Abstract
Primary liver cancer (PLC) includes hepatocellular carcinoma and intrahepatic cholangiocarcinoma and is the sixth most common cancer worldwide with poor prognosis. PLC is characterized by an abundant stromal reaction in which cancer-associated fibroblasts (CAFs) are one of the major stromal components. Solid evidence has demonstrated the crucial role of CAFs in tumor progression, and CAF abundance is often correlated with poor clinical outcomes. Although CAFs are regarded as an attractive and promising target for PLC treatment, a poor understanding of CAF origins and heterogeneity and a lack of specific CAF markers are the major hurdles to efficient CAF-specific therapy. In this review, we examine recent advances in the understanding of CAF diversity in the context of biomarkers, subtypes, and functions in PLC. The regulatory roles of CAFs in extracellular matrix remodeling, metastasis, cancer stemness, and therapeutic resistance are summarized. With an increasing link between CAF abundance and reduced antitumor immune responses, we provide updated knowledge on the crosstalk between CAFs and immune cells within the tumor microenvironment, which leads to immune resistance. In addition, we present current CAF-targeted therapies and describe some future perspectives. A better understanding of CAF biology will shed light on a novel therapeutic strategy against PLC.
Keywords: Primary Liver Cancer, Hepatocellular Carcinoma, Cancer-Associated Fibroblasts, Cholangiocarcinoma, Origin, Immune Suppression
Summary.
Accumulating evidence provides the critical roles of cancer-associated fibroblasts in extracellular matrix remodeling, metastasis, cancer stemness, immune modulation, and therapeutic resistance. A better understanding of cancer-associated fibroblast biology will shed light on a novel therapeutic strategy against primary liver cancer.
Primary liver cancer (PLC) ranks sixth in cancer incidence and third worldwide as a leading cause of cancer-related death, with an estimated incidence of more than 1 million cases by 2025.1 Compared with other malignancies, hepatocellular carcinoma (HCC) with cholangiocarcinoma (CCA) are by far the second-most common, accounting for approximately 70% and 15% of cases, respectively, and represents both ends of the spectrum of primary malignant tumors.2
HCC is derived from the malignant transformation of hepatocytes. Chronic hepatitis B virus and hepatitis C virus infections are considered the most prominent risk factors for HCC development, accounting for more than half of cases.3 Additionally, alcohol intake or chronic inflammatory conditions, such as nonalcoholic steatohepatitis (NASH) associated with metabolic syndrome or diabetes mellitus, are becoming the fastest growing causes of HCC, particularly in western countries. Reports on mutational signatures have also demonstrated that aristolochic acid and tobacco may be potential pathogenetic factors associated with HCC.4 Because of the delayed diagnostic time of HCC, most patients are discovered and considered as inoperable.5
CCA is thought to originate from the malignant transformation of cholangiocytes and is the most aggressive malignant tumor of the biliary tract. CCA is generally classified as intrahepatic and extrahepatic.6 Among these, intrahepatic cholangiocarcinoma (iCCA) together with HCC is considered one of the most common PLC. Similar to HCC, CCA also develops from hepatitis B and C virus infection and unresolved inflammatory conditions, including obesity, diabetic mellitus, and congenital hepatic fibrosis. Specifically, the main predisposing condition for CCA in western countries is primary sclerosing cholangitis.7 Notably, in Southeast Asia, liver fluke infections have the highest correlated risk of CCA, which spreads to the bile ducts, gallbladder, and liver parenchyma and causes chronic infection and inflammation.8 Additionally, intrahepatic stones could also be one of the major causes of iCCA.
For patients with HCC receiving curative-intent treatments, the 5-year overall survival rate is more than 50%, whereas for patients with iCCA receiving curative-intent surgery, the 5-year overall survival rate is only approximately 5%–17%, suggesting that patients with iCCA might have a worse prognosis than those with HCC. Locoregional therapies could be an alternative option for patients who cannot receive surgical intervention. Systemic therapy is also recommended for advanced liver cancer.9 However, in combination with the high degree of malignancy and the severity of neighboring invasion, the optimal management of HCC and CCA remains an extreme challenge and is long in duration.
The Cellular Origins of Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma
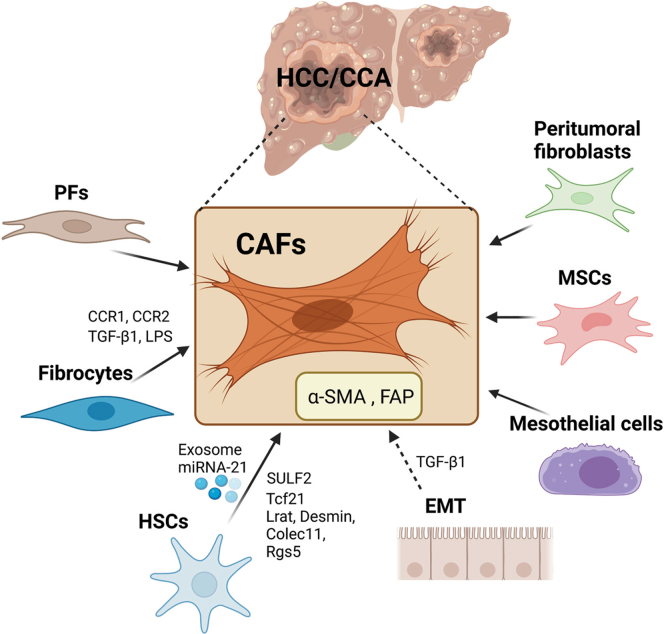
During hepatocarcinogenesis, persistent liver damage and advanced stage of liver fibrosis are key drivers. To be noted, more than 90% of HCC cases develop from a background of liver cirrhosis, and approximately one-third of patients with liver cirrhosis ultimately develop HCC.10 Indeed, an increasing number of studies suggest that the reconstruction of the tumor microenvironment (TME), a suitable environment for tumor cells, promotes tumor progression.11 In HCC, the reciprocal crosstalk among the TME, which includes cancer-associated fibroblasts (CAFs), immune cells, endothelial cells, extracellular matrix (ECM) elements, and HCC cells, significantly reinforces proliferation, migration, metastasis, and chemoresistance.11 CAFs, also called myofibroblasts, are a heterogeneous group of activated fibroblasts in the tumor stroma. Hepatic CAFs, commonly marked with smooth muscle alpha-actin (α-SMA) and fibroblast activation protein alpha (FAP) expression, are the main sources of collagen-producing cells, which is one of the critical and abundant components in the TME, and have been implicated in the progression of HCC.12 According to the studies, the origins of CAFs in HCC and CCA could be from a variety of cell types (Figure 1).
Figure 1.
Overview of the cellular origins of CAFs in HCC and CCA. CAFs can originate from multiple cell types including HSCs, PFs, fibrocytes, EMT, mesothelial cells, MSCs, and peritumoral fibroblasts. Several factors and exosome miRNA were reported as the drivers of those transdifferentiations.
