Abstract
A concise total synthesis of (±)-N-methyldibromoisophakellin, a member of the monomeric pyrrole–aminoimidazole alkaloid family isolated from the marine sponge Stylissa carbica, was achieved via a net [3+2] cycloaddition to install the cyclic guanidine. This ring annulation employs a 2-amido-1,3-aminoallyl cation obtained under oxidative conditions from variously N-substituted guanidines which in one instance led to isolation of a tetracycle bearing a carbinolamine center through subsequent benzylic oxidation. Finally, the serendipitous formation of a unique, related alkenyl guanidine, N-methylugibohlin, achieved via ring opening of cyclic guanidine under acidic conditions suggests that ugibohlin may be an artifact of isolation.
Keywords: pyrrole–aminoimidazole alkaloid, total synthesis, guanidine, isolation artifact, carbinolamine
Graphical Abstract

Introduction
Pyrrole-aminoimidazole alkaloids (PAIs) are one of the largest natural product families isolated from marine sponges derived from oroidin as a building block.1 This family is categorized based on the number of oroidin monomers incorporated and whether cyclization has occurred including linear monomers (e.g. hymenidin),2 cyclic monomers (e.g. dibromophakellin),3 cyclic dimers (e.g. palau’amine),4 cyclic tetramers (e.g. stylissadine A),5 and acyclic dimers (e.g. nagelamide A)6 PAIs. Biosynthetic studies have revealed that linear monomeric PAIs such as oroidin are the progenitors of more complex PAIs7 and oroidin is likely derived from the amino acids, ornithine proline and histidine.8 Moreover, PAIs have unique structures with broad bioactivities1a–c,9 and these features have attracted the attention of synthetic chemists leading to several synthetic studies and completed total syntheses1b, c, e, f,10 including those reported by our group.11
Dibromophakellin (1)3 is the first cyclic monomeric PAI isolated and subsequently (+)-dibromocantharelline (2, also known as (+)-dibromoisophakellin),12 (−)-dibromoisophakellin (5a),13 and cylindradine A (3)14 were reported differing in the connectivity of the pyrrole ring (N1, C4, or C5; Figure 1). A number of other unique, cyclized monomeric PAIs have been isolated including the further oxidized (−)-dibromohydroxyphakellin (4),15 the apparently rearranged dibromoagelaspongin (7),16 and the alkenyl guanidine-containing ugibohlin (6a).17 Furthermore, variations in the degree of pyrrole bromination and differences in N-alkylation are found in (−)-monobromoisophakellin (5a),18 monobromougibohlins (6b, c),19 (−)-N-methyldibromoisophakellin (8),20 and N-methylugibohlin (9).21 The first total synthesis of a monomeric PAI, (±)-dibromophakellin, was reported by Foley and Büchi in 1982.22 Subsequently, several total syntheses of cyclic monomeric PAIs have been reported,23 including an elegant biomimetic synthesis of (±)-dibromophakellstatin and (±)-dibromoisophakellin by Horne24 and synthesis of ugibohlin and (±)-N-methylisophakellin by Lindel25 building on Horne’s work. We previously described total syntheses of (+)-phakellin and (+)-monobromophakellin,26 (+)-dibromophakellstatin,27 and a bioinspired strategy to (±)-agelastatin A.11
Figure 1.
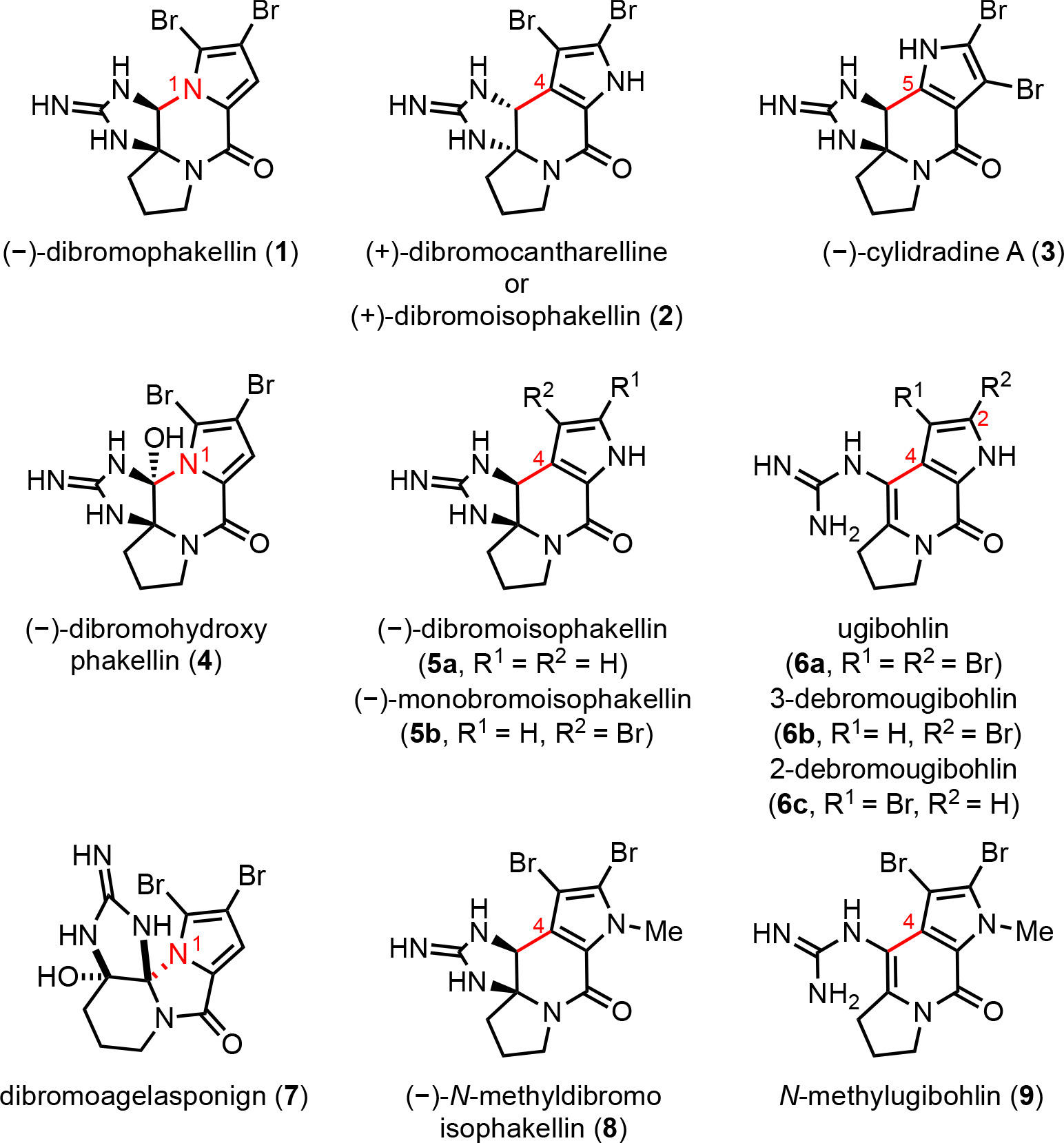
Tri- and tetracyclic pyrrole-aminoimidazole alkaloids (PAIs) with isomeric pyrrole connectivity and cyclic or acyclic guanidines.
In 1968, Sheehan described the thermal generation of aza-oxyallyl cations from α-lactams which engaged alkenes in [3+2] cycloadditions leading to γ-lactams.28 Building on this early precedent, the Jeffrey29 and Wu30 groups reported [3+2] cycloadditions with alkenes employing the aza-oxyallyl cations generated under oxidative conditions for the synthesis of γ-lactams. In addition, Jeffrey extended this chemistry to the oxidative generation of diaza-oxyallyl cations for the synthesis of cyclic ureas from indole derivatives.31 Building on these precedents, we recently described the development of a net [3+2] cycloaddition for direct cyclic guanidine annulation onto alkenes employing 2-amido-1,3-diaminoallyl cations as 1,3-dipoles generated under oxidative conditions leading to a concise synthesis of (±)-phakellin.32 Herein, we describe the first total syntheses of (±)-N-methyldibromoisophakellin (10) employing the 2-amido-1,3-diaminoallyl cation and identify an over-oxidized isophakellin structure bearing a carbinolamine. Further, our studies reveal the ease at which dibromoisophakellin isomerizes to N-methylugibohlin (9) under acidic conditions raising the possibility that the ughibohlins could be an artifact of isolation. This type of elimination was previously proposed by Mourabit33 and observed by Lindel.25
Our synthetic strategy toward (±)-N-methyldibromoisophakellin (10) would utilize the 2-amido-1,3-diaminoallyl cation 12 for direct guanidine annulation onto the tricyclic dibromoenamide 13 in turn derived from prolinol and a dibromo pyrrole trichloroketone 14 (Scheme 1).
Scheme 1.
