Abstract
Background:
Deficient mismatch repair (dMMR) or microsatellite instability is one of the well-established molecular biomarkers in colorectal cancer (CRC). The efficiency of neoadjuvant chemotherapy (NAC) in locally advanced colorectal cancer (LACC) patients with dMMR is unclear.
Objectives:
We assessed the tumor response and clinical outcome in LACC patients with dMMR received NAC.
Design:
Retrospective, single-center analysis.
Methods:
From 2013 to 2018, a total of 577 LACC patients with dMMR who underwent radical surgery were identified. Among them, 109 patients who received adjuvant chemotherapy were further screened out for analysis. According to whether receiving NAC or not, 109 patients were divided into two groups with the purpose of retrospectively analyzing their characteristics, treatment, and survival results, especially the 5-year disease-free survival (DFS) and 5-year overall survival.
Results:
Baseline characteristics were matched between the two groups. One of 40 patients in NAC group recurred, while 13 of 69 patients in non-NAC group recurred. Univariate and multivariate analyses showed that NAC (hazard ratio: 0.115; 95% confidence interval: 0.015–0.897; p = 0.039) was independent influence factor for DFS. In NAC group, there were 13/40 (32.5%) patients for tumor regression grade 1 and 27/40 (67.5%) patients converted clinical positive N-stage into negative N-stage.
Conclusion:
In this study, NAC was associated with better tumor downstaging and longer 5-year DFS in LACC patients with dMMR. Consequently, NAC might be an additional treatment choice when it comes to such patients in the future.
Keywords: deficient mismatch repair, locally advanced colorectal cancer, neoadjuvant chemotherapy, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors, which seriously threatens mankind’s health and life.1 For locally advanced colorectal cancer (LACC), neoadjuvant chemotherapy (NAC) combined with radical resection and adjuvant therapy was the standard treatment strategy.2,3 Compared with postoperative adjuvant chemotherapy, NAC can not only improve the local control rate, but also decrease the distant metastasis.4
DNA mismatch repair pathway was a crucial post-replicative repair process.5 Microsatellite status was one of the well-established molecular biomarkers in CRC6,7 and previous data showed that 10–20% of CRC were microsatellite instability (MSI), which was caused by MMR deficiency.8 As a result, deficient mismatch repair (dMMR) was considered equivalent to MSI-high (MSI-H).9 In recent years, MMR status has been proved to affect prognosis of CRC and guide treatment.10 Previous studies have demonstrated that immune checkpoint inhibitors (ICIs) have significantly therapeutic effects on CRC patients with dMMR.11 Nevertheless, there were still many LACC patients with dMMR who cannot benefit from immunotherapy on account of developed resistance and insensitivity. Although it is a remarkably clinical issue and urgent need to transform immunologically ‘cold’ immune microenvironment into the ‘hot’ one, it has existed as a tricky problem and been not well resolved at present.
When LACC patients are ineffective in immunotherapy, NAC might be the remaining potential option. But there were opinions that LACC patients with dMMR have the resistance to fluorouracil and are not sensitive to chemotherapy.12,13 However, Their results could be better convinced if they did not limit the objects for dMMR patients.12,13 Interestingly, Cercek A et al. gave evidence that the dMMR group was more likely to exhibit disease progression after receiving NAC.14 Thus, NAC has little relevance to dMMR status. In contrast, gastric cancer patients with MSI-H who received NAC had a better survival.15,16 That is, the resistance of fluoropyrimidine was not universal for patients with dMMR. Unfortunately, few previous investigations have directly reported if LACC patients with dMMR can benefit from NAC.
Therefore, we divided LACC patients with dMMR into two groups according to whether they received NAC, and compared their downstaging rate and oncological outcomes. This study aims to explore if NAC has residual value in the era of immunotherapy.
