Abstract
Lambert-Eaton myasthenic syndrome (LEMS) is a rare neuromuscular junction disorder. Underlying small cell lung cancer is found in more than half of patients. Proximal muscle weakness, autonomic features and areflexia are typical manifestations. However, LEMS is often misdiagnosed. We report a rare case of paraneoplastic LEMS, identified amid admission due to a different diagnosis. Our patient was initially admitted due to aspiration pneumonia. Further investigation revealed clinical and electrophysiological manifestations of LEMS. High clinical suspicion and early diagnostic workup were paramount in the patient outcome. Nevertheless, paraneoplastic aetiology was difficult to confirm and revealed itself a difficult challenge. Clinical awareness is crucial to diagnose LEMS and urge cancer screening and early treatment.
Keywords: Neuromuscular disease, Lung cancer (oncology)
Background
The first report of a patient presenting neurological signs which improved after small cell lung cancer (SCLC) surgical removal dates to 1953.1 In 1956, defective neuromuscular transmissions associated with malignancy were reported.2 In 1957, Lambert and Eaton described the electrophysiological abnormalities of the newly recognised neuromuscular transmission disorder, identifying a new clinical syndrome—Lambert-Eaton myasthenic syndrome (LEMS).3
LEMS is a rare neuromuscular junction (NMJ) disorder, which occurs as a paraneoplastic or primary autoimmune disorder. Proximal weakness and autonomic dysfunction are the characteristic features. More than half of the cases are associated with SCLC—SCLC–LEMS.4 LEMS is an often-overlooked diagnosis. Its annual incidence is 0.6 per million and its prevalence is 2.8 per million.5
We report a rare case of paraneoplastic LEMS (P-LEMS), with a challenging diagnosis workup.
Case presentation
A man in his 70s was admitted to the emergency department after choking while eating. He was a heavy active smoker and had a 2-month history of depression and anxiety. He had been hospitalised a year before due to a brief psychotic episode. It manifested by acute persecutory delusion. No triggering events or causes were identified. The patient remained asymptomatic after discharge. He reported progressive, constant, symmetric lower limb muscle weakness over the previous year. In the preceding months, dysphagia to liquids and solid food, dysarthria and constipation developed. The patient had no cognitive impairment or additional psychiatric symptoms.
At admission, the patient was hypoxemic and pulmonary sounds were diminished through both hemithoraces. He presented with ptosis, poor palatal elevation, tetraparesis and hyporeflexia. Bicipital reflexes became normal after bicipital muscle contraction. A dysautomia revealed severely reduced laryngeal sensitivity. Cerebral CT showed no relevant lesions. An aspiration pneumonia diagnosis was made. The patient was admitted to the internal medicine ward.
Investigations
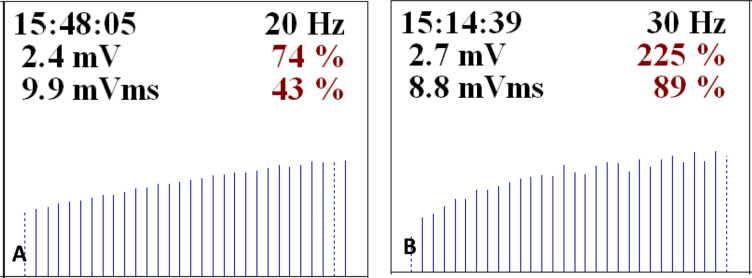
Electromyography showed a typical LEMS pattern (figure 1). Antibodies against presynaptic membrane P/Q-type voltage-gated calcium channels (VGCC) were positive. Evaluation of the cerebrospinal fluid (CSF) revealed 12 mononuclear leucocytes per µL, normal glycose and protein levels. Immunoglobulin G (IgG) index was 0.62 and no oligoclonal bands were detected. Microbiological culture and viral tests were negative. Anti-SOX-1 and anti-CV2/collapsin response mediator protein (CRMP) 5 antibodies were positive. CT of the thorax revealed a paravertebral 9 mm pulmonary nodule in the right lower lobe. Positron emission tomography (PET) was suggestive of hilar lymph node metastasis—initial SUVmax 10.6 and delayed SUVmax 14.3. Abdominopelvic CT, upper endoscopy and colonoscopy showed no evidence of neoplasms. A diagnosis of P-LEMS was made. Endobronchial ultrasound-guided bronchoscopies (EBUS) ganglion biopsies were performed, negative for malignant cells.
