Abstract
Background:
Autoimmune thyroid diseases (AITD) represent the most common autoimmune diseases. However, current therapies focus on relieving the symptoms instead of curing AITD, and new therapies to reverse the autoimmune attack on the thyroid are needed. HLA-DRβ1-Arg74 is the key HLA class II allele that triggers AITD by presenting pathogenic thyroglobulin (Tg) peptides that activate thyroid self-reactive T cells. We hypothesized that blocking the presentation of Tg peptides to T cells within the HLA-DRβ1-Arg74 peptide binding cleft could reverse the autoimmune response to the thyroid in AITD.
Methods:
The approach we used to block Tg peptide presentation within HLA-DRβ1-Arg74 is to design retro-inverso D-amino acid (RID) peptides that have high affinity to the HLA-DRβ1-Arg74 peptide binding pocket.
Results:
By using computational approaches and molecular dynamics simulations, we designed two RID peptides, RT-15 and VT-15, that blocked peptide binding to recombinant HLA-DRβ1-Arg74 molecule, as well as T cell activation in vitro. Furthermore, RT-15 and VT-15 blocked in vivo T cell activation by thyroglobulin in humanized NOD-DR3 mice induced with experimental autoimmune thyroiditis.
Conclusions:
In summary, we discovered two RID peptides that block thyroglobulin peptide binding to HLA-DRβ1-Arg74 and their presentation to T cells in AITD. These findings set the stage for a personalized medicine therapeutic approach for AITD patients who carry the DRβ1-Arg74 allele. This antigen-specific therapeutic strategy can potentially be extended to other autoimmune diseases.
Keywords: autoimmune thyroiditis, Graves'disease, Hashimoto's thyroiditis, peptides, HLA-DR3
Introduction
Autoimmune thyroid diseases (AITD), comprising both Graves' disease (GD) and Hashimoto's thyroiditis (HT), share common immunopathogenic mechanisms.1 In AITD, T and B cell responses targeting the thyroid result in lymphocytic infiltration of the thyroid and generation of antibodies against thyroid antigens.2 Despite their similar pathoetiology clinically GD manifests by hyperthyroidism, while HT is characterized by hypothyroidism. AITD are the most common autoimmune disorders afflicting up to 5% of the U.S. population.3 Epidemiological data suggest that both genetic and environmental factors play an important role in the development of AITD.4 It is hypothesized that epigenetic interactions between extrinsic factors (such as viral infections) and susceptibility genes cause the loss of immune tolerance leading to the autoimmune targeting of the thyroid.5–7
Current treatments for AITD focus on relieving symptoms instead of reversing the autoimmune response and thus for many patients managing the disease is challenging. Thioamides used to block thyroid hormone synthesis in GD can cause significant complications, such as hepatitis and agranulocytosis.8,9 Other treatment options include thyroidectomy that poses surgical risks, and radioactive iodine that may worsen or trigger Graves' ophthalmopathy and was recently shown to be associated with increased cancer risk.10,11 Levothyroxine treatment is effective in normalizing thyroid functions. However, close to 30% of HT patients continue to have significant symptoms such as foggy brain even when their thyrotropin (TSH) levels are normalized.12–14 In view of this, there is an unmet need for new immune-modulating treatments to block the immune attack on the thyroid.
A large body of literature has confirmed a very strong association between AITD and HLA-DR3.15 We sequenced HLA-DR3 and identified an amino acid signature pocket containing arginine at position β74 that is critical for development of AITD (designated HLA-DRβ1-Arg74).16 In addition, we and others have identified the major thyroglobulin (Tg) and thyroid-stimulating hormone receptor (TSHR) T cell epitopes that trigger HT and GD.17–20 Our goal is to block presentation of pathogenic peptides within HLA-DRβ1-Arg74 to autoreactive T cells that escaped tolerance as a novel antigen-specific treatment for AITD. Previously, we have shown that a small molecule inhibitor, Cepharanthine, can prevent binding of pathogenic thyroid peptides to HLA-DRβ1-Arg74 and block their presentation to T cells.19
In this study, we employed a novel strategy to block thyroid peptide presentation using retro-inverso D-amino acid peptides (RID peptides). RID peptides are composed of D-amino acids (inverso), and their sequence is the reverse of the corresponding pathogenic L-amino acid peptide sequence (retro).21,22 They have been shown to have very similar three-dimensional structure to the corresponding L-amino acid peptide.23 In previous work, we have shown that RID peptides can block an insulin peptide (InsB:9–23) binding to HLA-DQ8 and its presentation to T cells in type 1 diabetes (T1D).24 Our hypothesis is that blocking HLA-DRβ1-Arg74 (a subtype of HLA-DR3) using D-peptides will block autoreactive T cell activation in AITD. In this study, we show for the first time that specially designed RID peptides can block antigen presentation and activation of thyroid autoreactive T cells.
Materials and Methods
Design the RID peptides (RT-15 and VT-15)
We have previously shown that the Tg peptides, Tg.726 (CPTPCQLQAEQAFLRTV) and Tg.202 (VNTTDMMIFDLVHSYNRFPD), are major Tg T cell epitopes in autoimmune thyroiditis.19 These peptides therefore are good candidates for the design of inhibitors of T cell stimulation. In DR3, the canonical pockets that bind the peptides are specific for hydrophobic residues in P1 and P9 and a negatively charged residue in P4. In Tg.726 and Tg.202, both central sequences satisfy the canonical conditions. Using the selectivity properties of these peptides, that is, the binding of residues in DR pockets, we designed RID peptides based on the sequences of the two Tg peptides. If the side chains of the RID peptide reside in the same specificity pockets, the inverted stereochemistry forces the backbone to run in the opposite direction. This may give rise to the formation of new interaction between the backbone and the groove of HLA-DR3 that do not exist in the complexes with the L-amino acid peptides.