Methods
Patient selection
Through retrospective analysis, we collected 6028 CRC patients who underwent radical surgery in the Sixth Affiliated Hospital of Sun Yat-sen University from 2013 to 2018. A total of 577 LACC patients were pathologically diagnosed with dMMR after surgery. In all, 109 patients who received adjuvant chemotherapy were enrolled (Figure 1). The inclusion criteria were as follows: (1) CRC patients with preoperative staging of stage II (T3 to 4, N0) or stage III (T1 to 4, N1 to 2) with a positive node defined as 1.0 cm or larger in diameter on imaging; (2) patients received radical surgery and postoperative adjuvant chemotherapy; and (3) postoperative pathological specimens were diagnosed as dMMR. The exclusion criteria for patients included the following: (1) patients with other malignant tumors at the same time or in the past, (2) patients with distant or peritoneal metastasis, (3) concurrent multiple CRCs, and (4) patients with incomplete follow-up or collection of data. The information of demographic, treatment characteristics, and oncology outcomes were collected. Considering the impact of adjuvant chemotherapy on survival outcomes, 206 patients who did not receive adjuvant chemotherapy were excluded. In addition, 56 patients were excluded due to incomplete data, 35 patients did not receive 12 cycles of chemotherapy, and 19 patients who lost follow-up were excluded.
Figure 1.
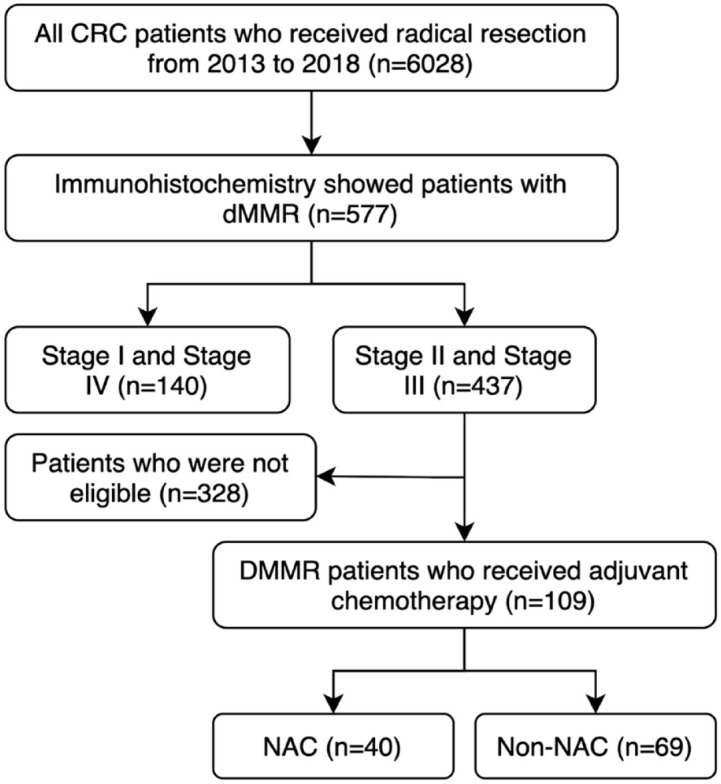
Flowchart representing the selection of eligible patients for the present study.
CRC, colorectal cancer; dMMR, deficient mismatch repair; NAC, neoadjuvant chemotherapy.
Pathological assessment
Two experienced pathologists analyzed the expression of MMR protein by immunohistochemistry (IHC) to test the MMR status in surgical pathological specimen. When the IHC result was uncertain, it can be further confirmed by the PCR-based MSI testing. Because of National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology guidelines, dMMR was defined as any lack of expression of MMR gene-related proteins (MLH1, MSH2, MSH6, and PMS2) in immunohistochemical detection. Pathological staging: The patients were staged by the American Joint Committee on Cancer staging /International Union Against Cancer TNM staging. Tumor regression grade (TRG) after preoperative treatment was evaluated semi-quantitatively according to NCCN guidelines: TRG1, TRG2, and TRG3, which corresponded to <10%, 10–50%, and >50% residual tumor cells within the tumor bed, respectively.
