Abstract
Alcohol use disorder (AUD) is a chronic, relapsing disorder characterized by an escalation of drinking and the emergence of negative affective states over time. Within this framework, alcohol may be used in excessive amounts to alleviate withdrawal-related symptoms, such as hyperalgesia. Future effective therapeutics for AUD may need to exhibit the ability to reduce drinking as well as alleviate co-morbid conditions such as pain, as well as take mechanistic sex differences into consideration. Agmatine is an endogenous neuromodulator that has been previously implicated in the regulation of reward and pain processing. In the current set of studies, we examined the ability of agmatine to reduce escalated ethanol drinking in complementary models of AUD where adult male and female mice and rats were made dependent via chronic, intermittent ethanol vapor exposure (CIE). We also examined the ability of agmatine to modify thermal and mechanical sensitivity in alcohol-dependent male and female rats. Agmatine reduced alcohol drinking in a dose-dependent fashion, with somewhat greater selectivity in alcohol-dependent female mice (versus non-dependent female mice) but equivalent efficacy across male mice and both groups of male and female rats. In mice and female rats, this efficacy did not extend to sucrose drinking, indicating some selectivity for ethanol reinforcement. Female rats made dependent on alcohol demonstrated significant hyperalgesia symptoms, and agmatine produced dose-dependent antinociceptive effects across both sexes. While additional mechanistic studies into agmatine are necessary, these findings support the broad-based efficacy of agmatine to treat co-morbid excessive drinking and pain symptoms in the context of AUD.
Keywords: Alcohol Dependence, Analgesia, Ethanol, Pain, Self-Administration
Introduction
Excessive alcohol consumption and alcohol use disorder (AUD) lead to serious health and social consequences. AUD is characterized by a loss of control over alcohol drinking that is accompanied by neuroadaptations in brain regions that control motivated and affective behaviors (Koob, 2021). Chronic alcohol exposure also enhances pain sensitivity and prolonged use is associated with the development of alcohol-related neuropathy (Egli, Koob, & Edwards, 2012). Long-term use of alcohol causes adaptive changes in several neurotransmitters systems, including GABA, glutamate, serotonin, and norepinephrine, among many others (Trudell, Messing, Mayfield, & Harris, 2014). Only three drugs are currently approved by the U.S. Food and Drug Administration (FDA) for use in AUD. Thus, the treatment options for AUD are limited and many individuals with severe AUD follow a chronic relapsing course. Targeting both excessive drinking and co-morbid symptoms (such as enhanced pain sensitivity, or hyperalgesia) may represent a particularly valuable therapeutic avenue (Edwards, Vendruscolo, Gilpin, Wojnar, & Witkiewitz, 2020).
Agmatine is an endogenous guanido amine whose presence in the mammalian brain was first discovered in 1994 (Li et al., 1994). Since then, a wide range of studies has shown that agmatine can modulate various receptors and ion channels (Barua, Kim, Kim, Kim, & Lee, 2019). Supporting a broad-based mechanism of action, functional studies have shown that agmatine exerts beneficial effects in animal models of multiple neuropsychiatric diseases such as ischemic stroke, traumatic injury, pain, Alzheimer’s Disease, anxiety, depression, and substance use disorder (SUD) (Laube & Bernstein, 2017; Neis, Rosa, Olescowicz, & Rodrigues, 2017).
Several brain regions (such as the ventral tegmental area, nucleus accumbens, and amygdala that are involved in both reward and pain behaviors) express high agmatine levels (Otake et al., 1998; Reis & Regunathan, 2000). This has led to studies investigating the effects of agmatine within specific behavioral models of SUD. Agmatine has been shown to attenuate ethanol, nicotine, and morphine withdrawal symptoms (Aricioglu-Kartal & Uzbay, 1997; N. R. Kotagale, Ali, Chopde, Umekar, & Taksande, 2018; N. R. Kotagale, Chopde, Umekar, & Taksande, 2015; Regunathan, 2006; Uzbay, Yesilyurt, Celik, Ergun, & Isimer, 2000). Further, agmatine reduces impaired performance on a cerebellar-dependent balance test in a rat model of third-trimester binge-like ethanol exposure (Lewis, Wellmann, & Barron, 2007), corrects ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol (Wellmann, Lewis, & Barron, 2010), and lessens behavioral and cognitive deficits induced by ethanol exposure during the entire gestation in rats (Aglawe et al., 2021). Agmatine also decreases morphine, cocaine, and fentanyl self-administration (Morgan, Campbell, Fons, & Carroll, 2002) and inhibits ethanol-induced locomotor sensitization (Ozden, Kayir, Ozturk, & Uzbay, 2011).
Previous studies have investigated the effects of agmatine on certain behavioral responses to alcohol exposure. However, crucial studies on alcohol intake and co-morbid conditions using alcohol-dependent animal models that model human AUD (H. C. Becker & Lopez, 2016; Vendruscolo & Roberts, 2014) have not been conducted. Therefore, the present study aimed to evaluate the effect of systemic administration of agmatine on voluntary ethanol consumption in well-established mouse and rat models of ethanol dependence and relapse drinking. The chronic intermittent ethanol (CIE) model affords the opportunity to examine agmatine effects on ethanol consumption and pain sensitivity at both alcohol-dependent and non-dependent levels. We also investigated sex as a factor in these relationships, given the substantial human sex differences in alcohol reward and pain (Becker & Koob, 2016).
Materials and Methods
Subjects
Adult male and female C57BL/6J mice were used in these studies. Mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were 10 weeks old upon arrival. Mice were individually housed in standard polycarbonate cages with corncob bedding in a temperature- and humidity-controlled vivarium within an AAALAC-accredited facility. Subjects were maintained on a 12-hr modified light/dark cycle (lights on at 10:00 PM) with ad libitum access to food (Diet #2918; Harland Teklad, Madison, WI) and water throughout the duration of the experiments. Procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council, 2011) under protocols approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Adult male and female Wistar rats (N=84) were 10 weeks old upon arrival and were purchased from Charles River (Raleigh, NC). Rats were pair-housed and given ad libitum access to food (Purina Rat Chow, Ralston Purina, St. Louis, MO) and water throughout all experimental procedures. Rats were maintained on a reverse 12-hour light/dark cycle (lights off at 8:00 am) and were handled regularly. Rats were given one week to acclimate to the colony room prior to the start of experimental procedures. All animal care, use, and procedures in the rat study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center (LSUHSC) and were in accordance with the National Institute of Health guidelines.
Overall Mouse Study Design and Procedure
Adult male and female C57BL/6J mice (N=96/sex) were trained to drink ethanol in a limited access (2-hr/day) free-choice (15% v/v ethanol vs. water) drinking procedure starting 3 hours after lights off. The ethanol solution was prepared fresh every day and bottles were weighed before and after the drinking session (± 0.01 g) to measure intake. Sentinel bottles placed in empty cages were used to control for leaks and evaporation as a source of error. The volume of daily ethanol solution consumed, and body weights of mice recorded weekly were used to calculate ethanol intake in g/kg. After 4 weeks, a stable baseline level of intake was established, and mice were separated into CIE and CTL groups (N= 48/group). CIE mice were exposed to chronic intermittent ethanol (CIE) vapor exposure in inhalation chambers (16-hr/day × 4 days). CTL mice were treated similarly but were exposed to air in inhalation chambers (detailed below). After a 72-hr forced abstinence period, all mice resumed ethanol drinking in the same limited access paradigm for a 5-day test period. This pattern of weekly CIE (or air) exposure cycles alternating with weekly test drinking cycles was repeated for several cycles following procedures previously published (Becker & Lopez, 2004; Griffin, Lopez, & Becker, 2009; Lopez & Becker, 2005). All mice received intraperitoneal (IP) administration of vehicle (saline) 30-min prior to the start of daily drinking sessions during Baseline and the early Test cycles to acclimate animals to the handling procedure. The experiments with male and female subjects were conducted separately (at different times and housing). After the second ethanol intake testing cycle for males (Experiment 1) or the third cycle for female subjects (Experiment 2), mice were further divided into drug treatment dosage groups (Vehicle, 40, 80, 120 mg/kg agmatine). There was no administration of agmatine during the week of CIE (or air) exposure.
Additionally, the effect of agmatine administration on the voluntary intake of a sucrose solution was evaluated (Experiment 3). In this study, male and female mice (N= 40/sex) were allowed to drink sucrose (0.5% w/v) vs. water using the same limited access procedure used to evaluate ethanol intake. After three weeks of baseline sucrose drinking after vehicle administration, mice were divided into groups to receive agmatine (Vehicle, 40, 80, 120 mg/kg) 30-min before a test drinking session.
CIE Exposure in Mice
Mice in the CIE group received chronic intermittent vapor exposure in inhalation chambers (16 h/day for 4 days) while control mice (CTL) were similarly handled but exposed to air in control chambers. CIE exposure was administered in inhalation chambers according to procedures previously described (Becker & Lopez, 2004; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, Middaugh, & Becker, 2009; Lopez & Becker, 2005). Briefly, mice were placed in Plexiglas inhalation chambers (60 × 36 × 60 cm) and exposed to ethanol vapor at levels set to yield stable blood ethanol concentrations (BEC) in C57BL/6J mice in the range of 175–225 mg/dl. At the beginning of a 16-hr exposure cycle, ethanol intoxication was initiated by intraperitoneal (IP) administration of 1.6 g/kg alcohol (8% w/v), and the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg; IP) to stabilize blood ethanol levels. CTL mice received an injection of pyrazole along with saline. Housing conditions were like those in the colony room. Blood samples (40 μl) were obtained from the retro-orbital sinus with heparinized capillary tubes during each CIE cycle of exposure. Blood samples were centrifuged, and plasma was processed in an Analox Instrument analyzer (Lunenburg, MA), with blood alcohol levels (BAL) expressed as mg/dl.
Operant Self-Administration and CIE Exposure in Rats
Prior to alcohol/water self-administration (Experiment 4), male (n=5–6/group) and female (n=8–12/group) rats were given 2-bottle choice access to 10% (w/v) ethanol and water in the home cage for 24 hours to habituate them to the taste of alcohol. The following day rats were given an overnight session in the operant chambers (Med Associates) with access to one lever that delivers water. Regular food was available ad libitum in the operant chambers during this overnight session. One day later rats were placed in the operant chambers with access to one lever that delivers 10% (w/v) ethanol for 2 hours, 1 hour, and 30 minutes over the course of three days. All subsequent sessions were 30-minute sessions with access to one lever that delivers 10% (w/v) ethanol and one lever that delivers water. Operant alcohol self-administration was conducted under a fixed-ratio 1 (FR-1) schedule of reinforcement, in which each lever press results in a 0.1 ml fluid delivery. Rats were trained to self-administer 10% (w/v) alcohol and water solution in operant chambers (Med Associates) until stable responding was maintained, as previously described (Pahng et al., 2019).
