Abstract
Gel bait formulations of insecticides have been shown to be highly effective in managing German cockroach (Blattella germanica L. [Blattodea: Ectobiidae]) populations. Three potential reasons for this are high palatability of baits, the use of slow-acting insecticides, and their horizontal transfer within aggregations, a phenomenon known as ‘secondary mortality’. Our objective was to determine whether horizontal transfer can go beyond secondary, to tertiary and quaternary effects, and to compare various gel baits with different active ingredients. We fed adult females a bait and recorded their bait consumption, moribundity, and mortality. Groups of first instars were then exposed to the dead females and their feces, secondary mortality was quantified, and a new cohort of nymphs was then exposed to the feces and dead nymphs (for tertiary mortality); this process was repeated for quaternary mortality. This design did not distinguish among the major mechanisms of horizontal transfer of insecticides, namely coprophagy and contact with feces, exposure to regurgitated fluids, and cannibalism and necrophagy of nymphs. All the tested baits caused 100% mortality of the adult females that directly fed on the bait and high secondary mortality (average of >85%) within 48 hr. Baits containing either dinotefuran, emamectin benzoate, fipronil, or indoxacarb caused tertiary mortality (average of 15–70%), but only the fipronil and indoxacarb baits caused some quaternary mortality. The relative importance of secondary, tertiary, and quaternary transfer of the active ingredient remains to be determined in field populations of the German cockroach.
Keywords: secondary mortality, tertiary mortality, quaternary mortality, horizontal transfer, gel bait
Bait formulations of insecticides have revolutionized urban pest management, especially interventions to eliminate indoor infestations of cockroaches. Compared to residual sprays, baits offer greater efficacy, greater specificity, lower nontarget hazards, a greater assortment of active ingredients with diverse modes of action, and utility in insecticide-sensitive areas (Buczkowski et al. 2001, Appel 2003, Gondhalekar et al. 2011, Schal 2011, Appel and Rust 2021). Another reason why baits are highly effective is often attributed to their translocation to aggregations where they can cause mortality of individuals that did not visit the bait. Secondary mortality, also known as ‘secondary kill,’ results from the horizontal transfer of insecticides that occurs when an individual exposed to an insecticide passes the toxicant to other individuals in the population, resulting in death (Silverman et al. 1991; Kopanic Jr and Schal 1997; Durier and Rivault 2000; Buczkowski and Schal 2001a, b; Buczkowski et al. 2008). This often occurs when foraging individuals interact with an insecticidal bait or spray, and return to the aggregation, where they contact others. Baits are particularly effective at causing secondary mortality because large amounts of active ingredient are ingested, and depending on the insecticide, large amounts of undigested insecticide are often defecated, unlike with spray formulations.
Secondary mortality was first reported in the German cockroach, Blattella germanica L. (Blattodea: Ectobiidae), using radiolabeled hydramethylnon (Silverman et al. 1991). Four mechanisms have been shown to facilitate the horizontal transfer of insecticides in the German cockroach, including contact (Durier and Rivault 2000, Buczkowski and Schal 2001a), coprophagy (Silverman et al. 1991; Kopanic Jr and Schal 1997, 1999, Buczkowski et al. 2001), emetophagy (Buczkowski and Schal 2001a), and necrophagy and cannibalism (Gahlhoff Jr et al. 1999, le Patourel 2000, Buczkowski et al. 2008). Only one study reported the horizontal transfer of insecticide beyond secondary mortality. Buczkowski et al. (2008) offered Advion bait, which contained 0.6% indoxacarb, to an adult male, quantified secondary mortality of first instars exposed to the adult male, and then offered the dead nymphs to starved adult males; 81% of the males died (tertiary mortality) largely from eating the dead nymphs. Nymphs exposed to the dead adults had no significant quaternary mortality, at least in part because small nymphs do not engage in necrophagy on adult males nearly as much as adult males do on first instars (Guthrie and Tindall 1968, Gahlhoff Jr et al. 1999).
The overall goal of our study was to quantify the amount of secondary, tertiary, and quaternary mortality obtained from various cockroach gel baits. We hypothesized that all the baits would cause secondary mortality and that the amount of tertiary mortality would vary depending on the type and quantity of active ingredient in the bait. We also hypothesized that there would be low quaternary mortality among the baits. To minimize the impact of cannibalism and necrophagy and instead highlight the role of coprophagy, emetophagy, and contact, we designed a sequence of experiments that examined the transfer of insecticide only among first instar cockroaches.
Materials and Methods
Cockroaches
An insecticide-susceptible strain of B. germanica (Orlando Normal, also known as American Cyanamid, collected in a Florida apartment in 1947) was used in this study. Cockroaches were reared on food pellets (Purina 5001 Rodent Diet, PMI Nutrition International, St. Louis, MO) and were provided water in cotton-stoppered vials. Cockroach colonies were maintained at 27 ± 1°C, 40–70% relative humidity, and a photoperiod of 12:12 (L:D) hr. Adult females were used as donors (primary mortality) because they eat more than any other stage (Cochran 1983, Silverman 1986, Hamilton and Schal 1988), and first instars were used to determine secondary, tertiary, and quaternary mortality because they engage in more coprophagy than other nymphal stages (Silverman et al. 1991, Kopanic Jr and Schal 1999, Kopanic et al. 2001). To promote feeding, food was withheld from cockroaches for 24 hr before the start of assays.
Insecticide Baits
The six commercial gel baits used in these experiments and their active ingredients are listed in Table 1. All baits were purchased from a local distributor.
Table 1.
Primary moribundity and mortality of B. germanica adult females after consuming various baits
| Bait | Active ingredient (%) | Manufacturer | n | Moribundity MST ± SEM (h)a | Moribundity Cox proportional hazards modelb | Mortality MST ± SEM (h)a | Mortality Cox proportional hazards modelb |
|---|---|---|---|---|---|---|---|
| Rodent diet (control) | NA | NA | 100 | >336 | f | >336 | f |
| Alpine Rotation 1 | Dinotefuran (0.5%) | BASF | 100 | 0.6 ± 0.6 | a | 1.9 ± 0.1 | a |
| Maxforce Magnum FC | Fipronil (0.05%) | Bayer | 100 | 3.9 ± 0.1 | c | 6.2 ± 0.5 | b |
| Maxforce Impact | Clothianidin (1.0%) | Bayer | 100 | 2.2 ± 0.1 | b | 15.2 ± 1.9 | c |
| Vendetta Nitro | Clothianidin (0.5%) + pyriproxyfen (0.5%) | MGK | 100 | 2.4 ± 0.3 | b | 17.4 ± 2.4 | c |
| Optigard | Emamectin benzoate (0.1%) | Syngenta | 100 | 23.5 ± 1.8 | e | 30.4 ± 1.3 | d |
| Advion | Indoxacarb (0.6%) | Syngenta | 100 | 12.4 ± 0.6 | d | 44.6 ± 2.4 | e |
a Moribundity and mortality MSTs represent the mean survival time (hr) of the 100 females in each treatment.
b Moribundity and mortality were statistically compared across all treatments (baits) using the Cox proportional hazards model (α < 0.05).
