Abstract
Objective
In most cases, complete resection of the intradural tumor is accompanied by long‐term neurological complications. Postoperative spinal deformity is the most common complication after surgical resection of intradural tumors, and posterior longitudinal ligament complex (PLC) plays an important role in postoperative spinal deformity. In this study, we investigated the role of PLC in spinal deformity after the surgical treatment of intradural tumors.
Methods
We analyzed the data of 218 consecutive patients who underwent intradural tumor resection from 2000 to 2018 in this retrospective study. Before 2010, patients underwent laminoplasty without maintaining the integrity of PLC (laminoplasty group, n = 155). After 2010, patients performed single‐port laminoplasty to maintain the integrity of PLC (laminoplasty retain posterior ligament complex group, n = 63). The score of quality of life, painful cortex, spinal cord movement, progressive kyphosis or scoliosis, perioperative morbidity, and neurological results were analyzed in the laminoplasty group and laminoplasty retain posterior ligament complex group. The distributed variable was shown as mean ± standard deviation and an independent t‐test or one‐way analysis of variance was calculated.
Results
There are 155 patients (71.1%) included in the laminoplasty group, and 63 patients (28.9%) in the laminoplasty retain posterior ligament complex group. The average age of patients was 42 ± 2.3 years, and the average modified McCormick score was 2. There were 158 (72.4%) patients with intramedullary tumors and 115 (52.7%) patients with extramedullary tumors. The length of hospital stays (8 days vs. 6 days; p = 0.023) and discharge to inpatient rehabilitation (48.4% vs. 26.9%; p = 0.012) were significantly lower in the laminoplasty retain posterior ligament complex group than the laminoplasty group. There was no significant difference in the risk of progressive deformity between the two groups at 18 months after surgery (relative risk 0.12; 95% confidence interval [CI] 0.43–1.25; p = 0.258) and at 20 months after surgery (relative risk 0.24; 95% CI 0.21–2.1).
Conclusion
Laminoplasty retains posterior ligament complex showed no impact on the spinal deformities compared with laminoplasty, but significantly improved the postoperative spinal activity, alleviated pain symptoms, and reduced hospital recovery time.
Keywords: Intradural Spinal Tumor, Laminoplasty, Pain, Posterior Ligaments Complex, Spinal Deformity
A female patient, 60 years old, neurofibromatoma in laminoplasty with posterior ligament complex retention (A shows that preoperative lumbar MRI examination T2W1 shows that the tumor is located in the chest 10‐lumbar four vertebral tube, completely under the epidural membrane; B shows that postoperative lumbar vertebrae positive side plate shows a fixed titanium plate).

Introduction
The intradural tumor accounted for 30% of all primary vertebral body tumors. The main symptom of an intradural tumor is pain, headstand, and nocturnal seizures. 1 , 2 As microsurgery technology advances, the surgical treatment of intramedullary tumors has made great progress 3 which may improve patients' survival rates and better quality of life. 4 , 5 , 6 However, despite the promotion of minimally invasive surgical techniques, spine deformity remains one of the serious postoperative complications of intradural tumor surgery. 7
Spinal deformity presents with severe, rigid, angular kyphoscoliosis or kyphosis with severe clinical symptoms, which often arise in surgical management. 8 The spinal deformity was also a common complication in intradural spinal tumor resection, with an incidence rate of about 10% in adults and 22%–100% in children. 9 , 10 , 11 Retaining harmonious coronal and sagittal spinopelvic alignment ligaments are one of the most important methods to prevent spinal deformities. 12 Besides, during intradural spinal tumor resection, it was very important to relieve nerve compression by removing all or part of the posterior elements, including the lamina, spinous process, supraspinous ligament and interspinous ligament, ligamentum flavum, and facet joints. However, posterior longitudinal ligament complex (PLC) (including the spinous process) plays an important role in maintaining spinal activity and preventing postoperative spinal deformity which acts as the posterior tension band and can be effective in preventing adjacent segment degeneration. 13 The intact PLC did not significantly correct non‐surgically induced spinal deformities, but it did significantly improve the integrity of the patient's intervertebral discs in the first 3 months. 14 However, the stability of the spine was also found to be significantly reduced by progressive resection of the PLC in an in vitro biomechanical investigation. 15 However, there are no clinical studies on the results of PLC in spinal deformities. Therefore, the role of laminoplasty retaining the PLC in improving spinal malformation after surgery in patients with intradural spinal tumor resection needs further study.
In our study, the purpose of our studies was to (i) investigate the effect of PLC preservation on improving the quality of life of patients during hospitalization, in terms of pain scores, and (ii) to analyze the effect on patients' long‐term spinal deformity by a 20‐month follow‐up, and we hypothesized that retaining the posterior ligament complex can reduce the incidence of spinal deformity after resection of intradural spinal tumors.
Materials and Methods
Patients
This was a retrospective study and included patients who underwent intradural tumor resection at our hospital from 2000 to 2018. Before 2010, all patients were undergoing laminoplasty without maintaining the integrity of the PLC (n = 155). After 2010, they underwent single‐port laminoplasty to maintain the integrity of the PLC (n = 63).
The criteria for patient selection were: (1) all patients have been diagnosed with intradural cancer which includes intramedullary and extramedullary tumors but not an extradural tumor; (2) preoperative and postoperative T1 and T2 ± axial, relaxation, and coronary magnetic resonance imaging, and if necessary, spinal computed tomography were used to the defined intradural tumor; (3) preoperative radiographs were assessed for loss of cervical lordosis, loss of lumbar lordosis, or scoliosis (Cobb >10°) for all patients. Postoperative follow‐up consisted of serial imaging and clinical assessments according to the schedule above for neurological exams. Patients with evidence of preoperative focal coronal or sagittal deformity underwent postoperative standing scoliosis film with a 36‐in cassette to quantify the degree of scoliosis or kyphosis at scheduled radiographic follow‐up.
Patients who met any of the following criteria were excluded: (1) cervical kyphosis; (2) segmental instability of the cervical spine; and (3) a history of anterior decompression and fusion surgery or posterior fusion surgery (Figure 1). This study was approved by our hospital's Ethics Committee (NO 2302223).