Hepatic Stellate Cells
Activated hepatic stellate cells (HSCs) have been reported as the predominant origin of hepatic myofibroblasts in HCC.13 HSCs are liver-specific mesenchymal cells that maintain interactions with sinusoidal endothelial cells and hepatic epithelial cells.14 During chronic liver injury, HSCs downregulate vitamin A–constraining lipid droplets and neural markers; migrate to the pericentral areas; and transdifferentiate into proliferative, fibrogenic, myofibroblasts.15 Fate-tracing revealed that HSCs gave rise to more than 80% of collagen-producing myofibroblasts in carbon tetrachloride (CCl4)-induced toxic liver fibrosis and cholestatic-induced biliary liver fibrosis.16 Zhou et al17 showed that HCC cells release exosomal miRNA-21, which mediates the differentiation of HSCs into α-SMA and FAP-positive CAFs, and activated CAFs express high levels of angiogenic cytokines that further promote HCC progression in subcutaneously transplanted tumors. Wang et al18 cocultured HSCs with HCC cells and revealed that sulfatase 2 secreted by HCC cells promoted HSC-CAF differentiation, which was associated with the upregulation of the CAF markers tenascin-C and stromal cell-derived factor 1 (SDF1) and promoted epithelial-to-mesenchymal transition (EMT) in HCC cells, and eventually led to HCC development. According to The Cancer Genome Atlas database, a 12-marker panel of CAFs in HCC has been identified. When the HSC cell line LX2 was cocultured with conditioned medium (CM) from the HCC cell line Huh7, LX2 cells showed high levels of 12 marker proteins in additional to α-SMA and FAP expression.19 By performing single-cell RNA sequencing (scRNA-seq), Wang et al20 identified that transcriptional factor 21 was preferentially expressed by periportal and pericentral HSCs that generated the most CAFs in a mouse liver fibrosis model, supporting the functional contribution of HSC-associated liver fibrosis to the progression of HCC. Tracing in combination with single-cell transcriptomics was used, Bhattacharjee et al21 illustrated that most CAFs showed abundant expression of the HSC signature, suggesting that HSCs are the primary source of liver metastasis–associated CAFs. Interestingly, in an iCCA model, HSCs have been also confirmed to be the main source of CAFs, and HSC-derived CAFs are the dominant tumor-interacting populations associated with iCCA progression.22 Analysis of scRNA-seq CAF data showed that most CAFs expressed an HSC signature, including lecithin retinol acyltransferase, Desmin, collectin subfamily member 11, and regulator of G protein signaling 5, suggesting an HSC origin, whereas less than 10% expressed the markers of portal fibroblasts (PFs), another fibrogenic population in iCCA. Additionally, scRNA-seq data of human and murine iCCA suggested that HSCs-CAFs represented the subpopulation with the most ligand-receptor interactions with tumor cells, and were associated with decreased survival and increased recurrence risk in iCCA.22
Portal Fibroblasts
PFs are located in the periportal area and maintain the integrity of the biliary tree and portal tract.23 PFs can be distinguished from HSCs by expressing of Thy1, fibulin (Fbln) 2, elastin (Eln), gremlin1, mesothelin, mucin 16, and ecto-ATPase nucleoside triphosphate diphosphohydrolase-2. Activated PFs with high expression of Thy1 and CD34 contributed to the development of hepatic myofibroblasts during the early progression of cholestatic fibrosis, although HSCs give rise to the major sources of CAFs during the late stages of cholestatic liver injury and during hepatotoxic liver injury.24 Yang et al25 performed single-cell and lineage-tracing analysis and revealed that PFs primarily contributed to collagen-producing myofibroblasts in BDL-liver, which was associated with the expression of PF-specific markers including Thy1, Fbln1, Eln, dermatopontin, microfibril-associated glycoprotein 4, and growth arrest specific 6. The number of PFs was also dramatically elevated in the bile duct ligation-induced liver fibrosis.25 A subset of PFs with mesenchymal stem cell features (PMSCs) was initially discovered by using scRNA-seq and could generate myofibroblasts.26 Researchers built oligogene signatures and found that slit homolog 2 (SLIT2) was a prototypical gene of the PMSCs signature, which promoted HSC survival and largely expanded with hepatic fibrosis progression.26 Itou et al27 characterized CAFs in the metastatic lymph nodes and showed that PFs were the sources of CAFs at primary sites associated with positive expression of platelet-derived growth factor receptor-β (PDGFR-β), FBLN2, and Thy1 in human iCCA.
Fibrocytes
Fibrocytes originate from hematopoietic stem cells and are characterized by the expression of fibroblast markers (collagen type I, vimentin, and fibronectin) and hematopoietic cell markers (cluster of differentiation [CD] 45, CD34, CD11b, CD54, CD80, CD86; major histocompatibility complex [MHC] II; Ly-6G/Ly-6C; C-C motif chemokine receptor [CCR] 2, CCR1, CCR7, and CCR5).28 Fibrocytes were first described as “CD45 and collagen type I expressing leukocytes that mediate tissue repair and are capable of antigen presentation to naive T cells.”29 Studies have suggested that in response to liver injury, fibrocyte migration from bone marrow was detected in both the liver and spleen, and these cells gave rise to myofibroblasts, which were regulated by the CCR2 and CCR1. In response to CCL4-damaged liver, bone marrow–derived fibrocyte was also regulated by transforming growth factor-beta 1 (TGF-β1) and the intestinal permeability marker lipopolysaccharide (LPS), indicating that the release of TGF-β1 and LPS plays an important role in fibrocyte trafficking.30
Epithelial-to-Mesenchymal Transition
EMT is a process in which endothelial cells lose their characteristic markers, acquire a migratory phenotype, and gain enhanced levels of mesenchymal markers.31 A growing body of research has suggested that CAFs with high aggressiveness originate from HCC cells undergoing EMT. Zeisberg et al32 performed lineage-tracing experiments and demonstrated that hepatocytes undergoing EMT may contribute to the population of FSP-1 (fibroblast specific protein-1)-positive cells in CCL4-induced liver fibrosis. Consistently, Zou et al33 demonstrated that in patients with HCC, hypoxia correlated with increased expression of FAP, a typical CAF marker. In addition, increased FAP in HCC cell lines was parallel with EMT markers, including E-cadherin, Snail, and Twist-related protein. In another study, TGF-β, which promotes EMT and stimulates fibroblast maturation, has been demonstrated to induce α-SMA-mediated CAF differentiation and contribute to HCC invasion and metastasis.34,35 However, the contribution of EMT to fibrogenic CAFs remains controversial. Cell fate mapping experiments in mice suggested that hepatocytes and cholangiocytes do not undergo EMT during liver fibrosis and do not give rise to the generation of myofibroblasts or the markers of myofibroblasts.36,37 In a rat CCA model, CAFs in CCA do not originate from hematopoietic stem cells or bone marrow–derived fibrocytes, and whether they originate from endothelial cells through EMT remains unknown.38 Therefore, more studies are required to clarify this issue.
Others
In addition to these possible cell sources, mesothelial cells, mesenchymal stromal cells (MSCs), and peritumoral fibroblasts can also be transformed into CAFs. Based on the findings by Asahina, mesothelial cells undergo HSC and myofibroblast transdifferentiation during CCL4-induced liver fibrogenesis, whereas during cholestatic liver damage, mesothelial cells only give rise to HSCs but not myofibroblasts.39,40 Multipotent MSCs migrate to the area of liver fibrosis and the HCC microenvironment, and can influence tumor initiation and progression.41 A study revealed that after being cocultured with HCC cell line, MSCs exhibited the properties of CAFs and were associated with upregulated tenascin-C and chemokine (C-X-C motif) ligand (CXCL) 12.42 Another recent study suggested that human adipose MSCs had significantly increased the expression of CAF markers, including α-SMA, vimentin, c-MYC, matrix metalloproteinases (MMP) 2, vascular endothelial growth factor, fibroblast growth factor receptor1 (FGFR1), interleukin (IL)-6, IL-8, and Tenascin-C, when cocultured with HCC cells, thereby acquiring a CAF-like phenotype.43 Apart from that, peritumoral fibroblasts can be transdifferentiated into CAFs induced by lysophosphatidic acid (LPA), which is released from HCC cells.44
Biomarkers of Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma
Because the expression of CAF markers is extremely heterogeneous and varies strongly between different CAF subpopulations in HCC and CCA tissues, identifying unique CAF biomarkers remains challenging. Although a large number of CAF biomarkers have been reported, such as a-SMA, Thy, FAP, vimentin, and FSP-1,18,19,22,43 none of them are specific to CAFs, because they overlap with distinct subtypes. The present review summarizes CAF markers based on different subpopulations, as shown in Table 1.45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64
Table 1.
