Abstract
Gastric cancer (GC) is a leading cause of global mortality, but also a cancer whose footprint is highly unequal. This review aims to define global disease epidemiology, critically appraise strategies of prevention and disease attenuation, and assess how these strategies could be applied to improve outcomes from GC in a world of variable risk and disease burden. Strategies of primary prevention focus on improving the detection and eradication of the main environmental risk factor, Helicobacter pylori. In certain countries of high incidence, endoscopic or radiographic screening of the asymptomatic general population has been adopted as a means of secondary prevention. By contrast, identification and targeted surveillance of individuals with precancerous lesions (such as intestinal metaplasia) is being increasingly embraced in nations of low incidence. This review will also highlight existing knowledge gaps in GC prevention, as well as the role of emerging technologies for early detection and risk stratification.
Keywords: atrophic gastritis, gastric cancer, Helicobacter pylori, intestinal metaplasia
INTRODUCTION
Worldwide in 2020, 1.1 million individuals were diagnosed with and 770,000 died from gastric cancer (GC), making GC the sixth-most common cancer and third-leading cause of cancer death.1 The majority of GC cases worldwide2–4 are believed to originate from Helicobacter pylori (Hp) infection and an Hp-induced oncogenic progression termed Correa’s cascade.5 In this model, chronic inflammatory insult from Hp colonization results in the development of a series of precursors of increasing severity: non-atrophic gastritis, atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia, and finally carcinoma. Notably, other mechanisms of chronic inflammation (such as autoimmunity directed against gastric parietal cells) may also initiate oncogenic progression.
Despite the significant morbidity and mortality caused by GC, there have been only limited efforts by governments and healthcare systems to implement programs of prevention (mostly in East Asia). This review will examine the epidemiology of GC across the world, and appraise strategies of prevention across regions of variable risk. Within low-incidence regions, a particular emphasis will be placed on applying recently-published guidelines on surveillance of precursor lesions. Notably, while cancers of multiple histologic types (e.g. adenocarcinomas, stromal tumors, lymphoma) can occur in the human stomach, in this review GC will refer specifically to gastric adenocarcinoma; moreover, while certain strategies (such as endoscopic screening) may reduce morbidity and mortality from both cardia and non-cardia GCs, non-cardia GCs will be the focus of this review.
EPIDEMIOLOGY OF GASTRIC CANCER
Global Disease Burden
There exists marked worldwide variability in the incidence of GC.6 Incidence is highest in East Asia, Central Asia, and Andean Latin America, where age-standardized rates exceed 15 per 100,000 person-years; in year 2020, the three highest-incidence nations in the world were Mongolia (33 per 100,000 person-years), Japan (32 per 100,000), and South Korea (28 per 100,000).6 By contrast, incidence is lowest in eastern sub-Saharan Africa, the US, Canada, Western Europe, Australia, and New Zealand, where rates are below 6 per 100,000 person-years. Even within regions there can exist heterogeneity in incidence. In South America, higher incidence is seen among the Andean regions along the Pacific, such as Peru (15 per 100,000); by contrast, much lower incidence is observed in nations along the Atlantic (e.g. Guyana has an incidence of 4 per 100,000). Worldwide, half of all cases occur in China,7 reflective of both the nation’s high incidence (21 per 100,000) and large population. Regional patterns of mortality generally follow incidence,7 though with a few notable exceptions. Japan and South Korea rank 2nd and 3rd in incidence, respectively; however, they rank only 38th and 64th in respective mortality (Japan 8 per 100,000; South Korea 6 per 100,000).6 As will be discussed, these two nations are unique in having structured, nationwide GC screening programs. Worldwide, while five-year net survival from GC is generally below 35% (inclusive of wealthy nations of North America and Western Europe), survival exceeds 60% in both Japan and South Korea.8
Hp infection prevalence also demonstrates marked regional variability. Two recent systematic reviews reported on regional and national Hp infection rates in the general population.9, 10 Hp infection prevalence appears highest in Africa, Central Asia, Mesoamerica, South America, and Russia; prevalence is lowest in the US, Western Europe, and Oceania. East Asian nations are generally considered of moderate Hp prevalence. While Hp is undoubtedly the leading global carcinogenic agent for GC, the only modest correlation between national Hp prevalence and GC incidence suggest other environmental or host-related factors may contribute to cancer development. Moreover, current Hp infection patterns may not be reflective of prior colonization rates, especially in nations with recent industrialization. Importantly, after the development of precursor lesions (AG or IM), individuals remain at heightened risk for future GC even after Hp eradication.11
While the United States (US) as a whole is a nation of modest incidence (4 per 100,000), this burden is concentrated within certain racial and ethnic groups. Asian Americans, Hispanic Americans, African Americans, Alaskan Natives, and American Indians are at increased risk relative to non-Hispanic Whites.12 Certain high-risk Asian subgroups (such as Korean Americans) may be at 8–12 fold increased risk relative to non-Hispanic Whites.13, 14 First-generation immigrants to the US from regions of high GC incidence may also be a particularly vulnerable population.15
Environmental Risk and Protective Factors
A summary of both environmental and host-related risk/protective factors are depicted in Table 1. Hp was first associated with GC through epidemiologic studies dating from the early 1990s.16, 17 Since that time, the carcinogenic role of Hp has been established beyond doubt, and Hp has been classified as a definite carcinogen by the World Health Organization.18 Infection with Hp is estimated to be responsible for 6.2% of all cancers worldwide, and between 75–90% of non-cardia GCs specifically.2–4 Chronic colonization by Hp is the most common inciting event to Correa’s cascade of precursor lesions. Notably, the association between topographic extent of AG and increased risk for GC was described as early as 1969 by Kimura and Takemoto,19 even before the discovery of Hp. The relationship between histologic subtypes, severity, and topography of precancerous lesions and risk for GC is explored further in the Surveillance section. While precancerous lesions have been historically associated with the intestinal-subtype of GC, some observational studies have also found an association with the diffuse-subtype of GC.20
TABLE 1:
Risk and Protective Factors for Non-Cardia Gastric Cancer
| Factor | Direction of Association | |
|---|---|---|
|
| ||
| Environmental | ||
|
| ||
| Helicobacter pylori infection.16, 17 | ↑↑ | |
| Presence of precursor lesions (AG or IM) 48, 122–126 | ↑↑ | |
| Smoking21–23.28 | ↑ | |
| Salt intake24–26 | ↑ | |
| Total, red, and processed meat intake27, 29 | ↑ | |
| Alcohol consumption31–33 | ↑ (heavy) ↓ (moderate) |
|
| Obesity30 | ↔ | |
| Fresh fruit consumption34, 35 | ↓ | |
| Vegetable consumption34, 35 | ↓↔ | |
| Aspirin use36, 37 | ↓ | |
| NSAID use36, 37 | ↓↔ | |
|
| ||
| Host-related | ||
|
| ||
| Family history (first degree)39 | ↑ | |
| Hereditary gastric cancer syndromes • Hereditary diffuse gastric cancer40 • Gastric adenocarcinoma and proximal polyposis of the stomach41 • Familial intestinal gastric cancer42 |
↑ | |
| Other hereditary cancer syndromes • Lynch syndrome43 • Li-Fraumeni44 • Familial adenomatous polyposis45 • Peutz-Jeghers syndrome46 |
↑ | |
| Autoimmune gastritis48 and pernicious anemia49 | ↑ | |
Table 1: Association between known risk or protective factors and non-cardia gastric cancer. ↑indicates association, ↓ indicates inverse association, ↔ indicates no clear association or conflicting studies. References listed are selected and non-comprehensive. AG, atrophic gastritis; IM, intestinal metaplasia; NSAID, non-steroidal anti-inflammatory drug.
