Abstract
Background:
Melanoma is a malignant tumor that originates from the skin’s melanocytes and has the highest death rate from skin cancer. Developing more efficacious anticancer medications with fewer adverse effects is the key to effective cancer management. Natural products are considered relevant and cost-effective sources of treatment. The plant (Polypodium vulgare) is a small and evergreen fern. One of the most important chemical compounds in the extract of this herb is flavonoids, which are thought to have beneficial effects in the treatment of melanoma through antioxidant properties.
Objectives:
Due to the limitations of current cancer management and cytotoxic drugs available in the country, the need to study drugs of natural origin has become more prominent. In this regard, the present study aims to investigate the cytotoxic effects of the ethanolic extract of Polypodium vulgare on A375 melanoma cells.
Methods:
Polypodium vulgare was extracted in 80% ethanol by the maceration. Then, its effects on the cell death of the melanoma cell line A375 compared to the AGO-1522 cell line as control were measured using the MTT-assay technique. The amount of cellular lipid peroxidation was estimated by TBARS assay. The amount of cellular ROS was calculated by fluorescent reagent 2,7-dichlorofluorescein diacetate. Cytochrome c concentration was measured by a cytochrome c immunoassay kit.
Results:
In this experiment, the anticancer effects of Polypodium vulgare ethanolic extract on human melanoma cell lines were investigated for the first time. Herb extract with a concentration of 0.123 mg/ml significantly increased the death of A375 melanoma cells (p < 0.001), lipid peroxidation (p < 0.01), and reactive oxygen species (ROS) (p < 0.01) and cytochrome c concentration (p < 0.001). Meanwhile, the same amount was ineffective and safe on AGO-1522 normal fibroblast cells.
Conclusion:
A 0.123 mg/ml concentration of Polypodium vulgare increases apoptosis in melanoma cells. Meanwhile, the same amount was safe on healthy cells. So, it could be considered an effective treatment without side effects in human melanoma.
Key Words: Melanoma, polypodium vulgare, flavonoids, antioxidant, reactive oxygen species
Introduction
The prevalence of skin cancer has increased noticeably. Considering the thinning of the ozone layer and the amount of UV radiation that reaches the earth’s surface, we will continue to face a growing trend (Guy et al., 2011). Melanoma is a malignant skin cancer that causes the most mortality rates (Afzali et al., 2013). Melanoma originates from skin melanocytes (Mackie et al., 2004). Several factors are involved in melanocytes becoming cancerous and causing melanoma (Jizba et al., 1971). More than 65% of melanomas are related to ultraviolet radiation from sunlight. When melanocytes have exposed to UV rays, they release melanin, a dark brown to the black pigment that protects the skin from the sun’s rays. However, melanin in melanocyte stem cells that have reached and crossed the threshold of genetic mutations can cause tumor growth (Emmerson et al., 2012). Other factors such as light skin, immune system suppression, genetic factors, and family history also are involved in causing this disease (Bandarchi et al., 2010).
The human body is constantly responding to oxygen. Free radicals react with oxygen in cells and form reactive oxygen species (ROS) (Kruidenier et al., 2002). UV radiation to the skin can also lead to the creation of ROS that can act as tumor initiators and promoters by damaging cellular macromolecules and activating cellular signaling molecules, which causes many diseases, including skin cancer (Moodycliffe et al., 1994).
Many biochemical systems in the cells prevent the chain reactions of free radicals in the body (Kruidenier et al., 2002). Antioxidants help stymie many oxidation reactions and inhibit or delay damage to cells and tissues (Michael et al., 2007). An imbalance between antioxidants and oxidative stress can damage sensitive biological tissues (Deby et al., 1986). Oxidative stress occurs when the body’s antioxidant defenses are deficient and can no longer neutralize various free radicals. The result is severe cellular damage, which leads to cell mutation and immunological damage (Fuller et al., 1988). If DNA damage evolves significantly and becomes irreversible, an apoptosis mechanism activates, and the cell destroys itself to prevent the replication of the damaged DNA. But if the immune system is suppressed, it can cause cancer (Moodycliffe et al., 1994).
