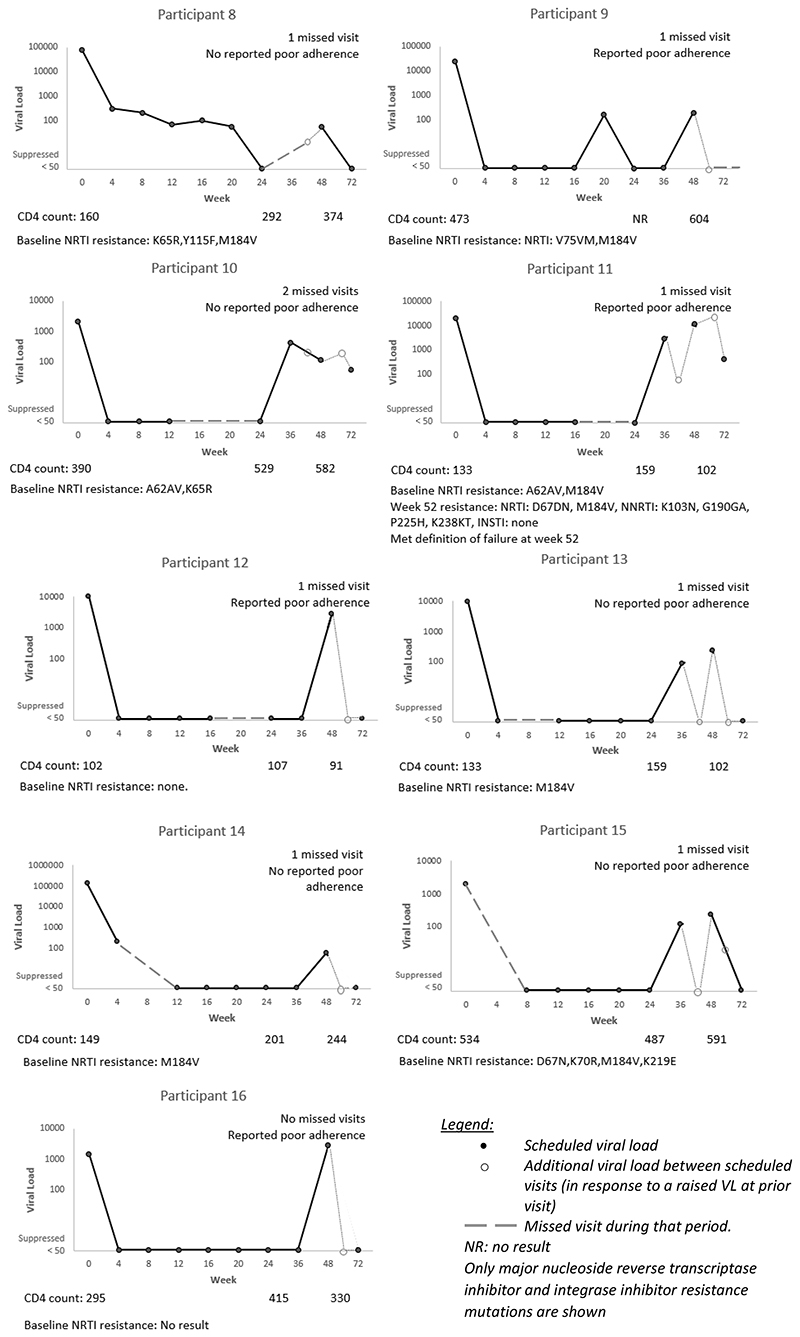
Of the nine not suppressed at week 24, four were also not suppressed at week 48. Sixteen participants in total were not suppressed at week 48 (Table 3). By week 72, two had developed virologic failure, seven re-suppressed, three had a VL <200 copies/mL, one had switched regimen and three did not have a VL at week 72 (two of these were lost to follow up). Figure 2 describes the VL trajectories of the nine participants who had a VL ≥50 copies/mL at week 48 (excluding the five who were not suppressed at week 24 as they are described in Figure 1, and excluding two who were classified as not suppressed due to a missing VL result at week 48).