Following stable acquisition of operant alcohol self-administration, rats were split into two groups, which were matched for alcohol self-administration over the last three sessions of self-administration. One group was designated as “alcohol-dependent” and the other as “non-dependent”. For chronic, intermittent ethanol exposure to ethanol vapor (CIE), rats were pair-housed in plexiglass vapor delivery chambers. CIE and control groups (air-exposed) were housed in separate rooms in individually ventilated cages. CIE procedures in which animals undergo cycles of alcohol exposure and abstinence over several weeks were the same as previously described (Edwards, Guerrero, Ghoneim, Roberts, & Koob, 2012; Gilpin, Richardson, Cole, & Koob, 2008). Here, the intermittent procedure entails daily cycles of alcohol vapor exposure (14 hrs, producing target blood alcohol levels (BALs) of 150–250 mg/dL) and alcohol withdrawal (10 hrs, where BALs are reduced to near zero, and physical and negative motivational symptoms emerge). Tail blood samples were collected and analyzed one to two times per week to maintain BALs of 150–250 mg/dL, as previously described (Gilpin et al., 2008). This procedure has been reliably used to produce both somatic and motivational-like symptoms of dependence (Gilpin et al., 2008; Rogers, Wiener, & Bloom, 1979; Vendruscolo & Roberts, 2014). To assess blood alcohol levels (BALs), tail blood (0.2 ml) was collected and centrifuged to extract the plasma. The plasma was injected into an oxygen-rate alcohol analyzer (Analox Instruments, London, UK) for Blood Alcohol Level (BAL) determination via an alcohol oxidation reaction as previously described (McGinn et al., 2016). Single point calibrations were done for each set of samples with reagents provided by Analox Instruments (25–400 mg%).
Following the acquisition of alcohol self-administration and induction of alcohol dependence by at least eight weeks of CIEV exposure, alcohol-dependent and non-dependent (air-exposed) animals were tested until post-vapor intake stabilization was achieved. Responses for both ethanol and water were recorded. For post-vapor alcohol drinking behavior, the number of alcohol rewards (0.1 ml fluid delivery) and the number of lever presses from the last three 30-minute sessions were recorded. All post-vapor testing for alcohol self-administration was conducted when alcohol-dependent animals were in acute withdrawal (8 hrs after removal from vapor when BALs are approximately zero). After stable responding for alcohol was achieved, rats were treated with agmatine (0, 40, 80, 120 mg/kg) in a counterbalanced fashion 30-min before a test operant self-administration session.
Additionally, the effect of agmatine administration on self-administration of a sucrose solution was evaluated (Experiment 5). In this study, male (n=15) and female (n=6) rats were trained to self-administer sucrose (0.5% w/v) vs. water using the same limited access procedure used to evaluate alcohol and water self-administration. After stable responding for sucrose was achieved, rats were treated with agmatine (0, 40, 80, 120 mg/kg) in a counterbalanced fashion 30-min before a test operant self-administration session.
Mechanical Sensitivity Testing in Rats
Mechanical sensitivity was determined in separate groups of animals (n=6–11/group) by obtaining mean hind paw withdrawal thresholds using the electronic von Frey test (TopCat Metrology Ltd., Little Downham, UK). To reduce stress before testing, animals were first acclimated to the testing room under white light for 30 minutes prior to the start of behavioral testing and then placed into elevated clear Plexiglas compartments on a mesh stand to acclimate for 5 minutes. Following this acclimation period, the electronic von Frey filament was applied to the mid-plantar region of the hind paw, and withdrawal thresholds were recorded as grams of force necessary to elicit paw withdrawal. The filament was alternatingly applied to the left and right hind paws at 1–3 minute intervals for a total of 2 measurements per paw. The average score of these tests per paw served as the dependent measure. Tests occurred 8 hours after removal from vapor.
Thermal Sensitivity Testing in Rats
Immediately following the completion of electronic von Frey testing, thermal sensitivity was quantified using the thermal probe test (TopCat Metrology Ltd.). The thermal probe (2 mm diameter) was applied to the mid-plantar region of the hind paw and began heating at 2.5°C per second at a force of 1 gram, with a maximum temperature threshold of 60°C. Each hind paw was tested one time, and the temperature (°C) necessary to elicit paw withdrawal served as the dependent measure. Tests occurred 8 hours after removal from vapor.
Drugs
190 proof ethyl alcohol (mice: Warner Graham CO., Cockeysville, MD, rats: LSUHSC-New Orleans General Stores) was used to expose rodents to ethanol vapors and to prepare solutions (15% v/v for mice and 10% w/v for rats) to drink. Pyrazole, sucrose, and agmatine sulfate salt were obtained from Sigma-Aldrich (St. Louis, MO). The doses of agmatine were selected based on previous publications (Aricioglu & Regunathan, 2005; Utkan, Gocmez, Regunathan, & Aricioglu, 2012).
Data Analysis
Preliminary analysis of mouse data failed to indicate statistically significant variation on ethanol intake or sucrose intake across days of baseline or test cycles. Therefore, a decision was made to analyze these data averaged over the last five days of baseline and the five days of each test cycle. Ethanol intake (g/kg), sucrose intake (ml), and blood ethanol concentrations (mg/dl) were evaluated using ANOVA followed by post-hoc comparisons using the Newman-Keuls test as detailed in each mouse experiment. Mouse data were analyzed using Statistica (Tibco Software Inc.; Palo Alto, CA). Rat data were analyzed using Prism Version 9 (GraphPad Software, Inc; La Jolla, CA), where lever presses (responding for ethanol, sucrose, or water) or paw withdrawal temperatures/thresholds were evaluated using ANOVA (incorporating Greenhouse-Geisser corrections) followed by post-hoc comparisons using Bonferroni’s multiple comparison tests (2-way analyses) or Dunnett’s multiple comparison tests (1-way analyses). For all the analyses, alpha was set at 0.05 to assess significant effects. Importantly, post-hoc comparisons were conducted with protections for multiple comparisons to avoid Type I errors. For both mice and rats, the sexes were run at separate times and thus were analyzed separately.
Results
Experiment 1: Effect of Agmatine Treatment on Ethanol Intake in Male Mice
Weekly average ethanol intake (g/kg) during Baseline and early Test cycles were analyzed using ANOVA, with Group (CTL, CIE) as a between-subjects factor and Phase (Baseline, Test-1, Test-2) as a repeated measure. ANOVA indicated a significant main effect of Group [F(1,94)= 27.42; p< 0.001, partial η2 = 0.23] and a significant Group × Phase interaction [F(2,188)= 22.50; p< 0.001, partial η2 = 0.19]. Post-hoc (Newman-Keuls) comparisons indicated that there was no difference in ethanol intake between groups during Baseline, since mice were separated into groups based on their intake during this phase of the study. As expected, CIE mice consumed significantly more ethanol than CTL mice and their own baseline during the two test cycles (#; p< 0.001; Figure 1A). Mice in the CTL group showed a small but significant decrease in ethanol intake compared to their own baseline during Test-2 (*; Figure 1A).
Figure 1:
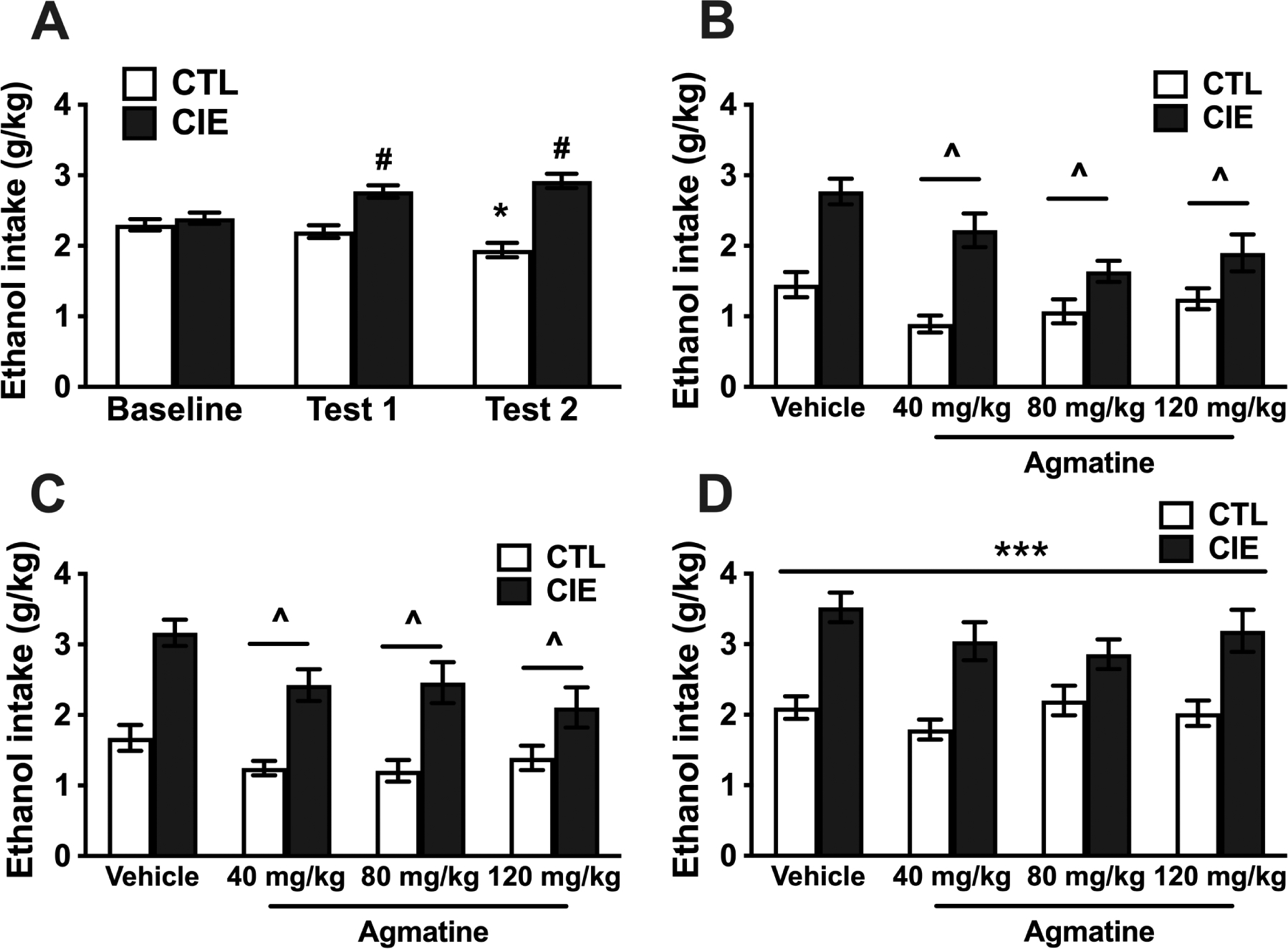
A) Voluntary ethanol intake (g/kg) for CIE exposed and CTL (air-exposed) male mice during baseline and the first two ethanol drinking test cycles while all mice received vehicle injections before drinking (N=48/group). * Indicates lower intake compared to their own baseline level (p<0.001). # Indicates higher ethanol intake in CIE mice compared to CTL mice and their own baseline level of intake (p<0.001). B) Ethanol intake (g/kg) for male mice that received pretreatment with different doses of agmatine during Test Cycle 3 (N=11–12/group). The ANOVA indicated a significant main effect of CIE vs. CTL condition due to significantly higher ethanol intake in CIE mice. ^ Indicates a main effect of agmatine dose with lower intake in mice that received agmatine compared to vehicle independently of CIE or CTL condition (p<0.05). C) Ethanol intake (g/kg) for male mice that received pretreatment with different doses of agmatine during Test Cycle 4 (second cycle of treatment) (N=11–12/group). The ANOVA indicates a significant main effect of CIE vs. CTL condition due to significantly higher ethanol intake in CIE mice. ^ Indicates lower intake in mice that received agmatine compared to vehicle independently of CIE or CTL condition (p<0.01). D) Ethanol intake (g/kg) for male mice during Test Cycle 5 (washout test) that received pretreatment with different doses of agmatine during Test cycles 3 and 4 (N=11–12/group). The ANOVA indicated a significant main effect of CIE vs. CTL condition due to significantly higher ethanol intake in CIE mice (***p<0.001). Values are mean ±SEM
CIE and CTL mice were then separated based on their intake level during Test-2 to receive treatment with agmatine during Test-3 (N= 11–12/group). Mice received IP injections of vehicle (saline) or 40, 80, 120 mg/kg of agmatine 30-min before the start of the drinking sessions. Ethanol intake during Test-3 was averaged for the week and analyzed by with Group (CTL, CIE) and agmatine Dose (0, 40, 80, 120 mg/kg) as between-subject factors. ANOVA indicated a significant main effect of Group [F(1,85)= 55.63; p< 0.001, partial η2 = 0.40], reflecting a higher level of voluntary ethanol intake in CIE mice compared to CTL mice. ANOVA also indicated a significant effect of agmatine treatment [F(3,85)=6.29; p<0.001, partial η2 = 0.18]. Post-hoc comparisons indicated that all doses of agmatine (averaged across Group) showed lower levels of intake compared to the vehicle subjects (^, Figure 1B). ANOVA also indicated that the Group × Dose interaction was near the significance threshold [F(3,85)= 2.54; p=0.06, partial η2 = 0.08] (Figure 1B).
