Abstract
Introduction
Hurler-Scheie syndrome is a type of mucopolysaccharidosis I (MPS). In MPS I the decreased activity of alpha-L-iduronidase lysosomal enzyme leads to glycosaminoglycan (GAG) deposition in the intra- and extracellular matrix. Excessive amounts of GAG can accumulate in most layers of the cornea, including epithelial cells, stromal keratocytes, and endothelial cells.
Case Presentation
A 25-year-old female patient suffering from Hurler-Scheie syndrome with multiple ocular manifestations is reported. Due to significant bilateral corneal opacification, penetrating keratoplasty was performed on both eyes. Histopathologic examination of the corneal buttons showed disorganized collagen fibers with heterogenous thickness and many granule-containing keratocytes with excessive cytoplasm. Despite receiving enzyme replacement therapy, in vivo confocal microscopy revealed characteristic vacuoles in the basal epithelium and corneal stroma 96 months after transplantation. High resolution anterior segment optical coherence tomography demonstrated hyperreflective opacities superficial and deeper in the stroma which was consistent with recurrence of host disease in the graft.
Conclusion
To the best of our knowledge, this is the first documented Hurler-Scheie syndrome case of recurrence after penetrating keratoplasty demonstrated by in vivo confocal microscopy. Additionally, this patient manifested severe ocular involvement of MPS which might be an explanation of the progressive course of corneal opacification after transplantation.
Key Words: Hurler-Scheie syndrome, In vivo confocal microscopy, Lysosomal storage disease, Mucopolysaccharidosis, Recurrence
Established Facts
Hurler-Scheie syndrome is associated with accumulation of glycosaminoglycans (GAG) in multiple organs.
Excessive amount of GAG can accumulate in the cornea, affecting epithelial cells, stromal keratocytes, and endothelial cells.
Novel Insights
Recurrence of Hurler-Scheie syndrome in the corneal transplant was demonstrated by in vivo confocal microscopy.
The correlation between histopathology and in vivo imaging modalities in revealing accumulation of abnormal material in the cornea is also demonstrated.
Introduction
Hurler−Scheie syndrome is a rare autosomal recessive storage disease belonging to type I mucopolysaccharidosis (MPS). MPS I is a group of disorders which can be divided into 3 distinct syndromes [Ashworth et al., 2006a]. Mutations in the alpha-L-iduronidase (IDUA) gene located on the short arm of chromosome 4 lead to the impairment of IDUA lysosomal enzyme. This enzyme is responsible for the digestion of glycosaminoglycans (GAG). Impaired IDUA enzyme activity leads to the lysosomal accumulation of GAG (dermatan sulfate and heparan sulfate) within cells throughout the body [Tomatsu et al., 2010]. Excessive accumulation of these catabolic substrates in MPS I is present in multiple organs including ocular tissues. The severity and clinical characteristics of different MPS I subdivisions vary greatly among affected patients. Hurler syndrome is the most severe form of MPS I with multiorgan manifestations presenting in the first year of life. Scheie syndrome is at the milder end of the severity spectrum; the onset of clinical signs occurs around the age of 5. The intermediate subdivision of MPS I is Hurler-Scheie syndrome. Patients suffering from Hurler-Scheie syndrome have an average intelligence quotient or slight intellectual impairments, mild facial distortions, and skeletal, cardiac, and respiratory abnormalities [Ashworth et al., 2006a]. Patients with Hurler-Scheie syndrome often develop corneal clouding, high hypermetropia, secondary glaucoma, retinopathy, and optic nerve abnormalities [Ashworth et al., 2006a, b; Bothun et al., 2011; Fahnehjelm et al., 2012].
Corneal transplantation is an option to restore corneal transparency. However, recurrence of host disease in the graft with other storage diseases has been reported [Bothun et al., 2011]. We report an MPS I patient with multiple ocular manifestations who underwent penetrating keratoplasty on both eyes. This is the first reported recurrence of Hurler-Scheie syndrome in the corneal transplant demonstrated by in vivo confocal microscopy. The correlation between histopathology and in vivo imaging modalities in revealing accumulation of abnormal material in the cornea is also demonstrated.
