Abstract
STUDY QUESTION
What is the impact of cancer or hematological disorders on germ cells in pediatric male patients?
SUMMARY ANSWER
Spermatogonial quantity is reduced in testes of prepubertal boys diagnosed with cancer or severe hematological disorder compared to healthy controls and this reduction is disease and age dependent: patients with central nervous system cancer (CNS tumors) and hematological disorders, as well as boys <7 years are the most affected.
WHAT IS KNOWN ALREADY
Fertility preservation in pediatric male patients is considered based on the gonadotoxicity of selected treatments. Although treatment effects on germ cells have been extensively investigated, limited data are available on the effect of the disease on the prepubertal male gonad. Of the few studies investigating the effects of cancer or hematologic disorders on testicular function and germ cell quantity in prepuberty, the results are inconsistent. However, recent studies suggested impairments before the initiation of known gonadotoxic therapy. Understanding which diseases and at what age affect the germ cell pool in pediatric patients before treatment is critical to optimize strategies and counseling for fertility preservation.
STUDY DESIGN, SIZE, DURATION
This multicenter retrospective cohort study included 101 boys aged <14 years with extra-cerebral cancer (solid tumors), CNS tumors, leukemia/lymphoma (blood cancer), or non-malignant hematological disorders, who were admitted for a fertility preservation programme between 2002 and 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In addition to clinical data, we analyzed measurements of testicular volume and performed histological staining on testicular biopsies obtained before treatment, at cryopreservation, to evaluate number of spermatogonia per tubular cross-section, tubular fertility index, and the most advanced germ cell type prior to chemo-/radiotherapy. The controls were data simulations with summary statistics from original studies reporting healthy prepubertal boys’ testes characteristics.
MAIN RESULTS AND THE ROLE OF CHANCE
Prepubertal patients with childhood cancer or hematological disorders were more likely to have significantly reduced spermatogonial quantity compared to healthy controls (48.5% versus 31.0% prevalence, respectively). The prevalence of patients with reduced spermatogonial quantity was highest in the CNS tumor (56.7%) and the hematological disorder (55.6%) groups, including patients with hydroxyurea pre-treated sickle cell disease (58.3%) and patients not exposed to hydroxyurea (50%). Disease also adversely impacted spermatogonial distribution and differentiation. Irrespective of disease, we observed the highest spermatogonial quantity reduction in patients <7 years of age.
LIMITATIONS, REASONS FOR CAUTION
For ethical reasons, we could not collect spermatogonial quantity data in healthy prepubertal boys as controls and thus deployed statistical simulation on data from literature. Also, our results should be interpreted considering low patient numbers per (sub)group.
WIDER IMPLICATIONS OF THE FINDINGS
Cancers, especially CNS tumors, and severe hematological disorders can affect spermatogonial quantity in prepubertal boys before treatment. Consequently, these patients may have a higher risk of depleted spermatogonia following therapies, resulting in persistent infertility. Therefore, patient counseling prior to disease treatment and timing of fertility preservation should not only be based on treatment regimes, but also on diagnoses and age.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by Marie Curie Initial Training Network (ITN) (EU-FP7-PEOPLE-2013-ITN) funded by European Commision grant no. 603568; ZonMW Translational Adult stem cell research (TAS) grant no. 116003002. No competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: fertility preservation, spermatogonia, prepubertal boys, pediatric oncology, hematological disorders, male fertility, testicular tissue cryopreservation
Introduction
The current 5-year survival rate for childhood cancer patients is over 80% (Robison and Hudson, 2014). As most patients survive into adulthood (Smith et al., 2010; Winther et al., 2015), disease and treatment side effects, including fertility impairment, and timely fertility preservation are increasingly important (Magelssen et al., 2006; Nathan et al., 2008; Diller et al., 2009; Green et al., 2014).
For prepubertal boys, conditioning therapies for hematopoietic stem cell transplantation, abdominal/pelvic radiotherapy, and high-cumulative dose alkylating agent-based chemotherapy are major risks for germ cell depletion and infertility (Poganitsch-Korhonen et al., 2017; Mulder et al., 2021). These therapies are used in both cancer and non-malignant hematological disorders patients (Loren and Senapati, 2019). In addition, prepubertal boys with severe hematological disorders, i.e. sickle cell disease (SCD), receiving hydroxyurea (HU) treatment to prevent vaso-occlusive crises (Stukenborg et al., 2018; Portela et al., 2020; Benninghoven-Frey et al., 2022), and the ones who do not receive HU (Gille et al., 2021), were recently acknowledged to face germ cell loss with possible long-term fertility consequences and are currently more frequently counseled for fertility preservation.
Although gonadotoxicity has been extensively investigated, limited data are available on the disease’s effect on prepubertal male gonad development. Of the few studies investigating the effects of cancer or a hematologic disorder on testicular function and germ cell quantity in prepuberty (Crofton et al., 2003; Krawczuk-Rybak et al., 2012; Wigny et al., 2016; Stukenborg et al., 2018), the results are inconsistent. However, a few recent studies indicate impairments before initiation of known gonadotoxic therapy (Wigny et al., 2016; Stukenborg et al., 2018; Gille et al., 2021). Understanding which diseases at what age affect the germ cell pool in pediatric patients before treatment is critical to optimize strategies and counseling for fertility preservation.
Here, we determine disease effects on the quantity, distribution, and differentiation of spermatogonia, the precursors of spermatozoa, in the testes of boys diagnosed with cancer or a severe hematological disorder prior to mainline treatment.
Materials and methods
Ethical approval
Ethical approvals came from the Dutch Central Committee on research involving human subjects (CCMO) (approval NL27690.000.09), Medical Ethics Review Committee of Amsterdam UMC (AUMC) (approval 2009-132), and the Universitair Ziekenhuis Brussel (approval 2000/149D) to use prepubertal tissue for research, with parents/caregivers’ (and patients, if applicable) written informed consent.
Patients
Testicular tissue samples and clinical data from prepubertal boys aged up to 14 years, who participated in fertility preservation programmes prior to chemo-/radiotherapy treatment at Amsterdam UMC (AUMC) between 2011 and 2018 and Universitair Ziekenhuis Brussel (UZB) between 2002 and 2017 were included. The older boys in our cohort were offered testicular tissue cryopreservation, because they could not produce an ejaculate. At AUMC, prepubertal boys were offered fertility preservation before the initiation of known gonadotoxic therapy, irrespective of the infertility risk. At UZB, only children at high risk for infertility (above 80%), which was estimated based on the selected treatment, were included in the fertility preservation programme. Prepubertal patients considered to be at high risk of infertility include the ones receiving high-dose alkylating and platinum-based agents, total body irradiation, or testicular radiotherapy, yet the criteria are not strictly established (Goossens et al., 2020; Delgouffe et al., 2022).
At both centers, children with a history of testicular torsion or cryptorchidism or with testicular malignancies were excluded from the fertility preservation programmes. Testicular malignancies were excluded because of the risk of reintroducing cancer cells during auto-transplantation of the testicular biopsy and because testicular malignancies are known to negatively affect gonadal function (Petersen et al., 1998) and can possibly negatively affect future fertility treatment. Patients diagnosed with Klinefelter syndrome were also excluded from this study.
Clinical data, classification and grouping
Patient data were identified through AUMC and UZB records, including age at time of testicular biopsy, testicular volume by ultrasound, SD of height- and weight-for-age z-score (WHO, 2006), general health condition, potential pre-treatment, and disease diagnosis. Based on diagnoses, we classified patients into four groups: extra-cerebral cancer (solid tumors), central nervous system cancer (CNS tumors), leukemia and lymphoma (blood cancer), and non-malignant hematological disorders.