Mitochondria is the cell’s main source of oxygen-free radical formation (Circu et al., 2010; Circu et al., 2012). Oxygen-free radicals can act as internal signals to induce apoptosis (Wang et al., 2005). In the presence of these signals, particular proteins from the Bcl-2 family increase the permeability of the mitochondrial outer membrane, and cytochrome c protein enters the cytosol and starts the formation of a complex called apoptosome that leads to the activation of caspases cascade and directs the cell to programmed death (Köhler et al., 2002). Caspases are from the cysteine proteases family, and after being activated, they hydrolyze many vital cell proteins and cause the cells to enter the irreversible stage of cell death (Würstle et al., 2012). Cells that experience apoptotic death show several morphological changes. Such as shrinking, organized disintegration of the nucleus, fragmentation of the cell, and appearance of apoptotic bodies that are swallowed by esophagus cells of the immune system (Ekshyyan et al., 2004).
Effective cancer management requires the development of better anticancer agents with higher efficacy and fewer side effects (Sharma et al., 2010). Recently, much attention has been paid to herbal medicine worldwide, and many researchers have collected more information on this matter daily (Yuan et al., 2016).
In Iran, the climate diversity and specific geographical location provide a favorable environment to investigate plants for their therapeutic properties (Hassanpouraghdam et al., 2022). Medical herbs have minor side effects, prevent and treat diseases by trapping free radicals, and have antioxidant properties (Zeng et al., 2011). On the other hand, due to the limitations of current cancer management to surgery and chemotherapy and their high side effects, it is remarkable to investigate substances that can be naturally replaced (Afrin et al., 2016).
The history of ferns backs millions of years ago (Chang et al., 2011). Polypodium vulgare from the Polypodiaceae family is an evergreen plant with thick horizontal rhizomes that leads to thin roots at their ends (I, 2016). The best type of rhizome for medical targets has a sweet taste and is dark in color and green inside (Haufler et al., 1995).
The leaves of the plant are placed in the form of numerous alternating parts in front of each other. Polypodium vulgare lacks flowers and reproduces by spores. This plant grows in forests, among rocks, and temperate and humid areas. It is propagated by rhizome pieces in light, sandy, and shaded lands (I, 2016).
Among the natural compounds in Polypodium vulgare, polyphenols play a significant role in cancer prevention and treatment (Ramos, 2008). Flavonoids are the most abundant compounds among polyphenols (Bonfili et al., 2008). They have hydroxyl groups that exert their antioxidant and anti-inflammatory activity in various ways, such as inhibiting cell proliferation, cell cycle arrest, and increasing apoptosis (Dhillon et al., 2007; Vauzour et al., 2010). These compounds are water soluble and can be classified into six classes based on their chemical structure namely as Flavanols, Anthocyanidins, Flavonols, Flavanones, Flavones, and Isoflavones. These compounds are found almost in all families of plants(Popa et al., 2008; Quideau et al., 2011). They can modulate the Nrf2 and NF-κB cells (Gopalakrishnan et al., 2008). An in-vitro study shows that flavonoids can significantly affect the MAPK and PI3K cells, which shows their participation in cancer cell proliferation (Dhillon et al., 2007).
Objectives
Different types of therapies are available to treat melanoma, but they can induce toxicity and side effects (Hasima et al., 2012). Today about 25% of all traditions bear one or more components from plants (Ansari et al., 2010). Considering the investigation of the antioxidant effects of the Polypodium vulgare, we decided to examine the cytotoxic effect of its ethanolic extract on the A375 melanoma cell line in-vitro for the first time.
Materials and Methods
Chemicals
2,4,6-Trinitrobenzene sulfonic acid (TNBS) and rhodanine from Sigma-Aldrich Chemie (GmbH, Munich, Germany); thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol, hexadecyl trimethyl ammonium bromide (HETAB), 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ), diphenyl-2-picryl hydrazyl (DPPH), methanol, hydrochloric acid (HCl), malondialdehyde (MDA), ethylenediamine tetra-acetic acid (EDTA), O-di anisidine hydrochloride, hydrogen peroxide, acetic acid, sodium acetate, Coomassie reagent, bovine serum albumin (BSA), ferric chloride (FeCl3 · 6 H2O),sodium sulfate (Na2SO4), sulfuric acid (H2SO4),phosphoric acid (H3PO4), potassium dihydrogen phosphate (KH2PO4), potassium hydrogen diphosphate (K2HPO4), peroxide hydrogen (H2O2) and sodium carbonate (Na2CO3) from Merck (Germany).