Treatment
The tumor and the entire pelvis were treated with radiotherapy 23–25 doses, with a total dose of 46.0–50.4 Gy from Monday to Friday. Patients received four to six cycles of fluorouracil (leucovorin 400 mg/m2 intravenously followed by fluorouracil 400 mg/m2 intravenously and fluorouracil 2.4 g/m2 by 48-h continuous intravenous infusion) with the oxaliplatin 85 mg/m2 intravenously each chemotherapy treatment cycle and underwent radiotherapy during the 2–4 cycles. For postoperative chemotherapy, patients accepted 6–8 cycles of mFOLFOX6, with the addition of radiation after surgery on the physicians’ decision.
Following up
The follow-up was carried out by outpatient or telephone every 6 month and the contents included: the patient’s current general condition, physical examination including digital rectal examination, carbohydrate antigen 19-9 and carcinoembryonic antigen (CEA), chest and abdomen computed tomography (CT), pelvic magnetic resonance imaging (MRI) and other examinations. If the review result were abnormal, further abdominal MRI and Positron emission tomography-CT examination were performed if necessary. The duration from radical resection to the date of last follow-up or tumor recurrence was defined as disease-free survival (DFS), and the duration from radical resection to the date of last follow-up or death caused by any reason was defined as overall survival (OS). This study was followed up until the date of death of the patient or the end of the study follow-up (January 2022).
Statistical analysis
SPSS 25.0 for data analysis was used. Chi-square test was used to analyze the classification parameters, and the number and percentage of cases were recorded. The Kaplan–Meyer log-rank test was used to compare the effects of influencing factors on 5-year DFS and 5-year OS. Moreover, the prognostic factors were analyzed by Cox univariate analysis. When the p value was less than 0.05, the multivariate Cox analysis was included, and the hazard ratio (HR) value, 95% confidence interval (CI), and p value were output. When p < 0.05, the difference was statistically significant.
The reporting of this study conforms to the STROBE statement.17
Results
Clinicopathological factors
The characteristics, treatment, and pathological characteristics of the patients are summarized in Table 1. Baseline characteristics were matched between the NAC group and non-NAC group. From 2013 to 2018, there were 577 dMMR underwent radical resection in 6028 CRC patients. Among 577 dMMR patients, 352/3272 (10.8%) were colon cancer patients with dMMR and 252/2756 (8.2%) were rectal cancer patients with dMMR (Figure 2(a)). A total of 109 LACC patients with dMMR received adjuvant chemotherapy, of which 76 males and 33 females were 47.61 ± 13.00 years old. In all, 40 LACC patients with dMMR (24.9%) received NAC while 69 (75.1%) without NAC. The MLH1/PMS2 deficient rate was 43.1%, the MSH2/MSH6 deficient rate was 32.1%, the MLH1/MSH6/PMS2 deficient rate was 1.0%, and the deficient rates of single MLH1 protein, MSH2 protein, MSH6 protein, and PMS2 protein were 3.7%, 2.8%, 8.3%, and 9.2%, respectively (Figure 2(b)).
Table 1.
Clinical characteristics and treatment of dMMR LACC patients.
