Abstract.
Several excellent guidelines and expert opinions on congenital hypothyroidism (CH) are currently available. Nonetheless, these guidelines do not address several issues related to CH in detail. In this review, the authors chose the following seven clinical issues that they felt were especially deserving of closer scrutiny in the hope that drawing attention to them through discussion would help pediatric endocrinologists and promote further interest in the treatment of CH.
1. How high should the levothyroxine (L-T4) dose be for initial treatment of severe and permanent CH?
2. What is the optimal method for monitoring treatment of severe CH?
3. At what level does maternal iodine intake during pregnancy affect fetal and neonatal thyroid function?
4. Does serum thyroglobulin differ between patients with a dual oxidase 2 (DUOX2) variants and those with excess iodine?
5. Who qualifies for a genetic diagnosis?
6. What is the best index for distinguishing transient and permanent CH?
7. Is there any cancer risk associated with CH?
The authors discussed these topics and jointly edited the manuscript to improve the understanding of CH and related issues.
Keywords: congenital hypothyroidism, the dual oxidase 2 (DUOX2), iodine, levothyroxine, molecular genetics
Introduction
Several excellent guidelines and expert opinions on congenital hypothyroidism (CH) (1,2,3,4) are currently available. However, clinicians may find it difficult to judge the quality of evidence for specific issues related to CH based on these guidelines and expert opinions.
In this review, we focused on seven clinical issues that have not been addressed in detail in the existing guidelines and are particularly important for clinical practice in Japan (e.g., iodine excess and dual oxidase 2 [DUOX2] variants) or have to do with advances in the study and treatment of CH. These clinical issues do not cover all aspects of CH, but the authors have attempted to address issues for which there is currently no clear-cut, high-evidence support. These discussions may be of practical value because they address concerns relevant to clinical practice.
1. How high should the levothyroxine (L-T4) dose be for the initial treatment of severe and permanent CH?
Long-term complications of severe CH include developmental delay and growth retardation, even with prompt L-T4 replacement (4,5,6). Together with the potential impact of hypothyroidism in utero, the initial L-T4 dose is an important issue.
Several studies suggest that patients with severe CH may not be adequately treated with the previously recommended dose of 6–8 μg/kg/d of L-T4 (7, 8). Thereafter, high-dose and early L-T4 replacement have been shown to achieve adequate development in severe CH (8, 9), and the guidelines since 2000 recommend an initial dose of 10–15 µg/kg/d. However, even early and high-dose L-T4 replacement resulted in a slightly lower intelligence quotient (IQ) in patients with severe CH than in controls (10). Early L-T4 replacement revealed no significant difference between the age at the start of treatment and intelligence prognosis, the latter of which was associated with low T4 levels at the initial visit (10). Thus, the optimal initial L-T4 dose remains an open question in patients with severe CH. This chapter summarizes reports published since 2010 on the initial dose of L-T4 and the long-term prognosis of patients with severe CH.
Since 2010, five neurodevelopmental studies on the initial L-T4 dosage in patients with CH have been published (Table 1) (5, 11,12,13,14). This review classifies the severity of CH by the free T4 (FT4) concentration as severe (< 0.39 ng/dL), moderate (0.39–0.78 ng/dL), and mild (0.78–1.17 ng/dL) in accordance with the most recent guidelines (4). Patients with severe CH had more subtle cognitive and motor impairments than those with mild or moderate CH despite an average initial L-T4 dose of 12.3 μg/kg/d (5). In severe CH, such as athyreosis, poor motor development was reported even with 15 µg/kg/d L-T4 (11), while others showed no difference from healthy siblings, with the equal doses (12). Favorable results have been reported with 10–15 µg/kg/d L-T4 in patients with CH due to ectopic or dyshormonogenesis (11, 12).
Table 1. Summary of neurodevelopmental studies with initial doses of L-T4 in CH patients since 2010.
A previous meta-analysis assessed the relationship between IQ and the initial L-T4 dosage based on CH severity (14). There were 438 patients with CH: 156 with severe CH (initial serum T4 ≤ 2 μg/dL or FT4 ≤ 3 pmol/L), and 282 with moderate or mild CH (initial serum T4 > 2 μg/dL or FT4 > 3 pmol/L). In 157 patients (36%; 47 with severe CH and 110 with mild/moderate CH), treatment was started with an initial dose of >10 μg/kg/d. The meta-analysis assessing found that with an initial dosage < 10 μg/kg/d, patients with severe CH showed a significantly greater decrease in IQ (< 8 μg/kg/d: –6.03; 95% confidence interval [CI]: –9.10, –2.96; 8–10 μg/kg/d: –9.2; 95% CI: –15.07, –3.33) than those with mild to moderate CH. In contrast, a dosage > 10 μg/kg/d was not associated with decreased IQ or quality of life (QOL), even in patients with severe CH (14). Oral administration of high-dose L-T4 shortened the time required for FT4 levels to normalize (12, 14).
However, overtreatment with high-dose L-T4 replacement during initial treatment may have adverse neurodevelopmental consequences. Seven previous studies evaluated the effects of overtreatment with L-T4, although the definition of overtreatment varied, and three of these studies (13, 15, 16) were conducted at the same institution (13,14,15,16,17,18,19). Craven et al. (17) found that an L-T4 dose above 12.5 μg/kg/d may lead to overtreatment, while Tuhan et al. and Vaidyanathan et al. (18, 19) similarly found a risk of overtreatment in patients receiving 12–17 μg/kg/d. In studies on the effects of L-T4 overtreatment, long-term follow-up after a 2-yr postnatal overtreatment period demonstrated lower IQ and increased incidence of attention deficit hyperactivity disorder at 11 yr of age (13, 16). However, Aleksander et al. (14) showed no difference in IQ between patients and their siblings, despite a similar duration of overtreatment. As few studies have compared the initial L-T4 dosage in patients with severe CH, the adverse effects of overtreatment with L-T4 for severe CH are not clearly known.
According to the studies mentioned above and the current guidelines (1,2,3,4), L-T4 10–15 μg/kg/d should be administered as the initial therapy for severe CH during the neonatal period. Patients with severe CH with athyreosis may require 15 μg/kg/d (11, 20). Clinicians should determine the dosage based on the severity of hypothyroidism, history of iodine exposure, maternal thyroid disease, prenatal medications, and the presence of thyroid structural abnormalities. Clinicians should monitor laboratory data and clinical symptoms after the start of L-T4 treatment to avoid underdosing or overdosing.
Answering the present question requires a study of two L-T4 dosages (10–15 and 15 μg/kg/d), ideally in a cohort of patients with comparable disease severity. The endpoints should include not only short-term thyroid function but also long-term neurodevelopmental outcomes.
2. What is the optimal method of monitoring the treatment of severe CH?
Maintaining serum FT4 levels between the mean and upper limit of the normal range in each age group while maintaining serum TSH levels within the normal range has been proposed as a goal of L-T4 replacement therapy for CH (2, 3). The rationale for this recommendation is the negative correlation between serum TSH and FT4 levels. However, T3 is more bioactive than T4, and conversion of T4 into T3 occurs in TSH-producing cells. In type 2 selenodeiodinase gene (DIO2) knockout mice, serum T3 levels did not differ from those of wild-type mice whereas serum T4 and TSH levels were higher, indicating that the conversion from intracellular T4 into T3 is crucial in the feedback regulation of TSH secretion (21). Moreover, approximately 20% of serum T3 is directly derived from the thyroid gland, whereas the remaining 80% is produced by converting T4 (22). Therefore, the regulation of serum TSH levels involves not only the serum T4 levels, but also the serum and local (in TSH-producing cells) T3 levels.
The recommendation to maintain T4 in the upper range is not entirely evidence based (2, 3). Approximately 43% of infants with CH and 10% of children with CH reportedly had high TSH levels relative to FT4, suggesting negative feedback resistance of the hypothalamic–pituitary–thyroid (HPT) axis to serum T4 (23). Indeed, patients with severe CH, such as athyrosis or ectopic thyroid, fail to achieve a normal serum TSH value despite their serum FT4 level exceeding the upper limit of the normal range and their FT3 level being at the lower limit of the normal range. It is still unclear whether monitoring treatment for severe CH should be based on serum TSH or FT4 levels in patients with high TSH levels relative to FT4.
The mechanism underlying the imbalance between serum FT4 and TSH levels in patients with severe CH remains unclear. One possibility is that fetal hypothyroidism may be associated with impaired development of the HPT axis (24). Epigenetic changes in feedback control of the HPT axis have been observed in humans (25). Second possibility is the involvement of T3 in the negative feedback of the HPT axis. Thyroid aplasia or severe hypoplasia may downregulate T3 secretion from the thyroid gland, resulting in a low FT3 level and inadequate TSH suppression, even after L-T4 replacement normalizes the serum FT4 levels (26). Other possibilities include the effect of the timing of blood sampling on the results after oral L-T4 administration. Serum FT4 levels can reportedly increase by up to 20% two–nine hours after L-T4 administration (27).