Figure 2.
Individual trajectories of the nine participants on tenofovir, lamivudine and dolutegravir with a viral load result at week 48, who were suppressed at week 24 but not suppressed at week 48
Overall, of the 20 participants who had not been suppressed at week 24 and/or week 48 (four at week 24 only, five at both time points, and 11 at week 48), six had a VL ≥50 copies/mL, three were missing VL results, one had switched due to an adverse event and ten were suppressed <50 copies/mL at week 72 (Supplementary Figure 2).
Discussion
Our study demonstrated that virologic suppression is maintained above 70% to 72 weeks, despite almost 90% of participants having resistance to one or more NRTI drugs at baseline. The COVID-19 pandemic restricted access to facilities for many participants over the follow-up period: a sensitivity analysis that accounted for this and included only those on TLD with a VL result, also demonstrated high proportions with sustained virologic suppression: 88%, 79% and 84% with VL <50 copies/mL and 98%, 89% and 85% with VL <400 copies/mL at weeks 24, 48 and 72 respectively. Three quarters of the participants with episodes of viraemia re-suppressed to <50 copies/mL after enhanced adherence counselling, while two participants remained viraemic at low levels and only two went on to meet criteria for virological failure.
The outcomes we report are similar to the NADIA trial, where 92% were suppressed <400 copies/mL on a second-line regimen of dolutegravir or darunavir with a recycled tenofovir/lamivudine backbone at 48 and 96 weeks, and tenofovir/lamivudine was found to be superior to zidovudine/lamivudine at 96 weeks as a second-line backbone 5,7. Differences in suppression rates could be due to differences in patterns of adherence between the study populations. NADIA found suppression rates over 90% in those with no predicted NRTI activity5. We found a similar proportion suppressed in those with baseline NRTI resistance, and the Malawian observational study found no evidence for increased risk of viraemia or virological failure for participants with baseline NRTI resistance 14. This supports the conclusion from the NADIA trial that dolutegravir in combination with a recycled NRTI backbone is an effective second-line regimen. Most of our participants who were not suppressed had low-level viraemia (VL <200 copies/mL), and most of these resuppressed at later time points with enhanced adherence counselling. While there was little self-reported poor adherence, this may be due to a social desirability bias. Those with missed visits reflect gaps in medication on hand, leading to poor adherence. The NADIA trial also postulated that poor adherence was prevalent in their population5. We hypothesise that suboptimal adherence resulted in low-level viraemia and subsequent re-suppression with improved adherence.
Despite the substantial proportion with low-level viraemia, only two participants fulfilled the study definition of virological failure over 72 weeks and neither of these participants (nor an additional patient who qualified for genotype resistance testing) had integrase resistance mutations. Resistance has been identified in NADIA (three cases in the TLD group)7 and the Malawian observational study (two cases)14. The relatively low median baseline VL at switching and the small sample size in ARTIST may have contributed to our finding that no patients developed resistance to dolutegravir over 72 weeks. However, as resistance to dolutegravir has been shown in dolutegravir monotherapy trials to generally develop between 24 and 48 weeks10,11, these results are reassuring. This finding in participants using recycled tenofovir in the NRTI backbone, is important considering the high prevalence of tenofovir resistance in patients failing first-line ART in sub-Saharan Africa18. Cycling in and out of care with periods of viraemia is to be expected over a lifetime of ART 19,20. Thus, sustaining virologic suppression over time requires a regimen that is robust to the development of resistance despite fluctuating adherence. Dolutegravir has a particularly high barrier to resistance, comparable to protease inhibitors and much higher than efavirenz21. Low level viraemia has also been shown not to be associated with subsequent virologic failure in patients on a dolutegravir regimen, nearly half of whom had previous virologic failure22.