Preparation of Plant Sample
The dried rhizome of Polypodium vulgare was supplied from the grand bazaar of Tehran, Iran, in July 2020. A voucher specimen was preserved in the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Shahid Beheshti University of Medical Science (SBMU), Tehran, Iran, with Herbarium’s registration number of SBMU-8237.
All ethical themes of the studies on animals were considered carefully, and the experimental protocol was approved by the Ethical Committee of Iran University of Medical Science (IUMS) with the code number IR.IUMS.REC.1401.289.
In order to make sure that prepared herbs were of the finest quality and fresh, the ones with ochre coats and green-yellow cores were separated and then finely powdered by milling. The extract was prepared by maceration, using 300 g of powder of the rhizome with a mixture of ethanol/water (80%:20%), and the outcome was 112g of the dried extract (Deliu et al., 2013; Farràs et al., 2021).
Cell culture
Human malignant melanoma, A375 cell line, and normal human fibroblast, AGO-1522 cell line, were gathered from the Iranian biological resource center (Tehran, Iran). In this investigation, as healthy control cells, we used the AGO-1522 cell line, which was taken from a 3-day-old male newborn mouse skin. Besides, the A375 human melanoma cell line originated from a 54-year-old woman with malignant melanoma in 1973.
These cell lines were grown as a monolayer culture in an RPMI medium supplemented with 1% nonessential amino acids, 1% L-glutamine, 100 IU per mL penicillin, 100 IU per mL streptomycin, 20 mg/mL glutamine, and 10% fetal bovine serum at 37°C in a 5% CO2 humidified atmosphere and 95% air in a CO2 incubator. Cells were passaged twice a week under sterile conditions.
Cell lines at the exponential growth phase were washed, trypsinized, and resuspended in a fresh medium. Cells were seeded at a concentration of 104 cells/well in the 96-microtitre plate. The cells were treated with different concentrations of Polypodium vulgare extract for 24 hours.
MTT assay
The anti-proliferation effect is the first factor evaluated in the review of new antitumor agents. The MTT test measures the viability of cells using a colorimetric method. MTT powder is a yellow tetrazolium salt that turns into an insoluble formazan precipitate when the tetrazolium ring separates by living cells.
After treating the cells with different concentrations of herb extract, MTT solution was poured into each well, placed on the culture medium of the cells, and put inside the incubator for 4 hours. The absorbance was measured, and the IC50, in which 50% of the cells lost their ability to proliferate excessively, was defined by regression analysis and related models with a probit regression model. This procedure was measured using the PRISM program. Then, the cytotoxic effects of Polypodium vulgare extract on melanoma cells compared to normal human fibroblasts had read by ELISA (Iranzadasl et al., 2021).
Lipid peroxidation assay
Cellular lipid peroxidation was estimated from the concentration of malondialdehyde (MDA), a thiobarbituric acid-reactive substance (TBARS) formed during the decomposition of lipid hydroperoxides. The absorbance was measured spectrophotometrically (sadat Yousefsani et al., 2020).
Determination of reactive oxygen species
To determine the free radical scavenging activity of Polypodium vulgare, dichlorofluorescein diacetate (1.6 μM) was added to cell plates. ROS was determined spectrofluorometrically by the measurement of highly florescent DCFH. The results were expressed as fluorescent intensity per 106 cells (Kiani et al., 2017).
Cytochrome-c release assay
Cytochrome-c Immunoassay kit, provided by R & D Systems, Inc. (Minneapolis, Minn), was used to determine the concentration of cytochrome-c, and a spectrophotometer set to 450 nm was used to determine the optical density of each well (Waseem et al., 2017).
Statistical tests
Regression analysis and related models were measured with the probit regression model procedure by the PRISM program(Li et al., 2015).