Although high TSH is often observed in the presence of high T4 as discussed above, the acceptable upper limit of the serum FT4 level required to normalize TSH has yet to be established. A TSH level below the lower detection limit of an assay likely indicates L-T4 overdose. Previous studies have reported conflicting results on this topic. L-T4 overdosing during CH replacement has a negative effect on cognitive function and intellectual development in children (13, 28). However, in their study of 104 patients with permanent CH (P-CH), Aleksander et al. found no association between the frequency of high T4 up to the age of 2 yr and IQ at the age of 10 yr or older (14). They further suggested that the higher T4 levels in CH patients may not represent a state of “overtreatment” but may indicate that more T4 is needed by thyrotrophs to achieve normal T3 levels (14).
Normalization of serum FT3 levels may be important for suppressing serum TSH levels during the treatment of severe CH. In adult post-total thyroidectomy patients, suppressing TSH to a range of 0.03–0.3 μIU/mL during L-T4 replacement reportedly resulted in a normal levels of serum FT3 and metabolic markers equivalent to the preoperative values (29). In patients with CH caused by thyroid aplasia, as in patients undergoing total thyroidectomy, suppression of TSH levels to the range described above may be necessary to achieve an acceptable level of FT3 and metabolic markers. However, Bagattini et al. compared the amount of L-T4 replacement and thyroid function test results in 13 patients with congenital thyroid aplasia (mean age: 21.5 ± 2.1 yr) and 23 patients after a total thyroidectomy (mean age: 24.0 ± 2.7 yr) (24) by examining the daily, weight-based L-T4 dosage and reported that serum TSH and FT4 were significantly higher in patients with athyrosis than in patients after a total thyroidectomy (TSH 1.8 ± 0.8 vs 1.03 ± 0.67 μIU/mL, FT4 1.32 ± 0.18 vs 1.18 ± 0.19 ng/dL) and FT3 levels were not different, despite a higher L-T4 dosage per body weight (2.16 ± 0.36 vs 1.73 ± 0.24 μg/kg/d). This difference was thought to be due to fetal thyroid hormone deficiency, resulting in a shift in the HPT axis setpoint for T4, but the detailed mechanism underlying this difference is still unknown (24).
In summary, the optimal monitoring parameters for severe CH are unknown, and it is unclear whether normalization of serum TSH is the highest priority, what the upper limit of an acceptable FT4 level is, and how high the FT3 level should be maintained. To identify an optimal method for monitoring treatments for severe CH, it is necessary to evaluate the impact of each parameter (TSH, FT4, FT3, FT4 to FT3 ratio, etc.) using intellectual development, anthropometric indicators, and metabolic markers as outcomes.
3. At what level does maternal iodine intake during pregnancy affect fetal and neonatal thyroid function?
Maternal iodine intake affects fetal and neonatal thyroid functions because iodine can cross the placental barrier. Although maternal iodine deficiency is a well-known cause of high TSH levels in newborn screening (NBS) worldwide, neonates with high TSH levels in NBS caused by maternal iodine excess have also been reported in Japan (30, 31).
Excess iodine causes hypothyroidism by downregulating thyroid hormone synthesis, a phenomenon known as the Wolff–Chaikoff effect, which generally resolves within several days in adults whereas fetuses and neonates with immature thyroid glands suffer longer lasting damage (32). Iodine-related fetal and neonatal hypothyroidism is suspected if a goiter is present and may be confirmed with a blood test (33). Because fetal exposure to maternal iodine excess is limited by the gestational period, the resulting hypothyroidism theoretically has a transient clinical course, with P-CH being a rare exception (31, 34).
Transplacental passage of iodine occurs with 1) administration of iodine-containing medicines, 2) intake of iodine-containing supplements or 3) intake of iodine-rich food alone. First, amiodarone, a typical iodine-containing medication, contains 37.5 mg of iodine in a 100 mg tablet formulation. The incidence of neonatal hypothyroidism caused by the administration of 200–1,600 mg of amiodarone during pregnancy is reportedly 23% (35). Since amiodarone is metabolized in the liver and approximately 3.5 mg of inorganic iodine is released into the systemic circulation per 100 mg tablet formulation, 200–1,600 mg of amiodarone is equivalent to 7–56 mg of iodine (36). Potassium iodide is administered to pregnant women with mild Graves’ disease; however, it is not an established treatment. Momotani et al. reported that when pregnant women with mild Graves’ disease were treated with 6–40 mg/d iodine, a relatively low dosage range for adults, only two of 35 fetuses showed elevated TSH with normal FT4 (37).
Another iodine-containing medical substance common in Japan is an oil-soluble iodinated contrast medium (ethiodized oil) used in hysterosalpingography (HSG). Post-HSG hypothyroidism has been reported in more than 20 Japanese fetuses and neonates over the past 30 yr (38,39,40). In Japan, the frequency of neonatal hypothyroidism after the use of this contrast medium is 2.4% (39). The low incidence of similar cases of HSG-related fetal or neonatal hypothyroidism in other countries (41) indicates that certain factors may influence hypothyroidism development. The higher incidence reported in Japan is thought to be associated with a high consumption of iodine-rich foods such as seaweed, although this association has not been established. Water-soluble iodinated contrast medium, which has a shorter iodine half-life than the oil-soluble version, is currently recommended for use with HSG in infertile women in Japan (39) although it may still not eliminate the risk of developing thyroid dysfunction. The use of povidone-iodine mouthwash during pregnancy and iodine-containing disinfectants during delivery is also known to cause neonatal hypothyroidism (3). In view of the risk to neonatal thyroid function, iodine-free formulations such as azulene mouthwash and chlorhexidine disinfectant should be used instead.
Second, fetal and neonatal hypothyroidism resulting from the maternal ingestion of nutritional supplements containing large quantities of iodine has also been reported worldwide (33, 42,43,44). In the United States, the use of iodine-containing supplements for healthy infant brain development is encouraged because pregnant women are generally deficient in dietary iodine (45). However, the amount of iodine in health food supplements is not strictly controlled by regulatory authorities such as the Food and Drug Administration and can be quite high (46). Moreover, supplements containing large amounts of potassium iodide (e.g., 12.5 mg in a single tablet) are commercially available in some developed countries. In contrast, pregnant women in Japan normally have sufficient amount of dietary iodine for the reasons described below.
Third, maternal dietary iodine excess alone may lead to fetal and neonatal hypothyroidism. In Japan, where iodine-rich seaweed is consumed in large quantities, Nishiyama et al. reported that maternal iodine excess during pregnancy was observed in 15 of 34 infants who tested positive on CH screening (31). Similar findings have been reported by Asakura et al. (30). However, these reports failed to consider the possibility of co-occurrence of a genetic abnormality causing CH in the infants studied. Further research is needed to investigate whether the frequency of CH increases when excess iodine is added to genetic predisposition.
The upper limit of the daily iodine intake varies by country. The upper limit in healthy adults is 1,100 μg in the United States (47). In contrast, since the daily iodine intake of 1,000–3,000 μg in Japanese adults is not associated with any adverse effects, the upper limit is 3,000 μg for healthy adults (48). A possible reason for the difference between the two countries is that the absorption rate of iodine contained in kelp, a major dietary source of iodine for Japanese people, may be lower than the absorption rate of iodine contained in other foods (49).
The amount of iodine intake in the previously cited study by Nishiyama et al. (31) was 820–3,200 μg/d, suggesting that even a daily maternal iodine intake below the upper limit of 2,000 μg, which is the upper limit for pregnant women in Japan (48), may affect thyroid function in the fetus or neonate. Further research is needed to determine an appropriate upper limit for pregnant Japanese women in the context of post-delivery neonatal thyroid function.
In summary, maternal iodine excess may cause transient fetal and neonatal hypothyroidism. Although the upper intake level in pregnant women is unclear, pregnant women should be made aware of the risks of fetal and neonatal hypothyroidism caused by excessive dietary iodine.
4. Does serum thyroglobulin (Tg) differ between patients with a DUOX2 variant and those with excess iodine?
Both DUOX2 variants and iodine excess are common causes of transient CH (T-CH) with goiter in Japan (31, 50). However, determining which of the two is the cause in a particular case based on physical findings and thyroid function test results alone is difficult. In addition, the diagnosis of iodine excess is not straightforward because iodine excess cannot always be diagnosed by the urinary iodine value due to individual differences in sensitivity to iodine, as hypothyroidism develops only in a small percentage of patients who have received iodinated contrast medium (ethiodized oil) during HSG (39). However, differentiating between DUOX2 variants and iodine excess is important for predicting subsequent follow-ups. Thyroid goiter and hypothyroidism have been reported in adult patients with DUOX2 variants, suggesting a need for long-term follow-up (51).