CAF Signature/Biomarkers in HCC and CCA
| CAF origin | Signature/biomarkers | References |
|---|---|---|
| HSCs | α-SMA, FAP | 17 |
| VEGF, MMP2, MMP9, bFGF, TGF-β | 17 | |
| Tenascin-C, SDF1 | 18 | |
| CXCL5, IGFL1, IGFL2, MMP1, ADAM32, ADAM18, FGF5, FGF8, FGF17, FGF19, FGF4, FGF23 | 19 | |
| Tcf21 | 20 | |
| Lrat, Desmin, Colec11, Rgs5 | 22 | |
| Positive: Lrat, Reln, Rgs5; Negative: Acta2, Col1a1, Col8a1, Col15a1, Crlf1 Fbn2, Desmin, GFAP, Glialfibrillary acidic protein | 22 | |
| VEGF, MMP9 | 45 | |
| Thy1, α-SMA, FAP | 46 | |
| LSD1 | 47 | |
| Desmin, GFAP, Glialfibrillary acidic protein | 48 | |
| PFs | Msln, Upk1b, Upk3b, Gpm6a | 22 |
| Thy1, CD34 | 24 | |
| Thy1, Fbln1, Eln, Dpt, Mfap4, Gas6 | 25 | |
| SLIT2 | 26 | |
| PDGFR-β, fibulin-2, Thy-1 | 27 | |
| Elastin, Thy1, Ntpdase 2 | 49 | |
| Fibrocytes | Collagen type I, Vimentin, fibronectin, CD45, CD34, MHCII, CD11b, Gr-1, CD54, CD80, CD86, CCR2, CCR1, CCR7, CCR5 | 28 |
| HCC cells | α-SMA, FAP | 17 |
| FAP | 19 | |
| FSP-1 | 32 | |
| ITGA2, ITGAV, ITGA1 | 50 | |
| CXCL11 | 51 | |
| ICC cells | CD146, IL-6, EZH2 | 52 |
| α-SMA, FSP-1, PDGFRβ | 53 | |
| PDGFD | 54 | |
| Mesothelial cells | Msln, Upk1b, Upk3b, Gpm6a | 22 |
| Gpm6a | 39 | |
| Msx2 | 55 | |
| Wilms | 56 | |
| ITGA8 | 57 | |
| Mesenchymal stromal cells | Tenascin-C, CXCL12 | 42 |
| α-SMA, Vimentin, c-MYC, MMP2, VEGF, IL-6, FGFR1, IL-8, Tenascin-C | 43 | |
| Positive: CD73, CD90, CD105, CD44, CD13, CD29, CD166 Negative: CD31, CD34, CD45, CD117, HLA-DR |
58 | |
| HCC-associated CAFs | COL1A1, COL4A1, COL6a2 | 50 |
| Versican | 59 | |
| ACTA2, COL1a1 | 60 | |
| CCA-associated CAFs | Podoplanin | 61, 62 |
| CD10 | 63 | |
| Periostin | 64 |
Acta2, actin alpha 2; ADAM, a disintegrin and metallopeptidase domain; bFGF, basic fibroblast growth factor; CAF, cancer-associated fibroblasts; CCA, cholangiocarcinoma; Colec11, collectin subfamily member 11; Crlf1, cytokine receptor like factor 1; Dpt, dermatopontin; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; Fbn, fibrillin; FSP-1, fibroblast-specific protein 1; Gas6, growth arrest specific 6; GFAP, glial fibrillary acidic protein; Gpm6a, glycoprotein m6a; HCC, hepatocellular carcinoma; HSC, hepatic stellate cells; ICC, intrahepatic cholangiocarcinoma; IGFL, insulin growth factor-like family member; ITGA, integrin subunit alpha; Lrat, lecithin retinol acyltransferase; LSD1, lysine-specific demethylase 1; Mfap4, microfibril-associated glycoprotein 4; MHC, major histocompatibility complex; Msln, mesothelin; Msx2, msh homeobox 2; PDGFR, platelet-derived growth factor receptor; PF, portal fibroblasts; Rgs5, regulator of G protein signaling 5; SDF1, stromal cell-derived factor 1; Tcf21, transcriptional factor 21; TGF-β1, transforming growth factor-beta 1; Upk, uroplakin; VEGF, vascular endothelial growth factor.
Recently, scRNA-seq has provided a wealth of data on CAF biomarkers in HCC and CCA. Chiavarina et al65 used proteomics and scRNA-seq analysis and unveiled 3 major CAF subpopulations in human HCC that expressed biomarkers including COL1A1, Thy1, RGS5, and NDUFA4 mitochondrial complex associated like 2 (NDUFA4L2) (HSC markers, CAF_HSC), PDGFRA, and MMP23B (PF markers, CAF_Port), and myosin heavy chain 11 (MYH11) and calponin 1 (vascular smooth muscle markers, CAF_VSMC). Specifically, their team showed that prolargin was a novel biomarker in the subpopulation of PF-derived CAFs and that CAF-derived prolargin interferes with interaction of tumor cells with endothelial cells, which suppresses tumor angiogenesis.65 Meng et al50 assessed the scRNA-seq data of patients with HCC and observed a significant distinction among CAFs associated with differential genes COL1A1, COL4A1, and COL6A2, which are enriched in the ECM-receptor interaction pathway. Further analysis revealed that COL1A1 and ITGA2 have the highest interaction mediated by crosstalk between CAFs and tumor cells.50 According to the analysis of scRNA-seq in human iCCA, another recent study discovered 6 distinct fibroblast subpopulations including vascular CAFs defined by CD146, MYH11, gap junction protein alpha 4, and RGS5 expression and upregulated IL-6 expression; matrix CAFs defined by collagen molecules (COL5A1, COL5A2, and COL6A3), periostin, fibronectin 1, lumican, decorin, and versican expression; inflammatory CAFs defined by FBLN1, IGF1, CXCL1, IGF-binding protein 6, secretory leukocyte peptidase inhibitor, serum amyloid a1 expression and associated with inflammatory response regulation and complement activation; antigen-presenting CAFs defined by CD74, HLA-DRA, and HLA-DRB1 expression; EMT-like CAFs defined by the epithelium-specific marker genes keratin (KRT) 19, KRT8, and serum amyloid a1; and lipofibroblasts defined by lipid metabolism and processing-related genes, such as apolipoprotein a2, fatty acid binding protein (FABP) 1, FABP4, and frizzled related protein.52
In summary, the expression of CAF biomarkers is very heterogeneous and mainly depends on the CAF subtypes analyzed in these studies. Thus, investigations of CAF-specific markers are still vital.
The Role of Cancer-Associated Fibroblasts in Extracellular Matrix Modelling
ECM is a collection of proteins that provide critical signals to preserve tissue scaffold structure and organ homeostasis, and regulate cell growth and apoptosis. During cancer progression, alterations in ECM drive tumor cell initiation, invasion, metastasis, and resistance.66 Many solid tumors, such as HCC and CCA, express large amounts of ECM components, including fibrillar collagen, laminin, proteoglycans, and fibronectin, which mostly come from activated CAFs.67 Santamato et al68 reported that activated HSC could produce and secrete the ECM component laminin-5, which contributes the migration and invasion of HCC cells via the mitogen-activated protein kinase ERK kinase/extracellular-signal-regulated kinase pathway. Increased and reorganized ECM protein secreted by CAFs reinforce the deposition of fibrillar collagen, thereby mechanically giving rise to ECM stiffening and promoting tumor cell proliferation and invasion.66 Schrader et al69 used an in vitro system of “mechanically tenable” matrix-coated polyacrylamide gels and revealed that enhanced matrix stiffness promoted proliferation and chemotherapeutic resistance of HCC cells via hepatocyte growth factor (HGF)-induced signaling responses. Interestingly, Olsen et al70 demonstrated that mechanically stiff substrates (coated with different ECM proteins or poly-L-lysine) were required by primary rat HSCs to differentiate into myofibroblasts, indicating that liver stiffness-dependent HSC differentiation required matrix proteins and the generation of mechanical tension, which is probably a key factor driving the progression of liver fibrosis. Abundant studies have confirmed that liver stiffness is closely correlated with the risk of developing HCC in patients, and its assessment can serve as a potential predictor of HCC development.71, 72, 73 In addition, MMPs are key mediators of ECM degradation.74 MMP9 was reported to induce EMT in human kidney fibrosis.75 Of interest, coculturing activated primary rat HSCs with type I collagen could stimulate the ECM remodeling properties of HSCs by inducing MMP9 expression.76 Furthermore, reconstruction of the fibrous structure by adjusting the alignment of collagen fiber is an alternative mechanism in ECM remodeling. Jung et al77 found that high forces generated by premetastatic cancer cells have the ability to align ECM fibrils and therefore enhance the delivery of CAF-promoting factors toward the stroma, resulting in the activation of myofibroblasts. Moreover, the contractility of CAFs is considered as another physical mechanism that remodels the ECM by generating mechanical forces by widening the pores of the ECM or aligning collagen fibers, contributing to directional invasion of tumor cells.78 However, whether this is true in HCC or CCA models is still unknown.