Marking Cockroach Nymphs
To quantify tertiary and quaternary mortality, our design (below) required first instar donors to interact with first instar recipients. Although genetic markers (e.g., orange-body) could be used to distinguish these two groups of nymphs, we marked the new first instars with a blue dye. Groups of first instars were starved for 24 hr and allowed to drink blue-colored sugar water (0.5 mmol/liter erioglaucine disodium salt in 1 mol/liter sucrose) for 2 hr before being introduced into the assay. The ingested, blue-colored water could be seen in the nymph’s gut for more than 4 hr. When the blue-colored nymphs were provided nondyed water, they would lose their blue coloring and a new group of blue-colored nymphs could be added to the assay in the next step.
Experimental Design
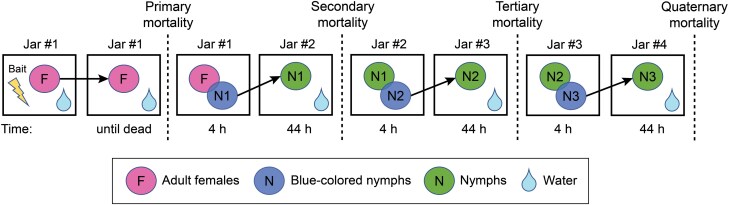
The design for this experiment is illustrated in Fig. 1. No-choice assays were performed in glass jars (88 mm diameter, 95 mm height) the inner walls of which were coated with petroleum jelly to prevent escape and the jars were covered with cheesecloth secured with rubber bands. Ten adult females (3–4-d-old) were placed in glass jar #1 along with ~0.5 g of fresh pre-weighed bait in a petri dish and water in a microcentrifuge tube. The females were kept in this jar until all died; mortality was recorded as primary mortality and the bait was removed. Twenty blue-colored nymphs were then added to jar #1, which contained the dead females, feces, and other residues they excereted. The water was removed for the 4-h assay so that nymphs would retain their blue color. The nymphs were then transferred to a new, clean glass jar (jar #2) with water and kept in this jar for 44 hr. The number of dead nymphs was recorded as secondary mortality. Twenty new blue-colored nymphs were added into jar #2 with no water for 4 hr. This jar contained the dead nymphs from secondary mortality and their feces and other excreted residues. The nymphs were then transferred to a new, clean glass jar (jar #3) with water and were kept in this jar for 44 hr. The number of dead nymphs was recorded as tertiary mortality. This process was repeated until no mortality was observed in the jar. Exposure of nymphs to fecal residues was limited to 4 hr to minimize starvation, ensure their retention of the blue dye, and prevent defecation in the jar. Later, nymphs remained in a clean jar for 44 hr to maximize their excretion of feces.
Fig. 1.
Experimental design demonstrating the horizontal transfer of insecticides. Diagram of the experimental design illustrating primary mortality in adult females fed various gel baits, the horizontal transfer of insecticides from adult females to nymphs (secondary mortality) and from nymphs to nymphs (tertiary and quaternary mortality). Ten adult females fed on a bait, the bait was removed once all the females were dead, and primary mortality was recorded. Twenty blue-dyed first instars were added to the same jar (#1) for 4 hr and then moved to a clean glass jar (#2) and secondary mortality was recorded after 44 hr. By this time, the nymphs had lost their color and 20 new blue-dyed first instar nymphs were added to jar #2 for 4 hr. The new nymphs were moved to jar #3 and tertiary mortality was recorded 44 hr later. The process of adding 20 blue-dyed nymphs to the jar for 4 hr, then moving them to a new jar and recording mortality after 44 hr was repeated until there was no mortality.
Primary Mortality
We assessed primary mortality in adult females in separate assays without nymphs to minimize interference with the overall design described above. We performed no-choice assays in glass jars, as above, but with 20 adult females (3–4 d old). There were 5 replicates per bait or rodent diet. Moribundity and mortality were recorded in each jar at 0.5, 1, 3, 6, 12, 24, 48, 72, and 96 hr. We defined moribundity as showing evidence of intoxication, such as uncoordinated movement or tremors. Bait consumption would cease closer to the time of moribundity than mortality. Mortality was defined as insects unable to right themselves within 30 s of being probed with forceps.
Bait Consumption
To measure consumption of the six commercial gel baits, adult females (4 d old) were starved, but given access to water, for 24 hr before the start of the experiment. Each individual female was weighed three times on a digital balance (1712 MP8, Sartorius, Göttingen, Germany) and the average initial weight was calculated. Each female was then placed in a small polypropylene container (57 mm diameter, 70 mm high; Consolidated Plastics, Stow, OH) with the inner wall smeared with petroleum jelly and provisioned with gel bait in a small vial cap and water in a microcentrifuge tube. The container was then covered with cheesecloth and the cockroach was allowed to feed on the bait. The weight of each individual female was again recorded three times 1 and 2 hr later, and the respective averages were calculated. The amount of bait consumed was calculated by subtracting the average initial weight of the female from the average weight of the female at 1 or 2 hr. There were 20 replicates (20 adult females) per treatment. Based on the average amount of bait ingested by each B. germanica female in 1 h, and the percentage active ingredient in each bait, we calculated the amount of each active ingredient ingested by adult females (Table 2).
Table 2.