Other well-established environmental risk factors include smoking,21–23 diets rich in salt and salt-preserved or salt-cured foods,24–26 and consumption of total, red, and processed meat.27 There may exist important synergistic interactions between these environmental exposures. One study found that high-salt diet was only associated with cancer risk in individuals with Hp infection and existing AG.24 A systematic review focused on understanding interactions between risk factors suggested an overall positive interaction between cigarette smoking and Hp infection on GC risk.28 A case-control study from Hawaii suggested a positive association between processed meat intake and GC, but only in individuals infected with Hp.29 Obesity has demonstrated an inconsistent relationship with GC risk. A systematic review demonstrated an overall association between excess body weight and all GCs; however, this effect was demonstrated only for cardia GCs, with no association seen for non-cardia GCs.30 Observational studies on the association between alcohol consumption and non-cardia GC have provided mixed results; two meta-analyses suggested that heavy (but not moderate) consumption of alcohol was associated with increased risk for cancer.31, 32 By contrast, one cohort study found that moderate alcohol intake (one drink per day) to be protective against GC.33
Dietary fruit and vegetable intake may be protective against GC risk. A systematic review of mostly case-control studies found that high consumption of both fruits and vegetables was associated with decreased risk for both non-cardia GCs and intestinal-type GCs.34 A systematic review of cohort studies incorporating over 2.4 million individuals found consumption of fruit (but not vegetables) was associated with reduced risk.35 Anti-inflammatory drugs may also have a protective role. A cohort study found that aspirin use was protective against intestinal-type non-cardia GC; this effect was not observed for consumption of non-aspirin non-steroidal anti-inflammatory drugs.36 Another cohort study with accompanying meta-analysis (17 published studies) found both use of aspirin and other non-steroidal anti-inflammatory drugs to be protective against non-cardia GC.37
Host-related Risk Factors
While the majority of cases of GC are sporadic, aggregation within families is estimated to occur in about 10% of cases.38 A systematic review of 32 studies found a positive first-degree family history of GC to be associated with an approximate 2.4 increase in relative risk for GC; this effect was seen in both Asian and non-Asian populations.39 In regions of the world with high incidence, the majority of cases of familial aggregation likely represent common and shared risk factors (such as Hp infection). However, in regions of low incidence, some if not most cases of familial aggregation are due to hereditary conditions. Worldwide, it is estimated that up to 3% of GCs may be truly hereditary,38 with three main syndromes described: hereditary diffuse gastric cancer, gastric adenocarcinoma and proximal polyposis of the stomach, and familial intestinal gastric cancer. While a genetic basis of disease has been established for hereditary diffuse gastric cancer (truncating germline mutations in the E-cadherin gene), a known pathogenic variant can be identified in fewer than 50% of affected family members who meet clinical criteria for testing.40 The genetic basis for gastric adenocarcinoma and proximal polyposis of the stomach41 and familial intestinal gastric cancer42 have not yet been identified. A number of other hereditary cancer syndromes have been associated with GC risk, including Lynch syndrome,43 Li-Fraumeni syndrome,44 familial adenomatous polyposis (mediated through fundic gland polyposis-associated dysplasia),45 and Peutz-Jeghers syndrome.46
Autoimmune gastritis represents a distinct form of gastritis characterized by immune-mediated destruction of oxyntic mucosa, and production of autoantibodies against parietal cell antigens and intrinsic factor. In severe cases, parietal-cell destruction and loss of intrinsic factor may progress to B12-deficiency anemia, a condition known as pernicious anemia. Similar to Hp-induced inflammation, autoimmune gastritis can progress to AG, IM, dysplasia, and ultimately adenocarcinoma. Unlike Hp gastritis however, the inflammation in autoimmune gastritis is focused in the corpus.47 While worldwide autoimmune gastritis is significantly less common than Hp-induced gastritis, in Western nations a significant proportion of individuals with precancerous lesions may have an autoimmune etiology; the estimated prevalence of autoimmune gastritis may be as high as 2% of the population.48 Individuals with autoimmune gastritis are both at risk for gastric adenocarcinomas, as well as neuroendocrine tumors driven by hypergastrinemia.48 The development of pernicious anemia may serve as a marker of particular high risk,49 as it represents both a late manifestation of disease and indicates disease severity.
PRIMARY PREVENTION: HELICOBACTER PYLORI TESTING AND ERADICATION
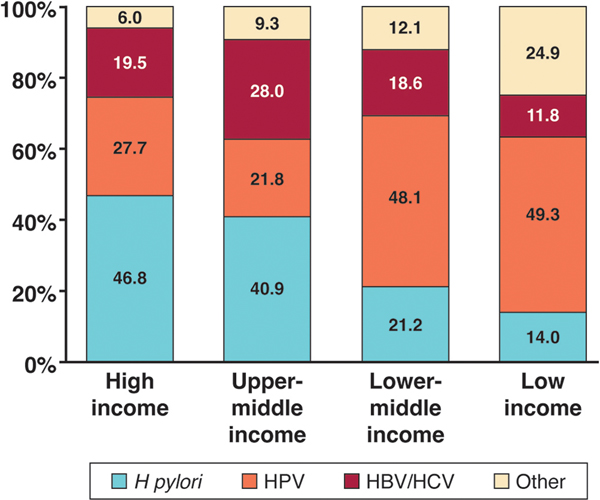
Unlike many cancer-causing infectious agents, the burden of Hp-associated cancer predominates in high- and upper-middle- income countries (Figure 1), where there are resources available for Hp testing and treatment.4 However, despite the ubiquity of this bacteria, and its clear role in carcinogenesis, optimal strategies for testing and treatment of populations of variable risk remain unclear.
Figure 1: Proportion of cancer cases attributable to different infectious agents, by World Bank income group.
The burden of Helicobacter pylori-associated cancer predominates in high- and upper-middle- income countries, where there are resources available for Helicobacter pylori testing and treatment.
Eradication trials among health subjects
To date, seven randomized controlled trials to determine the protective effect of Hp eradication therapy on GC incidence among healthy individuals have been reported. Six of the seven took place in East Asia (four in China,50–55 one in Japan,56 and one in South Korea57); the seventh was conducted in Colombia.58 While still considered “healthy,” it should be noted that the trial in South Korea was among individuals with a first-degree family history of GC, and thus considered higher-risk.57 A recent systematic review and meta-analysis of these seven trials found a 46% reduced risk of subsequent GC following eradication therapy (RR, 0.54; 95% CI, 0.40–0.72), with no heterogeneity in the association between studies, despite considerable variations in the treatments administered and the eradications rates (ranging from 55.6% to 83.7%).59 This is particularly relevant as a number of these studies used older, less effective regimens, so that the use of modern day eradication therapies would be expected to produce an even stronger protective effect. In addition, the protection provided by Hp eradication therapy for healthy individuals in these studies also extended to reduced GC mortality (RR, 0.61; 95% CI, 0.40–0.92), and translated into a number needed to treat to prevent one GC of 72 individuals, and a number needed to treat of 135 to prevent one death from GC.59
It should be noted that all but one of these trials took place in East Asia, a region of high incidence, and thus higher baseline risk even among healthy individuals. The one study that took place outside of East Asia, albeit in a high incidence area of South America, did not find that eradication therapy significantly reduced risk of GC incidence, but did find a significant reduction in the long-term incidence of histological progression (an outcome that they were more well-powered to observe).58, 60 While there have yet to be any randomized controlled trials of Hp eradication among healthy individuals in lower incidence countries like the US, there have been some retrospective studies suggesting a protective effect. Specifically, a retrospective cohort study of 371,813 patients with a diagnosis of Hp infection from the US Veterans Health Administration found that overall treatment was not associated with a reduced risk of GC incidence, but among those who underwent eradication testing, those with successful eradication had a 76% reduction in risk of developing GC compared to those whose Hp was not successfully eradicated (HR, 0.24; 95% CI, 0.15–0.41).61
Eradication trials among high-risk individuals with resected EGC
Three clinical trials, all in East Asia (one in Japan62 and two in South Korea63, 64), examined the association of Hp eradication therapy on subsequent GC incidence among individuals with early GC (EGC, defined as GC that invades no more deeply than the submucosa, irrespective of lymph node metastasis) undergoing endoscopic submucosal dissection. All three observed significant reduction of a future GC with Hp eradication therapy, resulting in a combined 51% reduced risk (RR, 0.49; 95% CI, 0.34–0.70) and a number needed to treat of 21.59 These studies are also important, as individuals with gastric neoplasia are not consistently tested and treated for Hp, although, as this evidence shows, there could be significant benefit from doing so.
Potential Risks of eradication
A major concern regarding a concerted effort to increase rates of Hp testing and eradication relates to potential risks, including increasing prevalence of gastroesophageal reflux disease (GERD), antimicrobial resistance, and alterations to the stomach microbiome. A number of studies have found an inverse association of Hp prevalence with GERD,65, 66 with concerns that Hp may then also be inversely associated with esophageal adenocarcinoma. However, the translation of this observational association to a finding that Hp eradication causes an increased risk of esophageal adenocarcinoma has not been shown from the Hp eradication randomized controlled trial with longest duration and power to capture such an outcome,53 nor from the mass eradication campaign in Matsu Island, Taiwan.67
Recognizing that Hp is an ancient microbe, there is also concern over the removal of this bacteria and subsequent alterations to the gastric microbiome. However, there have now been many studies of the change in the gastric microbiome after Hp eradication, with the finding that individuals with Hp have less bacterial richness than those without,68 and that removal of this dominant bacteria in the stomach ultimately increases bacterial diversity.69, 70 Another concern of broader application of Hp eradication is the emergence of antibiotic resistance. Long-term cohort study data following a mass eradication campaign in Matsu Island, Taiwan found no increase in Hp resistance over the 14 years of the campaign (during which some individuals received multiple rounds of treatment).67 More recently, data from a multi-center, open-label trial also from Taiwan showed that while the initial result of eradication therapy is to increase antibiotic resistance, these effects diminish within a year of treatment.71
It is important to consider Hp recurrence after eradication. The most recent systematic review, in 2017, examined 132 studies and calculated the annual global Hp recurrence, reinfection, and recrudescence rates at 4.3% (95% CI, 4–5), 3.1% (95% CI, 2–5) and 2.2% (95% CI, 1–3), respectively.72 Moreover, higher recurrence rates were strongly related to both countries with lower human development index score and higher underlying Hp prevalence.72, 73 More recently, a study comprising 15 provinces in China found a much lower annual reinfection rate of 1.5% (95% CI, 1.2–1.8), but also noted that higher rates were found among individuals with a GC family history, of a minority group, and with lower levels of education.74 Thus, when implementing greater coverage of Hp testing and eradication, it is vital to consider groups at highest risk of recurrence, and the methods needed to reduce recurrence rates.