Fig. 1.

CONSORT 2010 flow diagram.
Surgical Technique
For laminoplasty without retaining PLC described in the previous study, 16 only the subperiosteal paravertebral muscles were removed, and PLC was not retained to expose the medial joints. We try to protect the joint capsule in all cases. The Leksell rongeur was used to remove the tail prosthesis and intervertebral ligaments from the laminoplasty segment.
Laminoplasty
During laminoplasty, the patient with posterior ligament syndrome was completely anesthetized. Mayfield's pin‐fixing head holder was used, and his hands were placed on the operating table. The central incision of the left lumbar spine was about 12 cm long, exposing the scapula regularly. This swing was based on the cross‐section of the central part of the claw. This area was incised and rotated clockwise, completely right musculoskeletal syndrome, leaving the right spine in the joint.
Laminoplasty with Posterior Longitudinal Ligament Complex
The intact PLC maintained the intervertebral and yellow ligament during the laminoplasty procedure. Then a small hole was made on the tail plate with a 2‐mm Kerrison puncture, and to identify the hard shell before drilling. The width of the laminoplasty was adjusted relative to the spinal canal. 1‐mm Kerrison Bite forceps were removed by the yellow bandage, and the curette removes the surrounding hyperplasia of bone and completely exposes the outer membrane of the tumor. The tumor was removed through an incision along the midline of the tumor length. After the tumor was removed and the blade was re‐approached and fixed with a titanium microplate. Finally, the paravertebral muscles were closed with a blade and sutured to the deep part of the intervertebral ligament.
Remove the cortex of the right spine to create a V‐shaped skeleton and then the entire bone layer of the left spine was cut. Lift the vertebrae to the right to complete the opening process. Drill holes on the left and right sacrum at the end of each chest. Drill holes on the left side of each spine, and the titanium wire to drill the corresponding needle‐shaped blade, squeeze the titanium wire, and fix the growth of the thorns separately from the blade (Figure 2).
Fig. 2.
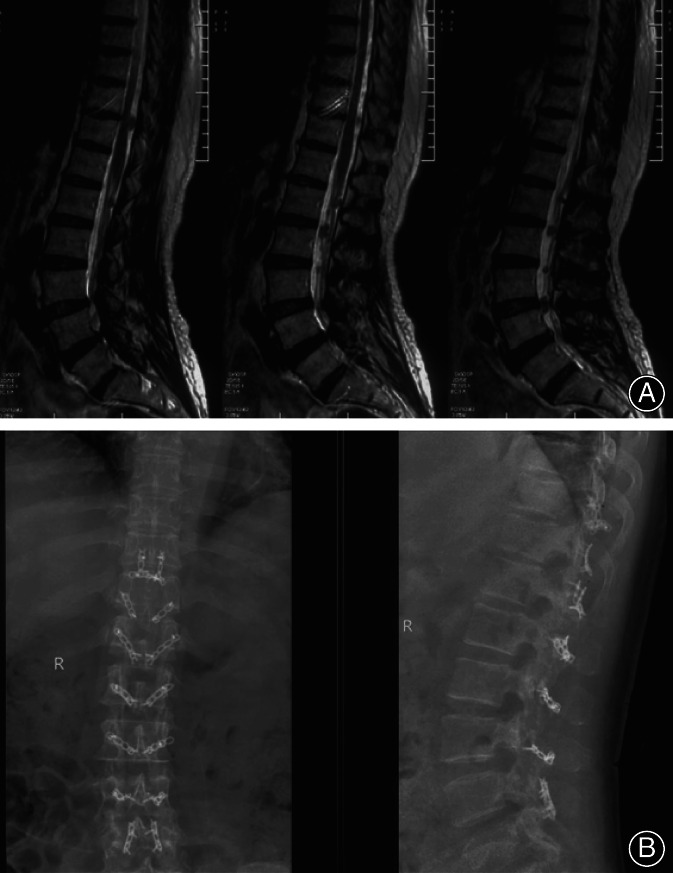
A female patient, 60 years old, neurofibromatoma in laminoplasty with posterior ligament complex retention (A shows that preoperative lumbar MRI examination T2W1 shows that the tumor is located in the chest 10‐lumbar four vertebral tube, completely under the epidural membrane; B shows that postoperative lumbar vertebrae positive side plate shows a fixed titanium plate).
Outcome Measures
At 3, 6, 12, 18, and 24 months after surgery, the patient's neurological function was examined and recorded. The functional measurement used a modified McCormick scale to assess the degree of cervical lordosis, lumbar lordosis, or scoliosis atrophy (Cobb angle>10°). Front, back, left, and right flexion and visual analog scale (VAS; 0–10) at 3, 6, 12, 18, and 24 months after surgery were applied. Feedback questionnaires and the quantitative quality of life scales (expressed as a percentage of the total score) were used to assess the patient's quality of life.
The primary result of this study was the progressive deformities of the spine (Figure 2). It was defined as a scoliosis or kyphosis curve that progresses at least 10° through X‐rays (Fa. Philips, Netherlands) and records the time of occurrence of spinal deformity. In addition, symptoms of progressive deformation were also observed during the observation period and an X‐ray test was an exam for the development of progressive deformities.
Statistical Analysis
For statistical data, we used SAS version 9.3 (SAS 9.3, SAS Institute, Cary, NC). The continuous variables data were represented by mean ± standard deviation. The χ 2 test or exact Fisher test was used to calculate determined variables and processed by independent t‐test or one‐way analysis of variance. The Kaplan–Meier method was used to determine the relationship between postoperative deformation frequency and time, and the logarithmic test was used to compare the laminoplasty group and the laminoplasty retain posterior ligament complex group. A p‐value less than 0.05 was considered statistically significant. 17 , 18
Results
Patients' Characteristics
In this study, 218 spinal canal tumor patients were included. Among them, 155 patients underwent laminoplasty, and 63 patients in the laminoplasty retain posterior ligament complex group.