Figure 1.
Electromyography displaying a typical Lambert-Eaton myasthenic syndrome pattern. (A) Left trapezius muscle—20 Hz high-frequency stimulation of the homolateral accessory nerve resulting in 74% and 43% incremental response in CMAP amplitude and area, respectively. (B) Left fifth finger abductor muscle—30 Hz high-frequency stimulation of the homolateral cubital nerve resulting in 225% and 89% incremental response in CMAP amplitude and area, respectively. CMAP; compound muscle action potential.
Differential diagnosis
The main differential diagnosis when considering LEMS is myasthenia gravis (MG). Both disorders of the NMJ often present fatigable weakness. While LEMS presents more arm and leg weakness, MG is typically associated with ocular or bulbar symptoms. Different from MG, LEMS patients present an incremental response to high-frequency repetitive nerve stimulation in electromyography. Progressive muscle weakness should also prompt myopathies consideration. Unlike LEMS, deep tendon reflexes are preserved in myopathies. Neuromyotonia associated with Isaacs and Morvan syndromes may also cause motor weakness. However, it is typically manifested by sustained and visible muscle contractions, frequently prompting cramps. Amyotrophic lateral sclerosis presents with asymmetric limb weakness and electromyographic signs of denervation and reinnervation, absent in LEMS.
Treatment
After multidisciplinary discussion including the oncology department, due to successive biopsies without malignant cells, the uncertainty of the lung nodule neoplastic nature, poor surgical conditions to perform an excisional biopsy and considering P-LEMS could be preceding cancer presentation, a decision was made not to initiate antineoplastic treatment, keeping the patient under close follow-up.
3,4-Diaminopyridine was unavailable during the period of admission. Treatment with IV immunoglobulin (IVIg), prednisone and pyridostigmine was started with a satisfactory response. Dysphagia and hypophonia were improved, and autonomous gait was recovered.
Outcome and follow-up
A month after discharge, the patient was diagnosed with pulmonary thromboembolism associated with IVIg. Anticoagulation was started. IVIG therapy was suspended. He was readmitted multiple times during the following months due to pneumonia and pyelonephritis. Neurological symptoms and signs gradually worsened. The patient evolved with a severe functional decline during that period.
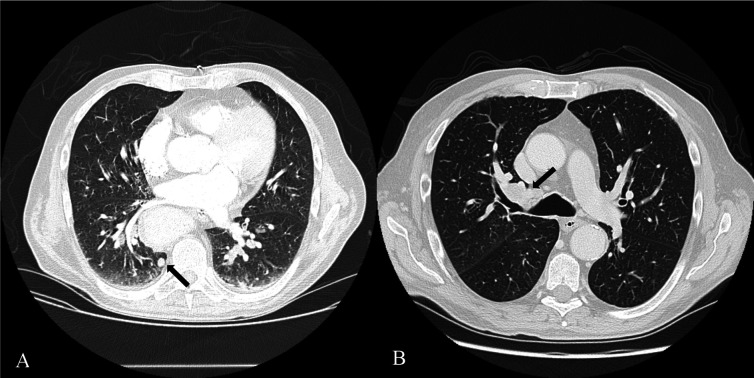
Six months after discharge, follow-up CT showed mediastinic ganglion dimensional and numerical increase and a de novo hilar mass (figure 2). A new EBUS-guided ganglion biopsy revealed SCLC metastasis.
Figure 2.
(A) CT of the thorax at the time of admission, showing 9 mm subpleural, perivertebral, in the right inferior pulmonary lobe. (B) Six-month follow-up CT, showing a 35 mm (long axis), heterogenous lesion in the right hilum, non-existing in previous exams.
Due to his frailty, cancer treatment was not started. The patient died about a year after the LEMS diagnosis.