Molecular Tests
Nucleic acid sequencing may alter the practice of Hp testing and treatment. 75 Sequencing-based tests can readily and reliably detect known Hp genetic mutations which confer resistance to various antibiotics.76, 77 As they can be performed on formalin-fixed tissue, sequencing-based tests offer considerable advantage with regards to convenience relative to traditional time-intensive culture-based susceptibility testing. In the near future, sequencing-based susceptibility testing may also be performed on stool, obviating the need for gastric biopsies.78 Such tests would allow for targeted antibiotic therapy to both improve eradication rates and decrease community-wide resistance.
Sequencing-based tests can also improve the detection of Hp. Both next-generation sequencing79, 80 and polymerase chain reaction81, 82 studies have shown that Hp genetic material can be isolated from gastric biopsy samples that otherwise have tested ‘negative’ for Hp by conventional histology.79, 80 Interestingly, molecular Hp in histology-negative samples is seen more commonly in moderate or severe IM compared to mild IM or normal gastric tissue.80 Molecular Hp titers may thus serve as a marker for residual infection, and also as a risk factor for recrudescence. These data suggest an emerging role for molecular Hp detection to drive strategies of ‘deep’, molecular-grade eradication—especially in patients harboring precancerous lesions.
GASTRIC CANCER SCREENING IN HIGH-INCIDENCE REGIONS
Population-based screening around the world
Currently, both Japan and South Korea have structured, national screening programs (summarized in Table 2). GC has historically been a leading cause of mortality in Japan; in 1960, GC accounted for 52% of deaths in men and 38% of deaths in women.83 In response to this substantial public health burden, local (provincial-level) screening programs were initiated in the 1960s, consisting of double-contrast radiography examinations for individuals over the age of 40, with abnormal radiographic results pursued by further endoscopy.84 As a result of early successes of local screening programs, Japan implemented a radiography-based, population-wide, national GC screening program for those aged 40 and older beginning in year 1983.83 in practice however, participation in radiographic screening through government-run clinics was rather low (10–15%).84, 85 In response, some municipalities introduced endoscopic screening even prior to a change in guidelines; moreover, many patients pursued opportunistic endoscopic screening in private clinics.84 With growing evidence supporting the mortality-protective benefit of endoscopy, the Japanese national guidelines were amended in 2018 to allow for either endoscopic or radiographic screening on a biennial basis and beginning at age 50 (compared to previously at age 40).84 No definite stopping age was defined.
Table 2:
National Gastric Cancer Screening Recommendations of Japan and South Korea (year 2022)
| Japan84, 85, 170 | Republic of Korea (South Korea)90 | |
|---|---|---|
|
| ||
| Recommending Body | Systematic Review Group and Guideline Development Group of the National Cancer Center | Multidisciplinary Gastric Cancer Screening Recommendation Committee |
| Age to start screening | 50 years of age | 40 years of age |
| Age to end screening | No upper age limit identified | Screening is recommended up to 74 years of age; between 74 to 85 years of age, there is insufficient evidence to assess the benefits and harms of screening; above 85 years of age, the Committee recommends against screening. |
| Frequency of screening | Every 2 years | Every 2 years |
| Modality of screening | Equal recommendation to endoscopy and radiography | For individuals 40 to 74 years of age, endoscopy is recommended over radiography. Radiography may be recommended based on clinician judgement of patient’s risk and patient preference. |
Similar to Japan, South Korea is a nation of high GC incidence and mortality. GC was first declared a public health concern in 1983.86 National-level, organized cancer screening in South Korea began in 2002 following publication of the National Cancer Screening Program Guidelines.87 The Korean guidelines recommend biennial screening of asymptomatic adults between 40 and 75 years of age, with individual choice between radiography and endoscopy.88 Over time, the proportion of individuals choosing endoscopy has increased markedly (from 31% in 2002 to 73% in 2011).88 There has also been increased acceptance and participation in GC screening in Korea over time; whereas 40% of the population participated in screening in 2005, this value grew to 75% by 2015.89 Updated Korean multidisciplinary expert committee guidelines from 2015 recommend upper endoscopy over radiography as the first-line screening modality.90
While Japan and South Korea are the most prominent examples of national screening programs targeting the asymptomatic general population, smaller-scale screening programs have been described elsewhere. In Chile between 1978 and 1986, a radiographic survey program based on the Japanese model was implemented, targeting both asymptomatic and symptomatic 40-year old individuals. While the program was based in Santiago, a mobile unit for radiography allowed screening to be conducted along the length of the country. Over this eight-year period, 42,492 individuals were screened, 33,184 asymptomatic and 9,308 symptomatic.91 As a result of screening, 261 cancers were diagnosed: 143 among asymptomatic individuals (0.43%), and 118 among symptomatic individuals (1.27%). The percentage of EGC was higher in the asymptomatic group (15%) compared to the symptomatic group (11%). In 1980, a radiographic screening program was implemented in Tachira, Venezuela using mobile fluoroscopy units.92 From 1981–1989, over 110,000 examinations were performed and 445 cancers were detected. The program suffered from low coverage of the population resulting in significant bias, as mostly symptomatic persons presented for screening. In 2012, the Chinese government began the Cancer Screening Program in Urban China initiative, targeting five common cancers including GC. In this program, urban residents belonging to targeted cities between the ages of 40 and 75 are recruited through phone call or personal contact. For GC screening, a two-step screening program is employed: an initial survey-based risk factor assessment, followed by endoscopy for high-risk individuals. In the first four years of the program (2013–2017) in Henan province, 179,000 individuals were invited to participate and 7,826 individuals underwent endoscopy. In individuals undergoing endoscopy, seven GCs (0.09%) were detected.93
Endoscopic vs radiographic screening
Within the South Korean national screening program, residents have been able to select between radiographic and endoscopic screening since 2002.88 A number of observational studies nested within the Korean screening program have been conducted comparing endoscopic with radiographic screening. In a nested case-control study comparing 54,418 GC decedents with 217,672 controls (matched by age, sex, and economic status), a reduction in GC-specific mortality was found only for endoscopic screening (OR, 0.53; 95% CI, 0.51–0.56), and not for radiographic screening (OR, 0.98; 95% CI, 0.95–1.01). Similarly, results from a community-based prospective cohort study with 10-year follow-up found endoscopic screening to be associated with reduced cancer-specific mortality (HR, 0.58; 95% CI, 0.36–0.94); radiographic screening was not associated with reduced mortality (HR, 0.91; 95% CI, 0.36–2.33).94 A population-based study ascertained development of interval cancer (defined as within one year of screening) by linking screening recipients with the Korean Central Cancer Registry;95 endoscopic screening was found to be significantly more sensitive (69.0%) for detection of GC compared to radiographic screening (36.7%). Also notably, in 2015 a Korean multidisciplinary expert committee placed a higher recommendation for endoscopic screening (recommendation level B) compared to radiographic screening (recommendation level C).90
The relative performance of endoscopic and radiographic screening has also been considered by the Japanese Guidelines Development Committee in their updated 2018 statement.84 While the statement maintained equal recommendation for endoscopic and radiographic screening (both at 2+ evidence level), several comparative studies suggesting superior performance of endoscopy were reviewed. A case-control study based in Tottori and Niigata Prefectures in Japan of 410 GC decedents between 40–79 years of age along with matched controls found that endoscopic screening (OR, 0.70; 95% CI, 0.49–0.99) was associated with reduced cancer-specific mortality, but not radiographic screening (OR, 0.87; 95% CI, 0.63–1.19).96 In a cohort study of Yonago, Japan, 56,676 individuals who underwent either endoscopic or radiographic screening between 2002 and 2007 were linked to a regional cancer database, allowing for determination of interval cancer development (defined as within 1 year of screening) and calculation of test sensitivity;97 endoscopic screening was found to be more sensitive compared to radiographic screening. A subsequent cohort study of the screened population of Yonago and Tottori, Japan over six years of follow-up (2007–2013) found endoscopic screening reduced GC mortality by 67% compared to radiographic screening.98
However, not all evidence has universally demonstrated endoscopy to be superior to radiography for screening. Moreover, the advent of digital radiography offers improvements in testing characteristics over conventional analogue radiography.99 Beginning in 2006, Maebashi City in Japan introduced endoscopy as a part of their mass screening program but allowed residents to choose between digital radiography or endoscopy; cancer detection and cancer mortality rates were compared between groups by linkage with a cancer registry. The GC detection rate was 0.20% for radiography and 0.48% for endoscopy; however, no difference in survival from GC-related death was noted by screening group..100
Based on trends in public utilization of governmental screening, it appears endoscopic screening is preferred by individuals over radiographic screening.89 Moreover, endoscopy also confers several important advantages not available to radiography including detection of precancerous lesions, detection of Hp from biopsies, and elimination of need for endoscopic confirmation (as is required for radiography). In low-incidence nations (such as the US), the detection of precancerous lesions (such as AG and IM) on screening endoscopy may even be the principal benefit, as it would allow for targeted surveillance of higher-risk individuals. In modeling studies, such a strategy of screening followed by surveillance in appropriate individuals was found to be cost-effective among Asian, Hispanic, and African Americans.101