Case Report
A 25-year-old female patient with bilateral corneal opacities presented to the Department of Ophthalmology University of Pecs. The patient was diagnosed with Hurler−Scheie syndrome (compound heterozygous mutation of NM_000203.1:c.134_145del and NM_000203.1:c.1421_1423del) at the age of 5 years. The first corneal manifestations (bilateral mild opacifications) were detected at age 7, the best corrected visual acuity (BCVA) was 20/20 OU (manifest refraction: +4.0D OU). Impaired respiratory functions, mitral valve disease, joint stiffness, atopic dermatitis, and mild hepatomegaly also developed during early and middle childhood. Cognitive and psychological functions were not impaired during the follow-up. She has been receiving enzyme replacement therapy laronidase (Aldurazyme®), a recombinant formulation of alpha-L-iduronidase since 17 years of age. There was no clear evidence of neutralizing antibodies against enzyme replacement therapy.
During her first ophthalmic examination at our institution, BCVA was 20/300 OD and 20/125 OS with a manifest refraction of +6.0D and +6.5D, respectively. Slit lamp examination revealed diffuse opacification of both corneas (Fig. 1). Applanation tonometry showed elevated intraocular pressure (30 mmHg OD and 32mm Hg OS [normal value: 10−21 mmHg]). Ultrasound examination detected thickened sclera and a widened optic nerve (Fig. 1). Electroretinogram (ERG) revealed abnormal rod and cone responses on both eyes. The patient underwent penetrating keratoplasty on both eyes and the corneas were sent for routine histological and electron microscopic evaluation. Histopathologic examination of the corneal button showed disorganized collagen fibers with heterogenous thickness and many granule-containing keratocytes with excessive cytoplasm (Fig. 2). Electron microscopy revealed vacuole-containing basal epithelial cells. Stromal keratocytes contained numerous vacuoles, and collagen fibers were randomly arranged with inhomogeneous diameter in the outer two-third of the stroma; the inner one-third was not affected (Fig. 3, 4).
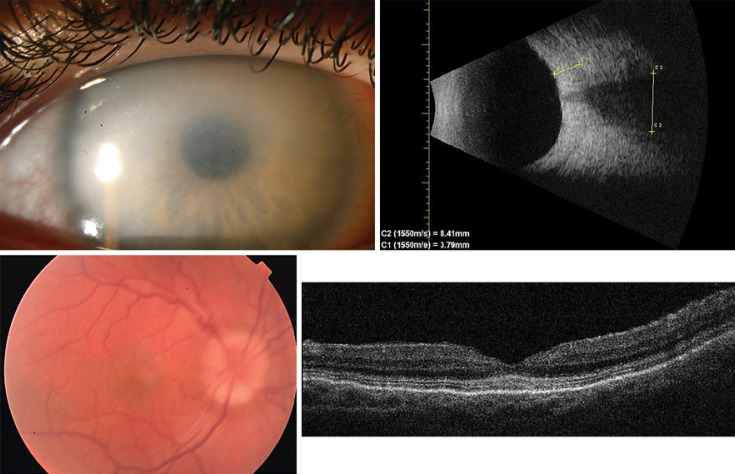
Fig. 1.
Preoperative slit lamp photo of the right eye showing diffuse corneal opacification (upper left). B-scan ultrasound demonstrating thickened sclera (3.79 mm) and broad optic nerve (8.41 mm in diameter) (upper right). Fundus photo showing optic disc swelling (lower left). Optical coherence tomography of the macula showing accumulation of granular material in the photoreceptor layer (lower right).
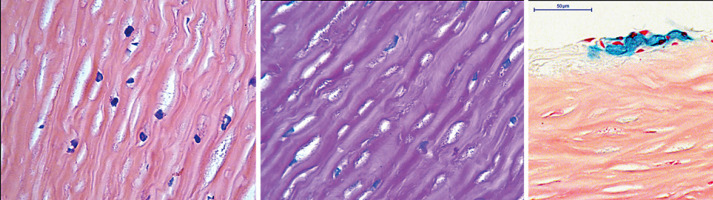
Fig. 2.
Corneal stroma showed numerous, periodic acid Schiff and Alcian blue positive granule containing keratocytes with excessive cytoplasm. Hematoxyllin-Eosin, ×40 (left). Periodic acid-Schiff ×40 (middle). Alcian blue, ×40 (right).