We analyzed the patient data in the age groups 0 to <4, 4 to <7, 7 to <11, and 11 to <14 years, to reflect the prepubertal testicular and germ cell developmental periods, namely, gonocyte differentiation into spermatogonia and dispersion throughout elongating seminiferous tubules; proliferation of spermatogonia to populate the testes; gradual maturation of somatic testicular environment; and finally Sertoli cell maturation allowing for spermatogonial differentiation into spermatocytes and later spermatids, respectively (Masliukaite et al., 2016).
Tissue preparation and immunohistochemistry
Following testicular biopsy, a portion of fresh tissue was fixed in formalin-based fixative for the UZB samples (VWR International, Leuven, Belgium) or diluted Bouin’s or modified methacarn (Jan et al., 2017) for the AUMC samples and embedded in paraffin. For immunohistological analysis to identify spermatogonia, 5 µm thick sections were dewaxed, rehydrated, and washed in PBS. Endogenous peroxidase was blocked with 0.3% (v/v) hydrogen peroxide in methanol (Merck KGaA, Darmstadt, Germany), and heat-mediated antigen retrieval was performed in 0.01 M citric acid (pH 6.0; J.T.Baker, NJ, USA). Sections were blocked against non-specific binding with 4% normal goat serum (Tebu-bio, Boechout, Belgium) or Superblock (SkyTek laboratories, UT, USA) and incubated with the primary antibody against MAGE-A4 (mouse monoclonal anti-melanoma-associated antigen 4, 1:100–1:200; gift from Dr. Spagnoli), which is a specific marker for spermatogonia (bright immunostaining) (Takahashi et al., 1995), overnight at 4°C. Following washing, sections were incubated with a peroxidase-conjugated anti-mouse antibody (Dako, Heverlee, Belgium or Immunologic, Duiven, The Netherlands) for 1 h. Staining was visualized with 3,3′-diaminobenzidine (Dako or Immunologic) as chromogen and hematoxylin as counterstain.
Determining spermatogonial quantity, distribution and differentiation
Two or more sections of each sample, at least 25 µm apart, were analyzed by microscope (Olympus IX81 or BX41, Tokyo, Japan). Between 30 and 100 round/oval shaped tubular cross-sections (tubules with a diameter maximum 1.5 times the perpendicular diameter) (Ntemou et al., 2019) per patient were analyzed to quantify spermatogonia per tubular cross-section (S/T) by counting MAGE-A4bright germ cells at the basal membrane of the seminiferous tubules. To analyze the distribution of spermatogonia in the testes, the percentage of tubular cross-sections containing spermatogonia, namely the tubular fertility index (TFI), was recorded. To analyze the differentiation capacity of spermatogonia, the most advanced germ cell type for each patient was noted.
Control data, simulation and modeling
Considering we could not collect S/T data from healthy prepubertal boys as controls for ethical reasons, we simulated control S/T values (n = 310) using the summary statistics (age group range and the number of subjects, and S/T mean and SD) from original studies (Hedinger, 1982; Paniagua and Nistal, 1984; Hadziselimovic et al., 1987; Cinti et al., 1993) that were selected in a systematic literature review and meta-analysis (Masliukaite et al., 2016) as reporting spermatogonial quantity throughout healthy prepuberty (thus excluding children with cryptorchidism, testicular tumors, or other health conditions that might influence spermatogenesis).
Control data simulations were performed in R (i386 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) using general functions and the ‘truncnorm’ package. ‘Truncnorm’ creates a requested spreading of subjects to fit the original study mean and SD per each age group (Supplementary Data File S1). In this way, even though we could not obtain the original datasets, we created a simulated dataset with the same summary statistics for each parameter of interest (spermatogonial quantity, TFI, and testicular volume) from each original study. We then pooled the simulated data from different studies together to form a control dataset (model). To account for model uncertainty, data simulation was repeated to create five independent control datasets (models): each having different subject distribution within the SD. To validate the models, we plotted these simulated controls of spermatogonial quantity against the regression fit with 95% CI from meta-analysis (Masliukaite et al., 2016) and indicated the four age groups used in this study (Supplementary Fig. S1). When performing statistical analysis, a statistical significance was only considered when the difference between samples and controls was significant within at least 4 out of 5 control models. If the observed significant difference was repeated in the majority of models, the likelihood of such significant difference in the original dataset was regarded as highly likely.
Similarly, we simulated testicular volume control values based on data available from healthy Dutch boys (Goede et al., 2011; Joustra et al., 2015) and TFI control values based on summary statistics in boys without known health conditions affecting testes histology (Farrington, 1969; Paniagua and Nistal, 1984).
Finally, control data on germ cell differentiation were based on a descriptive study of healthy prepubertal boys’ autopsies, excluding cases with testicular or endocrine pathologies or samples with complete spermatogenesis (Nistal and Paniagua, 1984), with no data simulation.
Calculating the prevalence
We deployed age-specific S/T normative values (Masliukaite et al., 2016) to calculate the prevalence of patients having reduced spermatogonial quantity. We selected a lower limit of 95% CI of the normative reference curve as the cutoff for S/T below the normative quantity and calculated the proportion of patients falling below this cutoff. In the same way, we estimated the prevalence of decreased spermatogonia in the simulated S/T controls in all five models separately.
Statistical analysis
Statistical analysis for baseline characteristics used a Pearson chi-square test between different disease groups per variable. To evaluate the disease effect on spermatogonial quantity throughout prepuberty, we assessed patients’ likelihood of having spermatogonial quantity below the normative cutoff value by performing Fisher’s exact test comparing the prevalence of patients versus controls having reduced spermatogonial quantity. We repeated the testing five times with each simulated control dataset and compared the patient data with each simulated control dataset per disease group and per selected age group using an independent sample T-test or Mann–Whitney U test as appropriate; we repeated the latter analysis with testicular volume and the TFI variables. All analyses were performed using SPSS (v24.0.0.1; IBM corp., NY, USA), and P < 0.05 was set for significance.
Results
Patient data included in the study
A total of 130 prepubertal boys were included in fertility preservation programmes prior to gonadotoxic cancer therapy at AUMC and UZB and underwent testicular biopsy (Supplementary Fig. S2). For 121 (93%) patients, a small fragment of their testicular tissue was analyzed (S/T, TFI and most advanced germ cell type), while from nine patients no tissue was processed for histological analysis. In 20 cases, we could not count S/T because the testicular sample’s morphology was damaged, or the material was insufficient to evaluate ≥30 cross-sections of seminiferous tubules. The data of 101 patients (78%) with sufficient material to count MAGE-A4 positive spermatogonia were included in the analysis.
Patient characteristics
Among the 101 included cases, 34.7% had solid tumor, 29.7% CNS tumor, 17.8% blood cancer, and 17.8% hematological disorder (Table I). Rhabdomyosarcoma and Ewing sarcoma patients comprised 60.0% of the solid tumor group. Medulloblastomas were the most frequent in the CNS tumor group (43.3%) and Hodgkin’s lymphoma in the blood cancer group (27.8%). In all three cancer groups, most presented without metastatic disease (68.6% in solid tumor, 83.3% in CNS tumor, and 77.8% in blood cancer patients). Of the CNS tumor patients, 73.3% had hydrocephalus at diagnosis, and 53.3% had tumors in the posterior fossa area. All patients with posterior fossa tumors had hydrocephalus (n = 16). In the hematological disorders group, SCD was the most common diagnosis (66.7%). All SCD patients were treated with HU 25 mg/kg/day until the day before biopsy. The mean patient age at diagnosis (±SD) was 7.3 ± 4.1 years (range 0.5–14.0). Most patients had normal height (80.2%) and weight (81.2%) for their age, and 54.5% displayed no general health problems (i.e. nausea, vomiting, unwillingness to eat, headaches, tiredness, irritability, neurological symptoms, fever, infections, or endocrine problems). We found no differences in age at diagnosis between all four disease groups or between patient frequency counts in the SD of height- and weight-for-age z-score categories or general health condition categories between the cancer groups.