The average age was 42 ± 2.3 years and 34 children and 184 adults participated. There were 102 males. The average duration of symptoms is 6 months and motor weakness is 57.3%. The percentages of patients who had previously received biopsy, surgical resection, radiotherapy, and chemotherapy were 8.25%, 14.7%, 8.7%, and 5.50%, respectively. Other comparative patient data were shown in Table 1.
TABLE 1.
Baseline data of pediatric and adult patients undergoing laminoplasty versus laminoplasty retain posterior ligament complex for intradural spinal tumors
| Variable | All patients (n = 218), n (%) | Laminoplasty (n = 155), n (%) | Laminoplasty retain posterior ligament complex (n = 63), n (%) | T value | p value |
|---|---|---|---|---|---|
| Mean age, year | 42 ± 2.3 | 46 ± 3 | 41 ± 2 | 0.66 | 0.362 |
| Pediatric (age < 18 years), n (%) | 34 (18) | 23 (17) | 11 (10) | 0.25 | 0.388 |
| Male, n (%) | 102 (46.8) | 77 (46.7) | 45 (71.4) | 0.14 | 0.125 |
| Median preoperative MMS | 3 (1–3) | 2 (1–2) | 2 (2–3) | 0.58 | 0.625 |
| Myelopathy, n (%) | 152 (69.7) | 116 (74.8) | 46 (73.0) | 0.35 | 0.359 |
| Radiculopathy, n (%) | 80 (36.7) | 48 (30.9) | 32 (50.7) | 0.62 | 0.115 |
| Duration of symptoms, month | 6 (2.75) | 7 (4.51) | 6 (9.52) | 0.88 | 0.852 |
| Motor weakness, n (%) | 125 (57.3) | 80 (51.6) | 45 (71.4) | 0.41 | 0.325 |
| Intramedullary, n (%) | 158 (72.4) | 105 (67.7) | 53 (84.1) | 0.52 | 0.152 |
| Intradural‐extramedullary, n (%) | 115 (52.7) | 85 (54.8) | 30 (47.6) | 0.15 | 0.635 |
| Median tumor spinal levels | 3 (1–2) | 2 (1–2) | 2 (1–2) | 0.58 | 0.362 |
| Abnormal preoperative alignment, n (%) | 45 (20.6) | 31 (20.0) | 14 (22.2) | 0.62 | 0.122 |
| Syrinx, n (%) | 42 (19.2) | 30 (19.3) | 12 (19.0) | 0.84 | 0.082 |
| Previous biopsy, n (%) | 18 (8.25) | 12 (7.75) | 6 (9.5) | 0.36 | 0.362 |
| Previous resection, n (%) | 32 (14.7) | 21 (13.5) | 11 (17.5) | 0.48 | 0.369 |
| Previous radiotherapy, n (%) | 19 (8.7) | 15 (9.7) | 4 (6.3) | 0.24 | 0.075 |
| Previous chemotherapy, n (%) | 12 (5.50) | 8 (5.16) | 4 (6.30) | 0.14 | 0.521 |
Abbreviation: MMS, modified McCormick Scale.
Pathology results show that 72 cases (33%) in ependymoma, 24 cases (11%) in low‐grade astrocytoma, 25 cases (11%) in hemangioblastoma, 12 cases (5%) in ganglioglioma, three cases (1%) in malignant astrocytoma, two cases (0.9%) in metastasis, five cases (2%) in cavernoma, three cases (1%) in medulloblastoma, 26 cases (11%) in schwannoma, 25 cases (11%) in meningioma, eight cases (3%) in lipoma, seven cases (3%) in neurofibroma, and eight cases (3%) in dermoid.
Perioperative Results
One hundred and eighty‐five cases (84.8%) were completely resected, 33 cases (15.1%) were partially resected, and 11 cases had wound infection (5.04%). Incisional CSF leaks occurred in 16 cases (7.33%). There were two cases (0.5%) of deep vein thrombosis and three cases (1%) of pulmonary embolism and the average length of hospital stay was 6 ± 2 days. Ninety‐two patients (42.2%) were discharged to inpatient rehabilitation. Length of hospitalization and discharge to inpatient rehabilitation were shorter significantly in the laminoplasty group than laminoplasty retain posterior ligament complex group. This may indicate that laminoplasty retaining the posterior ligament complex may help early recovery (Table 2).
TABLE 2.
Perioperative data of pediatric and adult patients undergoing laminoplasty versus laminoplasty retain posterior ligament complex for intradural spinal tumors
| Variable | All patients (n = 218), n (%) | Laminoplasty (n = 155), n (%) | Laminoplasty retain posterior ligament complex (n = 63), n (%) | T value | p value |
|---|---|---|---|---|---|
| >3 Operative levels, n (%) | 86 (39.4) | 56 (36.1) | 30 (47.6) | 0.57 | 0.632 |
| Subtotal resection, n (%) | 82 (37.6) | 63 (40.6) | 19 (30.3) | 0.42 | 0.621 |
| Surgical site infection, n (%) | 11 (5.04) | 8 (5.16) | 3 (4.76) | 0.85 | 0.215 |
| Incisional CSF leak, n (%) | 16 (7.33) | 14 (9.03) | 2 (3.17) | 0.44 | 0.325 |
| Length of hospitalization, day | 6 ± 2 | 8 ± 3 | 6 ± 2 | 0.18 | 0.023 |
| Discharge to inpatient rehabilitation, n (%) | 92 (42.2) | 75 (48.4) | 17 (26.9) | 0.25 | 0.012 |
| Postoperative radiotherapy, n (%) | 16 (7.34) | 12 (7.74) | 4 (6.34) | 0.62 | 0.625 |
| Median MMS at last follow‐up | 2 (1–3) | 2 (1–4) | 3 (2–4) | 0.11 | 0.255 |
| Last follow‐up, month | 20.6 ± 2.6 | 21.6 ± 1.2 | 23.6 ± 3.6 | 0.35 | 0.361 |
Abbreviations: CSF, cerebrospinal fluid; MMS, modified McCormick Scale.
