Abstract:
Cardiopulmonary bypass (CPB) is routinely used for performing congenital heart operations. While most congenital heart operations can be performed with bypass times under 2 hours, complex pulmonary artery reconstructions require longer periods of CPB to facilitate the surgical repair. This article is intended to summarize the surgical and perfusion techniques utilized in patients undergoing complex pulmonary artery reconstructions at our institution. The initial portion of this manuscript provides an in-depth description of the surgical techniques employed for pulmonary artery reconstructions. This information is important in order to understand why prolonged CPB is a necessary requirement. The manuscript then provides a detailed description of the perfusion techniques and the modifications to the CPB circuit. Finally, the manuscript provides a summary of data from a clinical study evaluating the application of these techniques in 100 consecutive children undergoing complex pulmonary artery reconstruction. The data from this study demonstrated that there was a poor correlation between duration of CPB and both the number of postoperative complications and hospital length of stay. Major adverse cardiac events occurred in 11 (11%) patients with one hospital mortality. These results suggest that prolonged CPB does not predispose to adverse outcomes in this select population of patients.
Keywords: cardiopulmonary bypass, outcomes, congenital heart surgery, congenital heart disease, pulmonary arteries, major aortopulmonary collateral arteries.
Cardiopulmonary bypass (CPB) has been utilized since 1953 for the repair of congenital heart defects (1). Over the subsequent years, the safety and sophistication of CPB circuits have led to a vast improvement in clinical outcomes for patients undergoing cardiac surgery (2–4). The reliability of the modern CPB circuits has resulted in an important paradigm shift from speed to precision (5,6). This has also permitted an expansion in the complexity of cases that can be undertaken given the safety of the CPB circuits.
Our surgical group at Stanford Children’s Hospital has pioneered many of the techniques used for complex pulmonary artery reconstructions (7–13). This includes unifocalization procedures for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (PA/VSD/MAPCAs) and the surgical repair of peripheral pulmonary artery stenosis (PPAS). These procedures are technically challenging and often require extremely long periods of CPB (14). In this circumstance, prolonged CPB has become an accepted norm within our institution in order to facilitate the repair of these complex forms of congenital heart defects.
The current literature would suggest that prolonged CPB is associated with a much higher incidence of adverse outcomes (15–24). However, prolonged CPB is often a surrogate marker for either a high-risk procedure or one in which significant intraoperative complications have occurred (or both). Stated differently, it is the high-risk procedure and/or intraoperative complications that account for the higher incidence of adverse outcomes and prolonged CPB is simply a marker of a very difficult procedure.
In contrast to the literature cited in the previous paragraph, complex pulmonary artery reconstructions offer an opportunity to assess the impact of prolonged CPB in a setting where this is the expected situation and not the product of a higher risk population and/or intraoperative complications (25). The purpose of this review article is to summarize our surgical and perfusion approach and review data on the postoperative complication rate as a function of bypass time in patients undergoing unifocalization or pulmonary artery reconstructions.
SURGICAL AND PERFUSION APPROACH
Both unifocalization procedures for PA/VSD/MAPCAs and pulmonary artery reconstructions for PPAS require extensive dissection prior to going on bypass. This portion of the procedure may last several hours and result in intermittent periods of desaturation and/or hemodynamic instability (7–13). This dissection is preferably performed off bypass (in order to obtain hemostasis of the operative field prior to heparinization) with the perfusionist on stand-by in the event of significant bleeding, desaturation, or hemodynamic collapse.
Once the dissection has been completed, CPB is instituted. For patients with MAPCAs, it is imperative to ligate all MAPCAs at their proximal origin immediately after going on bypass to eliminate this source of run-off. The perfusion pressure will initially be quite low but will rise commensurately with the size of the MAPCAs as they are ligated. The unifocalization or pulmonary artery reconstruction procedures typically require between 5 and 7 hours of CPB to permit precise surgical correction.