Table I.
Baseline characteristics in prepubertal boys admitted to fertility preservation programmes at AUMC and UZB.
| Disease group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 101) |
Solid tumors (n = 35) |
CNS tumors (n = 30) |
Blood cancers (n = 18) |
Hema-tological disorders (n = 18) |
P | ||||||
| Baseline characteristic | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Diagnosis | |||||||||||
| Rhabdomyosarcoma | 13 | 12.9% | 13 | 37.1% | — | — | — | — | — | — | NA |
| Ewing sarcoma | 8 | 7.9% | 8 | 22.9% | — | — | — | — | — | — | |
| Osteosarcoma | 5 | 5.0% | 5 | 14.3% | — | — | — | — | — | — | |
| Soft tissue sarcoma | 4 | 4.0% | 4 | 11.4% | — | — | — | — | — | — | |
| Hepatoblastoma | 2 | 2.0% | 2 | 5.7% | — | — | — | — | — | — | |
| Neuroblastoma | 2 | 2.0% | 2 | 5.7% | — | — | — | — | — | — | |
| Nasopharyngeal carcinoma | 1 | 1.0% | 1 | 2.9% | — | — | — | — | — | — | |
| Medulloblastoma | 13 | 12.9% | — | — | 13 | 43.3% | — | — | — | — | NA |
| Extragonadal germ cell tumor | 5 | 5.0% | — | — | 5 | 16.7% | — | — | — | — | |
| Glioma | 5 | 5.0% | — | — | 5 | 16.7% | — | — | — | — | |
| Ependymoma | 3 | 3.0% | — | — | 3 | 10.0% | — | — | — | — | |
| Brain tumor (unspecified) | 2 | 2.0% | — | — | 2 | 6.7% | — | — | — | — | |
| Astrocytoma | 1 | 1.0% | — | — | 1 | 3.3% | — | — | — | — | |
| Intracranial CNS tumor located parieto occipital | 1 | 1.0% | — | — | 1 | 3.3% | — | — | — | — | |
| Hodgkin’s lymphoma | 5 | 5.0% | — | — | — | — | 5 | 27.8% | — | — | NA |
| ALL | 4 | 4.0% | — | — | — | — | 4 | 22.2% | — | — | |
| Non-Hodgkin’s lymphoma | 3 | 3.0% | — | — | — | — | 3 | 16.7% | — | — | |
| AML | 2 | 2.0% | — | — | — | — | 2 | 11.1% | — | — | |
| Histiocytosis | 2 | 2.0% | — | — | — | — | 2 | 11.1% | — | — | |
| Lymphoma (unspecified) | 1 | 1.0% | — | — | — | — | 1 | 5.6% | — | — | |
| MDS | 1 | 1.0% | — | — | — | — | 1 | 5.6% | — | — | |
| Sickle cell disease | 12 | 11.9% | — | — | — | — | — | — | 12 | 66.7% | NA |
| Granulomatous disease | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Idiopathic medullary aplasia | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Inherited bone marrow failure syndrome | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Primary immune deficiency | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Severe aplastic anemia | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Thalassemia | 1 | 1.0% | — | — | — | — | — | — | 1 | 5.6% | |
| Metastatic disease | |||||||||||
| Present | — | — | 11 | 31.4% | 4 | 13.3% | 2 | 11.1% | — | — | 0.145 |
| Absent | — | — | 24 | 68.6% | 25 | 83.3% | 14 | 77.8% | — | — | |
| Missing data | — | — | 0 | 0.0% | 1 | 3.3% | 2 | 11.1% | — | — | |
| Hydrocephalus at diagnosis | |||||||||||
| Present | — | — | — | — | 22 | 73.3% | — | — | — | — | NA |
| Absent | — | — | — | — | 7 | 23.3% | — | — | — | — | |
| Missing data | — | — | — | — | 1 | 3.3% | — | — | — | — | |
| Fossa tumor | |||||||||||
| Present | — | — | — | — | 16 | 53.3% | — | — | — | — | NA |
| Absent | — | — | — | — | 9 | 30.0% | — | — | — | — | |
| Missing data | — | — | — | — | 5 | 16.7% | — | — | — | — | |
| Hydroxyurea pre-treatment | |||||||||||
| None | — | — | — | — | — | — | — | — | 6 | 33.3% | NA |
| 25 mg/kg/day | — | — | — | — | — | — | — | — | 12 | 66.7% | |
| Age at diagnosis (year) | |||||||||||
| 0–4 | 31 | 30.7% | 13 | 37.1% | 10 | 33.3% | 4 | 22.2% | 4 | 22.2% | 0.962 |
| 4–7 | 17 | 16.8% | 5 | 14.3% | 5 | 16.7% | 3 | 16.7% | 4 | 22.2% | |
| 7–11 | 33 | 32.7% | 11 | 31.4% | 8 | 26.7% | 7 | 38.9% | 7 | 38.9% | |
| 11–14 | 20 | 19.8% | 6 | 17.1% | 7 | 23.3% | 4 | 22.2% | 3 | 16.7% | |
| Height (SD) | |||||||||||
| Stunted (<−2 SD of z-score) | 2 | 2.0% | — | — | — | — | 1 | 5.6% | 1 | 5.6% | 0.114* |
| Normal (−2 SD to 2 SD of z-score) | 81 | 80.2% | 35 | 100.0% | 27 | 90.0% | 16 | 88.9% | 3 | 16.7% | |
| Over (>2 SD of z-score) | 2 | 2.0% | — | — | 2 | 6.7% | — | — | — | — | |
| Missing data | 16 | 15.8% | — | — | 1 | 3.3% | 1 | 5.6% | 14 | 77.8% | |
| Weight (SD) | |||||||||||
| Underweight (<−2 SD of z-score) | 1 | 1.0% | — | — | 1 | 3.3% | — | — | — | — | 0.580* |
| Normal (−2 SD to 2 SD of z-score) | 82 | 81.2% | 33 | 94.3% | 27 | 90.0% | 17 | 94.4% | 5 | 27.8% | |
| Overweight (>2 SD of z-score) | 3 | 3.0% | 2 | 5.7% | 1 | 3.3% | — | — | — | — | |
| Missing data | 15 | 14.9% | — | — | 1 | 3.3% | 1 | 5.6% | 13 | 72.2% | |
| General health | |||||||||||
| Affected | 16 | 15.8% | 5 | 4.3% | 7 | 23.3% | 2 | 11.1% | 2 | 11.1% | 0.485* |
| Not affected | 55 | 54.5% | 27 | 77.1% | 18 | 60.0% | 10 | 55.6% | — | — | |
| Missing data | 30 | 29.7% | 3 | 8.6% | 5 | 16.7% | 6 | 33.3% | 16 | 88.9% | |
P is significant if <0.05 as measured by Pearson’s chi-square test comparing patient distribution between different disease groups per category of a variable.