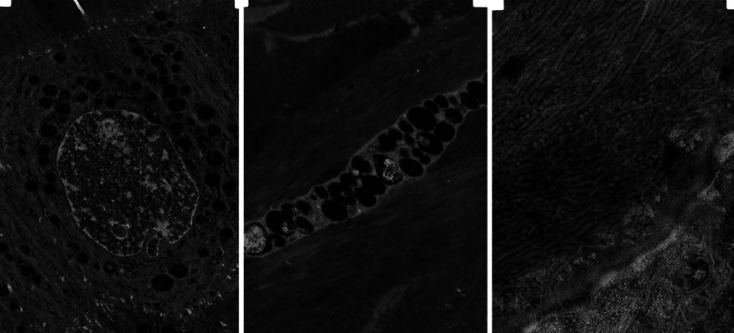
Fig. 3.
Electron microscopy showing that most of the basal epithelial cells contained vacuoles from which GAG were lost during histological processing (left). Keratocytes have numerous vacuoles (middle), and collagen fibers are randomly arranged. They cross each other, and the diameter of the fibers are inhomogeneous in the outer two-third of the stroma (right).
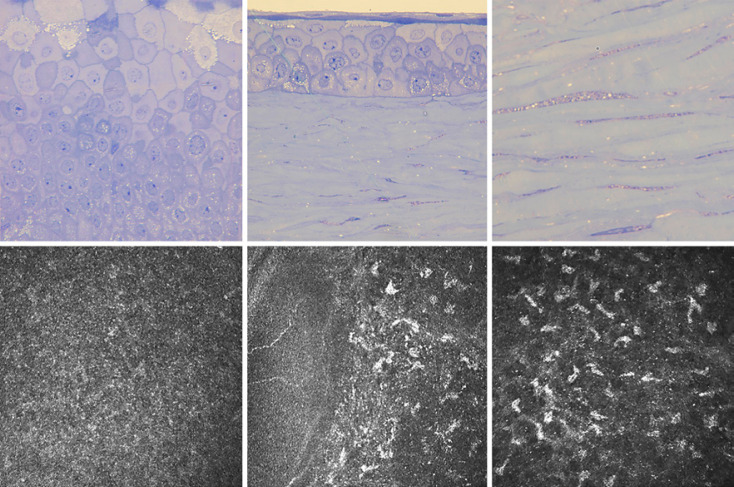
Fig. 4.
Toluidine blue stained thick sections showed fibrogranular material containing vacuoles in the basal epithelial cells (upper left, upper middle) and stromal keratocytes (upper middle, upper right) in the excised corneal button. In vivo confocal corneal microscopy demonstrating accumulation of hyperreflective material in the basal epithelial cells (lower left), Bowman membrane (lower middle), and anterior stroma (lower right) of the graft.
The postoperative course after corneal transplantation of the right eye was uneventful. Very short axial length (18.92 mm [normal value: 22−25 mm]), narrow anterior chamber (1.91 mm [normal value: 2.4−4.4 mm]), and endothelial trauma during surgery resulted in a more rapid endothelial decompensation after keratoplasty on the left eye. After keratoplasty, posterior segment optical coherence tomography (OCT) (Topcon 3D-2000 OCT, Topcon Corporation, Tokyo, Japan) showed accumulation of granular material in the retinal photoreceptor layer, a slightly elevated optic disc with blurred margins and choroidal folds (Fig. 1). 96 months after keratoplasty, best corrected visual acuity was 20/50 OD and 20/200 secondary to graft failure OS. Slit lamp examination showed fine stromal refractile lines on the right cornea and stromal edema with loss of transparency on the left cornea. The patient underwent in vivo anterior segment imaging assessments. Essentially, in vivo confocal corneal microscopy (Heidelberg Retina Tomograph II Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany) showed that accumulation of hyperreflective material was involved in the full thickness of the corneal graft (Fig. 4). Accumulated punctate deposits were observed in the basal epithelial cells and in the level of Bowman membrane, and hyperreflective extracellular material was seen throughout the stroma (Fig. 4). Swept source high resolution anterior segment OCT (ANTERION; Heidelberg Engineering GmbH, Heidelberg, Germany) demonstrated hyperreflective opacities superficial and deeper in the stroma which was consistent with recurrence of host disease in the graft (Fig. 5). The pachymetry was 589 microns on the right eye, and there was no significant increase in central corneal thickness. There was no evidence of endothelial rejection and no keratic precipitates and anterior chamber reaction were detected. OCT biometry revealed 19.41 ± 0.008 mm and 18.92 ± 0.013 mm axial length in the right and left eye with a shallow anterior chamber (2.27 mm and 1.61 mm). The lens thickness was 4.38 and 4.33 mm.