Tg, along with iodine and peroxidase, is essential for thyroid hormone synthesis. Serum Tg levels increase when thyroid hormone synthesis is impaired because of a congenital organification defect, such as that observed in patients with a TPO or DUOX2 variant. Neonatal hypothyroidism caused by iodine excess also reportedly increases Tg transiently. (42). Serum Tg level is frequently measured at CH diagnosis as a marker of dyshormonogenesis (DH) and iodine excess. However, no previous study has directly compared Tg levels in the two pathological conditions. In this section, we will discuss whether serum Tg levels during the neonatal period can distinguish between these two diseases.
Although there are several ways to answering this question, we reviewed studies analyzing the serum Tg levels in patients with a biallelic DUOX2 variant and those with evident iodine excess-related CH were reviewed. Patients with a DUOX2 variant show a wide clinical spectrum (50, 52, 53); those with a biallelic variant who often present more severe manifestations were considered here. Patients with post-HSG CH were included in the present analysis, as in a previous study by one of the authors (39). Most CH cases detected by HSG using an oil-based contrast medium have been reported in Japan; the patients had severe CH clearly stemming from iodine excess. Finally, patients whose Tg levels were measured during the neonatal period were included, as the Tg levels are normally age-dependent, particularly during the early months of life.
Studies on the characteristics of patients with CH with a DUOX2 variant have reported a high serum Tg level upon diagnosis. Maruo et al. measured Tg levels in 20 of 32 patients with CH with a DUOX2 variant and found it to be above 800 ng/mL in 17 patients (54). Jin et al. reported that serum Tg levels in five of ten patients with CH with a biallelic DUOX2 variant were 20.2, 101, 453, 500, and 1,560 ng/mL (55). Narumi et al. reported that the serum Tg level at diagnosis in three of nine patients with CH with a biallelic DUOX2 variant were 1,600, 1,500, and 710 ng/mL, respectively (56). Muzza et al. examined CH with elevated serum Tg levels or a hyperplastic thyroid gland on ultrasonography (US) and found that 11 of 30 patients harbored a biallelic variant impairing DUOX2 activity. Although the serum Tg value in patients with a DUOX2 variant overlapped with that in patients without the variant, the former had more severe symptoms along with a higher serum Tg level at diagnosis (median: 655 vs 426 ng/mL; range: 403–1,991 vs 54.4–3,000) (57).
With respect to iodine excess, Satoh et al. reported that hypothyroidism developed in five of 212 infants born after HSG (39). The urinary iodine concentration (UIC) in four of the five infants was 1,150, 940, 1,570, and 319 μg/L, which showed a rather large variation but was nonetheless higher than the median UIC of 121.0 μg/L in the first voided urine of Japanese neonates (58). The serum Tg level in two of the five infants was 178.9 and 1,219 ng/mL, respectively (39). Tachibana et al. reported that among 48 infants with a high TSH level on NBS, 15 with high urinary total iodine (> 500 μg/L) and hypothyroidism had Tg levels ranging from 111–7,089 ng/mL (59). This report shows a relationship between urinary iodine and serum Tg level in infants with CH but does not describe the cause of iodine excess or diagnostic criteria.
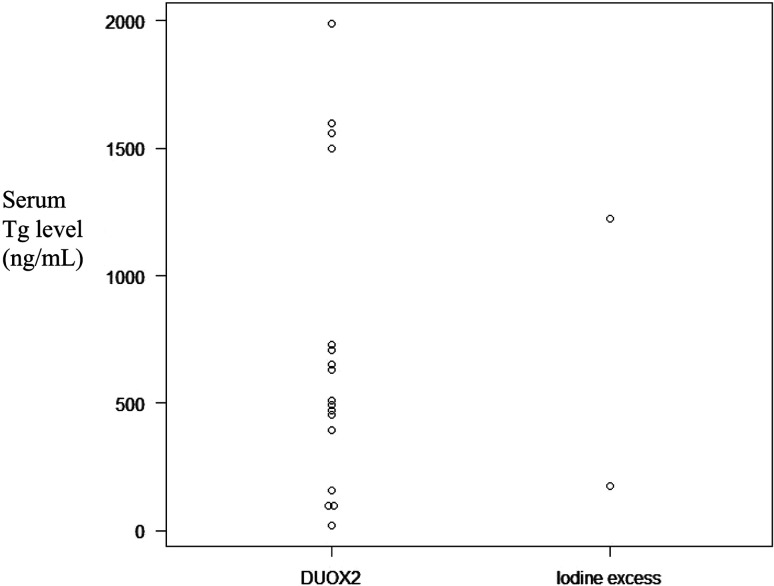
Previous studies have demonstrated the difficulty in distinguishing between hypothyroidism resulting from a DUOX2 variant or excess iodine based on the Tg level alone. The serum Tg level in neonates with a homozygous or compound heterozygous variant of DUOX2 ranged from 20.2, an extremely low value, to 1,991 ng/mL, and the serum Tg levels in the two neonates with iodine excess were 178.9 and 1,219 ng/mL, respectively, showing that the two causes of hypothyroidism can have overlapping Tg levels (Fig. 1). The differences in the serum Tg levels between the two infants with hypothyroidism due to iodine excess may indicate that the Wolff–Chaikoff effect and/or transfer of iodine to the fetus varies from case to case. The cause of the wide variation in Tg levels associated with DUOX2 variants is currently unknown. Multiple factors are possible, including differences in the severity of DUOX2 variants, presence of other genetic variants, and iodine intake. Since there are few reports of the serum Tg levels in neonates with hypothyroidism caused by excess iodine or a DUOX2 variant, further research is needed before any definitive conclusions can be drawn.
Fig. 1.
Dot chart of serum Tg in patients with hypothyroidism with biallelic DUOX2 variants or iodine excess. The left chart shows the DUOX2 variant, and the right chart shows iodine excess. The values were drawn from previous reports (39, 54,55,56,57).
5. Who qualifies for a genetic diagnosis?
Molecular studies focusing on a limited number of candidate genes have been conducted in patients with specific clinical characteristics or family histories. Identification of causative genes (Table 2) helped us to understand the mechanisms of thyroid development and hormone synthesis. Most P-CH cases are subclassified as thyroid dysgenesis (TD) or DH. The overall detection rate of potentially pathogenic variants in the analysis of CH candidate genes in Japan is approximately 20% (56, 60,61,62).
Table 2. Summary of causative genes in congenital hypothyroidism and clinical features.
Most cases of TD are sporadic, and their etiology is unclear. Less than 5% of TD cases are attributable to potentially pathogenic variants of the known genes that regulate thyroid gland development (60). Some patients have extrathyroidal complications. For example, patients with an NKX2-1 variant may experience extra-thyroidal complications, such as benign chorea, whereas those with a PAX8 variant may have urogenital tract malformations (Table 2). Careful phenotypic description of CH patients from both thyroidal and non-thyroidal perspectives is important for identifying a candidate gene.
CH is also associated with several other malformations. Recent genetic studies have identified candidate genes associated with syndromic CH, such as DYRK1A (Down syndrome), TBX1 (22q11.2 deletion syndrome), JAG1 (Alagille syndrome) and KAT6B (Ohdo syndrome, Genitopatellar syndrome) (63,64,65,66,67).
In contrast, most individuals with DH harbor variants in genes encoding known components of the thyroid hormone biosynthesis machinery. These variants cause loss of function, resulting in inadequate thyroid hormone synthesis with or without compensatory goiter. The most frequent gene in the DH group was DUOX2, and the phenotype was usually transient (54, 56). In most cases of DH, the CH is isolated. Pendred syndrome (SLC26A4 variant), in which patients experience sensorineural hearing loss, is an exception to this.
Next-generation sequencing (NGS) has brought about a major change in the conventional approach for diagnosing and understanding the molecular basis of CH by detecting several novel genes involved in the organogenesis of thyroid tissue (68). Furthermore, targeted NGS panels have already provided an efficient means of identifying gene variants in the coding regions of known CH genes (61, 62, 69). Although most cases are monogenic, oligogenic inheritance of CH has also been confirmed using this technology (62, 70). Further studies are required to establish a method for diagnosing oligogenic disorders. NGS approaches have also enabled the screening of genes in large CH populations (irrespective of TD or DH), demonstrating overlapping genetic etiologies in the TD and DH subgroups (71).