Hematological disorders group was excluded from comparison due to the large number of missing values.
CNS, central nervous system; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; NA, not applicable; AUMC, Amsterdam UMC; UZB, Universitair Ziekenhuis Brussel.
Prevalence and likelihood of patients with reduced spermatogonial quantity
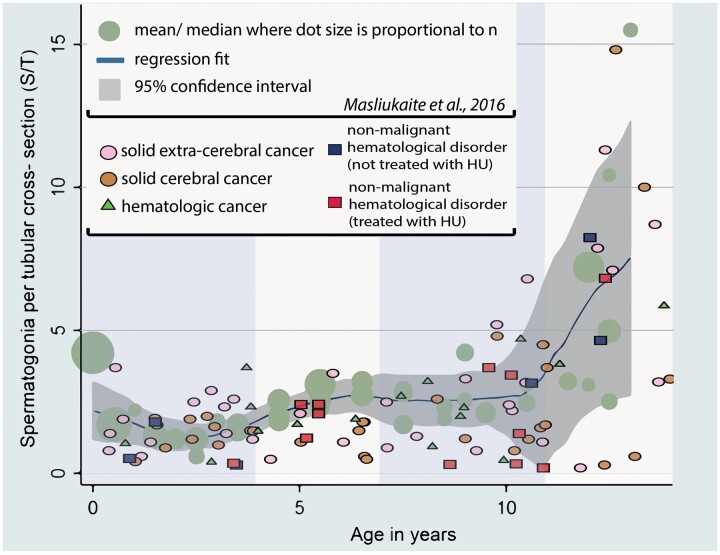
In general, patients were more likely to have reduced S/T quantity compared to the simulated controls: 48.5% versus 31.0%, respectively (P < 0.05) (Table II, based on simulated Model 1 (Supplementary Table SI and Fig. 1)). This was also true when HU pre-treated patients were excluded from the patient cohort (47.2% versus 31.0%, P < 0.05). This trend was confirmed throughout five simulation models (Supplementary Table SI). In addition, 2.0% of the patients (or 1.1% of patients when HU-pretreated cases were excluded) had complete absence of spermatogonia compared to 0.6–1.0% in the control simulation models (significance calculations were omitted owing to the low number of cases) (Supplementary Table SII). Per disease group, CNS tumor and hematological disorder patients were more likely to present with reduced S/T quantity compared to simulated controls (P < 0.05 in 5/5 models and P < 0.05 in 4/5 models, respectively), with the highest prevalence of reduced S/T quantity in HU-treated SCD patients (58.3%).
Table II.
The prevalence of patients with reduced spermatogonial quantity within each disease group and sub-group compared to simulated controls.
| Patients with decreased S/T (below reference 95% CIa) |
|||||
|---|---|---|---|---|---|
| n cases | % per (sub)group | OR (95% CI) | P rangec | ||
| Simulated controlsb (n = 310) | 96 | 31.0% | |||
| Patients total (n = 101) | 49 | 48.5% | 2.1 (1.3–3.3) | <0.05** | |
| Patients excluding HU treated (n = 89) | 42 | 47.2% | 2.0 (1.2–3.2) | <0.05** | |
| Patients per disease group | |||||
| Solid tumors (n = 35) | 14 | 40.0% | 1.5 (0.7–3.0) | 0.25–0.45 | |
| CNS tumors (n = 30) | 17 | 56.7% | 2.9 (1.4–6.2) | <0.05** | |
| Blood cancers (n = 18) | 8 | 44.4% | 1.8 (0.7–4.7) | 0.19–0.31 | |
| Hematological disorders (n = 18) | 10 | 55.6% | 2.8 (1.1–7.3) | 0.03–0.07* | |
| Patients per hematologic disorders sub-group | |||||
| HU-treated SCD (n = 12) | 7 | 58.3% | 3.1 (1.0–10.1) | 0.05–0.11 | |
| Non-treated other diagnosis (n = 6) | 3 | 50.0% | 2.2 (0.4–11.2) | 0.37–0.40 | |
The prevalence of cases below 95% CI of normative values as in Masliukaite et al. (2016).
Values from simulated Model 1.
P values from five models when the prevalence of patients having reduced S/T were compared to simulated controls having reduced S/T.
S/T, spermatogonia per tubular cross-section; OR, odds ratio when prevalence in patients were compared to prevalence in simulated controls in Model 1; CNS, central nervous system; HU, hydroxyurea; SCD, sickle cell disease.
Significance in 5/5 models.
Significance in 4/5 models.
Figure 1.
Spermatogonia per tubular cross-section in a patient population with cancer or hematological disorders and in simulated control datasets. S/T per patient per disease category and per hydroxyurea (HU) treatment against the reference values of spermatogonia per tubular cross-section in healthy prepubertal boys from the meta-analysis (Masliukaite et al., 2016). The vertical marking divides the graph to age groups of 0–4, 4–7, 7–11, and 11–14 years.
Testicular volume, spermatogonial quantity, distribution and differentiation per age group
As testicular development depends on prepubertal boys’ age, we compared testicular volume, S/T and TFI per age group (0–4, 4–7, 7–11, and 11–14 years) with the control data in five simulation models. The mean testicular volume in all patient and age groups was comparable to the simulated controls (Table III), yet it is important to mention that testicular volume data were missing for all SCD patients.
Table III.
Spermatogenic parameters per age group per disease group.