Unifocalization procedures are performed at a core temperature of 25°C. The initial cooling phase is performed at 200 cc/kg/min until the target temperature is reached at which point a flow rate of 100 cc/kg/min is used. pH Stat blood gas management is utilized. The left heart is routinely vented through the right superior pulmonary vein. Importantly, the use of volatile gasses (such as isoflurane) is avoided, as a higher-than-expected incidence of multisystem organ failure (specifically liver and kidney failure) has been noted when these gasses are used (26). The hematocrit (HCT) is maintained between 30 and 40% throughout the duration of CPB, and the goal is to achieve a hematocrit of 45–55% at the conclusion of bypass (14).
To support the prolonged lengths of CPB, the CPB circuit is modified to include upsizing of the oxygenator, reservoir, cannulae, vent catheter, and tubing (Figure 1). Our group utilizes a Sorin S5 pump (LivaNova Group, London, UK) with Terumo oxygenators (Terumo Corporation, Tokyo, Japan) and a custom Medtronic tubing pack (Medtronic, Inc., Dublin, Ireland). The conversion from a conventional bypass circuit to one for a unifocalization or pulmonary artery reconstruction procedures is summarized in Table 1.
Figure 1.
Diagram of the CPB circuit for unifocalization or pulmonary artery reconstruction procedures. The circuit size is upsized in anticipation of the need for longer periods of CPB (from Margetson et al. (14), reproduced with permission).
Table 1.
Upsizing of tubing and oxygenators—conversion standard versus modification.
| Weight Range (kg) | Oxygenator | Boot | Venous Line × Arterial Line |
|---|---|---|---|
| Standard circuit | |||
| 0–15 | RX-05 | ¼″ | ¼″ × ¼″ |
| 15–40 | RX-15RE30 | 3/8″ | 3/8″ × ¼″ |
| >40 | RX-25 | ½″ | 3/8″ × 3/8″ |
| Modified circuit for unifocalizations | |||
| 0–8 | RX-05 | ¼″ | ¼″ × ¼″ |
| 8–30 | RX-15RE30 | 3/8 | 3/8″ × ¼″ |
| >30 | RX-25 | ½″ | 3/8″ × 3/8″ |
The prime volumes for the three circuits shown in Table 1 are 300 mL for the RX-05 package, 500 mL for the RX-15RE30 package, and 1,000 mL for the RX-25 package. The fact that the circuits are upsized for certain weight ranges means that some patients will have additional hemodilution compared to a standard circuit. Specifically, patients between 8 and 15 kg fall in the upsized range and thus have a 67% greater prime volume compared to the standard circuit. Similarly, patients between 30 and 40 kg would also fall in the upsized range and have 100% greater prime volume. All of our circuits are coated with Medtronic Balance Biosurface coating. Our prime constituents include Normosol, sodium bicarbonate, mannitol, heparin, methylprednisolone (30 mg/kg), and tranexamic acid. We would add blood to the prime solution for all patients under 30 kg to avoid excessive hemodilution and to maintain an HCT between 30 and 40% during the bypass run. All patients undergoing complex pulmonary artery reconstructions will ultimately require several units of blood given the amount of blood loss from the extensive suture lines. In addition, platelets, concentrated human fibrinogen (Riastap, CSL Behring, Marburg, Germany) and fresh frozen plasma are used to help restore the clotting system.
DETERMINING WHETHER THE VSD CAN BE CLOSED
A critical cross-road in the unifocalization procedures comes after the unifocalization is completed and the assessment of whether the VSD can be closed or not. This decision is based on the adequacy of the unifocalized bed. The goal is to close the VSD if the resultant right ventricular pressure would be less than half systemic (7). The advantages of closing the VSD include complete normalization of the circulation and fully saturated oxygen levels. All of our CPB circuits are modified for unifocalization procedures to include the capability of performing an intraoperative flow study (Figure 2). A separate arterial line is primed and a cannula is placed in the reconstructed pulmonary artery. The systemic perfusion is then decreased to half flow (i.e., 50 cc/kg/min), and flow to the pulmonary arteries is gradually increased to a goal of 3 L/min/m2 (15). A maximal mean pulmonary artery pressure of 25 mmHg is considered acceptable (Table 2). Patients who pass this test proceed to VSD closure. For this portion of the procedure, the aorta is cross-clamped and the heart arrested using del Nido cardioplegia solution (27). Our policy is to redose cardioplegia at 1-hour intervals following the initial dose. Following closure of the VSD, the aortic cross-clamp is removed with subsequent placement of a right ventricle to pulmonary artery conduit. Patients who do not pass the intraoperative flow study do not have the VSD closed but instead undergo placement of a systemic-to-pulmonary artery shunt.