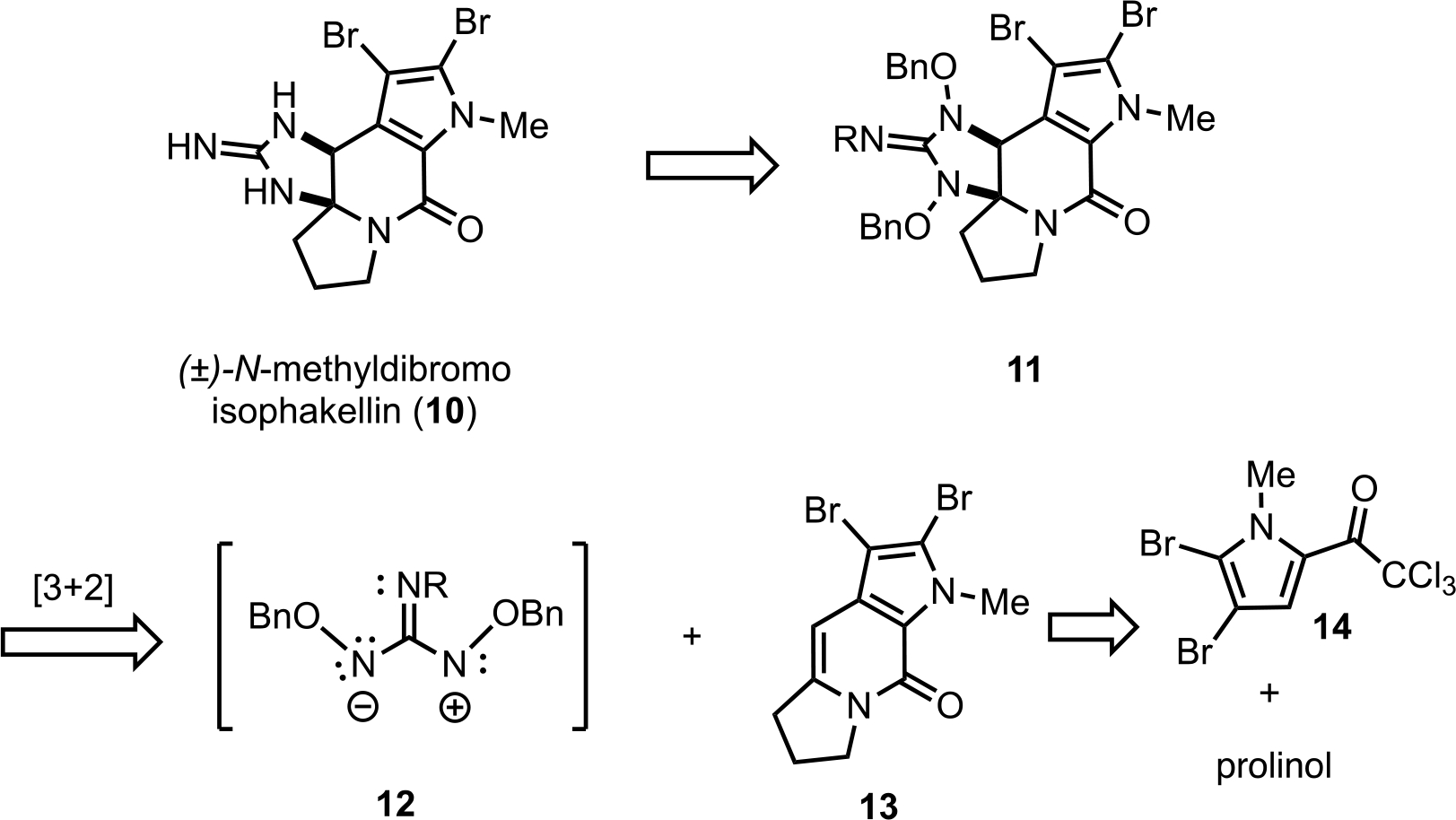
Retrosynthetic analysis of (±)-N-methyldibromoisophakellin (10) employing a [3+2] cycloguanidinylation.
Results and Discussion
The dibromopyrrole trichloroketone 14 was prepared from the commercially available 2-(trichloroacetyl)pyrrole in 2 steps according to the reported procedure34 and was converted to alcohol 15 by treatment with L-prolinol under reflux conditions in CH3CN (Scheme 2). The resulting alcohol was oxidized to the corresponding aldehyde by Dess–Martin oxidation35 and converted to the tricyclic dibromoacylenamide 13 via an acid-catalyzed Friedel–Crafts alkylation and subsequent elimination.33, 36 With the desired substrate in hand, we studied the [3+2] annulation with the 2-amido-1,3-diaminoallyl cation generated from N-Ts guanidine 16 upon treatment with PhI(OAc)2 (1.5 equiv) under our previously optimized conditions.32 However, the only isolable product (13%) was not the desired cyclic guanidine (cf. 18) but rather a compound that was missing one benzyloxy moiety and did not possess the expected downfield C6-proton based on NMR and MS. Ultimately, single crystal X-ray analysis of this adduct (see inset, Scheme 2).37 revealed this product to be the carbinolamine 17, presumably arising from a benzylic oxidation followed by an elimination. While a sterically-driven elimination of benzyl alcohol and capture of water is possible, we hypothesize that under the mildly basic conditions, the OBn group at N-7 is likely oxidized since the corresponding methoxy substituted-guanidine did not eliminate under identical conditions (vide infra), the N-9 OBn group remains intact, and the potential for a charge-transfer complex38 with the pyrrole ring. The pyrrole ring’s two bromine atoms through mesomeric effects and despite the weakly electron-withdrawing vinylogous acyl urea, may provide a sufficiently electron-rich aromatic to assist in this oxidation leading to selective loss of benzaldehyde at N-7 following hydrolysis of the resulting oxocarbenium. The exposed hydroxylamine in guanidine 19 can then undergo an elimination, perhaps facilitated by the hypervalent iodine reagent as shown, to generate an iminium species 20 that can capture water to provide carbinolamine 17 (Scheme 2). Interestingly, carbinolamine 17 is reminiscent of the previously isolated natural product, (−)-dibromohydroxyphakellin (4) (see Figure 1).
Scheme 2.