The Role of Cancer-Associated Fibroblasts in the Regulation of Metastasis
Accumulating evidence has shown that CAFs control tumor growth, angiogenesis, and metastasis in liver cancer.51,79, 80, 81 Paracrine signaling involving the release of chemokine (C-C motif) ligand (CCL) 5, CXCL11, HGF, and follistatin like 1 (FSTL1) is exploited by CAFs to facilitate pulmonary metastasis in HCC.51,79,82,83 Various CAF-derived mediators, including CCL2, CCL7, CXCL16, IL-6, cartilage oligomeric matrix protein, heparin-binding endothelial growth factor (EGF)-like growth factor, and periostin, have been shown to encourage the invasion of HCC or CCA cells in coculture models, but their prometastatic roles have yet to be validated in vivo.35,64,80,81,84,85
Chemotactic Cytokines
In 2022, Xu et al79 described a key role of CAF-secreted CCL5 in supporting HCC malignancy. Ablation of CCL5 or its cognate receptor CCR3/5 has been shown to impede CAF-driven cell aggressiveness and in vivo lung metastasis. Mechanistically, CCL5 stabilizes hypoxia-inducible factor 1 alpha (HIF1α) in HCC cells, which in turn increases zinc finger e-box binding homeobox 1 expression for EMT and hence induces metastasis.79 CXCL11 represents another key CAF-derived cytokine that fosters lung metastasis via paracrine activation of the circUBAP2/IFIT1/3 cascade.51 CXCL11 is enriched in metastatic HCC tissues, and its knockdown in CAFs weakens tumor migration in coculture and orthotopic models.51 miR-4756 was identified as a downstream factor that is repressed by CXCL11 and inactivates IFIT1/3, thus counteracting the migratory-promoting effect.51 Of note, circUBAP2 depletion reduces murine tumor migration and lung metastasis, whereas simultaneous blockade of miR-4756 antagonizes this suppression, indicating the significance of the CXCL11-circUBAP2/IFIT1/3 axis in HCC malignancy.51
Growth Factors and Proteins
Early studies of liver myofibroblasts in CCA suggested that fibroblastic heparin-binding-EGF and periostin foster CCA cell invasion, potentially through endothelial growth factor receptor and ITGα5β1/PI3K/AKT pathways, respectively.81,85,64 In HCC, HGF is enriched in primary CAFs and can accelerate tumor progression and dissemination.82,84 HGF acts by stimulating the cMet/Erk1/2/FRA1/HEY1 axis in HCC cells, and fos-related antigen 1 (FRA1) silencing effectively retards HGF-induced tumor growth and metastasis.82 FSTL1 represents another CAF mediator with prometastatic ability, as illustrated by the elevated intrahepatic and lung metastasis in mice engrafted with HCC cells cultured in FSTL1-overexpressing CM and the reversed phenotypes in response to FSTL1 blockade.83 In vitro migratory and invasion assays verified these findings and showed that these aggressive features were enhanced by FSTL1 overexpression and repressed by its neutralization.83 Clinically, FSTL1 is primarily expressed in HCC CAFs and correlates with advanced cancer stage, reiterating its importance in HCC progression.83
The Role of Cancer-Associated Fibroblasts in the Regulation of Cancer Stemness and Drug Resistance
Emerging work is revealing the stemness-driving capacities of CAFs in liver cancer. In fact, CAFs are a substantial source of cytokines and proteins that can promote cancer stemness, which is characterized by the gain of self-renewal and tumor-initiating abilities and drug resistance.80,82,83,86, 87, 88, 89, 90, 91 These CAF-derived mediators, along with the underlying stemness-driving mechanisms, have been extensively examined, some of which will be discussed next.
Interleukin-6, Interleukin-33, and Cardiotrophin Like Cytokine Factor-1 Cytokines
CAF-derived IL-6 is suggested to play a key role in stemness induction in HCC in a signal transducer and activator of transcription 3 (STAT3)-dependent manner.87,88 Exogenous addition of IL-6 stimulated stem cell properties (marker gene expression and spheroid formation) and sorafenib resistance in HCC cells, whereas neutralizing IL-6 in CAF-CM or depleting hepatic STAT3 alleviated these effects.87,88 Recently, FAP+ CAFs from iCCA tumors were shown to secrete large amounts of IL-6 and IL-33 to induce stemness in an myeloid-derived suppressor cells (MDSC)-dependent manner.91 Specifically, these cytokines act on MDSCs by triggering STAT3-mediated expression and hyperactivation of 5-lipoxygenase, resulting in the release of leukotriene B4 mediator that supports iCCA cell stemness.91 Cardiotrophin-like cytokine factor (CLCF) 1 is also a stemness-stimulatory cytokine in HCC.86 In HCC cells, stemness marker gene levels and spheroid growth are supported by exogenous CLCF and repressed by fibroblastic CLCF1 or hepatic CLCF1 receptor (ciliary neurotrophic factor receptor [CNTFR]) silencing.86 Likewise, in a murine model, CLCF1/CNTFR depletion hampered tumorigenesis and the SRY-box transcription factor 2 score. Downstream CXCL6/E2F and TGFβ/MAPK signaling axes have been shown to mediate the stemness-enhancing effects, in which E2F transcriptionally controls stemness-associated gene expression.86
Follistatin Like-1 and Hepatocyte Growth Factor
As discussed previously, FSTL1 and HGF independently promote HCC metastatic dissemination, a phenotype that can be attributed to stemness acquisition in cancer cells.82,83 Indeed, FSTL1 has been demonstrated to equip HCC cells with tumor-initiating and drug resistance abilities.83 FSTL1-overexpressing CM enhances self-renewal abilities and apoptotic resistance to sorafenib in HCC cells, and supports tumorigenicity in murine model and ex vivo culture of mice tumors.83 Consistently, FSTL1 blockade reverses these tumor-initiating phenotypes, sensitizes tumors to sorafenib, and prolongs mice survival.83 HGF is another major CAF mediator that elicits potent self-renewal abilities and chemoresistance in HCC cells, as indicated by increased spheroid formation and reduced apoptosis induced by cisplatin or doxorubicin in response to HGF administration.82 FRA1 acts downstream of HGF paracrine signaling in HCC cells, and its knockdown abrogates the HGF-induced stemness properties.82 Collaborating with these findings, another group showed that HGF could increase stemness marker expression, colony- and spheroid-forming capacities, and cisplatin and sorafenib resistance in HCC cells.84,90
The Role of Cancer-Associated Fibroblasts in Nonalcoholic Steatohepatitis–Induced Hepatocellular Carcinoma
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide; the global prevalence is around 20%–25% in adults.92 About 25% of patients with NAFLD progress to NASH, a more severe form of NAFLD characterized by steatosis-driven inflammation, hepatocellular injury, and liver fibrosis.93, 94, 95 NASH emerges as a key underlying risk factor of multiple end-stage liver cancer diseases, including cirrhosis, liver failure, and HCC.94,96 In fact, NASH has become the fastest growing cause of HCC according to a global epidemiology study from 2010 to 2019.97 Moreover, NASH might represent an important etiology accounting for failure to immunotherapies in HCC. In murine model, immune checkpoint inhibitor, anti-PD-1, failed to control tumor growth in NASH-related HCC; and in clinical studies, patients with NASH-HCC had worse prognosis and lower disease control rate on receiving immunotherapies compared with patients with other HCC aetiologies.98,99
In clinical and animal models of NASH, HSC is believed to be the origin of scar-forming myofibroblast. Single cell transcriptomic analysis of human NASH samples showed that injured hepatocytes stimulate myofibroblast differentiation and activation through TGFβ-1/2, Sonic hedgehog, and PDGFRBB signaling, which implicates the interplay between hepatocytes and activated fibroblasts in development of NASH-induced HCC.100 By examining the single cell expression profiles of HSCs extracted from a NASH model derived from foz/foz mice fed with western diet, heterogeneity of HSC was observed with 4 well-defined clusters with distinct functions.101 In patients with NASH-induced HCC, increased senescence and senescence-associated secretory phenotype were observed in CAFs, as evidenced by the increase in expression of α-SMA, p21, γ-H2AX, and IL-6. This senescence-associated secretory phenotype feature was correlated with higher incidence of NAFLD.102 Through analysis of gene expression omnibus database (GEO no. GDD3087), Myosin IC (MYO1C) expression was found to be upregulated in fibrotic liver of patients with NAFLD-induced HCC. Pharmacologic and genetic deletion of Myo1c attenuated TGFβ signaling of activated fibroblasts. Myo1c-knockout mice showed refractory to CCl4-induced liver fibrosis.103 Using 2 mouse NASH-induced fibrosis models, Asakawa et al. have analyzed the expression of CAFs by RNA sequencing analysis and showed that FGF9 was markedly upregulated in CAF, which was found to confer antiapoptotic and promigratory roles in hepatoma cells.104 In a stelic animal model NASH-HCC mouse model, a stepwise increase in HGF in plasma from the normal to the fibrosis and subsequent to HCC stage was demonstrated, which is in parallel with accumulation of activated fibroblasts.82 Collectively, these molecules might represent novel targets for alleviating liver fibrosis or delaying the clinical progression from NASH to HCC; but the actual therapeutic benefits in NASH-induced HCC require further investigations.
Crosstalk Between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment
CAFs can shape the immune microenvironment toward a tolerant milieu through extensive crosstalk with various immune cell types. An early in vitro study illustrated that CAFs deactivate natural killer (NK) cells and impair their cytotoxicity against HCC cells by secreting prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO), and interestingly, the release of these factors depends on the presence of NK cells, implying plausible crosstalk between CAFs and NK cells in the HCC TME.56 Other coculture studies demonstrated that CAFs secreted SDF1 alpha to recruit neutrophils, monocytes, or dendritic cells by interacting with their surface C-X-C chemokine receptor type (CXCR) 4.105, 106, 107 IL-6 is additionally released from CAFs and stimulates the JAK/STAT3 pathway in these immune cells, potentiating their differentiation into immunosuppressive subtypes, which in turn represses T-cell proliferation and antitumor activity.105, 106, 107 These early in vitro studies provided insights into the immunomodulatory roles of CAFs in liver cancer.