Fig. 5.
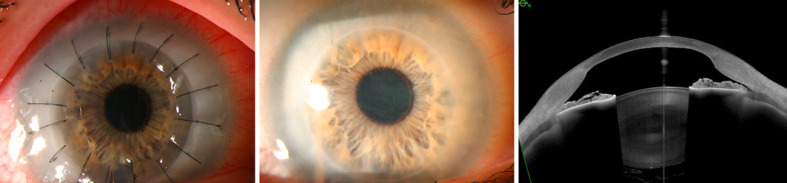
Slit lamp photo of the clear corneal transplant OD 1 month after penetrating keratoplasty (left). Slit lamp photo of the right eye 96 months after penetrating keratoplasty showing multiple, fine refractile lines in the corneal stroma (middle). Anterior segment optical coherence tomography showing hyperreflective material in the superficial and deeper stroma of the transplanted cornea 8 years after keratoplasty (right).
Discussion
Hurler-Scheie syndrome is a progressive multisystem disorder, and management of affected patients requires a multidisciplinary approach. The most common ocular manifestation is cloudiness of the cornea. Excessive amounts of GAG can accumulate in most layers of the cornea, including epithelial cells, stromal keratocytes, and endothelial cells. Typical histopathologic findings are fibrogranular deposit-containing vacuoles in the epithelial and stromal cells [Aragona et al., 2014]. Cytoplasmic accumulation of catabolic substrates (dermatan sulfate and heparan sulfate) is the consequence of the lack of IDUA enzyme activity in the lysosomes. The intracellular GAG deposits may impact the size of keratocytes and interfere with the regular arrangement of the collagen fibers and lamellae, leading to loss of corneal transparency [Ashworth et al., 2006a, b; Bothun et al., 2011; Fahnehjelm et al., 2012]. In the healthy human cornea, both the average diameter of collagen fibers and the average distance between them are highly homogeneous which might be responsible for corneal transparency [Davson, 1984]. In cases of abundant GAG accumulation, collagen fibers in the corneal stroma become heterogeneous, incident light rays may be scattered randomly, and the cornea loses its transparency [Huang et al., 1996]. We detected scattered hyperreflective punctate material in the basal epithelial and stromal layers during in vivo confocal microscopy. Previous authors also observed intracytoplasmic deposition and extracellular accumulation of GAG in keratocytes [Aragona et al., 2014].
GAG deposition in the trabecular meshwork may impact the aqueous outflow causing elevated intraocular pressure [Summers and Ashworth, 2011]. However, a thickened and rigid cornea is thought to influence the measured intraocular pressure. GAG can be accumulated in the dura, sclera, and certain layers of the retina. Edema and secondary atrophy of the optic nerve head may be caused by compression from the thickened dura and sclera [Fahnehjelm et al., 2012]. Deposits in the ganglion cell layer may also contribute to the optic nerve atrophy. Accumulation of GAG in the retinal pigment epithelial cells and in the photoreceptor layer may lead to progressive photoreceptor loss and impaired visual functions [Summers and Ashworth, 2011; Fahnehjelm et al., 2012]. Disturbed retinal function can be diagnosed with ERG [Leung et al., 1971; Ashworth et al., 2006b; Tzetzi et al., 2007; Fahnehjelm et al., 2012] which was abnormal in our case. Thickening and increased rigidity of the cornea and sclera secondary to GAG accumulation may lead to flattening of the cornea, shortening of axial length with accompanying hyperopic shift [Summers and Ashworth, 2011; Fahnehjelm et al., 2012]. Choroidal folds can also be seen in patients with moderate or high hyperopia due to their shorter axial length and thickened sclera [Gass, 1997].