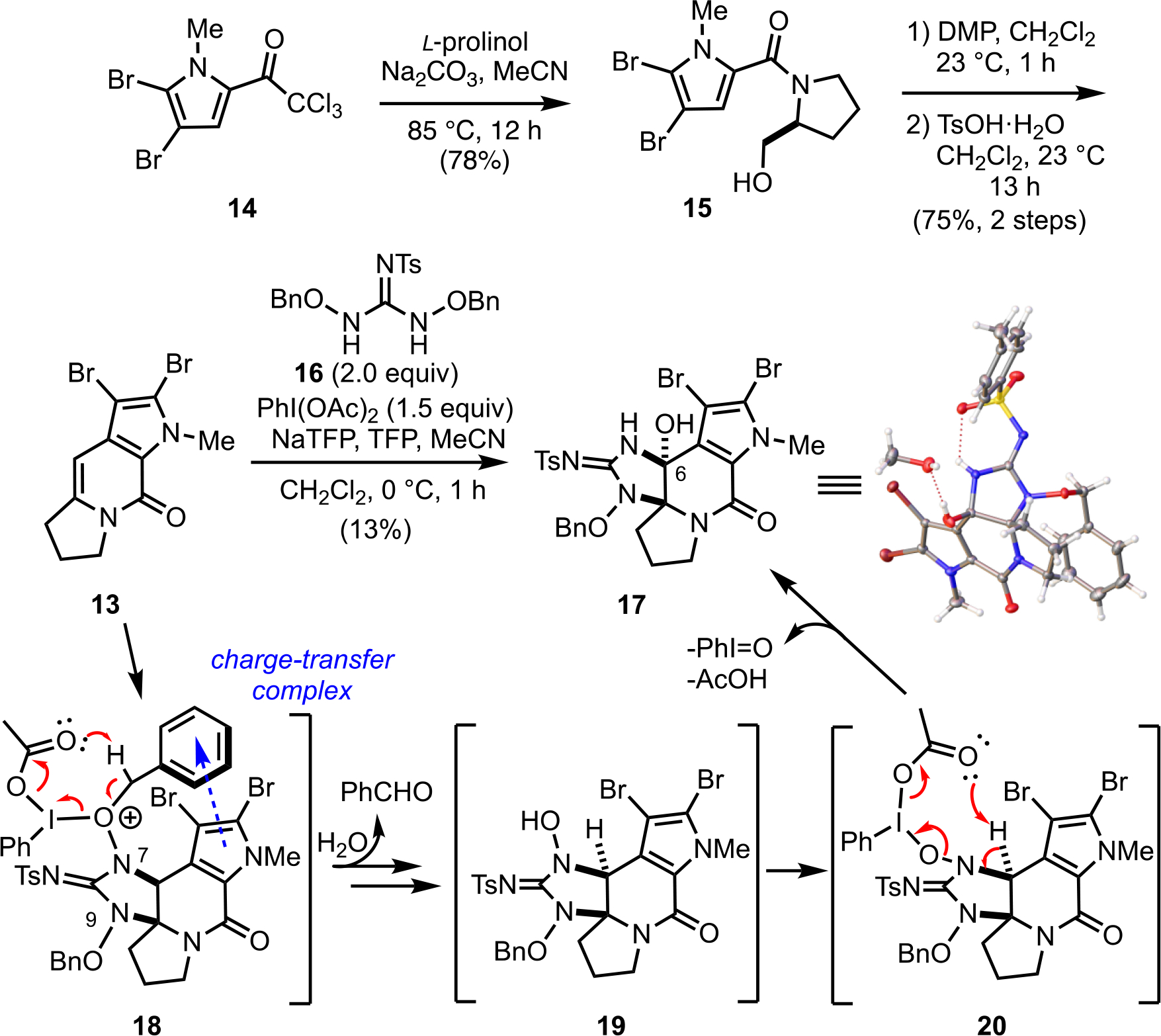
Attempted cycloguanidinylation with tricyclic dibromoenamide 13 and N-Ts guanidine 16. Proposed benzylic oxidation and elimination sequence leading to the observed carbinolamine 17 (inset: single crystal X-ray structure of carbinolamine 17•MeOH)
Given the observed benzylic oxidation with guanidine 16 leading to unexpected product 17 (Scheme 2), we sought to circumvent this issue by studying alternative 1,3-dipole precursors devoid of benzyl ethers such as bis-N-methoxy guanidine 22 prepared as previously described from thioimidate 21 (Scheme 3).32,39 The chemical shifts observed by 1H-15N HMBC correlations (compared to typical chemical shifts of hydroxylamines40 and oximes41) suggested that guanidine reagent 22 exists primarily as the oxime isomer as shown versus the imine-containing isomer (iso-22).
Scheme 3.