Figure 2.
Diagram of the flow study modification to the CPB circuit. An additional pump-head is required that will be dedicated to perfusing the reconstructed pulmonary artery bed following unifocalization.
Table 2.
Flow study chart demonstrating flow by absolute amount and percentage of the goal.
| Flow (%) | Flow at an Index of 3.0 |
|---|---|
| 25 | 225 |
| 50 | 450 |
| 75 | 675 |
| 100 | 900 |
This example is for a patient with a body surface area of 0.30 m2.
Over the years we realized that many patients with PA/VSD/MAPCAs are “ideal” candidates and will pass the flow study. The best preoperative indicators that a patient will pass the flow study include the oxygen saturations (>84%) and the cardiac catheterization data (Qp:Qs >1.5). If a patient meets these criteria, then it is good evidence of an adequate pulmonary vascular bed, and an intraoperative flow study would no longer be performed. We also make an assessment of the quality of the MAPCAs while they are being harvested and unifocalized, and this may influence our decision regarding the flow study. As a consequence of this change in philosophy, flow studies are now performed in only about 20% of the patients. By inference, this also means that the failure rate for the flow study is higher than it was when all patients underwent a flow study.
One additional study that can be performed in patients with PA/VSD/MAPCAs is an assessment of the residual collateral flow in patients with MAPCAs (26). Patients who met the eligibility criteria (i.e., primary midline unifocalization, candidate for two ventricle repair) had a diversion loop added to the left ventricular vent system prior to surgery (see Figure 3). After the initiation of CPB, a left ventricular vent catheter was placed through the right superior pulmonary veins into the left ventricle. This left ventricular vent return would represent a sum of the pulmonary collateral flow, coronary collateral flow, and potentially any flow entrained through the atrial or ventricular septal defect. It is only when the aorta is cross-clamped that the latter two sources of flow are fully eliminated. During the cross-clamp period, the left ventricular diversion loop was opened for 1-minute intervals and the amount of residual collateral flow measured during this interval. The percentage of residual collateral flow is the amount of residual collateral flow divided by the total pump flow to the patient is the flow rate (100 mL/kg/min) times the body weight.
Figure 3.
Diagram of the collateral flow study modification to the CPB circuit. This modification permits the measurement of the residual collateral flow once the MAPCAs have been ligated (from Mainwaring et al. (28), reproduced with permission).
CLINICLAL OUTCOMES IN PATIENTS UNDERGOING PULMONARY ARTERY RECONSTRUCTION
To evaluate the clinical outcomes of patients undergoing pulmonary artery reconstruction, we subsequently performed a study of 100 consecutive patients who were undergoing procedures with CPB times in excess of 5 hours (25). This time definition was utilized as a marker for the complexity of surgical repair. Specifically excluded were patients undergoing unifocalizations or pulmonary artery reconstructions with CPB times <5 hours as this would be a surrogate marker for less complex repairs.