Strategy for computational design
See the Supplementary Information S1.
Recombinant HLA-DRβ1-Arg74 protein production
The baculovirus vector system was utilized to produce recombinant HLA-DRβ1-Arg74, as described previously (Life Technologies custom services).19
Peptide synthesis
The peptides used in this study were synthesized by GenScript (Piscataway, NJ). Two RID peptides (RT-15 and VT-15), thyroglobulin peptide (Tg.2098), and control peptide (APO) were used in this study. APO was used in our enzyme-linked immunosorbent assay (ELISA) (described in section Dose response of RID peptides blocking peptide binding to HLA-DRB1-Arg74) because it is a well-known strong binder to HLA-DR3.25 APO was used as a negative control peptide in our experimental autoimmune thyroiditis (EAT) model (see section Induction of EAT) because according to our previous studies it does not elicit T cell responses.19 It is the best negative control for T cell experiments because it binds HLA-DR3 but does not elicit T cell responses. Tg.2098 was shown to be a major thyroglobulin epitope by us and other groups.17,18,26,27 The sequences of the peptides are shown in Table 1.
Table 1.
Sequences of Peptides Used in This Study
| Peptide | Sequence |
|---|---|
| RT-15 | {d-ARG}{d-ASN}{d-ARG}{d-LEU}{d-PHE}{d-VAL}{d-LEU}{d-ASP}{d-PHE}{d-GLN}{d-LEU}{d-GLN}{d-CYS}{d-PRO}{d-THR} |
| VT-15 | {d-VAL}{d-THR}{d-ARG}{d-LEU}{d-PHE}{d-VAL}{d-GLN}{d-ASP}{d-PHE}{d-ILE}{d-LEU}{d-GLN}{d-CYS}{d-PRO}{d-THR} |
| Tg.2098 | LSSVVVDPSIRHFDV |
| APO | IPDNLFLKSDGRIKYTLNK |
Tg, thyroglobulin.
Dose response of RID peptides blocking peptide binding to HLA-DRβ1-Arg74
RID peptides (RT-15 and VT-15) were synthesized in powder form and dissolved in water. DELFIA (dissociation-enhanced lanthanide fluorescence immunoassay) was performed, as described previously19,20,28 (Supplementary Information S1).
Mice
We used the NOD-DR3 transgenic mouse line in our experiments; in this line, the murine major histocompatibility complex II (MHC II) was deleted and a human DR3 (DRβ1-Arg74) transgene is expressed, as previously described in detail.18,19
Induction of EAT
Fifteen female humanized NOD-DR3 mice 4–6 weeks old were injected subcutaneously with human thyroglobulin (hTg) with purity ≥96% by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Cell Sciences, Canton, MA) in Complete Freund's Adjuvant (Sigma) to induce EAT, as previously described.19 Mice were immunized with 100 μg in each leg on day 0 and boosted on day 7, and sacrificed on day 21.
T cell stimulation and carboxyfluorescein succinimidyl ester analysis
Immunized mice lymphocyte isolation and T cell stimulation and carboxyfluorescein succinimidyl ester (CFSE) cell proliferation analysis were performed, as described previously19,20,28 (Supplementary Information S1).
Inhibition of T cell proliferation by RID peptides ex vivo
The ability of RID peptides to block T cell proliferation in response to hTg and Tg.2098 was assessed. CFSE-labeled cells were incubated with hTg (10 μg/mL) or Tg.2098 (20 μg/mL) and exposed to 20 μg/mL RID peptides (RT-15 or VT-15) and then analyzed by flow cytometry. We previously tested different concentrations (40, 20, 10 μg/mL) of hTg used in this assay and found no significant difference in hTg-induced interferon gamma (IFNγ) production (Supplementary Fig. S1).
Cytokine assays
The ability of RID peptides to block T cell activation as manifested by IFNγ, IL-4, and IL-5 production was also assessed. To measure IFNγ, IL-4, and IL-5 secretion by T cells into the medium, we used the Milliplex magnetic bead system (EMD Millipore Corporation, Billerica, MA), as described19,20,28,29 (Supplementary Information S1).
Inhibition of cytokine production by RID peptides ex vivo
The ability of RID peptides to block T cell activation and cytokine production in response to hTg and Tg.2098 was assessed. We isolated lymphocytes from the spleens of 15 mice that were induced with EAT by hTg immunization. Splenic lymphocytes were stimulated with hTg (10 μg/mL) or Tg.2098 (20 μg/mL) and exposed to RID peptides at 20 μg/mL. After 48 hours of incubation, we collected supernatants and analyzed IFNγ, IL-4, and IL-5 levels, as described previously.
Testing the ability of RID peptides to suppress EAT in vivo
Eight female humanized NOD-DR3 mice were injected with a cocktail mix of RID peptides, RT-15 and VT-15 (100 μg each), 2 hours and 1 hour before immunization with human thyroglobulin (100 μg) on day 0 and 7. Control mice were injected with phosphate-buffered saline (PBS) 2 hours and 1 hour before immunization with human thyroglobulin. Mice were sacrificed on day 21. Spleens and draining lymph nodes were processed to isolate lymphocytes as described previously, and lymphocytes were stimulated with human thyroglobulin and Tg.2098 to test for T cell activation.