| Number of spermatogonia (S/T) |
Testes size (ml) |
TFI (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n (missing) | P rangeb | Mean | SD | n (missing) | P rangeb | Mean | SD | n (missing) | P rangeb | ||
| Simulated controlsa | |||||||||||||
| 0–4 | 2.17 | 1.34 | 143 | 0.51 | 0.12 | 133 | 51.64 | 11.72 | 55 | ||||
| 4–7 | 2.40 | 1.08 | 63 | 0.62 | 0.2 | 135 | 67.37 | 10.29 | 38 | ||||
| 7–11 | 2.41 | 1.06 | 61 | 0.96 | 0.64 | 224 | 76.11 | 20.62 | 62 | ||||
| 11–14 | 6.76 | 4.46 | 43 | 3.61 | 2.92 | 152 | 86.40 | 11.83 | 10 | ||||
| Patients per age group | |||||||||||||
| Total patients | |||||||||||||
| 0–4 | 1.37 | 0.90 | 31 | <0.001** | 0.55 | 0.24 | 19 (12) | 0.21–0.42 | 51.65 | 23.76 | 31 | <0.05** | |
| 4–7 | 1.43 | 0.78 | 17 | ≤0.001** | 0.67 | 0.41 | 11 (6) | 0.64–0.74 | 51.88 | 22.21 | 17 | <0.05* | |
| 7–11 | 2.13 | 1.61 | 33 | 0.18–0.47 | 1.09 | 0.74 | 18 (15) | 0.16–0.46 | 57.62 | 26.26 | 33 | ≤0.001** | |
| 11–14 | 7.41 | 5.57 | 20 | 0.43–0.79 | 3.68 | 2.47 | 13 (7) | 0.75–0.99 | 75.05 | 29.64 | 20 | 0.02–0.42 | |
| Total patients without HU exposure | |||||||||||||
| 0–4 | 1.41 | 0.89 | 30 | <0.001** | 0.55 | 0.24 | 19 (12) | 0.21–0.42 | 53.07 | 22.79 | 30 | <0.05** | |
| 4–7 | 1.31 | 0.81 | 13 | ≤0.001** | 0.67 | 0.41 | 11 (6) | 0.64–0.74 | 46.62 | 21.45 | 13 | <0.05** | |
| 7–11 | 2.30 | 1.59 | 27 | 0.34–0.88 | 1.09 | 0.74 | 18 (15) | 0.16–0.46 | 61.89 | 21.37 | 27 | <0.05** | |
| 11–14 | 7.45 | 5.72 | 19 | 0.43–0.79 | 3.68 | 2.47 | 13 (7) | 0.75–0.99 | 74.00 | 30.07 | 19 | 0.02–0.32 | |
| Patients per age group per disease group | |||||||||||||
| Solid tumors | |||||||||||||
| 0–4 | 1.64 | 0.91 | 13 | 0.12–0.18 | 0.55 | 0.11 | 11 (2) | 0.04–0.21 | 62.00 | 19.64 | 13 | 0.46–0.89 | |
| 4–7 | 1.60 | 1.14 | 5 | 0.06–0.11 | 0.86 | 0.56 | 5 | 0.38–0.46 | 53.40 | 23.64 | 5 | 0.16–0.63 | |
| 7–11 | 2.50 | 1.88 | 11 | 0.58–0.63 | 0.97 | 0.37 | 8 (3) | 0.23–0.50 | 62.73 | 19.90 | 11 | <0.05** | |
| 11–14 | 6.20 | 4.02 | 6 | 0.52–0.96 | 4.27 | 2.4 | 5 (1) | 0.36–0.58 | 74.00 | 36.62 | 6 | 0.27–0.96 | |
| CNS tumors | |||||||||||||
| 0–4 | 1.22 | 0.52 | 10 | <0.05** | 0.58 | 0.47 | 5 (5) | 0.25–0.43 | 47.80 | 16.05 | 10 | ≤0.001** | |
| 4–7 | 0.90 | 0.56 | 5 | <0.001** | 0.51 | 0.15 | 5 | 0.11–0.24 | 39.00 | 25.05 | 5 | <0.05* | |
| 7–11 | 2.10 | 1.54 | 8 | 0.11–0.18 | 1.43 | 1.21 | 6 (2) | 0.25–0.40 | 55.88 | 21.76 | 8 | <0.05* | |
| 11–14 | 7.03 | 7.06 | 7 | 0.46–0.76 | 2.94 | 2.55 | 7 | 0.26–0.52 | 66.71 | 36.12 | 7 | 0.09–0.60 | |
| Blood cancers | |||||||||||||
| 0–4 | 1.67 | 1.46 | 4 | 0.40–0.43 | 0.53 | 0.25 | 2 (2) | 0.89–0.99 | 55.50 | 35.45 | 4 | 0.41–0.61 | |
| 4–7 | 1.50 | 0.21 | 3 | 0.02–0.06* | 0.56 | — | 1 (2) | — | 48.00 | 11.14 | 3 | <0.05** | |
| 7–11 | 2.13 | 1.42 | 7 | 0.47–0.64 | 0.83 | 0.14 | 4 (3) | 0.63–0.93 | 63.57 | 24.30 | 7 | 0.02–0.07 | |
| 11–14 | 10.67 | 7.02 | 4 | 0.17–0.33 | 6.00 | — | 1 (3) | — | 80.00 | 15.08 | 4 | 0.20–0.84 | |
| Hematological disorders | |||||||||||||
| 0–4 | 0.55 | 0.71 | 4 | <0.05** | 0.50 | — | 1 (3) | — | 23.75 | 22.44 | 4 | <0.001** | |
| 4–7 | 1.84 | 0.55 | 4 | 0.14–0.32 | — | — | (4) | — | 69.00 | 16.71 | 4 | 0.44–0.92 | |
| 7–11 | 1.59 | 1.59 | 7 | 0.26–0.39 | — | — | (7) | — | 45.66 | 40.20 | 7 | 0.07–0.10 | |
| 11–14 | 6.37 | 1.81 | 3 | 0.58–0.88 | — | — | (3) | — | 90.00 | 10.44 | 3 | 0.37–0.94 | |
| Hematological disorders patients per age group by HU exposure | |||||||||||||
| HU treated | |||||||||||||
| 0–4 | 0.16 | — | 1 | — | — | — | (1) | — | 9.00 | — | 1 | — | |
| 4–7 | 1.84 | 0.55 | 4 | 0.14–0.32 | — | — | (4) | — | 69.00 | 16.71 | 4 | 0.44–0.70 | |
| 7–11 | 1.37 | 1.61 | 6 | 0.10–0.97 | — | — | (6) | — | 38.43 | 38.74 | 6 | <0.05** | |
| 11–14 | 6.62 | — | 1 | — | — | — | (1) | — | 95.00 | — | 1 | — | |
| Non-treated | |||||||||||||
| 0–4 | 0.68 | 0.81 | 3 | 0.04–0.06 | 0.50 | — | 1 (2) | — | 28.67 | 24.7 | 3 | <0.05** | |
| 4–7 | — | — | — | — | — | — | — | — | — | — | — | — | |
| 7–11 | 2.96 | — | 1 | — | — | — | (1) | — | 89.00 | — | 1 | — | |
| 11–14 | 6.25 | 2.54 | 2 | 0.69–1.00 | — | — | (2) | — | 87.50 | 13.44 | 2 | 0.58–1.00 | |
Values from simulated Model 1.
P values from five models when simulated controls were compared to patient data.
CNS, central nervous system; HU, hydroxyurea; S/T, spermatogonia per tubular cross-section; TFI, tubular fertility index.
Significance in 5/5 models.
Significance in 4/5 models.
However, the average S/T was significantly lower at 0–4 years (P < 0.001 in 5/5 models) and at 4–7 years (P ≤ 0.001 in 5/5 models) in our patient cohort compared to the simulated controls. The mean TFI was reduced in patients compared to simulated controls for age groups up to 11 years old (P < 0.05; in 5/5 models for age groups 0–4 and 7–11 and in 4/5 models for 4–7 years). All these results remained unchanged when HU-pretreated cases were excluded from the analysis (Table III).