In 2016, Yang et al108 provided the first in vivo evidence, to our knowledge, showing that CAFs promoted HCC tumor growth by attracting immunosuppressive cells. Using a coimplantation model, the researchers revealed that FAP+ CAFs were indispensable for fostering MDSC recruitment and tumor growth, and importantly, FAP overexpression was sufficient to equip normal fibroblasts with tumor-promoting abilities.108 Mechanistically, FAP stimulated uPAR/FAK/Src/JAK2 autocrine signaling in CAFs and activated STAT3, inducing the expression and release of CCL2, which in turn recruited MDSCs in a CCR2-dependent manner.108 Depleting fibroblastic STAT3 or CCL2, or murine CCR2 attenuated the FAP-induced MDSC infiltration and tumor-promoting effects in vivo.108 Extending this work, the group later described that the MDSC-recruiting and protumorigenic features of FAP/STAT3/CCL2 signaling were conserved in iCCA, reiterating the importance of CAFs and FAP signaling in generating an immunosuppressive stroma that is permissive for tumor progression.109 Apart from MDSCs, CAFs also exert immunosuppressive effects on macrophages via the endosialin/GAS6 axis in HCC.110 Endosialin, a transmembrane protein highly expressed in HCC CAFs, has been revealed to enhance tumor growth and macrophage infiltration in vivo by interacting with its surface CD68.110,111 And M2 polarization of macrophages is achieved through GAS6 release.110 To target this CAF-macrophage crosstalk, the group generated an anti-endosialin human antibody (IgG78) that tags glycosylated endosialin for lysosomal degradation, which was shown to successfully deplete CAF-induced M2 infiltration and tumorigenesis in subcutaneous and orthotopic xenografts, verifying the immunoregulatory and protumoral abilities of endosialin and rendering IgG78 an appealing therapeutic candidate.110
Recently, Song et al86 elucidated an intricate crosstalk among CAFs, HCC cells, and tumor-associated neutrophils triggered by CAF-derived CLCF1 cytokines. The interaction of CLCF1 with its receptor on HCC cells (CNTFR) contributes to N2 neutrophil infiltration and tumor propagation.86 Using a murine model engrafting CAFs-HCC cells followed by tail-vein infusion of human neutrophils, they showed that CLCF1 or CNTFR depletion abrogated neutrophil recruitment and tumor proangiogenic growth triggered by CAFs.86 CLCF1/CNTFR induction in HCC cells activates the release of CXCL6 and TGF-β, which function to attract neutrophils via CXCR1/2, and polarize them to the N2 protumorigenic subtype, respectively.86 It is noteworthy that ERK1/2 seems to be a key regulator of the CLCF1-CXCL6/TGFβ axis. In HCC cells, ERK1/2 acts downstream of CNTFR induction and controls CXCL6 and TGFβ secretion. Meanwhile, in CAFs, it is activated by CXCL6 and TGFβ to reinforce CLCF1 release, forming a positive feedback loop to foster HCC progression. ERK1/2 blockade reverses N2 phenotype in vitro and in vivo and mitigates the protumorigenic effects imposed by CLCF1.86
Collectively, CAFs promote immunosuppression by direct or indirect crosstalk with immune cells and educating them to harbor tolerant traits. Several key axes, including SDF1a-CXCR4, FAP/STAT3/CCL2-CCR2, Endosialin-CD68, and CLCF1-CNTFR/CXCL6, are deployed by CAFs for immune cell recruitment.86,105, 106, 107, 108, 109, 110 Notably, CLCF1 and endosialin are primarily expressed on HCC CAFs and associated with poor clinical outcome in HCC, whereas heightened expression of FAP predicts poor prognosis in iCCA patients, suggesting the clinical relevance of these signaling cascades.86,109,110 As shown by coculture studies, CAF-derived soluble factors (IL-6, PGE2, and IDO) seems to induce suppressive phenotypes in NK cells, neutrophils, monocyte, or dendritic cells.58,105, 106, 107 In murine models, CAF-derived GAS6, TGF-β, and CCL2 induce immunosuppressive M2 and N2 differentiation and MDSC recruitment, thereby augmenting HCC propagation or metastasis.86,108, 109, 110
Therapeutic Implications
As described in previous sections, CAFs drive immunosuppression, stemness, and metastasis in liver cancer. These protumorigenic features, along with their abundance in the TME and genetic stability, render them attractive targets for anticancer therapies.112 Therapeutic approaches against CAFs have been extensively reviewed.52,113, 114, 115 Here, we briefly discuss the recent advancements in CAF-targeting therapies, particularly those applicable to liver cancer.
Cancer-Associated Fibroblasts Elimination
In the last decade, studies have engrossed on diminishing the prominent FAP+ CAFs subset to control tumorigenesis. DNA vaccines, CAR-T-cell adoptive transfer, and oncolytic virus-based approaches deplete FAP+ CAFs and restrain the tumor growth of mesothelioma, and colon, pancreatic, and lung cancers in murine models.116, 117, 118, 119 Of note, a research group generated a novel FAP immunogen that synergies with antitumor antigen vaccines in eliciting antitumor immunity, thus attenuating lung and prostate tumoral growth with increased mice survival.120 Nevertheless, the applicability of these approaches might be limited because of the widespread expression of FAP, especially in bone marrow and muscle, and systemic targeting of FAP+ cells might result in cachexia, bone toxicity, muscle loss, or even death.121,122 To avoid systemic toxicity, recent studies have focused on developing strategies that can specifically eradicate FAP+ CAFs.123,124 Zhen et al123 developed a nanoparticle-based photoimmunotherapy that enables selective elimination of FAP+ CAFs, which efficaciously retarded tumor growth without endangering other FAP+ populations. Another approach involves the expression of FAP-bispecific T-cell engagers (FAP-BiTE), a bispecific antibody directed against fibroblastic FAP and CD3ε on T cells using the adenoviral major late promoter system.124 This system allows tumor-restricted expression of FAP-BiTE, which elicits potent T-cell cytotoxicity against CAFs in human biopsies (malignant ascites and prostate cancer tissue).124 In fact, in HCC, mere expression of FAP can imbue normal fibroblasts with a protumorigenic niche.108 Moreover, FAP autocrine signaling in CAFs recruits MDSCs (via CCL2) and endows MDSCs with stemness-enhancing abilities (via IL-6/33), thus augmenting propagation and initiation of HCC or iCCA tumors.108,109 As such, FAP-targeting approaches might offer therapeutic benefits and seem warranted in liver cancer.