Dolutegravir with recycled tenofovir and lamivudine seems to fulfil this need for a tolerable, robust second-line regimen and could help in achieving the third UNAIDS target of 95% of those on treatment being virologically suppressed23. Low-level viraemia at the primary end-point did not predict later virologic failure in participants on first-line TLD in the ADVANCE study, resulting in a recommendation that the goal of treatment on dolutegravir regimens could be shifted to less stringent targets than a VL <50 copies/mL24. The low proportion of participants meeting criteria for virologic failure on TLD despite ongoing low-level viraemia in our study seems to support this recommendation.
The demonstrated efficacy of recycling tenofovir and lamivudine with dolutegravir in patients failing first-line NNRTI regimens in our study and the NADIA trial4,7 addresses two key concerns facing HIV services in this phase of the HIV pandemic: first, patients on second-line ART are in need of more tolerable regimens than the currently-recommended zidovudine/lamivudine/dolutegravir combination, and the fixed-dose, once-daily TLD provides a safe alternative to make treatment more acceptable and effective for individual patients.
Second, scale up of ART is complicated by resource constraints and many programmes are opting for universal TLD regimens, frequently without VL testing before switching patients due to high cost and low availability25,26. The growing evidence from this study, the NADIA trial, and other studies, suggests that switching all patients on NNRTI-based first line to TLD regardless of VL is effective and safe, with the caveat that monitoring and interventions to reduce the development of dolutegravir resistance should be implemented alongside widespread switching. This approach could facilitate less expensive and simpler strategies for wider TLD use 21.
Suboptimal adherence in a number of participants in this study did not result in virological failure with the development of integrase-inhibitor resistance mutations. This is compatible with dolutegravir’s high resistance barrier, though larger numbers and longer follow-up are needed to verify this. However, adherence challenges remain an issue regardless of regimen. While TLD represents a tolerable and robust second-line that may be helpful in improving adherence and outcomes in those who have previously failed treatment, individuals on this regimen will still benefit from adherence support27. Effective monitoring and management of adherence issues is required to sustain the population health benefits that TLD promises.
Limitations of this study include the higher proportion of missing VL results at week 48 and 72. COVID-19 reduced access to the primary care facilities where the study took place, and while we widened the visit window and used telephonic follow-up to mitigate this, the pandemic may still be responsible for missing VL data. Interpretation of our study is also limited by the small sample size, not having a control arm and lack of long-term therapeutic drug monitoring. While the frequent VL monitoring is not pragmatic for clinical practice, it allowed exploration of virologic trajectories following episodes of viraemia. A strength of our study is that it was embedded within a primary care ART clinical service, increasing generalisability
Conclusion
Most of our participants on a regimen of dolutegravir with a recycled tenofovir and lamivudine NRTI backbone were virologically suppressed at 72 weeks, despite substantial baseline NRTI resistance. Most of those who did not suppress had low-level viraemia or missed visits. Only two participants with raised VL developed virologic failure, and no participants were found to have developed dolutegravir resistance. With enhanced adherence counselling most participants re-suppressed or continued to have low-level viraemia. These findings, together with those of the NADIA trial and other studies, support the routine switching of all patients on tenofovir and NNRTI-based first-line therapy to TLD regardless of viral load, alongside measures to mitigate and detect the development of dolutegravir resistance. This would simplify rollout and make this effective, tolerable, and inexpensive regimen available to millions more patients.
Supplementary Material
Acknowledgements
We thank the patients who participated in this study and the staff at Ubuntu Clinic and Michael Mapongwana Community Health Centre for facilitating the recruitment and care of patients, and colleagues at the Western Cape Department of Health. We acknowledge other members of the study team for their hard work: Rene Goliath, Zimasa Gcwabe, Sydney Ncoko, Sihle Nqina, Zimkhitha Asare, Meagan McMaster, Ethel Saul, Antoinetta Mashinyira, Celeste Worship, Matilda Nduna and Lameze Witbooi, as well as Christine Kriel, Neide Cossa and Tasanya Chinsamy from Médecins Sans Frontières. We thank the data safety monitoring committee members for oversight of the study: Joseph Jarvis, David Meya, Daniela Garone and Catherine Orrell, as well as the trial steering committee: Richard Kaplan and Francois Venter. We thank the Médecins Sans Frontières Welcome Service team for their assistance with recruitment: Thembisa Makeleni-Leteze, Thembelihle Dutyulwa, Nombasa Dumile, Xoliswa Nxiba, Patiswa Shumane, Nokwanda Pani, Thando Kamati, Mthura Dutyulwa and Kirsten Arendse.