Spine Deformity
Thirty‐one patients (12%) developed progressive image deformities an average of 20 months after surgery. Of these 31 patients, seven cases had cervical lordosis and three cases had mild cervical kyphosis (Table 3). Three patients had progressive kyphosis. The majority of patients with radiographic progression (23, 74%) were asymptomatic, whereas eight cases (25%) had associated mechanical neck or back pain. Six cases (19%) subsequently underwent lumbar spine fusion. At the last follow‐up, the median modified McCormick score was unchanged from the preoperative value: three scores (interquartile range, 2–4) versus two scores (interquartile range, 1–3).
TABLE 3.
Comparison of postoperative rates of deformity in pediatric and adult patients undergoing laminoplasty versus laminoplasty retain posterior ligament complex for intradural spinal tumors at 24 months after surgery
| Incidence of postoperative deformity | All patients (n = 218), n (%) | Laminoplasty (n = 155), n (%) | Laminoplasty retain posterior ligament complex (n = 63), n (%) | T value | p value |
|---|---|---|---|---|---|
| Adult patients | 13/184 (7.06) | 11/132 (8.33) | 2/52 (3.84) | 0.22 | 0.221 |
| Pediatric patients | 6/34 (17.6) | 2/23 (8.69) | 2/11 (18.1) | 0.18 | 0.512 |
| Cord and C‐function | 85.36 ± 7.32 | 84.32 ± 6.25 | 85.66 ± 12.25 | 0.75 | 0.625 |
| C‐function | 86.36 ± 10.23 | 88.62 ± 11.25 | 82.62 ± 11.25 | 0.39 | 0.255 |
| QOL (%) | 62.36 ± 12.62 | 60.25 ± 11.25 | 65.11 ± 6.25 | 0.48 | 0.251 |
| VAS | 2.33 ± 0.362 | 2.02 ± 0.62 | 1.25 ± 0.25 | 0.08 | 0.021 |
Abbreviations: QOL, quality of life; VAS, visual analog scale.
In the laminoplasty group, eight patients (3%) developed progressive spinal malformations, of which two cases (0.06%) were symptomatic. In the laminoplasty retain posterior ligament complex group, three patients (1%) developed progressive spinal deformity, and one patient (0.04%) had symptoms. Over time, the laminoplasty group and the laminoplasty retain posterior ligament complex groups showed similar changes in the risk of progressive deformity (relative risk 0.12; 95% confidence interval [CI] 0.43–1.25; p = 0.258, Figure 3). This similarity also occurred in adults (relative risk 0.24; 95% CI 0.21–2.1; Figure 3) and children (relative risk 0.52; 95% CI 0.31–2.8; Figure 3). In group analysis, the progressive deformity (25% vs. 20%), preoperative scoliosis or cervical/lumbar lordosis loss in patients under 18 years of age was similar between the laminoplasty retain posterior ligament complex group and laminoplasty group. In addition, neither of these two surgery methods showed spondylolisthesis.
Fig. 3.

Incidence of progressive radiographic deformity as a function of time after tumor resection via the Kaplan–Meier method in the all patients (A), children (B), adult population (C).
Quality of Life
We also analyzed the quality of life and long‐term results of the VAS and found that maintaining the PLC can relieve pain symptoms, but there is a significant difference in long‐term quality of life at 24 months after surgery. In addition, we further analyzed the spinal flexion test and found no significant difference between the two groups, such as front (68.62 ± 6.36 vs. 71.52 ± 7.62, p = 0.025) and right flex (15.62 ± 5.62 vs. 19.36 ± 5.61, p = 0.021) at 6 months after surgery. Front (68.62 ± 6.36 vs. 71.52 ± 7.62, p = 0.025), back (15.25 ± 6.25 vs. 20.61 ± 10.25, p = 0.045), left flex (19.25 ± 5.25 vs. 26.36 ± 3.62, p = 0.035), and right flex in 12 months, front (68.62 ± 6.36 vs. 71.52 ± 7.62, p = 0.025), back (15.25 ± 6.25 vs. 20.61 ± 10.25, p = 0.045), left flex (19.25 ± 5.25 vs. 26.36 ± 3.62, p = 0.035) at 12 months after surgery. Front (p = 0.011), back (p = 0.035), left flex (p = 0.025) and right flex (p = 0.025) at 18 months after surgery and front (p = 0.001), back (p = 0.025), left flex (p = 0.012) and right flex (p = 0.021) at 24 months after surgery (Table 4).
TABLE 4.