Due to this boderline effect, a decision was made to repeat the evaluation of agmatine during another test cycle. Mice were exposed to a fourth cycle of CIE (or air) exposure and evaluated again for voluntary ethanol intake after treatment with agmatine. Ethanol intake during Test-4 was analyzed by ANOVA, with Group (CTL, CIE) and agmatine Dose (0, 40, 80, 120 mg/kg) as between-subject factors. ANOVA indicated a significant main effect of Group [F(1,85)= 64.15; p< 0.001, partial η2 = 0.43], reflecting a higher level of voluntary ethanol intake in CIE mice compared to CTL mice. ANOVA also indicated a significant effect of agmatine [F(3,85)= 4.70; p< 0.0043, partial η2 = 0.14]. Post-hoc comparisons indicated that mice that received any dose of agmatine showed lower levels of intake compared to the vehicle subjects (^, Figure 1C). However, the Group × Dose interaction was not significant [F(3,85)= 1.26; p=0.29, partial η2 = 0.04] (Figure 1C).
Mice were then evaluated after a 5th and final CIE or Air exposure cycle. In this test cycle, all mice received vehicle injections before drinking to evaluate any long-lasting effect of previous treatment with agmatine. ANOVA indicated a main effect of Group [F(1,85)= 57.06; p< 0.001, partial η2 = 0.40] due to overall higher intake in CIE mice compared to CTL mice (***, Figure 1D). However, ANOVA failed to indicate a significant main effect of agmatine Dose [F(3,85)= 1.28; p=0.29, partial η2 = 0.04] or a significant Group × Dose interaction [F(3,85)= 1.18; p=0.32, partial η2 = 0.04 (Figure 1D).
Finally, blood alcohol concentration (mg/dl) during CIE exposure were within the expected range and were analyzed in an ANOVA using agmatine dose as between factor and cycle (1–5) as repeated measure. The ANOVA indicated a significant main effect of cycle [F(4,164)=27.20; p<0.0001, partial η2 = 0.40] due to significantly lower BAL during the first CIE exposure cycle compared to cycles 2–5 (Table 1). Dose of agmatine [F(3,41)=0.25; p=0.86, partial η2 = 0.01] or the interaction between dose and cycle [F(12,164)=1.49; p=014, partial η2 = 0.10] were not significant in this ANOVA.
Table 1:
Blood alcohol concentration (BAL) during cycles of CIE in male and female mice
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | |
|---|---|---|---|---|---|---|
| Males | 152.55 *±6.02 | 214.75±7.69 | 222.98±5.71 | 212.13±5.89 | 231.06±7.53 | |
| Females | 206.27 ^±3.38 | 215.21±4.17 | 230.87±5.72 | 238.67±6.36 | 223.66±5.01 | 222.35±5.63 |
Blood alcohol concentrations recorded during each CIE cycle for male and female subjects averaged across agmatine dose conditions.
Indicates BAL lower than those registered for cycles 2–5 in males and
indicates lower BAL compared to cycles 3–6 in females (p<0.05).
Values are mean ± SEM
Experiment 2: Effect of Agmatine Treatment on Ethanol Intake in Female Mice
Ethanol intake (g/kg) averaged over the last five days of Baseline and the five days of each early Test cycle were analyzed by ANOVA, with Group (CTL, CIE) as a between-subjects factor and Phase (Baseline, Test-1, Test-2, Test-3) as a repeated measure. ANOVA indicated a significant main effect of Group [F(1,92)= 18.11; p< 0.001, partial η2 = 0.16]; a significant main effect of Phase [F(3,276)= 12.47; p< 0.001, partial η2 = 0.12] and a significant Group × Phase interaction [F(3,276)= 10.31; p< 0.001, partial η2 = 0.10]. Post-hoc (Newman-Keuls) comparisons based on the interaction term indicated that there was no difference in ethanol intake between groups during Baseline. As expected, CIE mice consumed significantly more ethanol than CTL mice and their own baseline during the three test cycles (#; p< 0.001; Figure 2A). Mice in the CTL group showed a stable level of intake from baseline and across the three test cycles.
Figure 2:
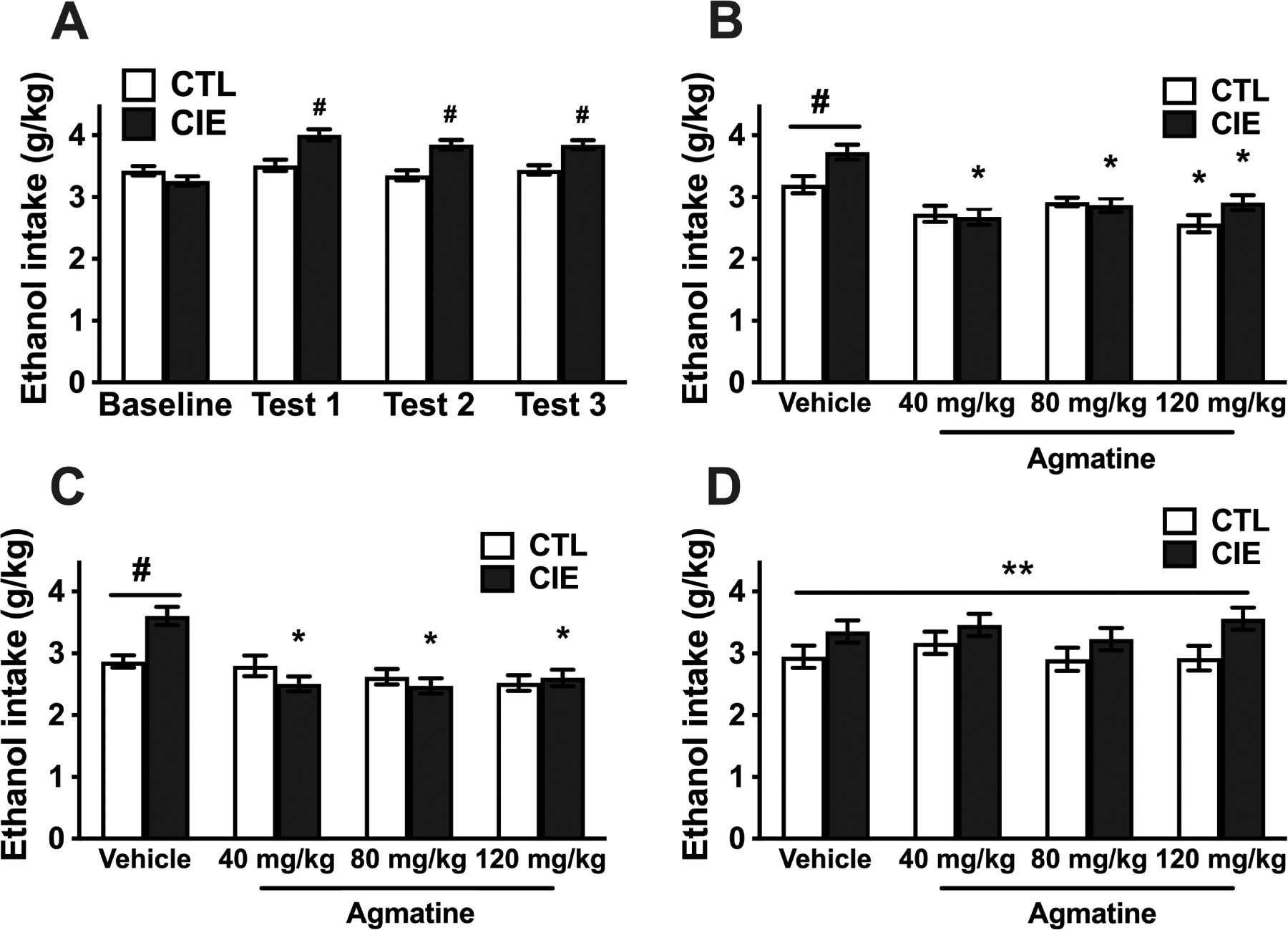
A) Voluntary ethanol intake (g/kg) for CIE-exposed and CTL (air-exposed) female mice during baseline and the first three ethanol drinking test cycles (all mice received vehicle injections) (N=46 CTL, 48 CIE). # Indicates higher ethanol intake in CIE mice compared to CTL mice and their own baseline level of intake (p<0.001). B) Ethanol intake (g/kg) for female mice that received different doses of agmatine during Test Cycle 4 (N=11–12/group). # Indicates higher intake in CIE vs. CTL mice that received vehicle injections (p<0.01). * Indicates lower intake in CIE mice that receive agmatine pre-treatment (any dose) and CTL mice that received the highest dose compared to their respective vehicle condition (p<0.01). C) Ethanol intake (g/kg) for female mice that received different doses of agmatine during Test Cycle 5 (second cycle of agmatine treatment) (N=11–12/group). # Indicates higher intake in CIE vs. CTL mice that received vehicle injections (p<0.001). * Indicates lower intake in CIE mice that receive agmatine pre-treatment compared to CIE mice that received vehicle (p<0.001). D) Ethanol intake (g/kg) for female mice during Test Cycle 6 (washout test) that received pretreatment with different doses of agmatine during Test cycles 4 and 5 (N=10–12/group). The ANOVA indicated a significant main effect of CIE vs. CTL condition due to significantly higher ethanol intake in CIE mice (**p<0.01). Values are mean ±SEM
For Test 4, CIE and CTL mice were then separated based on their intake level during Test 3 to receive treatment with agmatine (N= 11–12/group). Mice received IP injections of vehicle (saline) or agmatine (40, 80, 120 mg/kg) 30-min before the start of the drinking sessions. Ethanol intake during Test 4 was analyzed by ANOVA, with Group and agmatine Dose as between-subject factors. ANOVA indicated a significant main effect of Group [F(1,86)= 5.15; p< 0.026, partial η2 = 0.06], reflecting a higher level of ethanol intake in CIE mice compared to CTL mice. The analysis also indicated a significant effect of agmatine Dose [F(3,86)= 16.83; p< 0.001, partial η2 = 0.37] and a significant Group × Dose interaction [F(3,86)= 2.87; p< 0.041 partial η2 = 0.09]. Post-hoc comparisons based on the interaction term indicated that CIE mice that received vehicle injections had significantly higher levels of intake compared to CTL mice that received vehicle (#; Figure 2B). Further, while all agmatine doses reduced voluntary ethanol intake in CIE-exposed mice, only the highest dose (120 mg/kg) reduced ethanol intake in CTL mice compared to vehicle subjects (*; Figure 2B).
