Abstract
The California quail (Callipepla californica) is an iconic native bird of scrub and oak woodlands in California and the Baja Peninsula of Mexico. Here, we report a draft reference assembly for the species generated from PacBio HiFi long read and Omni-C chromatin-proximity sequencing data as part of the California Conservation Genomics Project (CCGP). Sequenced reads were assembled into 321 scaffolds totaling 1.08 Gb in length. Assembly metrics indicate a highly contiguous and complete assembly with a contig N50 of 5.5 Mb, scaffold N50 of 19.4 Mb, and BUSCO completeness score of 96.5%. Transposable elements (TEs) occupy 16.5% of the genome, more than previous Odontophoridae quail assemblies but in line with estimates of TE content for recent long-read assemblies of chicken and Peking duck. Together these metrics indicate that the present assembly is more complete than prior reference assemblies generated for Odontophoridae quail. This reference will serve as an essential resource for studies on local adaptation, phylogeography, and conservation genetics in this species of significant biological and recreational interest.
Keywords: California Conservation Genomics Project, Odontophoridae, transposable elements, upland game bird
Introduction
The charismatic California quail (Callipepla californica) is the state bird of California. The “chi-ca-go” rally calls given by both sexes can be heard year-round from throughout its native distribution in the Baja peninsula of Mexico, California, southern Oregon, and western Nevada (Fig. 1; Calkins et al. 2014). Starting in the mid-1800s, successful introductions were carried out widely across western North America (e.g. Washington, Idaho, Montana, Utah), Chile, Argentina, New Zealand, Australia, Hawaii, and Corsica (Calkins et al. 2014). California quail are typically found in a variety of oak woodland, chaparral, and sage habitats within its native range. The species spans broad thermal and precipitation gradients, but in the most arid regions of southeastern California it is replaced by its sister species, the Gambel’s quail (Callipepla gambeli). The 2 form a narrow hybrid zone in the Coachella valley in Riverside County, California (Zonana et al. 2019).
Fig. 1.
Female (a) and male (b) California quail (Callipepla californica). (c) Distribution of California quail (red) across western North America. Map includes both native and introduced populations. Distribution data from eBird (https://science.ebird.org/). Photos taken at Blue Oak Ranch Reserve, Santa Clara County, California and courtesy of Jackie Childers.
California and Gambel’s quail comprise half the members of the genus Callipepla, a lineage within the family Odontophoridae. Most of the 33 Odontophoridae quail species occupy tropical forest environments, but Callipepla and its sister genus, Colinus bobwhites, inhabit more open and arid habitats (Hosner et al. 2015; Winkler et al. 2020; Salter et al. 2022). Within California quail, 5 subspecies are currently accepted (Calkins et al. 2014). Callipepla c. californica is the most widespread subspecies ranging from southern Oregon south through much of California to northern Baja California, Mexico. It is replaced south of central Baja California by C. c. achrustera and in northwestern California by C. c. brunnescens. Callipepla c. canfieldae is restricted to the Owen’s valley of eastern California and C. c. catalinensis is native only to Catalina Island off the coast of southern California. Population genetic data are largely lacking for California quail, although an early allozyme analysis suggested that some subtle population structure may exist between populations in California and Baja California (Zink et al. 1987).
California quail are generally considered widespread and of least conservation concern. An exception is the endemic Catalina Island population (C. c. catalinensis), which is classified as a California Bird Species of Special Concern due to the narrowness of its distribution (Collins 2008). Quail populations across the state of California also have declined precipitously from historic highs in the 1800s. These declines were driven by unregulated hunting and trapping that supplied tens of thousands of quail annually to markets in San Francisco and Los Angeles (e.g. 177,366 quail in 1895; Grinnell et al. 1918; Leopold 1977). Bag limits and anti-trapping laws were established in 1901, but ongoing habitat degradation from overgrazing, mechanized agriculture, and urbanization have all contributed to a constricted quail distribution with lower densities than historically reported. Furthermore, persistent drought conditions and the increasing severity of wildfires in the western United States continue to pose significant threats to California quail across its distribution (Calkins et al. 2014). Finally, free-ranging (feral) cats also can reduce the likelihood of habitat occupancy by California quail, especially in urban environments (Iknayan et al. 2021).
In light of these historic and future threats to California quail populations, the species has been included in the California Conservation Genomics Project (CCGP; Shaffer et al. 2022). The goals of the CCGP are to better understand shared patterns of genetic diversity and population structure across over 150 California plant and animal species. A key component of each CCGP project is the generation of high-quality reference genomes for each species. Here, we report on the reference assembly of the California quail generated by the CCGP using PacBio HiFi long-read and Omni-C chromatin-proximity sequencing data. This represents the first long-read-based genome assembly for the family Odontophoridae. Along with high-quality reference genomes for the domestic chicken (Gallus gallus; Warren et al. 2017) and mallard duck (Anas platyrhynchos; Zhu et al. 2021) the California quail genome generated for the CCGP will serve as an important resource for the intensively studied and harvested Galloanserae (ducks, upland game birds, and relatives).
Methods
Biological materials
A liver tissue sample was obtained from a male California quail (C. californica brunnescens) collected on 13 October 2020. This bird was captured at Cow Mountain Recreation Management Area, Lake Co., California (39.08005°N; 122.08910°W). The individual was collected with approval of the California Department of Fish and Wildlife (permit #: SCP-458) and the U.S. Fish and Wildlife Service (permit #: MB153526-0). The bird was captured and euthanized using methods approved by the University of California, Berkeley IACUC (AUP-2016-04-8665-1). A voucher specimen has been deposited at the Museum of Vertebrate Zoology, Berkeley, California (https://arctos.database.museum/guid/MVZ:Bird:193975).