Results
MTT assay
The MTT test was used to investigate the cytotoxic effects of Polypodium vulgare extract and the viability of A375 melanoma and normal AGO-1522 cell lines to find the IC50. The results show that the IC50 of the ethanolic extract of Polypodium vulgare for the human melanoma cell line was 0.0319 mg/ml and for the normal fibroblast cell line was 0.123 mg/ml. Therefore, all of the tests were done at 0.0319 mg/ml concentration.
As shown in Figure 1, melanoma cell line treated with Polypodium vulgare ethanolic extract after 24 and 48 hours shows a significant decrease in cell viability compared to normal fibroblast cells (p < 0.001) (Figure 1).
Figure 1.
Effects of Ethanolic Extract of Polypodium vulgare at the Concentration of 0.123 mg/ml on Induction of Cytotoxicity Using Human Melanoma Cell Line (HMM) and Normal Fibroblast Cell Line (Control). Cytotoxicity was measured using MTT dye. Values are shown as mean ± SD of three separate experiments (n = 3). *** p < 0.001, significant difference in comparison with control cells
Lipid peroxidation assay
The results of spectrophotometry showed that the treatment of human melanoma cell line with the ethanol extract of Polypodium vulgare with a concentration of 0.123 mg/ml after 24 hours has significantly increased the formation of MDA compared to normal fibroblast cells (p < 0.01) (Figure 2).
Figure 2.
Effect of Ethanolic Extract of Polypodium vulgare at the Concentration of 0.123 mg/ml on Induction of Lipid Peroxidation Using Human Melanoma Cell Line (HMM) and Normal Fibroblast Cell Line (Control). TBARS formation was measured spectrophotometrically and expressed as μM concentrations. Values are shown as mean ± SD of three separate experiments (n = 3). ** p < 0.01, significant difference in comparison with control cells
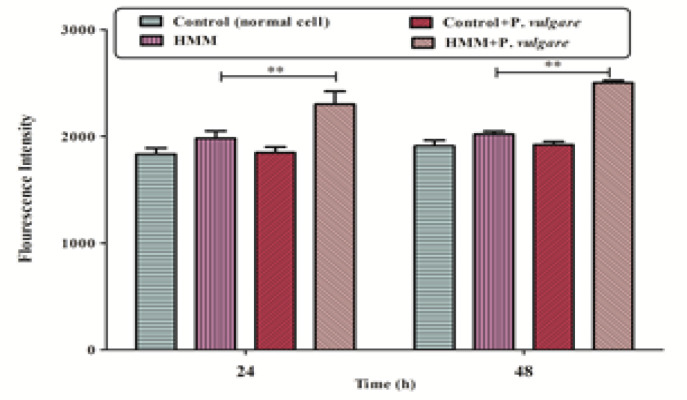
ROS formation
The data obtained from the fluorescent intensity of cells after 24 and 48 hours showed that the ethanolic extract of Polypodium vulgare with a concentration of 0.123 mg/ml has significantly induced the production of ROS in human melanoma cell line compared to normal human fibroblast cells (p < 0.01) (Figure 3).
Figure 3.
Effects of Ethanolic Extract of Polypodium vulgare at the Concentration of 0.123 mg/ml on the Formation of Reactive Oxygen Specious Using Human Melanoma Cell Line (HMM) and Normal Fbroblast Cell Line (Control). Reactive oxygen specious were determined spectrofluorometrically by the measurement of highly fluorescent DCF. Values are shown as mean ± SD of three separate experiments (n = 3). ** p < 0. 01, significant difference in comparison with control cells
Determination of Cytochrome-c Release
The results of spectrophotometry showed that the treatment of human melanoma cell line with ethanolic extract of Polypodium vulgare with a concentration of 0.123 mg/ml after 48 hours has significantly increased the concentration of cytochrome c compared to normal fibroblast cells, which is the result of the collapse of the mitochondrial outer membrane on melanoma cells (p < 0.001).