As was done with male subjects, evaluation of agmatine treatment continued after mice were exposed to a fifth cycle of CIE (or air) exposure. Analysis of ethanol intake during Test 5 revealed a significant main effect of agmatine Dose [F(3,86)= 12.41; p< 0.001, partial η2 = 0.30] and a significant Group × Dose interaction [F(3,86)= 6.03; p< 0.001, partial η2 = 0.17]. Post-hoc comparisons from this interaction indicated that CIE mice that received vehicle injections had significantly higher levels of intake compared to CTL mice that received vehicle (#; Figure 2C). This analysis also indicated that the three agmatine doses evaluated reduced voluntary ethanol intake in CIE exposed mice (*; Figure 2C) without affecting ethanol intake in CTL mice.
Mice were then evaluated after a 6th and final CIE or Air exposure cycle. In this test cycle, all mice received vehicle injections before drinking to evaluate any long-lasting effect of previous treatment with agmatine. ANOVA of these data indicated a main effect of Group [F(1,85)= 10.24; p< 0.0019, partial η2 = 0.11] due to overall higher intake in CIE mice compared to CTL mice (**, Figure 2D). ANOVA did not indicate a significant main effect of agmatine Dose [F(3,85)= 0.70; p= 0.56, partial η2 = 0.02] or a significant interaction between Group and Dose [F(3,85)= 0.35; p=0.79, partial η2 = 0.01] (Figure 2D).
As indicated above for male subjects, blood alcohol concentration (mg/dl) during CIE exposure for females was analyzed in an ANOVA using agmatine dose as between factor and cycle (1–6) as repeated measure. The ANOVA indicated a significant main effect of cycle [F(5,220)=5.29; p<0.001, partial η2 = 0.11] due to lower BAL during the first CIE exposure cycle compared to cycles 3–6 (Table 1). Dose of agmatine [F(3,44)=0.52; p=0.67, partial η2 = 0.03] or the interaction between dose and cycle [F(15,220)=0.62; p=0.86, partial η2 = 0.04] did not have a significant effect on BAL.
Experiment 3: Effect of Agmatine on Sucrose Intake in Male and Female Mice
Sucrose intake (g/kg) was average over the last five days of baseline and the five days of treatment with agmatine. To match the analyses conducted for ethanol intake separate ANOVAs were conducted for male and female subjects. The mixed model ANOVA was conducted with agmatine Dose (0, 40, 80, 120 mg/kg) as a between-subject factor, and Phase (Baseline, Test) as a repeated measure. For males, the ANOVA indicated a main effect of Phase [F(1,34)=7.72; p<0.008, partial η2 = 0.18] due to overall lower sucrose intake during Test week compared to baseline. There was no effect of agmatine dose [F(3,34)=0.18; p=0.91, partial η2 = 0.02] or interaction between Phase and agmatine dose [F(3,34)=1.08; p=0.37, partial η2 = 0.09] (Figure 3). For females the ANOVA failed to indicate any significant effect of Phase [F(1,35)=2.95; p=0.10, partial η2 = 0.08], agmatine dose [F(3,35)=0.02; p=0.99, partial η2 = 0.01], or interaction between these factors [F(3,35)=0.13; p=0.94, partial η2 = 0.01] (Figure 3).
Figure 3:
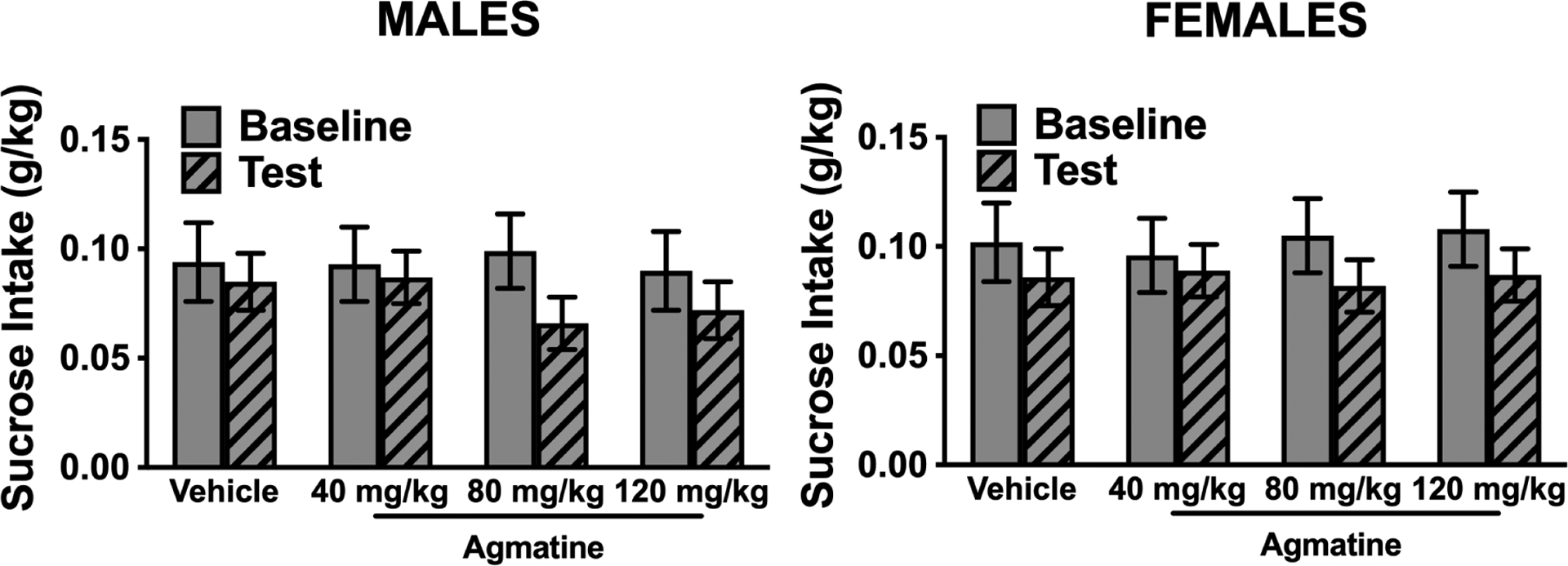
Sucrose intake (g/kg) in male (left) and female (right) mice during baseline (all mice received vehicle injections) and during the test cycle when subjects received different doses of agmatine (N=9–10/group). The ANOVA failed to indicate any effect of agmatine dose or interaction of dose and phase (baseline vs. test). Values are mean ± SEM
Experiment 4: Effect of Agmatine on Operant Alcohol Self-Administration in Male and Female Rats
We first examined the efficacy of agmatine (0–120 mg/kg, IP, 30-minute pretreatment) to alter operant self-administration of alcohol in male rats exposed to chronic, intermittent alcohol vapor (alcohol-dependent group) vs. air (non-dependent group). Agmatine dose-dependently reduced alcohol self-administration in both groups (Figure 4, main effect of agmatine dose [F(2.331,20.98)= 13.04; p<0.001; partial η2 = 0.66] without a group × dose interaction [F(3,27)= 0.2741; p=0.8435; partial η2 = 0.04], indicating a general effect of agmatine to reduce alcohol drinking in both groups. In female rats, we also discovered that agmatine dose-dependently reduced drinking across both alcohol-dependent and non-dependent groups (Figure 5, main effect of dose [F(2.678,48.21)= 21.11; p<0.001; partial η2 = 0.50]. Again, there was no group × dose interaction [F(3,54)= 0.0099; p=0.9986; partial η2 = 0.01]. We also examined the effects of agmatine to alter self-administration of water (tests co-occurring with alcohol self-administration), representing responding for a natural reward and/or a general indicator of locomotor activity and impairment. Self-administration of water was relatively low in all groups and not significantly altered by agmatine treatment (males [F(1.777,15.99)= 3.132; p=0.0759; partial η2 = 0.17]; females [F(1.238,22.29)= 2.331; p=0.1367; partial η2 = 0.10]). We also found no group × dose interactions with regard to water responding (males [F(3,27)= 0.5633; p=0.6439; partial η2 = 0.04]; females [F(3,54)= 0.4681; p=0.7058; partial η2 = 0.02).