Comparison of lumbar activity in pediatric and adult patients undergoing laminoplasty versus laminoplasty retain posterior ligament complex for intradural spinal tumors at 24 months after surgery
| Lumbar activity | Laminoplasty (n = 155), n (%) | Laminoplasty retain posterior ligament complex (n = 63), n (%) | T value | p value |
|---|---|---|---|---|
| Baseline—Front | 55.53 ± 2.61 | 52.61 ± 3.68 | 0.55 | 0.562 |
| Baseline—Back | 9.36 ± 3.61 | 11.25 ± 2.36 | 0.61 | 0.225 |
| Baseline—Left flex | 10.25 ± 2.36 | 11.25 ± 3.25 | 0.41 | 0.125 |
| Baseline—Right flex | 9.36 ± 2.61 | 10.36 ± 3.62 | 0.85 | 0.263 |
| 3 months after surgery—Front | 56.25 ± 3.36 | 59.62 ± 6.32 | 0.25 | 0.352 |
| 3 months after surgery—Back | 10.25 ± 3.62 | 13.62 ± 3.62 | 0.11 | 0.158 |
| 3 months after surgery—Left flex | 12.36 ± 1.25 | 14.52 ± 3.62 | 0.36 | 0.114 |
| 3 months after surgery—Right flex | 9.33 ± 3.61 | 10.25 ± 2.36 | 0.25 | 0.582 |
| 6 months after surgery—Front | 68.62 ± 6.36 | 71.52 ± 7.62 | 0.36 | 0.025 |
| 6 months after surgery—Back | 13.52 ± 2.62 | 19.62 ± 6.32 | 0.15 | 0.125 |
| 6 months after surgery—Left flex | 18.62 ± 3.62 | 22.62 ± 6.36 | 0.25 | 0.251 |
| 6 months after surgery—Right flex | 15.62 ± 5.62 | 19.36 ± 5.61 | 0.32 | 0.021 |
| 12 months after surgery—Front | 70.52 ± 6.25 | 82.25 ± 2.36 | 0.22 | 0.015 |
| 12 months after surgery—Back | 15.25 ± 6.25 | 20.61 ± 10.25 | 0.10 | 0.045 |
| 12 months after surgery—Left flex | 19.25 ± 5.25 | 26.36 ± 3.62 | 0.25 | 0.035 |
| 12 months after surgery—Right flex | 16.65 ± 3.25 | 21.22 ± 2.61 | 0.66 | 0.085 |
| 18 months after surgery—Front | 75.25 ± 5.61 | 83.62 ± 6.65 | 0.28 | 0.011 |
| 18 months after surgery—Back | 16.25 ± 6.25 | 23.25 ± 6.25 | 0.36 | 0.035 |
| 18 months after surgery—Left flex | 20.25 ± 6.33 | 26.35 ± 10.32 | 0.55 | 0.025 |
| 18 months after surgery—Right flex | 21.25 ± 2.62 | 26.35 ± 5.61 | 0.25 | 0.025 |
| 24 months after surgery—Front | 80.25 ± 10.25 | 88.62 ± 6.99 | 0.36 | 0.001 |
| 24 months after surgery—Back | 18.36 ± 3.36 | 26.35 ± 5.66 | 0.21 | 0.025 |
| 24 months after surgery—Left flex | 21.25 ± 6.25 | 28.66 ± 6.35 | 0.44 | 0.012 |
| 24 months after surgery—Right flex | 22.36 ± 6.99 | 28.36 ± 10.25 | 0.48 | 0.021 |
Abbreviations: Front, front flexion; Back, back flexion; Left flex, left flexion; Right flex, right flexion.
Discussion
Our research results show that laminoplasty with retained posterior ligament complex surgery does show no impact on spinal deformities compared with patients with laminoplasty, However, it needs to be emphasized that maintaining the PLC can improve the patient's spinal mobility after long‐term surgery and shorten the patient's hospital stay. This may be important for the early recovery of patients after surgery.
Posterior Longitudinal Ligament Complex Plays Important Role in Spinal Surgery
Spinal deformity and abnormal movement were common complications after intradural spinal tumor resection. In order to further reduce patient complications, we studied the effect of laminoplasty preservation of the PLC in spinal surgery to improve spinal deformity. The PLC runs along the dorsal plexus of the vertebral body down the sacrum which increases cervical stability by providing posterior support to the vertebral body and limiting flexion and lateral bending and rotation. 19 , 20 What's more, there are also many complications associated with PLC resection, including symptoms such as cerebrospinal fluid leakage and spinal cord compression. 21 , 22 Therefore, PLC is important in maintaining spinal stability and preventing complications, so the decision to perform surgical resection needs to be considered holistically.
Preservation of the PLC significantly improves the sagittal balance of the postoperative cervical spine. The damage of PLC resulting from surgery can lead to changes in spinal stability. 20 , 23 , 24 This segmental ROM can lead to an increased risk of pain and postoperative deformity. 25 , 26 In addition, preservation of the PLC improved the patient's axial symptoms significantly. 27
Posterior Longitudinal Ligament Complex in Laminoplasty
The earliest surgical method of laminoplasty was according to Kirita's technique. 28 Compared with laminoplasty, laminoplasty can prevent complications such as postoperative vertebral instability, kyphosis, perineural adhesions, and delayed nerve injury. 29 Although long‐term results indicate that cervical laminoplasty was safe and effective, it was still necessary to study the development of new surgery methods to improve clinical outcomes such as reducing lordosis. 28 Shiraishi et al. found that the exposure of the nipple plate didn't affect the semicircular and separating muscles associated with the thoracic. Provide conservative exposure, allowing various operations on the back of the cervical spine. 30 Kotani et al. 31 found that cervical laminoplasty using reserved deep extensors can improve spinal function and quality of life. Our results also showed that maintaining the PLC can reduce pain symptoms, However, there is no significant effect on the quality of life. We consider that it may be related to the short observation period.
Laminoplasty is suggested as an alternative option because of its advantages in maintaining the motion of the cervical spine while maintaining the integrity of the posterior neck muscles and preventing epidural scar formation. 26 , 32 However, many reports have highlighted that laminoplasty can result in radiological kyphotic changes after surgery, although the risk is lower than that with laminoplasty alone. 33 , 34 The surgical technique allows wide exposure of the spinal canal, and it can easily be extended intraoperatively caudally, or rostrally. On the other hand, the disadvantages of this technique include possible extradural scar formation, loss of posterior spinal column integrity, and spinal instability and deformity. Thus, laminoplasty may avoid many complications, because the spinal cords posterior elements are replaced after removing the laminae completely. It is presumed that an intact posterior element provides for spine stabilization and theoretically prevents instability. However, its effectiveness in preventing postoperative spinal deformity is yet to be demonstrated. 35
Postoperative Complications in Laminoplasty with Posterior Longitudinal Ligament Complex
The postoperative cervical sagittal deformityis is the most serious complication after laminoplasty with removing spinal tumors. 36 , 37 Compared with laminoplasty to treat stenosis caused by degenerative spinal diseases, these deformities were more common after the removal of intramedullary spinal cord injuries. Typical complications for surgery were progressive deformities, axial pain in the area, and neurological symptoms associated with the deformity. 38 Post‐laminoplasty deformities were more common in children with immature bones; however, they were also more common in young people (<25 years of age) than in older people. The integrity of the PLC also plays an important role in predicting spinal stability, spine deformities, and progressive nerve damage. 39 Laminoplasty means that removing intradural spinal cord tumors has nothing to do with reducing the incidence of short‐term progressive spinal deformities or improving neurological function. 16 However, the resection of intramedullary spinal cord tumors was associated with a reduction in the incidence of spinal deformities which requires progressive anastomosis in children undergoing bone plastic laminoplasty and does not affect long‐term functional outcomes. 40 , 41 Similar results also have been found in our results. Therefore, it was very important to pay attention to the occurrence of spinal malformation in intradural tumors. 42
Microsurgical resection of spinal ependymomas is associated with a considerable risk of postoperative neurological deterioration. The final risk score consisted of the following independent predictors: preoperative MMCS >1 (1 point), proximal tumor level at Th 10 and higher (1 point), and tumor extension ≥3 vertebrae (1 point). McCormick scores revealed improved symptoms, particularly of gait disturbance, sensory deficits, and general performance in spinal arachnoid cysts. However, many studies found that there was no statistically significant difference between the scores on the modified McCormick Scale preoperatively and at the 3‐month follow‐up in patients undergoing surgical treatment for the intradural spinal tumor.