Targeting Cancer-Associated Fibroblasts Precursors or Secretome
Methods to suppress CAF functions are also in development for combating liver tumorigenesis. One way is to prevent the conversion from precursor cells to CAFs. Metformin and curcumin were recently illustrated to inactivate HSCs, the most common CAF precursor in liver cancer.125,126 Metformin, a commonly used antidiabetic drugs, can impede murine tumorigenesis in a liver fibrosis model.125 Natural compound curcumin can suppress EMT and alleviate cell aggressiveness induced by HSCs through abrogating the HIF-1α/CTGF axis in HCC cells.126 Peritumoral fibroblasts represent another source of CAFs in HCC and are capable of supporting tumor growth in vivo.44 LPA is released from HCC cells that foster peritumoral fibroblasts transdifferentiation, and using an LPA inhibitor (α-bromomethylene phosphonate [BrP]-LPA) can readily block the transdifferentiation and slow tumorigenesis.44
Another way to impair CAF function is to ablate their paracrine secretion. FSTL1 drives stemness acquisition and malignant progression in HCC cells, patient-derived organoids, and tumors, and effective tumor control is accomplished by FSTL1 blockade.83 Critically, FSTL1 is tightly linked to sorafenib resistance, and combining an anti-FSTL1 antibody with sorafenib prolonged mice survival with reduced tumor stemness, illustrating the potential clinical benefits of FSTL1 targeting, either as a single agent or in combination with sorafenib.83 Some other mediators, CCL5 and CXCL11, confer HCC cells’ ability to disseminate to lung.51,79 Abrogation of these mediators, their interacting receptor (CCR3/5), or downstream signaling molecules (HIF1α, circUBAP2) ameliorates tumor malignancy, and thereby representing actional targets for HCC treatment.51,79 In addition to soluble factors, CAFs also secrete exosomes to the TME. miR-320a is found to exhibit antitumor properties, and its expression is reduced in CAF-derived exosomes.113 Elevating the level of miR-320a by overexpression in CAFs or exogenous addition to HCC cells restores antitumoral effects and prevents metastasis, suggesting its use in HCC treatment.127
Targeting Cancer-Associated Fibroblasts–Mediated Crosstalk
In tumor stroma, CAFs actively communicate with immune cells to elicit their protumorigenic capacities. Weakening CAF-immune cells crosstalk prevents CAF-driven tumor growth, thus representing an alternative modality for cancer treatment. As discussed previously, in liver cancer, CAFs crosstalk with immune cells through CCL2, CLCF1, or endosialin release, favoring tumoral infiltration of MDSCs, N2 neutrophils, or M2 macrophages with resultant tumor propagation.86,108,109 Depleting these mediators or receptors on respective immune cells (ie, CCR2 and CNTFR) can reignite antitumor immunity and constrain tumor growth, rendering these factors attractive therapeutic targets.86,108,109 Furthermore, IL-6, an abundant CAF-secreted cytokine, has been documented to indirectly crosstalk with T cells via PD-1/PD-L1 signaling, hence restricting their proliferation and antitumor immunity in HCC.105 Consistent with this finding, combinational treatment involving IL-6 and PD-L1 neutralization effectively controlled tumor size and improved survival in HCC mouse model, suggestive of plausible targeting of this CAF-T-cell crosstalk in liver cancer.128
TGFβ signaling represents an important cascade that mediates crosstalk of CAFs, and the possibilities of therapeutically targeting this factor have been extensively investigated. In HCC, TGFβ signaling mediates CAF-cancer cell crosstalk to control tumor phenotype.83 TGFβ is emanate from both CAFs and HCC cells, acting as autocrine and paracrine factors to stimulate FSTL1 signaling in CAFs, eventually leading to increased HCC stemness and metastatic dissemination.83 In fact, a prior phase II clinical trial has investigated the use of the TGFβ inhibitor, galunisertib, in patients with advanced HCC and revealed an improved prognosis in TGFβ responders.129 However, fibroblastic TGFβ signaling is also implicated in immune evasion in a reconstituted colon cancer model with liver metastases.116 Coadministration of galunisertib and anti-PD-L1 antibodies elicited a potent Tc antitumor response, attenuating most metastases and prolonging mice survival.130 A similar finding was reported in another study using antibodies against TGFβ and PD-L1, which inactivated the fibroblastic TGFβ pathway and profoundly inhibited tumor growth in murine mammary and colorectal carcinoma models.131 These preclinical studies further support the notion that CAF-mediated crosstalk controls tumorigenesis, and their disruption might offer therapeutic value. Additionally, although combination modalities involving in CAF-targeting and anti-PD-L1 drugs deemed effective in other cancers, it is crucial to investigate whether or not CAF-directed agents can overcome anti-PD-L1 resistance and yield clinical benefits in patients with liver cancer.
Conclusions and Future Perspectives
Taken together, accumulating evidence supports the tumor-promoting role of CAFs in PLC via their direct effects on cancer cells or indirectly via other cell types within the TME. In this review, we summarized the roles of CAFs in ECM remodeling, metastasis, cancer stemness, and the induction of an immunosuppressive microenvironment (Figure 2). It is critical to further investigate the mechanisms involved in CAF-induced tumor progression to identify new therapeutic targets for PLC treatment. Notably, it is critical to determine the crosstalk between CAFs and other immune cells to increase the current response rate of immune checkpoint therapies. For a better understanding of CAF biology in PLC, it is crucial to identify the origin of CAFs in PLC. With the development of scRNA sequencing, several origins of CAFs in PLC have been proposed, including HSCs, PFs, fibrocytes, mesothelial cells, and MSC. Investigations are currently underway to evaluate the therapeutic implications of blocking the transition of these precursor cells to CAFs. Among these studies, CAF heterogeneity exists within PLC. The identification of different subsets of CAFs will provide insight into the development of accurate and efficient strategies against CAFs. However, the identification of subsets of CAFs in PLC requires fresh tumor tissues, which may result in bias in cell preparation with the loss of rare cell populations. With the advent of single-nucleus RNA sequencing, researchers have been able to better identify the subsets of CAFs and their interactions with immune cells in frozen tissues. In addition, scRNA sequencing identified specific subsets of CAFs based on transcriptional gene expression signatures, but this analysis does not represent the protein level. To overcome this limitation, scRNA or single-nucleus RNA sequencing analysis coupled with spatial transcriptomics will provide a better resolution of CAF subsets, and their crosstalk with microenvironment components in PLC. Because of the existence of CAF heterogenicity, the markers of CAFs are also heterogeneous. To precisely target CAF populations, the current research direction is focused on identifying specific CAF markers without hampering normal tissues. Regarding the functional studies of CAFs in PLC, researchers have relied on in vitro coculture systems that have evolved from simple 2-dimensional monocultures to 3-dimensional systems with CAFs derived from mouse and primary PLC patients in the past decade. Recently, increasing efforts have been made to understand CAF biology to elucidate the complex interaction among cancer cells, CAFs, and other immune cells using organoid cultures that closely mimic the in vivo TME. To improve the viability of cells and maintain biologic activity in this coculture system, researchers have used hyaluronan-gelatine hydrogel as a matrix for potential in vitro drug testing.132 Apart from this, in vivo marker-based CAF-specific transgenic mouse models and patient-derived xenograft models are also used in CAF research. With a better understanding of CAF biology in the TME, we believe that new therapeutic strategies against CAFs for PLC therapy lies ahead.
Figure 2.
CAFs and their secretome remodel the stromal environment and support immune evasion, metastasis, and stemness acquisition of PLC. Secretory functions of CAFs regulate ECM remodeling (top left); tumor immunity (bottom left); metastatic dissemination (top right); and self-renewal, tumor-initiating, and drug resistance capacities (bottom right) of PLC cells. CAFs secrete ECM proteins that contribute to matrix degradation, deposition, and stiffness (top left). ECM protein is also required to drive HSCs transdifferentiate into CAFs. ECM degradation and alignment of collagen fiber as alternative mechanisms in ECM remodeling could help to deliver CAF-promoting factors and results in CAFs activation. As a feedback loop, the activated CAFs could guide the cancer cells via alignment of collagen fiber for migration. In PLC TME, CAFs release various cytokines to tumor stroma, directly or indirectly foster immune cell infiltration, and polarize them to inhibitory subtypes, thus generating an environment favorable for tumor propagation (bottom left). CAF secretome also actively engages in HCC aggressive behaviors and metastatic colonization in lung, predominantly by inducing stemness features in HCC cells (top and bottom right). CAF mediators directly enhance renewal, tumorigenic, and therapeutic resistance properties in HCC cells, and indirectly promote these characteristics in iCCA cells in an MDSC-dependent manner (bottom right).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by the RGC General Research Fund (15102722) and Research Impact Fund (R7022-20).