Finally, the cost and time required for NGS have decreased over the past several years, and bioinformatics analysis in silico is now widely used (although the accuracy of the analysis is not yet optimal). The authors believe that these trends will continue for some time. Thus, in clinical practice, genetic testing using NGS will soon become a powerful tool for clinicians to identify the genetic etiology of CH.
Although the molecular and genetic mechanisms underlying CH have been elucidated, the exact cause of CH remains obscure in many cases, especially in TD. Whole genome analysis, such as exome analysis, may be considered for research involving undiagnosed cases with multiple congenital anomalies or a family history. The contribution of other genetic and environmental factors should also be considered when attempting to clarify the etiology. The former includes regulatory regions, intronic mutations, and copy number variants in the genes of interest. In the future, alternative mechanisms, such as epigenetic modifications, should be further explored to understand their contribution to CH (72).
6. What is the best index for distinguishing transient and permanent CH?
Clinically, the distinction between P-CH and T-CH is one of the most important concerns for parents of affected children, excluding cases of TD that can be easily diagnosed by US or scintigraphy. Usually, thyroid function test findings are re-evaluated after discontinuing L-T4 treatment at age 3 yr of age or older to differentiate between P-CH and T-CH (2, 3). The latest European consensus guidelines for CH stated that the clinician may consider stopping treatment after 1 yr of age (2). However, these guidelines do not clarify the characteristics of patients in whom treatment may be stopped before the age of 3 yr.
Many studies have reported that the L-T4 dosage is a useful predictor of the clinical course of hypothyroidism (Table 3) (73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89). Most reports revealed a significant difference at the age of 1 yr, at which the mean L-T4 dosage for P-CH and T-CH was 3.1–4.5 µg/kg/d and 1.9–3.4 µg/kg/d, respectively (74, 76, 78, 80, 83, 84, 86, 87). Because of the variations among the studies and the overlap in the mean values of the two groups, complete distinction between the groups based on the L-T4 dosage was not possible. Three reports suggested the possibility of predicting the clinical course of hypothyroidism at the age of 1 yr based on receiver operating characteristic (ROC) analysis (77, 84, 85). The area under the curve (AUC) of the ROC curves was 0.61–0.73 at the age of 1 yr. A L-T4 dosage exceeding 4.7–4.9 µg/kg/d and below 1.7–1.8 µg/kg/d at the age of 1 yr may help predict P-CH and T-CH, respectively (77, 85). The specificity of these cut-off values was 97–100%. At older ages, both the sensitivity and specificity were higher, with the AUC of the ROC curves being 0.73–0.80 at the age of 2 yr and 0.82–0.83 at the age of 3 yr (77, 85).
Table 3. L-T4 dosage per body weight as predictor of P-CH and T-CH.
Some researchers have reported that predicting P-CH and T-CH was possible at the age of 90–180 d (79, 83). However, the sensitivity and specificity of the L-T4 dosage as a predictor tended to be lower in younger populations. As age 90–180 d is too early to discontinue L-T4, this conclusion may be difficult to apply in clinical practice.
Two additional parameters for discriminating between P-CH and T-CH have been reported. First, Yamamura et al. suggested that an increase in the L-T4 dosage at age ≥ 3 yr is useful for distinguishing P-CH from T-CH (89). Second, the TSH level at diagnosis also reportedly had a predictive value (76, 83), although other reports showed no significant difference in TSH levels between P-CH and T-CH (73,74,75, 77, 80, 84, 85, 87, 89). Some studies have examined the Tg levels at diagnosis, albeit in only in a few subjects (75, 78).
Recently, Mehran et al. developed a model for predicting P-CH and T-CH development in patients up to 1 yr of age based on a forward stepwise multivariable logistic regression analysis of 1,047 patients with CH (90). The equation for predicting P-CH risk included confirmed TSH levels, total T4 < 8.2 μg/dL, increased L-T4 requiring dosage up to the age of 1 yr, 6-mo duration of TSH >10 μIU/mL, parental consanguinity, and a family history of thyroid disease. This prediction model had a significantly greater diagnostic value than TSH or total T4 level alone.
It is unclear for how long patients with suspected T-CH should be followed up after L-T4 discontinuation. In our previous study, patients with CH were followed up till the age of 15 yr or older (85), whereas the duration of observation after L-T4 discontinuation was < 1 yr in almost all other studies (73,74,75,76,77,78,79,80,81, 83,84,85,86,87). In patients with P-CH in our study, the longest L-T4-free period after discontinuation was 1.2 yr (85). This indicates that some patients may require L-T4 administration again within approximately 1 yr. The length of follow-up after L-T4 discontinuation should be longer than 1 yr. However, few studies besides ours have been designed to observe patients for more 1 yr after L-T4 discontinuation.
To better understand whether the L-T4 dosage is a useful prediction, a prospective study investigating not only thyroid function, but also the physical and mental development, of patients with CH is warranted. The increased use of genetic testing has clarified the spectrum of clinical expressions and the genotype–phenotype correlation. In particular, patients with a DUOX2 variant should be analyzed separately because they are usually treated for short periods despite having severe hypothyroidism during the neonatal and early postnatal periods (54).
In conclusion, the L-T4 dosage is one of the best means of differentiating P-CH from T-CH. The L-T4 dosage at 1 yr of age had an especially high diagnostic accuracy with high sensitivity and specificity. The addition of some other factors may improve the diagnosis of P-CH and T-CH.
7. Is there any cancer risk associated with CH?
Several cases of thyroid cancer as a complication of CH with or without neonatal hypothyroidism were reviewed, including patients with DH or TD, and the mechanism of carcinogenesis. There are a few previous reports of thyroid cancer in patients with CH caused by DH, which did not include genetic analysis (91,92,93,94,95). Recently, thyroid cancer complicated by DH stemming from variants of TG (96,97,98,99,100), TPO (101, 102), NIS (103), and PDS (104, 105) have been reported (Table 4). These cancers can develop at various ages but are most common in middle-aged individuals and can be aggressive (91,92,93,94,95,96,97,98,99,100,101,102,103,104,105). Few cancer-driver gene variants have been identified in thyroid cancer associated with DH (Table 4), although the presence of such variants is generally required for carcinogenesis (106).
Table 4. Summary of case reports of thyroid dyshormonogenesis with gene variants associated with thyroid cancer.
The mechanisms implicated in the development of thyroid cancer in patients with thyroid DH are not yet fully understood. Constant and prolonged TSH stimulation may result in goiter, thyroid nodules, or thyroid cancer (91, 97, 100, 107,108,109). TSH is a growth factor of thyroid epithelial cells that can promote thyroid nodule formation and cancer progression (100). A higher serum TSH concentration was found in children and adolescents with differentiated thyroid cancer than in those with benign thyroid nodules (109). In contrast, a large, multinodular goiter can develop despite early treatment with L-T4, resulting in normal TSH levels (99, 102). Cancer in the absence of elevated serum TSH levels indicates that genetic and environmental factors other than TSH levels may play a role in oncogenesis, and the precise cancer risk in CH patients who receive L-T4 replacement therapy from early infancy is currently unknown.
The frequency of thyroid cancer in patients with a TG variant is higher than that in the general populations (96). Hishinuma et al. reported thyroid cancer in 11 of 25 patients with a TG variant (44%) (110). Most of these patients were diagnosed before the initiation of NBS and had normal thyroid function at diagnosis. Some patients with TG variants may have TSH levels in the normal range, possibly because of sustained TSH-stimulated thyroid growth, which partially compensates for thyroid hormone production (96, 111). It is not yet clear whether early L-T4 treatment can prevent the development of thyroid cancer; however, early L-T4 treatment in patients with TG abnormalities who were diagnosed positive as positive for NBS did not develop goiter, suggesting that early LT4 treatment may prevent thyroid cancer development (96). As a mechanism of carcinogenesis in TG abnormalities, abnormal folding and transport of Tg protein to the Golgi apparatus has been suggested to result in retention of the abnormal proteins in the endoplasmic reticulum (ER), which may trigger carcinogenesis due to ER stress in patients with TG variants. (110, 112).
Biochemical evidence suggests that thyroid peroxidase (TPO) may promotes oncogenesis. TPO reduces hydrogen peroxide (H2O2) and attaches iodine to the tyrosyls residues in Tg. In the context of pathological TPO deficiency, H2O2 produced by DUOXs can diffuse through the apical membrane of thyrocytes and reach the nucleus directly or via redox signaling pathways, possibly activating intracellular NADPH oxidase 4 (NOX4) in the nucleus and ER. The presence of NOX4 in the perinuclear region might increase nuclear oxidative stress and promote DNA damage and genomic instability (113, 114). However, there are no reports of a higher frequency of cancer in TPO variant cases than that in the general population.