Statistics
Due to non-normality of the distribution of the paired differences, statistical significance of the inhibitory effect was assessed using the Wilcoxon sign rank test.30 Calculations were performed using Stata version 17.0 (StataCorp, College Station, TX).
Study approval
The protocol for these studies was reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of Icahn School of Medicine and Albert Einstein College of Medicine.
Results
Construction of RID peptides (RT-15 and VT-15) that block Tg/Tg.2098 binding to the HLA-DRβ1-Arg74 pocket
The MM-GBSA analyses of the simulations of retro-inverso (RI)-Tg.726 and RI-Tg.202 show that the overall interaction energies of the two peptides with HLA-DR3 are not the same, although they are within 10% of each other. Not surprisingly, the interactions of the individual residues with the groove pockets of DR3 depend both on position and on the nature of the specific residue. If we assume that the total interaction energy is simply a sum of the individual contributions, we can use the two peptides to design a hybrid peptide that optimizes the interactions.
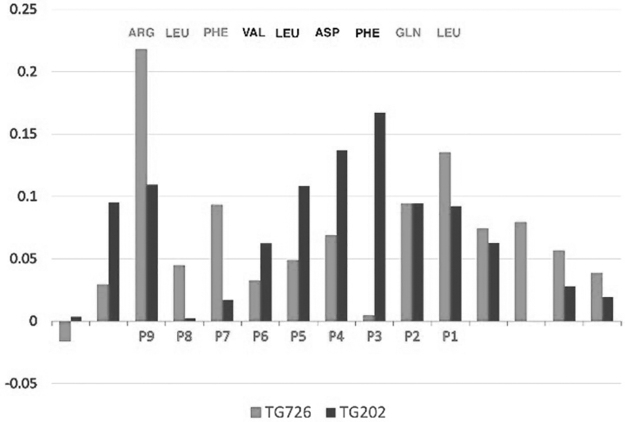
To be able to compare the two sequences, we represent the specific contribution of each residue as a fraction of the total interaction energy. A side-by-side comparison of the two peptides, Table 2, shows that certain residues are preferred in specific pockets. The preferred residues based on the two sequences are highlighted in gray. Thus, in P1 and P2, Leu and Gln from Tg.726 have larger fractional contributions than Met and Ile from Tg.202. In pockets P3 through P6, the contributions from Tg.202 are more than from Tg.726. Finally, the contributions from Tg.726 in pockets P7-P9 are more than from Tg.202.
Table 2.
Fractional Interactions of Retro-Inverso Peptides Tg.726 and Tg.202 with HLA-DR3
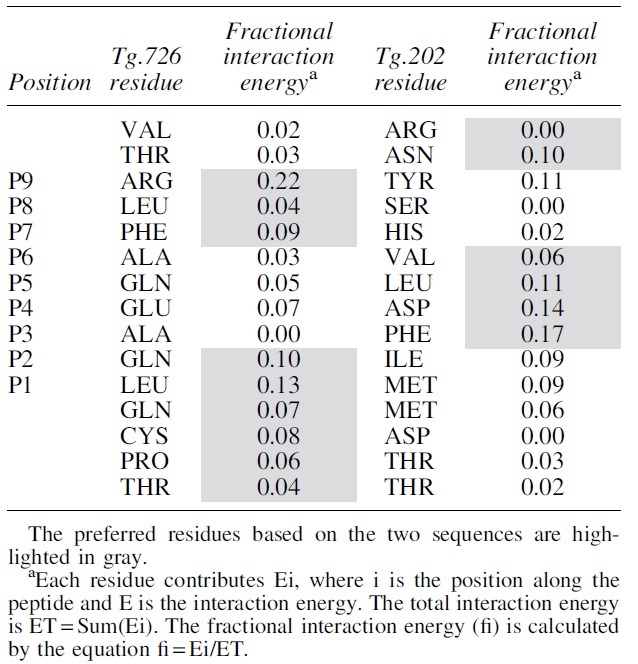
|
Clearly selecting the highlighted residues creates a putative better peptide than either of the original, we name it RT-15. Likewise, selecting the remaining residue should create a peptide, which will bind less strongly to HLA-DR3 than the two originals, we name it ANTI. The resulting peptide sequences are shown in Figure 1. The contributions from each peptide are color coded (gray for Tg.726 and black for Tg.202). The RT-15 constructed by the considerations described above is shown above the graph with the corresponding color coding of their origin: RT-15: {d-ARG}{d-ASN}{d-ARG}{d-LEU}{d-PHE}{d-VAL}{d-LEU}{d-ASP}{d-PHE}{d-GLN}{d-LEU}{d-GLN}{d-CYS}{d-PRO}{d-THR} and ANTI: {d-VAL}{d-THR}{d-TYR}{d-SER}{d-HIS}{d-ALA}{d-GLN}{d-GLN}{d-ALA}{d-ILE}{d-MET}{d-MET}{d-ASP}{d-THR}{d-TYR}.
FIG. 1.
The fractional contributions of the residues of Tg.726 (gray) and Tg.202 (black) to the interaction energy with DR3. The composite sequence at the top represents the sequence with the better fractional contribution to binding (RT-15) [see results section “Construction of RID peptides (RT-15 and VT-15) that block Tg/Tg.2098 binding to the HLA-DRB1-Arg74 pocket”]. Tg, thyroglobulin.