References
- 1.Fitzmaurice C., Allen C., Barber R.M., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales J.M., Marin J.J., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet J.M., Castet F., Heikenwalder M., et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 4.Schulze K., Imbeaud S., Letouzé E., et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trifylli E.M., Koustas E., Papadopoulos N., et al. An insight into the novel immunotherapy and targeted therapeutic strategies for hepatocellular carcinoma and cholangiocarcinoma. Life. 2022;12:665. doi: 10.3390/life12050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondossola D., Ghidini M., Grossi F., et al. Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma. World J Gastroenterol. 2020;26:3542–3561. doi: 10.3748/wjg.v26.i25.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paillet J., Plantureux C., Lévesque S., et al. Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma. J Exp Med. 2021;218 doi: 10.1084/jem.20200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian M.B., Chen Y.D., Liang S., et al. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty. 2012;1:1–12. doi: 10.1186/2049-9957-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.T., Wang J.J., Luu M., et al. Comparison of clinical features and outcomes between intrahepatic cholangiocarcinoma and hepatocellular carcinoma in the United States. Hepatology. 2021;74:2622–2632. doi: 10.1002/hep.32007. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag H.B. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Gu C., Song Q., et al. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10:1–15. doi: 10.1186/s13578-020-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Z., Dong C., Jiang K., et al. Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma. J Hematol Oncol. 2019;12:1–9. doi: 10.1186/s13045-019-0782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia W., Liang S., Cheng B., et al. The role of cancer-associated fibroblasts in hepatocellular carcinoma and the value of traditional Chinese medicine treatment. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.763519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin C., Evason K.J., Asahina K., et al. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Investig. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 16.Mederacke I., Hsu C.C., Troeger J.S., et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:1–11. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Ren H., Dai B., et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:1–18. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C., Shang C., Gai X., et al. Sulfatase 2-induced cancer-associated fibroblasts promote hepatocellular carcinoma progression via inhibition of apoptosis and induction of epithelial-to-mesenchymal transition. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.631931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou B., Liu X., Gong Y., et al. A novel 12-marker panel of cancer-associated fibroblasts involved in progression of hepatocellular carcinoma. Cancer Manag Res. 2018;10:5303. doi: 10.2147/CMAR.S176152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S.S., Tang X.T., Lin M., et al. Perivenous stellate cells are the main source of myofibroblasts and cancer-associated fibroblasts formed after chronic liver injuries. Hepatology. 2021;74:1578–1594. doi: 10.1002/hep.31848. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharjee S., Hamberger F., Ravichandra A., et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest. 2021;131 doi: 10.1172/JCI146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Affo S., Nair A., Brundu F., et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866–882. doi: 10.1016/j.ccell.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dranoff J.A., Wells R.G. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishio T., Hu R., Koyama Y., et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol. 2019;71:573–585. doi: 10.1016/j.jhep.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Yang W., He H., Wang T., et al. Single-cell transcriptomic analysis reveals a hepatic stellate cell–activation roadmap and myofibroblast origin during liver fibrosis in mice. Hepatology. 2021;74:2774–2790. doi: 10.1002/hep.31987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei L., Bruneau A., El Mourabit H., et al. Portal fibroblasts with mesenchymal stem cell features form a reservoir of proliferative myofibroblasts in liver fibrosis. Hepatology. 2022;76:1360–1375. doi: 10.1002/hep.32456. [DOI] [PubMed] [Google Scholar]
- 27.Itou R.A., Uyama N., Hirota S., et al. Immunohistochemical characterization of cancer-associated fibroblasts at the primary sites and in the metastatic lymph nodes of human intrahepatic cholangiocarcinoma. Hum Pathol. 2019;83:77–89. doi: 10.1016/j.humpath.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Xu J., Cong M., Park T.J., et al. Contribution of bone marrow-derived fibrocytes to liver fibrosis. Hepatobiliary Surg Nutr. 2015;4:34–47. doi: 10.3978/j.issn.2304-3881.2015.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucala R., Spiegel L.A., Chesney J., et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 30.Scholten D., Reichart D., Paik Y.H., et al. Migration of fibrocytes in fibrogenic liver injury. Am J Clin Pathol. 2011;179:189–198. doi: 10.1016/j.ajpath.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi K.J., Nam J.K., Kim J.H., et al. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp Mol Med. 2020;52:781–792. doi: 10.1038/s12276-020-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeisberg M., Yang C., Martino M., et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 33.Zou B., Liu X., Zhang B., et al. The expression of FAP in hepatocellular carcinoma cells is induced by hypoxia and correlates with poor clinical outcomes. J Cancer. 2018;9:3278–3286. doi: 10.7150/jca.25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morén A., Bellomo C., Tsubakihara Y., et al. LXRα limits TGFβ-dependent hepatocellular carcinoma associated fibroblast differentiation. Oncogenesis. 2019;8:1–14. doi: 10.1038/s41389-019-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Chen S., Wang W., et al. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Scholten D., Österreicher C.H., Scholten A., et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu A.S., Diaz R., Hui J.J., et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzanares M.Á., Usui A., Campbell D.J., et al. Transforming growth factors α and β are essential for modeling cholangiocarcinoma desmoplasia and progression in a three-dimensional organotypic culture model. Am J Clin Pathol. 2017;187:1068–1092. doi: 10.1016/j.ajpath.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lua I., Li Y., Zagory J.A., et al. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. 2016;64:1137–1146. doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Wang J., Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A. 2013;110:2324–2949. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin Z., Jiang K., Li R., et al. Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Mol Cancer. 2018;17:1–17. doi: 10.1186/s12943-018-0926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya S.D., Mi Z., Talbot L.J., et al. Human mesenchymal stem cell and epithelial hepatic carcinoma cell lines in admixture: concurrent stimulation of cancer-associated fibroblasts and epithelial-to-mesenchymal transition markers. Surgery. 2012;152:449–454. doi: 10.1016/j.surg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salah R.A., Nasr M.A., El-Derby A.M., et al. Hepatocellular carcinoma cell line-microenvironment induced cancer-associated phenotype, genotype and functionality in mesenchymal stem cells. Life Sci. 2022;288 doi: 10.1016/j.lfs.2021.120168. [DOI] [PubMed] [Google Scholar]
- 44.Mazzocca A., Dituri F., Lupo L., et al. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology. 2011;54:920–930. doi: 10.1002/hep.24485. [DOI] [PubMed] [Google Scholar]
- 45.Coulouarn C., Corlu A., Glaise D., et al. Hepatocyte–stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma hepatocyte–stellate cell cross-talk in liver cancer. Cancer Res. 2012;72:2533–2542. doi: 10.1158/0008-5472.CAN-11-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong P.F., Wei W., Gupta S., et al. Multiplex quantitative analysis of cancer-associated fibroblasts and immunotherapy outcome in metastatic melanoma. J Immunother Cancer. 2019;7:1–10. doi: 10.1186/s40425-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C., Liu L., Chen X., et al. LSD1 stimulates cancer-associated fibroblasts to drive notch3-dependent self-renewal of liver cancer stem-like cells. Cancer Res. 2018;78:938–949. doi: 10.1158/0008-5472.CAN-17-1236. [DOI] [PubMed] [Google Scholar]
- 48.Okabe H., Beppu T., Hayashi H., et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–2564. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 49.Fausther M., Goree J.R., Lavoie E.G., et al. Establishment and characterization of rat portal myofibroblast cell lines. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng Y., Sang Y., Liao J., et al. Single cell transcriptional diversity and intercellular crosstalk of human liver cancer. Cell Death Dis. 2022;13:1–14. doi: 10.1038/s41419-022-04689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu G., Sun J., Yang Z.F., et al. Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 2021;12:1–19. doi: 10.1038/s41419-021-03545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M., Yang H., Wan L., et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X.F., Dong M., Pan Y.H., et al. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum Pathol. 2017;65:92–100. doi: 10.1016/j.humpath.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Cadamuro M., Brivio S., Mertens J., et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70:700–709. doi: 10.1016/j.jhep.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinkevich Y., Mori T., Sahoo D., et al. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251–1260. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asahina K., Zhou B., Pu W.T., et al. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa T., Li Y., Lua I., et al. Isolation of a unique hepatic stellate cell population expressing integrin α8 from embryonic mouse livers. Dev Dyn. 2018;247:867–881. doi: 10.1002/dvdy.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T., Yang Y., Hua X., et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Kato K., Fukai M., Hatanaka K.C., et al. Versican secreted by cancer-associated fibroblasts is a poor prognostic factor in hepatocellular carcinoma. Ann Surg Oncol. 2022;29:7135–7146. doi: 10.1245/s10434-022-11862-0. [DOI] [PubMed] [Google Scholar]
- 60.Mano Y., Yoshio S., Shoji H., et al. Bone morphogenetic protein 4 provides cancer-supportive phenotypes to liver fibroblasts in patients with hepatocellular carcinoma. J Gastroenterol. 2019;54:1007–1018. doi: 10.1007/s00535-019-01579-5. [DOI] [PubMed] [Google Scholar]
- 61.Hu G., Wang S., Xu F., et al. Tumor-infiltrating podoplanin+ fibroblasts predict worse outcome in solid tumors. Cell Physiol Biochem. 2018;51:1041–1050. doi: 10.1159/000495484. [DOI] [PubMed] [Google Scholar]
- 62.Obulkasim H., Shi X., Wang J., et al. Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncol Lett. 2018;15:137–146. doi: 10.3892/ol.2017.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishihara Y., Aishima S., Hayashi A., et al. CD10+ fibroblasts are more involved in the progression of hilar/extrahepatic cholangiocarcinoma than of peripheral intrahepatic cholangiocarcinoma. Histopathology. 2009;55:423–431. doi: 10.1111/j.1365-2559.2009.03398.x. [DOI] [PubMed] [Google Scholar]