Several studies of patients with thyroid hemi-agenesis (THA), defined as the absence of one thyroid lobe, reported thyroid cancer as a complication of this condition (Supplementary Table 1), even in patients as young as 14 yr of age (115). Cancer occurs more frequently in female, than in male, probably because the frequency of THA is higher among female patients (116) and develops at approximately the same rate in the right and left residual lobes. To the best of our knowledge, no case of hypothyroidism has ever been reported in a patient with cancer associated with THA. It is unclear whether the frequency of thyroid cancer in THA patients is higher than that in the general population.
Many cases of thyroid cancer were associated with ectopic thyroid (Supplementary Table 2). Massine et al. summarized 28 cases of lingual thyroid cancer that occurred between the ages of 18 and 86 yr, before 2000 (117). Carcinoma arising from the lingual thyroid are rare, with an incidence of approximately 1% (117, 118). The risk of malignant transformation is no more likely than that in orthotopic thyroid, and the risk factors (ionizing radiation, family history, etc.) are the same (117). Thus far, genetic analysis of transcription factors in patients with ectopic thyroid cancer has failed to yield any significant findings.
In conclusion, the risk of thyroid cancer may be higher in patients with a TG variant, but it remains unknown whether the occurrence rate of cancer in other DH and TD patients is not higher than that in the general population. Careful follow-up with physical examination and US is necessary, especially in patients with poorly controlled disease and resulting prolongation of TSH elevation or in patients with thyroid nodules or a goiter. Determining the cancer risk associated with CH with thyroid DH and TD is difficult because of the rarity of pediatric thyroid cancer and the requirement for long-term follow-up to achieve meaningful endpoints.
Conflict of interests
The authors have no conflicts of interest.
Supplementary Tables
Acknowledgments
We would like to thank Mr. James R. Valera for his assistance with editing this manuscript.
References
- 1.Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, et al. American Academy of PediatricsSection on Endocrinology and Committee on Genetics, American Thyroid AssociationPublic Health Committee, Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006;117: 2290–303. doi: 10.1542/peds.2006-0915 [DOI] [PubMed] [Google Scholar]
- 2.Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAECongenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab 2014;99: 363–84. doi: 10.1210/jc.2013-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagasaki K, Minamitani K, Anzo M, Adachi M, Ishii T, Onigata K, et al. Mass Screening CommitteeJapanese Society for Pediatric EndocrinologyJapanese Society for Mass Screening. Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin Pediatr Endocrinol 2015;24: 107–33. doi: 10.1297/cpe.24.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Trotsenburg P, Stoupa A, Léger J, Rohrer T, Peters C, Fugazzola L, et al. Congenital hypothyroidism: a 2020-2021 consensus guidelines update-an ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021;31: 387–419. doi: 10.1089/thy.2020.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Sluijs Veer L, Kempers MJ, Wiedijk BM, Last BF, Grootenhuis MA, Vulsma T. Evaluation of cognitive and motor development in toddlers with congenital hypothyroidism diagnosed by neonatal screening. J Dev Behav Pediatr 2012;33: 633–40. doi: 10.1097/DBP.0b013e3182690727 [DOI] [PubMed] [Google Scholar]
- 6.Ng SM, Anand D, Weindling AM. High versus low dose of initial thyroid hormone replacement for congenital hypothyroidism. Cochrane Database Syst Rev 2009; CD006972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubuis JM, Glorieux J, Richer F, Deal CL, Dussault JH, Van Vliet G. Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine treatment. J Clin Endocrinol Metab 1996;81: 222–7. [DOI] [PubMed] [Google Scholar]
- 8.Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr 2000;136: 292–7. doi: 10.1067/mpd.2000.103351 [DOI] [PubMed] [Google Scholar]
- 9.Salerno M, Militerni R, Bravaccio C, Micillo M, Capalbo D, Di MS, et al. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid 2002;12: 45–52. doi: 10.1089/105072502753451968 [DOI] [PubMed] [Google Scholar]
- 10.Dimitropoulos A, Molinari L, Etter K, Torresani T, Lang-Muritano M, Jenni OG, et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr Res 2009;65: 242–8. doi: 10.1203/PDR.0b013e31818d2030 [DOI] [PubMed] [Google Scholar]
- 11.Hauri-Hohl A, Dusoczky N, Dimitropoulos A, Leuchter RH, Molinari L, Caflisch J, et al. Impaired neuromotor outcome in school-age children with congenital hypothyroidism receiving early high-dose substitution treatment. Pediatr Res 2011;70: 614–8. doi: 10.1203/PDR.0b013e3182321128 [DOI] [PubMed] [Google Scholar]
- 12.Albert BB, Heather N, Derraik JG, Cutfield WS, Wouldes T, Tregurtha S, et al. Neurodevelopmental and body composition outcomes in children with congenital hypothyroidism treated with high-dose initial replacement and close monitoring. J Clin Endocrinol Metab 2013;98: 3663–70. doi: 10.1210/jc.2013-1903 [DOI] [PubMed] [Google Scholar]
- 13.Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J Clin Endocrinol Metab 2013;98: 4499–506. doi: 10.1210/jc.2013-2175 [DOI] [PubMed] [Google Scholar]
- 14.Aleksander PE, Brückner-Spieler M, Stoehr AM, Lankes E, Kühnen P, Schnabel D, et al. Mean high-dose l-thyroxine treatment is efficient and safe to achieve a normal IQ in young adult patients with congenital hypothyroidism. J Clin Endocrinol Metab 2018;103: 1459–69. doi: 10.1210/jc.2017-01937 [DOI] [PubMed] [Google Scholar]
- 15.Bongers-Schokking JJ, Resing WC, Oostdijk W, de Rijke YB, de Muinck Keizer-Schrama SM. Individualized treatment to optimize eventual cognitive outcome in congenital hypothyroidism. Pediatr Res 2016;80: 816–23. doi: 10.1038/pr.2016.159 [DOI] [PubMed] [Google Scholar]
- 16.Bongers-Schokking JJ, Resing WCM, Oostdijk W, de Rijke YB, de Muinck Keizer-Schrama SMPF. Relation between early over- and undertreatment and behavioural problems in preadolescent children with congenital hypothyroidism. Horm Res Paediatr 2018;90: 247–56. doi: 10.1159/000494056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craven M, Frank GR. Does initial dosing of levothyroxine in infants with congenital hypothyroidism lead to frequent dose adjustments secondary to iatrogenic hyperthyroidism on follow-up? J Pediatr Endocrinol Metab 2018;31: 597–600. doi: 10.1515/jpem-2017-0513 [DOI] [PubMed] [Google Scholar]
- 18.Tuhan H, Abaci A, Cicek G, Anik A, Catli G, Demir K, et al. Levothyroxine replacement in primary congenital hypothyroidism: the higher the initial dose the higher the rate of overtreatment. J Pediatr Endocrinol Metab 2016;29: 133–8. doi: 10.1515/jpem-2015-0047 [DOI] [PubMed] [Google Scholar]
- 19.Vaidyanathan P, Pathak M, Kaplowitz PB. In congenital hypothyroidism, an initial L-thyroxine dose of 10-12 μg/kg/day is sufficient and sometimes excessive based on thyroid tests 1 month later. J Pediatr Endocrinol Metab 2012;25: 849–52. doi: 10.1515/jpem-2012-0025 [DOI] [PubMed] [Google Scholar]
- 20.Uyttendaele M, Lambert S, Tenoutasse S, Boros E, Ziereisen F, Van Vliet G, et al. Congenital hypothyroidism: Long-term experience with early and high levothyroxine dosage. Horm Res Paediatr 2016;85: 188–97. doi: 10.1159/000443958 [DOI] [PubMed] [Google Scholar]
- 21.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 2001;15: 2137–48. doi: 10.1210/mend.15.12.0740 [DOI] [PubMed] [Google Scholar]
- 22.Peeters RP, Visser TJ. Metabolism of Thyroid Hormone. 2017. 2017/01/01. In: Endotext [Internet]. MDText.com, Inc. Available from: https://www.ncbi.nlm.nih.gov/pubmed/.