Funding
This research was funded in whole, or in part, by the Wellcome Trust (212265/Z/18/Z, 214321/Z/18/Z, and 203135/Z/16/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Footnotes
Conflicts of interest and sources of support and funding: This work was supported by the Wellcome Trust [212265/Z/18/Z] (GaM) and the Médecins Sans Frontières Khayelitsha project. The Wellcome Centre for Infectious Diseases Research in Africa is supported with core funding from the Wellcome Trust [203135/Z/16/Z]. GrM was supported by the Wellcome Trust [214321/Z/18/Z], and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa [Grant No 64787]. GaM received fees from ViiV Healthcare for a consultancy in 2018. TC was a contractor for Gilead Sciences during the time of this analysis. Funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding and last author had full access to all the data in the study and had final responsibility for the decision to submit for publication. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission.
Previous data presentation: The Southern African HIV Clinicians Society 24th International Workshop on HIV Drug Resistance and Treatment Strategies (September 2021) and the 5th Southern African HIV Clinicians Society Conference (October 2021)
Author contributions
Conceptualisation: Graeme Meintjes, Gary Maartens, Claire Keene, Rulan Griesel, Ying Zhao, Tali Cassidy
Data curation: Amanda Jackson, Rulan Griesel, Ying Zhao, Zaayid Omar, Kaneez Sayed
Formal analysis: Tali Cassidy, Claire Keene, Graeme Meintjes, Gary Maartens
Funding acquisition: Graeme Meintjes, Gary Maartens, Claire Keene, Eric Goemaere
Methodology: Graeme Meintjes, Gary Maartens, Claire Keene, Rulan Griesel, Ying Zhao, Gert van Zyl, Andrew Hill, Tali Cassidy, Tracy Flowers
Project administration: Ying Zhao, Rulan Griesel
Writing – original draft: Claire Keene, Tali Cassidy, Ying Zhao, Graeme Meintjes, Gary Maartens
Writing – review & editing: Rulan Griesel, Zaayid Omar, Kaneez Sayed, Andrew Hill, Olina Ngwenya, Amanda Jackson, Gert van Zyl, Tracy Flowers, Eric Goemaere
References
- 1.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–264. doi: 10.1016/S1473-3099(19)30036-2. [DOI] [PubMed] [Google Scholar]
- 2.The World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. 2019.
- 3.Stanford University. NRTI Resistance Notes. HIV Drug Resistance Database; [Accessed September 25, 2020]. Published 2016. https://hivdb.stanford.edu/dr-summary/resistance-notes/NRTI/ [Google Scholar]
- 4.Keene CM, Griesel R, Zhao Y, et al. Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line in adults failing a tenofovir-based first-line regimen: a prospective cohort study. AIDS. 2021;35(9):1423–1432. doi: 10.1097/QAD.0000000000002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N Engl J Med. 2021;385:330–341. doi: 10.1056/NEJMoa2101609. [DOI] [PubMed] [Google Scholar]
- 6.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, Emtricitabine, and Efavirenz vs. Zidovudine, Lamivudine, and Efavirenz for HIV. New England Journal of Medicine. 2006;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Musaazi J, Kityo C, et al. Efficacy and safety of dolutegravir or darunavir in combination with lamivudine plus either zidovudine or tenofovir for second-line treatment of HIV infection (NADIA): week 96 results from a prospective, multicentre, open-label, factorial, randomised, non. Lancet HIV. :1–13. doi: 10.1016/S2352-3018(22)00092-3. Published online 2022. [DOI] [PubMed] [Google Scholar]
- 8.Mulenga LB, Fwoloshi S, Mweemba A, et al. 135: Dolutegravir with recycles NRTIs is noninferior to PI-based ART: VISEND trial; Conference on Retroviruses and Opportunistic Infections (CROI 2022); February 12-16; 2022. p. 54. [Google Scholar]
- 9.Ross L, Parkin N, Lanier R. The number of HIV major NRTI mutations correlates directly with other antiretroviral-associated mutations and indirectly with replicative capacity and reduced drug susceptibility. AIDS Res Hum Retroviruses. 2008;24(4):617–620. doi: 10.1089/aid.2007.0188. [DOI] [PubMed] [Google Scholar]