Laminoplasty often results in loss of mobility after surgery. 43 , 44 The loss of range of motion may be due to interlayer fusion between adjacent open layers, rupture of the posterior neck extensor muscles, and/or prolonged use of the collar after surgery. 45 Chen et al. 46 found that the modified unilateral laminoplasty that preserves the PLC can effectively treat cervical spondylotic myelopathy, restore nerve function, and retain cervical curvature and range of motion. As the strength of the postoperative musculoskeletal complex increases, the loss of balance in the sagittal plane of the cervical spine increases. Huang et al. used a finite element analysis to investigate the biomechanical effects of the lumbar posterior complex on the adjacent segments after posterior lumbar interbody fusion (PLIF) surgeries. Studies have shown that the PLC involved acts as a posterior bundle, resulting in lower forces on the range of motion and proximal segment during flexion. Preserving the PLC during decompression can effectively prevent the adjacent segment degeneration after PLIF. 13 This was also consistent with the results of our study, which showed that retaining PLC had a significant effect on improved spinal activity.
Limitations and Strengths of this Study
The limitations of this study include the following. Firstly, the relatively small sample size of our study and the lack of long‐term follow‐up results. If the sample size and follow‐up time could be expanded, it would be useful to clarify the role of the PLC on spinal deformity and spine motor after spinal intraspinal tumors in adult and pediatric patients. Secondly, although the results of children and adults were analyzed in our study, further research is needed to investigate the differences between the two groups. And then we also found that preservation of the PLC did not significantly improve MMCS for spinal tumor surgery. This may be related to the short follow‐up period in our study. Finally, the study of the PLC requires in‐depth research in terms of mechanisms, including animal experiments, mechanical experiments, and molecular biology level. The strength of studies found that treatment of the PLC during laminoplasty significantly improves postoperative spinal activity, pain symptoms, and hospital recovery time.
Conclusion
Laminoplasty with retain posterior ligament complex surgery does show no impact on the spinal deformities compared with than patients with laminoplasty alone, but significantly improves postoperative spinal activity, pain symptoms, and hospital recovery time. This has important clinical significance for the subsequent Preservation of the post‐ligand complex during spinal tumor laminoplasty have important clinical significance.
Author Contributions
Conceptualization: Xiang Yin, Keyu Luo. Data curation: Xiang Yin, Keyu Luo. Formal analysis: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu. Funding acquisition: Yufei Jin, Yinbo Wang, Mingyong Liu. Investigation: Yufei Jin, Yinbo Wang, Mingyong Liu. Methodology: Yufei Jin, Yinbo Wang, Mingyong Liu. Project administration: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu. Resources, Software: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu. Supervision and validation: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu. Visualization: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu. Roles/writing—original draft: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu, Peng Liu. Writing—review and editing: Xiang Yin, Keyu Luo, Yufei Jin, Yinbo Wang, Mingyong Liu, Peng Liu.
Funding Information
This research was no fund.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethics statement
This study was approved by our hospital's Ethics Committee (NO2302223).
Acknowledgements
We would like to thank all participants and our hospital.
References
- 1. Jin MC, Ho AL, Feng AY, Medress ZA, Pendharkar AV, Rezaii P, et al. Prediction of discharge status and readmissions after resection of intradural spinal tumors. Neurospine. 2022;19(1):133–45. 10.14245/ns.2143244.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noordhof RK, Vinke S, Kurt E. Spinal cord stimulation in patients suffering from chronic pain after surgery for spinal intradural tumors: a case report and literature summary. Pain Pract. 2022;22(8):746–52. 10.1111/papr.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommer F, Hussain I, Kirnaz S, Goldberg J, McGrath L, Navarro‐Ramirez R, et al. Safety and feasibility of augmented reality assistance in minimally invasive and open resection of benign intradural extramedullary tumors. Neurospine. 2022;19(3):501–12. 10.14245/ns.2244222.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebrahimi R, Sohi ASM, Mirsardoo A, Moosavi N, Khonji MS. Primary intradural extramedullary Ewing sarcoma in the lumbar area: a case report. Radiol Case Rep. 2022;17(12):4617–21. 10.1016/j.radcr.2022.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elsamadicy AA, Koo AB, Reeves BC, Pennington Z, Sarkozy M, Hersh A, et al. Hospital frailty risk score and healthcare resource utilization after surgery for primary spinal Intradural/cord tumors. Global Spine J. 2022;21925682211069937. 10.1177/21925682211069937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsumoto Y, Saiwai H, Iida K, Okada S, Endo M, Setsu N, et al. Shape factor of the spinal cord: a possible predictor of surgical outcome for intradural extramedullary spinal tumors in the thoracic spine. Global Spine J. 2022;12(7):1462–7. 10.1177/2192568220982571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desai B, Hobbs J, Hartung G, Xu G, Gokaslan ZL, Linninger A, et al. Image‐guidance technology and the surgical resection of spinal column tumors. J Neurooncol. 2017;131(3):425–35. 10.1007/s11060-016-2325-4 [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Xie J, Wang Y, Bi N, Li T, Zhang J, et al. Intraspinal neural axis abnormalities in severe spinal deformity: a 10‐year MRI review. Eur Spine J. 2019;28(2):421–5. 10.1007/s00586-018-5522-3 [DOI] [PubMed] [Google Scholar]