| Variables | Total (n = 109) | NAC (n = 40) | Non-NAC (n = 69) | p Value |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 98 (89.9) | 37 (92.5) | 61 (88.4) | 0.494 |
| ⩾65 | 11 (10.1) | 3 (7.5) | 8 (11.6) | |
| Gender | ||||
| Male | 76 (69.7) | 27 (67.5) | 49 (71.0) | 0.700 |
| Female | 33 (30.3) | 13 (32.5) | 20 (29.0) | |
| Clinical T stage | ||||
| 2–3 | 65 (59.6) | 26 (65.0) | 39 (56.5) | 0.385 |
| 4 | 44 (40.4) | 14 (35.0) | 30 (43.5) | |
| Clinical N stage | ||||
| 0 | 32 (29.4) | 13 (32.5) | 19 (27.5) | 0.583 |
| 1–2 | 77 (70.6) | 27 (67.5) | 50 (72.5) | |
| Tumor differentiation | ||||
| Poor or moderate | 69 (63.3) | 25 (62.5) | 35 (50.7) | 0.234 |
| Well | 40 (36.7) | 15 (37.5) | 34 (49.3) | |
| Preoperative radiotherapy | ||||
| − | 97 (89.0) | 28 (70.0) | 69 (100.0) | <0.001 |
| + | 12 (11.0) | 12 (30.0) | 0 (0.00) | |
| Tumor location | ||||
| Colon | 72 (66.1) | 13 (32.5) | 59 (85.5) | <0.001 |
| Rectum | 37 (33.9) | 27 (67.5) | 10 (14.5) | |
| Histologic type | ||||
| Adenocarcinoma | 75 (68.8) | 30 (75.0) | 45 (65.2) | 0.288 |
| Mucinous | 34 (31.2) | 10 (25.0) | 24 (44.8) | |
| Vascular invasion infiltration | ||||
| − | 97 (89.0) | 38 (95.0) | 59 (85.5) | 0.127 |
| + | 12 (11.0) | 2 (5.0) | 10 (14.5) | |
| Perineural invasion | ||||
| − | 103 (94.5) | 38 (95.0) | 65 (94.2) | 0.860 |
| + | 6 (5.5) | 2 (5.0) | 4 (5.8) | |
| CEA (ng/mL) | ||||
| <5 | 82 (75.2) | 30 (75.0) | 52 (75.4) | 0.966 |
| ⩾5 | 27 (24.8) | 10 (25.0) | 17 (24.6) | |
| CA19-9 (U/mL) | ||||
| <34 | 94 (89.5) | 37 (92.5) | 57 (82.6) | 0.149 |
| ⩾34 | 15 (10.5) | 3 (7.5) | 12 (17.4) | |
CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; dMMR, deficient mismatch repair; LACC, locally advanced colorectal cancer; NAC, neoadjuvant chemotherapy.
Figure 2.
(a) The proportion of mismatch repair proteins deficient patients in CRC patients. (b) The mismatch repair proteins deficient of 109 LACC patients.
CRC, colorectal cancer; LACC, locally advanced colorectal cancer.
Oncological outcomes
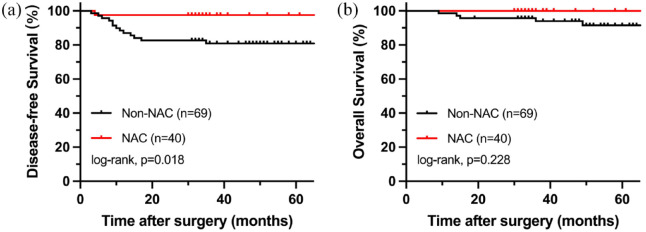
After a median follow-up of 49 months (9–99 months), 14 of the 109 patients (12.8%) had tumor recurrence, of which 3 patients occurred local recurrence and 11 patients occurred distant metastasis. In the end, six patients (5.5%) died of disease deterioration. In the NAC group, there were 13 patients for TRG1, 16 patients for TRG2, and 11 patients for TRG3. In addition, 27/40 (67.5%) patients converted clinical positive N-stage into negative N-stage after NAC. Comparing the 5-year DFS rate of LACC patients with dMMR with or without NAC (97.5% versus 81.2%, p = 0.014), there was a significant statistical difference in the DFS rate between two group (Figure 3(a)). For the 5-year OS rate, there was not statistical difference between LACC patients with dMMR treated with or without NAC (97.5% versus 92.8%, p = 0.295) (Figure 3(b)). Besides, the effects of neoadjuvant radiotherapy and tumor location on DFS and OS were not statistically different (p > 0.05).
Figure 3.
Oncological outcomes LACC patients with dMMR. (a) Kaplan–Meier estimates for DFS. (b) Kaplan–Meier estimates for OS.
DFS, disease-free survival; dMMR, deficient mismatch repair; LACC, locally advanced colorectal cancer; OS, overall survival.