The peptides were used to construct two simulations as described above, followed by an MM-GBSA analysis. RT-15 was predicted to be nearly 40% better than the ANTI peptide. Interestingly, the comparison of the contributions of the residues between RT-15 and ANTI creates a second generation of predicted sequences—VT-15 and ANTI2. Their sequences are as follows: VT-15: {d-VAL}{d-THR}{d-ARG}{d-LEU}{d-PHE}{d-VAL}{d-GLN}{d-ASP}{d-PHE}{d-ILE}{d-LEU}{d-GLN}{d-CYS}{d-PRO}{d-THR} and ANTI2: {d-ARG}{d-ASN}{d-TYR}{d-SER}{d-HIS}{d-ALA}{d-GLN}{d-ASP}{d-ALA}{d-GLN}{d-MET}{d-MET}{d-ASP}{d-THR}{d-THR}.
RT-15 and VT-15 block peptide binding to HLA-DRβ1-Arg74 in vitro in a dose-dependent manner
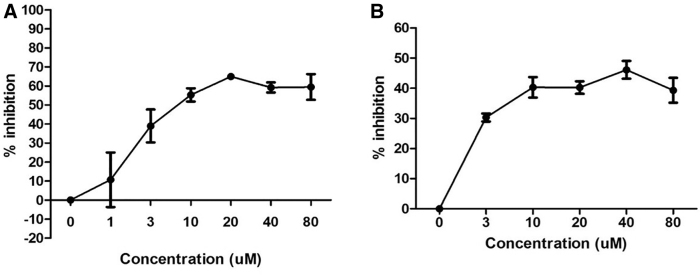
HLA-DRβ1-Arg74 protein was incubated with biotinylated APO peptide and with increasing concentrations of RT-15 (1–80 μM) or VT-15 (3–80 μM) for 48 hours at 37°C. DELFIA assay (see the Materials and Methods section) was performed. Both RT-15 and VT-15 blocked biotinylated APO binding to HLA-DRβ1-Arg74 in a dose-dependent manner, with the highest inhibition observed at 20 and 40 μM for RT-15 (Fig. 2A) and VT-15 (Fig. 2B), respectively.
FIG. 2.
Dose response of RT-15 and VT-15 blocking biotinylated APO peptide binding to recombinant HLA-DRβ1-Arg74 protein in vitro. Ten micromolars biotinylated APO peptide was incubated with 0.012 mg/mL HLA-DRβ1-Arg74 protein, with increasing concentrations (0–80 μM) of RT-15 and VT-15. RT-15 blocked APO binding to DRβ1-Arg74 in a dose-dependent manner, with a peak that plateaued at 20 μM (A). Similarly, VT-15 blocked the binding dose dependently, with a peak that plateaued at 40 μM (B).
RT-15 and VT-15 block T cell activation ex vivo by Tg and Tg.2098
T cell proliferative responses
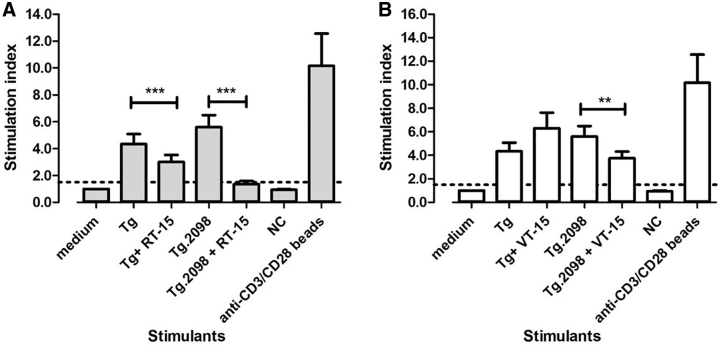
To test if RT-15 and VT-15 can block T cell activation by Tg and Tg.2098 presented within HLA-DRβ1-Arg74, we induced EAT in humanized NOD-DR3 mice. Splenocytes were isolated from the immunized mice at sacrifice and incubated with hTg or Tg.2098 with or without RID peptides. An unrelated peptide was used as negative control, and anti-CD3/CD28 beads (which activate T cells in an antigen-independent manner) were used as positive control. RT-15 (20 μg/mL) blocked the activation of EAT-induced mouse T cells by hTg (stimulation index decreased by 31%; p-exact <0.001, Wilcoxon sign rank test).
Similarly, RT-15 blocked the activation of T cells by Tg.2098 (stimulation index decreased by 76%; p-exact <0.001, Wilcoxon sign rank test) (Fig. 3A). VT-15 (20 μg/mL) suppressed the activation of EAT-induced mouse T cells by Tg.2098, with stimulation index decreased by 33% (p-exact = 0.003, Wilcoxon sign rank test). VT-15 did not block the activation of T cells by hTg (Fig. 3B). CFSE plots showing the blockade of Tg and Tg.2098 by RT-15 and VT-15 are shown in Supplementary Figure S2.
FIG. 3.
Effect of RT-15 and VT-15 on hTg- and Tg.2098-induced T cell proliferation in the EAT model. Humanized NOD-DR3 mice were induced with EAT, and splenocytes were isolated and incubated with Tg/Tg.2098, with or without RID peptides RT-15 and VT-15. RT-15 blocked hTg- and Tg.2098-induced T cell proliferation significantly (A). VT-15 only blocked Tg.2098-induced T cell proliferation, but not hTg (B). Anti-CD3/CD28 beads, positive control. **p-Exact <0.01; ***p-exact <0.001. EAT, experimental autoimmune thyroiditis; hTg, human thyroglobulin; NC, negative control peptide; RID, retro-inverso D-amino acid.