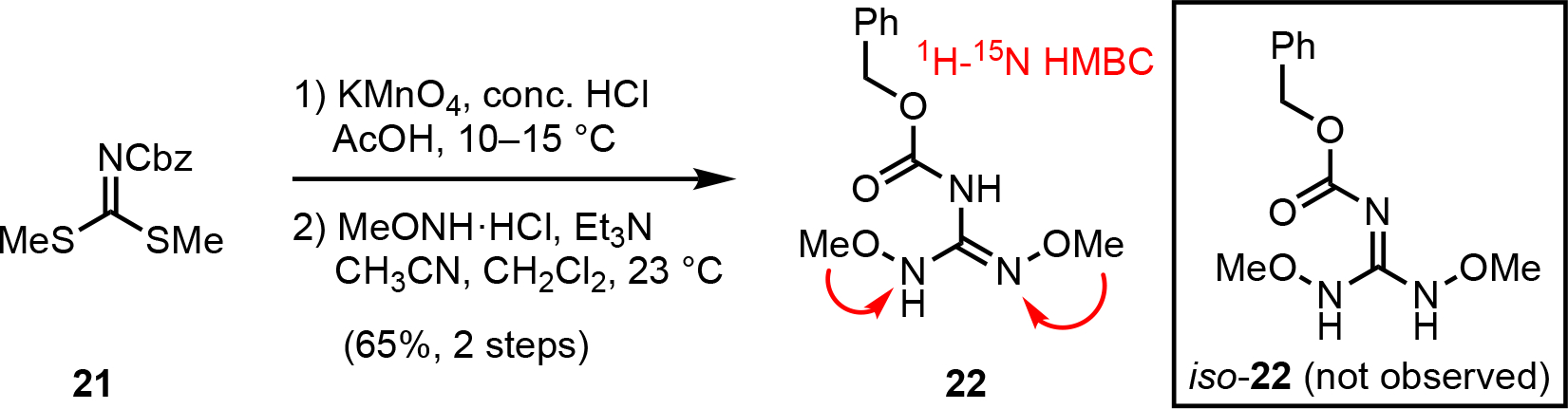
Synthesis of a bis-OMe, Cbz-guanidinylating reagent 22.
With guanidine 22 in hand, we again attempted the cycloguanidinylation however this time with alkene 2336b since the brominated pyrrole substrate alkene 13 displayed reduced reactivity likely due to the inductive effects of the bromine atoms (Scheme 4). We were pleased to find that this cycloguanidinylation proceeded smoothly under typical oxidative conditions to efficiently provide a mixture of the separable (MPLC), isomeric cyclic guanidine compounds 26 and 27 in 69% and 30% yield, respectively, without production of the previously observed carbinolamine. The major cyclic guanidine 26 was isolated as an ~1:1 mixture of E/Z oxime isomers (26a/b) which could be separated by RP-HPLC (for tentative assignment of each isomer, see SI). The structure of the isomeric guanidine 27 was supported by the observed nOe correlations indicated (N7-OCH3 → C3-pyrrole NH and N9-OCH3 → H11) with the latter nOe absent from the oxime isomers 26a/b in conjunction with 15N-NMR chemical shifts and 1H-15N HMBC (see SI for details). Both cyclic guanidines 26 and 27 showed expected 1H-15N HMBC correlations with N7 (δ 170 ppm; consistent with previously reported chemical shifts for hydroxylamine nitrogens).40 Oximes 26 exhibited a 1H-15N HMBC correlation between a 15N resonance (δ 310.0 and 318.0) and the N16-OCH3 group which is consistent with literature 15N values for oxime-type nitrogens.41 In the case of isomer 27, the second methoxy group was assigned as the N9-OCH3 based on a NOESY correlation with the C11 proton and a 1H-15N HMBC correlation with a nitrogen resonance at δ 183.0. Based on this NMR analysis, it was determined that compound 26 has the oxime-type cyclic guanidine structure and compound 27 has the Cbz-imine-type cyclic guanidine structure. It should be noted that imine 27 appears as a single geometrical isomer by NMR indicating that it exists as a single isomer or rapidly interconverts relative to the NMR time scale. These two regioisomeric adducts arise from either hindered rotation about the N7-C8 bond (blue arrows, Scheme 4) or differential electron density at N9 and N16 (cf. 24 and 25) leading to the adducts 26 and 27 in this presumed stepwise cycloguanidinylation.
Scheme 4.