Prognostic analysis of clinical factors
Univariate analysis revealed that stage T4 (HR: 2.892; 95% CI: 1.014–8.249; p = 0.047) and poor tumor differentiation (HR: 4.964; 95% CI: 1.384–17.806; p = 0.014) would lead to shorter DFS, while longer DFS was associated with NAC (HR: 0.126; 95% CI: 0.017–0.965; p = 0.046). In the multivariate analysis, stage T4 (HR: 1.845; 95% CI: 1.090–3.149; p = 0.025) and NAC (HR: 0.115; 95% CI: 0.015–0.897; p = 0.039) resulted in longer DFS (Table 2). In addition, the analysis of the influencing factors on OS showed that age >65 years (HR: 8.372; 95% CI: 1.685–41.607; p = 0.009), stage T4 (HR: 5.619; 95% CI: 1.057–29.881; p = 0.043), and CEA > 5 ng/mL (HR: 8.653; 95% CI: 1.547–48.399; p = 0.014) were related to shorter OS. But there was no statistical difference in each factor during multivariate analysis (Table 3). In addition, neoadjuvant radiotherapy is not an influencing factor for DFS (HR: 0.623; 95% CI: 0.081–4.761; p = 0.648) and OS (HR: 1.216; 95% CI: 0.139–10.622; p = 0.860).
Table 2.
Cox regression analysis of prognostic factors for the DFS of dMMR LACC patients.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age > 65 years | 1.619 (0.362–7.239) | 0.528 | ||
| Gender (female) | 0.916 (0.287–2.922) | 0.883 | ||
| pT stage 4 | 2.892 (1.014–8.249) | 0.047 | 1.845 (1.090–3.149) | 0.025 |
| pN stage positive | 3.060 (0.684–13.682) | 0.143 | ||
| Poor tumor differentiation | 4.964 (1.384–17.806) | 0.014 | 3.594 (0.978–13.199) | 0.054 |
| NAC | 0.126 (0.017–0.965) | 0.046 | 0.115 (0.015–0.897) | 0.039 |
| Neoadjuvant radiotherapy | 0.623 (0.081–4.761) | 0.648 | ||
| Tumor location (rectum) | 0.516 (0.144–1.850) | 0.310 | ||
| Histologic type (mucinous type) | 0.601 (0.168–2.153) | 0.434 | ||
| No. of lymph nodes excised > 12 | 0.717 (0.100–2.571) | 0.610 | ||
| Vascular invasion infiltration | 2.349 (0.655–8.424) | 0.190 | ||
| Perineural invasion | 3.574 (0.417–30.623) | 0.245 | ||
| CEA > 5 ng/mL | 0.836 (0.233–2.997) | 0.783 | ||
| CA19-9 > 34 U/mL | 1.035 (0.232–4.627) | 0.964 | ||
CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; DFS, disease-free survival; dMMR, deficient mismatch repair; HR, hazard ratio; LACC, locally advanced colorectal cancer NAC, neoadjuvant chemotherapy.
Table 3.
Cox regression analysis of prognostic factors for the OS of dMMR LACC patients.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age > 65 years | 8.372 (1.685–41.607) | 0.009 | 4.353 (0.770–24.611) | 0.096 |
| Gender (female) | 1.268 (0.230–7.002) | 0.785 | ||
| pT stage 4 | 5.619 (1.057–29.881) | 0.043 | 3.393 (0.579–19.886) | 0.176 |
| pN stage positive | 2.250 (0.260–19.430) | 0.461 | ||
| Poor tumor differentiation | 6.097 (0.712–52.200) | 0.099 | ||
| NAC | 0.354 (0.041–3.041) | 0.344 | ||
| Neoadjuvant radiotherapy | 1.216 (0.139–10.622) | 0.860 | ||
| Tumor location (rectum) | 0.909 (0.165–5.015) | 0.912 | ||
| Histologic type (Mucinous type) | 0.499 (0.058–4.289) | 0.527 | ||
| No. of lymph nodes excised > 12 | 0.491 (0.088–2.736) | 0.417 | ||
| Vascular invasion infiltration | 4.318 (0.789–23.620) | 0.092 | ||
| Perineural invasion | 3.574 (0.417–30.623) | 0.245 | ||
| CEA > 5 ng/mL | 8.653 (1.547–48.399) | 0.014 | 3.735 (0.583–23.951) | 0.165 |
| CA19-9 > 34 U/mL | 3.263 (0.597–17.835) | 0.172 | ||
CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; dMMR, deficient mismatch repair; HR, hazard ratio; LACC, locally advanced colorectal cancer NAC, neoadjuvant chemotherapy; OS, overall survival.