Discussion
Melanoma is a malignant form of skin cancer that initiates from melanocyte cells. Melanocytes react to environmental stimuli such as UV radiation by producing pigments (Samulitis et al., 2011). World Health Organization estimates that melanoma is increasing rapidly compared to other types of skin cancer (Lin et al., 2007). Common melanoma treatments such as immunotherapy, chemotherapy, radiotherapy, and surgery have not shown optimistic results and have high side effects, long treatment duration, and high cost (Burke et al., 2009). Due to these problems, alternative treatments have received much attention (Menaa, 2013). In the present study, we tried to reach an effective treatment with fewer side effects for melanoma using Polypodium vulgare. A similar study in Korea showed that the ethanolic extract of the fruit of Gardenia jasminoides Ellis inhibited metastasis and migration of melanoma tumors and reduced angiogenesis (Debnath et al., 2011).In another study, a medicinal plant from the ginseng family was introduced as an effective treatment for melanoma. This plant could inhibit the mediators involved in tumor growth and accelerate the apoptosis of melanoma cells (Lee et al., 2009).
Recently, an article was published about the cytotoxic effects of Iris germanica L. on melanoma cells, which showed that this plant could increase the death of cancer cells (Iranzadasl et al., 2021).
On the other hand, a wide range of research has been conducted on Polypodium vulgare extract and its therapeutic and anticancer properties. In 2015, a study was conducted on the antioxidant, antibacterial and anti-inflammatory properties of Polypodium vulgare. So, the flavonoid and tannin of this plant were extracted. And the DPPH method proved the activity of plant extract against free radicals. Also, the polyphenol extract of the plant prevented the denaturation of proteins and showed weak anti-inflammatory properties (Sofiane et al., 2015).
An article published by Nouioua Wafa investigated the antioxidant and anti-inflammatory properties of the methanolic extract of the Polypodium vulgare. DPPH method and bacterial culture in an agar medium were used to check the antioxidant properties, and they proved the effectiveness of the plant extract against Aspergillus fungal species (Wafa et al., 2017).
In 2019, another study was conducted on the anti-tyrosinase activity of four different plant species, including Polypodium vulgare. Melanin, produced by tyrosine-catalyzed reactions, is a fundamental epidermal factor that blocks UV radiation. The high activity of the tyrosinase enzyme increases the risk of skin cancer. Therefore, the anti-tyrosinase activity of methanolic and hexanoic extracts of the plant was investigated. For methanol extract, the effects were more evident (Farràs et al., 2019).
However, there has been no investigation on the extract of this plant on the A375 melanoma cell line. The present study examined the effects of ethanolic extract of Polypodium vulgare on cell proliferation and apoptosis of the A375 melanoma cell line compared to the AGO-1522 cell line as a control for the first time. Melanoma cells were treated with different doses of Polypodium vulgare extract, and IC50 was measured. According to the obtained results, the amount of 0.123 mg/ml of Polypodium vulgare extract eradicates 50% of A375 cancer cells while repeating the same experiment on healthy AGO-1522 cells at a dose of 0.319 mg/ml caused the death of 50% of the cells.
Therefore, in the rest of the experiments, only the IC50 dose was used because lower doses did not show adequate performance and higher doses risk damaging healthy cells.
The spectrophotometry results showed that the number of ROS in melanoma cells treated with 0.123 mg/ml of Polypodium vulgare extracts increased, such as the concentration of MDA, which indicated lipid peroxidation. The augmentation of factors above in A375 cells provided apoptotic pathways and led to cell death.
Measuring the concentration of cytochrome c could be proof of apoptosis from the intracellular pathway. The results showed a significant increase in the concentration of cytochrome c in A375 melanoma cells and confirmed our hypothesis.
On the other hand, repeating these experiments on normal human fibroblast cells did not show significant changes in the amount of cellular ROS, lipid peroxidation, and cytochrome c concentration, which implied that the concentration of 0.123 mg/ml of the ethanolic extract of Polypodium vulgare indicatively eradicated cancer cells.
In conclusions, the present study evaluated the anticancer effects of the ethanolic extract of Polypodium vulgare on a human melanoma cell line. The results showed that the concentration of 0.123 mg/ml of herb extract significantly increased apoptosis in melanoma cells. Meanwhile, the same amount was ineffective and safe on healthy cells. So, it could be considered an effective treatment without side effects in human melanoma. Despite the encouraging results in in-vitro experiments, clinical studies will still be needed to further explore the therapeutic effect of Polypodium vulgare in malignant melanoma.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
This project was supported by a grant from the Iran University of Medical Sciences. The authors would like to acknowledge the generous assistance of the staff of the Iran University of Medical Sciences. (Number of Grant: 1401-1-75-20037).