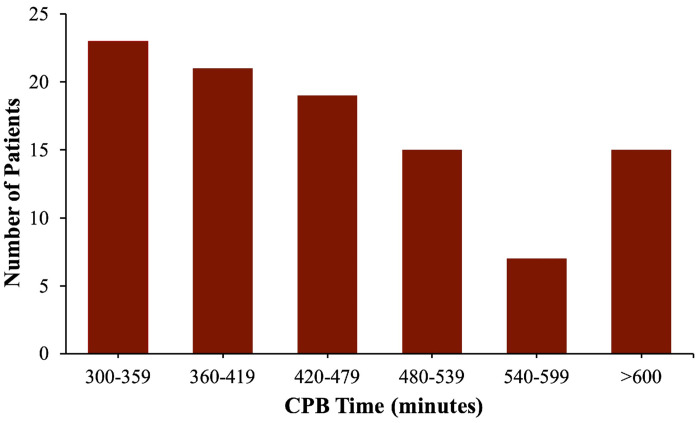
The median age of the patients at surgery was 15 months (range: 1 month–21 years). Sixty-six of the 100 patients had an underlying anatomic diagnosis of PA/VSD/MAPCAs while 34 patients had the diagnosis of PPAS. The median duration of CPB for these procedures was 473 minutes, with a range of 305–965 minutes (Figure 4). Fifty-seven patients (57%) had a period of aortic cross-clamp, with a median duration of 26 minutes (range: 18–108 minutes). Three (3%) patients required a second dose of cardioplegia. Forty-three patients (43%) underwent pulmonary artery reconstruction procedures that did not require aortic cross-clamp.
Figure 4.
Histogram demonstrating the CPB times for the patient cohort. The median CPB time was 473 minutes (from Mainwaring et al. (25), reproduced with permission).
SUMMARY OF DATA
The median number of postoperative complications was four with a range of 0–19. These data are shown in Figure 5. The most common postoperative complications were low cardiac output in 43 patients (43%), open sternum in 40 (40%), re-intubation in 24 (24%), bronchoscopy in 18 (18%), and arrhythmias in 18 (18%). Neurologic complications occurred in five (5%) patients, including three who had seizures and two who experienced a stroke with full recovery.
Figure 5.
Histogram demonstrating the number of complications sustained per patient. The median number of complications was four with a range of 0–19 (from Mainwaring et al. (25), reproduced with permission).
The median length of hospital stay was 24 days, with a range of 4–130 days. The relationship between the total number of complications and the hospital length of stay is shown in Figure 6A. There was a good correlation between these two variables (R-squared = 0.64). The relationship between total number of complications and CPB time is shown in Figure 6B, and the relationship between hospital length of stay and CPB time is shown in Figure 6C. Both of these analyses demonstrated a poor correlation by linear regression (R-squared = 0.002 and 0.005, respectively).
Figure 6.
Scatter plot demonstrating: (A) the relationship between total number of complications (y-axis) and the length of hospital stay (x-axis). Linear regression analysis demonstrated an R-squared = 0.64. (B) Total number of complications (y-axis) and CPB time (x-axis). Linear regression analysis demonstrated an R-squared = 0.002. (C) The length of hospital stay (y-axis) and CPB time (x-axis). Linear regression analysis demonstrated an R-squared = 0.005 (from Mainwaring et al. (25), reproduced with permission).
Major adverse cardiac events (MACE) (ECMO, Death, and/or Cardiac arrest) occurred in 11 patients (11%). Nine patients were placed on ECMO and six patients sustained a cardiac arrest. There was one mortality (1%). This patient had a relatively short CPB time (365 minutes) but sustained a cardiac arrest postoperatively. MACE were more common in patients with extremely long periods of CPB (>540 minutes) compared to shorter periods of CPB (Figure 7).
Figure 7.
Graph demonstrating the prevalence of MACE in patients with CPB times of 5–6 hours, 7–8 hours, and >9 hours (from Mainwaring et al. (25), reproduced with permission).
The impact of a specific complication on the hospital length of stay was calculated by comparing the median value of patients with and without that complication. MACE had the greatest impact on hospital length of stay with a differential of +35 days. Other complications that had a demonstrable impact on hospital length of stay were unplanned re-operation (+20), pneumonia (+15), re-intubation (+14), bleeding (+11), and pleural effusion (+6).
DISCUSSION
The study cited above was performed to evaluate the impact of prolonged CPB on outcomes in patients undergoing unifocalization or complex pulmonary artery reconstructions. This patient cohort is expected to require very lengthy periods of CPB to facilitate the pulmonary reconstructions. The data demonstrate that there was a poor correlation between the length of CPB and either the total number of complications or the hospital length of stay. There was a good correlation between the total number of complications and hospital length of stay. MACE occurred in 11% of the patients and did portend a longer hospital length of stay. Based on both previously published papers and the current set of data, one can conclude that prolonged CPB is not a risk factor for adverse outcomes in this specific patient population.