Discussion
LEMS underlying mechanism is mediated by anti-VGCC antibodies. Calcium diffusion into the presynaptic terminal occurs through VGCC. Anti-VGCC antibodies lead to decreased acetylcholine (ACh) release and muscle weakness.4
About 60% of LEMS cases present underlying cancer.4 In P-LEMS, most patients are men (65%), and the median age of presentation is 60 years.5 Most patients are affected by SCLC, a smoking-related lung carcinoma with neuroendocrine features, which expresses VGCC. The initial humoral autoimmune response is presumed to be produced against the tumour VGCC subunit antigens.6
LEMS major clinical manifestation is proximal muscle weakness (96%), mostly in the lower limbs. Weakness usually spreads proximally to distally.6 Other main clinical findings are fatigue (18%), autonomic dysfunction (80%–96%) and areflexia.6 7 Oculobulbar symptoms and signs (78%), such as ptosis, diplopia, dysphagia and dysarthria, are often mild and transient. Dry mouth, impotence and constipation are the most common dysautomic symptoms. Deep tendon reflexes are usually absent or diminished but can be amplified after muscular contraction—postexercise facilitation.5 This is a characteristic feature that occurs in 40% of patients. Clinical onset is usually insidious.6 Anti-CRMP5 are well-characterised antibodies with a strong cancer association. Anti-CRMP5-associated paraneoplastic encephalitis is rare. Most cases are associated with SCLC, occurring in 5% of patients.8 Although the patient presented pleocytosis, he had no symptoms suggestive of encephalitis. CSF had no increased protein concentration, oligoclonal bands or elevated IgG index. The paraneoplastic encephalitis diagnosis was dismissed. Anti-CRMP5 positivity was considered in the LEMS alone.
LEMS diagnosis is based on clinical findings, characteristic electromyographic features (low compound muscle action potential at rest; decremental response to low-frequency stimulation; incremental response after brief exercise or high-frequency stimulation) and serology. On characteristic symptoms, typical electromyographic patterns are enough to establish a diagnosis. Anti-VGCC antibodies occur in 90% of patients and strongly support the diagnosis. Anti-SOX-1 antibodies are an independent predictor of SCLC and may be useful as a serological tumour marker.5 Their specificity for SCLC–LEMS is 95% and sensitivity is 65%.6
In most cases, LEMS diagnosis precedes cancer diagnosis by many months and up to 6 years. However, a cancer diagnosis may occur up to 5 years before LEMS onset. LEMS diagnosis should be followed by an extensive malignancy search. CT scan, MRI or a PET of the chest is recommended. Cancer screening should be carried out every 3–6 months for a minimum 2-year period.6
Management includes underlying tumour treatment and symptomatic control.6 Successful cancer treatment can be curative. 3,4-Diaminopyridine, a voltage-gated potassium channel blocker, is an effective symptomatic, first-line medical therapy. It induces longer motor nerve terminal depolarisation, providing more time for calcium channels to remain open. Consequently, calcium concentration at the motor nerve terminal increases and presynaptic release of ACh is augmented. Pyridostigmine, a cholinesterase inhibitor, offers limited benefit. However, it is safe, inexpensive and widely available. In patients showing limited improvement from 3,4-diaminopyridine treatment, immune therapy is used—prednisone, azathioprine, rituximab, IVIg and plasma exchange.9
LEMS is consistently responsive to immunotherapy or anticancer therapy.10 The prognosis varies according to the underlying aetiology. In P-LEMS, survival is limited by neoplastic progression. In the absence of malignancy, the survival rate is nearly normal.11 In SCLC, LEMS confers a significant, independent survival advantage, possibly due to an active immune response against cancer.12
Approximately 58% of patients get a misdiagnosis before LEMS diagnosis.13 High clinical suspicion is necessary for diagnosis, prompt neoplastic screening and optimal survival. Even in the absence of cancer in the preliminary work-up, patients with SOX-1 antibodies should be closely monitored.6
This clinical report illustrates unique LEMS diagnosis circumstances. Although the patient reported persistent manifestations of LEMS, the primary reason for admission was aspiration pneumonia. Furthermore, confirmation of paraneoplastic aetiology was particularly difficult. This case is a key reminder of the importance of a complete clinical approach. It is essential to raise clinical awareness of LEMS, a rare medical condition.