Figure 4:
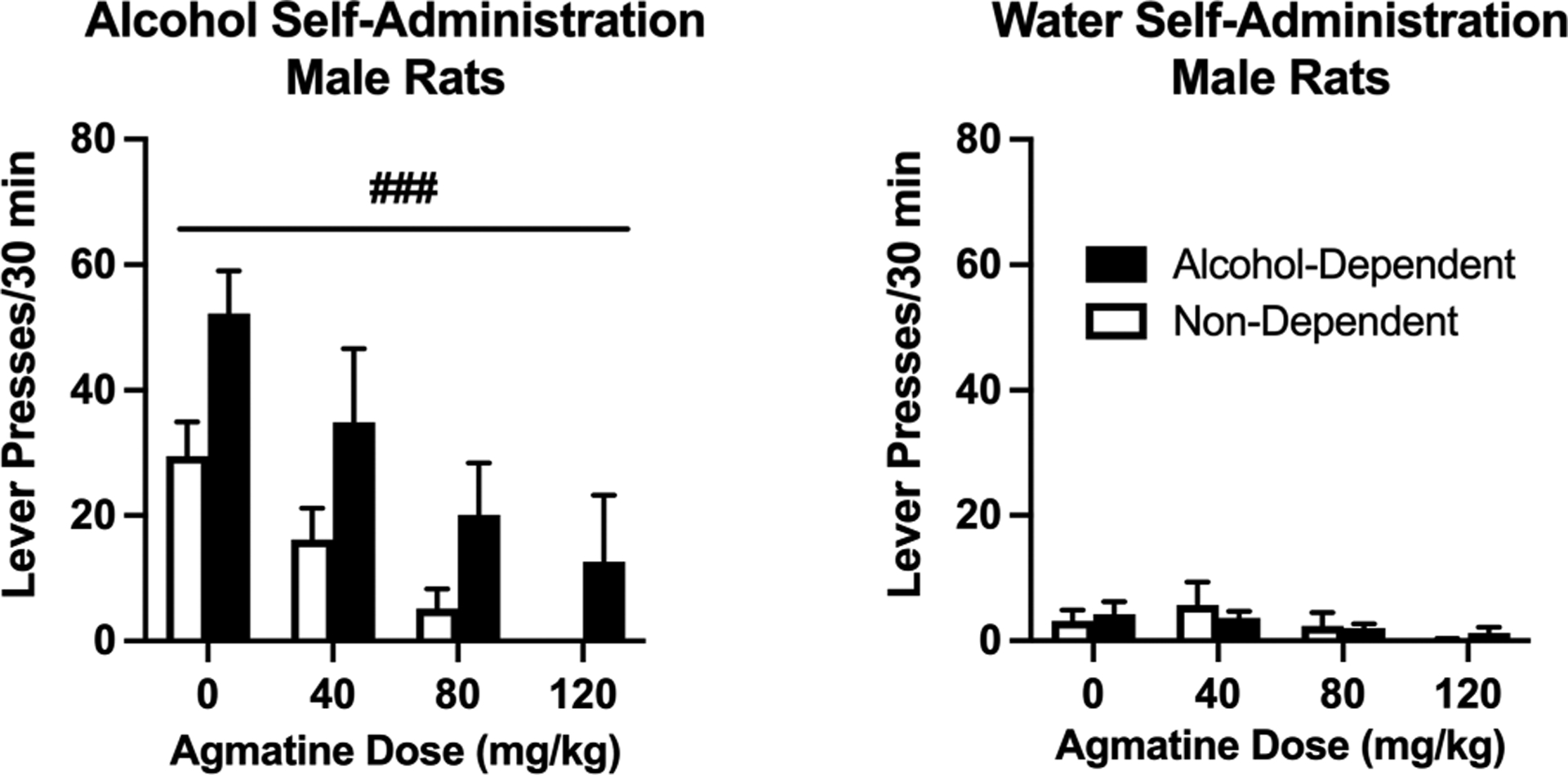
Operant alcohol (left) and water (right) self-administration in male Wistar rats. Agmatine reduces alcohol self-administration in a dose-dependent manner across both alcohol-dependent and non-dependent male Wistar rats. Responding for water was not significantly altered in either group. N=5–6/group. ###p<0.001 main effect of agmatine dose in male rats. Values are mean ± SEM
Figure 5:
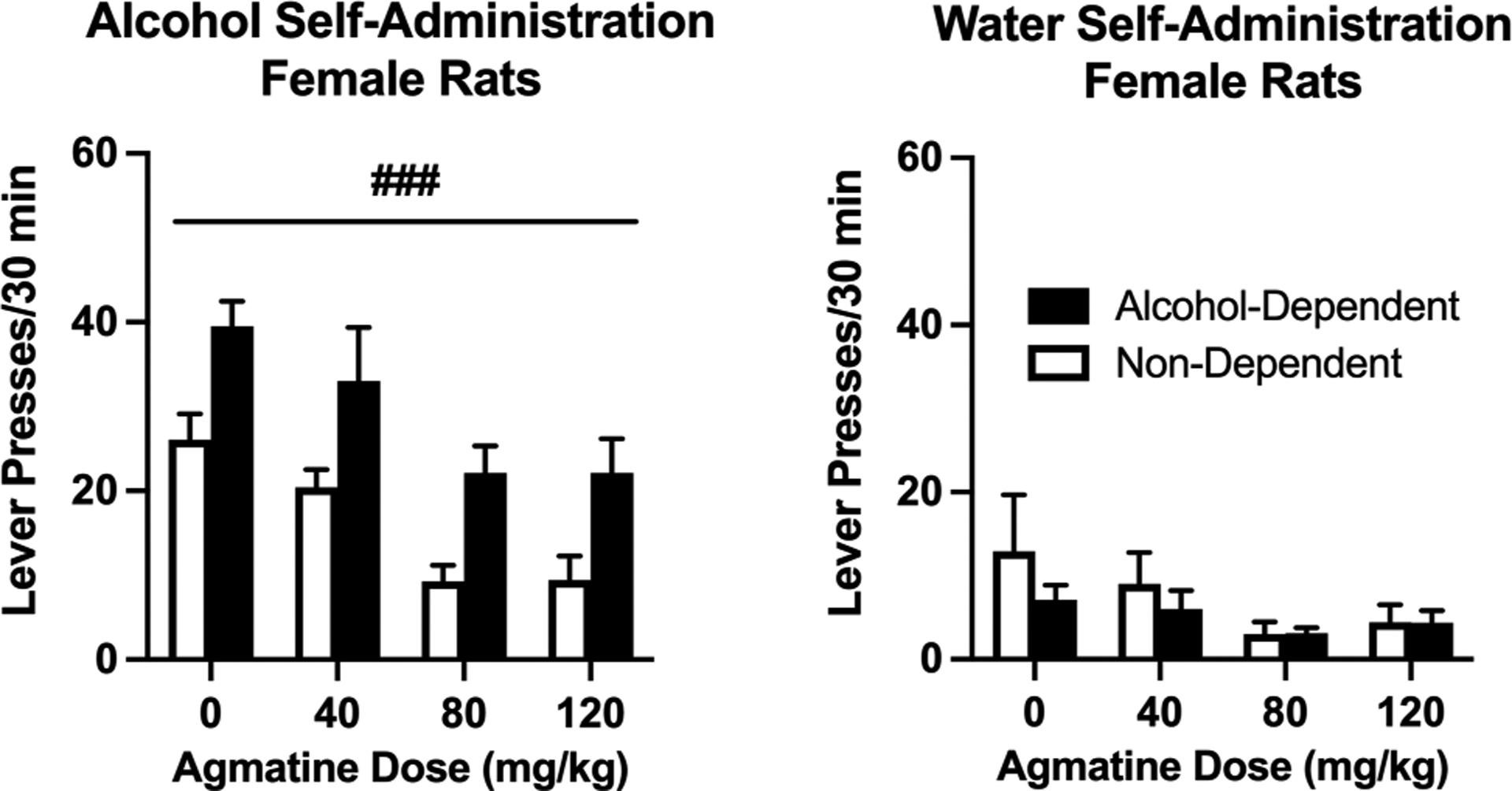
Operant alcohol (left) and water (right) self-administration in female Wistar rats. Agmatine reduces alcohol self-administration in a dose-dependent manner across both alcohol-dependent and non-dependent female Wistar rats. N=8–12/group. ###p<0.001 main effect of agmatine dose in female rats. Values are mean ± SEM
Experiment 5: Effect of Agmatine on Sucrose Self-Administration in Male and Female Rats
Based on the striking ability of agmatine to reduce alcohol self-administration in both sexes, we also wanted to examine the efficacy of this compound on an additional measure of natural reward responding. Using a similar operant paradigm, separate groups of female and male Wistar rats were trained to self-administer sucrose (0.5% w/v) in 30-minute sessions. Agmatine (0–120 mg/kg, IP, 30-minute pretreatment) did not alter sucrose drinking in female rats ([F(2.285,11.43)= 0.3340; p=0.7496; partial η2 = 0.06]; Figure 6), suggesting that its effects in females are specific to alcohol reward and reinforcement. In comparison, agmatine dose-dependently reduced sucrose self-administration in male rats similar to patterns of responding to alcohol [F(1.953,27.34)= 8.605; p=0.0014; partial η2 = 0.38].