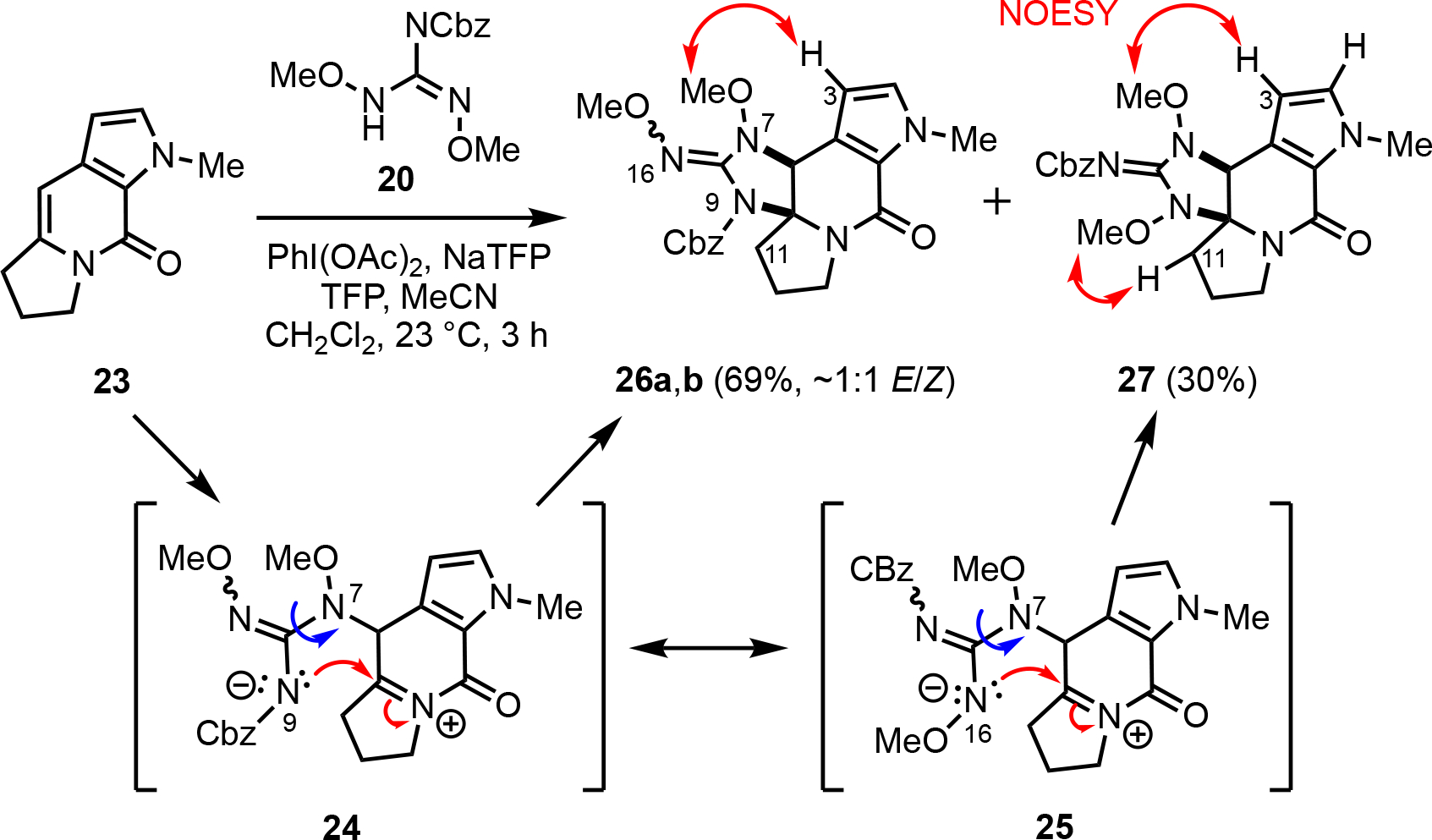
Guanidine [3+2] annulation toward (±)-N-methyldibromoisophakellin (10) leading to isomeric cyclic guanidines 26a,b and 27.
We next attempted N–O bond cleavage of the resulting cyclic guanidines 26 and 27 via treatment with SmI2 (Scheme 5).42 Interestingly, the isomeric cyclic guanidines 26 and 27 showed divergent reactivity as they each gave rise to different products during attempted deprotections. Surprisingly, cyclic guanidine 26 returned the cycloaddition substrate 23 as the major product through an overall deguanidinylation with SmI2. In addition, alkenyl guanidine 28 was obtained through a presumed ring-opening/elimination of the cyclic guanidine as a minor product possibly initiated by Lewis acidic Sm(III) species. On the other hand, cyclic guanidine 27 was readily converted to the desired N–O bond cleaved cyclic guanidine 29 (74%). The synthesis of (±)-N-methyldibromoisophakellin (10) was then achieved in 2 steps by dibromination of the pyrrole and deprotection of the Cbz group under basic hydrolytic conditions rather than hydrogenolysis.43 Characterization data obtained for synthetic N-methyldibromoisophakellin matched that previously reported.20
Scheme 5.