- 23.Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI, et al. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab 2000;85: 2722–7. doi: 10.1210/jcem.85.8.6718 [DOI] [PubMed] [Google Scholar]
- 24.Bagattini B, Cosmo CD, Montanelli L, Piaggi P, Ciampi M, Agretti P, et al. The different requirement of L-T4 therapy in congenital athyreosis compared with adult-acquired hypothyroidism suggests a persisting thyroid hormone resistance at the hypothalamic-pituitary level. Eur J Endocrinol 2014;171: 615–21. doi: 10.1530/EJE-14-0621 [DOI] [PubMed] [Google Scholar]
- 25.Cavaliere H, Medeiros-Neto GA, Rosner W, Kourides IA. Persistent pituitary resistance to thyroid hormone in congenital versus later-onset hypothyroidism. J Endocrinol Invest 1985;8: 527–32. doi: 10.1007/BF03348554 [DOI] [PubMed] [Google Scholar]
- 26.Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinol 2014;10: 164–74. doi: 10.1038/nrendo.2013.258 [DOI] [PubMed] [Google Scholar]
- 27.Ain KB, Pucino F, Shiver TM, Banks SM. Thyroid hormone levels affected by time of blood sampling in thyroxine-treated patients. Thyroid 1993;3: 81–5. doi: 10.1089/thy.1993.3.81 [DOI] [PubMed] [Google Scholar]
- 28.Alvarez M, Iglesias Fernández C, Rodríguez Sánchez A, Dulín Lñiguez E, Rodríguez Arnao MD. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm Res Paediatr 2010;74: 114–20. doi: 10.1159/000313370 [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Miyauchi A, Hisakado M, Yoshioka W, Ide A, Kudo T, et al. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid 2017;27: 484–90. doi: 10.1089/thy.2016.0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asakura Y, Adachi M, Tachibana K. Influence of iodine excess on neonatal thyroid function (in Japanese). J Jpn Pediatr Soc 2002;106: 644–9. [Google Scholar]
- 31.Nishiyama S, Mikeda T, Okada T, Nakamura K, Kotani T, Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid 2004;14: 1077–83. doi: 10.1089/thy.2004.14.1077 [DOI] [PubMed] [Google Scholar]
- 32.Theodoropoulos T, Braverman LE, Vagenakis AG. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science 1979;205: 502–3. doi: 10.1126/science.451615 [DOI] [PubMed] [Google Scholar]
- 33.Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr 2012;161: 760–2. doi: 10.1016/j.jpeds.2012.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid 2001;11: 501–10. doi: 10.1089/105072501300176462 [DOI] [PubMed] [Google Scholar]
- 35.Lomenick JP, Jackson WA, Backeljauw PF. Amiodarone-induced neonatal hypothyroidism: a unique form of transient early-onset hypothyroidism. J Perinatol 2004;24: 397–9. doi: 10.1038/sj.jp.7211104 [DOI] [PubMed] [Google Scholar]
- 36.Rao RH, McCready VR, Spathis GS. Iodine kinetic studies during amiodarone treatment. J Clin Endocrinol Metab 1986;62: 563–8. doi: 10.1210/jcem-62-3-563 [DOI] [PubMed] [Google Scholar]
- 37.Momotani N, Hisaoka T, Noh J, Ishikawa N, Ito K. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves’ disease complicated by pregnancy. J Clin Endocrinol Metab 1992;75: 738–44. [DOI] [PubMed] [Google Scholar]
- 38.Omoto A, Kurimoto C, Minagawa M, Shozu M. A case of fetal goiter that resolved spontaneously after birth. J Clin Endocrinol Metab 2013;98: 3910–1. doi: 10.1210/jc.2013-1066 [DOI] [PubMed] [Google Scholar]
- 39.Satoh M, Aso K, Katagiri Y. Thyroid dysfunction in neonates born to mothers who have undergone hysterosalpingography involving an oil-soluble iodinated contrast medium. Horm Res Paediatr 2015;84: 370–5. doi: 10.1159/000439381 [DOI] [PubMed] [Google Scholar]
- 40.Sasaki Y, Kikuchi A, Murai M, Kanasugi T, Isurugi C, Oyama R, et al. Fetal goiter associated with preconception hysterosalpingography using an oil-soluble iodinated contrast medium. Ultrasound Obstet Gynecol 2017;49: 275–6. doi: 10.1002/uog.15902 [DOI] [PubMed] [Google Scholar]
- 41.van Welie N, Roest I, Portela M, van Rijswijk J, Koks C, Lambalk CB, et al. H2Oil Study GroupThyroid function in neonates conceived after hysterosalpingography with iodinated contrast. Hum Reprod 2020;35: 1159–67. doi: 10.1093/humrep/deaa049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas JV, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res 2009;72: 344–7. [DOI] [PubMed] [Google Scholar]
- 43.Overcash RT, Marc-Aurele KL, Hull AD, Ramos GA. Maternal iodine exposure: a case of fetal goiter and neonatal hearing loss. Pediatrics 2016;137: e20153722. doi: 10.1542/peds.2015-3722 [DOI] [PubMed] [Google Scholar]
- 44.Hardley MT, Chon AH, Mestman J, Nguyen CT, Geffner ME, Chmait RH. Iodine-induced fetal hypothyroidism: diagnosis and treatment with intra-amniotic levothyroxine. Horm Res Paediatr 2018;90: 419–23. doi: 10.1159/000488776 [DOI] [PubMed] [Google Scholar]
- 45.Rogan WJ, Paulson JA, Baum C, Brock-Utne AC, Brumberg HL, Campbell CC, et al. Council on Environmental Health. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics 2014;133: 1163–6. doi: 10.1542/peds.2014-0900 [DOI] [PubMed] [Google Scholar]
- 46.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009;360: 939–40. doi: 10.1056/NEJMc0807851 [DOI] [PubMed] [Google Scholar]
- 47.Food and Nutrition Board. 8. Iodine. In: Institute of Medicine (US) Panel on Micronutrients, editor. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. p. 258-89. [PubMed] [Google Scholar]
- 48.Study Group for dietary reference intakes. Dietary reference intakes for Japanese 2020 ver. Japan: Ministry of Health, Labor and Welfare; 2020. (in Japanese). [Google Scholar]
- 49.Takamura N, Hamada A, Yamaguchi N, Matsushita N, Tarasiuk I, Ohashi T, et al. Urinary iodine kinetics after oral loading of potassium iodine. Endocr J 2003;50: 589–93. doi: 10.1507/endocrj.50.589 [DOI] [PubMed] [Google Scholar]
- 50.Maruo Y, Takahashi H, Soeda I, Nishikura N, Matsui K, Ota Y, et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J Clin Endocrinol Metab 2008;93: 4261–7. doi: 10.1210/jc.2008-0856 [DOI] [PubMed] [Google Scholar]
- 51.Ohye H, Fukata S, Hishinuma A, Kudo T, Nishihara E, Ito M, et al. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with large goiter. Thyroid 2008;18: 561–6. doi: 10.1089/thy.2007.0258 [DOI] [PubMed] [Google Scholar]
- 52.Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 2002;347: 95–102. doi: 10.1056/NEJMoa012752 [DOI] [PubMed] [Google Scholar]
- 53.Pfarr N, Korsch E, Kaspers S, Herbst A, Stach A, Zimmer C, et al. Congenital hypothyroidism caused by new mutations in the thyroid oxidase 2 (THOX2) gene. Clin Endocrinol (Oxf) 2006;65: 810–5. doi: 10.1111/j.1365-2265.2006.02672.x [DOI] [PubMed] [Google Scholar]
- 54.Maruo Y, Nagasaki K, Matsui K, Mimura Y, Mori A, Fukami M, et al. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur J Endocrinol 2016;174: 453–63. doi: 10.1530/EJE-15-0959 [DOI] [PubMed] [Google Scholar]
- 55.Jin HY, Heo SH, Kim YM, Kim GH, Choi JH, Lee BH, et al. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediatr 2014;82: 252–60. doi: 10.1159/000362235 [DOI] [PubMed] [Google Scholar]
- 56.Narumi S, Muroya K, Asakura Y, Aachi M, Hasegawa T. Molecular basis of thyroid dyshormonogenesis: genetic screening in population-based Japanese patients. J Clin Endocrinol Metab 2011;96: E1838–42. doi: 10.1210/jc.2011-1573 [DOI] [PubMed] [Google Scholar]
- 57.Muzza M, Rabbiosi S, Vigone MC, Zamproni I, Cirello V, Maffini MA, et al. The clinical and molecular characterization of patients with dyshormonogenic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J Clin Endocrinol Metab 2014;99: E544–53. doi: 10.1210/jc.2013-3618 [DOI] [PubMed] [Google Scholar]
- 58.Fuse Y, Ogawa H, Fujita M, Arata N, Harada S, Ohashi T, et al. Maternal-neonatal relationship of iodine metabolism in perinatal period: Changes in urinary iodine excretion in Japanese mothers and newborn infants. Presented at 15th International & 14th European Congress of Endocrinology. 2012. Florence, Italy. Endocrine Abstracts 2012;29:P1640. [Google Scholar]
- 59.Tachibana M, Miyoshi Y, Fukui M, Onuma S, Fukuoka T, Satomura Y, et al. Urinary iodine and thyroglobulin are useful markers in infants suspected of congenital hypothyroidism based on newborn screening. Journal of pediatric endocrinology & metabolism. J Pediatr Endocrinol Metab 2021. doi: 10.1515/jpem-2021-0205 [DOI] [PubMed] [Google Scholar]
- 60.Narumi S, Muroya K, Asakura Y, Adachi M, Hasegawa T. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J Clin Endocrinol Metab 2010;95: 1981–5. doi: 10.1210/jc.2009-2373 [DOI] [PubMed] [Google Scholar]