Figure 6:
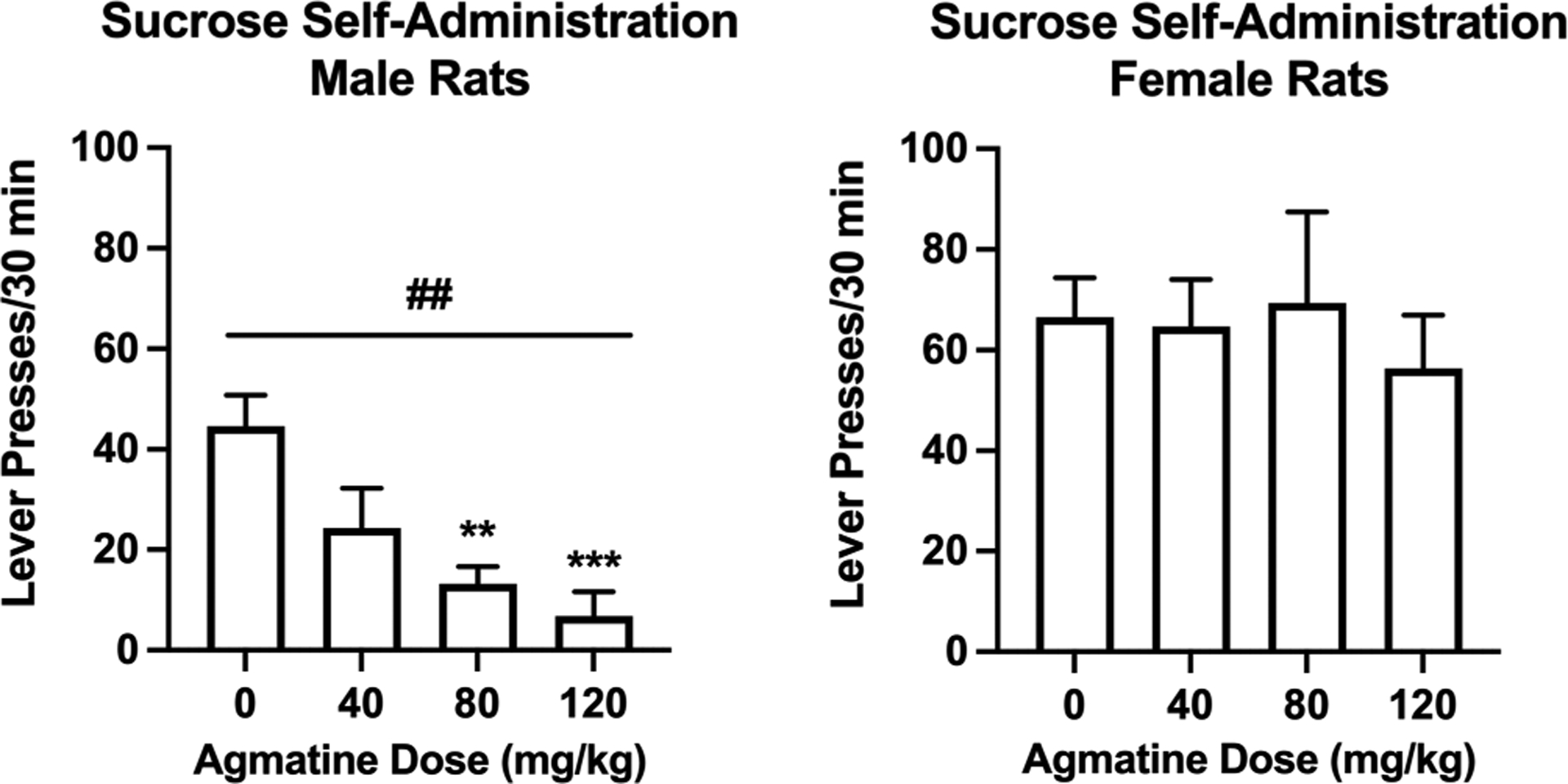
Operant sucrose self-administration in male (left) and female (right) Wistar rats. Agmatine reduces responding for sucrose in a dose-dependent fashion in male Wistar rats but does not alter operant sucrose self-administration in female Wistar rats. N=15 (males) and N=6 (females). ##p<0.01 main effect of agmatine dose in male rats. **p<0.01 and ***<0.001 difference in agmatine dose from vehicle in male rats. Values are mean ± SEM
Experiment 6: Agmatine Antinociceptive Efficacy in Male and Female Rats
We next determined the antinociceptive effects of agmatine in separate groups of alcohol-dependent and non-dependent rats. Agmatine (0–120 mg/kg, IP, 30-minute pretreatment) produced group-dependent effects with regard to thermal sensitivity in both male and female Wistar rats (Figure 7A, group × dose interaction in males: [F(3,33)= 4.856; p=0.0066; partial η2 = 0.42]; group × dose interaction in females: [F(3,51)= 7.284; p=0.0004; partial η2 = 0.65]. Agmatine also produced similar group-dependent effects with regard to mechanical sensitivity in both sexes (Figure 7B, group × dose interaction in males: [F(3,33)= 4.244; p=0.0121; partial η2 = 0.27; group × dose interaction in females: [F(3,51)= 2.790; p=0.0498; partial η2 = 0.30]. In general, these interactions would seem to indicate that agmatine may exhibit anti-hyperalgesic efficacy in alcohol-dependent rats. Importantly, Bonferroni post hoc analyses indicated significant differences in baseline hypersensitivity (CIE-induced) only in females (thermal: t(4.144= 7.200; p=0.0163; partial η2 = 0.96); mechanical: t(4.762= 12.99; p= 0.0015; partial η2 = 0.72), possibly indicating greater hypersensitivity in females versus males resulting from CIE. For mechanical sensitivity, agmatine also produced significant main effects of dose across both alcohol-dependent and non-dependent rats (dose effect in males: [F(1.932,21.26)= 11.06; p=0.0006; partial η2 = 0.49 dose effect in females: [F(2.471,42.01)= 9.763; p=0.0001; partial η2 = 0.60]), indicating a more general antinociceptive effect across all animals.
Figure 7:
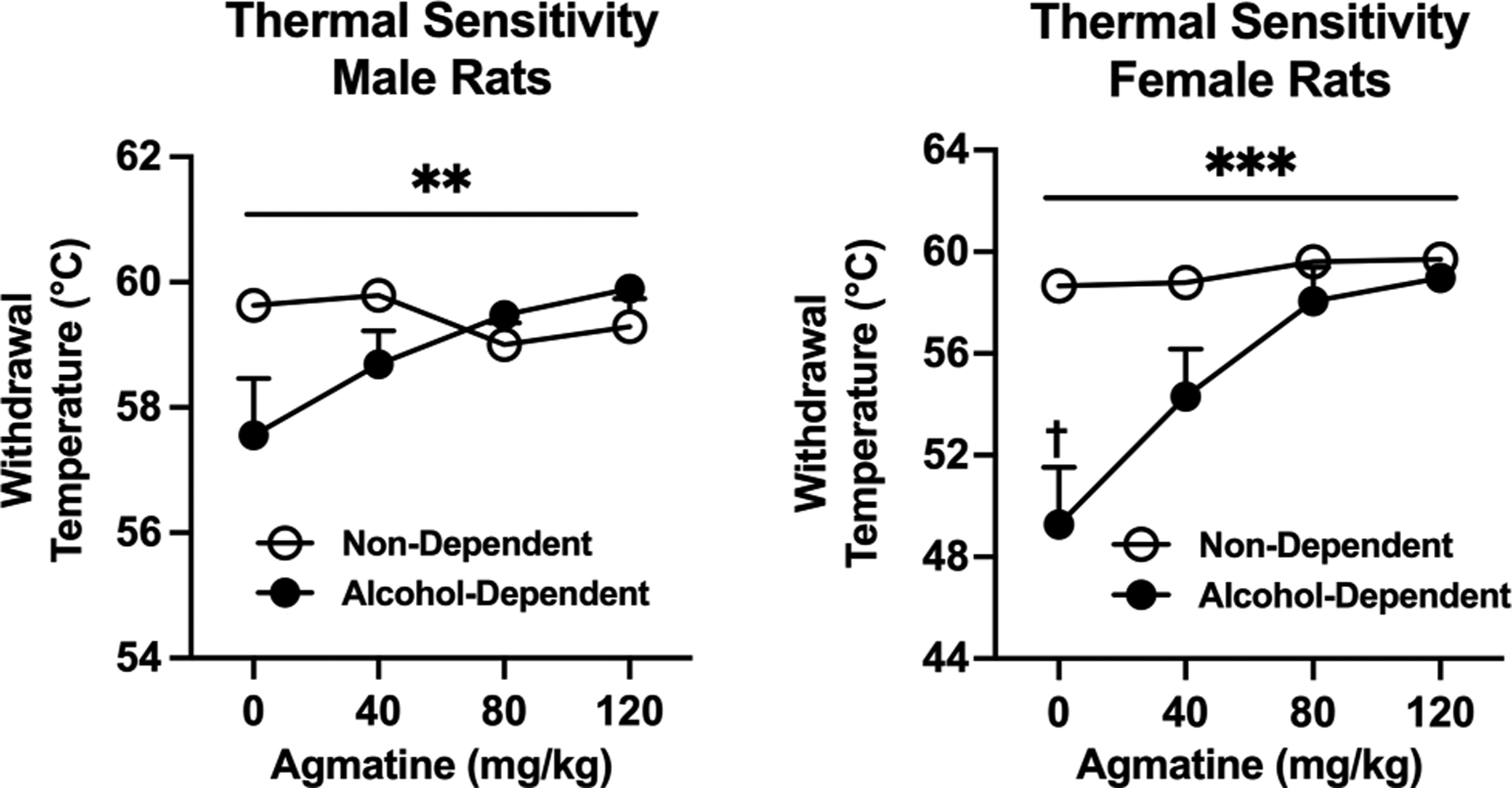
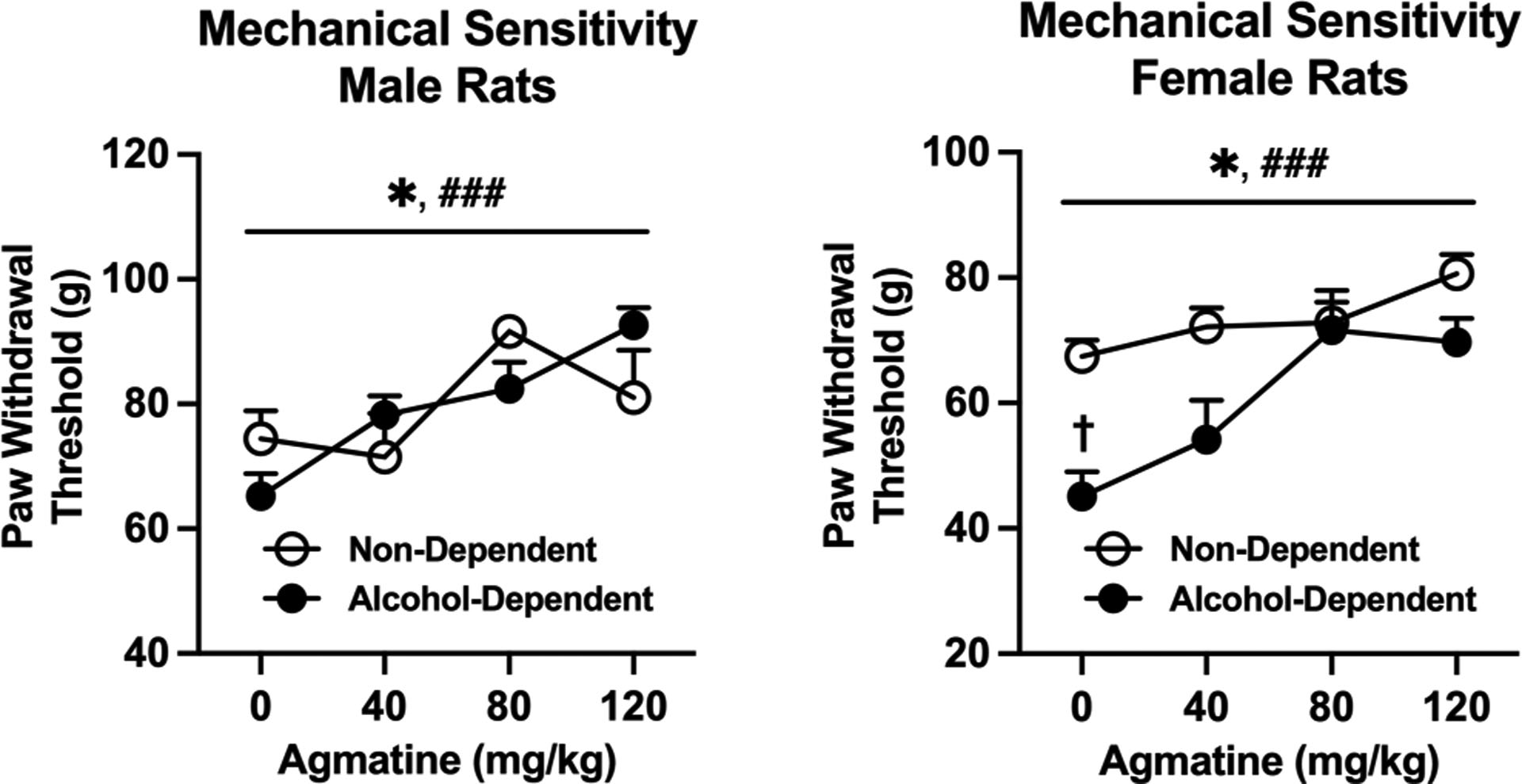
A) Thermal sensitivity in male (left, n=6–7/group) and female (right, n=8–11/group) Wistar rats. Agmatine produces thermal antinociception in both male and female rats. †p<0.05 lower paw withdrawal thresholds in alcohol-dependent group. **p<0.01 and ***<0.001 interaction of dose and dependence state in male and female rats (respectively). B) Mechanical sensitivity in male (left) and female (right) Wistar rats. Agmatine produces mechanical antinociception in both male and female rats. N= 6–11/group. †p<0.05 lower paw withdrawal thresholds in alcohol-dependent group. *p<0.05 interaction of dose and dependence state in male and female rats. Values are mean ± SEM
Discussion
There is an urgent need to understand the neurobiological mechanisms of escalated alcohol drinking and hyperalgesia in the context of alcohol use disorder (AUD), as well as a continuing need for preclinical medication development targeted to novel systems to treat these devastating conditions. As hyperalgesia represents a form of chronic stress and negative affect, we expect that this condition plays a critical role in negative reinforcement mechanisms underlying excessive drinking in alcohol-dependent animals. Following this conceptualization, the interrogation of new targets that are effective against both escalated drinking and pain would likely represent a prolific avenue for medication development. The effect of systemic administration of agmatine on voluntary ethanol consumption was evaluated in well-established mouse and rat models of AUD. As expected, based on our previously published work, ethanol intake escalated over successive CIE exposure cycles in dependent mice and while ethanol consumption in non-dependent mice remained relatively stable throughout the study (Becker & Lopez, 2004; Griffin, Lopez, & Becker, 2009; Lopez & Becker, 2005). Similarly, alcohol-dependent rats displayed both escalation of drinking and development of mechanical and thermal hypersensitivity, consistent with previous observations (Edwards, Guerrero, et al., 2012; Edwards, Vendruscolo, et al., 2012), although in the current study, significant hyperalgesia symptoms appeared only in females.