The prototypical patient in this study had a CPB time of 473 minutes, sustained 4 postoperative complications and was in the hospital for 24 days following surgery. It is interesting to note that there were 10 (10%) patients who had no complications and nine (9%) who had just one complication despite prolonged CPB. Paralleling these data, there were 24 (24%) patients who were discharged within 10 days of surgery. The data also show that as the number of complications increased the duration of hospital stay increased in a linear relationship. However, the data demonstrate a lack of correlation between CPB time and total number of complications or length of stay. These data do not necessarily indicate that CPB is benign or that CPB time is irrelevant, since the entry criteria for CPB time was 300 minutes. We would interpret these data to indicate that once patients are on the heart–lung machine for 300 minutes there appears to be no incremental increase in the number of postoperative complications or length of hospital stay with longer periods of CPB.
For the 90 (90%) patients who did have one or more complications there were a total of 485 postoperative complications. The most common complications were low cardiac output syndrome in 43 patients (43%) and open sternum in 40 (40%). While these two complications were the most prevalent, they did not have an impact on hospital length of stay. Conversely, there were 11 (11%) patients who experienced MACE and these patients had a median hospital length of stay of 56 days compared to 21 days for those who did not have MACE (net +35 days). It is evident that MACE will have a very significant impact on the overall hospital course.
One question is whether there were any distinguishing features that predisposed to MACE. For the MACE cohort, the median CPB time was 498 minutes, or just 25 minutes longer than the group as a whole. However, MACE was more frequent in patients who could not achieve complete repair (i.e., VSD left open) compared with those who underwent (or had previously undergone) complete septation. Specifically, there were three patients who had MACE in the unrepaired group out of 12 patients in this category (i.e., 25%). Eight patients experienced MACE in the repaired group out of 88 patients in this category (i.e., 9%). This finding is consistent with previous data indicating that failure to achieve complete repair following unifocalization procedures was associated with a 3.5-fold increased risk of mortality (7).
SUMMARY OF COLLATERAL FLOW
In patients undergoing a unifocalization procedure, we have previously demonstrated that there can be a significant amount of residual collateral flow following ligation of the MAPCAs (28). The residual collateral flow was assessed by measuring the left ventricular vent return during the aortic cross-clamp time. The average residual collateral flow return was 5.5% of the total pump flow and ranged from 0.8 to 15.2%. This collateral flow is siphoned off from the systemic perfusion while on-pump but might not be detected based on perfusion pressures alone. Patients with lower preoperative oxygen saturations have more residual collateral flow than patients who had higher oxygen saturations and supports the concept that hypoxemia stimulates the growth of collaterals. It is a matter of conjecture whether 5 or 6 hours of systemic perfusion at less than expected effective flow rates would have an adverse effect. We err on the side of higher flow rates and accept higher perfusion pressures during these procedures. This strategy was recently affirmed in a clinical study evaluating the incidence of renal injury after pediatric heart surgery (29).
There are two theoretical reasons why surgeons and perfusionists should consider performing a collateral flow study for clinical (rather than experimental) reasons in cases involving MAPCAs. First, the collateral flow study provides an objective measurement of the amount of flow that is being “lost” or “stolen” from the systemic perfusion. This measurement may provide additional guidance regarding adequate flow rates. The second theoretical reason is that an abnormally high value (i.e., >20%) may signal that an important MAPCA has not been identified. This scenario is more likely to occur at the inception of a program performing these complex procedures. It is imperative that surgeons and perfusionists communicate effectively to assure adequate perfusion during longer CPB cases.