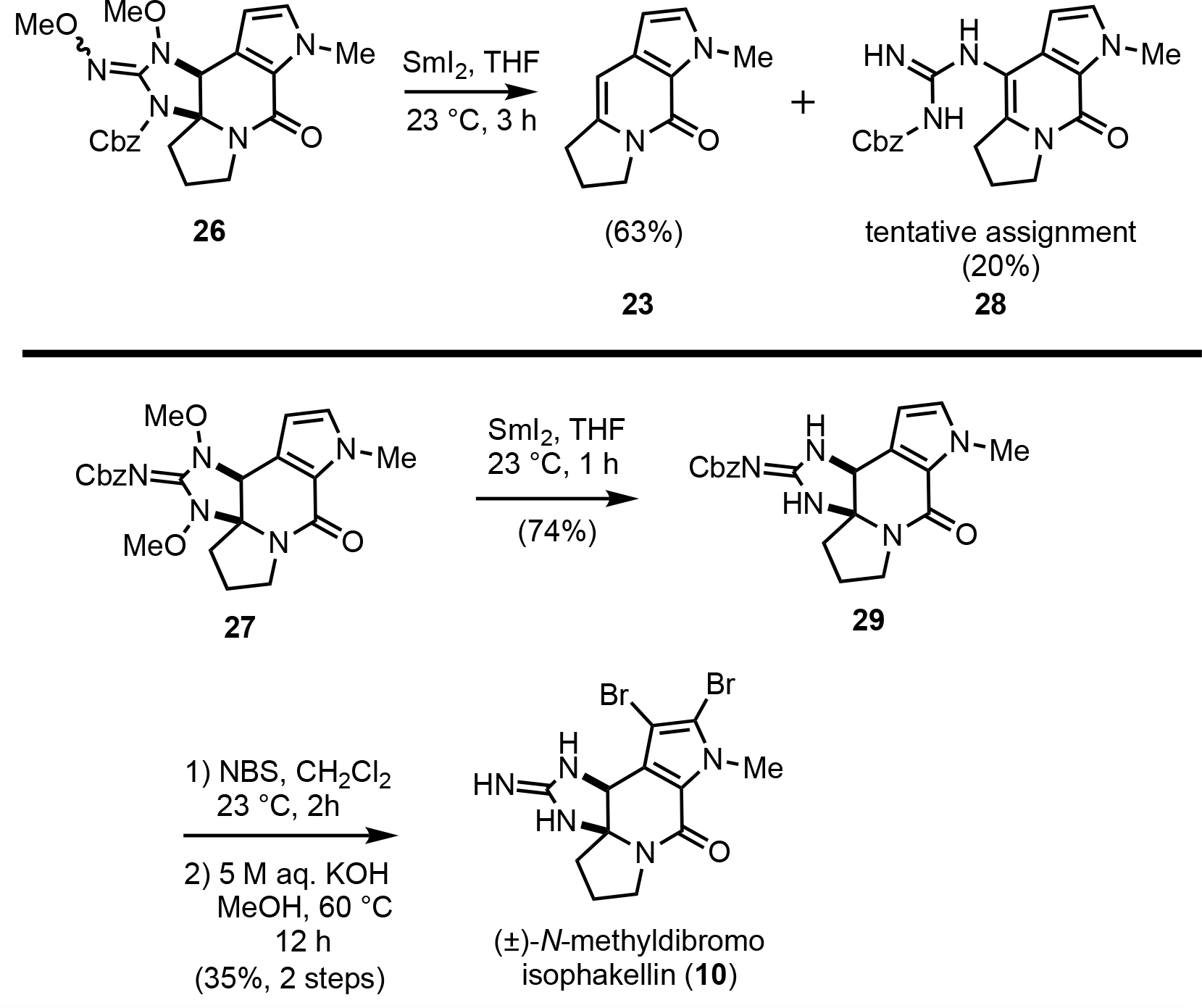
Divergent reactivity of the isomeric guanidine annulation adducts 26 and 27: synthesis of (±)-N-methydibromoisophakellin (10).
We also studied an alternative route to (±)-N-methyldibromoisophakellin (10) from cyclic guanidine 26 which ultimately led to a synthesis of an ugibohlin (Scheme 6). Protected guanidine 26 was converted to Boc-protected N-methyldibromoisophakellin 30 in 3 steps involving hydrogenolysis leading to N–O bond cleavage and cleavage of the Cbz group,44 transient Boc protection of the guanidine and finally dibromination of the pyrrole ring. Attempted Boc group deprotection under acidic conditions (TFA) led to a single compound which was identified as N-methylugibohlin (9) with spectral data matching that previously reported.21 The synthesis of ugibohlin from ring cleavage of dibromoisophakellin was previously proposed by Mourabit33 and subsequently described by the Lindel group25 under strongly acidic conditions (6M aqueous HCl). We propose that Boc-protected N-methyldibromoisophakellin 30 is readily converted to N-methylugibohlin (9) via guanidium ion 31 leading to the N-acyl iminium species 32 and elimination. Based on these results, we hypothesize that ugibohlins may be artifacts of isolation of the corresponding isophakellins, as originally posited by Mourabit,33 since silica gel chromatography and/or HPLC purification with TFA-containing solvents may lead to ring-cleavage to ugibohlin framework which were co-isolated with the corresponding isophakellins.17, 19b, 21
Scheme 6.