Effects of Agmatine in Male and Female Mice
Treatment with agmatine had different effects on voluntary ethanol intake in male and female subjects. In males, the three doses of agmatine produced a reduction in ethanol intake in both CIE-exposed (dependent) and non-dependent mice. In contrast, this effect appeared more selective for CIE-exposed female mice. Specifically, all three doses of agmatine under evaluation reduced ethanol intake in CIE female mice while only the highest dose (120 mg/kg) reduced ethanol intake in non-dependent females. This apparent selective of agmatine reducing ethanol intake in dependent (CIE-exposed) female mice was confirmed in a second Test evaluation. Examination of drug washout testing indicated that the effects of agmatine in both males and females were not long-lasting. That is, once subjects returned to receiving vehicle injections during a final test cycle (washout), ethanol intake returned to pre-treatment levels. In future studies, we will investigate if chronic agmatine treatment will produce sustained reduction in drinking after CIE. Finally, agmatine did not affect the consumption of a different palatable substance (sucrose solution). This suggests that the effect of agmatine administration appears to be selective for ethanol intake, and in the case of female mice, more selective for elevated ethanol intake in subjects that experienced repeated chronic intermittent ethanol exposure. Taken together, results from this series of studies suggest that agmatine may have potential as a therapeutic in reducing ethanol consumption.
Effects of Agmatine in Male and Female Rats
We discovered very similar effects of agmatine treatment to reduce operant alcohol self-administration across both alcohol-dependent and non-dependent rats, a pattern of efficacy observed in this model for several current and potential medications for AUD (Tunstall, Carmack, Koob, & Vendruscolo, 2017), including naltrexone (Walker & Koob, 2008). We also examined agmatine’s ability to regulate operant responding for sucrose and revealed an interesting sex-dependent outcome. While sucrose self-administration was reduced in male rats, this effect was not observed in females. These results may have implications for targeting potential agmatine therapy for specific reward-related disorders in a sex-dependent fashion. While there is considerable overlap in results across both rat and mouse models of AUD with regard to agmatine efficacy, the discrepancy in ethanol versus sucrose selectivity may relate to differences in procedures employed (e.g., home cage drinking versus operant responding). Consistent with reports in other pain models (e.g., Kotagale et al., 2013), we also revealed an efficacy for agmatine in reducing both thermal and mechanical hypersensitivity in alcohol-dependent female rats. Interestingly, regarding mechanical sensitivity, agmatine appeared to exhibit a broader antinociceptive activity across all animals, raising the threshold in both alcohol-dependent and non-dependent groups (Figure 7). Future studies are warranted to determine if agmatine treatment during alcohol withdrawal-related hyperalgesia will prevent the development of neuropathic pain and whether chronic agmatine will produce long-lasting analgesic effects or tolerance.
Conclusions and Future Directions
Based on our results demonstrating strong dose-dependent efficacy across multiple behaviors, a potential concern of medicating with agmatine is motor side effects that may influence behavior. However, doses of agmatine up to 100 mg/kg were previously shown to reduce immobility in the forced swim test in male rats (indicative of an antidepressant-like effect, (Aricioglu & Altunbas, 2003). Chronic administration of lower doses (10–20 mg/kg) in rats also produces antidepressant-like effects without evident side effects (Kale et al., 2020). Oral administration of even higher doses in mice (900 mg/kg daily for one week or 300 mg/kg for fifteen weeks) was well tolerated and produced no behavioral impairments or organ pathology upon necropsy (Bergin et al., 2019). Previous studies have shown that agmatine has a short half-life and poor CNS penetrance. Systemic administration of agmatine rapidly results in significant levels in serum but its concentration is very low 180 min later (Roberts et al., 2005). In the studies presented here, the IP administration of agmatine occurred 30 min before the 2-hour drinking session. Despite the reported short half-life of agmatine, it significantly reduced voluntary ethanol intake in mice. Several studies have shown that adult C57BL/6J that drink ethanol in limited access protocols consume about 1/3 of the total amount within the first 15–20 min of the 2-hr session (Bauer, McVey, Boehm II 2022; Maphis, Huffman, Lisenbardt 2022). This pattern of intake is accelerated in CIE-exposed mice (Griffin, Lopez, Yanke, Middaugh, & Becker, 2009; see also Ardinger, Lapish, Czachowski, Grahame 2022). Therefore, it is possible that the amount of agmatine in the system during the first minutes of the limited access session was sufficient to significantly modulate ethanol intake, particularly in CIE-exposed mice. Current studies focused on an acute single administration of agmatine just before the drinking protocol and the data in mice showed that drinking returned to baseline after the washout, suggesting that the acute effect is not long-lasting. Future studies will investigate whether chronic administration of agmatine is effective in producing a sustained reduction/reversal in drinking.
Agmatine has demonstrated analgesic efficacy across a host of pain models. Most relevant to the current investigation, a recent study found that central administration of agmatine potentiates the antinociceptive effect of alcohol in mice, while also attenuating analgesic tolerance to alcohol over time (N. Kotagale et al., 2022). Although we did not examine nociceptive sensitivity in mice, a recent mouse study discovered that agmatine prevents the development of tolerance to the antinociceptive effects of alcohol (N. Kotagale et al., 2022). Together, these findings indicate that agmatine may both reduce alcohol drinking as well as spare the analgesic effects of alcohol in individuals using alcohol to manage their pain (Cucinello-Ragland & Edwards, 2021). Agmatine regulates several cellular targets in relevant brain regions that are also altered by alcohol suggesting that a multifunctional molecule such as agmatine could be ideal to counter alcohol behavioral effects (Laube & Bernstein, 2017; Barua, Kim, Kim, Kim, & Lee, 2019). Chronic relapsing neuropsychiatric diseases such as AUD arise due to neuroadaptations within the circuits that connect relevant brain regions through synaptic plasticity and altered gene and protein expressions. To reverse these cellular changes, it may be necessary to intervene in several target gene/signaling pathways rather than a specific target. Agmatine is known to regulate several targets in relevant brain regions that are also altered by alcohol. For example, in a neuropathic pain model, it has been shown that the GluN2B-containing functional NMDA receptors are required for the anti-nociceptive effect of agmatine (Waataja et al. 2019; Peterson et al. 2021). Moreover, agmatine exhibited anti-depressive effects and potentiated the antidepressant actions of ketamine by modulating AMPA receptors and mTOR signaling (Neiss et al. 2016). Our findings indicate that the use of agmatine as a lead compound for the reversal of alcohol-dependent induced drinking that might lead to identifying more biological stable agmatine analogs and novel neuronal signaling pathway targets as an effective treatment for AUD. While additional mechanistic work will be required to determine the specific neuroanatomical site(s) of action and neurobiological system(s) altered by agmatine therapy, the current results suggest a promising role of agmatine in the treatment of problematic drinking and pain symptoms in the context of AUD.
Highlights:
Agmatine reduced ethanol intake in alcohol-dependent and non-dependent mice.
Agmatine reduced ethanol self-administration in dependent and non-dependent rats.
Agmatine administration did not alter sucrose intake in mice.
Agmatine reduced sucrose intake in male rats.
Agmatine reduced thermal and mechanical pain sensitivity in alcohol-dependent rats.
Acknowledgments
This work was generously supported by research and training grants from the National Institute on Alcohol Abuse and Alcoholism (F31AA028445, R01AA025996, 75N94019C00008, U01AA014095, U24AA029968, P50 AA010761), the National Institute of Child Health and Human Development (NIH-NICHD-2018-05), and the Department of Veterans Affairs (BLRD BX000813).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
The authors declare no competing financial interests or potential conflicts of interest.