PacBio HiFi library preparation
High molecular weight (HMW) genomic DNA (gDNA) was extracted from 45 mg of liver tissue using the Nanobind Tissue Big DNA kit as per the manufacturer’s instructions (Pacific BioSciences—PacBio, Menlo Park, California). The DNA purity was estimated using absorbance ratios (260/280 = 1.80 and 260/230 = 2.06) on the NanoDrop ND-1000 spectrophotometer. The final DNA yield (17 µg) was quantified using the Quantus Fluorometer (QuantiFluor ONE dsDNA Dye assay; Promega, Madison, Wisconsin). The size distribution of the HMW DNA was estimated using the Femto Pulse system (Agilent, Santa Clara, California) and found that 71% of the fragments were 120 kb or more.
The HiFi SMRTbell library was constructed using the SMRTbell Express Template Prep Kit v2.0 (PacBio; Cat. #100-938-900) according to the manufacturer’s instructions. HMW gDNA was sheared to a target DNA size distribution between 15 and 20 kb using Diagenode’s Megaruptor 3 system (Diagenode, Belgium; Cat. B06010003). The sheared gDNA was concentrated using 0.45× of AMPure PB beads (PacBio; Cat. #100-265-900) for the removal of single-strand overhangs at 37 °C for 15 min, followed by further enzymatic steps of DNA damage repair at 37 °C for 30 min, end repair and A-tailing at 20 °C for 10 min and 65 °C for 30 min, ligation of overhang adapter v3 at 20 °C for 60 min and 65 °C for 10 min to inactivate the ligase, then nuclease treated at 37 °C for 1 h. The SMRTbell library was purified and concentrated with 0.45× Ampure PB beads for size selection using the BluePippin/PippinHT system (Sage Science, Beverly, Massachusetts; Cat. #BLF7510/HPE7510) to collect fragments greater than 7 to 9 kb. The 15 to 20 kb average HiFi SMRTbell library was sequenced at UC Davis DNA Technologies Core (Davis, California) using 2 8M SMRT cells, Sequel II sequencing chemistry 2.0, and 30-h movies each on a PacBio Sequel II sequencer.
Omni-C library preparation and sequencing
The Omni-C library was prepared using the Dovetail Omni-C Kit (Dovetail Genomics, California) according to the manufacturer’s protocol with slight modifications. First, specimen tissue was thoroughly ground with a mortar and pestle while cooled with liquid nitrogen. Subsequently, chromatin was fixed in place in the nucleus. The suspended chromatin solution was then passed through 100 and 40 µm cell strainers to remove large debris. Fixed chromatin was digested under various conditions of DNase I until a suitable fragment length distribution of DNA molecules was obtained. Chromatin ends were repaired and ligated to a biotinylated bridge adapter followed by proximity ligation of adapter containing ends. After proximity ligation, crosslinks were reversed and the DNA purified from proteins. Purified DNA was treated to remove biotin that was not internal to ligated fragments. An NGS library was generated using an NEB Ultra II DNA Library Prep kit (NEB, Ipswich, Massachusetts) with an Illumina compatible y-adaptor. Biotin-containing fragments were then captured using streptavidin beads. The post capture product was split into 2 replicates prior to PCR enrichment to preserve library complexity with each replicate receiving unique dual indices. The library was sequenced on an Illumina NovaSeq platform to generate approximately 100 million 2 × 150 bp read pairs per Gb genome size.
Nuclear genome assembly
We assembled the genome of the California quail following the CCGP assembly pipeline Version 4.0, as outlined in Table 1, which lists the tools and non-default parameters used in the assembly. The pipeline uses PacBio HiFi reads and Omni-C data to produce high-quality and highly contiguous genome assemblies minimizing manual curation. We removed remnant adapter sequences from the PacBio HiFi dataset using HiFiAdapterFilt (Sim et al. 2022) and obtained the initial dual or partially phased diploid assembly (http://lh3.github.io/2021/10/10/introducing-dual-assembly) using HiFiasm (Cheng et al. 2022) with the filtered PacBio HiFi reads and the Omni-C dataset. We tagged output haplotype 1 as the primary assembly, and output haplotype 2 as the alternate assembly. We identified sequences corresponding to haplotypic duplications, contig overlaps and repeats on the primary assembly with purge_dups (Guan et al. 2020) and transferred them to the alternate assembly. We aligned the Omni-C data to both assemblies following the Arima Genomics Mapping Pipeline (https://github.com/ArimaGenomics/mapping_pipeline) and then scaffolded both assemblies with SALSA (Ghurye et al. 2017, 2019).
Table 1.
Assembly pipeline and software used.
| Assembly | Software and optionsa | Version |
|---|---|---|
| Filtering PacBio HiFi adapters | HiFiAdapterFilt | Commit 64d1c7b |
| K-mer counting | Meryl (k = 21) | 1 |
| Estimation of genome size and heterozygosity | GenomeScope | 2 |
| De novo assembly (contiging) | HiFiasm (Hi-C Mode, –primary, output p_ctg.hap1, p_ctg.hap2) | 0.16.1-r375 |
| Identification of haplotypic duplications | purge_dups (Custom cutoffs: 5,17,25,25,55,99) | 1.2.5 |
| Scaffolding | ||
| Omni-C data alignment | Arima Genomics Mapping Pipeline | Commit 2e74ea4 |
| Omni-C scaffolding | SALSA (-DNASE, -i 20, -p yes) | 2 |
| Gap closing | YAGCloser (-mins 2 -f 20 -mcc 2 -prt 0.25 -eft 0.2 -pld 0.2) | Commit 0e34c3b |
| Omni-C contact map generation | ||
| Short-read alignment | BWA-MEM (-5SP) | 0.7.17-r1188 |
| SAM/BAM processing | Samtools | 1.11 |
| SAM/BAM filtering | Pairtools | 0.3.0 |
| Pairs indexing | Pairix | 0.3.7 |
| Matrix generation | Cooler | 0.8.10 |
| Matrix balancing | hicExplorer (hicCorrectmatrix correct --filterThreshold -2 4) | 3.6 |
| Contact map visualization | HiGlass | 2.1.11 |
| PretextMap | 0.1.4 | |
| PretextView | 0.1.5 | |
| PretextSnapshot | 0.0.3 | |
| Genome quality assessment | ||
| Basic assembly metrics | QUAST (--est-ref-size) | 5.0.2 |
| Assembly completeness | BUSCO (-m geno, -l aves) | 5.0.0 |
| Merqury | 2020-01-29 | |
| Contamination screening | ||
| Local alignment tool | BLAST+ | 2.1 |
| General contamination screening | BlobToolKit | 2.3.3 |
| Synteny visualization | ||
| Visualization of genome assembly consistency | JupiterPlot (ng = 80, m = 1,000,000) | 1.0 |
| Repeat analysis | ||
| Identification of LTR elements | RepeatModeler (ltrstruct) | 2 |
| Annotation of TE diversity | RepeatMasker | 4.1.2 |
Software citations are in the text.
aOptions detailed for non-default parameters.