Treatment options for patients with MPS include bone marrow transplant and enzyme replacement which may provide better life quality. In cases of Hurler-Scheie syndrome, parenteral IDUA enzyme replacement with laronidase is available and improves both functional and symptomatic respiratory and musculoskeletal performance; however, its effect on the ocular complications is controversial [Ashworth et al., 2006a; Bothun et al., 2011; Fahnehjelm et al., 2012]. Progression of corneal opacities has been reported in patients after bone marrow transplant suggesting that there might be intrinsic factors at the ocular level that escape enzymatic correction [Yuan et al., 2016; Gonzalez et al., 2020]. Penetrating or deep anterior lamellar keratoplasty is a treatment of choice for restoring corneal transparency in patients with MPS [Rosen et al., 1968; Maumenee, 1978; Schwartz et al., 1985; Iwamoto et al., 1990; Varssano et al., 1997; Käsmann-Kellner et al., 1999; Vajpayee et al., 2007; Bothun et al., 2011]. However, graft failure from recurrent GAG accumulation in MPS has been described in the literature [Schwartz et al., 1985; Varssano et al., 1997]. Previous authors reported clear grafts after keratoplasty in patients with Hurler-Scheie syndrome, although the follow-up period was shorter (0.5−6 years) than it was in our case (8 years) [Rosen et al., 1968; Ashworth et al., 2006b; Vajpayee et al., 2007; Bothun et al., 2011]. In our patient, corneal confocal microscopy detected accumulated punctate material in the basal epithelium and throughout the thickness of the stroma. High resolution anterior segment OCT demonstrated scattered hyperreflective opacities in the affected cornea. These in vivo imaging findings in the graft 8 years after penetrating keratoplasty highly correlated with the light and electron microscopical observations of the recipient cornea revealing recurrence of MPS I in the transplanted graft.
We reported a patient with Hurler-Scheie syndrome who underwent penetrating keratoplasty on both eyes. Despite receiving enzyme replacement therapy, in vivo confocal microscopy detected characteristic vacuoles in the basal epithelium and corneal stroma 8 years after transplantation. We believe that this is a recurrence of the host disease in the corneal transplant. To the best of our knowledge this is the first documented Hurler-Scheie syndrome case of recurrence after penetrating keratoplasty demonstrated by in vivo confocal microscopy. Additionally, this patient manifested severe ocular involvement of MPS I which might be an explanation of the progressive course of corneal opacification after transplantation.
Statement of Ethics
This case study was approved by the Ethical Committee of the Faculty of Medicine, University of Pecs (KK/842-2/2020, 8433-PTE 2020), and all procedures adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of the details of the medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by PTE ÁOK-KA-2021-14.
Authors Contributions
The authors confirm contribution to the paper as follows: study conception and design: Zsofia Kolkedi, Eszter Szalai, Adrienne Csutak; data collection: Zsofia Kolkedi; analysis and interpretation of results: Zsofia Kolkedi, Eszter Szalai; draft manuscript preparation: Zsofia Kolkedi, Eszter Szalai; supervision and review of the manuscript: Eszter Szalai, Adrienne Csutak. All authors reviewed the results and approved the final version of the manuscript.
Data Availability Statement
All data obtained and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgement
The authors would like to thank László Seress, MD, PhD, MSc, Emeritus Professor of Central Electron Microscope Laboratory, University of Pecs, for his technical assistance in the light and electron microscopy images.
Funding Statement
This work was supported by PTE ÁOK-KA-2021-14.
