Abstract
Purpose
Local ablative treatment (LAT) is increasingly combined with systemic therapy in oligometastatic breast cancer (OMBC), without a high-level evidence to support this strategy. We evaluated the addition of LAT to systemic treatment in terms of progression-free survival (PFS) and overall survival (OS). Secondary endpoints were local control (LC) and toxicity. We sought to identify prognostic factors associated with longer OS and PFS.
Methods and materials
We identified consecutive patients treated between 2014 and 2018 for synchronous or metachronous OMBC (defined as ≤ 5 metastases). LAT included stereotactic body radiation therapy (SBRT) and volumetric modulated arc therapy (VMAT), surgery, cryotherapy and percutaneous radiofrequency ablation (PRA). PFS and OS were calculated, and Cox regression models analyzed for potential predictors of survival.
Results
One hundred two patients were included (no-LAT, n = 62; LAT, n = 40). Sixty-four metastases received LAT. Median follow-up was 50.4 months (95% CI [44.4; 53.4]). One patient experienced grade 3 toxicity in the LAT group. Five-year PFS and OS were 34.75% (95% CI [24.42–45.26]) and 63.21% (95% CI [50.69–73.37]) respectively. Patients receiving both LAT and systemic therapy had longer PFS and OS than those with no-LAT ([HR 0.39, p = 0.002]) and ([HR 0.31, p = 0.01]). The use of LAT, HER2-positive status and hormone-receptor positivity were associated with longer PFS and OS whereas liver metastases led to worse PFS.
Conclusions
LAT was associated with improved outcomes in OMBC when added to systemic treatment, without significantly increasing toxicity. The prognostic factors identified to extend PFS and OS may help guide clinicians in selecting patients for LAT.
Keywords: Oligometastases, Breast cancer, Local ablative treatment, Standard of care, Systemic treatment
Highlights
-
•
Outcomes of oligometastatic breast cancer (OMBC) cohort without cranial metastatases treated by local ablative treatment and systemic treatment.
-
•
Patients with LAT had more favorable PFS and OS compared to noLAT.
-
•
HR+ and HER + immunohistochemical (HIC) subtype had a more favorable OS and PFS compared to triple negatives patients, liver metastases had a worse PFS.
1. Introduction
A number of observational metastatic breast cancer (mBC) studies have shown that long-term survivors tend to present with a lower tumor burden at diagnosis. This is often referred to as oligometastatic breast cancer (OMBC) [[1], [2], [3], [4]].
According to the North American and European Task Force consensus guidelines OMBC is defined as low-volume metastatic disease with a limited number of lesions (one to five) and organ involvement and excludes oversized metastases not amenable to surgery or localized therapies [2,5]. Twenty eight years ago, Hellmann and Weichselbaum [6,7] hypothesized that OMBCs are a distinct biological entity and an intermediate state between localized and polymetastatic breast cancers. They postulated that OMBCs may therefore have lost the capacity to develop widespread metastases and that local ablative treatment of the primitive tumor and distant metastases may reduce the risk of widespread dissemination.
Today, OMBC is still considered to be a complex entity, with heterogeneous presentations and behaviors and has been classified as part of the recent ESTRO-EORTC initiative [8]. Evidence from retrospective and prospective phase I/II studies has shown that surgery [9], percutaneous radiofrequency ablation (PRA) [10] and especially radiotherapy may be safely deployed for OMBC and can achieve high rates of local control [[11], [12], [13]] but there is currently no standard of care for OMBC patients.
We conducted a retrospective single-center study to evaluate long-term outcomes of adding local ablative treatment (LAT) to the current systemic OMBC treatment. We also sought to identity prognostic factors that may help define the risk of disease recurrence or progression.
2. Materials and methods
2.1. Patients
This retrospective monocentric study identified consecutive patients treated for synchronous or metachronous oligorecurrent metastatic breast cancer at our institution between January 2014 and December 2018. Patients had to have at least 1 and a maximum of 5 metastatic lesions. Only patients with synchronous de novo OMBC and oligorecurrent metastatic BC, according to the EORTC and ESTRO classifications [8], were finally included in the study. Metastatic relapses occurring >6 months with a controlled primary were considered oligorecurrent. In the case of synchronous OMBC, the primary breast cancer and lymph nodes both had to have been treated with the same modalities as non-metastatic breast cancer. We excluded patients with oligoprogressive disease, brain metastases and any uncontrolled loco-regional recurrences. The extent of disease was assessed by one of the following examinations: thoracic-abdo-pelvic computed tomography (CT) with bone scintigraphy, 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET/CT) or whole-body magnetic resonance imaging (WB-MRI). Other imaging tools such as liver, spine and brain MRIs were deployed at the discretion of the physician. We used the definition of Kelly et al. to determine the number of metastatic sites [14]. For lesions involving bone, lung or liver, each radiologically identifiable lesion was considered as one disease site. For lymph node lesions, radiologic involvement of an individual echelon of the lymphatic system was considered a distinct disease site, even if it involved multiple nodes of a single echelon.
Contralateral axillary lymph node involvement was evaluated by ultrasound and mammography, breast MRI and PET-CT [15]. In the absence of contralateral breast tumor, contralateral axillary lymph nodes were considered as distant metastases. For mediastinal nodes, we used the thoracic lymph node stations of Chapet et al. [16], and for cervical lymph nodes the consensus of Gregoire et al. [17].