- 61.Tanaka T, Aoyama K, Suzuki A, Saitoh S, Mizuno H. Clinical and genetic investigation of 136 Japanese patients with congenital hypothyroidism. J Pediatr Endocrinol Metab 2020;33: 691–701. doi: 10.1515/jpem-2019-0433 [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Nakamura A, Nakayama K, Hishimura N, Morikawa S, Ishizu K, et al. Targeted next-generation sequencing for congenital hypothyroidism with positive neonatal TSH screening. J Clin Endocrinol Metab 2020;105: e2825–33. doi: 10.1210/clinem/dgaa308 [DOI] [PubMed] [Google Scholar]
- 63.de Filippis T, Marelli F, Nebbia G, Porazzi P, Corbetta S, Fugazzola L, et al. JAG1 loss-of-function variations as a novel predisposing event in the pathogenesis of congenital thyroid defects. J Clin Endocrinol Metab 2016;101: 861–70. doi: 10.1210/jc.2015-3403 [DOI] [PubMed] [Google Scholar]
- 64.Gannon T, Perveen R, Schlecht H, Ramsden S, Anderson B, Kerr B, et al. DDD studyFurther delineation of the KAT6B molecular and phenotypic spectrum. Eur J Hum Genet 2015;23: 1165–70. doi: 10.1038/ejhg.2014.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu C, Luo S, Zhang Y, Fan X, D’Gama AM, Zhang X, et al. Chromosomal microarray and whole exome sequencing identify genetic causes of congenital hypothyroidism with extra-thyroidal congenital malformations. Clin Chim Acta 2019;489: 103–8. doi: 10.1016/j.cca.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 66.Fagman H, Liao J, Westerlund J, Andersson L, Morrow BE, Nilsson M. The 22q11 deletion syndrome candidate gene Tbx1 determines thyroid size and positioning. Hum Mol Genet 2007;16: 276–85. doi: 10.1093/hmg/ddl455 [DOI] [PubMed] [Google Scholar]
- 67.Kariyawasam D, Rachdi L, Carré A, Martin M, Houlier M, Janel N, et al. DYRK1A BAC transgenic mouse: a new model of thyroid dysgenesis in Down syndrome. Endocrinology 2015;156: 1171–80. doi: 10.1210/en.2014-1329 [DOI] [PubMed] [Google Scholar]
- 68.de Filippis T, Gelmini G, Paraboschi E, Vigone MC, Di Frenna M, Marelli F, et al. A frequent oligogenic involvement in congenital hypothyroidism. Hum Mol Genet 2017;26: 2507–14. doi: 10.1093/hmg/ddx145 [DOI] [PubMed] [Google Scholar]
- 69.Fan X, Fu C, Shen Y, Li C, Luo S, Li Q, et al. Next-generation sequencing analysis of twelve known causative genes in congenital hypothyroidism. Clin Chim Acta 2017;468: 76–80. doi: 10.1016/j.cca.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 70.Makretskaya N, Bezlepkina O, Kolodkina A, Kiyaev A, Vasilyev EV, Petrov V, et al. High frequency of mutations in ‘dyshormonogenesis genes’ in severe congenital hypothyroidism. PLoS One 2018;13: e0204323. doi: 10.1371/journal.pone.0204323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters C, van Trotsenburg ASP, Schoenmakers N. DIAGNOSIS OF ENDOCRINE DISEASE: Congenital hypothyroidism: update and perspectives. Eur J Endocrinol 2018;179: R297–317. doi: 10.1530/EJE-18-0383 [DOI] [PubMed] [Google Scholar]
- 72.Stoupa A, Kariyawasam D, Muzza M, de Filippis T, Fugazzola L, Polak M, et al. New genetics in congenital hypothyroidism. Endocrine 2021;71: 696–705. doi: 10.1007/s12020-021-02646-9 [DOI] [PubMed] [Google Scholar]
- 73.Eugster EA, LeMay D, Zerin JM, Pescovitz OH. Definitive diagnosis in children with congenital hypothyroidism. J Pediatr 2004;144: 643–7. doi: 10.1016/j.jpeds.2004.02.020 [DOI] [PubMed] [Google Scholar]
- 74.Unüvar T, Demir K, Abacı A, Büyükgebiz A, Böber E. The role of initial clinical and laboratory findings in infants with hyperthyrotropinemia to predict transient or permanent hypothyroidism. J Clin Res Pediatr Endocrinol 2013;5: 170–3. doi: 10.4274/Jcrpe.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rabbiosi S, Vigone MC, Cortinovis F, Zamproni I, Fugazzola L, Persani L, et al. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J Clin Endocrinol Metab 2013;98: 1395–402. doi: 10.1210/jc.2012-3174 [DOI] [PubMed] [Google Scholar]
- 76.Cho MS, Cho GS, Park SH, Jung MH, Suh BK, Koh DG. Earlier re-evaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses. Ann Pediatr Endocrinol Metab 2014;19: 141–5. doi: 10.6065/apem.2014.19.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Messina MF, Aversa T, Salzano G, Zirilli G, Sferlazzas C, De Luca F, et al. Early discrimination between transient and permanent congenital hypothyroidism in children with Eutopic gland. Horm Res Paediatr 2015;84: 159–64. doi: 10.1159/000435811 [DOI] [PubMed] [Google Scholar]
- 78.Kara C, Günindi F, Can Yılmaz G, Aydın M. Transient congenital hypothyroidism in Turkey: an analysis on frequency and natural course. J Clin Res Pediatr Endocrinol 2016;8: 170–9. doi: 10.4274/jcrpe.2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu C, Luo S, Li Y, Li Q, Hu X, Li M, et al. The incidence of congenital hypothyroidism (CH) in Guangxi, China and the predictors of permanent and transient CH. Endocr Connect 2017;6: 926–34. doi: 10.1530/EC-17-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park IS, Yoon JS, So CH, Lee HS, Hwang JS. Predictors of transient congenital hypothyroidism in children with eutopic thyroid gland. Ann Pediatr Endocrinol Metab 2017;22: 115–8. doi: 10.6065/apem.2017.22.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zdraveska N, Zdravkovska M, Anastasovska V, Sukarova-Angelovska E, Kocova M. Diagnostic re-evaluation of congenital hypothyroidism in Macedonia: predictors for transient or permanent hypothyroidism. Endocr Connect 2018;7: 278–85. doi: 10.1530/EC-17-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saba C, Guilmin-Crepon S, Zénaty D, Martinerie L, Paulsen A, Simon D, et al. Early determinants of thyroid function outcomes in children with congenital hypothyroidism and a normally located thyroid gland: a regional cohort study. Thyroid 2018;28: 959–67. doi: 10.1089/thy.2018.0154 [DOI] [PubMed] [Google Scholar]
- 83.Oron T, Lazar L, Ben-Yishai S, Tenenbaum A, Yackobovitch-Gavan M, Meyerovitch J, et al. Permanent vs transient congenital hypothyroidism: assessment of predictive variables. J Clin Endocrinol Metab 2018;103: 4428–36. doi: 10.1210/jc.2018-00362 [DOI] [PubMed] [Google Scholar]
- 84.Higuchi S, Hasegawa Y. Levothyroxine dosages less than 2.4 μg/kg/day at 1 year and 1.3 μg/kg/day at 3 years of age may predict transient congenital hypothyroidism. Clin Pediatr Endocrinol 2019;28: 127–33. doi: 10.1297/cpe.28.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Itonaga T, Higuchi S, Shimura K, Nagasaki K, Satoh M, Takubo N, et al. Levothyroxine dosage as predictor of permanent and transient congenital hypothyroidism: a multicenter retrospective study in Japan. Horm Res Paediatr 2019;92: 45–51. doi: 10.1159/000502418 [DOI] [PubMed] [Google Scholar]
- 86.Park ES, Yoon JY. Factors associated with permanent hypothyroidism in infants with congenital hypothyroidism. BMC Pediatr 2019;19: 453. doi: 10.1186/s12887-019-1833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asena M, Demiral M, Unal E, Öcal M, Demirbilek H, Özbek MN. Validity of six month L-thyroxine dose for differentiation of transient or permanent congenital hypothyroidism. J Clin Res Pediatr Endocrinol 2020;12: 275–80. doi: 10.4274/jcrpe.galenos.2020.2019.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matejek N, Tittel SR, Haberland H, Rohrer T, Busemann EM, Jorch N, et al. Predictors of transient congenital primary hypothyroidism: data from the German registry for congenital hypothyroidism (AQUAPE “HypoDok”). Eur J Pediatr 2021;180: 2401–8. doi: 10.1007/s00431-021-04031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamamura H, Kokumai T, Furuya A, Suzuki S, Tanahashi Y, Azuma H. Increase in doses of levothyroxine at the age of 3 years and above is useful for distinguishing transient and permanent congenital hypothyroidism. Clin Pediatr Endocrinol 2020;29: 143–9. doi: 10.1297/cpe.29.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehran L, Azizi F, Mousapour P, Cheraghi L, Yarahmadi S, Amirshekari G, et al. Development of a risk prediction model for early discrimination between permanent and transient congenital hypothyroidism. Endocrine 2021;73: 374–83. doi: 10.1007/s12020-021-02641-0 [DOI] [PubMed] [Google Scholar]
- 91.Cooper DS, Axelrod L, DeGroot LJ, Vickery AL, Jr, Maloof F. Congenital goiter and the development of metastatic follicular carcinoma with evidence for a leak of nonhormonal iodide: clinical, pathological, kinetic, and biochemical studies and a review of the literature. J Clin Endocrinol Metab 1981;52: 294–306. doi: 10.1210/jcem-52-2-294 [DOI] [PubMed] [Google Scholar]
- 92.Medeiros-Neto G, Stanbury J. Thyroid malignancy and dyshormonogenetic goiter. Inherited disorders of the thyroid system. 1st ed. Boca Raton, FL1994. p. 207-18.