Relation among the amount of active ingredient ingested in 1 hr by B. germanica adult females and bait toxicity
| Active ingredient | Bait | % active ingredient | Mean AI ingested (µg) ± SEMa | Statistical analysis of AI ingesteda | Topical LD50 (µg/male) | Fold AI (ingested/topical LD50) |
|---|---|---|---|---|---|---|
| Dinotefuran | Alpine Rotation 1 | 0.5 | 16.85 ± 3.09 | bc | NA | NA |
| Fipronil | Maxforce FC Magnum | 0.05 | 1.08 ± 0.13 | d | 0.0020b | 550 |
| Clothianidin | Maxforce Impact | 1.0 | 23.60 ± 2.91 | b | 0.0199c | 1,186 |
| Clothianidin | Vendetta Nitro | 0.5 | 9.25 ± 1.28 | cd | 0.0199c | 465 |
| Emamectin benzoate | Optigard | 0.1 | 5.71 ± 0.69 | d | NA | NA |
| Indoxacarb | Advion | 0.6 | 46.62 ± 4.16 | a | 0.1746d | 267 |
NA = not available
a Letters associated with each mean represent the results of ANOVA followed by Tukey’s HSD test. Means that do not share letters are significantly different from each other (P < 0.05).
b Value represents the mean of 17 studies between 1997 and 2022, ranging from 0.96 to 5.31 ng/male: Kaakeh et al. (1997), Scott and Wen (1997), Buczkowski and Schal (2001a), Holbrook et al. (2003), Wang et al. (2004), Kristensen et al. (2005), Nasirian et al. (2006), Wang et al. (2006), Chai and Lee (2010), Gondhalekar et al. (2011), Ang et al. (2013), Ko et al. (2016b), Liang et al. (2017), Wu and Appel (2017), DeVries et al. (2019), Gonzalez-Morales et al. (2022), Lee et al. (2022). For values reported as µg/g body mass with no data on body mass, we assumed a male body mass of 50 mg to convert to µg/male.
c Value from Lee et al. (2022).
d Value represents the mean of 4 studies between 2010 and 2022, ranging from 79.8 to 318.6 ng/male: Chai and Lee (2010), Gondhalekar et al. (2011), Ko et al. (2016b), Lee et al. (2022).
Statistical Analysis
Data analyses were performed using JMP Pro 16.0 software (SAS Institute 2021). Survival analysis was performed to compare moribundity times and mortality times of adult females fed various baits. The Cox proportional hazards model was used to compare different treatments. When possible, the mean survival times (MSTs ± SEM) were estimated. Percentages of secondary, tertiary, and quaternary mortality were arcsine-square root transformed and one-way analysis of variance (ANOVA) was used to compare mortality across the baits. Each ANOVA was followed by Tukey’s honestly significant difference (HSD) test (α = 0.05) to identify differences in mortality among the baits. Bait consumption (mg bait ingested) was checked for normality (Shapiro-Wilk) and analyzed by ANOVA and Tukey’s HSD tests. Means ± SEM are reported.
Results
Primary Moribundity and Mortality
All six gel baits caused 100% mortality in adult females that fed on the baits and there was no mortality in the rodent diet control group. The bait containing dinotefuran (Alpine Rotation 1) caused 100% mortality within 6 hr, fipronil bait (Maxforce FC Magnum) caused 100% mortality in 24 hr, clothianidin baits (Maxforce Impact and Vendetta Nitro) caused 100% mortality in 72–96 hr, and baits containing indoxacarb (Advion) and emamectin benzoate (Optigard) caused 100% mortality in 96 hr (Fig. 2B). The Cox proportional hazards model indicated significant differences in the estimated MST of adult females among some of the treatment groups (Table 1).
Fig. 2.
Primary moribundity and mortality of adult females, represented by survival plots. Time-course of (A) moribundity over 24 hr and (B) mortality over 96 hr of adult females feeding on gel baits or rodent diet (control). There were 5 replicates per treatment with n = 20 females per replicate. Thus, each survival plot consists of 100 females. Moribundity and mortality were subjected to survival analysis; MSTs ± SEM and statistical analyses are shown in Table 1.
We also examined moribundity of adult females because bait consumption would cease closer to the moribundity than mortality times. Most females showed evidence of intoxication within 1–4 hr (Fig. 2A, Table 1). Indoxacarb and emamectin benzoate were significantly slower, suggesting that some bait consumption could occur beyond 24 hr.
Bait Consumption by Adult Females
Goodness of fit tests (Shapiro-Wilk) indicated that the distributions of bait consumption across all the baits and at both time points (n = 20 for each bait-time combination) did not depart from normal distributions. The amount of bait consumed by adult females at 1 hr varied significantly among the gel baits and ranged from 1.85 to 7.77 mg (ANOVA, F5,114 = 22.1198, P < 0.0001); minimal consumption occurred between the first and second h, with cumulative consumption ranging from 1.85 to 7.96 mg (Fig. 3). Overall, the indoxacarb and emamectin benzoate baits were consumed significantly more than the other four baits, which were not significantly different from each other (Tukey’s HSD test, P > 0.05). These results suggest that the indoxacarb and emamectin benzoate baits were the most palatable and the baits containing fipronil and clothianidin (and pyriproxyfen) were least palatable.
Fig. 3.
Bait consumption of adult females. Mean (± SEM) amount of various gel baits consumed by adult females in 1 and 2 hr. There were 20 replicates per treatment (n = 20 females per replicate). Different letters above the bars indicate significant differences among the treatments (ANOVA, Tukey’s HSD test, P < 0.05).
We calculated the amount of each active ingredient ingested by adult females (Table 2). There were significant differences among the six baits (ANOVA, F5,114 = 43.6908, P < 0.0001). Significantly more indoxacarb (Advion) was ingested than any other active ingredient (Tukey’s HSD test, P < 0.001), followed by clothianidin (Maxforce Impact), dinotefuron (Alpine), clothianidin (Vendetta Nitro), emamectin benzoate (Optigard), and fipronil (Maxforce FC Magnum) (Table 2).
Secondary, Tertiary, and Quaternary Mortality
There was high secondary mortality with all the baits (Fig. 4A). All first instars exposed to adult females that fed on dinotefuran- or fipronil-containing baits died within 48 hr. Secondary mortality of first instars on other baits ranged from 85 to 92%. There was no mortality in the control groups of first instars exposed to females that fed on rodent diet. Thus, all the baits caused significantly more secondary mortality than observed in the control (ANOVA, F6,28 = 62.779, P < 0.0001; Tukey’s HSD test), and only the Vendetta Nitro (clothianidin) and Advion (indoxacarb) baits caused significantly lower secondary mortality than Alpine Rotation 1 (dinotefuran) and Maxforce FC Magnum (fipronil) (Fig. 4A).
Fig. 4.
Mortality of first instar nymphs. Mean (±SEM) percent (A) secondary, (B) tertiary, and (C) quaternary mortality of first instar nymphs. Each bar represents a different gel bait (n = 20 nymphs per replicate, 5 replicates per treatment). Different letters above the bars indicate significant differences among the treatments within each panel (ANOVA, Tukey’s HSD test, P < 0.05). Statistical analyses were conducted on arcsine-square root-transformed percentages, but figures show the untransformed percentages.