Biopsy of a metastasis was not required but was deemed to be preferable. For synchronous OMBC, pathology data used in this analysis was obtained from either the primary tumor or the metastasis. For metachronous OMBC, pathology data was obtained from the last site of the recurrence or from metastases. Tumors were defined as hormone receptor-positive (HR+) when estrogen receptor or progesterone expression was ≥10%. Breast cancers with a HER2 (Epidermal Growth Factor Receptor 2) immunohistochemical score (IHC) of 3 or 2 and a positive in situ hybridization (ISH) test were considered to be HER2-positive. All cancers with an IHC score of 0, 1 or 2 and with a negative ISH test, as well as patients with a negative ISH test without IHC data, were considered to be HER2-negative [18].
The study was approved by our multidisciplinary breast committee and our institutional board committee (BEC–FO–0227).
2.2. Local ablative treatment (LAT)
LAT included surgery, stereotactic body radiation therapy (SBRT) and volumetric modulated arc therapy (VMAT), cryotherapy and percutaneous radiofrequency ablation (PRA). At least one metastatic site had to have been treated. Patients that had received palliative irradiation were excluded from this study.
2.2.1. Radiotherapy
All patients underwent computed tomography (CT)-based planning including either a free breathing CT scan or a 4-dimensional CT for SBRT (thoracic, liver or rib metastases), with or without contrast, as required. Patients were immobilized with a thermoplastic head and shoulder mask, SBRT base plate, thermoplastic molds (Orfit Industries NV, Wijnegem, Belgium) and vacuum bags (CIVCO, Coralville, Iowa, USA). Contouring of the gross tumor volume (GTV) was based on all available clinical, metabolic and respiratory motion information including PET-CT or MRI. The planning target volume (PTV) was the GTV with an additional 2–5 mm margin. Radiation was either delivered by Volumetric Modulated Arc Therapy (VMAT) or SBRT. The dose was prescribed based on the volume and the localization of the metastasis. The radiation dose was prescribed to the PTV edge, typically to the 80% isodose line or with 95% of the PTV required to receive 95% of the planned dose. Various dose fractionation schedules were used: 27 Gy in 3 fractions for bone lesions, 50–55Gy in 5 fractions for lung tumors or 50 Gy in 25 fractions for lymph node metastases after surgical excision. Patients with synchronous OMBC and an untreated primary received definitive radiotherapy to the primary tumor for 63.8 Gy en 29 fractions as well as LAT for oligometastasis. Conventional fractionated radiotherapy schedule was used in VMAT. Patients receiving palliative radiation were excluded from our study.
2.2.2. Other local ablative therapy
Cryotherapy, PRA and surgery were performed in accordance with good medical practice and curative intent.
3. Outcomes
The primary endpoint was progression-free survival (PFS). Secondary endpoints were OS, local control and toxicity. Toxicity was scored on the NCI CTCAE v5.0 toxicity scale [19]. Best overall response was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [20].
3.1. Statistical analysis
Comparisons between groups were assessed using the Chi-square or Fisher's exact test for categorical variables and the Mann-Whitney test for continuous variables.
PFS and OS were computed from initiation of the first treatment and survival rates were estimated by the Kaplan-Meier method with 95% confidence intervals (CI), using the following first-event definition: progression or death from any cause for PFS and death from any cause for OS. Patients who did not experience the event of interest were censored at their last follow-up. Univariate and multivariate analyses were performed using the Logrank test and the Cox proportional hazards models. To avoid the guarantee-time bias, a Cox model with a time-dependent variable was used to investigate the impact of LAT on PFS and OS. Sensitivity landmark analyses were also performed 3 and 6-month after the start of the initial treatment. Groups were defined according to treatments received before the landmark time and patients who progressed, died, or were censored before the landmark time were excluded. All statistical tests were two-sided and p-values <0.05 were considered statistically significant. Statistical analyses were conducted using Stata Software®, version 16.1.
4. Results
4.1. Population characteristics
A total of 160 patients with OMBC were consecutively screened between January 2014 and December 2018, with 102 patients finally included in the current analysis. Sixty-two patients only received systemic treatment whilst 40 received both LAT and systemic treatment which corresponded to 124 and 64 metastases in the no-LAT and LAT group, respectively. Patient and tumor characteristics are listed in Table 1 and a flowchart of the selection of the study population is shown in Fig. 1.
Table 1.
Patient and tumor characteristics at oligometastatic disease diagnosis (n = 102).