REVIEW OF THE LITERATURE
There are numerous reports in the adult cardiac surgery literature suggesting that prolonged CPB may be associated with a higher prevalence of adverse outcomes (15–21). It is also acknowledged in this literature that prolonged CPB is inextricably linked with higher complexity and, in some circumstances, with intraoperative complications. Either one of these situations will generally require extended periods of aortic cross-clamp as well. This means that prolonged CPB in adult patients is almost always a surrogate marker for a higher risk patient and accounts for the association with adverse outcomes.
The literature on the relationship between CPB and outcomes in congenital heart surgery is currently much more limited in scope. Andropoulos et al. identified prolonged CPB as a predisposing factor to worse neurologic outcome and longer intensive care unit lengths of stay in patients undergoing neonatal arterial switch (22). Mastropietro et al. found an association between prolonged CPB (>150 minutes) and MACE in patients undergoing truncus arteriosus repair (23). This manuscript acknowledged that longer periods of CPB were often associated with more complex (truncal valve) issues and intertwined with considerably longer periods of aortic cross-clamp time. This relationship among complexity, CPB time, and aortic cross-clamp time is well summarized by Dr. Richard Jonas . . .. “This is the most obvious explanation for the finding that total bypass time continues to emerge regularly as a risk factor for morbidity and mortality after many operations” (24).
CONCLUSION
In summary, this review article was written to summarize our surgical and perfusion approach to patients undergoing complex pulmonary artery reconstructions requiring prolonged CPB. This review article also includes a summary of a study that we performed to evaluate the impact of prolonged CPB on outcomes. Our data demonstrate that there was a poor correlation between CPB time and both the total number of postoperative complications and hospital length of stay. Based on previously published papers and the current set of data, one can conclude that prolonged CPB is not a risk factor for adverse outcomes in this specific patient population. This finding is markedly different compared to the majority of existing literature in the field for other complex congenital or adult cardiac operations. It is likely that this difference is attributable to the unique aspects of pulmonary artery reconstruction procedures.
ACKNOWLEDGEMENT
There were no sources of funding.
REFERENCES
- 1.Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–85. [PubMed] [Google Scholar]
- 2.DeWall RA, Gott VL, Lillehei CW, et al. Total body perfusion for open cardiotomy utilizing the bubble oxygenator: Physiologic responses in man. J Thorac Cardiovasc Surg. 1956;32:591–603. [PubMed] [Google Scholar]
- 3.Radegran K. The early history of cardiac surgery in Stockholm. J Card Surg. 2003;18:564–72. [DOI] [PubMed] [Google Scholar]
- 4.Stoney WS. Evolution of cardiopulmonary bypass. Circulation. 2009;119:2844–53. [DOI] [PubMed] [Google Scholar]
- 5.McCracken C, Spector LG, Menk JS, et al. Mortality following pediatric congenital heart surgery: An analysis of the causes of death derived from the national death index. J Am Heart Assoc. 2018;7:e010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma M, Gauvreau K, Allan CK, et al. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83:1436–45. [DOI] [PubMed] [Google Scholar]
- 7.Mainwaring RD, Patrick WL, Roth S, et al. Surgical algorithm and results in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J Thorac Cardiovasc Surg. 2018;156:1194–204. [DOI] [PubMed] [Google Scholar]
- 8.Mainwaring RD, Patrick WL, Rosenblatt TR, et al. Surgical results of unifocalization revision. J Thorac Cardiovasc Surg. 2019;158:534–45. [DOI] [PubMed] [Google Scholar]
- 9.Monge M, Mainwaring RD, Sheikh AY, et al. Surgical reconstruction of peripheral pulmonary artery stenosis in Williams and Alagille syndromes. J Thorac Cardiovasc Surg. 2013;145:476–81. [DOI] [PubMed] [Google Scholar]