We generated Omni-C contact maps for both assemblies by aligning the Omni-C data with BWA-MEM (Li 2013), identified ligation junctions, and generated Omni-C pairs using pairtools (Goloborodko et al. 2018). We generated a multiresolution Omni-C matrix with cooler (Abdennur and Mirny 2020) and balanced it with hicExplorer (Ramírez et al. 2018). We used HiGlass (Kerpedjiev et al. 2018) and the PretextSuite (https://github.com/wtsi-hpag/PretextView; https://github.com/wtsi-hpag/PretextMap; https://github.com/wtsi-hpag/PretextSnapshot) to visualize the contact maps and then checked the contact maps for major misassemblies. In detail, if in the proximity of a join that was made by the scaffolder we identified a strong off-diagonal signal and a lack of signal in the consecutive genomic region, we dissolved it by breaking the scaffolds at the coordinates of the join. After this process, no further manual joins were made. Some of the remaining gaps (joins generated by the scaffolder) were closed using the PacBio HiFi reads and YAGCloser (https://github.com/merlyescalona/yagcloser). Finally, we checked for contamination using the BlobToolKit Framework (Challis et al. 2020).
Mitochondrial genome assembly
We assembled the mitochondrial genome of C. californica from the PacBio HiFi reads using the reference-guided pipeline MitoHiFi (Allio et al. 2020; Uliano-Silva et al. 2021). The mitochondrial sequence of C. douglasii (https://www.ncbi.nlm.nih.gov/nuccore/MW574356.1; Kimball et al. 2021) was used as the starting reference sequence. After completion of the nuclear genome, we searched for matches of the resulting mitochondrial assembly sequence in the nuclear genome assembly using BLAST+ (Camacho et al. 2009) and filtered out contigs and scaffolds from the nuclear genome with a percentage of sequence identity >99% and size smaller than the mitochondrial assembly sequence.
Genome assembly assessment
We generated k-mer counts from the PacBio HiFi reads using meryl (https://github.com/marbl/meryl) and then used the k-mer database in GenomeScope2.0 (Ranallo-Benavidez et al. 2020) to estimate genome features including genome size, heterozygosity, and repeat content. We ran QUAST (Gurevich et al. 2013) to obtain general contiguity metrics, and used BUSCO (Manni et al. 2021) with the Aves ortholog database (aves_odb10, which contains 8,338 genes) to evaluate genome quality and functional completeness. Assessment of base level accuracy (QV) and k-mer completeness was performed using the previously generated meryl database and merqury (Rhie et al. 2020). We further estimated genome assembly accuracy via BUSCO gene set frameshift analysis using the pipeline described in Korlach et al. (2017). Measurements of the size of the phased blocks are based on the size of the contigs generated by HiFiasm on HiC mode. We follow the quality metric nomenclature established by Rhie et al. (2021), with the genome quality code x·y·P·Q·C, where x = log10[contig NG50]; y = log10[scaffold NG50]; P = log10[phased block NG50]; Q = Phred base accuracy QV (quality value); C = % genome represented by the first “n” scaffolds, following a known karyotype of 2n = 84 for C. californica (Bird Chromosome Database, Chromosome number data V3.0/2022, Degrandi et al. 2020). Quality metrics for the notation were calculated on the primary assembly.
To visualize higher-level synteny between quail scaffolds and chromosomes of the chicken genome, we generated a jupiter plot using the JupiterPlot pipeline (Chu 2018). We mapped the longest scaffolds representing 80% of the draft assembly to chicken chromosomes exceeding 1 Mb in length.
Repeat annotation
We performed de novo repeat annotation of the draft California quail reference assembly using the program RepeatModeler2 with the ltrstruct option selected to improve identification of LTR (Long Terminal Repeat) elements (Flynn et al. 2020). The resulting repeat library contained 496 elements and was used to annotate transposable element (TE) diversity in the California quail assembly using RepeatMasker (Smit et al. 2013–2015). To assess temporal patterns of TE activity in the quail genome, we used the calcDivergenceFromAlign.pl script in the RepeatMasker package to estimate the Kimura 2-parameter (K2P) distance of each TE element from the consensus sequence. K2P distances were used to generate barplots for the LTR, SINE, LINE, and DNA classes of TE elements found in the genome.
Results
Sequencing data
The Omni-C and PacBio HiFi sequencing libraries generated 132.2 million read pairs and 3.1 million reads, respectively. The latter yielded ~45-fold coverage based on the Genomescope2.0 genome size estimation of 1.09 Gb (N50 read length of 15,835 bp, minimum read length 44 bp; mean read length 15,667 bp, maximum read length 50,092 bp). Based on the PacBio HiFi reads, we estimated 0.195% sequencing error rate and 0.7% heterozygosity rate. The k-mer spectrum, also based on the PacBio HiFi reads, shows a bimodal distribution with major peaks at ~22- and ~45-fold coverage, where peaks correspond to heterozygous and homozygous states of a diploid species, respectively (Fig. 2a). The distribution presented in this k-mer spectrum supports that of a low heterozygosity profile.
Fig. 2.