Serologic Screening
In high-incidence regions of the world, serum pepsinogen (PG), gastrin, and anti-Hp antibody titers have been proposed individually and in combination as GC screening tools. These tests detect inciting events in the carcinogenic sequence.102 PGs are the inactive pro-enzymes of pepsin, and can be classified as either PG I or PG II. Both are detectable in the blood and can offer information on the functional and morphological status of the gastric mucosa.103 With progression of Hp-induced inflammation and atrophy toward the corpus over time, PG I levels decrease and the PG I to PG II ratio decreases. Either a level of PG I ≤ 70 μg/L or a PG I to II ratio ≤ 3.0 have been adopted by some as indicative of a positive PG test.104 PG testing has been evaluated in Asia as well as other parts of the world for its potential as a biomarker for GC; however, test characteristics remain suboptimal, with sensitivities ranging from 23–57% and specificities from 71–97%.105–111 The combined use of PG testing and Hp antibody may result in greater discrimination than use of either marker individually.112 This method has been termed the ABCD method in East Asia, with individuals in group A (Hp-negative/PG-negative) being the lowest risk, and other groups (group B, Hp-positive/PG-negative; group C, Hp-positive/PG-positive; group D, Hp-negative/PG-positive) demonstrating progressively higher risk; notably at very advanced stages of atrophy, Hp antibody becomes negative in some individuals. The ABCD method has been evaluated across numerous populations with variable performance.105, 107–110, 113–115
A recent US-based case-control study nested within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found PG test positivity to be associated with a nearly 10-fold increased risk of developing GC.116 A test combining Hp antibody, PG levels, and gastrin-17 levels (known as GastroPanel®) may further improve testing characteristics.117 Notably, these serologic tests detect the stomach environment that predispose to GC, and thus individuals at heightened risk for cancer; however, they do not directly detect GC itself. It should also be noted that PG testing is currently not in routine clinical use in the US. One recent study evaluated the ability of GastroPanel® to identify precancerous lesions (AG and IM) in a multiethnic US cohort, with findings of low discrimination and sensitivity.118 Notably, the population included in this study had low rates of Hp infection, and high rates of anti-secretory therapy use. These data highlight the need for additional confirmatory studies before serologic testing can be more thoroughly implemented in the US.
Currently, efforts are underway to develop blood-based tests that can detect biomarkers produced by cancer cells for screening. Among the most promising are the use of circulating tumor DNA and microRNA. The multi-cancer targeted ctDNA methylation assay for cancer detection Galleri® reported a sensitivity of 66.7% and a specificity of 99.5% overall for multiple cancers; however, its performance for early-stage GC was poor.119 Another multi-cancer detection test, CancerSEEK, reported sensitivity of approximately 72% and a specificity of greater than 99% for multiple cancers combined; however, the test did not separately report on performance for GC.120 Tests developed specifically for the detection of GC show greater promise. One such test, GastroClear™, is based on microRNA. The prospective validation study conducted on 4,566 persons in Singapore reported overall sensitivity of 87.0% and specificity of 68.4%; these testing characteristics remained robust even for early-stage GC.121 Further examination is warranted to explore if GastroClear™ can be validated in Western populations.
SURVEILLANCE OF PRECANCEROUS LESIONS IN LOW-INCIDENCE REGIONS
In low-incidence nations such as the US, screening of the general population is unlikely to be cost-effective.101 Identification and appropriate surveillance of individuals with precancerous lesions may be one avenue by which to improve early cancer detection. The optimal approach toward the management of these lesions remains a topic of debate, though recent guidelines from both the US and Europe have provided some much-needed guidance.48, 122–126
Performance of High-quality Endoscopic Examination
Most guidelines and expert panels agree that biopsies in patients in whom Hp testing is indicated and/or who have endoscopic findings suspicious for premalignant lesions should be performed according to the updated Sydney protocol.47, 48, 123, 127, 128 In addition to targeted biopsies from suspicious areas, one-to-two standard biopsies should be taken from each of five sites (the greater and lesser curvature of antrum, the incisura angularis, and the greater and lesser curvature of the corpus) to map the extent of mucosal changes for risk stratification. These biopsies can be collected separately, or combined in two jars, with antrum and incisura biopsies in one jar and corpus biopsies in the other. Advanced tools such as high-definition endoscopy with virtual chromoendoscopy and narrow-band imaging (NBI) can enhance detection of precancerous lesions compared with white light alone.129–131 NBI is a high-resolution imaging technique, where light of specific blue wavelengths is used to enhance fine structural visualization of the mucosal surface without the use of dyes. NBI has been shown to be superior to standard, ‘white-light’ endoscopy for the detection of IM.132 During NBI exam, features of IM include the presence of regular ridge or tubule-villous-mucosa,133, 134 and the presence of blue-whitish patchy areas which have been termed ‘light blue crests’.131, 133–135 Other quality improvement measures such as adequate mucosal cleaning and air insufflation, a seven-minute examination time, and systematic photo-documentation of mucosal visualization are important steps to improve yield.123, 124, 129–131, 136–139
Prevalence and Progression of Precancerous Lesions
While ascertainment of the true prevalence of gastric precursor lesions has been challenging in the absence of population-based screening programs, these lesions have been shown to impact a large portion of the US population. The prevalence of AG is estimated around 15% and the prevalence of gastric IM is estimated around 5–7%, though these may be significantly greater in high-risk populations such as racial/ethnic minority groups, immigrants from high-risk countries, and those with a family history of GC.48, 140–145 It has been suggested that once gastric IM has developed, individuals are at risk for subsequent GC regardless of race or ethnicity.146 If so, differences in underlying prevalence of precursors may partially explain differences in cancer incidence between groups.
The annual progression rate from IM to GC is estimated to be 0.1% per year, though certain subtypes of IM are considered to have a higher risk of progression.146–148 Studies have shown individuals with incomplete IM (which resembles the colonic epithelium and lacks a brush border) have a higher progression rate compared to those with complete IM (which resembles small intestinal epithelium, with a brush border and goblet cells).146 The risk of GC has also been demonstrated to be higher among individuals with extensive IM, defined as IM in both the antrum and corpus, than limited IM confined to the antrum.143, 146, 148–150 The Operative Link for Gastritis Assessment (OLGA) and the Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM) staging systems have emerged as key tools in assessing the severity and extent of these mucosal changes and aiding in the prediction of cancer risk.128, 151, 152 In both OLGA and OLGIM, Stages III and IV represent more severe, extensive disease and correlate with a higher risk of progression.152–156 OLGIM shows less interobserver variability and is prognostically useful, though it may be less sensitive than OLGA at identifying high-risk gastritis.153, 157 Some experts recommend using a combination of OLGA and OLGIM for staging of chronic gastritis, or prioritizing OLGIM as the more reliable predictor.124, 153 Both OLGA and OLGIM are not commonly used in current clinical practice in the US, and its broader adoption would require for 1) gastroenterologists to routinely adopt the 5-biopsy protocol, and 2) pathologists to routinely interpret histologic severity per the updated Sydney protocol.47
US Guidelines
Guidance from professional societies in the US on the management of AG and IM has historically been limited, though several notable position statements have emerged in recent years to fill this important void. All guidelines advocate for eradication of Hp if it is identified.48, 122, 123, 127, 158 In 2015, the American Society for Gastrointestinal Endoscopy updated its guidelines on management of premalignant conditions and suggested surveillance should be pursued in individuals with a family history of GC or of high-risk racial/ethnic background, though they did not provide an optimal surveillance interval.122 While this guidance did not comment on management of AG specifically, it suggested endoscopy should be pursued within six months of the diagnosis of pernicious anemia.122
In 2020, the American Gastroenterological Association (AGA) developed the first US guidelines dedicated specifically to the management of IM.123 The AGA guidelines recommended against routine surveillance in patients with gastric IM; however, they did recommend that surveillance could be considered at three-to-five year intervals in high-risk groups, including individuals with the incomplete or extensive subtypes of IM, and individuals with any IM who are racial/ethnic minorities (including Hispanics, Asians, African Americans, and Native Americans/Alaska Natives), immigrants from high risk regions for GC, and those with family history of GC.123 The AGA guidelines are notable in emphasizing the importance of shared decision making between patient and physician, and the importance of discussion of not only the potential benefits of surveillance, but also the potential risks of endoscopy. A recent AGA clinical practice update48 published in 2021 further extended the recommendations for endoscopic surveillance to patients with advanced stages of AG (including severe atrophic changes or IM in both the antrum and corpus, corresponding to OLGA/OLGIM III/IV classifications). This panel suggested surveillance at three-year intervals in those without a family history of GC and more intensive follow up (every one-to-two years) in those with a family history of GC. In patients with pernicious anemia, the review recommended an upper endoscopy at the time of diagnosis to stage the severity of autoimmune gastritis, with endoscopic follow-up to be considered depending on burden of neuroendocrine tumors. Notably US guidelines do not recommend screening of asymptomatic populations for the presence of precancerous lesions.