Cytokine production by T cells
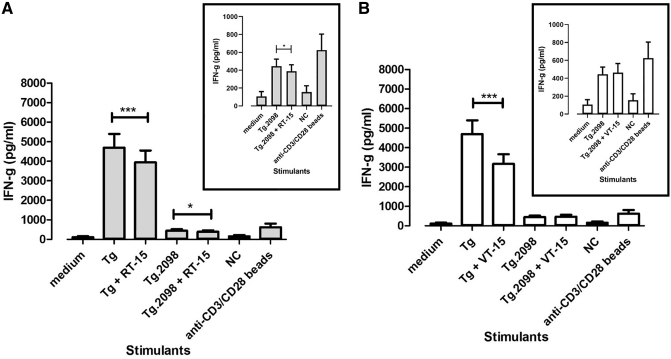
We also measured cytokine production by T cells isolated from NOD-DR3 mice induced with EAT to test the effects of RT-15 and VT-15 on T cell activation. RT-15 inhibited IFNγ production by T cells stimulated with Tg (p-exact = 0.001, Wilcoxon sign rank test). Similarly, RT-15 also inhibited IFNγ production by T cells stimulated with Tg.2098 (p-exact = 0.042, Wilcoxon sign rank test) (Fig. 4A). VT-15 inhibited IFNγ production by T cells stimulated with Tg (p-exact <0.001, Wilcoxon sign rank test). VT-15 did not inhibit IFNγ production by T cells stimulated with Tg.2098 (Fig. 4B). IL-4 and IL-5 (Th2 cytokines) production was also measured. NOD-DR3 mice induced with EAT produced very low levels of IL-4 and IL-5 (Supplementary Fig. S3).
FIG. 4.
Effect of RT-15 and VT-15 on hTg- and Tg.2098-induced T cell activation in the EAT model as measured by IFNγ production. Splenocytes were isolated from humanized NOD-DR3 mice induced with EAT and incubated with Tg/Tg.2098, with or without RID peptides RT-15 and VT-15. RT-15 significantly reduced IFNγ production induced by hTg and Tg.2098 (A), while VT-15 only reduced hTg-induced IFNγ production (B). Anti-CD3/CD28 beads, positive control. *p-Exact <0.05; ***p-exact <0.001. IFNγ, interferon gamma.
RID peptides blocked T cell responses in vivo
To test the in vivo effects of RT-15 and VT-15 in the EAT model, we injected humanized NOD-DR3 mice (n = 8) with a cocktail of RT-15 and VT-15 (or PBS as control) intraperitoneally before immunizing them with hTg. Upon sacrifice, splenocytes were isolated and tested for their response to Tg. Results showed that mice injected with a cocktail of RT-15 and VT-15 had decreased T cell proliferative responses to Tg compared with controls, although it was not statistically significant (stimulation index was decreased by 45%; p-exact = 0.152, Wilcoxon sign rank test).
Similarly, mice injected with RID-peptide cocktail showed less T cell proliferation in response to Tg.2098 compared with controls (stimulation index was decreased by 36%), although this effect did not reach statistical significance (p-exact = 0.094) (Fig. 5). The decreasing trend of T cell response is more evident for Tg.2098 than Tg. The response of both sets of mice to negative peptide control was similar. CFSE plots showing the blockade of Tg and Tg.2098 by RT-15 and VT-15 compared with PBS are shown in Supplementary Figure S4. EAT mice injected with RID peptide cocktail did not have significant difference in Tg antibody levels compared with controls (Supplementary Information S1 and Supplementary Fig. S5).
FIG. 5.
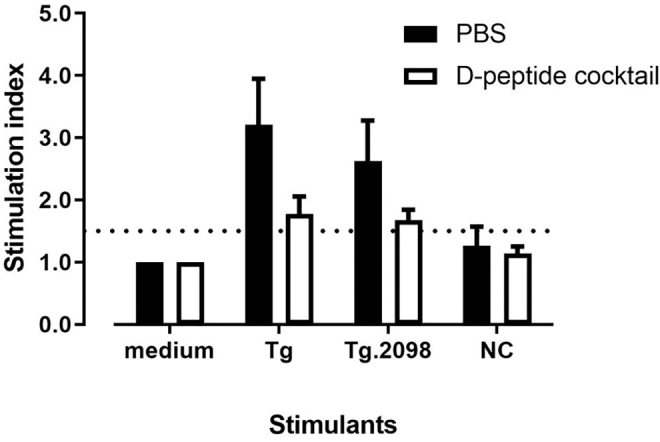
Treatment of EAT in vivo with RID peptide cocktail. Humanized NOD-DR3 mice were pretreated with a mix of RID peptide cocktail RT-15 and VT-15, or PBS as control, before immunization with Tg to induce EAT. Upon sacrifice, splenocytes were isolated and incubated with Tg and Tg.2098. Mice injected with RID peptide cocktail (white bar) showed less hTg-induced T cell proliferation compared with PBS control (black bar), although not statistically significant (p-exact = 0.152). Similarly, splenocytes isolated from mice pretreated with D-peptide cocktail showed less T cell proliferation in response to Tg.2098, compared with PBS control, although not statistically significant (p-exact = 0.094). PBS, phosphate-buffered saline.