References
- 1.Aragona P, Wylegala E, Wroblewska-Czajka E, Smedowski A, Nowinska A, Roszkowska AM, et al. Clinical, confocal, and morphological investigations on the cornea in human mucopolysaccharidosis IH-S. Cornea. 2014;33:35–42. doi: 10.1097/ICO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 2.Ashworth JL, Biswas S, Wraith E, Lloyd IC. Mucopolysaccharidoses and the eye. Surv Ophthalmol. 2006a;51:1–17. doi: 10.1016/j.survophthal.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Ashworth JL, Biswas S, Wraith E, Lloyd IC. The ocular features of the mucopolysaccharidoses. Eye (Lond) 2006b;20:553–563. doi: 10.1038/sj.eye.6701921. [DOI] [PubMed] [Google Scholar]
- 4.Bothun ED, Decanini A, Summers CG, Orchard PJ, Tolar J. Outcome of penetrating keratoplasty for mucopolysaccharidoses. Arch Ophthalmol. 2011;129:138–144. doi: 10.1001/archophthalmol.2010.341. [DOI] [PubMed] [Google Scholar]
- 5.Davson H. The Eye. Orlando: Academic Press; 1984. [Google Scholar]
- 6.Fahnehjelm KT, Ashworth JL, Pitz S, Olsson M, Törnquist AL, Lindahl P, et al. Clinical guidelines for diagnosing and managing ocular manifestations in children with mucopolysaccharidosis. Acta Ophthalmol. 2012;90:595–602. doi: 10.1111/j.1755-3768.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Gass JD. Stereoscopic Atlas of Macular Disease: Diagnosis and treatment. ed 4. St. Louis: Mosby; 1997. [Google Scholar]
- 8.Gonzalez EA, Visioli F, Pasqualim G, de Souza CFM, Marinho DR, Giugliani R, et al. Progressive eye pathology in mucopolysaccharidosis type I mice and effects of enzyme replacement therapy. Clin Exp Ophthalmol. 2020;48:334–342. doi: 10.1111/ceo.13713. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Bron AJ, Meek KM, Vellodi A, McDonald B. Ultrastructural study of the cornea in a bone marrow-transplanted Hurler syndrome patient. Exp Eye Res. 1996;62:377–387. doi: 10.1006/exer.1996.0043. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto M, Nawa Y, Maumenee I, Young-Ramsaran J, Matalon R, Green W. Ocular histopathology and ultrastructure of Morquio syndrome (systemic mucopolysaccharidosis IV A) Graefes Arch Clin Exp Ophthalmol. 1990;228:342–349. doi: 10.1007/BF00920060. [DOI] [PubMed] [Google Scholar]
- 11.Käsmann-Kellner B, Weindler J, Pfau B, Ruprecht KW. Ocular changes in mucopolysaccharidosis IV A (Morquio A syndrome) and long-term results of perforating keratoplasty. Ophthalmologica. 1999;213:200–205. doi: 10.1159/000027420. [DOI] [PubMed] [Google Scholar]
- 12.Leung L, Weinstein G, Hobson H. Further electroretinographic studies of patients with mucopolysaccharidoses. Birth Defects Orig Artic Ser. 1971;7:32–40. [PubMed] [Google Scholar]
- 13.Maumenee I. The cornea in connective tissue diseases. Ophthalmology. 1978;85:1014–1017. doi: 10.1016/s0161-6420(78)35591-3. [DOI] [PubMed] [Google Scholar]
- 14.Rosen D, Haust MD, Yamashita T, Bryans AM. Keratoplasty and electron microscopy of the cornea in systemic mucopolysaccharidosis (Hurler's disease) Can J Ophthalmol. 1968;3:218–230. [PubMed] [Google Scholar]
- 15.Schwartz M, Werblin T, Green W. Occurrence of mucopolysaccharide in corneal grafts in the Maroteaux-Lamy syndrome. Cornea. 1985;4:58–66. [PubMed] [Google Scholar]
- 16.Summers CG, Ashworth JL. Ocular manifestations as key features for diagnosing mucopolysaccharidoses. Rheumatology (Oxford) 2011;50 Suppl 5:v34–40. doi: 10.1093/rheumatology/ker392. [DOI] [PubMed] [Google Scholar]
- 17.Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, et al. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 18.Tzetzi D, Hamilton R, Robinson PH, Dutton GN. Negative ERGs in mucopolysaccharidoses (MPS) Hurler-Scheie (I-H/S) and Hurler (I-H)-syndromes. Doc Ophthalmol. 2007;114:153–158. doi: 10.1007/s10633-007-9047-z. [DOI] [PubMed] [Google Scholar]
- 19.Vajpayee RB, Tyagi J, Sharma N, Kumar N, Jhanji V, Titiyal JS. Deep Anterior Lamellar Keratoplasty by Big-Bubble Technique for Treatment Corneal Stromal Opacities. Am J Ophthalmol. 2007;143:954. doi: 10.1016/j.ajo.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Varssano D, Cohen E, Nelson L, Eagle R., Jr Corneal transplantation in Maroteaux-Lamy syndrome. Arch Ophthalmol. 1997;115:428–429. doi: 10.1001/archopht.1997.01100150430024. [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Bothun ED, Hardten DR, Tolar J, McLoon LK. A novel explanation of corneal clouding in a bone marrow transplant-treated patient with Hurler syndrome. Exp Eye Res. 2016;148:83–89. doi: 10.1016/j.exer.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data obtained and analyzed during the current study are available from the corresponding author on reasonable request.