European Guidelines
These US surveillance guidelines can be compared and contrasted to recently-published European guidance (Table 3).124–126 The European second guideline on the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) was published in 2019, and stratified individuals based on histologic extent and severity, family history, and Hp status.124 MAPS II does not recommend routine surveillance in patients with mild or topographically limited AG/IM in the absence of family history. MAPS II recommends surveillance with three-year intervals for individuals with advanced AG/IM (in both antrum and corpus or OLGA/OLGIM III/IV), or any IM with the presence of certain risk factors (a family history of GC, incomplete IM, persistent Hp gastritis), and more intensive one-to-two year intervals for those with advanced AG or IM and a family history of GC. The British gastroenterological society guideline similarly recommends a three-year surveillance interval for individuals with extensive AG/IM (involving both antrum and corpus), and in cases of limited AG/IM if there is either a family history of GC or persistent Hp infection.125 Of note, the British guideline also recommends endoscopic screening for precancerous lesions among asymptomatic individuals aged ≥50 with multiple risk factors. The Italian multi-society position paper recommends a three-year surveillance interval for individuals with advanced AG/IM (defined as AG/IM involving both antrum and body, or OLGA/OLGIM stages III/IV).126 The European recommendations are concordant with each other, and offer more structured recommendations for surveillance based on histologic attributes (such as anatomic location and severity) as compared to US guidelines. While both US and European guidelines incorporate family history, there is greater emphasis on race, ethnicity, and national origin in the US guidelines. The US guidelines utilize incomplete phenotyping, whereas European guidelines utilize OLGA/OLGIM scoring.
Table 3:
Comparison of United States and European Guidelines for the Surveillance of Precancerous Lesions
| United States Surveillance Guidelines |
European Surveillance Guidelines |
||
|---|---|---|---|
| American Gastroenterological Association Clinical Practice Update on Atrophic Gastritis (2021) 48 | The optimal endoscopic surveillance interval for patients with AG is not well-defined. A surveillance endoscopy every 3 years should be considered in individuals with advanced atrophic gastritis, defined based on anatomic extent and histologic grade. Optimal surveillance interval for individuals with autoimmune gastritis is unclear; consider surveillance at 1–2 years depending on burden of neuroendocrine tumors. |
European Society of Gastrointestinal Endoscopy, European Helicobacter and Microbiota Study Group, European Society of Pathology, and Sociedade Portuguesa de Endoscopia Digestiva second guideline on the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) (2019) 124 | Mild-to-moderate AG or IM limited to antrum and no GC family history: no surveillance IM at a single location + family history of GC, incomplete IM, or persistent Hp gastritis: 3 year surveillance Advanced AG or advanced IM (in both antrum and corpus; OLGA/OLGIM III/IV): 3 year surveillance Advanced AG or advanced IM (in both antrum and corpus; OLGA/OLGIM III/IV) + family history of GC: 1–2 year surveillance Autoimmune gastritis: 3–5 year surveillance |
|
|
|||
| American Gastroenterological Association Guidelines on management of Gastric Intestinal Metaplasia (2020) 123 | Recommends against routine use of endoscopic surveillance in patients with IM. Patients with IM at higher risk for GC who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance. Patients with IM specifically at higher risk of GC include those with: • Incomplete vs complete IM • Extensive vs limited IM • Family history of GC Patients at overall increased risk for GC include: • Racial/ethnic minorities • Immigrants from high incidence regions Upper endoscopy every 3–5 years with careful mucosal visualization and gastric biopsies of the antrum, body, and any concerning lesions could be considered if shared decision making favors surveillance. |
||
|
| |||
| British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma (2019) 125 | Extensive AG or IM (in both antrum and corpus): 3 years AG or IM limited to antrum + family history of GC or persistent Hp infection: 3 years |
||
|
| |||
| Italian Multi-Society Position Paper: Chronic atrophic gastritis - Natural history, diagnosis and therapeutic management (2019) 126 | Advanced AG or IM (in both the antrum and corpus; OLGA/OLGIM III/IV): 3 years Autoimmune gastritis: 3–5 years |
||
GC, gastric cancer; AG, atrophic gastritis; IM, intestinal metaplasia, Hp, Helicobacter pylori; OLGA, operative link on gastritis assessment; OLGIM, operative link on gastric intestinal metaplasia assessment.
Taken as a whole, the European and US guidelines are more similar than they are dissimilar. All guidelines recommend a stratified approach to surveillance, with an emphasis on recalling individuals at heightened risk due to either histologic features (e.g. extent, severity) or demographic/familial features (e.g. family history, race, origin). The recommended intervals for surveillance (generally from three-to-five years in higher-risk individuals) are also consistent across guidelines. This emerging consensus in low-incidence nations of structured surveillance for high-risk individuals represents a major and recent paradigm shift, and for the first time makes gastric precancerous lesion surveillance a normative practice in line with established guidelines for the esophagus and colon.
REMAINING KNOWLEDGE GAPS
While significant progress has been made toward attenuation of GC morbidity and mortality, GC survival remains unacceptably low worldwide8 In particular, there exist a number of significant knowledge gaps in low-incidence nations which need to be closed.
Defining optimal surveillance intervals for precancerous lesions
The optimal surveillance interval for gastric precancerous lesions is unclear. Both the AGA guidelines for IM123 and the clinical practice update for AG48 base their recommendations on expert opinion. European guidance124–126 incorporates both expert opinion and modeling in their rationale for surveillance intervals. There exist no direct data derived from the US or Europe on the effect of surveillance intervals on patient-important outcomes of early cancer detection, reduction in GC morbidity/mortality, endoscopic complications, or psychological harms. By contrast, from East Asia there have been a small number of studies evaluating the effect of different screening intervals on diagnostic stage (EGC vs advanced stage), with several studies suggesting a screening interval of less than three years to be associated with increased likelihood of EGC diagnosis.159–162 This is notable as US and European guidance for surveillance intervals are generally for three years or greater. Whether these East Asian data can be translated to low-incidence populations remains unknown.
Risk stratifying asymptomatic individuals
Given the emerging consensus in low-incidence nations on a stratified approach to surveillance, an important future question is to determine which members of the asymptomatic population need to be screened. Most individuals with precancerous lesions in the US are diagnosed during endoscopies performed for symptomatic indications.163 Based on the estimated prevalence of AG and IM in the general population,141–145 the majority of individuals in low-incidence nations likely remain undiagnosed. There exists a need for diagnostic risk stratification tools to identify the segments of the asymptomatic population to endoscopically screen. This likely will involve a combination of demographic features (such as age, sex, race), familial history, and perhaps serology. Incorporating these features into a comprehensive, non-invasive risk assessment that allows for high-yield endoscopic screening should be an area of future focus.
Helicobacter pylori eradication trials in low-incidence nations
There exists no clinical trials assessing the efficacy of Hp testing or treatment on cancer incidence or mortality in low-incidence nations. While a bevy of modeling studies in US populations have been published over the preceding two decades,164 these results has not translated into prospective trials.165 The need for Western-derived primary data is essential, given the variabilities in Hp prevalence, treatment regimens, eradication and recrudescence rates across regions of the world.