Discussion
Although AITD are the most common autoimmune diseases, the treatment remains unsatisfactory with emphasis on relieving symptoms instead of reversing autoimmune response. Peptide therapies for autoimmunity have been tried before, mostly using altered peptide ligands (APLs). APLs are L-amino acid peptides that are based on major T cell epitopes that were modified to render them non-immunogenic. However, attempts to use APLs as therapeutics for autoimmune diseases were unsuccessful.31,32 In one of the studies, APL was poorly tolerated, leading to a halt in the clinical trial.31 In another study, the use of APL resulted in unexpected therapeutic outcomes.32
However, there are ongoing clinical trials testing the new generation of TSHR APLs in GD.33 In this study, we use RID peptides, which consist of D-amino acids, as a novel strategy to treat AITD. RID peptides have higher binding affinity to the HLA-DRβ1-Arg74 pocket compared with L-peptides because their backbone forms alternate hydrogen bonding with the HLA pocket.24 Another significant advantage of RID peptides over L-peptides is that they are more resistant to protease degradation and have longer half-life than L-peptides.34 In addition, the contact residues of RID peptides are less likely to activate T cell receptors24 and thus less likely to be pathogenic in vivo. RID peptide sequence can be easily manipulated to improve stability, affinity, and pharmacokinetic profiles to reach maximum effects.35 Multiple RID peptides can also be combined to maximize their blocking effect.36
We focused on blocking thyroglobulin peptide Tg.2098 binding to HLA-DRβ1-Arg74 since HLA-DRβ1-Arg74 is the major HLA allele associated with AITD and Tg.2098 is the major epitope in AITD.17–19 Previously, we have discovered that Cepharanthine, a plant alkaloid, blocked antigen presentation of thyroid peptides to T cells within DRβ1-Arg74, suppressing both HT and GD in humanized mouse models.19,20,37 D-amino acid peptides present attractive alternative molecules for blocking antigen presentation within HLA-DRβ1-Arg74. In our current study, we identified two RID peptides, RT-15 and VT-15, that were able to block peptide binding to HLA-DRβ1-Arg74 in vitro in a dose-dependent manner. In the EAT model, RT-15 had a stronger effect than VT-15 in blocking T cell activation by Tg and Tg.2098. When we pretreated EAT mice in vivo with a cocktail of RT-15 and VT-15, there was a clear trend showing decreased T cell proliferation in response to Tg and Tg.2098 compared with control mice.
Our model was a short three-week EAT model. The EAT mice developed thyroglobulin antibodies, but not thyroid lymphocytic infiltration. However, the RID peptides did not have significant effect on thyroid antibodies production on the EAT mice (Supplementary Fig. S5). Previously, we have identified a small molecule, Cepharanthine, that could also block antigen presentation within HLA-DRβ1-Arg74.19 The advantage of RID peptides over small molecules is that RID peptides are more specific, and their sequence can be easily modified to achieve maximum efficacy. Another advantage is that various RID peptides can be combined to target multiple epitopes, as we have done in this study combining RT-15 and VT-15. However, the disadvantage of using RID peptides is that they can be only administered parenterally. It is possible that in the future complementary therapy, using both small molecules and RID peptides may be given to fully block the HLA-DRβ1-Arg74 pocket.
RID peptides have been shown as a promising approach in several diseases. For example, RID peptides have been tested in Alzheimer's disease for their ability to eliminate toxic amyloid β (Aβ), which are important elements in development and progression of Alzheimer's disease.38–41 In addition, RID peptides have been designed to target p53-MDM2 interaction as potential cancer therapies,42 or to target α-synuclein as a therapy for Parkinson's disease.43 RID peptides that can inhibit 3-chymotrypsin-like protease, the SARS-CoV-2 main protease, have been identified by computational strategy.44 In particular, RID peptides have been tested in their potential to treat autoimmune diseases. One study showed that an RID peptide designed to block B7-CD28 interaction was able to suppress the expansion of encephalitogenic T cells in vitro.45
Another study showed the use of RID peptides to target HLA-DQ8 as a potential therapy for T1D.24 Our study presents an approach to use RID peptides to block HLA class II molecules as a potential therapy for AITD. Our approach is to block an HLA-DR allele—HLA-DRβ1-Arg74 (a subtype of HLA-DR3). Humans have three MHC class II genes—DR, DQ, and DP with three to six alleles, depending on whether they are homozygotes or heterozygotes for one or more MHC genes. Therefore, blocking HLA-DRβ1-Arg74 will block only one of three to six alleles, which will leave two to five additional MHC class II alleles to present antigens needed during bacterial infection. This is the advantage of our approach—that it will not cause significant immune suppression.
Even though blocking only one HLA class II allele (i.e., HLA-DRβ1-Arg74) would not be expected to cause generalized immunosuppression, it should be noted that it can impair immune responses to external pathogens that are dependent on HLA-DRβ1-Arg74. There are some limitations to our study. It is possible that RID peptides may block only Tg.2098 binding to HLA-DRβ1-Arg74, and due to epitope spreading, other epitopes may propagate the autoimmune response. However, studies have shown that autoimmune responses can be reversed even after epitope spreading began (e.g., in Celiac disease46 and in mouse models of multiple sclerosis47). Another limitation of the study is that we did not use in our T cell activation experiments thyroid draining lymph nodes, but only lymphocytes from lymph nodes at the immunization site, since the lymph nodes from thyroid were too small to purify enough lymphocytes. Finally, since the two RID peptides we designed aim to block antigen presentation by HLA-DRβ1-Arg74, they may only benefit patients who carry this allele.
In summary, we have developed a novel antigen-specific approach to block the autoimmune responses to thyroid antigens in AITD. Using two RID peptides, RT-15 and VT-15, we were able to block antigen presentation by HLA-DRβ1-Arg74. This provides a proof-of-principle that RID peptides can be used to block antigen presentation. More studies are needed to show the promise of using RID peptides as therapeutic treatment. Our study provides a personalized medicine approach to treat AITD patients carrying the DRβ1-Arg74 allele. Moreover, our approach be expanded to treat other autoimmune diseases.