Tertiary mortality was defined as mortality in the second cohort of nymphs that were exposed to the first cohort of nymphs that were previously exposed to adult females that fed on a bait and died (Fig. 1). The level of tertiary mortality varied significantly across the six baits (ANOVA, F6,28 = 18.758, P <0.0001) (Fig. 4B). On average, the fipronil bait caused 70% tertiary mortality of first instars, indoxacarb bait, and emamectin benzoate bait caused 34 and 32% mortality, respectively, and the dinotefuran bait caused 15% of the nymphs to die. There was no tertiary mortality in the clothianidin bait treatment nor in the Vendetta Nitro bait that contained both clothianidin and pyriproxyfen. All the nymphs in the control group survived. Tertiary mortality was highest in the fipronil bait, followed by indoxacarb, emamectin benzoate, and dinutefuron baits (Tukey’s HSD test, P < 0.05) (Fig. 4B).
There was low quaternary mortality (Fig. 4C), with only 15.0 ± 5.2% (SEM) mortality in the indoxacarb bait treatment, 5.0 ± 2.7% in the fipronil bait treatment, and no mortality in the other bait treatments and the rodent diet control. The indoxacarb bait caused significantly more quaternary mortality than the control and the other baits (ANOVA, F6,28 = 7.9419, P < 0.0001; Tukey’s HSD test, P = 0.0004), but not the fipronil bait (P = 0.1652).
Discussion
Experimental Design
The experimental design of this study is the first of its kind used to investigate the transfer of insecticides within cockroach aggregations. It was used to maximize the amount of insecticide-contaminated material (feces, regurgitated liquids, and dead bodies) that cockroaches could contact, and focused on interactions between adults and nymphs and between groups of nymphs. German cockroach females were offered various baits (primary mortality) and used to deliver active ingredient to nymphs. Nongravid females tend to eat more than males (Cochran 1983, Silverman 1986, Hamilton and Schal 1988), so that more active ingredient can be excreted that nymphs could then contact (Silverman et al. 1991). First instars were used as targets of secondary, tertiary, and quaternary mortality, because they forage outside their aggregation less frequently than other stages (Sommer 1975, Cloarec and Rivault 1991, Demark et al. 1993), and readily ingest adult excretions (Silverman et al. 1991, Kopanic Jr and Schal 1997, Buczkowski and Schal 2001a), increasing their exposure to insecticides.
Our design was substantially different from a previous study that used indoxacarb bait, where tertiary mortality was demonstrated by allowing adult males to contact and feed on dead nymphs for 72 hr, which underscored the role of necrophagy (Buczkowski et al. 2008). The latter design showed significant tertiary mortality compared to the control (no mortality), but no quaternary mortality. We demonstrated mortality at the secondary, tertiary, and quaternary levels for different gel baits. Overall, we found that all baits caused 100% mortality of the adult females (primary mortality) and an average of ≥85% secondary mortality of first instars. Four of the baits caused tertiary mortality at an average level of 15–70%, but only the indoxacarb bait resulted in quaternary mortality (15%).
It is important to note that these and related experiments were conducted in small arenas that maximized contact with the insecticides. A major caveat is that the processes and mechanisms we discuss below are expected to be different in larger arenas and in the field.
Mechanisms of Horizontal Transfer of Insecticides
The association of neonates with older conspecifics is common in cockroaches, and it ranges from short-term contact with the mother (brooding) to long-term membership in aggregations (Gautier et al. 1988, Nalepa and Bell 1997, Nalepa et al. 2001). Four major nonmutually exclusive mechanisms have been shown to facilitate the horizontal transfer of insecticides in German cockroach aggregations: ingestion of feces (coprophagy) and various other excretions (e.g., emetophagy), contact with dead or dying cockroaches and their excretions, necrophagy, and cannibalism.
Cannibalism is not common in cockroaches and tends to occur under stressful conditions, such as elevated temperature, high population density, and limited resources (Guthrie and Tindall 1968). Some cannibalism may occur in all stages and both sexes of the German cockroach even in the presence of sufficient food, water, and harborage (Aparicio 1996), but it is not common in this species. Nevertheless, cannibalism can be a major factor in a carefully manipulated experimental design – starved adult males readily cannibalized indoxacarb-intoxicated first instars, resulting in tertiary transfer of the insecticide and significant mortality (Buczkowski et al. 2008).
Necrophagy appears to be important in the nutritional ecology of cockroaches, with crop contents of cockroaches in natural forest populations found to contain arthropod cuticle (Schal et al. 1984), presumably from scavenged dead insects and related organisms. Necrophagy occurs with both fast- and slow-acting insecticides and various life stages (Gahlhoff Jr et al. 1999, Durier and Rivault 2000).
Coprophagy is common in insects and it provides two major adaptive benefits: (1) inoculation with mutualistic microbes that assist in digestion, immune responses, and behavior, and (2) nutritional benefits from ingesting undigested food, enzymes, and other metabolites (Stevenson and Dindal 1987, Nalepa et al. 2001, Weiss 2006). Coprophagy and emetophagy have been documented as prominent mechanisms in horizontal transfer of baits in cockroaches. Using radiolabeled hydramethylnon, Silverman et al. (1991) documented that nymphs readily ingest the feces of adults that fed on hydramethylnon-containing baits. Other studies documented the importance of coprophagy in both small and large (>1 m) arenas using various exclusion methods to prevent either adults or first instars from directly consuming the bait (Kopanic Jr and Schal 1999). Coprophagy was shown to be especially adaptive to first instars that forage less and can develop into second instars solely on adult feces (Kopanic et al. 2001). Nevertheless, the relative importance of coprophagy varied across different baits with various active ingredients (Silverman et al. 1991; Kopanic Jr and Schal 1997, 1999; Buczkowski et al. 2001). Emetophagy was first reported with fipronil, where cockroaches that ingested bait were found to exude liquid excretions that appeared attractive and lethal to conspecifics within the aggregation (Buczkowski and Schal 2001a). This behavior could also occur with other neurotoxic insecticides, including relatively slow-acting indoxacarb (Buczkowski et al. 2008).