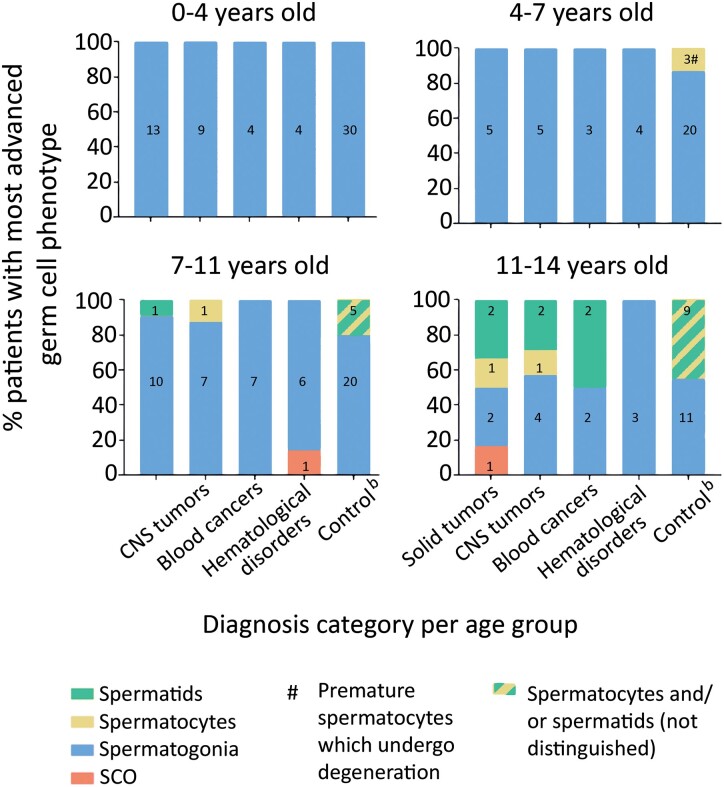
We also compared testes volume, S/T, TFI, and the most advanced germ cell type per age group in each disease group. No significant differences were found in testis volume in any age group compared to the controls (Table III). The patient group with solid tumors was the least affected group with regards to S/T, TFI, or germ cell development. The CNS tumor patients had significantly reduced S/T and TFI compared to the controls between ages 0–4 (P < 0.05 and P ≤ 0.001 in 5/5 models, respectively) and 4–7 years (P < 0.001 in 5/5 models and P < 0.05 in 4/5 models, respectively). The blood cancer patients were mostly affected in S/T and TFI compared to the controls at ages 4–7 years (P < 0.05 in 4/5 models and P < 0.05 in 5/5 models, respectively). The hematological disorder patients had severely reduced S/T and TFI compared to the controls at ages 0–4 years (P < 0.05 and P = 0.001 in 5/5 models, respectively). When this group was split in HU-treated and non-treated sub-groups, the sample numbers became too low to perform statistical calculations, but the same trend of results remained. In the aforementioned patient groups per age group where S/T reduction was significant, TFI below 50% was recorded. When comparing the most advanced germ cell development for each age group per disease group, the differentiation of spermatogonia into spermatocytes and spermatids was not observed even at ages 11–14 years in the hematological disorders group, as opposed to healthy controls where spermatocytes were seen from the age group 7–11 years onwards (Fig. 2).
Figure 2.
Most advanced germ cell type by age group and disease group. Most advanced germ cell type in prepubertal boys at the time of testicular biopsy (prior to mainline therapy) per age group for each disease group (years) compared with age-matched controls. Numbers inside bars represent the number of patients. Control values in healthy prepubertal boys are from Nistal and Paniagua (1984) where spermatocytes and spermatids are presented in one category. SCO, Sertoli cell only; CNS, central nervous system.
Discussion
We demonstrated that spermatogonial quantity is likely to be reduced in the testes of prepubertal boys diagnosed with cancer or a severe hematological disorder compared to healthy controls and that the reduction appears to be disease dependent. Patients with CNS tumors and hematological disorders were most affected (56.7% and 55.6%, respectively), with the highest prevalence in HU-treated SCD patients (58.3%) in the latter group. Our findings complement previous evidence that testicular function in pediatric cancer patients is already altered prior to treatment (Wigny et al., 2016; Stukenborg et al., 2018). The highest reduction in spermatogonial quantity was in the youngest patient groups (0–4 and 4–7 years). The disease adversely impacted spermatogonial quantity, distribution (TFI), and differentiation (most advanced germ cell type); these effects differed per disease group and age group similarly to the prevalence.
The pathophysiology and pathogenesis per disease group may be attributed to spermatogonial quantity reduction. In patients with solid tumors, tumor location outside the CNS and not near the pituitary or gonads, as recorded in most of the patients, could be the main factors for the least adverse outcome. In contrast, physical brain changes in CNS tumor patients, such as hydrocephalus, may impact endocrine regulation, affecting testicular function and the germ cell pool. Lin and Riva-Cambrin (2015) note that approximately 70–90% of patients with a posterior fossa tumor present with hydrocephalus at diagnosis, as in our cohort (100%, n = 16). Increased intracranial pressure from hydrocephalus could be a possible cause of hypothalamic–pituitary dysfunction in pediatric patients (Löppönen et al., 1998). In the blood cancer group, reduction in spermatogonial quantity may be attributed to the disease’s systemic effects (Magelssen et al., 2006), such as overproduction of pro-inflammatory cytokines or impaired oxygen and nutrient transport to target organs. Among Hodgkin’s disease patients, fever is thought to be associated with increased cases of infertility (Rueffer et al., 2001). Based on studies in cryptorchid patients, the increase in testicular temperature (Mieusset et al., 1993) could be associated with cellular, histologic, and hormonal changes in the testis that further impair spermatogenesis and fertility. Thus, systemic effects of the disease in prepubertal boys could be linked to the development of a suboptimal testicular environment, disturbing the germ cell pool and, as documented in adult blood cancer cases, inducing tubular hyalinization and Sertoli and Leydig cell atrophy or degeneration (Chapman et al., 1981). This hypothesis is supported by studies reporting germ cell loss and impaired fertility in adult blood cancer patients (Chapman et al., 1981; Rueffer et al., 2001; Sieniawski et al., 2008). Concurrently, in pediatric patients, germline mutations might be another factor contributing to germ cell loss. Recent studies have demonstrated that up to 10% of children who develop cancer may have a predisposition owing to germline mutations, including pathways of DNA repair, RASopathy, telomeropathy, or a recognized syndrome (e.g. the Li Fraumeni syndrome) (Zhang et al., 2015). For instance, 9% of patients with acute myeloid leukemia carry potential pathogenic variants (Wartiovaara-Kautto et al., 2018). One of the most frequent mutations in children is the germline GATA2 mutation, which causes a high propensity for myeloid diseases (Hasle, 2016). The GATA2 germline mutation causes several diseases, including monocytopenia, congenital deafness, lymphedema (Hasle, 2016), and also affects cell response to androgens (He et al., 2014) required for normal testicular development.
The hematological disorders group in our study, primarily SCD patients, often presented with severely reduced and focally distributed spermatogonia. This indicates the poor prognosis of spermatogenic recovery even after several years of follow-up (Wallace et al., 1991; Masliukaite et al., 2016). We also noted that hematological disorder patients presented with delayed maturation of the seminiferous epithelium, shown by the absence of spermatocytes and spermatids up to the age of 14 years. This concurs with the previously described ∼2-year delayed sexual maturation in SCD patients (Zemel et al., 2007). Such adverse outcomes may be associated with hypothalamic–pituitary–gonadal axis dysfunction, altered spermatogenesis-relevant growth factor production, germline mutations, or, in SCD patients, vaso-occlusion (Sieniawski et al., 2008; Hofmann, 2015). Furthermore, SCD patients, who all received HU pre-treatment, had the highest prevalence of reduced spermatogonial quantity (58.3%) compared to other hematological diseases not receiving HU (50.0%). Our results are in accordance with previously published results on decreased spermatogonial quantity in prepubertal HU-treated SCD patients (Stukenborg et al., 2018; Benninghoven-Frey et al., 2022). The HU mechanism of action involves inhibition of DNA synthesis and induction of cell cycle arrest or apoptosis in the S-phase (Timson, 1975), possibly contributing to observed germ cell reduction. Studies in adult men show reduced sperm counts following HU administration (Debaun, 2014) and a possibility of a spermatogenic recovery upon HU withdrawal (Grigg, 2007; Stukenborg et al., 2018). However, a recent study in prepubertal patients could not find an effect on spermatogonial numbers when comparing SCD patients with or without HU treatment (Gille et al., 2021), showing that the decrease in germ cell numbers is also related to the disease itself. Although it is unclear if the SCD or the treatment causes this effect in our patient cohort owing to the low number of patients, we hypothesize that it is a combination of both, yet the effect of the HU is not much stronger than the effect of the disease itself. More research in large cohorts is required to reach a definite conclusion on the effect of HU on spermatogonial quantity in perpuberty, taking into account the possible functional recovery of synchronized spermatogonia upon HU withdrawal.