| Total (patients) N = 102 |
No-LAT N = 62 |
LAT N = 40 |
p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (Range) | 55 (28–90) | 56.5 (28–90) | 53.5 (30–76) | 0.207 |
| ECOG | ||||
| 0–1 2–3 |
99 (97.1%) 3 (2.9%) |
59 (95.2%) 3 (4.8%) |
40 (100%) 0 (0%) |
– |
| Menopause | ||||
| No Yes Missing |
42 (42.0%) 58 (58.0%) 2 |
24 (39.3%) 37 (60.7%) 1 |
18 (46.2%) 21 (53.8%) 1 |
0.5 |
| Oligometastatic | ||||
| Synchronous Oligorecurrent |
53 (52.0%) 49 (48.0%) |
34 (54.8%) 28 (45.2%) |
19 (47.5%) 21 (52.5%) |
0.47 |
| T (n = 100) | ||||
| T0/T1/T2 T3/T4 Missing |
68 (68.0%) 32 (32.0%) 2 |
39 (62.9%) 23 (37.1%) 0 |
29 (76.3%) 9 (23.7%) |
0.16 |
| N (n = 101) | ||||
| N0 N+ Missing |
27 (26.7%) 74 (73.3%) 1 |
17 (27.4%) 45 (72.6%) 0 |
10 (25.6%) 29 (74.4%) 1 |
0.84 |
| IHC Subtype | ||||
| HR + HR + /HER2 -HER2 + TNBC |
78 (76.5%) 66 (64.7%) 18 (17.6%) 18 (17.6%) |
48 (77.4%) 43 (69.4%) 9 (14.5%) 10 (16.1%) |
30 (75.0%) 23 (57.5%) 9 (22.5%) 8 (20.0%) |
0.446 |
| Histologic type | ||||
| NST Lobular Other |
83 (81.4%) 12 (11.8%) 7 (6.9%) |
48 (77.4%) 9 (14.5%) 5 (8.1%) |
35 (87.5%) 3 (7.5%) 2 (5.0%) |
– |
| Grade | ||||
| Well differentiated (G1) Moderately differentiated (G2) Poorly differentiated (G3) Missing |
5 (5.0%) 53 (52.5%) 43 (42.6%) 1 |
2 (3.3%) 31 (50.8%) 28 (45.9%) 1 |
3 (7.5%) 22 (55.0%) 15 (37.5%) 0 |
0.49 |
| Diagnostic imaging | ||||
| PET/CT Whole body MRI Liver MRI Bone MRI Chest/abdo/pelvis CT Bone scintigraphy |
72 (70.6%) 7 (6.9%) 16 (15.7%) 23 (22.6%) 91 (89.2%) 69 (67.6%) |
40 (64.5%) 3 (4.8%) 13 (21%) 14 (22.6%) 57 (92%) 42 (67.7%) |
32 (80%) 4 (10%) 3 (7.5%) 9 (22.5%) 34 (85%) 27 (67.5%) |
– |
| Number of metastases per patient | ||||
| 1 2 3 4 5 |
54 (52.9%) 22 (21.6%) 15 (14.7%) 10 (9.8%) 1 (1.0%) |
30 (48.4%) 13 (21%) 9 (14.5%) 9 (14.5%) 1 (1.6%) |
24 (60%) 9 (22%) 6 (15%) 1 (2.5%) 0 (0.0%) |
– |
| Size of metastases (cm) | ||||
| <3 3–5 >5 Missing |
71 (71.7%) 20 (20.2%) 8 (8.1%) 3 |
41 (68.3%) 13 (21.7%) 6 (10.0%) 2 |
30 (76.9%) 7 (17.9%) 2 (5.1%) 1 |
0.66 |
| Organs involved | ||||
| 1 2 3 and more |
88 (86.3%) 14 (13.7%) 0 |
54 (87.1%) 8 (12.9%) 0 |
34 (85%) 6 (15%) 0 |
0.76 |
| Number of metastases per organ | ||||
| Bone Node Liver Lung Skin Pancreas Adrenal Choroid |
188 (100%) 100 (53.2%) 37 (19.7%) 35 (18.2%) 12 (6.4%) 1 (0.5%) 1 (0.5%) 1 (0.5%) 1 (0.5%) |
123 (100%) 65 (52.9%) 18 (14.6%) 29 (23.6%) 9 (7.3%) 0 (0%) 1 (0.8%) 1 (0.8%) 0 (0%) |
65 (100%) 35 (53.9%) 19 (29.2%) 6 (9.2%) 3 (4.6%) 1 (1.5%) 0 (0%) 0 (0%) 1 (1.5%) |
– |
HR: hormone receptors; HER2 (human epidermal receptor 2); PET (positron emission tomography); CT (computed tomography); MRI (magnetic resonance imaging); NST (no special type).
Fig. 1.
Study flowchart.
Fifty-three patients had oligometastatic disease at diagnosis (de novo/synchronous) and 49 had recurrent OMBC. Fifty-four patients (52.9%) only had one metastasis. Most metastases wereocated in bone structures (n = 100), lymph nodes (n = 37) and liver (n = 35) and most were under 3 cm in size. Eighty-eight patients (86.3%) only had one organ involvement and 14 (13.7%) patients had two organs involved.
Treatment characteristics are shown in Table 2. Estrogen-receptor positivity was more common in the group of patients with LAT (62.5% vs 40.3% p = 0.029, data not shown). Seventy-two percent of patients had an FDG-PET/CT examination which identified metastatic spread, based on the recommendations from the European Organization for Research and Treatment of Cancer imaging group [21].
Table 2.
Treatment characteristics.
| Total (patients) N = 102 |
No-LAT N = 62 |
LAT N = 40 |
p-value | |
|---|---|---|---|---|
| Systemic treatment | 102 (100%) | 62 (100%) | 40 (100%) | |
| Hormonotherapy (HT) Aromatase inhibitor Tamoxifen Fulvestrant |
74 (72.5%) 52 (70.3%) 13 (17.6%) 9 (12.2) |
43 (69.4%) 31 (72.1%) 5 (11.6%) 7 (16.3%) 26 (41.9%) 9 (34%) 10 (38.5%) 3 (11.5%) 3 (11%) |
31 (77.5%) 21 (67.7%) 8 (25.8%) 2 (6.5%) 24 (60.0%) 4 (16.7%) 8 (33.3%) 11 (45.8%) 1 (4.2%) |
0.368 |
| Chemotherapy (CT) Anthracycline Taxane Anthracycline + taxane Other |
50 (49%) 8 (16%) 17 (34%) 21 (42%) 4 (8%) |
0.075 | ||
| Anti-CDK4/6 | 25 (34.7%) | 20 (46.5%) | 5 (17.2%) | 0.011 |
| Anti-HER2 | 18 (17.8%) | 9 (14.8%) | 9 (22.5%) | 0.320 |
| Systemic treatment combined | ||||
| HT±anti CDK4/6 CT exclusive CT + HT CT + Anti-HER2 HT + Anti-HER2 CT + HT + Anti-HER2 |
51 (50%) 20 (19.6%) 13 (12.7%) 8 (7.8%) 1 (1.0%) 9 (8.8%) |
36 (58.1%) 13 (21.0%) 4 (6.5%) 6 (9.7%) 0 (0.0%) 3 (4.8%) |
15 (37.5%) 7 (17.5%) 9 (22.5%) 2 (5.0%) 1 (2.5%) 6 (15.0%) |
– |
| Local ablative treatment | ||||
| Radiotherapy SBRT VMAT Surgery PRA Cryotherapy |
24 (23.5%) 11 15 19 (18.6%) 6 (5.8%) 1 (1.0%) |
0 0 0 0 |
24 (60.0%) 11 15 19 (47.5%) 6 (15.0%) 1 (2.5%) |
– |
| All metastases treated | ||||
| No Yes |
67 (65.7%) 35 (34.3%) |
62 (100%) 0 (0%) |
5 (12.5%) 35 (87.5%) |
– |
| Time between start of 1st treatment and local ablative treatment (month) Median (Range) |
– | – | 4.4 (0.0; 47.1) | – |
HR: hormone receptors; HT hormone therapy; HER2 human epidermal receptor2; CDK cyclin-dependent kinases; CT chemotherapy; PRA percutaneous radiofrequency ablation, SBRT stereotactic body radiation therapy, VMAT volumetric modulated arc therapy.