Improved Education and Training of Gastroenterologists
Prior to widespread adoption of precancerous lesion surveillance in the US and Europe, improvements in endoscopic training are needed. In particular, examination with use of NBI must become standardized, and education on use of NBI should begin during clinical gastroenterological training. Gastric biopsies using the updated Sydney protocol should become standardized in the diagnostic work-up for dyspepsia and gastritis, a step shown to increase detection of Hp and IM,166 and a practice recommended by the AGA.167 Use of Sydney protocol biopsies during index endoscopies will both improve detection of lesions, and also allow for staging (by extent or OLGA/OLGIM scoring) if AG/IM is discovered—potentially avoiding need for a second staging examination to determine risk level. In Japan, a quality assurance manual of endoscopic screening has been published, with recommendations such as standardized interpretation of endoscopic images and creation of adverse event reporting systems.168 While particular to Japan (where biopsies are less common and procedures are performed often unsedated), a similar manual would help to standardize practice in the US and Europe. It also behooves gastroenterologists to partner with pathologists to ensure histologic scoring of gastric biopsies. Many pathologists in the West do not report severity scoring routinely on gastric biopsies. These are critical data without which calculation of OLGA and OLGIM stages are not possible.
Validating and integrating novel technologies for detection and surveillance
There have been numerous exciting and rapidly-changing developments in the field of imaging-enhanced detection and risk stratification that may revolutionize precancerous lesion management in the future. For instance, there has been a flurry of research focused on use of artificial intelligence to aid detection of precancerous lesions and Hp gastritis.169 While not readily used yet in clinical practice, use of imaging assistance may help to narrow the experience gap between endoscopists in low- vs high-incidence regions and improve detection. Also of great promise is the development of next generation sequencing-based tests to risk stratify IM-affected mucosa at highest risk for cancer progression.80 Molecular risk stratification through genomic, epigenomic, or transcriptomic profiling may serve as a future compliment to histologic severity or topographic extent as a means to risk stratify individuals.
CONCLUSIONS
When designing public health programs to combat GC, challenges lie in the significant differences in incidence between regions of the world. In high-incidence nations, most notably in Northeast Asia, structured endoscopic and radiographic screening programs of the general population have yielded early though impressive results. By contrast, in low-incidence nations a bespoke strategy of first identifying individuals harboring precancerous lesions, and then rational surveillance of a subset of these individuals based on subsequent cancer risk, may be more appropriate. Recent guidelines published in the US and Western Europe suggest a consensus toward targeted surveillance based on demographic, familial, or histologic features. There is also now high-quality data to suggest that testing and treatment for Hp in asymptomatic individuals may reduce GC incidence and mortality, though these data are mostly derived from high-incidence regions. Exciting technologies including sequencing-based detection and susceptibility testing of Hp, artificial intelligence in endoscopy, and molecular profiling of IM may alter future practice.
Financial Support:
RJH is supported by the NIH/NCI under Award Number K08CA252635, the Rutgers University Asian Resource Centers for Minority Aging Research Center under NIH/NIA Grant P30-AG0059304, the Stanford University Department of Medicine Chair Diversity Investigator Award, and the American College of Gastroenterology North American International GI Training Grant. ML is supported by the NIH/NIDDK under Award Number K08DK125876, the NCI [U01 CA265729], a Memorial Sloan Kettering Division of Subspecialty Medicine Award, and the NCI Cancer Center Support Grant [P30 CA008748]. HI is supported by NCI Cancer Center Support Grant [5P30CA072720-23]. ME receives support from the NCI under Award Number P20CA251657 and R01CA267842.
Abbreviations:
- AG
atrophic gastritis
- AGA
American Gastroenterological Association
- EGC
early gastric cancer
- GC
gastric cancer
- GERD
gastroesophageal reflux disease
- Hp
Helicobacter pylori
- IM
intestinal metaplasia
- MAPS II
second guideline on the management of epithelial precancerous conditions and lesions in the stomach
- NBI
narrow-band imaging
- OLGA
operative link on gastritis assessment
- OLGIM
operative link on gastric intestinal metaplasia assessment
- PG
pepsinogen
- US
United States
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
REFERENCE
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487–90. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–15. [DOI] [PubMed] [Google Scholar]
- 4.de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8:e180–e190. [DOI] [PubMed] [Google Scholar]
- 5.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 6.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today, accessed [3 August 2022]. Lyon, France, 2020. [Google Scholar]
- 7.Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020;5:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 10.Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–876. [DOI] [PubMed] [Google Scholar]
- 11.Take S, Mizuno M, Ishiki K, et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol 2020;55:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RJ, Koh H, Hwang JH, et al. A Summary of the 2020 Gastric Cancer Summit at Stanford University. Gastroenterology 2020;159:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang RJ, Sharp N, Talamoa RO, et al. One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol Biomarkers Prev 2020;29:903–909. [DOI] [PubMed] [Google Scholar]
- 14.Shah SC, McKinley M, Gupta S, et al. Population-Based Analysis of Differences in Gastric Cancer Incidence Among Races and Ethnicities in Individuals Age 50 Years and Older. Gastroenterology 2020;159:1705–1714 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabla BS, Shah SC, Corral JE, et al. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:347–359 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991;325:1132–6. [DOI] [PubMed] [Google Scholar]
- 17.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- 18.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Schistosomes, Liver Flukes and Helicobacter pylori. Vol 61 of IARC monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer, Lyon, 1994. [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura K, T. T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy 1969;1:87–97. [Google Scholar]
- 20.Wang JE, Kim SE, Lee BE, et al. The risk of diffuse-type gastric cancer following diagnosis with gastric precancerous lesions: a systematic review and meta-analysis. Cancer Causes Control 2022;33:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomura AM, Wilkens LR, Henderson BE, et al. The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes Control 2012;23:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001;12:721–32. [DOI] [PubMed] [Google Scholar]
- 23.Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008;19:689–701. [DOI] [PubMed] [Google Scholar]
- 24.Shikata K, Kiyohara Y, Kubo M, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. International Journal of Cancer 2006;119:196–201. [DOI] [PubMed] [Google Scholar]
- 25.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 2007;10:75–83. [DOI] [PubMed] [Google Scholar]
- 26.Tsugane S, Sasazuki S, Kobayashi M, et al. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. British Journal of Cancer 2004;90:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez CA, Jakszyn P, Pera G, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). Journal of the National Cancer Institute 2006;98:345–54. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez CA, Lopez-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scand J Gastroenterol 2010;45:6–14. [DOI] [PubMed] [Google Scholar]
- 29.Epplein M, Nomura AM, Hankin JH, et al. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control 2008;19:869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer 2009;45:2867–73. [DOI] [PubMed] [Google Scholar]
- 31.Tramacere I, Negri E, Pelucchi C, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol 2012;23:28–36. [DOI] [PubMed] [Google Scholar]
- 32.Wang PL, Xiao FT, Gong BC, et al. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget 2017;8:99013–99023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Freedman ND, Loftfield E, et al. Alcohol consumption and risk of gastric cardia adenocarcinoma and gastric noncardia adenocarcinoma: A 16-year prospective analysis from the NIH-AARP diet and health cohort. Int J Cancer 2018;143:2749–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunet N, Valbuena C, Vieira AL, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev 2007;16:312–27. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Chen Y, Wang X, et al. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer 2014;50:1498–509. [DOI] [PubMed] [Google Scholar]
- 36.Epplein M, Nomura AM, Wilkens LR, et al. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am J Epidemiol 2009;170:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abnet CC, Freedman ND, Kamangar F, et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer 2009;100:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol 2015;16:e60–70. [DOI] [PubMed] [Google Scholar]
- 39.Yaghoobi M, McNabb-Baltar J, Bijarchi R, et al. What is the quantitative risk of gastric cancer in the first-degree relatives of patients? A meta-analysis. World J Gastroenterol 2017;23:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020;21:e386–e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worthley DL, Phillips KD, Wayte N, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 2012;61:774–9. [DOI] [PubMed] [Google Scholar]