Discussion
To our knowledge, this study directly illustrated that NAC might have to do with better histological regression and longer DFS in LACC patients with dMMR for the first time, which provided some evidence to fill the gap in this field.
Microsatellite status was considered to be a prognostic biomarker.18,19 Previous studies manifested that dMMR status represented a better prognosis for CRC patients, but the benefits of fluorouracil for dMMR patients were limited.20,21 Namely, the prognostic role of NAC in dMMR patients was controversial. De Rosa N et al. proved that NAC had not improve the progression-free survival and clinical response rates of dMMR rectal cancer patients compared with proficient MMR (pMMR) rectal cancer patients.22 Similarly, Elizabeth C Smyth et al. found that MSI-H had to do with a negative prognostic effect in gastric cancer patients treated with NAC.23 As the backbone of systemic therapy, the resistance of fluoropyrimidine raised concern on primary chemotherapy resistance in dMMR CRC patients. On the contrary, the study from Cercek A et al. made clear that the resistance of fluoropyrimidine was not universal for dMMR CRC patients, which displayed that a fraction of such patients may be salvaged with chemoradiation.14 Therefore, the resistance of dMMR CRC to NAC might be only relative. Also, Shu-Biao Ye reported that CRC patients with dMMR could profit by NAC, while patients with stage III and pMMR can benefit from neoadjuvant chemoradiotherapy.24 Undoubtedly, past researches provided some evidence to demonstrate the influence of MMR status on NAC. However, such studies were centered on the comparison of MMR states, which made it ignored whether dMMR patients could be helped by NAC. As many LACC patients with bulky and clinically symptomatic tumors, choosing the first-line treatment of local control appropriately and promptly is essential.
An ideal study to determine the benefits of NAC in dMMR CRC would require distribution of NAC group versus non-NAC group. This study analyzed this problem in a retrospective way. Our study found 13 (32.5%) patients achieved TRG1 and 27/40 (67.5%) patients converted clinical positive N-stage into negative N-stage after receiving NAC. In addition, only one of 40 LACC patients with dMMR who received NAC occurred distant metastases. But three patients occurred local recurrence and 10 patients occurred distant metastasis in the 69 dMMR LACC patients who did not receive NAC. Furthermore, our study also revealed that NAC was associated with longer 5-year DFS. Since not all patients received neoadjuvant radiotherapy, it is important to consider whether it influenced the outcome. Previous research conducted that dMMR rectal cancer patients were not sensitive to radiotherapy.25 Moreover, the article from Shu-Biao Ye et al. observed that the prognosis of rectal cancer patients with dMMR received neoadjuvant chemoradiotherapy was not significantly better than that of the ones received NAC.24 Our results also revealed that neoadjuvant radiotherapy was not a prognostic factor for dMMR patients. In addition, radical resection of T4 stage tumor was more difficult, so the value of NAC was more vital. Previous research from Federica Papaccio et al. indicated that NAC could improve the prognosis of pMMR rectal cancer patients with stage T4.24 Our study demonstrated the stage T4 was an independent risk factor on DFS for LACC patients with dMMR. That is to say, NAC will be required to reduce the T stage before radical surgery in the future.