Visual overview of genome assembly metrics. a) K-mer spectra output generated from PacBio HiFi data without adapters using GenomeScope2.0. The bimodal pattern observed corresponds to a diploid genome and the k-mer profile matches that of low (<1%) heterozygosity. K-mers covered at lower coverage and lower frequency correspond to differences between haplotypes, whereas the higher coverage and higher frequency k-mers correspond to the similarities between haplotypes. b) BlobToolKit Snail plot showing a graphical representation of the quality metrics presented in Table 2 for the Callipepla californica primary assembly (bCalCai1). The plot circle represents the full size of the assembly. From the inside-out, the central plot covers length-related metrics. The red line represents the size of the longest scaffold; all other scaffolds are arranged in size-order moving clockwise around the plot and drawn in gray starting from the outside of the central plot. Dark and light orange arcs show the scaffold N50 and scaffold N90 values. The central light gray spiral shows the cumulative scaffold count with a white line at each order of magnitude. White regions in this area reflect the proportion of Ns in the assembly; the dark versus light blue area around it shows mean, maximum, and minimum GC versus AT content at 0.1% intervals (Challis et al. 2020). Hi-C contact maps for the primary (c) and alternate (d) genome assembly generated with PretextSnapshot. Hi-C contact maps translate proximity of genomic regions in 3D space to contiguous linear organization. Each cell in the contact map corresponds to sequencing data supporting the linkage (or join) between 2 such regions.
Assembly metrics
The final assembly (bCalCai1) consists of 2 pseudo haplotypes, here tagged as primary and alternate, with the genome size of the primary assembly similar to the estimated genome size from Genomescope2.0 (Fig. 2a). The primary assembly consists of 321 scaffolds spanning 1.08 Gb with contig N50 of 5.5 Mb, scaffold N50 of 19.4 Mb, largest contig N50 of 25.2 Mb, and largest scaffold N50 of 121.9 Mb. On the other hand, the alternate assembly consists of 1,855 scaffolds spanning 1.39 Gb with contig N50 of 3.4 Mb, scaffold N50 of 12.5 Mb, largest contig of 30.6 Mb, and largest scaffold of 94.9 Mb. Detailed assembly statistics are reported in Table 2 and graphical representation for the primary assembly is shown in Fig. 2b (see Supplementary Fig. S1 for the alternate assembly). Hi-C contact maps for the primary and alternate assemblies are shown in Fig. 2c and d, respectively. The primary assembly has a BUSCO completeness score of 96.5% using the Aves gene set, a per-base quality (QV) of 63.63, a k-mer completeness of 89.72, and a frameshift indel QV of 42.85; the alternate assembly has a BUSCO completeness score of 96.7% using the Aves gene set, a per-base quality (QV) of 62.61, a k-mer completeness of 90.41, and a frameshift indel QV of 42.86.
Table 2.
Metrics for the primary (left column) and alternate (right column) assemblies of the California quail (Callipepla californica) genome.
| BioProjects & Vouchers | CCGP NCBI BioProject | PRJNA720569 | |||||
| Genera NCBI BioProject | PRJNA766282 | ||||||
| Species NCBI BioProject | PRJNA777150 | ||||||
| NCBI BioSample | SAMN26894057 | ||||||
| Specimen voucher | MVZ:Bird:193975 | ||||||
| NCBI Genome accessions | Primary | Alternate | |||||
| Assembly accession | JALIRH000000000 | JALIRI000000000 | |||||
| Genome sequences | GCA_023055505.1 | GCA_023055725.1 | |||||
| Genome Sequence | PacBio HiFi reads | Run | 1 PACBIO_SMRT (Sequel II) run: 3.2M spots, 49.6G bases, 35.9 Gb | ||||
| Accession | SRX15651218 | ||||||
| Omni-C Illumina reads | Run | 2 ILLUMINA (Illumina NovaSeq 6000) runs: 132.2M spots, 39.9G bases, 12.8 Gb | |||||
| Accession | SRX15651219, SRX15651220 | ||||||
| Genome Assembly Quality Metrics | Assembly identifier (quality codea) | bCalCai1(6.7.P6.Q63.C78) | |||||
| HiFi read coverageb | 45.28× | ||||||
| Primary | Alternate | ||||||
| Number of contigs | 608 | 1,855 | |||||
| Contig N50 (bp) | 5,506,990 | 3,481,594 | |||||
| Contig NG50b | 5,478,314 | 5,669,995 | |||||
| Longest contigs | 25,292,651 | 30,636,471 | |||||
| Number of scaffolds | 321 | 1,607 | |||||
| Scaffold N50 | 19,408,658 | 12,585,889 | |||||
| Scaffold NG50b | 18,906,995 | 16,621,059 | |||||
| Largest scaffold | 121,926,015 | 94,958,808 | |||||
| Size of final assembly | 1,085,347,722 | 1,399,796,956 | |||||
| Phased block NG50b | 5,365,876 | 5,773,240 | |||||
| Gaps per Gbp (# Gaps) | 264 (287) | 177 (248) | |||||
| Indel QV (frameshift) | 42.85789529 | 42.86055461 | |||||
| Base pair QV | 63.6319 | 62.6168 | |||||
| Full assembly = 63.0313 | |||||||
| K-mer completeness | 89.7222 | 90.4135 | |||||
| Full assembly = 99.4094 | |||||||
| BUSCO completeness (aves_odb10), n = 8,338 | C | S | D | F | M | ||
| Pc | 96.50% | 96.20% | 0.30% | 0.60% | 2.90% | ||
| Ac | 96.70% | 96.20% | 0.50% | 0.60% | 2.70% | ||
| Organelles | 1 Partial mitochondrial sequence | JALIRH010000321.1 | |||||
(a) Assembly quality code x.y.P.Q.C derived notation, from (Rhie et al. 2021). x = log10[contig NG50]; y = log10[scaffold NG50]; P = log10 [phased block NG50]; Q = Phred base accuracy QV (Quality value); C = % genome represented by the first ‘n’ scaffolds, following a known karyotype for SPECIES of 2n = 82 following a known karyotype of 2n =84 for C. californica (Bird Chromosome Database, Chromosome number data V3.0/2022, Degrandi et al. 2020). Quality code for all the assembly denoted by primary assembly (bCalCai1.0.p).
(b) Read coverage and NGx statistics have been calculated based on the estimated genome size of 1.09 Gb.
(c) (P)rimary and (A)lternate assembly values.