Learning points.
Lambert-Eaton myasthenic syndrome (LEMS) is a rare neuromuscular junction disorder, which occurs as a paraneoplastic or primary autoimmune disorder.
More than half of the cases are associated with small cell lung cancer.
LEMS is an often-overlooked diagnosis. Clinicians should look out for characteristic postexercise facilitation and discrepancy between the degree of muscle weakness and functional limitation.
Clinical awareness is crucial to diagnose LEMS and urge cancer screening and early treatment.
Acknowledgments
The authors would like to thank Márcio Cardoso MD and Sara Duarte MD, from the department of neurophysiology and neurology, Hospital de Santo António, Centro Hospitalar Universitário do Porto, for their collaboration.
Footnotes
Contributors: LV was involved in drafting, writing, literature review and final approval. SRM and SXP were involved in literature review and final approval. JN was involved in editing and final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Anderson HJ, Churchill-Davidson HC, Richardson AT. Bronchial neoplasm with myasthenia; prolonged apnoea after administration of succinylcholine. Lancet 1953;265:1291–3. 10.1016/s0140-6736(53)91358-0 [DOI] [PubMed] [Google Scholar]
- 2.Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasms. Am J Physiol 1956;187:612–3. [Google Scholar]
- 3.EATON LM, LAMBERT EH. Electromyography and electric stimulation of nerves in diseases of motor unit; observations on myasthenic syndrome associated with malignant tumors. J Am Med Assoc 1957;163:1117–24. 10.1001/jama.1957.02970480021005 [DOI] [PubMed] [Google Scholar]
- 4.Jayarangaiah A, Theetha Kariyanna P. StatPearls. In: Lambert Eaton Myasthenic Syndrome. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 5.Ivanovski T, Miralles F. Lambert-Eaton myasthenic syndrome: early diagnosis is key. Degener Neurol Neuromuscul Dis 2019;9:27–37. 10.2147/DNND.S192588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesner VG, Oh SJ, Dimachkie MM, et al. Lambert-eaton myasthenic syndrome. Neurol Clin 2018;36:379–94. 10.1016/j.ncl.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirtz PW, Smallegange TM, Wintzen AR, et al. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg 2002;104:359–63. 10.1016/s0303-8467(02)00054-9 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim Ismail I, K John J, Ibrahim M, et al. Paraneoplastic limbic encephalitis associated with anti-CV2/CRMP5 antibodies secondary to thymoma in an adolescent. Case Rep Neurol 2020;12:50–5. 10.1159/000505232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodkin C, Pascuzzi RM. Update in the management of myasthenia gravis and lambert-eaton myasthenic syndrome. Neurol Clin 2021;39:133–46. 10.1016/j.ncl.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Chalk CH, Murray NM, Newsom-Davis J, et al. Response of the Lambert-Eaton myasthenic syndrome to treatment of associated small-cell lung carcinoma. Neurology 1990;40:1552–6. 10.1212/wnl.40.10.1552 [DOI] [PubMed] [Google Scholar]
- 11.Anwar A, Saleem S, Ahmed MF, et al. Recent advances and therapeutic options in Lambert-Eaton myasthenic syndrome. Cureus 2019;11:e5450. 10.7759/cureus.5450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titulaer MJ, Verschuuren JJGM. Lambert-Eaton myasthenic syndrome: tumor versus nontumor forms. Ann N Y Acad Sci 2008;1132:129–34. 10.1196/annals.1405.030 [DOI] [PubMed] [Google Scholar]
- 13.Wirtz PW, van Dijk JG, van Doorn PA, et al. The epidemiology of the Lambert-Eaton myasthenic syndrome in the Netherlands. Neurology 2004;63:397–8. 10.1212/01.wnl.0000130254.27019.14 [DOI] [PubMed] [Google Scholar]