- 10.Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4:e547–54. doi: 10.1016/S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 11.Hocqueloux L, Allavena C, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for HIV-1-infected virologically suppressed patients: Results from the randomized non-inferiority MONCAY trial; AIDS2018; 2018. [Google Scholar]
- 12.Lepik KJ, Harrigan PR, Yip B, et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS. 2017;31(10):1425–1434. doi: 10.1097/QAD.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 13.Fulcher JA, Du Y, Zhang TH, Sun R, Landovitz RJ. Emergence of Integrase Resistance Mutations during Initial Therapy Containing Dolutegravir; Conference on Retroviruses and Opportunistic Infections (CROI); 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schramm B, Temfack E, Descamps D, et al. Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study. Lancet HIV. 2022;9(8):e544–e553. doi: 10.1016/S2352-3018(22)00136-9. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. HIV Drug Resistance Report. 2021
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Human Immunodeficiency Virus-1 Infection : Developing Antiretroviral Drugs for Treatment Guidance for Industry Human Immunodeficiency Virus-1 Infection : Developing Antiretroviral Drugs for Treatment Guidance for Industry. 2015.
- 17.Stanford University. DRM penalty scores and resistance interpretation. HIV Drug Resistance Database; [Accessed September 4, 2020]. Published 2020. https://hivdb.stanford.edu/page/release-notes/ [Google Scholar]
- 18.The TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended fi rst-line regimens for adult HIV-1 infection : a multicentre retrospective cohort study. Lancet Infectious Diseases. 2016;16:565–575. doi: 10.1016/S1473-3099(15)00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan SR, Oosthuizen C, Stinson K, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study. PLoS Med. 2017;14(11):1–24. doi: 10.1371/journal.pmed.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenkranz P, Rosen S, Boulle A, et al. The revolving door of HIV care: Revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLoS Med. 2021;18(5):1–10. doi: 10.1371/journal.pmed.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips AN, Bansi-Matharu L, Venter F, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. 2020;7:e193–200. doi: 10.1016/S2352-3018(19)30400-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen GJ, Sun HY, Chang SY, et al. Incidence and impact of low-level viremia among people living with HIV who received protease inhibitor- or dolutegravir-based antiretroviral therapy. International Journal of Infectious Diseases. 2021;105:147–151. doi: 10.1016/j.ijid.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 23.UNAIDS. Understanding Fast-Track Targets Accelerating. Action to End the AIDS Epidemic by 2030. 2015.
- 24.Pepperrell T, Venter WDF, Moorhouse M, et al. Time to rethink endpoints for new clinical trials of antiretrovirals? Long-term re-suppression of HIV RNA with integrase inhibitors. AIDS. 2020;34(2):321–324. doi: 10.1097/QAD.0000000000002422. [DOI] [PubMed] [Google Scholar]
- 25.Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–e127. doi: 10.1016/S2352-3018(18)30317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitoria M, Hill A, Ford N, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: What are the issues? AIDS. 2018;32(12):1551–1561. doi: 10.1097/QAD.0000000000001845. [DOI] [PubMed] [Google Scholar]
- 27.Jennings L, Kellerman T, Spinelli M, et al. Drug Resistance, Rather than Low Tenofovir Levels in Blood or Urine, Is Associated with Tenofovir, Emtricitabine, and Efavirenz Failure in Resource-Limited Settings. AIDS Res Hum Retroviruses. 2022;38(6):455–462. doi: 10.1089/aid.2021.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.