BUSCO Scores. (C)omplete and (S)ingle; (C)omplete and (D)uplicated; (F)ragmented and (M)issing BUSCO genes. n, number of BUSCO genes in the set/data base. bp, base pairs.
We identified 16 misassemblies, 11 on the primary and 5 on the alternate, and broke the corresponding joins made by SALSA. We were able to close 16 gaps, 10 on the primary and 6 on the alternate. Finally, we removed 2 contigs, one per assembly, corresponding to mitochondrial contaminants. No further sequences were removed. We have deposited both assemblies on NCBI (see Table 2 and Data Availability for details).
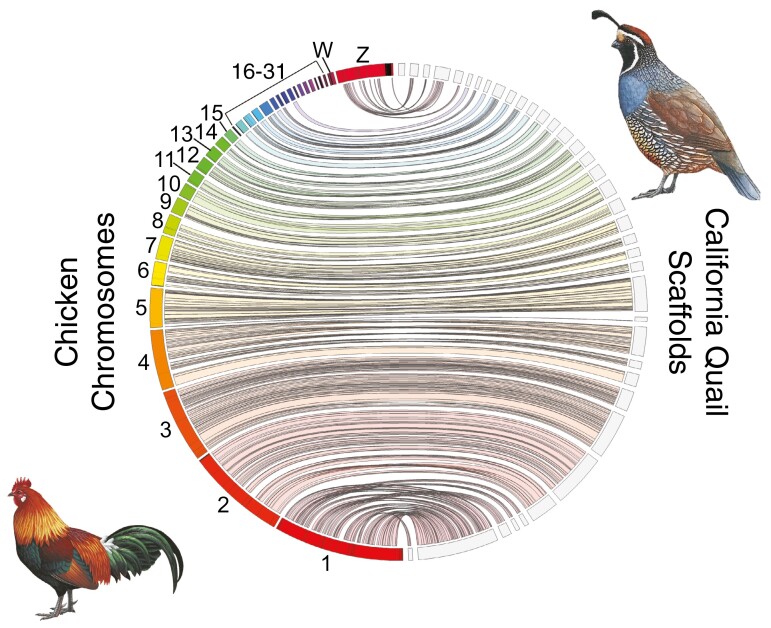
All metrics for the California quail assembly exceed those reported for other Odontophoridae quail genomes with the exception of a higher scaffold N50 in the most recent northern bobwhite genome (Table 3). A Jupiter plot showed quail scaffolds mapping to most chromosomes of the chicken genome with little evidence for major inversions or translocations (Fig. 3). The Jupiter plot also shows 2 large scaffolds (Scaffold 2: 80 Mb; Scaffold 6: 42 Mb) mapping to chicken chromosome 2. This likely corresponds to a previously reported fission of chicken chromosome 2 into chromosomes 3 and 6 of the California quail (Shibusawa et al. 2004).
Table 3.
A comparison of assembly metrics and BUSCO scores for the California quail, 3 other Odontophoridae quail genomes (scaled quail, northern bobwhite, marbled wood-quail) and the chicken assembly.
| California quail (Callipepla californica) | Scaled quail (Callipepla squamata) | Northern bobwhite (Colinus virginianus) | Marbled wood-quail (Odontophorus gujanensis) | Chicken (Gallus gallus) | |
|---|---|---|---|---|---|
| Assembly | GCA_023055505.1 | GCA_002218305.1 | GCA_008692595.2 | GCA_013399175.1 | GCA_000002315.5 |
| Total length | 1,085,347,722 | 1,045,281,893 | 948,540,911 | 977,719,362 | 1,065,348,650 |
| # contigs | 608 | 59,818 | 39,169 | 52,876 | 1,402 |
| Contig N50 | 5,506,990 | 154,132 | 106,817 | 74,578 | 17,655,422 |
| Contig L50 | 53 | 1,950 | 2,337 | 3,724 | 19 |
| # scaffolds | 321 | 34,302 | 6,035 | 26,662 | 524 |
| Scaffold N50 | 19,408,658 | 1,035,259 | 66,825,924 | 343,789 | 20,785,086 |
| Scaffold L50 | 12 | 278 | 5 | 781 | 12 |
| BUSCO results (%): | |||||
| Complete | 96.5 | 94.2 | 90.8 | 93.5 | — |
| Single copy | 96.2 | 92.8 | 89.8 | 92.5 | — |
| Duplicate | 0.3 | 1.4 | 0.9 | 1 | — |
| Fragmented | 0.6 | 3.6 | 3.5 | 3.7 | — |
| Missing | 2.0 | 2.2 | 5.8 | 2.8 | — |
BUSCO scores for the California quail genome were based on the aves_odb10 database, scores for the other quail genomes were based on earlier aves databases. Data for scaled quail and marbled wood-quail from Feng et al. (2020), northern bobwhite from Salter et al. (2019), and chicken from Warren et al. (2017).
Fig. 3.
Jupiter plot comparing higher-level synteny and completeness between the chicken (Gallus gallus) genome (GRCg6a) and the California quail draft assembly. Chicken chromosomes are on the (colored) and quail scaffolds are on the right (light gray). Twists represent reversed orientation of scaffolds between assemblies. Bird illustrations reproduced from https://birdsoftheworld.org with permission from Lynx Edicions.
Mitochondrial assembly
We assembled a mitochondrial genome for C. californica with MitoHiFi. The final mitochondrial sequence has a size of 16,706 bp. The base composition is A= 30.52%, C = 31.99, G = 13.26%, T = 24.23, and the sequence consists of 22 unique transfer RNAs and 13 protein-coding genes.