In the group of 40 patients treated with both systemic therapy and LAT, median time between the start of systemic therapy and LAT was 4.4 months (range 0; 47.1). Thirteen patients received LAT before systemic treatment, particularly for spinal bone tumors. Fourteen patients were treated with radiotherapy alone, 11 patients had surgery, 8 patients had both surgery and radiotherapy, 5 patients had PRA alone, 1 patient received PRA and SBRT, and 1 patient cryotherapy and SBRT. Thirty-five of 40 patients in the LAT group (87.5%) received a local ablative treatment that targeted all metastases and sites. All metastases were treated, in patients with multiple lesions in a single organ. In the remaining patients (5/40; 12.5%), not all metastases were treated. This was either due to regression of some metastatic lesions after systemic treatment, fast disease progression or no access to LAT for other types of lesions (choroidal metastases) because our center does not offer proton therapy.
CDK4/6 inhibitors were more often prescribed for patients without LAT than patients with LAT (46.5% and 16.7% respectively, p = 0.011). The hormone therapy (HT) was more longer prescribed in the group of patients with LAT than patients with no-LAT [24.2 months (range 6.6; 70.2) and 12.8 months (range 2.0; 76.8) (data not shown).
4.2. Tumor assessment and patient survival
Median follow-up for the entire population was 50.4 months (95% CI [44.4–53.4]). Among patients who were treated by the addition of LAT (n = 40), 34 (85%) achieved a complete overall response (CR), whilst 6 (14.5%) achieved a partial overall response (PR) or had stable disease (SD). Among patients without LAT (n = 62), 12 patients (19.4%) achieved CR whilst 42 patients (67.7%) achieved PR or SD (data not shown).
The median PFS for the entire population was 27.6 months (95% CI [17.2; 48.4]). The 2-and 5-year PFS rates for our entire population were 52.7% (95% CI [42.57; 61.88]) and 34.8% (95% CI [24.42; 45.26]) respectively. Fifteen patients (37.5%) in the LAT group and 47 patients (75.8%) in the group without LAT progressed. Twelve of the 15 patients (80%) in the LAT group had an isolated failure involving a single metastatic site compared to 30 patients (63.8%) with no-LAT. Disease in 4 LAT group patients progressed beyond the treated site but remained within the same organ, and 3 of these patients had salvage local ablative treatment. There was only one progression within the treated site.
The median OS was not reached. The 2-and 5-year OS rates for our entire population were 86.2% (95% CI [77.80; 91.58]) and 63.2% (95% CI [50.69; 73.37]) respectively. At the time of analysis, 5 (12.5%) and 22 patients (35.5%) in the LAT and no-LAT group, respectively, died of their metastatic disease.
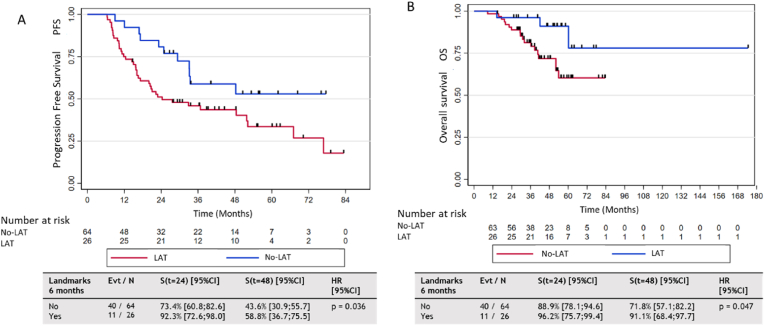
Results from the unavailable and multivariable analysis are detailed in Table 3. In the univariable analysis, a significant increase in PFS was observed in patients with LAT compared the no-LAT group (HR 0.39, 95% CI [0.22; 0.72], p = 0.002). There was also a significant increase in OS in patients with LAT compared to the no-LAT group (HR 0.31, 95% CI [0.13; 0.78], p = 0.01). However, there was no significant difference in OS or PFS between patients with LAT and 1 vs. ≥2 metastases. In addition, landmark analyses were carried out at 3 and 6 months after the start of the initial treatment to identify any differences in PFS or OS between patients with LAT and those with no-LAT (Fig. 2).
Table 3.