Limitations of study
- Limitation of research on only one type of cancer cells
- The high cost of more tests such as flow cytometry, western blot, etc.
Suggestions for future studies
- Determining the exact path of apoptosis of cancer cells
- Investigating the anticancer effects of Polypodium vulgare extract in in-vivo studies and further clinical studies
- Investigating the Polypodium vulgare extract’s exact effect on other types of cancer cells such as liver, lung, etc.
- Comparing the anticancer effects of Polypodium vulgare extract with anticancer drugs available in the pharmaceutical market
References
- Afrin S, Giampieri F, Gasparrini M, et al. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules, 2016:169. doi: 10.3390/molecules21020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzali M, Mirzaei M, Saadati H, et al. Epidemiology of skin cancer and changes in its trends in Iran. Feyz J Kashan University Med Sci, 2013:17. [Google Scholar]
- Ansari J, Inamdar N. The promise of traditional medicines. Int J Pharmacol. 2010;6:808–12. [Google Scholar]
- Bandarchi B, Ma L, Navab R, et al. From melanocyte to metastatic malignant melanoma. Dermatol Res Pract. 2010:2010. doi: 10.1155/2010/583748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Amici M, et al. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008;275:5512–26. doi: 10.1111/j.1742-4658.2008.06696.x. [DOI] [PubMed] [Google Scholar]
- Burke K, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25:219–24. doi: 10.1177/0748233709106067. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res. 2012;1823:1767–77. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath T, Park P-J, Nath NCD, et al. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011;128:697–703. [Google Scholar]
- Deby C, Pincemail J. Toxicity of oxygen, free radicals and defense mechanisms. Presse Med. 1986;15:1468–74. [PubMed] [Google Scholar]
- Deliu I, Bejan C, Vişoiu E, et al. The antimicrobial activity of some extracts of fern gametophytes. Curr Trends Nat Sci. 2013;2:10–3. [Google Scholar]
- Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY. Apoptosis: a key in neurodegenerative disorders. Curr Neurovasc Res. 2004;1:355–71. doi: 10.2174/1567202043362018. [DOI] [PubMed] [Google Scholar]
- Emmerson E, Hardman MJ. The role of estrogen deficiency in skin ageing and wound healing. Biogerontology. 2012;13:3–20. doi: 10.1007/s10522-011-9322-y. [DOI] [PubMed] [Google Scholar]
- Farràs A, Cásedas G, Les F, et al. Evaluation of anti-tyrosinase and antioxidant properties of four fern species for potential cosmetic applications. Forests. 2019;10:179. [Google Scholar]
- Farràs A, Mitjans M, Maggi F, et al. Polypodium vulgare L (Polypodiaceae) as a source of bioactive compounds: Polyphenolic profile, cytotoxicity and cytoprotective properties in different cell lines. Front Pharmacol. 2021;12:727528. doi: 10.3389/fphar.2021.727528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller B, Gower J, Green C. Free radical damage and organ preservation: Fact or fiction?: A Review of the interrelationship between oxidative stress and physiological ion disbalance. Cryobiology. 1988;25:377–93. doi: 10.1016/0011-2240(88)90046-6. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Kong A-NT. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-κB and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46:1257–70. doi: 10.1016/j.fct.2007.09.082. [DOI] [PubMed] [Google Scholar]
- Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer. Pharmacoeconomics. 2011;29:863–74. doi: 10.2165/11589300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3:328. [PMC free article] [PubMed] [Google Scholar]
- Hassanpouraghdam MB, Ghorbani H, Esmaeilpour M, et al. Diversity and Distribution Patterns of Endemic Medicinal and Aromatic Plants of Iran: Implications for Conservation and Habitat Management. Int J Environ Res Public Health. 2022;19:1552. doi: 10.3390/ijerph19031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler CH, Soltis DE, Soltis PS. Phylogeny of the Polypodium vulgare complex: insights from chloroplast DNA restriction site data. Syst Bot. 1995;1995:110–9. [Google Scholar]
- I P. A community-derived classification for extant lycophytes and ferns. J Syst Evol. 2016;54:563–603. [Google Scholar]