- 64.Utispan K., Sonongbua J., Thuwajit P., et al. Periostin activates integrin α5β1 through a PI3K/AKT-dependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- 65.Chiavarina B., Ronca R., Otaka Y., et al. Fibroblast-derived prolargin is a tumor suppressor in hepatocellular carcinoma. Oncogene. 2022;41:1410–1420. doi: 10.1038/s41388-021-02171-z. [DOI] [PubMed] [Google Scholar]
- 66.Levental K.R., Yu H., Kass L., et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jurj A., Ionescu C., Berindan-Neagoe I., et al. The extracellular matrix alteration, implication in modulation of drug resistance mechanism: friends or foes? J Exp Clin Cancer Res. 2022;41:1–21. doi: 10.1186/s13046-022-02484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santamato A., Fransvea E., Dituri F., et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci. 2011;121:159–168. doi: 10.1042/CS20110002. [DOI] [PubMed] [Google Scholar]
- 69.Schrader J., Gordon-Walker T.T., Aucott R.L., et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olsen A.L., Bloomer S.A., Chan E.P., et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akima T., Tamano M., Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. J Hepatol. 2011;41:965–970. doi: 10.1111/j.1872-034X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 72.Kumada T., Toyoda H., Yasuda S., et al. Prediction of hepatocellular carcinoma by liver stiffness measurements using magnetic resonance elastography after eradicating hepatitis C virus. J Clin Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serenari M., Han K.H., Ravaioli F., et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73:855–862. doi: 10.1016/j.jhep.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 74.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y., Qiao X., Wang L., et al. Matrix metalloproteinase 9 induces endothelial-mesenchymal transition via Notch activation in human kidney glomerular endothelial cells. BMC Cell Biol. 2016;17:1–11. doi: 10.1186/s12860-016-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takahra T., Smart D.E., Oakley F., et al. Induction of myofibroblast MMP-9 transcription in three-dimensional collagen I gel cultures: regulation by NF-κB, AP-1 and Sp1. Int J Biochem Cell Biol. 2004;36:353–363. doi: 10.1016/s1357-2725(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 77.Jung W.H., Yam N., Chen C.C., et al. Force-dependent extracellular matrix remodeling by early-stage cancer cells alters diffusion and induces carcinoma-associated fibroblasts. Biomaterials. 2020;234 doi: 10.1016/j.biomaterials.2020.119756. [DOI] [PubMed] [Google Scholar]
- 78.Attieh Y., Vignjevic D.M. The hallmarks of CAFs in cancer invasion. Eur J Cell Biol. 2016;95:493–502. doi: 10.1016/j.ejcb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Xu H., Zhao J., Li J., et al. Cancer associated fibroblast–derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1α/ZEB1 axis. Cell Death Dis. 2022;13:1–13. doi: 10.1038/s41419-022-04935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun L., Wang Y., Wang L., et al. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J Exp Clin Cancer Res. 2019;38:1–17. doi: 10.1186/s13046-019-1163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clapéron A., Mergey M., Aoudjehane L., et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013;58:2001–2011. doi: 10.1002/hep.26585. [DOI] [PubMed] [Google Scholar]
- 82.Lau E.Y.T., Lo J., Cheng B.Y.L., et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Loh J.J., Li T.W., Zhou L., et al. FSTL1 secreted by activated fibroblasts promotes hepatocellular carcinoma metastasis and stemness. Cancer Res. 2021;81:5692–5705. doi: 10.1158/0008-5472.CAN-20-4226. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Wang R., Xiong S., et al. Cancer-associated fibroblasts promote the stemness of CD24+ liver cells via paracrine signaling. J Mol Med. 2019;97:243–255. doi: 10.1007/s00109-018-1731-9. [DOI] [PubMed] [Google Scholar]
- 85.Utispan K., Thuwajit P., Abiko Y., et al. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:1–20. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song M., He J., Pan Q.Z., et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73:1717–1735. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 87.Xiong S., Wang R., Chen Q., et al. Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. Am J Cancer Res. 2018;8:302–316. [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y., Chen G., Han Z., et al. IL-6/STAT3 signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells. Onco Targets Ther. 2020;13:9721–9730. doi: 10.2147/OTT.S262089. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Tsai Y.T., Li C.Y., Huang Y.H., et al. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene. 2022;41:3011–3023. doi: 10.1038/s41388-022-02309-7. [DOI] [PubMed] [Google Scholar]
- 90.Peng H., Xue R., Ju Z., et al. Cancer-associated fibroblasts enhance the chemoresistance of CD73+ hepatocellular carcinoma cancer cells via HGF-Met-ERK1/2 pathway. Ann Transl Med. 2020;8:856. doi: 10.21037/atm-20-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin Y., Cai Q., Chen Y., et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75:28–42. doi: 10.1002/hep.32099. [DOI] [PubMed] [Google Scholar]
- 92.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 93.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 95.Samuel V.T., Shulman G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018;27:22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pais R., Barritt 4th A.S., Calmus Y., et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65:1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang D.Q., Singal A.G., Kono Y., et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pfister D., Núñez N.G., Pinyol R., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang J., Ryan J.S., Ajmera V., et al. Responses to immunotherapy in hepatocellular carcinoma patients with nonalcoholic steatohepatitis cirrhosis. Clin Oncol. 2022;40 389–389. [Google Scholar]
- 100.Wallace S.J., Tacke F., Schwabe R.F., et al. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenthal S.B., Liu X., Ganguly S., et al. Heterogeneity of HSC s in a mouse model of NASH. Hepatology. 2021;74:667–685. doi: 10.1002/hep.31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Asakawa M., Itoh M., Suganami T., et al. Upregulation of cancer-associated gene expression in activated fibroblasts in a mouse model of non-alcoholic steatohepatitis. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-019-56039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arif E., Wang C., Swiderska-Syn M.K., et al. Targeting myosin 1c inhibits murine hepatic fibrogenesis. Am J Physiol. 2021;320:G1044–G1053. doi: 10.1152/ajpgi.00105.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J.S., Yoo J.E., Kim H., et al. Tumor stroma with senescence-associated secretory phenotype in steatohepatitic hepatocellular carcinoma. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng Y., Li H., Deng Y., et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Discov. 2018;9:1–11. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng Y., Cheng J., Fu B., et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36:1090–1101. doi: 10.1038/onc.2016.273. [DOI] [PubMed] [Google Scholar]
- 107.Cheng J., Deng Y., Yi H., et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2016.7. e198–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang X., Lin Y., Shi Y., et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3–CCL2 signaling FAP via STAT3–CCL2 promote tumor immunosuppression. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 109.Lin Y., Li B., Yang X., et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21:1133–1142. doi: 10.1016/j.neo.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang F., Wei Y., Han D., et al. Interaction with CD68 and regulation of GAS6 expression by endosialin in fibroblasts drives recruitment and polarization of macrophages in hepatocellular carcinoma. Cancer Res. 2020;80:3892–3905. doi: 10.1158/0008-5472.CAN-19-2691. [DOI] [PubMed] [Google Scholar]
- 111.Christian S., Ahorn H., Koehler A., et al. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]
- 112.Fiori M.E., Di Franco S., Villanova L., et al. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:1–16. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahai E., Astsaturov I., Cukierman E., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gieniec K.A., Butler L.M., Worthley D.L., et al. Cancer-associated fibroblasts: heroes or villains? Br J Cancer. 2019;121:293–302. doi: 10.1038/s41416-019-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y., McAndrews K.M., Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wen Y., Wang C.T., Ma T.T., et al. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101:2325–2332. doi: 10.1111/j.1349-7006.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lo A., Wang L.C.S., Scholler J., et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L.C.S., Lo A., Scholler J., et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity FAP-redirected CAR T Cells. Cancer Immunol Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Sostoa J., Fajardo C.A., Moreno R., et al. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J Immunother Cancer. 2019;7:1–15. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duperret E.K., Trautz A., Ammons D., et al. Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice DNA vaccine for FAP alters immune stroma. Clin Cancer Res. 2018;24:1190–1201. doi: 10.1158/1078-0432.CCR-17-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roberts E.W., Deonarine A., Jones J.O., et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tran E., Chinnasamy D., Yu Z., et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhen Z., Tang W., Wang M., et al. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 124.Freedman J.D., Duffy M.R., Lei-Rossmann J., et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells oncolytic adenovirus expressing FAP BiTE. Cancer Res. 2018;78:6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 125.Shankaraiah R.C., Callegari E., Guerriero P., et al. Metformin prevents liver tumourigenesis by attenuating fibrosis in a transgenic mouse model of hepatocellular carcinoma. Oncogene. 2019;38:7035–7045. doi: 10.1038/s41388-019-0942-z. [DOI] [PubMed] [Google Scholar]
- 126.Shao S., Duan W., Xu Q., et al. Curcumin suppresses hepatic stellate cell-induced hepatocarcinoma angiogenesis and invasion through downregulating CTGF. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Z., Li X., Sun W., et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 128.Liu H., Shen J., Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486:239–244. doi: 10.1016/j.bbrc.2017.02.128. [DOI] [PubMed] [Google Scholar]
- 129.Faivre S., Santoro A., Kelley R.K., et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468–1477. doi: 10.1111/liv.14113. [DOI] [PubMed] [Google Scholar]
- 130.Tauriello D.V., Palomo-Ponce S., Stork D., et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 131.Mariathasan S., Turley S.J., Nickles D., et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo X., Fong E.L.S., Zhu C., et al. Hydrogel-based colorectal cancer organoid co-culture models. Acta Biomater. 2021;132:461–472. doi: 10.1016/j.actbio.2020.12.037. [DOI] [PubMed] [Google Scholar]