Univariable and multivariable analysis for OS and PFS.
| Characteristics | Univariable analysis HR and 95% CI |
p-value |
Multivariable analysis HR and 95% CI |
p-value |
||||
|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | OS | PFS | OS | PFS | |
| Metastasis-directed treatment No Yes |
1 | 1 | 1 | 1 | ||||
| -Cox model with time- dependent variable | 0.31 [0.13; 0.78] | 0.39 [0.22; 0.72] | 0.01 | 0.002 | 0.13 [0.04; 0.38] | 0.35 [0.19; 0.65] | <0.001 | 0.001 |
| -Landmark 3 months | 0.39 [0.12; 1.30] | 0.57 [0.28; 1.15] | 0.1 | 0.1 | – | – | – | – |
| -Landmark 6 months | 0.31 [0.09; 1.05] | 0.50 [0.25; 0.97] | 0.05 | 0.03 | – | – | – | – |
| ECOG | ||||||||
| 0 | 1 | 1 | 0.005 | 0.03 | - | - | - | - |
| ≥1 | 1.43 [0.70; 2.91] | 1.70 [1.03; 2.82] | ||||||
| Synchronous | 1 | 1 | 0.08 | 0.25 | - | - | - | - |
| Oligorecurrent | 1.07 [0.53; 2.17] | 1.33 [0.81; 2.19] | ||||||
| Number of metastases | ||||||||
| 1 | 1 | 1 | 0.23 | 0.27 | 1 | 1 | 0.9 | 0.77 |
| ≥2 | 1.54 [1.76; 3.13] | 1.32 [0.80; 2.17] | 0.98 [0.46; 2.10] | 1.08 [0.63; 1.86] | ||||
| Grade | ||||||||
| I/II | 1 | 1 | ||||||
| III | 2.33 [1.13; 4.81] | 1.65 [1.00; 2.73] | 0.02 | 0.047 | – | – | – | – |
| Bone metastases only | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.36 [0.17; 0.79] | 0.49 [0.29; 0.81] | 0.01 | 0.005 | 0.62 [0.26; 1.44] | 0.63 [0.34; 1.17] | 0.3 | 0.14 |
| Liver metastases | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 2.61 | 1.86 | 0.008 | 0.03 | – | 2.13 | – | 0.035 |
| [1.24; 5.47] | [1.05; 3.30] | [1.05; 4.31] | ||||||
| Visceral metastases only | ||||||||
| No | 1 | 1 | ||||||
| Yes | 2.16 | 1.48 | 0.03 | 0.13 | – | – | – | – |
| [1.06; 4.38] | [0.88; 2.47] | |||||||
| HT + CDK inhibitor | ||||||||
| No | 1 | 1 | ||||||
| Yes | 0.62 [0.12; 3.11] | 0.96 [0.47; 1.95] | 0.5 | 0.9 | – | – | – | – |
| HT only | ||||||||
| No | 1 | 1 | 0.005 | 0.6 | – | – | – | – |
| Yes | 0.26 [0.12; 0.59] | 0.88 [0.53; 1.44] | ||||||
| CT only | ||||||||
| No | 1 | 1 | ||||||
| Yes | 11.53 [5.54; 24.00] | 5.16 [2.88; 9.25] | <0.001 | <0.001 | – | – | – | – |
| Subtype | ||||||||
| TN | 1 | 1 | <0.001 | <0.001 | 1 | 1 | <0.001 | <0.001 |
| HR + /HER2- | 0.09 [0.04; 0.20] | 0.30 [0.16; 0.55] | 0.04 [0.02; 0.12] | 0.28 [0.14; 0.56] | ||||
| HER2+ | 0.12 [0.04; 0.38] | 0.14 [0.05; 0.36] | 0.05 [0.02; 0.18] | 0.07 [0.03; 0.20] | ||||
HR hormone receptors; HT hormone therapy; CT chemotherapy; HER human epidermal receptor; CDK cyclin-dependent kinases; PRA percutaneous radiofrequency ablation.
Fig. 2.
Progression-free survival (PFS) and overall survival according to local ablative treatment 6 months after initiation of initial treatment (landmark analysis). A: PFS analysis of groups treated with systemic treatment alone (no-LAT) (n = 64) or local ablative treatment (LAT) group (n = 26) (landmark 6-month analysis).B: OS analysis of groups treated with systemic treatment alone (no-LAT) (n = 64) or LAT (n = 26) (landmark 6-month analysis). Groups were defined according to treatments received before the landmark time and patients who progressed or were censored before the landmark time were excluded.evt: event.
Multivariable analysis revealed that LAT was significantly associated with longer PFS and OS compared to no-LAT. In addition, overexpression of HER2 and HR positivity were significant predictors of both PFS and OS. Liver metastases led to worse PFS rates. Bone metastases were associated with significantly longer PFS in the univariate analysis but did not reach significance in the multivariate analysis (Table 3).
4.3. Toxicity to anatomic sites of LAT
LAT was well tolerated, and no grade ≥3 toxicity was observed in patients with radiotherapy, surgery or cryotherapy. Three patients (13.6%) experienced grade 2 toxicity with pain and fatigue. Only one patient presented with a grade 3 toxicity (pneumothorax) after PRA. Conservative management involving chest tube drainage was successful in this patient.
5. Discussion
In our retrospective and observational study, patients who underwent LAT in addition to systemic therapy had improved rates of 2-and 5-year PFS and OS without experiencing significant toxicity. To the best of our knowledge, our study is to date one of the largest to examine the outcomes of OMBC patients treated with LAT and also has one of the longest follow-up period.
OMBC diagnosis is to date primarily based on morphological and functional imaging data. 18F-FDG-PET/CT and WB-MRI appear to be the most sensitive and specific imaging tools currently available [22] and were largely used in our study. Although more than two thirds of patients were staged by one of these imaging methods, it is likely that the two examinations may have been used more frequently in the group of patients with LAT. This may have affected both baseline staging as well as follow-up for analysis of local ablative treatments. Indeed, a comparison of these 2 groups using the Mann-Whitney test shows that 18F-FDG-PET/CT was more often prescribed in the LAT group when compared to no-LAT patients (84.2% vs 64.5%, p = 0.03, data not shown in table). Because invasive lobular carcinoma demonstrates lower conspicuity on 18F-FDG-PET/CT [23], WB-MRI was preferred for low proliferative or lobular carcinoma and 18F-FDG-PET/CT for other forms. Liver MRIs were used in cases of suspected liver metastases (16,5%).