- Iranzadasl M, Pasalar P, Kamalinejad M, et al. Cytotoxic effect of iris germanica l Rhizomes extract on human melanoma cell line. Int J Cancer Manag. 2021:14. [Google Scholar]
- Jizba J, Dolejš L, Herout V, et al. The structure of osladin-the sweet principle of the rhizomes of polypodium vulgare L. Tetrahedron Lett. 1971;12:1329–32. [Google Scholar]
- Kiani A, Yousefsani BS, Doroudian P, et al. The mechanism of hepatotoxic effects of sodium nitrite on isolated rat hepatocytes. Toxicol Environ Health Sci. 2017;9:244–50. [Google Scholar]
- Köhler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunological Methods. 2002;265:97–110. doi: 10.1016/s0022-1759(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Kruidenier La, Verspaget H. oxidative stress as a pathogenic factor in inflammatory bowel disease-radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Lee CC, Zia F, Olaku O, et al. Survey of complementary and alternative medicine practitioners regarding cancer management and research. J Soc Integr Oncol. 2009;7:26. doi: 10.2310/7200.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Liu XY, Xie JT, et al. A comparison of different estimation methods for fungicide EC50 and EC95 values. J Phytopathol. 2015;163:239–44. [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Mackie R, Quinn A. Non-melanoma skin cancer and other epidermal skin tumours. Rook’s textbook of dermatology. 2004: 1801–50. [Google Scholar]
- Menaa F. Latest approved therapies for metastatic melanoma: what comes next? J Skin Cancer. 2013:2013. doi: 10.1155/2013/735282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Hedayati B, Dalgleish A. Disease regression in malignant melanoma: Spontaneous resolution or a result of treatment with antioxidants, green tea, and pineapple cores? A case report. Integr Cancer Ther. 2007;6:77–9. doi: 10.1177/1534735406298897. [DOI] [PubMed] [Google Scholar]
- Moodycliffe A, Kimber I, Norval M. Role of tumour necrosis factor-alpha in ultraviolet B light-induced dendritic cell migration and suppression of contact hypersensitivity. Immunology. 1994;81:79. [PMC free article] [PubMed] [Google Scholar]
- Popa V, Dumitru M, Volf I, et al. Lignin and polyphenols as allelochemicals. Ind Crops Prod. 2008;27:144–49. [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, et al. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–26. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Sadat Yousefsani B, Akbarizadeh N, Pourahmad J. The antioxidant and neuroprotective effects of Zolpidem on acrylamide-induced neurotoxicity using Wistar rat primary neuronal cortical culture. Toxicol Rep. 2020;7:233–40. doi: 10.1016/j.toxrep.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulitis BK, Dorr RT, Chow HS. Interaction of dacarbazine and imexon, in vitro and in vivo, in human A375 melanoma cells. Anticancer Res. 2011;31:2781–5. [PubMed] [Google Scholar]
- Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010;10:241–53. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- Sofiane G, Wafa N, Ouarda D. Antioxidant, antimicrobial and anti-inflammatory activities of flavonoids and tannins extracted from Polypodium vulgare L. Asian J Biol Chem Pharm Res. 2015;5:114–22. [Google Scholar]
- Vauzour D, Rodriguez-Mateos A, Corona G, et al. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–31. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafa N, Sofiane G. Antioxidant and antimicrobial activities of methanolic extract of Polypodium vulgare L. J Int Res Med Pharm Sci. 2017;12:27–32. [Google Scholar]
- Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29:489–96. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Waseem M, Parvez S, Tabassum H. Mitochondria as the target for the modulatory effect of curcumin in oxaliplatin-induced toxicity in isolated rat liver mitochondria. Arch Med Res. 2017;48:55–63. doi: 10.1016/j.arcmed.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Würstle ML, Laussmann MA, Rehm M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res. 2012;318:1213–20. doi: 10.1016/j.yexcr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Yuan H, Ma Q, Ye L, et al. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LB, Zhang ZR, Luo ZH, et al. Antioxidant activity and chemical constituents of essential oil and extracts of Rhizoma Homalomenae. Food Chem. 2011;125:456–63. [Google Scholar]