Many experimental designs, including ours, cannot differentiate among the various mechanisms of horizontal transfer of insecticides. Cannibalism and necrophagy are often difficult to separate without real-time monitoring (e.g., video records), and emetophagy likewise requires careful real-time observations because the secretions of dying individuals quickly dry out. Contact is a component of all these mechanisms, and its relevance in insecticide transfer depends largely on characteristics of the active ingredient (Buczkowski and Schal 2001b, Buczkowski et al. 2001). For example, some insecticides, like hydramethylnon (Silverman et al. 1991), have little activity through contact, while most neurotoxic insecticides are effective by contact. Thus, in some cases, the presence of insecticide-contaminated dead cockroaches can cause mortality mainly through contact rather than by necrophagy or coprophagy (Durier and Rivault 2000).
Properties of Ingested Insecticides and Their Effects on Horizontal Transfer
Effective bait formulations must be attractive, palatable, easy to consume, slow-acting compared to most contact insecticides, and toxic to cockroaches in the amounts consumed (Appel and Rust 2021). Although fast-acting contact insecticides are generally thought to be more repellent than slow-acting toxicants (Ebeling et al. 1966), and the latter provide greater cockroach population reductions in field trials (Milio et al. 1986, Appel 1992, Appel and Benson 1995), this generalization appears to have been made for pyrethroids and older insecticide classes that are no longer used in indoor baits. Fast-acting insecticides used in modern bait formulations are often highly palatable and not repellent (Appel and Rust 2021). In most investigations of secondary mortality, including ours, repellency of the active ingredient likely played a minor role, if any, because cockroaches are usually restricted to small arenas and starved before the assay begins to maximize bait consumption. Therefore, assuming no repellency of the bait, three major features would significantly impact secondary mortality under these conditions: palatability and amount of bait ingested, transit time of the bait through the digestive system, and the speed-of-action of the insecticide. By starving adult females for 24 hr, we maximized ingestion of all baits, thus maximizing the amount of active ingredient available to be transferred in the arena. Our bait consumption experiments demonstrated that the majority of bait intake occurred during the first hr of the experiment (most likely during the first few minutes), before the onset of symptoms from most of the insecticides. The largest bait consumption occurred with the indoxacarb bait and the emamectin benzoate bait, both relatively slower-acting baits, which presumably allowed cockroaches more time to feed on the baits. The bait containing dinotefuron, the fastest acting insecticide, was consumed at intermediate levels, but not significantly different from the rest of the baits. Although the amount of bait consumed varied (Fig. 3), there were no major differences in the secondary mortality caused by these baits. These findings suggest that under these experimental conditions, modern bait formulations require relatively lower amounts of bait to be consumed to cause secondary mortality than older formulations that used organophosphates and carbamates.
As mentioned before, these results will likely differ in the field due to the availability of alternative food sources, larger foraging areas, and unique characteristics of insecticide-resistant populations (Wang et al. 2008). Previous studies have shown that the secondary kill properties of bait formulations are considerably affected by availability of alternative foods, the palatability of food and baits, and insecticide resistance, which can affect the ingestion of bait, survival, and the amount of feces produced (Ko et al. 2016a). At the tertiary and quaternary levels, bait consumption may be more important because nymphs are being exposed to lower amounts of active ingredient in the feces, liquids, or dead bodies of the insects that fed on the feces of adults that fed on the bait. The two baits that were consumed the most, the indoxacarb bait and the emamectin benzoate bait, caused 34 and 32% tertiary mortality, respectively (Fig. 4B). However, the fipronil bait caused significantly more tertiary mortality despite much less of it being consumed, which could be due to the insecticidal properties of the active ingredient. The fipronil bait also caused some quaternary mortality, although it was not significantly different from the amount of quaternary mortality caused by the indoxacarb bait, which was consumed more. These results show that while bait formulations must be palatable to effectively transfer enough insecticide, it is also important to consider the speed of action of the active ingredient. A highly palatable bait that contains a slow-acting active ingredient, like the indoxacarb bait, would give the foraging insect more time to return to the aggregation and defecate more material. In the case of the fipronil bait, although it was less palatable (i.e., less of it was ingested) than the indoxacarb and ememectin benzoate baits, we observed mortality at the secondary, tertiary, and quaternary levels because of the highly effective active ingredient. Although the features of the two baits were different, both caused high levels of mortality in our assays.
The transit time through the gut and the insecticide’s speed of action are closely related. Relatively faster-acting insecticides might interfere with secondary mortality for two reasons. First, the intoxicated cockroaches might not be able to return to the aggregation after they ingested the bait (Stejskal et al. 2004). Although this might be a significant concern in real-world situations and in large arenas, return to the harborage was not a component of our assays in small containers. Second, fast-acting insecticides might kill the insect before the bait transits through the alimentary canal, and thus compromise secondary mortality through coprophagy. For example, the transfer of insecticides through coprophagy was less effective with fast-acting insecticide baits that failed to traverse the alimentary tract before the forager died (Kopanic Jr and Schal 1999), but was effective with delayed action insecticides like hydramethylnon (Silverman et al. 1991; Buczkowski and Schal 2001a, b). Thus, residues of hydramethylnon killed most nymphs and adults in secondary mortality assays, whereas residues of fast-acting insecticides (like chlorpyrifos and fipronil) killed fewer cockroaches (Buczkowski and Schal 2001b).