Unsurprisingly, toxicity rates following LAT were low as reported in other studies. Three prospective non-randomized phase II trials including a total of 117 patients showed excellent tolerability rates without any grade 3 toxicities after ablative SBRT or VMAT [[24], [25], [26]]. Nevertheless, Palma et al. reported 3 cases (4.5%) of grade 5 toxicity in patients undergoing stereotactic radiotherapy, whilst respecting organs-at-risk-constraints [27].
The OS and specific survival rates in our population are in line with the literature and are comparable with studies listed in Table 4. These studies reported a 2-year PFS and OS ranging from 27% to 90% and 65%–95%, respectively. Five-year OS rates in the literature range from 49% to 83% [25].
Table 4.
Prospective and retrospective studies of curative local ablative treatment in OMBC.
| Author & study design | Period | Patients OMBC | Local ablative treatment | Comparison with systemic treatment | PFS |
OS |
Long-term outcomes in multivariate analysis (OS or PFS) | ||
|---|---|---|---|---|---|---|---|---|---|
| 2years | 5years | 2years | 5years | ||||||
| Glemarec et al. Retrospective |
2014–2018 | 102 Synchronous (52%) Metachronous (48%) |
SBRT Surgery PRA Cryotherapy |
Yes | 52.7% | 34.7% | 86.2% | 63.2% | Local ablative treatment (OS & PFS) Local ablative treatment (OS & PFS) Liver metastasis only (PFS) Luminal subtype (OS & PFS) HER2+ (OS & PFS) |
| Palma et al. Prospective |
2012–2016 | 18 | SBRT | Yes | Specific OMBC data not shown | ||||
| Trovo et al. Prospective |
2012–2015 | 54 Synchronous (74%) Metachronous (26%) |
SBRT | No | 53% | – | 95% | – | Not significant correlation. |
| Milano et al. Prospective |
2001–2011 | 48 Synchronous (86%) Metachronous (14%) |
SBRT | No | 42% (no BO) 30% (no BO) |
75% (BO) 67% (BO) |
– 31% (no BO) |
– 83% (BO) |
Bone only (OS & PFS) |
| David et al. Prospective |
2014–2016 | 15 Bone metastases only (100%) Synchronous (13%) Metachronous (87%) |
SBRT | No | 100% | – | 65% | – | No multivariate analysis |
| Scorsetti et al. Retrospective |
2010–2014 | 33 Liver and Lung metastases only (100%) Synchronous and metachronous not showed |
SBRT | No | 27% | – | 66% | – | No significant correlation |
| Kobayashi et Retrospective |
1980–2010 | 75 Synchronous (19%) Metachronous (81%) |
Radiotherapy Surgery |
Yes | – | 56.8% | – | 79.2% | No multivariate analysis |
| Yoo et al. Retrospective |
2004–2008 | 50 Metachronous (100%) |
Radiotherapy 34% with EQD2 ≥ 50Gy |
No | – | 24.5% | 85.2% | 49% | Luminal subtype (OS) pN stage (OS) Solitary bone metastasis (OS) |
| Tan et al. Retrospective |
2011–2017 | 66 Synchronous (100%) |
SBRT | No | 52% | – | 82.5% | - | Luminal subtype (OS) Start/change of CT or HT (PFS) Number lines of CT/HT after SBRT (OS) |
BO: bone only; OMBC: oligometastatic breast cancer, OS: overall survival, PFS: progression-free survival, SBRT: stereotactic body radiation therapy, EQD2: equivalent dose 2Gy. CT: chemotherapy, HT: hormonotherapy [42].
We identified several risk factors as potential predictors of survival. Significant findings from other, mostly retrospective studies are detailed in Table 4. Better OS in patients that only had bone lesions was notably reported in two older accounts series [25,28]. We found a significantly better OS and PFS when LAT was associated with systemic treatment of at least one metastatic deposit compared to no-LAT. However, there was no difference in the outcome of patients with synchronous vs. metachronous presentations. Furthermore, the number of lesions (1 vs.≥2) or of metastatic sites (1 vs.≥2) was not associated with PFS or OS. A series of 3447 patients found the number of metastases to be an independent prognostic factor with one to three metastases associated with better survival (OS) compared to patients with 4 or 5 metastases and patients with more than five metastases. We were unable to use this threshold because of the lack of patients with 4 or 5 metastases in our series [4]. This may be explained by the fact that patients with low metastatic burden possibly received LAT more often than those with multiple lesions. Secondly, HER2-positive and HR+/HER2- OMBCs had significantly longer PFS and OS than patients with triple-negative (TN) cancers. This seems intuitive as the aggressive behavior of TN metastatic disease has been associated with a poor prognosis and low survival rates when juxtaposed to all other tumor subtypes. Our results suggest that HR and HER2 positive patients may be ideal candidates for ablative therapies compared to TN patients. One may considere in some rares cases that eldery patients may be spared the anti-HER2 therapy. It should be noted that the earlier literature did not investigate HER2 positivity in metastatic breast cancer and that more recent studies have shown that HER2 positivity is not correlated with improved survival after ablative therapies [24,25,29,30]. In terms of estrogen-receptor positive breast cancers, we are, to the best of our knowledge, the first to report the use of newer systemic agents such as CDK4/6 inhibitors in OMBC patients. None of the series considered in the Van Omnen et al. [31] meta-analysis included patients treated with CDK4/6 inhibitors. Two additional recent publications also do not provide informative data in this regard because they each only included one patients treated with CDK4/6 inhibitors [32,33]. The association of local ablative treatment such as radiation with CDK4/6 inhibitors may present some advantages. In addition to cytotoxic effects on DNA [34], CDK4/6 inhibitors also induce cellular senescence and promote anti-tumor immunity, which may represent potential mechanisms for radiosensitization [35].These advantages must be weighed against the increased toxicity associated with concomitant radiotherapy especially digestive radiation therapy [36,37]. Our study was unfortunately unable to address this issue since most of our CDK4/6 inhibitor patients received a systemic treatment without LAT. Almost all of these patients were part of the phase III PALOMA 2 trial [38].