Repeat annotation
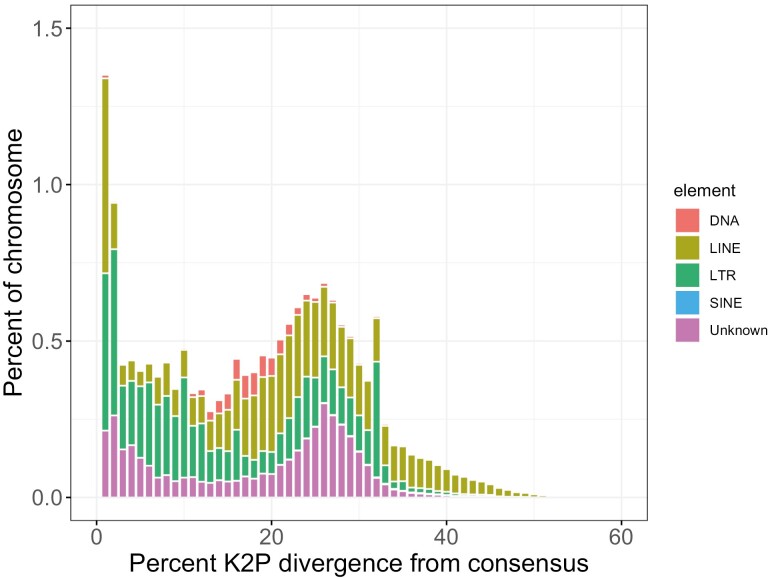
19.12% of the California quail genome was masked by RepeatMasker (Table 4). This included 178.7 Mb (16.46%) of the genome masked by interspersed repeats and 28.8 Mb (2.66%) masked by satellites and other simple repeats. Endogenous retroviral elements (5.25%) and LINE elements of the CR1 family (6.90%) were the most abundant TEs annotated in the quail genome. DNA transposons and SINE elements were each found in <1% of the genome. LINE elements exhibited a pattern of greater divergence from the consensus sequence (peak between 20% and 30% K2P divergence), indicating that these elements likely stem from a more ancient proliferation. In contrast, LTR elements exhibit a pattern of lower divergence from the consensus i.e. indicative of more recent activity (Fig. 4).
Table 4.
Percentage of each genome spanned by different classes of repeats.
| Number elements | Length (bp) | Percentage of genome (%) | |
|---|---|---|---|
| Retroelements | 273,188 | 135,663,026 | 12.5 |
| SINEs | 3,230 | 414,480 | 0.04 |
| LINEs | 203,697 | 74,892,484 | 6.9 |
| LTR elements | 66,261 | 60,356,062 | 5.56 |
| DNA transposons | 28,034 | 8,366,846 | 0.77 |
| Unclassified | 60,686 | 34,668,306 | 3.19 |
| Total interspersed repeats | 178,698,178 | 16.46 | |
| Other repeats | |||
| Small RNA | 885 | 106,771 | 0.01 |
| Satellites | 469 | 292,684 | 0.03 |
| Simple repeats | 299,705 | 24,793,328 | 2.28 |
| Low complexity | 54,030 | 3,734,179 | 0.34 |
Estimates of each class of repeat region identified within RepeatMasker using the quail TE libraries generated de novo with RepeatModeler2.
Fig. 4.
TE landscape for the California quail genome. Percent divergence on the x axis was calculated as the percent Kimura 2-parameter (K2P) distance with excluding CpG sites. The abundance of TEs in each percent divergence bin was normalized as percentage of the genome length on the y axis.
Discussion
We generated a highly contiguous genome for the California quail (C. californica). The contig N50 for this assembly exceeded 91% of all avian genome assemblies generated to date, and scaffold N50 was in the 86th percentile for all avian genomes (Bravo et al. 2021). Compared with 3 other Odontophoridae quail genomes, the California quail assembly exhibited the highest BUSCO completeness (96.5% of 8,338 orthologs present) and was the only assembly with a contig N50 exceeding 1 Mb in length. A high-quality northern bobwhite (Colinus virginianus) assembly had a much higher scaffold N50 of 66.8 Mb but a contig N50 of 0.11 Mb (Salter et al. 2019). Similarly, the California quail genome contains only 2.64 Ns per 100 kb, whereas the bobwhite genome has 1,363.4 Ns per 100 kb. These differences underscore the power of PacBio long reads for generating a more contiguous and complete genome.
TE sequence spans ~16.5% of the California quail genome. This is higher than estimates from previous quail assemblies based on short-read sequencing data, which included on average 9.2% TE content (Feng et al. 2020). However, the higher levels of TE content found in the quail genome more closely matches levels observed in other highly contiguous genomes generated from other lineages within Galloanserae (ducks and chickens). The galGal5 assembly for the domestic chicken (G. gallus; family Phasianidae; order Galliformes) found 16.5% of the genome to be repeats (Warren et al. 2017), whereas both wild and domestic mallard (A. platyrhynchos; family Anatidae; order Anseriformes) genomes were found to contain ~17% TE content (Zhu et al. 2021). In California quail, the increase in detectable TE content was primarily driven by newly discovered LTR element sequences. The ability to detect a greater abundance of LTR elements in long-read assemblies has been shown in a number of species, including the duck genome. LTR elements tend to be longer than other TE sequences and have been shown to be a major cause of gaps in avian assemblies (Peona et al. 2021). The first comparisons of TE diversity among the genomes of chicken, turkey, and zebra finch suggested a much greater abundance of LTRs in the zebra finch relative to galliform genomes (Warren et al. 2010). The California quail genome joins a growing number of highly contiguous genomes from within Galloanserae that together point to a much greater abundance of LTR elements than previously appreciated.
A high-quality reference genome for California quail will be an important resource for research and conservation of this popular upland game bird. First, California quail is a bird of significant recreational interest throughout its native and introduced distribution. For example, an estimated 315,268 quail were harvested by over 35,000 hunters in California during the 2018 to 2019 season (Miller and Meshriy 2019). However, management units are geographically broad and do not currently incorporate population genetic data that can inform patterns of population structure or genetic diversity. Second, population genetic data aligned to this reference genome will be important for determining the distinctiveness of the Catalina Island subspecies (C. c. catalinensis) and for testing speculations about human transport to the Channel Islands in the last 12,000 yr (Collins 2008). Third, this will facilitate research on speciation and hybridization genomics between Gambel’s and California quail. Of particular interest will be the impact of differences in chromosome number between the 2 species (Gambel’s: 2n = 80 chromosomes, California: 2n = 84; Degrandi et al. 2020) on hybrid zone dynamics. Finally, a major priority for improved quail management and conservation will be understanding local adaptation across rainfall gradients and the capacity of quail populations to adapt to future changes in rainfall patterns across the west (Zornes and Bishop 2009). The impacts of annual rainfall on reproductive success have been well established in quail, with aridland populations responding positively to increased annual rainfall but more mesic-adapted populations responding negatively (Leopold 1977). Generation of a high-quality reference genome is a critical step toward understanding the genetic basis of local adaptation across precipitation gradients in this species.