- 42.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873–80. [PMC free article] [PubMed] [Google Scholar]
- 43.Capelle LG, Van Grieken NC, Lingsma HF, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology 2010;138:487–92. [DOI] [PubMed] [Google Scholar]
- 44.Masciari S, Dewanwala A, Stoffel EM, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med 2011;13:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–13. [DOI] [PubMed] [Google Scholar]
- 46.van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol 2010;105:1258–64; author reply 1265. [DOI] [PubMed] [Google Scholar]
- 47.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 48.Shah SC, Piazuelo MB, Kuipers EJ, et al. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021;161:1325–1332.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375–82. [DOI] [PubMed] [Google Scholar]
- 50.Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53:1244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L, Lin S, Ding S, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl) 2014;127:1454–8. [PubMed] [Google Scholar]
- 52.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94. [DOI] [PubMed] [Google Scholar]
- 53.Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong BC, Zhang L, Ma JL, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012;61:812–8. [DOI] [PubMed] [Google Scholar]
- 56.Saito D, Boku N, Fujioka T, et al. Impact of H. pylori eradication on gastric cancer prevention: endoscopic results of the Japanese Intervention Trial (JITHP-Study). A randomized multi-center trial. Gastroenterology 2005;128. [Google Scholar]
- 57.Choi IJ, Kim CG, Lee JY, et al. Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med 2020;382:427–436. [DOI] [PubMed] [Google Scholar]
- 58.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881–8. [DOI] [PubMed] [Google Scholar]
- 59.Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113–2121. [DOI] [PubMed] [Google Scholar]
- 60.Piazuelo MB, Bravo LE, Mera RM, et al. The Colombian Chemoprevention Trial: 20-Year Follow-Up of a Cohort of Patients With Gastric Precancerous Lesions. Gastroenterology 2021;160:1106–1117 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Metz DC, Ellenberg S, et al. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020;158:527–536 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–7. [DOI] [PubMed] [Google Scholar]
- 63.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378:1085–1095. [DOI] [PubMed] [Google Scholar]
- 64.Choi JM, Kim SG, Choi J, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc 2018;88:475–485 e2. [DOI] [PubMed] [Google Scholar]
- 65.Zamani M, Alizadeh-Tabari S, Hasanpour AH, et al. Systematic review with meta-analysis: association of Helicobacter pylori infection with gastro-oesophageal reflux and its complications. Aliment Pharmacol Ther 2021;54:988–998. [DOI] [PubMed] [Google Scholar]
- 66.Han YM, Chung SJ, Yoo S, et al. Inverse correlation between gastroesophageal reflux disease and atrophic gastritis assessed by endoscopy and serology. World J Gastroenterol 2022;28:853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiang TH, Chang WJ, Chen SL, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gantuya B, El-Serag HB, Matsumoto T, et al. Gastric Microbiota in Helicobacter pylori-Negative and -Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liou JM, Lee YC, El-Omar EM, et al. Efficacy and Long-Term Safety of H. pylori Eradication for Gastric Cancer Prevention. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Y, Zhang Y, Gerhard M, et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020;69:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liou JM, Chen CC, Chang CM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis 2019;19:1109–1120. [DOI] [PubMed] [Google Scholar]
- 72.Hu Y, Wan JH, Li XY, et al. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 2017;46:773–779. [DOI] [PubMed] [Google Scholar]
- 73.Yan TL, Hu QD, Zhang Q, et al. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther 2013;37:963–8. [DOI] [PubMed] [Google Scholar]
- 74.Xie Y, Song C, Cheng H, et al. Long-term follow-up of Helicobacter pylori reinfection and its risk factors after initial eradication: a large-scale multicentre, prospective open cohort, observational study. Emerg Microbes Infect 2020;9:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am J Gastroenterol 2022;117:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang XY, Shen WX, Chen CF, et al. Detection of the clarithromycin resistance of Helicobacter pylori in gastric mucosa by the amplification refractory mutation system combined with quantitative real-time PCR. Cancer Med 2019;8:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Lv T, He C, et al. Evaluation of multiplex ARMS-PCR for detection of Helicobacter pylori mutations conferring resistance to clarithromycin and levofloxacin. Gut Pathog 2020;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moss SF, Dang LP, Chua D, et al. Comparable Results of Helicobacter pylori Antibiotic Resistance Testing of Stools vs Gastric Biopsies Using Next-Generation Sequencing. Gastroenterology 2022;162:2095–2097 e2. [DOI] [PubMed] [Google Scholar]
- 79.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 2006;103:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang KK, Ramnarayanan K, Zhu F, et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018;33:137–150 e5. [DOI] [PubMed] [Google Scholar]
- 81.Ramirez-Lazaro MJ, Lario S, Casalots A, et al. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS One 2011;6:e20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramirez-Lazaro MJ, Lario S, Quilez ME, et al. Droplet Digital PCR Detects Low-Density Infection in a Significant Proportion of Helicobacter Pylori-Negative Gastric Biopsies of Dyspeptic Patients. Clin Transl Gastroenterol 2020;11:e00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan YK, Fielding JW. Early diagnosis of early gastric cancer. Eur J Gastroenterol Hepatol 2006;18:821–9. [DOI] [PubMed] [Google Scholar]
- 84.Hamashima C. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018;48:673–683. [DOI] [PubMed] [Google Scholar]
- 85.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2008;38:259–67. [DOI] [PubMed] [Google Scholar]
- 86.Kweon SS. Updates on Cancer Epidemiology in Korea, 2018. Chonnam Med J 2018;54:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim Y, Jun JK, Choi KS, et al. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725–30. [PubMed] [Google Scholar]
- 88.Lee S, Jun JK, Suh M, et al. Gastric cancer screening uptake trends in Korea: results for the National Cancer Screening Program from 2002 to 2011: a prospective cross-sectional study. Medicine (Baltimore) 2015;94:e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee EY, Lee YY, Suh M, et al. Socioeconomic Inequalities in Stomach Cancer Screening in Korea, 2005–2015: After the Introduction of the National Cancer Screening Program. Yonsei Med J 2018;59:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park HA, Nam SY, Lee SK, et al. The Korean guideline for gastric cancer screening. J Korean Med Assoc. 2015;58:373–84. [Google Scholar]
- 91.Llorens P. Gastric cancer mass survey in Chile. Semin Surg Oncol 1991;7:339–43. [DOI] [PubMed] [Google Scholar]
- 92.Pisani P, Oliver WE, Parkin DM, et al. Case-control study of gastric cancer screening in Venezuela. Br J Cancer 1994;69:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo L, Zhang S, Liu S, et al. Determinants of participation and detection rate of upper gastrointestinal cancer from population-based screening program in China. Cancer Med 2019;8:7098–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H, Hwang Y, Sung H, et al. Effectiveness of Gastric Cancer Screening on Gastric Cancer Incidence and Mortality in a Community-Based Prospective Cohort. Cancer Res Treat 2018;50:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 2012;7:e50041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hamashima C, Ogoshi K, Okamoto M, et al. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One 2013;8:e79088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamashima C, Okamoto M, Shabana M, et al. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013;133:653–9. [DOI] [PubMed] [Google Scholar]
- 98.Hamashima C, Shabana M, Okada K, et al. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci 2015;106:1744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iinuma G, Ushio K, Ishikawa T, et al. Diagnosis of gastric cancers: comparison of conventional radiography and digital radiography with a 4 million-pixel charge-coupled device. Radiology 2000;214:497–502. [DOI] [PubMed] [Google Scholar]
- 100.Hagiwara H, Moki F, Yamashita Y, et al. Gastric cancer mortality related to direct radiographic and endoscopic screening: A retrospective study. World J Gastroenterol 2021;27:5595–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saumoy M, Schneider Y, Shen N, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018;155:648–660. [DOI] [PubMed] [Google Scholar]
- 102.Correa P. Gastric cancer: overview. Gastroenterol Clin North Am 2013;42:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cha JH, Jang JS. Clinical correlation between serum pepsinogen level and gastric atrophy in gastric neoplasm. Korean J Intern Med 2020;35:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Y-k, Yu J-c, Kang W-m, et al. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLOS ONE 2015;10:e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci 2010;55:3132–7. [DOI] [PubMed] [Google Scholar]
- 106.Castro C, Dinis-Ribeiro M, Rodrigues ANG, et al. Western long-term accuracy of serum pepsinogen-based gastric cancer screening. Eur J Gastroenterol Hepatol 2018;30:274–277. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Xue L, Xing L, et al. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer 2012;130:1614–9. [DOI] [PubMed] [Google Scholar]
- 108.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer 2014;134:1445–57. [DOI] [PubMed] [Google Scholar]