In recent years, immunotherapy has gradually become a crucial choice for CRC patients with dMMR.11 But the challenges of acquired and intrinsic resistance to ICI remain.26,27 The mechanism of immunotherapy resistance was unclear and there was no truly effective method.28 How to make it a very real clinical problem when immunotherapy is ineffective in dMMR CRC patients, and NAC may be an alternate option. However, the use of chemotherapy must take the ratio of drug toxicity to benefit in consideration, and it is still unknown whether patients with dMMR can benefit from NAC. Our results suggested that a subset of patients with dMMR can still benefit from NAC. Therefore, NAC should be considered as the second treatment option for LACC patients with dMMR. For LACC patients with dMMR, NAC still has residual value in this era of immunotherapy.
It was undeniable that there were some limitations in our study such as retrospective, single center, and limited sample quantity. In addition, some patients who reached pathological complete response after neoadjuvant chemoradiotherapy were unable to perform MMR status analysis due to the lack of tumor samples. Despite these limitations, we still hope that this study can provide useful information for the treatment in LACC patients with dMMR.
Conclusions
In conclusion, NAC might be correlated with better histological regression and longer DFS for LACC patients with dMMR. This study bridged the previous knowledge gaps and preferred NAC being an additional treatment choice for LACC patients with dMMR and improving their survival.
Supplemental Material
Supplemental material, sj-doc-1-tag-10.1177_17562848221150306 for Clinical significance of neoadjuvant chemotherapy for locally advanced colorectal cancer patients with deficient mismatch repair: possibly residual value in the era of immunotherapy by Mian Chen, Junguo Chen, Jun Huang, Huashan Liu, Wuteng Cao, Shuangling Luo, Zhanzhen Liu, Huanxin Hu, Sicong Lai, Yujie Hou, Liang Kang and Liang Huang in Therapeutic Advances in Gastroenterology
Acknowledgments
Thanks to the support of National Key Clinical Discipline.
Footnotes
ORCID iD: Mian Chen  https://orcid.org/0000-0001-8156-3283
https://orcid.org/0000-0001-8156-3283
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mian Chen, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Junguo Chen, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Jun Huang, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Huashan Liu, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Wuteng Cao, Department of Radiology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Shuangling Luo, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Zhanzhen Liu, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Huanxin Hu, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Sicong Lai, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Yujie Hou, Department of Colorectal Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China; Guangdong Institute of Gastroenterology, Guangzhou, Guangdong, China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Liang Kang, Department of Colorectal Surgery, Guangdong Institute of Gastroenterology, and Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510655, China.
Liang Huang, Department of Colorectal Surgery, Guangdong Institute of Gastroenterology, and Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510655, China.
Declarations
Ethics approval and consent to participate: The ethics committee of the Sixth Affiliated Hospital of Sun Yat-sen University approved the conduct of this study (E2021156). All patient information was kept confidential in this study, and the requirement for informed consent was waived because the data were anonymized.
Consent for publication: Not applicable.
Author contribution(s): Mian Chen: Conceptualization; Writing – original draft.
Junguo Chen: Methodology; Writing – original draft.
Jun Huang: Methodology; Writing – original draft.
Huashan Liu: Formal analysis; Writing – review & editing.
Wuteng Cao: Formal analysis; Writing – review & editing.
Shuangling Luo: Formal analysis; Writing – review & editing.
Zhanzhen Liu: Investigation; Writing – review & editing.
Huanxin Hu: Investigation; Writing – review & editing.
Sicong Lai: Investigation; Writing – review & editing.
Yujie Hou: Investigation; Writing – review & editing.
Liang Kang: Conceptualization; Writing – original draft.
Liang Huang: Conceptualization; Supervision; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Science and technology key research and development plan project of Guangzhou (China) (202103000072) and Natural Science Foundation of Guangdong Province (China) (2018A030313621).
The authors declare that there is no conflict of interest.
Availability of data and materials: Data can be made available upon reasonable request.