- 9. Arima H, Hasegawa T, Yamato Y, Yoshida G, Banno T, Oe S, et al. Incidence and predictors of postoperative kyphotic deformity after thoracic spinal cord tumor resection. Spine Surg Relat Res. 2022;6(1):17–25. 10.22603/ssrr.2021-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikwuezunma I, Beutler GJ, Margalit A, Jain A, Kebaish KM, Sponseller PD. Late spinal infections are more common after pediatric than after adult spinal deformity surgery. Spine Deform. 2022;10(4):817–23. 10.1007/s43390-022-00494-9 [DOI] [PubMed] [Google Scholar]
- 11. Shlobin NA, Le N, Scheer JK, Tan LA. State of the evidence for proximal junctional kyphosis prevention in adult spinal deformity surgery: a systematic review of current literature. World Neurosurg. 2022;161:179–189.e1. 10.1016/j.wneu.2022.02.063 [DOI] [PubMed] [Google Scholar]
- 12. Smith JS, Klineberg E, Schwab F, Shaffrey CI, Moal B, Ames CP, et al. Change in classification grade by the SRS‐Schwab adult spinal deformity classification predicts impact on health‐related quality of life measures: prospective analysis of operative and nonoperative treatment. Spine. 2013;38(19):1663–71. 10.1097/BRS.0b013e31829ec563 [DOI] [PubMed] [Google Scholar]
- 13. Huang YP, Du CF, Cheng CK, Zhong ZC, Chen XW, Wu G, et al. Preserving posterior complex can prevent adjacent segment disease following posterior lumbar interbody fusion surgeries: a finite element analysis. PloS One. 2016;11(11):e0166452. 10.1371/journal.pone.0166452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alanay A, Yazici M, Acaroglu E, Turhan E, Cila A, Surat A. Course of nonsurgical management of burst fractures with intact posterior ligamentous complex: an MRI study. Spine. 2004;29(21):2425–31. 10.1097/01.brs.0000143169.80182.ac [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Shen Z, Huang M, Wang X. Stepwise resection of the posterior ligamentous complex for stability of a thoracolumbar compression fracture: an in vitro biomechanical investigation. Medicine. 2017;96(35):e7873. 10.1097/md.0000000000007873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGirt MJ, Garcés‐Ambrossi GL, Parker SL, Sciubba DM, Bydon A, Wolinksy JP, et al. Short‐term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: analysis of 238 patients. Neurosurgery. 2010;66(5):1005–12. 10.1227/01.Neu.0000367721.73220.C9 [DOI] [PubMed] [Google Scholar]
- 17. He Z, Zheng J, Liu S, Guan Z, Zhou Q, Jin X, et al. The effect of whole‐body vibration in osteopenic patients after total knee arthroplasty: a randomized controlled trial. Aging Clin Exp Res. 2022;34(6):1381–90. 10.1007/s40520-021-02043-2 [DOI] [PubMed] [Google Scholar]
- 18. Guan Z, Luo L, Liu S, Guan Z, Zhang Q, Wu Z, et al. The role of TGR5 as an onco‐immunological biomarker in tumor staging and prognosis by encompassing the tumor microenvironment. Front Oncol. 2022;12:953091. 10.3389/fonc.2022.953091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aryal V, Jimenez A. Anatomy, Back, Posterior Longitudinal Ligament. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing LLC.; 2022. [PubMed] [Google Scholar]
- 20. Le HV, Wick JB, Van BW, Klineberg EO. Ossification of the posterior longitudinal ligament: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 2022;30(17):820–30. 10.5435/jaaos-d-22-00049 [DOI] [PubMed] [Google Scholar]
- 21. Kilitci A, Asan Z, Yuceer A, Aykanat O, Durna F. Comparison of the histopathological differences between the spinal material and posterior longitudinal ligament in patients with lumbar disc herniation: a focus on the etiopathogenesis. Ann Saudi Med. 2021;41(2):115–20. 10.5144/0256-4947.2021.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugita D, Nakajima H, Kokubo Y, Takeura N, Yayama T, Matsumine A. Cyclic tensile strain facilitates ossification of the cervical posterior longitudinal ligament via increased Indian hedgehog signaling. Sci Rep. 2020;10(1):7231. 10.1038/s41598-020-64304-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machino M, Sakai K, Yoshii T, Furuya T, Ito S, Segi N, et al. Treatment for the thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. J Clin Med. 2022;11(16):4690–704. 10.3390/jcm11164690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xue R, Liu D, Li Y, Zhang D. Different standing postures are the influencing factors for the efficacy of laminoplasty in the treatment of K‐line (−) patients with ossification of the posterior longitudinal ligament. Eur Spine J. 2022;31(9):2377–82. 10.1007/s00586-022-07300-0 [DOI] [PubMed] [Google Scholar]
- 25. Chang C, Zhu J, Li H, Yang Q. Enhanced magnetic resonance imaging manifestations of paediatric intervertebral disc calcification combined with ossification of the posterior longitudinal ligament: case report and literature review. BMC Pediatr. 2022;22(1):400. 10.1186/s12887-022-03461-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu H, Li X, Chen S, Zhang L, Yang G, Welle K, et al. Comparative effectiveness and safety of anterior cervical corpectomy with fusion, laminoplasty, and laminectomy and instrumented fusion for ossification of the posterior longitudinal ligament: a systematic review and network meta‐analysis. J Invest Surg. 2022;35(3):667–76. 10.1080/08941939.2020.1871535 [DOI] [PubMed] [Google Scholar]