- 109.Kwak MS, Chung GE, Chung SJ, et al. Predicting the Development of Gastric Neoplasms in a Healthcare Cohort by Combining Helicobacter pylori Antibodies and Serum Pepsinogen: A 5-Year Longitudinal Study. Gastroenterol Res Pract 2018;2018:8796165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choi HS, Lee SY, Kim JH, et al. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J Dig Dis 2014;15:293–8. [DOI] [PubMed] [Google Scholar]
- 111.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, et al. Serum pepsinogen test for early detection of gastric cancer in a European country. Eur J Gastroenterol Hepatol 2012;24:37–41. [DOI] [PubMed] [Google Scholar]
- 112.Sasazuki S. The ABC Method and Gastric Cancer: Evidence From Prospective Studies. J Epidemiol 2016;26:611–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004;109:138–43. [DOI] [PubMed] [Google Scholar]
- 114.Song M, Camargo MC, Weinstein SJ, et al. Serum pepsinogen 1 and anti-Helicobacter pylori IgG antibodies as predictors of gastric cancer risk in Finnish males. Aliment Pharmacol Ther 2018;47:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005;54:764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.In H, Sarkar S, Ward J, et al. Serum Pepsinogen as a Biomarker for Gastric Cancer in the United States: A Nested Case-Control Study Using the PLCO Cancer Screening Trial Data. Cancer Epidemiol Biomarkers Prev 2022;31:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Syrjänen K. A Panel of Serum Biomarkers (GastroPanel®) in Non-invasive Diagnosis of Atrophic Gastritis. Systematic Review and Meta-analysis. Anticancer Res 2016;36:5133–5144. [DOI] [PubMed] [Google Scholar]
- 118.Huang RJ, Park S, Shen J, et al. Pepsinogens and Gastrin Demonstrate Low Discrimination for Gastric Precancerous Lesions in a Multi-Ethnic United States Cohort. Clin Gastroenterol Hepatol 2022;20:950–952 e3. [DOI] [PubMed] [Google Scholar]
- 119.Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol 2021;32:1167–1177. [DOI] [PubMed] [Google Scholar]
- 120.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.So JBY, Kapoor R, Zhu F, et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut 2021;70:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evans JA, Chandrasekhara V, Chathadi KV, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 123.Gupta S, Li D, El Serag HB, et al. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 125.Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019;68:1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis 2019;51:1621–1632. [DOI] [PubMed] [Google Scholar]
- 127.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marcos-Pinto R, Carneiro F, Dinis-Ribeiro M, et al. First-degree relatives of patients with early-onset gastric carcinoma show even at young ages a high prevalence of advanced OLGA/OLGIM stages and dysplasia. Aliment Pharmacol Ther 2012;35:1451–9. [DOI] [PubMed] [Google Scholar]
- 129.Bansal A, Ulusarac O, Mathur S, et al. Correlation between narrow band imaging and nonneoplastic gastric pathology: a pilot feasibility trial. Gastrointest Endosc 2008;67:210–6. [DOI] [PubMed] [Google Scholar]
- 130.Tahara T, Shibata T, Nakamura M, et al. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc 2009;70:246–53. [DOI] [PubMed] [Google Scholar]
- 131.Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy 2006;38:819–24. [DOI] [PubMed] [Google Scholar]
- 132.Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, et al. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc 2017;86:857–865. [DOI] [PubMed] [Google Scholar]
- 133.Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012;44:236–46. [DOI] [PubMed] [Google Scholar]
- 134.Pimentel-Nunes P, Libanio D, Lage J, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016;48:723–30. [DOI] [PubMed] [Google Scholar]
- 135.Savarino E, Corbo M, Dulbecco P, et al. Narrow-band imaging with magnifying endoscopy is accurate for detecting gastric intestinal metaplasia. World J Gastroenterol 2013;19:2668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Emura F, Sharma P, Arantes V, et al. Principles and practice to facilitate complete photodocumentation of the upper gastrointestinal tract: World Endoscopy Organization position statement. Dig Endosc 2020;32:168–179. [DOI] [PubMed] [Google Scholar]
- 137.Machaca Quea NR, Emura F, Barreda Bolaños F, et al. Effectiveness of systematic alphanumeric coded endoscopy for diagnosis of gastric intraepithelial neoplasia in a low socioeconomic population. Endosc Int Open 2016;4:E1083–e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- 139.Emura F, Rodriguez-Reyes C, Giraldo-Cadavid L. Early Gastric Cancer: Current Limitations and What Can Be Done to Address Them. Am J Gastroenterol 2019;114:841–845. [DOI] [PubMed] [Google Scholar]
- 140.Altayar O, Davitkov P, Shah SC, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Epidemiology and Risk Factors. Gastroenterology 2020;158:732–744.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77.e1. [DOI] [PubMed] [Google Scholar]
- 142.Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol 2010;25:439–48. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen TH, Tan MC, Liu Y, et al. Prevalence of Gastric Intestinal Metaplasia in a Multiethnic US Veterans Population. Clin Gastroenterol Hepatol 2021;19:269–276.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi CE, Sonnenberg A, Turner K, et al. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig Dis Sci 2015;60:2070–6. [DOI] [PubMed] [Google Scholar]
- 145.Namekata T, Miki K, Kimmey M, et al. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol 2000;151:820–30. [DOI] [PubMed] [Google Scholar]
- 146.Gawron AJ, Shah SC, Altayar O, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Natural History and Clinical Outcomes. Gastroenterology 2020;158:705–731.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li D, Bautista MC, Jiang SF, et al. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol 2016;111:1104–13. [DOI] [PubMed] [Google Scholar]
- 148.Laszkowska M, Truong H, Faye AS, et al. Prevalence of Extensive and Limited Gastric Intestinal Metaplasia and Progression to Dysplasia and Gastric Cancer. Dig Dis Sci 2022;67:3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reddy KM, Chang JI, Shi JM, et al. Risk of Gastric Cancer Among Patients With Intestinal Metaplasia of the Stomach in a US Integrated Health Care System. Clin Gastroenterol Hepatol 2016;14:1420–5. [DOI] [PubMed] [Google Scholar]
- 150.Tan MC, Mallepally N, Liu Y, et al. Demographic and Lifestyle Risk Factors for Gastric Intestinal Metaplasia Among US Veterans. Am J Gastroenterol 2020;115:381–387. [DOI] [PubMed] [Google Scholar]
- 151.Rugge M, Fassan M, Pizzi M, et al. Autoimmune gastritis: histology phenotype and OLGA staging. Aliment Pharmacol Ther 2012;35:1460–6. [DOI] [PubMed] [Google Scholar]
- 152.Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis 2008;40:650–8. [DOI] [PubMed] [Google Scholar]
- 153.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez CA, Sanz-Anquela JM, Gisbert JP, et al. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer 2013;133:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.RUGGE M, DE BONI M, PENNELLI G, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Alimentary Pharmacology & Therapeutics 2010;31:1104–1111. [DOI] [PubMed] [Google Scholar]
- 156.den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2019;68:585–593. [DOI] [PubMed] [Google Scholar]
- 157.Zhou Y, Li HY, Zhang JJ, et al. Operative link on gastritis assessment stage is an appropriate predictor of early gastric cancer. World J Gastroenterol 2016;22:3670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang RJ, Epplein M, Hamashima C, et al. An Approach to the Primary and Secondary Prevention of Gastric Cancer in the United States. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jin S, Jeon SW, Kwon Y, et al. Optimal Endoscopic Screening Interval for Early Detection of Gastric Cancer: a Single-Center Study. J Korean Med Sci 2018;33:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim YS, Park HA, Kim BS, et al. Efficacy of screening for gastric cancer in a Korean adult population: a case-control study. J Korean Med Sci 2000;15:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee H, Min BH, Lee JH, et al. Survival outcome associated with the screening interval for gastric cancer in Korea. Digestion 2011;84:142–8. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto Y, Yoshida N, Yano T, et al. Assessment of Outcomes From 1-Year Surveillance After Detection of Early Gastric Cancer Among Patients at High Risk in Japan. JAMA Netw Open 2022;5:e2227667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77 e1. [DOI] [PubMed] [Google Scholar]
- 164.Lansdorp-Vogelaar I, Meester RGS, Laszkowska M, et al. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract Res Clin Gastroenterol 2021;50–51:101735. [DOI] [PubMed] [Google Scholar]
- 165.Parsonnet J, Harris RA, Hack HM, et al. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet 1996;348:150–4. [DOI] [PubMed] [Google Scholar]
- 166.Lash JG, Genta RM. Adherence to the Sydney System guidelines increases the detection of Helicobacter gastritis and intestinal metaplasia in 400738 sets of gastric biopsies. Aliment Pharmacol Ther 2013;38:424–31. [DOI] [PubMed] [Google Scholar]
- 167.Yang YX, Brill J, Krishnan P, et al. American Gastroenterological Association Institute Guideline on the Role of Upper Gastrointestinal Biopsy to Evaluate Dyspepsia in the Adult Patient in the Absence of Visible Mucosal Lesions. Gastroenterology 2015;149:1082–7. [DOI] [PubMed] [Google Scholar]
- 168.Hamashima C, Fukao A. Quality assurance manual of endoscopic screening for gastric cancer in Japanese communities. Jpn J Clin Oncol 2016;46:1053–1061. [DOI] [PubMed] [Google Scholar]
- 169.Dilaghi E, Lahner E, Annibale B, et al. Systematic review and meta-analysis: Artificial intelligence for the diagnosis of gastric precancerous lesions and Helicobacter pylori infection. Dig Liver Dis 2022;54:1630–1638. [DOI] [PubMed] [Google Scholar]
- 170.Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol 2018;48:278–286. [DOI] [PubMed] [Google Scholar]