- 93.Drut R, Moreno A. Papillary carcinoma of the thyroid developed in congenital dyshormonogenetic hypothyroidism without goiter: Diagnosis by FNAB. Diagn Cytopathol 2009;37: 707–9. doi: 10.1002/dc.20916 [DOI] [PubMed] [Google Scholar]
- 94.Şıklar Z, Berberoğlu M, Yağmurlu A, Hacıhamdioğlu B, Savaş Erdeve S, Fitöz S, et al. Synchronous occurrence of papillary carcinoma in the thyroid gland and thyroglossal duct in an adolescent with congenital hypothyroidism. J Clin Res Pediatr Endocrinol 2012;4: 30–3. doi: 10.4274/jcrpe.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eremija J, Milenković T, Mitrović K, Todorović S, Vuković R, Plavšić L. The first case of papillary thyroid carcinoma in an adolescent with congenital dyshormonogenetic hypothyroidism in Serbia. Vojnosanit Pregl 2014;71: 1078–80. doi: 10.2298/VSP1411078E [DOI] [PubMed] [Google Scholar]
- 96.Hishinuma A, Fukata S, Kakudo K, Murata Y, Ieiri T. High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid 2005;15: 1079–84. doi: 10.1089/thy.2005.15.1079 [DOI] [PubMed] [Google Scholar]
- 97.Alzahrani AS, Baitei EY, Zou M, Shi Y. Clinical case seminar: metastatic follicular thyroid carcinoma arising from congenital goiter as a result of a novel splice donor site mutation in the thyroglobulin gene. J Clin Endocrinol Metab 2006;91: 740–6. doi: 10.1210/jc.2005-2302 [DOI] [PubMed] [Google Scholar]
- 98.Raef H, Al-Rijjal R, Al-Shehri S, Zou M, Al-Mana H, Baitei EY, et al. Biallelic p.R2223H mutation in the thyroglobulin gene causes thyroglobulin retention and severe hypothyroidism with subsequent development of thyroid carcinoma. J Clin Endocrinol Metab 2010;95: 1000–6. doi: 10.1210/jc.2009-1823 [DOI] [PubMed] [Google Scholar]
- 99.Fukata S. A case of thyroglobulin mutations (In Japanese). Journal of the Japan Thyroid Association 2010;1: 53–5. [Google Scholar]
- 100.Yoon JH, Hong AR, Kim HK, Kang HC. Anaplastic thyroid cancer arising from dyshormonogenetic goiter: c.3070T>C and novel c.7070T>C mutation in the thyroglobulin gene. Thyroid 2020;30: 1676–80. doi: 10.1089/thy.2020.0248 [DOI] [PubMed] [Google Scholar]
- 101.Medeiros-Neto G, Gil-Da-Costa MJ, Santos CL, Medina AM, Silva JC, Tsou RM, et al. Metastatic thyroid carcinoma arising from congenital goiter due to mutation in the thyroperoxidase gene. J Clin Endocrinol Metab 1998;83: 4162–6. [DOI] [PubMed] [Google Scholar]
- 102.Chertok Shacham E, Ishay A, Irit E, Pohlenz J, Tenenbaum-Rakover Y. Minimally invasive follicular thyroid carcinoma developed in dyshormonogenetic multinodular goiter due to thyroid peroxidase gene mutation. Thyroid 2012;22: 542–6. doi: 10.1089/thy.2011.0478 [DOI] [PubMed] [Google Scholar]
- 103.Agretti P, Bagattini B, De Marco G, Di Cosmo C, Dionigi G, Vitti P, et al. Papillary thyroid cancer in a patient with congenital goitrous hypothyroidism due to a novel deletion in NIS gene. Endocrine 2016;54: 256–8. doi: 10.1007/s12020-015-0790-8 [DOI] [PubMed] [Google Scholar]
- 104.Camargo R, Limbert E, Gillam M, Henriques MM, Fernandes C, Catarino AL, et al. Aggressive metastatic follicular thyroid carcinoma with anaplastic transformation arising from a long-standing goiter in a patient with Pendred’s syndrome. Thyroid 2001;11: 981–8. doi: 10.1089/105072501753211073 [DOI] [PubMed] [Google Scholar]
- 105.Sakurai K, Hata M, Hishinuma A, Ushijima R, Okada A, Taeda Y, et al. Papillary thyroid carcinoma in one of identical twin patients with Pendred syndrome. Endocr J 2013;60: 805–11. doi: 10.1507/endocrj.EJ12-0396 [DOI] [PubMed] [Google Scholar]
- 106.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 2011;7: 569–80. doi: 10.1038/nrendo.2011.142 [DOI] [PubMed] [Google Scholar]
- 107.McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab 2012;97: 2682–92. doi: 10.1210/jc.2012-1083 [DOI] [PubMed] [Google Scholar]
- 108.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015;25: 716–59. doi: 10.1089/thy.2014.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng J, Li C, Lu W, Wang C, Ai Z. Quantitative assessment of preoperative serum thyrotropin level and thyroid cancer. Oncotarget 2016;7: 34918–29. doi: 10.18632/oncotarget.9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hishinuma A. Thyroglobulin gene abnormalities. Rinsho Byori 2005;53: 935–41 (in Japanese). [PubMed] [Google Scholar]
- 111.Hishinuma A, Ieiri T, Fukata S, Miyauchi A. Thyroglobulin gene abnormalities. Nihon Rinsho 2005;63(Suppl 10): 31–5 (in Japanese). [PubMed] [Google Scholar]
- 112.De Jaco A, Dubi N, Camp S, Taylor P. Congenital hypothyroidism mutations affect common folding and trafficking in the α/β-hydrolase fold proteins. FEBS J 2012;279: 4293–305. doi: 10.1111/febs.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 2011;711: 193–201. doi: 10.1016/j.mrfmmm.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 114.Ameziane El Hassani R, Buffet C, Leboulleux S, Dupuy C. Oxidative stress in thyroid carcinomas: biological and clinical significance. Endocr Relat Cancer 2019;26: R131–43. doi: 10.1530/ERC-18-0476 [DOI] [PubMed] [Google Scholar]
- 115.Hamburger JI, Hamburger SW. Thyroidal hemiagenesis. Report of a case and comments on clinical ramifications. Arch Surg 1970;100: 319–20. doi: 10.1001/archsurg.1970.01340210095026 [DOI] [PubMed] [Google Scholar]
- 116.Greening WP, Sarker SK, Osborne MP. Hemiagenesis of the thyroid gland. Br J Surg 1980;67: 446–8. doi: 10.1002/bjs.1800670621 [DOI] [PubMed] [Google Scholar]
- 117.Massine RE, Durning SJ, Koroscil TM. Lingual thyroid carcinoma: a case report and review of the literature. Thyroid 2001;11: 1191–6. doi: 10.1089/10507250152741055 [DOI] [PubMed] [Google Scholar]
- 118.Sturniolo G, Violi MA, Galletti B, Baldari S, Campennì A, Vermiglio F, et al. Differentiated thyroid carcinoma in lingual thyroid. Endocrine 2016;51: 189–98. doi: 10.1007/s12020-015-0593-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.