Supplementary Material
Acknowledgments
We would like to thank Joshua Ho for assistance with tissue subsampling, Jackie Childers for contributing photos of both male and female California quail, and Zachary Harlow for providing access to the Blue Oak Ranch Reserve, Santa Clara County, California. PacBio Sequel II library prep and sequencing were carried out at the DNA Technologies and Expression Analysis Cores at the UC Davis Genome Center, supported by NIH Shared Instrumentation Grant 1S10OD010786-01. Deep sequencing of Omni-C libraries used the Novaseq S4 sequencing platforms at the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by NIH S10 OD018174 Instrumentation Grant. We thank the staff at the UC Davis DNA Technologies and Expression Analysis Cores and the UC Santa Cruz Paleogenomics Laboratory for their diligence and dedication to generating high-quality sequence data.
Contributor Information
Phred M Benham, Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States; Department of Integrative Biology, University of California Berkeley, Berkeley, CA, United States.
Carla Cicero, Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States.
Merly Escalona, Department of Biomolecular Engineering, University of California Santa Cruz, Santa Cruz, CA, United States.
Eric Beraut, Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, Santa Cruz, CA, United States; Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States.
Mohan P A Marimuthu, DNA Technologies and Expression Analysis Core Laboratory, Genome Center, University of California-Davis, Davis, CA, United States; Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States.
Oanh Nguyen, DNA Technologies and Expression Analysis Core Laboratory, Genome Center, University of California-Davis, Davis, CA, United States; Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States.
Michael W Nachman, Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States; Department of Integrative Biology, University of California Berkeley, Berkeley, CA, United States.
Rauri C K Bowie, Museum of Vertebrate Zoology, University of California Berkeley, Berkeley, CA, United States; Department of Integrative Biology, University of California Berkeley, Berkeley, CA, United States.
Funding
This work was supported by the California Conservation Genomics Project, with funding provided to the University of California by the State of California, State Budget Act of 2019 [UC Award ID RSI-19-690224].
Data availability
Data generated for this study are available under the NCBI BioProject PRJNA720569. Raw sequencing data for the California quail (NCBI BioSample SAMN26894057) are deposited in the NCBI Short Read Archive (SRA) under SRS13350135. The transposable element library used to mask repeats has been submitted to dryad. Assembly scripts and other data used for the analyses are deposited in the following GitHub repository: www.github.com/ccgproject/ccgp_assembly.
References
- Abdennur N, Mirny LA.. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, Prosdocimi F, Nabholz B, Delsuc F.. MitoFinder: efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020;20(4):892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo GA, Schmitt CJ, Edwards SV.. What have we learned from the first 500 avian genomes? Annu Rev Ecol Evol Syst. 2021;52(1):611–639. doi: 10.1146/annurev-ecolsys-012121-085928 [DOI] [Google Scholar]
- Calkins JD, Gee JM, Hagelin JC, Lott DF.. California quail (Callipepla californica), version 1.0. In: Poole AF, editor. Birds of the world. Ithaca (NY): Cornell Lab of Ornithology; 2014. [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL.. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, Cochrane G, Blaxter M.. BlobToolKit—interactive quality assessment of genome assemblies. G3. 2020;10(4):1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jarvis ED, Fedrigo O, Koepfli KP, Urban L, Gemmell NJ, Li H.. Haplotype-resolved assembly of diploid genomes without parental data. Nature Biotechnology. 2022;40(9):1332–1335. 10.1038/s41587-022-01261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. Jupiter Plot: a Circos-based tool to visualize genome assembly consistency (1.0). Zenodo. 2018. doi: 10.5281/zenodo.1241235 [DOI] [Google Scholar]
- Collins PW. Catalina California Quail (Callipepla californica catalinensis). In: Shuford WD, Gardali T, editors. California bird species of special concern: a ranked assessment of species, subspecies, and distinct populations of birds of immediate conservation concern in California. Studies of Western Birds 1. Camarillo (CA), Sacramento: Western Field Ornithologists, California Department of Fish and Game; 2008. [Google Scholar]
- Degrandi TM, Barcellos SA, Costa AL, Garnero ADV, Hass I, Gunski RJ.. Introducing the bird chromosome database: an overview of cytogenetic studies in birds. Cytogenet Genome Res. 2020;160(4):199–205. doi: 10.1159/000507768 [DOI] [PubMed] [Google Scholar]
- Feng S, Stiller J, Deng Y, Armstrong J, Fang Q, Reeve AH, Xie D, Chen G, Guo C, Faircloth BC, et al. Dense sampling of bird diversity increases power of comparative genomics. Nature. 2020;587(7833):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Hubley R, Goubert C, Rosen J, Clark AG, Feschotte C, Smit AF.. RepeatModeler2 for automated genomic discovery of transposable element families. Proc Natl Acad Sci USA. 2020;117(17):9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghurye J, Pop M, Koren S, Bickhart D, Chin C-S.. Scaffolding of long read assemblies using long range contact information. BMC Genomics. 2017;18(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghurye J, Rhie A, Walenz BP, Schmitt A, Selvaraj S, Pop M, Koren S.. Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput Biol. 2019;15(8):e1007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloborodko A, Abdennur N, Venev S, Brandao HB, Fudenberg G.. Zenodo. 2018. doi: 10.5281/zenodo.1490831 [DOI] [Google Scholar]
- Grinnell JG, Bryant HC, Storer TI.. The game birds of California. Berkeley (CA): University of California Press; 1918. [Google Scholar]