Finally, irrespective of the disease, we showed for the first time that the patients aged 0–4 and 4–7 years were predominantly prone to reduced spermatogonial quantity compared to the normative reference values. Early age periods are highly important in testicular development, as proliferation of Sertoli cells, gonocyte migration and differentiation to spermatogonia, spermatogonial proliferation, and interstitial cell development set the basis of adult testis structure and function (Mancini et al., 1960; Paniagua and Nistal, 1984; Masliukaite et al., 2016; Allen et al., 2018). Whether these processes are compromised by germline mutations or by early adverse general biological conditions caused by the disease, e.g. changes in metabolism, hormonal regulation, and/or overproduction of pro-inflammatory cytokines (Rueffer et al., 2001; Magelssen et al., 2006; Zhang et al., 2015), is not known and still requires further investigation.
Limitations
Although we provide testicular tissue analysis data on the largest European multicenter cohort of male pre-pubertal patients who underwent fertility preservation before gonadotoxic cancer treatment, our study has limitations. First, for ethical reasons, we could not collect spermatogonial quantity data in healthy prepubertal boys as controls and thus deployed statistical simulation on data from literature. To reduce the role of chance, account for uncertainty, and increase reliability we performed multiple simulations of control datasets. Second, our results should be interpreted considering low patient numbers per (sub)group.
Conclusion
Our findings demonstrate that cancer and severe hematological disorders can affect prepubertal boys’ spermatogonial quantity and distribution in the testis, even before gonadotoxic therapy. Most affected are patients with CNS tumors and hematological disorders, including HU-pretreated SCD. Boys up to the age of 7 years are most susceptible to germ cell loss. These groups may have a high risk of depleted spermatogonia resulting in high risk for permanent infertility after potentially sterilizing therapies (Green et al., 2014; Poganitsch-Korhonen et al., 2017; Allen et al., 2018) and possible poor outcome of fertility preservation. Therefore, patient counseling and the timing of testicular biopsy for fertility preservation should be adjusted based on the diagnosis prior to treatment and patient’s age in parallel to the current criteria of the mainline treatment regime. For boys with SCD who need HU treatment, more research is needed on the timing of fertility preservation.
Supplementary Material
Acknowledgements
We would like to thank Dr Rebecca Holman, clinical statistician from Clinical Research Unit, Amsterdam UMC, for expert opinion on data simulation and choice of analysis methods. We also like to acknowledge Cindy de Winter-Korver, Saskia van Daalen, Mick Uijldert, and Lisa Catsburg for invaluable help in tissue processing and evaluation.
Contributor Information
Ieva Masliukaite, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Research Institute Amsterdam Reproduction & Development, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Elissavet Ntemou, Biology of the Testis Lab, Department of Reproduction, Genetics and Regenerative Medicine, Vrije Universiteit Brussel (VUB), Brussels, Belgium.
Elizabeth A M Feijen, Princess Maxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Marianne van de Wetering, Princess Maxima Center for Pediatric Oncology, Utrecht, The Netherlands.
Andreas Meissner, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Alexandre T Soufan, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Sjoerd Repping, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; The National Health Care Institute, Diemen, The Netherlands.
Leontien M C Kremer, Princess Maxima Center for Pediatric Oncology, Utrecht, The Netherlands; Department of Pediatric Oncology, Emma Children's Hospital, Amsterdam UMC, Location AMC, Amsterdam, The Netherlands.
Kirsi Jahnukainen, NORDFERTIL Research Lab Stockholm, Department of Women’s and Children’s Health, Karolinska Institutet and University Hospital, Stockholm, Sweden; New Children’s Hospital, Pediatric Research Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Ellen Goossens, Biology of the Testis Lab, Department of Reproduction, Genetics and Regenerative Medicine, Vrije Universiteit Brussel (VUB), Brussels, Belgium.
Ans M M van Pelt, Reproductive Biology Laboratory, Center for Reproductive Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Research Institute Amsterdam Reproduction & Development, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Authors’ roles
I.M., E.N., M.v.d.W., S.R., L.M.C.K., K.J., E.G., and A.M.M.v.P. created the concept and designed the study; E.N., M.v.d.W., A.M., and A.T.S. collected the clinical data; I.M. and E.N. collected laboratory data; I.M. and E.A.M.F. created data analysis pipeline and performed programming and statistical calculations; I.M. and E.N. performed initial data interpretation and drafted the manuscript; I.M., E.N., M.v.d.W., S.R., L.M.C.K., A.M., A.T.S., E.A.M.F., K.J., E.G., and A.M.M.v.P. performed the final interpretation of the data and critical reading of the manuscript.
Funding
This study was supported by Marie Curie Initial Training Network (ITN) (EU-FP7-PEOPLE-2013-ITN) funded by European Commision grant no. 603568; ZonMW Translational Adult stem cell research (TAS) grant no. 116003002.
Conflict of interest
The authors declare no conflict of interest.
References
- Allen CM, Lopes F, Mitchell RT, Spears N.. How does chemotherapy treatment damage the prepubertal testis? Reproduction 2018;156:R209–R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoven-Frey KM, Neuhaus N, Lahtinen AK, Krallmann C, Portela JMD, Jarisch A, Nordhoff V, Soave A, Ba Omar HAM, Sundin M. et al. Early testicular maturation is sensitive to depletion of spermatogonial pool in sickle cell disease. Haematologica 2022;107:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, Sutcliffe SB, Malpas JS.. Male gonadal dysfunction in Hodgkin’s disease: a prospective study. JAMA 1981;245:1323–1328. [PubMed] [Google Scholar]
- Cinti S, Barbatelli G, Pierleoni C, Caucci M.. The normal, cryptorchid and retractile prepubertal human testis: a comparative morphometric ultrastructural study of 101 cases. Scanning Microsc 1993;7:351–362. [PubMed] [Google Scholar]
- Crofton PM, Thomson AB, Evans AEM, Groome NP, Bath LE, Kelnar CJH, Wallace WHB.. Is inhibin B a potential marker of gonadotoxicity in prepubertal children treated for cancer? Clin Endocrinol (Oxf) 2003;58:296–301. [DOI] [PubMed] [Google Scholar]
- Debaun MR. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: a review. Expert Rev Hematol 2014;7:767–773. [DOI] [PubMed] [Google Scholar]
- Delgouffe E, Braye A, Goossens E.. Testicular tissue banking for fertility preservation in young boys: which patients should be included? Front Endocrinol 2022;13:854186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, Leisenring WM, Meacham LR, Mertens AC, Mulrooney DA. et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 2009;27:2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington GH. Histologic observations in crytorchidism: the congenital germinal-cell deficiency of the undescended testis. J Pediatr Surg 1969;4:606–613. [DOI] [PubMed] [Google Scholar]
- Gille AS, Pondarré C, Dalle JH, Bernaudin F, Chalas C, Fahd M, Jean C, Lezeau H, Riou L, Drouineaud V. et al. Hydroxyurea does not affect the spermatogonial pool in prepubertal patients with sickle cell disease. Blood 2021;137:856–859. [DOI] [PubMed] [Google Scholar]
- Goede J, Hack WWM, Sijstermans K, Van Der Voort-Doedens LM, Van Der Ploeg T, Meij-De Vries A, Delemarre-Van De Waal HA.. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr 2011;76:56–64. [DOI] [PubMed] [Google Scholar]