Supplementary Material
Acknowledgments
We thank Dr. Chella David from the Mayo Clinic for generously providing us with the humanized NOD-DR3 mice. This work was supported in part through the computational resources and staff expertise provided by the Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Authors' Contributions
C.W.L.: Investigation, methodology, funding acquisition, writing—original draft, and writing—review and editing. R.O.: Formal analysis, and writing—review and editing. F.M. and A.K.: Investigation and methodology. H.H.: Resources. C.S.: Formal analysis. Y.T.: Conceptualization, supervision, writing—original draft, writing—review and editing, and funding acquisition.
Author Disclosure Statement
Dr. Tomer declares that he was previously (2015–2017) the principal investigator on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current article is not related to that research project. Drs. Li, Osman, and Tomer declare that they submitted a patent application that is not related to the content of this article. All other authors have no potential conflict of interest to declare.
Funding Information
This work was supported in part by grants DK067555 and DK073681 from National Institute of Diabetes and Digestive and Kidney Diseases (to Y.T.) and by a research grant from the American Thyroid Association (to C.W.L.).
Supplementary Material
References
- 1. Tomer Y. Mechanisms of autoimmune thyroid diseases: From genetics to epigenetics. Annu Rev Pathol 2014;9:147–156; doi: 10.1146/annurev-pathol-012513-104713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasham A, Tomer Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res 2012;54(1–3):204–213; doi: 10.1007/s12026-012-8302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87(2):489–499; doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 4. Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun 2007;28(2–3):85–98; doi: 10.1016/j.jaut.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose NR, Bonita R, Burek CL. Iodine: An environmental trigger of thyroiditis. Autoimmun Rev 2002;1(1–2):97–103; doi: 10.1016/s1568-9972(01)00016-7 [DOI] [PubMed] [Google Scholar]
- 6. Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev 1993;14(1):107–120; doi: 10.1210/edrv-14-1-107 [DOI] [PubMed] [Google Scholar]
- 7. Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr Rev 2003;24(5):694–717; doi: 10.1210/er.2002-0030 [DOI] [PubMed] [Google Scholar]
- 8. Nakamura H, Miyauchi A, Miyawaki N, et al. Analysis of 754 cases of antithyroid drug-induced agranulocytosis over 30 years in Japan. J Clin Endocrinol Metab 2013;98(12):4776–4783; doi: 10.1210/jc.2013-2569 [DOI] [PubMed] [Google Scholar]
- 9. Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med 2009;360(15):1574–1575; doi: 10.1056/NEJMc0809750 [DOI] [PubMed] [Google Scholar]
- 10. Kitahara CM, Preston DL, Sosa JA, et al. Association of radioactive iodine, antithyroid drug, and surgical treatments with solid cancer mortality in patients with hyperthyroidism. JAMA Netw Open 2020;3(7):e209660; doi: 10.1001/jamanetworkopen.2020.9660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 1998;338(2):73–78; doi: 10.1056/NEJM199801083380201 [DOI] [PubMed] [Google Scholar]
- 12. Saravanan P, Chau WF, Roberts N, et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: Results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002;57(5):577–585; doi: 10.1046/j.1365-2265.2002.01654.x [DOI] [PubMed] [Google Scholar]
- 13. Bunevicius R, Kazanavicius G, Zalinkevicius R, et al. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999;340(6):424–429; doi: 10.1056/NEJM199902113400603 [DOI] [PubMed] [Google Scholar]
- 14. Groenewegen KL, Mooij CF, van Trotsenburg ASP. Persisting symptoms in patients with Hashimoto's disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J Transl Autoimmun 2021;4:100101; doi: 10.1016/j.jtauto.2021.100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ban Y, Davies TF, Greenberg DA, et al. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves' disease. Genes Immun 2004;5(3):203–208; doi: 10.1038/sj.gene.6364059 [DOI] [PubMed] [Google Scholar]
- 16. Menconi F, Monti MC, Greenberg DA, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U S A 2008;105(37):14034–14039; doi: 10.1073/pnas.0806584105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson EM, Yang H, Menconi F, et al. Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: A genetic, biochemical, and reverse immunological perspective. J Biol Chem 2009;284(49):34231–34243; doi: 10.1074/jbc.M109.041574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menconi F, Huber A, Osman R, et al. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun 2010;35(1):45–51; doi: 10.1016/j.jaut.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li CW, Menconi F, Osman R, et al. Identifying a small molecule blocking antigen presentation in autoimmune thyroiditis. J Biol Chem 2016;291(8):4079–4090; doi: 10.1074/jbc.M115.694687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li CW, Osman R, Menconi F, et al. Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease. J Autoimmun 2020;108:102402; doi: 10.1016/j.jaut.2020.102402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanke SR. Cell biology. Expanding functionality within the looking-glass universe. Science 2009;325(5947):1505–1506; doi: 10.1126/science.1180332 [DOI] [PubMed] [Google Scholar]
- 22. Doti N, Mardirossian M, Sandomenico A, et al. Recent applications of retro-inverso peptides. Int J Mol Sci 2021;22(16):8677; doi: 10.3390/ijms22168677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phan-Chan-Du A, Petit MC, Guichard G, et al. Structure of antibody-bound peptides and retro-inverso analogues. A transferred nuclear Overhauser effect spectroscopy and molecular dynamics approach. Biochemistry 2001;40(19):5720–5727; doi: 10.1021/bi001151h [DOI] [PubMed] [Google Scholar]