- Guan D, McCarthy SA, Wood J, Howe K, Wang Y, Durbin R.. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G.. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosner PA, Braun EL, Kimball RT.. Land connectivity changes and global cooling shaped the colonization history and diversification of New World quail (Aves: Galliformes: Odontophoridae). J Biogeogr. 2015;42(10):1883–1895. [Google Scholar]
- Iknayan KJ, Wheeler MM, Safran SM, Young JS, Spotswood EN.. What makes urban parks good for California quail? Evaluating park suitability, species persistence, and the potential for reintroduction into a large urban national park. J Appl Ecol. 2021;59(1):199–209. [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, McCallum C, Dinkla K, Strobelt H, Gehlenborg N.. HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball RT, Guido M, Hosner PA, Braun EL.. When good mitochondria go bad: cyto-nuclear discordance in landfowl (Aves: Galliformes). Gene. 2021 July;801:145841. [DOI] [PubMed] [Google Scholar]
- Korlach J, Gedman G, Kingan SB, Chin C-S, Howard JT, Audet J-N, Jarvis ED.. De novo PacBio long-read and phased avian genome assemblies correct and add to reference genes generated with intermediate and short reads. GigaScience. 2017;6(10):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AS. The California quail. Berkeley (CA): University of California Press; 1977. [Google Scholar]
- Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM, 2013, 10.48550/arXiv.1303.3997 [DOI]
- Manni M, Berkeley MR, Seppey M, Simao FA, Zdobnov EM.. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes, Molecular Biology and Evolution, 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Meshriy M.. Resident Upland Game Bird and Small Game Mammal Harvest Survey 2018–2019. Sacramento: California Department of Fish and Wildlife; 2019. [Google Scholar]
- Peona V, Blom MPK, Xu L, Burri R, Sullivan S, Bunikis I, Liachko I, Haryoko T, Jonsson KA, Zhou Q, et al. Identifying the causes and consequences of assembly gaps using a multiplatform genome assembly of a bird-of-paradise. Mol Ecol Resour. 2021;21(1):263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Bhardwaj V, Arrigoni L, Lam KC, Grüning BA, Villaveces J, Haberman B, Akhtar A, Manke T.. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat Commun. 2018;9(1):1–9. doi: 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranallo-Benavidez TR, Jaron KS, Schatz MC.. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat Commun. 2020;11(1):1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, Damas J, Formenti G, Koren S, Uliano-Silva M, Chow W, Fungtammasan A, Kim J, et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, Phillippy AM.. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter JF, Hosner PA, Tsai WLE, McCormack JE, Braun EL, Kimball RT, Faircloth BC.. Historical specimens and the limits of subspecies phylogenomics in the New World quails (Odontophoridae). Mol Phylogenet Evol. 2022. July;175:107559. [DOI] [PubMed] [Google Scholar]
- Salter JF, Johnson O, Stafford NJ, Herrin WF, Schilling D, Cedotal C, Faircloth BC.. A highly contiguous reference genome for northern bobwhite (Colinus virginianus). G3. 2019;9(12):3929–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HB, Toffelmier E, Corbett-Detig RB, Escalona M, Erickson B, Fiedler P, Wang IJ.. Landscape genomics to enable conservation actions: the California Conservation Genomics Project. J Hered. 2022;113(6):577–588. doi: 10.1093/jhered/esac020 [DOI] [PubMed] [Google Scholar]
- Shibusawa M, Nishida-Umehara C, Tsudzuki M, Masabanda J, Griffin DK, Matsuda Y.. A comparative karyological study of the blue-breasted quail (Coturnix chinensis, Phasianidae) and California quail (Callipepla californica, Odontophoridae). Cytogenet Genome Res. 2004;106(1):82–90. [DOI] [PubMed] [Google Scholar]
- Sim SB, Corpuz RL, Simmonds TJ, Geib SM.. HiFiAdapterFilt, a memory efficient read processing pipeline, prevents occurrence of adapter sequence in PacBio HiFi reads and their negative impacts on genome assembly. BMC Genomics. 2022;23(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P.. RepeatMasker Open-4.0. 2013–2015. http://www.repeatmasker.org. Accessed 15 October 2022.
- Uliano-Silva M, Ferreira Nunes JG, Krasheninnikova K, McCarthy SA.. marcelauliano/MitoHiFi: mitohifi_v2.0 (v2.0). Zenodo. 2021. doi: 10.5281/zenodo.5205678 [DOI] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, Searle S, White S, Vilella AJ, Fairley S, et al. The genome of a songbird. Nature. 2010;464(7289):757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Hillier LDW, Tomlinson C, Minx P, Kremitzki M, Graves T, Markovic C, Bouk N, Pruitt KD, Thibaud-Nissen F, et al. A new chicken genome assembly provides insight into avian genome structure. G3. 2017;7(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DW, Billerman SM, Lovette IJ.. New World Quail (Odontophoridae), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, editors. Birds of the world. Ithaca (NY): Cornell Lab of Ornithology; 2020. [Google Scholar]
- Zhu F, Yin ZT, Wang Z, Smith J, Zhang F, Martin F, Ogeh D, Hincke M, Lin FB, Burt DW, et al. Three chromosome-level duck genome assemblies provide insights into genomic variation during domestication. Nat Commun. 2021;12(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink RM, Lott DF, Anderson DW.. Genetic variation, population structure, and evolution of California quail. Condor. 1987;89(2):395–405. [Google Scholar]
- Zonana DM, Gee JM, Bridge ES, Breed MD, Doak DF.. Assessing behavioral associations in a hybrid zone through social network analysis: complex assortative behaviors structure associations in a hybrid quail population. Am Nat. 2019;193(6):852–865. [DOI] [PubMed] [Google Scholar]
- Zornes M, Bishop RA. In: Williamson SJ, editor. Western Quail Conservation Plan. Cabot (VT): Wildlife Management Institute; 2009. p. 92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated for this study are available under the NCBI BioProject PRJNA720569. Raw sequencing data for the California quail (NCBI BioSample SAMN26894057) are deposited in the NCBI Short Read Archive (SRA) under SRS13350135. The transposable element library used to mask repeats has been submitted to dryad. Assembly scripts and other data used for the analyses are deposited in the following GitHub repository: www.github.com/ccgproject/ccgp_assembly.