In our assays, all six baits caused significant secondary mortality, but the level of tertiary and quaternary mortality varied with the amount ingested, mode of action, and speed of kill of the active ingredients. Alpine bait (dinotefuran), which caused rapid moribundity and mortality (MSTs of 0.6 and 1.9 hr for moribundity and mortality, respectively; Table 1), resulted in low tertiary mortality, consistent with the idea that fast-acting insecticides are less effective in horizontal transfer. On the other hand, Maxforce FC Magnum (fipronil), also a fast-acting insecticide (MSTs of 3.9 and 6.2 hr for moribundity and mortality, respectively) compared to indoxacarb, had the highest tertiary mortality among the six baits. Clothianidin-containing baits (Vendetta Nitro and Maxforce Impact), which were slower-acting insecticides (MSTs of 2.2–2.4 and 15.2–17.4 hr for moribundity and mortality, respectively), presumably giving females more time to eat and defecate active ingredient, resulted in no discernible tertiary mortality. Finally, the slowest acting baits, Optigard (emamectin benzoate) and Advion (indoxacarb), with MST values of 23.5 and 12.4 hr for moribundity and 30.4 and 44.6 hr for mortality, resulted in similar levels of intermediate tertiary mortality; Advion, however, had the highest quaternary mortality of any bait. Overall, these results suggest a complex relationship between the palatability, speed-of-kill, and horizontal transfer of an insecticide. In assays in small containers, as in our assays, foraging distance is minimal, and transit time of the bait through the digestive system is probably more important than other variables listed above. However, insecticides with exceptionally low LD50s by both contact and ingestion (e.g., Maxforce FC Magnum [fipronil]) (Ko et al. 2016b) can be effectively transferred among cockroaches and cause secondary mortality and beyond, even if they are relatively fast acting. Table 2 shows that given the relatively low amount of Maxforce FC Magnum bait ingested (2.15 mg), each adult female ingested ~1.08 µg of fipronil, which is ~550-fold the topical LD50 of an adult male. Similar extrapolations with indoxacarb (Advion), the slowest of the insecticides we tested, estimate that the 7.77 mg of bait ingested by each female delivered ~46.62 µg of indoxacarb per female, which is 267-fold the topical LD50 of an adult male (Table 2). Although these two insecticide baits differ in various other features, they were nevertheless highly effective in our secondary and tertiary assays and the only baits with some quaternary effects with insecticide-susceptible cockroaches. While the performance of both baits can be compromised by physiological and behavioral resistance, >3-fold more Advion bait was consumed than Maxforce FC Magnum bait, suggesting that the palatability and thus performance of the fipronil bait, including its horizontal transfer, could be vastly improved. It is also important to note that whereas clothianidin from Maxforce Impact was ingested at 1,186-fold its LD50 value, we found no tertiary or quaternary effects with this bait.
Perspectives
Two related topics need to be considered in future investigations to understand the magnitude and significance of horizontal transfer of baits among cockroaches – there is a need to quantify secondary mortality in residential settings, and to consider how insecticide resistance might impede this process. Thus far, the only attempt to estimate secondary mortality in the field was a study of lab-reared cockroaches released into a vacant apartment. Using a reciprocal exclusion design, whereby adults or first instars were prevented from ingesting insecticide bait, Kopanic Jr and Schal (1999) demonstrated that horizontal transfer of insecticide to nymphs played a significant role in neonate mortality. We suspect that secondary effects might be substantial in the field, because small nymphs forage only short distances, and the relative magnitude of horizontal transfer of bait insecticides (compared to direct ingestion of bait) is positively related to distance of the bait from the aggregation (Silverman 1986, Kopanic Jr and Schal 1999). Unfortunately, thus far there has not been a satisfactory method to quantify secondary effects in the field. A promising approach could be the use of UV light to track insecticide-laden feces. Laboratory experiments revealed that fluorescent bait was accepted by cockroaches as a food source, feces produced after its consumption were also fluorescent, and consumption of fluorescent bait led to continuous fluorescent feces production, giving cockroaches enough time to deposit fluorescent feces within aggregations (Varadínová et al. 2015).
The transfer of insecticide from foragers to first instars undergoes a considerable dilution factor, in part related to insecticide degradation in the digestive system. Thus, high levels of insecticide resistance might lessen the significance of insecticide transfer within aggregations. Ko et al. (2016b) used nymphs of two color-morphs to distinguish unselected insecticide-susceptible nymphs from insecticide-resistant nymphs; this design allowed both strains to co-habit the same arena, which equally exposed them to adult feces. Nymphs that were multiresistant to fipronil, indoxacarb, and hydramethylnon survived significantly longer than insecticide-susceptible nymphs on residues of bait-fed males, demonstrating that resistance significantly lessened secondary mortality (Ko et al. 2016b). These findings raise an important question: Does the translocation of active ingredients expose cockroaches to moderate and sublethal doses that could accelerate the evolution of insecticide resistance and thus negate the benefits of secondary mortality in population suppression?
Overall, the magnitude of secondary and tertiary mortality that can be attained with different insecticides will depend on many factors, including the concentration of the active ingredient in the bait, stability of the active ingredient in the insect’s digestive tract, relative toxicity of the active ingredient, amount of the active ingredient excreted, amount of the active ingredient bioavailable after excretion, mechanisms of translocation, and insecticide resistance (Buczkowski and Schal 2001a, b). By understanding how these factors interact and by designing baits that incorporate life history traits and adaptations of the German cockroach, we could potentially develop gel baits that can attain higher levels of mortality to suppress cockroach populations.
Acknowledgments
We would like to thank Rick Santangelo for maintaining the cockroach colonies, Eduardo Hatano for help with statistical analyses, and Bayer CropScience for partial support of this study. Additional support for this study was provided by a fellowship from the NIH Initiative for Maximizing Student Diversity (IMSD) program at North Carolina State University, the National Science Foundation Graduate Research Fellowship Program (DGE-1746939), the Blanton J. Whitmire Endowment at North Carolina State University, and a grant from the US Department of Housing and Urban Development Healthy Homes program (NCHHU0053-19).
Contributor Information
Jamora A Hamilton, Department of Entomology and Plant Pathology, North Carolina State University, Raleigh NC 27695, USA.
Ayako Wada-Katsumata, Department of Entomology and Plant Pathology, North Carolina State University, Raleigh NC 27695, USA.
Coby Schal, Department of Entomology and Plant Pathology, North Carolina State University, Raleigh NC 27695, USA.