References
- 1. Xie Y, Shi L, He X, et al. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf) 2021; 9: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akgun E, Caliskan C, Bozbiyik O, et al. Randomized clinical trial of short or long interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2018; 105:1417–1425. [DOI] [PubMed] [Google Scholar]
- 3. Huang CM, Huang MY, Ma CJ, et al. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat Oncol 2017; 12: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang J, Jang SM, Baek JH, et al. Short-term results and long-term oncologic outcomes between neoadjuvant chemoradiotherapy and adjuvant postoperative chemoradiotherapy for stage III rectal cancer: a case-matched study. Ann Surg Oncol 2012; 19: 2494–2499. [DOI] [PubMed] [Google Scholar]
- 5. Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 2003; 21: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 6. Lee MKC, Loree JM. Current and emerging biomarkers in metastatic colorectal cancer. Curr Oncol 2019; 26: S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sugai T, Yoshida M, Eizuka M, et al. Analysis of the DNA methylation level of cancer-related genes in colorectal cancer and the surrounding normal mucosa. Clin Epigenetics 2017; 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poulogiannis G, Frayling IM, Arends MJ. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 2010; 56: 167–179. [DOI] [PubMed] [Google Scholar]
- 9. Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther 2018; 189: 45–62. [DOI] [PubMed] [Google Scholar]
- 10. Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 2013; 155: 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lizardo DY, Kuang C, Hao S, et al. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochim Biophys Acta Rev Cancer 2020; 1874: 188447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song H, Zeng J, Roychoudhury S, et al. Targeting histone chaperone FACT complex overcomes 5-fluorouracil resistance in colon cancer. Mol Cancer Ther 2020; 19: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao P, Ma YG, Zhao Y, et al. MicroRNA-552 deficiency mediates 5-fluorouracil resistance by targeting SMAD2 signaling in DNA-mismatch-repair-deficient colorectal cancer. Cancer Chemother Pharmacol 2019; 84: 427–439. [DOI] [PubMed] [Google Scholar]
- 14. Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res 2020; 26: 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai Z, Rui W, Li S, et al. Microsatellite status affects tumor response and survival in patients undergoing neoadjuvant chemotherapy for clinical stage III gastric cancer. Front Oncol 2020; 10: 614785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haag GM, Czink E, Ahadova A, et al. Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer 2019; 144: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miceli R, An J, Di Bartolomeo M, et al. Prognostic impact of microsatellite instability in Asian gastric cancer patients enrolled in the ARTIST trial. Oncology 2019; 97: 38–43. [DOI] [PubMed] [Google Scholar]
- 19. Marrelli D, Polom K, Pascale V, et al. Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol 2016; 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 20. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010; 28: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993; 260: 816–819. [DOI] [PubMed] [Google Scholar]
- 22. de Rosa N, Rodriguez-Bigas MA, Chang GJ, et al. DNA mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol 2016; 34: 3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol 2017; 3: 1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye SB, Cheng YK, Zhang L, et al. Association of mismatch repair status with survival and response to neoadjuvant chemo(radio)therapy in rectal cancer. NPJ Precis Oncol 2020; 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du C, Zhao J, Xue W, et al. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology 2013; 62: 723–730. [DOI] [PubMed] [Google Scholar]
- 26. Kather JN, Halama N, Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin Cancer Biol 2018; 52: 189–197. [DOI] [PubMed] [Google Scholar]
- 27. Thomas J, Leal A, Overman MJ. Clinical development of immunotherapy for deficient mismatch repair colorectal cancer. Clin Colorectal Cancer 2020; 19: 73–81. [DOI] [PubMed] [Google Scholar]
- 28. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tag-10.1177_17562848221150306 for Clinical significance of neoadjuvant chemotherapy for locally advanced colorectal cancer patients with deficient mismatch repair: possibly residual value in the era of immunotherapy by Mian Chen, Junguo Chen, Jun Huang, Huashan Liu, Wuteng Cao, Shuangling Luo, Zhanzhen Liu, Huanxin Hu, Sicong Lai, Yujie Hou, Liang Kang and Liang Huang in Therapeutic Advances in Gastroenterology