- 24. Lombardi A, Concepcion E, Hou H, et al. Retro-inverso D-peptides as a novel targeted immunotherapy for type 1 diabetes. J Autoimmun 2020;115:102543; doi: 10.1016/j.jaut.2020.102543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chicz RM, Urban RG, Gorga JC, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med 1993;178(1):27–47; doi: 10.1084/jem.178.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn JC, McCormick DJ, Brusic V, et al. Pathogenic human thyroglobulin peptides in HLA-DR3 transgenic mouse model of autoimmune thyroiditis. Cell Immunol 2004;229(2):79–85; doi: 10.1016/j.cellimm.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 27. Muixi L, Carrascal M, Alvarez I, et al. Thyroglobulin peptides associate in vivo to HLA-DR in autoimmune thyroid glands. J Immunol 2008;181(1):795–807; doi: 10.4049/jimmunol.181.1.795 [DOI] [PubMed] [Google Scholar]
- 28. Li CW, Osman R, Menconi F, et al. Cepharanthine blocks presentation of thyroid and islet peptides in a novel humanized autoimmune diabetes and thyroiditis mouse model. Front Immunol 2021;12:796552; doi: 10.3389/fimmu.2021.796552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li CW, Osman R, Menconi F, et al. Flexible peptide recognition by HLA-DR triggers specific autoimmune T-cell responses in autoimmune thyroiditis and diabetes. J Autoimmun 2017;76:1–9; doi: 10.1016/j.jaut.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilcoxon F. Individual comparisons by ranking methods. Biometrics 1945;1:80–83. [Google Scholar]
- 31. Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000;6(10):1167–1175; doi: 10.1038/80516 [DOI] [PubMed] [Google Scholar]
- 32. Spear TT, Wang Y, Smith TWJr., et al. Altered peptide ligands impact the diversity of polyfunctional phenotypes in T cell receptor gene-modified T cells. Mol Ther 2018;26(4):996–1007; doi: 10.1016/j.ymthe.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearce SHS, Dayan C, Wraith DC, et al. Antigen-specific immunotherapy with thyrotropin receptor peptides in Graves' hyperthyroidism: A phase I study. Thyroid 2019;29(7):1003–1011; doi: 10.1089/thy.2019.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu M, Li X, Xie Z, et al. D-peptides as recognition molecules and therapeutic agents. Chem Rec 2016;16(4):1772–1786; doi: 10.1002/tcr.201600005 [DOI] [PubMed] [Google Scholar]
- 35. Vlieghe P, Lisowski V, Martinez J, et al. Synthetic therapeutic peptides: Science and market. Drug Discov Today 2010;15(1–2):40–56; doi: 10.1016/j.drudis.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 36. Craik DJ, Fairlie DP, Liras S, et al. The future of peptide-based drugs. Chem Biol Drug Des 2013;81(1):136–147; doi: 10.1111/cbdd.12055 [DOI] [PubMed] [Google Scholar]
- 37. Nagayama Y, Kita-Furuyama M, Ando T, et al. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 2002;168(6):2789–2794; doi: 10.4049/jimmunol.168.6.2789 [DOI] [PubMed] [Google Scholar]
- 38. Schartmann E, Schemmert S, Niemietz N, et al. In vitro potency and preclinical pharmacokinetic comparison of all-D-enantiomeric peptides developed for the treatment of Alzheimer's disease. J Alzheimers Dis 2018;64(3):859–873; doi: 10.3233/JAD-180165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schartmann E, Schemmert S, Ziehm T, et al. Comparison of blood-brain barrier penetration efficiencies between linear and cyclic all-d-enantiomeric peptides developed for the treatment of Alzheimer's disease. Eur J Pharm Sci 2018;114:93–102; doi: 10.1016/j.ejps.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 40. Schemmert S, Camargo LC, Honold D, et al. In vitro and in vivo efficacies of the linear and the cyclic version of an all-d-enantiomeric peptide developed for the treatment of Alzheimer's disease. Int J Mol Sci 2021;22(12):6553; doi: 10.3390/ijms22126553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leithold LH, Jiang N, Post J, et al. Pharmacokinetic properties of tandem d-peptides designed for treatment of Alzheimer's disease. Eur J Pharm Sci 2016;89:31–38; doi: 10.1016/j.ejps.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 42. Liu M, Li C, Pazgier M, et al. D-peptide inhibitors of the p53-MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc Natl Acad Sci U S A 2010;107(32):14321–14326; doi: 10.1073/pnas.1008930107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shaltiel-Karyo R, Frenkel-Pinter M, Egoz-Matia N, et al. Inhibiting alpha-synuclein oligomerization by stable cell-penetrating beta-synuclein fragments recovers phenotype of Parkinson's disease model flies. PLoS One 2010;5(11):e13863; doi: 10.1371/journal.pone.0013863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez Gonzalez JE, Eberle RJ, Willbold D, et al. A computer-aided approach for the discovery of D-peptides as inhibitors of SARS-CoV-2 main protease. Front Mol Biosci 2021;8:816166; doi: 10.3389/fmolb.2021.816166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Srinivasan M, Wardrop RM, Gienapp IE, et al. A retro-inverso peptide mimic of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J Immunol 2001;167(1):578–585; doi: 10.4049/jimmunol.167.1.578 [DOI] [PubMed] [Google Scholar]
- 46. Green PH, Cellier C. Celiac disease. N Engl J Med 2007;357(17):1731–1743; doi: 10.1056/NEJMra071600 [DOI] [PubMed] [Google Scholar]
- 47. Ji N, Somanaboeina A, Dixit A, et al. Small molecule inhibitor of antigen binding and presentation by HLA-DR2b as a therapeutic strategy for the treatment of multiple sclerosis. J Immunol 2013;191(10):5074–5084; doi: 10.4049/jimmunol.1300407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.