- Goossens E, Jahnukainen K, Mitchell R, van Pelt A, Pennings G, Rives N, Poels J, Wyns C, Lane S, Rodriguez-Wallberg K. et al. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open 2020;2020:hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Charles A, Chemaitilly W, Pui C, Klosky JL, Spunt SL. et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg A. Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J 2007;37:190–192. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic F, Herzog B, Buser M.. Development of cryptorchid testes. Eur J Pediatr 1987;146(Suppl 2):S8–S12. [DOI] [PubMed] [Google Scholar]
- Hasle H. Myelodysplastic and myeloproliferative disorders of childhood. Hematology Am Soc Hematol Educ Program 2016;2016:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Lanz RB, Fiskus W, Geng C, Yi P, Hartig SM, Rajapakshe K, Shou J, Wei L, Shah SS. et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc Natl Acad Sci USA 2014;111:18261–18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedinger CR. Histopathology of undescended testes. Eur J Pediatr 1982;139:266–271. [DOI] [PubMed] [Google Scholar]
- Hofmann I. Myeloproliferative neoplasms in children. J Hematop 2015;8:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan SZ, Vormer TL, Jongejan A, Röling MD, Silber SJ, de Rooij DG, Hamer G, Repping S, van Pelt AMM.. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017;144:3659–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joustra SD, Van Der Plas EM, Goede J, Oostdijk W, Delemarre-Van De Waal HA, Hack WWM, Van Buuren S, Wit JM.. New reference charts for testicular volume in Dutch children and adolescents allow the calculation of standard deviation scores. Acta Paediatr 2015;104:e271–e278. [DOI] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Płonowski M, Solarz E, Sega-Pondel D, Kazanowska B, Zelazowska-Rutkowska B, Wysocka J.. Assessment of gonadal function in boys and adolescents at the diagnosis of neoplastic disease. J Pediatr Endocrinol Metab 2012;25:453–458. [DOI] [PubMed] [Google Scholar]
- Lin CT, Riva-Cambrin JK.. Management of posterior fossa tumors and hydrocephalus in children: a review. Childs Nerv Syst 2015;31:1781–1789. [DOI] [PubMed] [Google Scholar]
- Löppönen T, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Lanning P, Knip M.. Pituitary function in children with hydrocephalus before and after the first shunting operation. Eur J Endocrinol 1998;138:170–175. [DOI] [PubMed] [Google Scholar]
- Loren AW, Senapati S.. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 2019;134:746–760. [DOI] [PubMed] [Google Scholar]
- Magelssen H, Brydøy M, Fosså SD.. The effects of cancer and cancer treatments on male reproductive function. Nat Clin Pract Urol 2006;3:312–322. [DOI] [PubMed] [Google Scholar]
- Mancini RE, Narbaitz R, Lavieri JC.. Origin and development of the germinative epithelium and sertoli cells in the human testis: cytological, cytochemical, and quantitative study. Anat Rec 1960;136:477–489. [DOI] [PubMed] [Google Scholar]
- Masliukaite I, Hagen JM, Jahnukainen K, Stukenborg J-B, Repping S, van der Veen F, van Wely M, van Pelt AMM.. Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: a systematic review and meta-analysis. Fertil Steril 2016;106:1652–1657.e2. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Fouda PJ, Vaysse P, Guitard J, Moscovici J, Juskiewenski S.. Increase in testicular temperature in case of cryptorchidism in boys. Fertil Steril 1993;59:1319–1321. [DOI] [PubMed] [Google Scholar]
- Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, Yu R, Ginsberg JP, Mitchell RT, Byrne J. et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021;22:e57–e67. [DOI] [PubMed] [Google Scholar]
- Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, Gurney JG, Donaldson SS, Leisenring WM, Robison LL. et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2008;26:4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistal M, Paniagua R.. Occurrence of primary spermatocytes in the infant and child testis. Andrologia 1984;16:532–536. [DOI] [PubMed] [Google Scholar]
- Ntemou E, Kadam P, Van Laere S, Van Saen D, Vicini E, Goossens E.. Effect of recombinant human vascular endothelial growth factor on testis tissue xenotransplants from prepubertal boys: a three-case study. Reprod Biomed Online 2019;39:119–133. [DOI] [PubMed] [Google Scholar]
- Paniagua R, Nistal M.. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J Anat 1984;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Petersen PM, Skakkebaek NE, Giwercman A.. Gonadal function in men with testicular cancer: Biological and clinical aspects. APMIS 1998;106:24–36. [DOI] [PubMed] [Google Scholar]
- Poganitsch-Korhonen M, Masliukaite I, Nurmio M, Lähteenmäki P, Van Wely M, Van Pelt AMM, Jahnukainen K, Stukenborg J-B.. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017;31:1460–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela JMD, Heckmann L, Wistuba J, Sansone A, Pelt AMMV, Kliesch S, Schlatt S, Neuhaus N.. Development and disease-dependent dynamics of spermatogonial subpopulations in human testicular tissues. J Clin Med 2020;9:E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LL, Hudson MM.. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffer U, Breuer K, Josting A, Lathan B, Sieber M, Manzke O, Grotenhermen FJ, Tesch H, Bredenfeld H, Koch P. et al. Male gonadal dysfunction in patients with Hodgkin’s disease prior to treatment. Ann Oncol 2001;12:1307–1311. [DOI] [PubMed] [Google Scholar]
- Sieniawski M, Reineke T, Josting A, Nogova L, Behringer K, Halbsguth T, Fuchs M, Diehl V, Engert A.. Assessment of male fertility in patients with Hodgkin’s lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol 2008;19:1795–1801. [DOI] [PubMed] [Google Scholar]
- Smith MA, Seibel NL, Altekruse SF, Ries LAG, Melbert DL, O'Leary M, Smith FO, Reaman GH.. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 2010;28:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, Töhönen V, Bjarnason R, Romerius P, Sundin M. et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod 2018;33:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Shichijo S, Noguchi M, Hirohata M, Itoh K.. Identification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res 1995;55:3478–3482. [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res 1975;32:115–132. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Shalet SM, Lendon M, Morris-Jones PH.. Male fertility in long‐term survivors of childhood acute lymphoblastic leukaemia. Int J Androl 1991;14:312–319. [DOI] [PubMed] [Google Scholar]
- Wartiovaara-Kautto U, Hirvonen EAM, Pitkänen E, Heckman C, Saarela J, Kettunen K, Porkka K, Kilpivaara O.. Germline alterations in a consecutive series of acute myeloid leukemia. Leukemia 2018;32:2282–2285. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and Development. Geneva: World Health Organization, 2006. Coordinating Team: Mercedes de Onis et al., Geneva. [Google Scholar]
- Wigny KMGJ, Van Dorp W, Van Der Kooi ALLF, De Rijke YB, De Vries ACH, Smit M, Pluijm SMF, Van Den Akker ELT, Pieters R, Laven JSE. et al. Gonadal function in boys with newly diagnosed cancer before the start of treatment. Hum Reprod 2016;31:2613–2618. [DOI] [PubMed] [Google Scholar]
- Winther JF, Kenborg L, Byrne J, Hjorth L, Kaatsch P, Kremer LCM, Kuehni CE, Auquier P, Michel G, De Vathaire F. et al. Childhood cancer survivor cohorts in Europe. Acta Oncol 2015;54:655–668. [DOI] [PubMed] [Google Scholar]
- Zemel BS, Kawchak DA, Ohene-Frempong KO, Schall JI, Stallings VA.. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res 2007;61:607–613. [DOI] [PubMed] [Google Scholar]
- Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA. et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 2015;373:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.