This current study has some obvious limitations. Firstly, our analysis is retrospective and based on data from an institutional tumor board registry. The addition of LAT to the systemic treatment was therefore not randomized. The decision whether or not to add focal therapy was made for each individual patient after review of their file in our weekly tumor board meeting dedicated to metastatic BC patients. To date, results from 2 prospective randomized phase II trials were reported but only patients and treatment details from Palma et al. were published. SABRT-COMET trial reported on a prospective randomized phase II trial that explored systemic treatment with or without ablative irradiation of metastases from different primary tumors. This study however included only 18 metastatic breast cancer patients and only 5 patients in the control group [27]. The results nevertheless show that SBRT to all sites of metastatic disease significantly improves the 5-year OS rate from approximately 18%–42%. Secondly, the heterogeneity of the local ablative treatment which included four different modalities. The main use of PET/CT and whole-body MRI in the group of patients with LAT may have led to a better selection of low burden tumors. The choice of imaging technique for OMBC staging may of course have contributed to the analysis of the feasibility of different LAT treatments. This needs to be considered before interpreting our results. Thirdly, our statistical design did not include a matched-paired analysis between groups. This problem may have been offset by using a propensity score to adjust for confounding variables between the two populations (molecular subtypes, metastatic site, number of systemic lines, tumor size etc.). Our multivariate analysis nevertheless accounted for several confounding factors, including a comparison between patients with “systemic therapy + LAT” vs. “No-LAT”.
6. Conclusion
The optimal treatment strategy for OMBC continues to evolve and our results provide supportive evidence for the use of more aggressive strategies including LAT, particularly for estrogen receptor positive and HER2 positive tumors, without liver metastases. Further research is needed to confirm that these two subgroups of patients are potential candidates for ablative therapies. Despite the negative results of a recent phase II randomized study [39], some patients may benefit from the addition of local ablative treatments and other phase III trials and the OligoCare study are likely to provide robust evidence of the benefits in terms of OS [8,[39], [40], [41]].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Kontani K., Hashimoto S., Murazawa C., Norimura S., Tanaka H., Ohtani M., et al. Factors responsible for long-term survival in metastatic breast cancer. World J Surg Oncol. 2014;12:344. doi: 10.1186/1477-7819-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagani O., Senkus E., Wood W., Colleoni M., Cufer T., Kyriakides S., et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steenbruggen T.G., Schaapveld M., Horlings H.M., Sanders J., Hogewoning S.J., Lips E.H., et al. Characterization of oligometastatic disease in a real-world nationwide cohort of 3447 patients with de Novo metastatic breast cancer. JNCI Cancer Spectr. 2021;5 doi: 10.1093/jncics/pkab010. pkab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I., et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Hellman S., Weichselbaum R.R. Oligometastases. JCO. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Hellman S., Weichselbaum R. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 8.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M., et al. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 9.Yoo T.G., Cranshaw I., Broom R., Pandanaboyana S., Bartlett A. Systematic review of early and long-term outcome of liver resection for metastatic breast cancer: is there a survival benefit? Breast. 2017;32:162–172. doi: 10.1016/j.breast.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Barral M., Auperin A., Hakime A., Cartier V., Tacher V., Otmezguine Y., et al. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc Intervent Radiol. 2016;39:885–893. doi: 10.1007/s00270-016-1301-x. [DOI] [PubMed] [Google Scholar]
- 11.Tree A.C., Khoo V.S., Eeles R.A., Ahmed M., Dearnaley D.P., Hawkins M.A., et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 12.Salama J.K., Kirkpatrick J.P., Yin F.-F. Stereotactic body radiotherapy treatment of extracranial metastases. Nat Rev Clin Oncol. 2012;9:654–665. doi: 10.1038/nrclinonc.2012.166. [DOI] [PubMed] [Google Scholar]
- 13.Viani G.A., Gouveia A.G., Louie A.V., Korzeniowski M., Pavoni J.F., Hamamura A.C., et al. Stereotactic body radiotherapy to treat breast cancer oligometastases: a systematic review with meta-analysis. Radiother Oncol. 2021;164:245–250. doi: 10.1016/j.radonc.2021.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Kelly P., Ma Z., Baidas S., Moroose R., Shah N., Dagan R., et al. Patterns of progression in metastatic estrogen receptor positive breast cancer: an argument for local therapy. International Journal of Breast Cancer. 2017;2017:1–7. doi: 10.1155/2017/1367159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moossdorff M. Contralateral lymph node recurrence in breast cancer: regional event rather than distant metastatic disease. A systematic review of the literature n.d. https://www.clinicalkey.fr/#!/content/playContent/1-s2.0-S0748798315004734?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0748798315004734%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed February 25, 2021). [DOI] [PubMed]