- 10.Mainwaring RD, Ibrahimiye AN, Hanley FL. Surgical techniques for reconstruction of peripheral pulmonary artery stenosis and other complex peripheral reconstructions. Ann Thorac Surg. 2016;102:e181–3. [DOI] [PubMed] [Google Scholar]
- 11.Mainwaring RD, Hanley FL. Surgical reconstruction of peripheral pulmonary artery stenosis. Semin Thorac Cardiovasc Surg. 2017;29:198–205. [DOI] [PubMed] [Google Scholar]
- 12.Collins RT, Mainwaring RD, MacMillen KL, et al. Outcomes of pulmonary artery reconstruction in Williams syndrome. Ann Thorac Surg. 2019:108:146–53. [DOI] [PubMed] [Google Scholar]
- 13.Martin E, Mainwaring RD, Collins RT, et al. Surgical outcomes for peripheral pulmonary artery stenosis in patients without Williams or Alagille syndromes. Semin Thorac Cardiovasc Surg. 2020;32:973–9. [DOI] [PubMed] [Google Scholar]
- 14.Margetson TD, Sleasman J, Kollmann S, et al. Perfusion methods and modifications to the cardiopulmonary bypass circuit for midline unifocalization procedures. J Extra Corpor Technol. 2019;51:147–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Salsano A, Giacobbe DR, Sportelli E, et al. Aortic cross-clamp time and cardiopulmonary bypass time: Prognostic implications in patients operated on for infective endocarditis. Interact Cardiovasc Thorac Surg. 2018;27:328–35. [DOI] [PubMed] [Google Scholar]
- 16.Qiang J, Mei Y, Wang X, et al. Risk factors for pulmonary complications following cardiac surgery with cardiopulmonary bypass. Int J Med Sci. 2013;10:1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Surg. 2006;22:814–22. [DOI] [PubMed] [Google Scholar]
- 18.Rustum S, Fleissner F, Beckmann E, et al. Is there an upper limit to cardiopulmonary bypass times? Ann Circ. 2017. doi: 10.17352/ac.000004. [Google Scholar]
- 19.Adamik B, Kubler A, Gozdzik A, et al. Prolonged cardiopulmonary bypass is a risk factor for intestinal ischaemic damage and endotoxaemia. Heart Lung Circ. 2017;26:717–23. [DOI] [PubMed] [Google Scholar]
- 20.Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg. 2011;9:104–9. [DOI] [PubMed] [Google Scholar]
- 21.Nissinen J, Biancari F, Wistbacka J-O, et al. Limits of aortic cross-clamp and cardiopulmonary bypass in adult cardiac surgery. Perfusion. 2009;24:297–305. [DOI] [PubMed] [Google Scholar]
- 22.Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurologic outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012;94:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastropietro CW, Amula V, Sassalos P, et al. Characteristics and operative outcomes for children undergoing repair of truncus arteriosus: A contemporary multicenter analysis. J Thor Cardiovasc Surg. 2019;157:2386–96. [DOI] [PubMed] [Google Scholar]
- 24.Jonas RA. Comprehensive Surgical Management of Congenital Heart Disease, 2nd ed. Boca Raton, FL: Taylor and Francis Group, CRC Press; 2014:181. [Google Scholar]
- 25.Mainwaring RD, Dixit M, Palmon M, et al. Prevalence of complications following unifocalization and pulmonary artery reconstruction procedures. World J Pediatr Congenit Heart Surg. 2020;11:704–11. [DOI] [PubMed] [Google Scholar]
- 26.Quinonez ZA, Downey L, Abbasi RK, et al. Anesthetic management during surgery for tetralogy of Fallot with pulmonary atresia and major aortopulmonary collateral arteries. World J Pediatr Congenit Heart Surg. 2018;9:236–41. [DOI] [PubMed] [Google Scholar]
- 27.Matte GS, del Nido PJ. History and use of del Nido cardioplegia at Boston Children’s Hospital. J Extra Corpor Technol. 2012;43:98–103. [PMC free article] [PubMed] [Google Scholar]
- 28.Mainwaring RD, Margetson TD, McCarthy P, et al. Measurement of residual collateral flow during repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. Ann Thorac Surg. 2019;108:154–9. [DOI] [PubMed] [Google Scholar]
- 29.Tadphale SD, Ramakrishnan K, Spentzas T, et al. Impact of different cardiopulmonary bypass strategies on renal injury after pediatric heart surgery. Ann Thorac Surg. 2021;111:1374–9. [DOI] [PubMed] [Google Scholar]