References Cited
- Ang, L. -H., W. A. Nazni, M. -K. Kuah, A. C. Shu-Chien, and C. -Y. Lee. . 2013. Detection of the A302S rdl mutation in fipronil bait-selected strains of the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 106: 2167–2176. doi: 10.1603/ec13119 [DOI] [PubMed] [Google Scholar]
- Aparicio, M. L. 1996. Cannibalistic responses of the German cockroach, Blattella germanica (L.), to differential density and developmental stage of conspecifics, pp. 88. University of Florida. [Google Scholar]
- Appel, A. G. 1992. Performance of gel and paste bait products for German cockroach (Dictyoptera: Blattellidae) control: laboratory and field studies. J. Econ. Entomol. 85: 1176–1183. doi: 10.1093/jee/85.4.1176 [DOI] [PubMed] [Google Scholar]
- Appel, A. G. 2003. Laboratory and field performance of an indoxacarb bait against German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 96: 863–870. doi: 10.1093/jee/96.3.863 [DOI] [PubMed] [Google Scholar]
- Appel, A. G., and E. P. Benson. . 1995. Performance of abamectin bait formulations against German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 88: 924–931. doi: 10.1093/jee/88.4.924 [DOI] [PubMed] [Google Scholar]
- Appel, A. G., and M. K. Rust. . 2021. Management using baits, pp. 213–230. In C. Wang, C.-Y. Lee and M. K. Rust (eds.), Biology and management of the German cockroach. CSIRO Publishing, Australia. [Google Scholar]
- Buczkowski, G., and C. Schal. . 2001a. Emetophagy: fipronil-induced regurgitation of bait and its dissemination from German cockroach adults to nymphs. Pestic. Biochem. Physiol. 71: 147–155. doi: 10.1006/pest.2001.2572 [DOI] [Google Scholar]
- Buczkowski, G., and C. Schal. . 2001b. Method of insecticide delivery affects horizontal transfer of fipronil in the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 94: 680–685. doi: 10.1603/0022-0493-94.3.680 [DOI] [PubMed] [Google Scholar]
- Buczkowski, G., R. J. Kopanic, and C. Schal. . 2001. Transfer of ingested insecticides among cockroaches: effects of active ingredient, bait formulation, and assay procedures. J. Econ. Entomol. 94: 1229–1236. doi: 10.1603/0022-0493-94.5.1229 [DOI] [PubMed] [Google Scholar]
- Buczkowski, G., C. W. Scherer, and G. W. Bennett. . 2008. Horizontal transfer of bait in the German cockroach: indoxacarb causes secondary and tertiary mortality. J. Econ. Entomol. 101: 894–901. doi: 10.1093/jee/101.3.894 [DOI] [PubMed] [Google Scholar]
- Chai, R. -Y., and C. -Y. Lee. . 2010. Insecticide resistance profiles and synergism in field populations of the German cockroach (Dictyoptera: Blattellidae) from Singapore. J. Econ. Entomol. 103: 460–471. doi: 10.1603/ec09284 [DOI] [PubMed] [Google Scholar]
- Cloarec, A., and C. Rivault. . 1991. Age-related changes in foraging in the German cockroach (Dictyoptera: Blattellidae). J. Insect Behav. 4: 661–673. doi: 10.1007/bf01048077 [DOI] [Google Scholar]
- Cochran, D. G. 1983. Food and water consumption during the reproductive cycle of female German cockroaches. Entomol. Exp. Appl. 34: 51–57. doi: 10.1111/j.1570-7458.1983.tb03289.x [DOI] [Google Scholar]
- Demark, J. J., T. Kuczek, and G. W. Bennett. . 1993. Laboratory analysis of the foraging efficiency of nymphal German cockroaches (Dictyoptera: Blattellidae) between resource sites in an experimental arena. Ann. Entomol. Soc. Am. 86: 372–378. doi: 10.1093/aesa/86.3.372 [DOI] [Google Scholar]
- DeVries, Z. C., R. G. Santangelo, J. Crissman, A. Suazo, M. L. Kakumanu, and C. Schal. . 2019. Pervasive resistance to pyrethroids in German cockroaches (Blattodea: Ectobiidae) related to lack of efficacy of total release foggers. J. Econ. Entomol. 112: 2295–2301. doi: 10.1093/jee/toz120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durier, V., and C. Rivault. . 2000. Secondary transmission of toxic baits in German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 93: 434–440. doi: 10.1603/0022-0493-93.2.434 [DOI] [PubMed] [Google Scholar]
- Ebeling, W., R. Wagner, and D. A. Reierson. . 1966. Influence of repellency on the efficacy of blatticides. I. Learned modification of behavior of the German cockroach. J. Econ. Entomol. 59: 1374–1388. [DOI] [PubMed] [Google Scholar]
- Gahlhoff Jr, J., D. Miller, and P. Koehler. . 1999. Secondary kill of adult male German cockroaches (Dictyoptera: Blattellidae) via cannibalism of nymphs fed toxic baits. J. Econ. Entomol. 92: 1133–1137. [Google Scholar]
- Gautier, J.-Y., P. Deleporte, and C. Rivault. . 1988. Relationships between ecology and social behavior in cockroaches, pp. 335–351. In C. N. Slobodchikoff (ed.), The ecology of social behavior. Academic Press. [Google Scholar]
- Gondhalekar, A. D., C. Song, and M. E. Scharf. . 2011. Development of strategies for monitoring indoxacarb and gel bait susceptibility in the German cockroach (Blattodea: Blattellidae). Pest Manag. Sci. 67: 262–270. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Morales, M. A., Z. C. DeVries, R. G. Santangelo, M. L. Kakumanu, and C. Schal. . 2022. Multiple mechanisms confer fipronil resistance in the German cockroach: enhanced detoxification and Rdl mutation. J. Med. Entomol. 59: 1721–1731. doi: 10.1093/jme/tjac100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, D., and A. Tindall. . 1968. The biology of the cockroach, St. Martin’s, New York. [Google Scholar]
- Hamilton, R. L., and C. Schal. . 1988. Effects of dietary protein levels on reproduction and food consumption in the German cockroach (Dictyoptera: Blattellidae). Ann. Entomol. Soc. Am. 81: 969–976. doi: 10.1093/aesa/81.6.969 [DOI] [Google Scholar]
- Holbrook, G., J. Roebuck, C. Moore, M. Waldvogel, and C. Schal. . 2003. Origin and extent of resistance to fipronil in the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae). J. Econ. Entomol. 96: 1548–1558. [DOI] [PubMed] [Google Scholar]
- Kaakeh, W., B. L. Reid, and G. W. Bennett. . 1997. Toxicity of fipronil to German and American cockroaches. Entomol. Exp. Appl. 84: 229–237. doi: 10.1046/j.1570-7458.1997.00220.x [DOI] [Google Scholar]
- Ko, A. E., C. Schal, and J. Silverman. . 2016a. Diet quality affects bait performance in German cockroaches (Dictyoptera: Blattellidae). Pest Manag. Sci. 72: 1826–1836. doi: 10.1002/ps.4295 [DOI] [PubMed] [Google Scholar]