References
- Aglawe MM, Kale MB, Rahangdale SR, Kotagale NR, Umekar MJ, & Taksande BG (2021). Agmatine improves the behavioral and cognitive impairments associated with chronic gestational ethanol exposure in rats. Brain Res Bull, 167, 37–47. doi: 10.1016/j.brainresbull.2020.11.015 [DOI] [PubMed] [Google Scholar]
- Ardinger CE, Lapish CC, Czachowski CL, & Grahame NJ (2022). A critical review of front-loading: A maladaptive drinking pattern driven by alcohol’s rewarding effects. Alcohol Clin Exp Res, 46(10), 1772–1782. doi: 10.1111/acer.14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricioglu F, & Altunbas H (2003). Is agmatine an endogenous anxiolytic/antidepressant agent? Ann N Y Acad Sci, 1009, 136–140. doi: 10.1196/annals.1304.014 [DOI] [PubMed] [Google Scholar]
- Aricioglu F, & Regunathan S (2005). Agmatine attenuates stress- and lipopolysaccharide-induced fever in rats. Physiol Behav, 85(3), 370–375. doi: 10.1016/j.physbeh.2005.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricioglu-Kartal F, & Uzbay IT (1997). Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci, 61(18), 1775–1781. doi: 10.1016/s0024-3205(97)00801-1 [DOI] [PubMed] [Google Scholar]
- Barua S, Kim JY, Kim JY, Kim JH, & Lee JE (2019). Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors. Neurochem Res, 44(4), 735–750. doi: 10.1007/s11064-018-02712-1 [DOI] [PubMed] [Google Scholar]
- Bauer MR, McVey MM, & Boehm SL 2nd. (2022). Drinking history dependent functionality of the dorsolateral striatum on gating alcohol and quinine-adulterated alcohol front-loading and binge drinking. Alcohol, 105, 43–51. doi: 10.1016/j.alcohol.2022.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, & Lopez MF (2004). Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res, 28(12), 1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a [DOI] [PubMed] [Google Scholar]
- Becker HC, & Lopez MF (2016). An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. Int Rev Neurobiol, 126, 157–177. doi: 10.1016/bs.irn.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev, 68(2), 242–263. doi: 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin DH, Jing Y, Williams G, Mockett BG, Zhang H, Abraham WC, & Liu P (2019). Safety and neurochemical profiles of acute and sub-chronic oral treatment with agmatine sulfate. Sci Rep, 9(1), 12669. doi: 10.1038/s41598-019-49078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinello-Ragland JA, & Edwards S (2021). Neurobiological aspects of pain in the context of alcohol use disorder. Int Rev Neurobiol, 157, 1–29. doi: 10.1016/bs.irn.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, & Koob GF (2012). Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol, 17(1), 76–85. doi: 10.1111/j.1369-1600.2010.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Gilpin NW, Wojnar M, & Witkiewitz K (2020). Alcohol and Pain: A Translational Review of Preclinical and Clinical Findings to Inform Future Treatment Strategies. Alcohol Clin Exp Res, 44(2), 368–383. doi: 10.1111/acer.14260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, … Koob GF (2012). Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology, 62(2), 1142–1151. doi: 10.1016/j.neuropharm.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, & Edwards S (2012). Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev, 36(10), 2179–2192. doi: 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, & Koob GF (2008). Vapor inhalation of alcohol in rats. Curr Protoc Neurosci, Chapter 9, Unit 9 29. doi: 10.1002/0471142301.ns0929s44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, & Becker HC (2009). Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res, 33(11), 1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, & Becker HC (2009). Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl), 201(4), 569–580. doi: 10.1007/s00213-008-1324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale M, Nimje N, Aglawe MM, Umekar M, Taksande B, & Kotagale N (2020). Agmatine modulates anxiety and depression-like behaviour in diabetic insulin-resistant rats. Brain Res, 1747, 147045. doi: 10.1016/j.brainres.2020.147045 [DOI] [PubMed] [Google Scholar]
- Koob GF (2021). Drug Addiction: Hyperkatifeia/Negative Reinforcement as a Framework for Medications Development. Pharmacol Rev, 73(1), 163–201. doi: 10.1124/pharmrev.120.000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagale N, Bhondekar S, Bhad M, Pise S, Charpe A, Umekar M, & Taksande B (2022). Agmatine prevents development of tolerance to anti-nociceptive effect of ethanol in mice. Alcohol, 101, 1–8. doi: 10.1016/j.alcohol.2022.02.004 [DOI] [PubMed] [Google Scholar]
- Kotagale NR, Ali MT, Chopde CT, Umekar MJ, & Taksande BG (2018). Agmatine inhibits nicotine withdrawal induced cognitive deficits in inhibitory avoidance task in rats: Contribution of alpha2-adrenoceptors. Pharmacol Biochem Behav, 167, 42–49. doi: 10.1016/j.pbb.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Kotagale NR, Chopde CT, Umekar MJ, & Taksande BG (2015). Chronic agmatine treatment prevents behavioral manifestations of nicotine withdrawal in mice. Eur J Pharmacol, 754, 190–198. doi: 10.1016/j.ejphar.2015.02.033 [DOI] [PubMed] [Google Scholar]
- Kotagale NR, Shirbhate SH, Shukla P, & Ugale RR (2013). Agmatine attenuates neuropathic pain in sciatic nerve ligated rats: modulation by hippocampal sigma receptors. Eur J Pharmacol, 714(1–3), 424–431. doi: 10.1016/j.ejphar.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Laube G, & Bernstein HG (2017). Agmatine: multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem J, 474(15), 2619–2640. doi: 10.1042/BCJ20170007 [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, & Barron S (2007). Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav, 88(1), 114–121. doi: 10.1016/j.pbb.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, & Reis DJ (1994). Agmatine: an endogenous clonidine-displacing substance in the brain. Science, 263(5149), 966–969. doi: 10.1126/science.7906055 [DOI] [PubMed] [Google Scholar]
- Lopez MF, & Becker HC (2005). Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl), 181(4), 688–696. doi: 10.1007/s00213-005-0026-3 [DOI] [PubMed] [Google Scholar]
- Maphis NM, Huffman RT, & Linsenbardt DN (2022). The development, but not expression, of alcohol front-loading in C57BL/6J mice maintained on LabDiet 5001 is abolished by maintenance on Teklad 2920x rodent diet. Alcohol Clin Exp Res, 46(7), 1321–1330. doi: 10.1111/acer.14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MA, Paulsen RI, Itoga CA, Farooq MA, Reppel JE, Edwards KN, … Edwards S (2016). Withdrawal from Chronic Nicotine Exposure Produces Region-Specific Tolerance to Alcohol-Stimulated GluA1 Phosphorylation. Alcohol Clin Exp Res, 40(12), 2537–2547. doi: 10.1111/acer.13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, Campbell UC, Fons RD, & Carroll ME (2002). Effects of agmatine on the escalation of intravenous cocaine and fentanyl self-administration in rats. Pharmacol Biochem Behav, 72(4), 873–880. doi: 10.1016/s0091-3057(02)00774-8 [DOI] [PubMed] [Google Scholar]
- Neis VB, Moretti M, Bettio LE, Ribeiro CM, Rosa PB, Goncalves FM, … Rodrigues AL (2016). Agmatine produces antidepressant-like effects by activating AMPA receptors and mTOR signaling. Eur Neuropsychopharmacol, 26(6), 959–971. doi: 10.1016/j.euroneuro.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Neis VB, Rosa PB, Olescowicz G, & Rodrigues ALS (2017). Therapeutic potential of agmatine for CNS disorders. Neurochem Int, 108, 318–331. doi: 10.1016/j.neuint.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Otake K, Ruggiero DA, Regunathan S, Wang H, Milner TA, & Reis DJ (1998). Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res, 787(1), 1–14. doi: 10.1016/s0006-8993(97)01200-6 [DOI] [PubMed] [Google Scholar]
- Ozden O, Kayir H, Ozturk Y, & Uzbay T (2011). Agmatine blocks ethanol-induced locomotor hyperactivity in male mice. Eur J Pharmacol, 659(1), 26–29. doi: 10.1016/j.ejphar.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Farooq MA, Edwards KN, & Edwards S (2019). Dysregulation of c-Jun N-terminal kinase phosphorylation in alcohol dependence. Alcohol, 75, 11–18. doi: 10.1016/j.alcohol.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CD, Kitto KF, Verma H, Pflepsen K, Delpire E, Wilcox GL, & Fairbanks CA (2021). Agmatine requires GluN2B-containing NMDA receptors to inhibit the development of neuropathic pain. Mol Pain, 17, 17448069211029171. doi: 10.1177/17448069211029171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan S (2006). Agmatine: biological role and therapeutic potentials in morphine analgesia and dependence. AAPS J, 8(3), E479–484. doi: 10.1208/aapsj080356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, & Regunathan S (2000). Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci, 21(5), 187–193. doi: 10.1016/s0165-6147(00)01460-7 [DOI] [PubMed] [Google Scholar]
- Roberts JC, Grocholski BM, Kitto KF, & Fairbanks CA (2005). Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther, 314(3), 1226–1233. doi: 10.1124/jpet.105.086173 [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, & Bloom FE (1979). Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol, 27(4), 466–486. doi: 10.1016/s0163-1047(79)92061-2 [DOI] [PubMed] [Google Scholar]
- Trudell JR, Messing RO, Mayfield J, & Harris RA (2014). Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol Sci, 35(7), 317–323. doi: 10.1016/j.tips.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Carmack SA, Koob GF, & Vendruscolo LF (2017). Dysregulation of Brain Stress Systems Mediates Compulsive Alcohol Drinking. Curr Opin Behav Sci, 13, 85–90. doi: 10.1016/j.cobeha.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utkan T, Gocmez SS, Regunathan S, & Aricioglu F (2012). Agmatine, a metabolite of L-arginine, reverses scopolamine-induced learning and memory impairment in rats. Pharmacol Biochem Behav, 102(4), 578–584. doi: 10.1016/j.pbb.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Uzbay IT, Yesilyurt O, Celik T, Ergun H, & Isimer A (2000). Effects of agmatine on ethanol withdrawal syndrome in rats. Behav Brain Res, 107(1–2), 153–159. doi: 10.1016/s0166-4328(99)00127-8 [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, & Roberts AJ (2014). Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol, 48(3), 277–286. doi: 10.1016/j.alcohol.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waataja JJ, Peterson CD, Verma H, Goracke-Postle CJ, Seguela P, Delpire E, Wilcox GL, Fairbanks CA (2019). Agmatine preferentially antagonizes GluN2B-containing N-methyl-d-aspartate receptors in spinal cord. J Neurophysiol, 121(2), 662–671. doi: 10.1152/jn.00172.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, & Koob GF (2008). Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology, 33(3), 643–652. doi: 10.1038/sj.npp.1301438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann K, Lewis B, & Barron S (2010). Agmatine reduces ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol. Neurotoxicol Teratol, 32(2), 158–163. doi: 10.1016/j.ntt.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