- 27. Wang M, Luo XJ, Deng QX, Li JH, Wang N. Prevalence of axial symptoms after posterior cervical decompression: a meta‐analysis. Eur Spine J. 2016;25(7):2302–10. 10.1007/s00586-016-4524-2 [DOI] [PubMed] [Google Scholar]
- 28. Cho SK, Kim JS, Overley SC, Merrill RK. Cervical laminoplasty: indications, surgical considerations, and clinical outcomes. J Am Acad Orthop Surg. 2018;26(7):e142–52. 10.5435/jaaos-d-16-00242 [DOI] [PubMed] [Google Scholar]
- 29. Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open‐door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8(7):693–9. 10.1097/00007632-198310000-00003 [DOI] [PubMed] [Google Scholar]
- 30. Shiraishi T. A new technique for exposure of the cervical spine laminae. Technical Note. J Neurosurg. 2002;96(1 Suppl):122–6. 10.3171/spi.2002.96.1.0122 [DOI] [PubMed] [Google Scholar]
- 31. Kotani Y, Abumi K, Ito M, Sudo H, Takahata M, Ohshima S, et al. Minimum 2‐year outcome of cervical laminoplasty with deep extensor muscle‐preserving approach: impact on cervical spine function and quality of life. Eur Spine J. 2009;18(5):663–71. 10.1007/s00586-009-0892-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Wo J, Wen J, Zhang L, Xu W, Wang X. Laminoplasty versus laminectomy with fusion for treatment of multilevel cervical compressive myelopathy: an updated meta‐analysis. Postgrad Med J. 2022;98(1163):680–8. 10.1136/postgradmedj-2020-139667 [DOI] [PubMed] [Google Scholar]
- 33. Du W, Wang S, Wang H, Zhang J, Wang F, Zhang X, et al. Cervical alignment and clinical outcome of open‐door laminoplasty vs. laminectomy and instrumentation in kyphotic multilevel cervical degenerative myelopathy. Arch Orthop Trauma Surg. 2022. 10.1007/s00402-021-04316-x [DOI] [PubMed] [Google Scholar]
- 34. Pettersson SD, Skrzypkowska P, Ali S, Szmuda T, Krakowiak M, Počivavšek T, et al. Predictors for cervical kyphotic deformity following laminoplasty: a systematic review and meta‐analysis. J Neurosurg Spine. 2022;1–10. 10.3171/2022.4.Spine22182 [DOI] [PubMed] [Google Scholar]
- 35. Shi W, Wang S, Zhang H, Wang G, Guo Y, Sun Z, et al. Risk factor analysis of progressive spinal deformity after resection of intramedullary spinal cord tumors in patients who underwent laminoplasty: a report of 105 consecutive cases. J Neurosurg Spine. 2019;9:1–9. 10.3171/2018.10.Spine18110 [DOI] [PubMed] [Google Scholar]
- 36. Lin S, Lin T, Wu Z, Chen G, Shangguan Z, Wang Z, et al. Does the asymmetry and extension function of the preoperative cervical paraspinal extensor predict postoperative cervical sagittal deformity in patients who undergo modified laminoplasty. Spine J. 2022. 10.1016/j.spinee.2022.07.099 [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y, Hou J, Xiao R, Zheng J, Zou X, Zhu Y, et al. Cervical sagittal alignment in patients with basilar invagination. Spine. 2022;47(21):1515–24. 10.1097/brs.0000000000004423 [DOI] [PubMed] [Google Scholar]
- 38. Fassett DR, Clark R, Brockmeyer DL, Schmidt MH. Cervical spine deformity associated with resection of spinal cord tumors. Neurosurg Focus. 2006;20(2):E2. [PubMed] [Google Scholar]
- 39. Vaccaro AR, Lehman RA Jr, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30(20):2325–33. 10.1097/01.brs.0000182986.43345.cb [DOI] [PubMed] [Google Scholar]
- 40. Antkowiak L, Putz M, Sordyl R, Pokora S, Mandera M. Relevance of intraoperative motor evoked potentials and D‐wave monitoring for the resection of intramedullary spinal cord tumors in children. Neurosurg Rev. 2022;45(4):2723–31. 10.1007/s10143-022-01788-2 [DOI] [PubMed] [Google Scholar]
- 41. Cannizzaro D, Mancarella C, Nasi D, Tropeano MP, Anania CD, Cataletti G, et al. Intramedullary spinal cord tumors: the value of intraoperative neurophysiological monitoring in a series of 57 cases from two Italian centers. J Neurosurg Sci. 2022;66(5):447–55. 10.23736/s0390-5616.19.04758-1 [DOI] [PubMed] [Google Scholar]
- 42. Hong JT, Kim IS, Lee HJ, Park JH, Hur JW, Lee JB, et al. Evaluation and surgical planning for craniovertebral junction deformity. Neurospine. 2020;17(3):554–67. 10.14245/ns.2040510.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X, Yu H, Welle K, Gathen M, Zhang L, Xiao J, et al. Comparative effectiveness and safety of open‐door laminoplasty, French‐door laminoplasty, laminectomy and fusion, and laminectomy alone for multilevel degenerative cervical myelopathy: a Bayesian network analysis. Adv Ther. 2022;39(1):117–39. 10.1007/s12325-021-01980-8 [DOI] [PubMed] [Google Scholar]
- 44. Niu S, Anastasio AT, Rhee JM. Laminoplasty achieves improved outcomes despite leading to a more positive sagittal balance: neither preoperative nor postoperative sagittal balance correlated with spine‐specific outcome data. Clin Spine Surg. 2022;35(1):E150–4. 10.1097/bsd.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 45. Iizuka H, Iizuka Y, Nakagawa Y, Nakajima T, Toda N, Shimegi A, et al. Interlaminar bony fusion after cervical laminoplasty: its characteristics and relationship with clinical results. Spine. 2006;31(6):644–7. 10.1097/01.brs.0000203707.79269.6a [DOI] [PubMed] [Google Scholar]
- 46. Chen C, Yang C, Yang S, Gao Y, Zhang Y, Wu X, et al. Clinical and radiographic outcomes of modified unilateral open‐door laminoplasty with posterior muscle‐ligament complex preservation for cervical Spondylotic myelopathy. Spine. 2019;44(24):1697–704. 10.1097/brs.0000000000003158 [DOI] [PubMed] [Google Scholar]