- Ko, A. E., D. N. Bieman, C. Schal, and J. Silverman. . 2016b. Insecticide resistance and diminished secondary kill performance of bait formulations against German cockroaches (Dictyoptera: Blattellidae). Pest Manag. Sci. 72: 1778–1784. doi: 10.1002/ps.4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanic, R. J., G. L. Holbrook, V. Sevala, and A. C. Schal. . 2001. An adaptive benefit of facultative coprophagy in the German cockroach Blattella germanica. Ecol. Entomol. 26: 154–162. doi: 10.1046/j.1365-2311.2001.00316.x [DOI] [Google Scholar]
- Kopanic Jr, R. J., and C. Schal. . 1997. Relative significance of direct ingestion and adult-mediated translocation of bait to German cockroach (Dictyoptera: Blattellidae) nymphs. J. Econ. Entomol. 90: 1073–1079. [Google Scholar]
- Kopanic Jr, R. J., and C. Schal. . 1999. Coprophagy facilitates horizontal transmission of bait among cockroaches (Dictyoptera: Blattellidae). Environ. Entomol. 28: 431–438. [Google Scholar]
- Kristensen, M., K. K. Hansen, and K. V. Jensen. . 2005. Cross-resistance between dieldrin and fipronil in German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 98: 1305–1310. [DOI] [PubMed] [Google Scholar]
- Lee, S. H., D. H. Choe, M. K. Rust, and C. Y. Lee. . 2022. Reduced susceptibility towards commercial bait insecticides in field German cockroach (Blattodea: Ectobiidae) populations from California. J. Econ. Entomol. 115: 259–265. [DOI] [PubMed] [Google Scholar]
- le Patourel, G. 2000. Secondary transmission of fipronil toxicity between oriental cockroaches Blatta orientalis L in arenas. Pest Manag. Sci. 56: 732–736. doi: [DOI] [Google Scholar]
- Liang, D., J. McGill, and J. E. Pietri. . 2017. Unidirectional cross-resistance in German cockroach (Blattodea: Blattellidae) populations under exposure to insecticidal baits. J. Econ. Entomol. 110: 1713–1718. doi: 10.1093/jee/tox144 [DOI] [PubMed] [Google Scholar]
- Milio, J., P. Koehler, and R. Patterson. . 1986. Laboratory and field evaluations of hydramethylnon bait formulations for control of American and German cockroaches (Orthoptera: Blattellidae). J. Econ. Entomol. 79: 1280–1286. [DOI] [PubMed] [Google Scholar]
- Nalepa, C. A., and W. J. Bell. . 1997. Postovulation parental investment and parental care in cockroaches, pp. 26–51. In B. J. Crespi and J. C. Choe (eds.), The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge. [Google Scholar]
- Nalepa, C., D. Bignell, and C. Bandi. . 2001. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Soc. 48: 194–201. doi: 10.1007/pl00001767 [DOI] [Google Scholar]
- Nasirian, H., H. Ladonni, M. Shayeghi, H. Vatandoost, M. R. Yaghoobi-Ershadi, Y. Rassi, M. Abolhassani, and M. R. Abaei. . 2006. Comparison of permethrin and fipronil toxicity against German cockroach (Dictyoptera: Blattellidae) strains. Iran. J. Public Health. 35: 63–67. [Google Scholar]
- SAS Institute. 2021. JMP pro computer program, version 16.0.0. SAS Institute, Cary, NC, USA. [Google Scholar]
- Schal, C. 2011. Cockroaches, pp. 150–290. In S. A. Hedges and D. Moreland (eds.), Handbook of pest control, 10th ed. Mallis Handbook LLC, Cleveland, Ohio. [Google Scholar]
- Schal, C., J. Y. Gautier, and W. J. Bell. . 1984. Behavioural ecology of cockroaches. Biol. Rev. 59: 209–254. doi: 10.1111/j.1469-185x.1984.tb00408.x [DOI] [Google Scholar]
- Scott, J. G., and Z. Wen. . 1997. Toxicity of fipronil to susceptible and resistant strains of German cockroaches (Dictyoptera: Blattellidae) and house flies (Diptera: Muscidae). J. Econ. Entomol. 90: 1152–1156. doi: 10.1093/jee/90.5.1152 [DOI] [Google Scholar]
- Silverman, J. 1986. Adult German cockroach (Orthoptera: Blattellidae) feeding and drinking behavior as a function of density and harborage-to-resource distance. Environ. Entomol. 15: 198–204. doi: 10.1093/ee/15.1.198 [DOI] [Google Scholar]
- Silverman, J., G. Vitale, and T. Shapas. . 1991. Hydramethylnon uptake by Blattella germanica (Orthoptera: Blattellidae) by coprophagy. J. Econ. Entomol. 84: 176–180. [DOI] [PubMed] [Google Scholar]
- Sommer, S. 1975. Experimental investigation of circadian locomotor activity of Blattella germanica L. (Dictyoptera, Blattellidae). Biol. Zentralbl. 94: 455–467. [Google Scholar]
- Stejskal, V., J. Lukas, and R. Aulicky. . 2004. Speed of action of 10 commercial insecticidal gel-baits against the German cockroach, Blattella germanica. Int. Pest Control. 46: 185–189. [Google Scholar]
- Stevenson, B., and D. Dindal. . 1987. Functional ecology of coprophagous insects: a review. Pedobiologia. 30: 285–298. [Google Scholar]
- Varadínová, Z., D. Frynta, R. Aulický, and V. Stejskal. . 2015. Detection of cockroach faeces: consumption of fluorescent bait and production of UV‐light‐detectable faeces from G erman cockroach, Blattella germanica. Entomol. Exp. Appl. 155: 167–175. [Google Scholar]
- Wang, C., M. E. Scharf, and G. W. Bennett. . 2004. Behavioral and physiological resistance of the German cockroach to gel baits (Blattodea: Blattellidae). J. Econ. Entomol. 97: 2067–2072. doi: 10.1093/jee/97.6.2067 [DOI] [PubMed] [Google Scholar]
- Wang, C. L., M. E. Scharf, and G. W. Bennett. . 2006. Genetic basis for resistance to gel baits, fipronil, and sugar-based attractants in German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 99: 1761–1767. [DOI] [PubMed] [Google Scholar]
- Wang, C., X. Yang, M. A. El-Nour, and G. W. Bennett. . 2008. Factors affecting secondary kill of the German cockroach (Dictyoptera: Blattellidae) by gel baits, pp. 153–159. In W. H. Robinson and D. Bajomi (eds.), Proceedings of the Sixth International Conference on Urban Pests. OOK-Press, Weszprém, Europa Congress Center, Hungary. [Google Scholar]
- Weiss, M. R. 2006. Defecation behavior and ecology of insects. Annu. Rev. Entomol. 51: 635–661. doi: 10.1146/annurev.ento.49.061802.123212 [DOI] [PubMed] [Google Scholar]
- Wu, X., and A. G. Appel. . 2017. Insecticide resistance of several field-collected German cockroach (Dictyoptera: Blattellidae) strains. J. Econ. Entomol. 110: 1203–1209. doi: 10.1093/jee/tox072 [DOI] [PubMed] [Google Scholar]