- 16.Chapet O., Kong F.-M., Quint L.E., Chang A.C., Ten Haken R.K., Eisbruch A., et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63:170–178. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 17.Grégoire V., Ang K., Budach W., Grau C., Hamoir M., Langendijk J.A., et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Franchet C., Djerroudi L., Maran-Gonzalez A., Abramovici O., Antoine M., Becette V., et al. Annales de Pathologie; 2021. Mise à jour 2021 des recommandations du GEFPICS pour l’évaluation du statut HER2 dans les cancers infiltrants du sein en France. [DOI] [Google Scholar]
- 19.Common terminology Criteria for adverse events. CTCAE); 2017. p. 155. [Google Scholar]
- 20.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.deSouza N.M., Liu Y., Chiti A., Oprea-Lager D., Gebhart G., Van Beers B.E., et al. Strategies and technical challenges for imaging oligometastatic disease: recommendations from the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2018;91:153–163. doi: 10.1016/j.ejca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Lacaze J.-L., Aziza R., Chira C., De Maio E., Izar F., Jouve E., et al. Diagnosis, biology and epidemiology of oligometastatic breast cancer. Breast. 2021;59:144–156. doi: 10.1016/j.breast.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulaner G.A., Jhaveri K., Chandarlapaty S., Hatzoglou V., Riedl C.C., Lewis J.S., et al. Head-to-Head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326–331. doi: 10.2967/jnumed.120.247882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trovo M., Furlan C., Polesel J., Fiorica F., Arcangeli S., Giaj-Levra N., et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126:177–180. doi: 10.1016/j.radonc.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Milano M.T., Katz A.W., Zhang H., Huggins C.F., Aujla K.S., Okunieff P. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: some patients survive longer than a decade. Radiother Oncol. 2019;131:45–51. doi: 10.1016/j.radonc.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 26.David S., Tan J., Savas P., Bressel M., Kelly D., Foroudi F., et al. Stereotactic ablative body radiotherapy (SABR) for bone only oligometastatic breast cancer: a prospective clinical trial. Breast. 2020;49:55–62. doi: 10.1016/j.breast.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 28.Yoo G.S., Yu J.I., Park W., Huh S.J., Choi D.H. Prognostic factors in breast cancer with extracranial oligometastases and the appropriate role of radiation therapy. Radiat Oncol J. 2015;33:301. doi: 10.3857/roj.2015.33.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scorsetti M., Franceschini D., De Rose F., Comito T., Villa E., Iftode C., et al. Stereotactic body radiation therapy: a promising chance for oligometastatic breast cancer. Breast. 2016;26:11–17. doi: 10.1016/j.breast.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Tan H., Cheung P., Louie A.V., Myrehaug S., Niglas M., Atenafu E.G., et al. Outcomes of extra-cranial stereotactic body radiotherapy for metastatic breast cancer: treatment indication matters. Radiother Oncol. 2021;161:159–165. doi: 10.1016/j.radonc.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 31.van Ommen-Nijhof A., Steenbruggen T.G., Schats W., Wiersma T., Horlings H.M., Mann R., et al. Prognostic factors in patients with oligometastatic breast cancer - a systematic review. Cancer Treat Rev. 2020;91 doi: 10.1016/j.ctrv.2020.102114. [DOI] [PubMed] [Google Scholar]
- 32.Selvarajan G., Dhanushkodi M., Radhakrishnan V., Murali C.S., Ananthi B., Iyer P., et al. The continuing conundrum in oligometastatic breast carcinoma: a real-world data. Breast. 2022;63:140–148. doi: 10.1016/j.breast.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagasaki E., Kudo R., Tamura M., Hayashi K., Uwagawa T., Kijima Y., et al. Long-term outcomes of oligometastatic breast cancer patients treated with curative intent: an updated report. Breast Cancer. 2021 doi: 10.1007/s12282-021-01240-1. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein M., Kastan M.B. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y., Luo J., Chen X., Yang Z., Mei X., Ma J., et al. CDK4/6 inhibitors: a novel strategy for tumor radiosensitization. J Exp Clin Cancer Res. 2020;39:188. doi: 10.1186/s13046-020-01693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosacki C., Bouleftour W., Sotton S., Vallard A., Daguenet E., Ouaz H., et al. CDK 4/6 inhibitors combined with radiotherapy: a review of literature. Clinical and Translational Radiation Oncology. 2021;26:79–85. doi: 10.1016/j.ctro.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Rashdan A., Quirk S., Roumeliotis M., Abedin T., Amaro C.P., Barbera L., et al. Radiation therapy with cyclin-dependent kinase 4/6 inhibitors: a multi-institutional safety and toxicity study. Int J Radiat Oncol Biol Phys. 2022 doi: 10.1016/j.ijrobp.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 39.Chmura S.J., Winter K.A., Woodward W.A., Borges V.F., Salama J.K., Al-Hallaq H.A., et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer ( NCT02364557) J Clin Orthod. 2022;40:1007. doi: 10.1200/JCO.2022.40.16_suppl.1007. [DOI] [Google Scholar]
- 40.Olson R. clinicaltrials.gov; 2021. Phase III randomized controlled trial and economic evaluation of stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic (1-3 metastases) cancer (SABR-COMET-3) [Google Scholar]
- 41.Roussy Gustave, Cancer Campus, Paris Grand. clinicaltrials.gov; 2018. Multicentric phase III trial of superiority of stereotactic body radiation therapy in patients with metastatic breast cancer in first-line treatment. [Google Scholar]
- 42.Lan B., Abudureheiyimu N., Zhang J., Wang C., Jiang S., Wang J., et al. Clinical features and prognostic factors for extracranial oligometastatic breast cancer in China. Int J Cancer. 2020;147:3199–3205. doi: 10.1002/ijc.33152. [DOI] [PubMed] [Google Scholar]