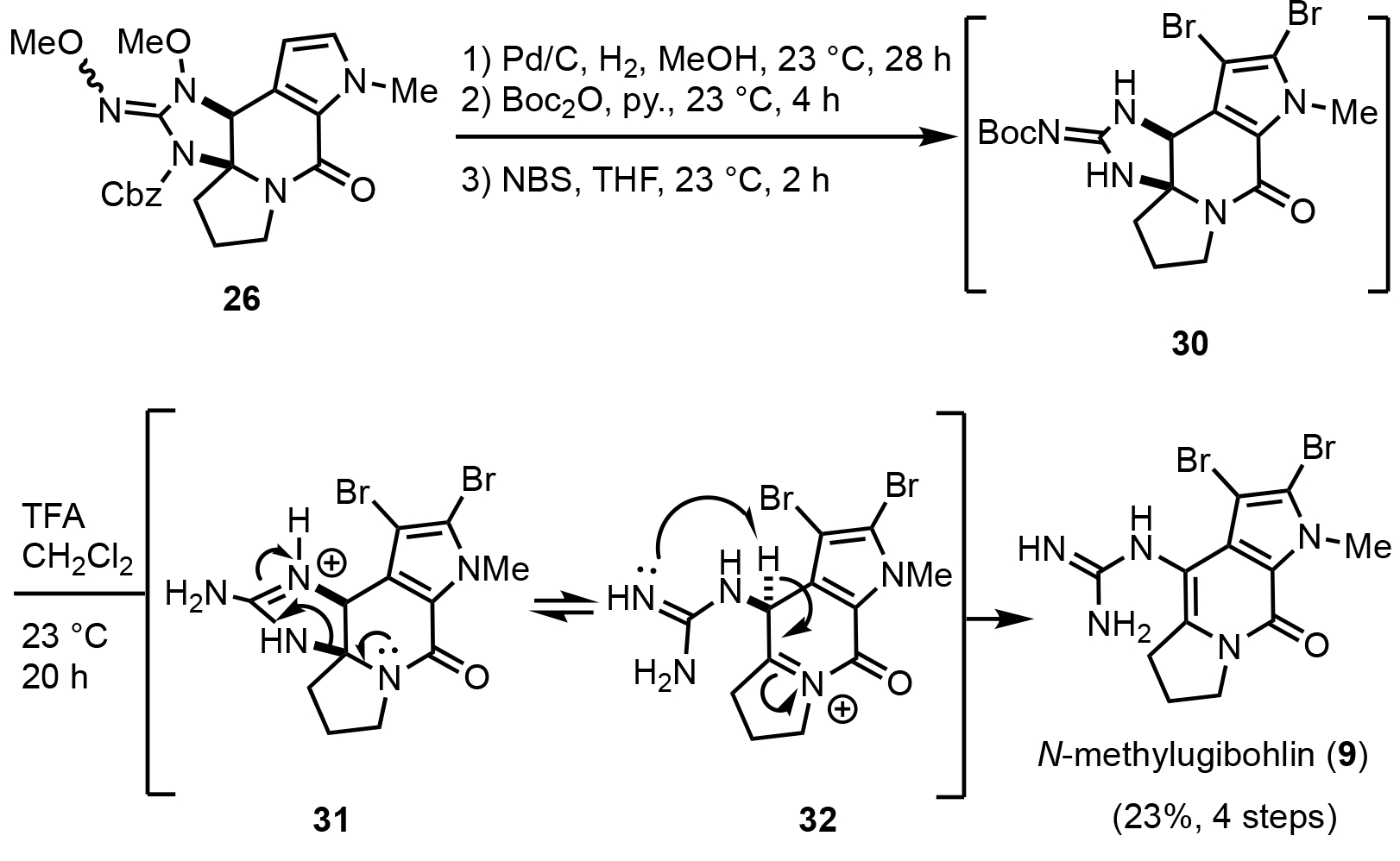
Synthesis of N-methylugibohlin (9) through ring cleavage of dibromoisophakellin core.
Conclusions
In summary, we report a total synthesis of (±)-N-methyldibromoisophakellin (10) in 7 steps from commercially available 2-trichloroacetyl-1-methylpyrrole and prolinol employing a net [3+2] cycloguanidinylation with a 2-amido-1,3-diaminoallyl cation generated under oxidative conditions. A novel carbinolamine-containing tetracycle 17 was also produced during an attempted cycloaddition with the 1,3-dipole derived from a Ts-protected bis-N-benzyloxy guanidine. This adduct was confirmed by X-ray analysis and presumably arises from a selective, oxidative benzyl cleavage of one N-OBn, an elimination, and water capture under the conditions of the cycloaddition. We found that a Cbz-protected bis-methoxy guanidinylating agent is useful for generation of the corresponding 2-amino-1,3-diaminoallyl cations and this was employed for the synthesis of (±)-N-methyldibromoisophakellin (10). Finally, the first total synthesis of N-methylugibohlin (9) is described and occurs by cyclic guanidine ring cleavage and elimination under mild acidic conditions suggesting that the ughibohlins may in fact be artifacts of isolation formed during purification of isophakellins when using trifluoroacetic acid as a modifier in RP-HPLC.
Supplementary Material
Acknowledgment
Support of this project from NIH NIGMS (R37 GM052964 and GM052964S1) and the Welch Foundation (AA-1280) is gratefully acknowledged. We are also grateful to Professor K. K. Klausmeyer (Baylor) for securing the X-ray structure of carbinolamine 17.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article includes (1) experimental procedures for all reactions described (2) full characterization data for all new compounds.
Dedicated to Prof. John L. Wood, a great friend and colleague to honor his selfless work and stewardship as Associate Editor of the Americas for Tetrahedron Letters (2001–2020).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).(a) Hoffmann H; Lindel T Synthesis of the Pyrrole-Imidazole Alkaloids. Synthesis 2003, 1753–1783. DOI: 10.1055/s-2003-41005. [DOI] [Google Scholar]; (b) Forte B; Malgesini B; Piutti C; Quartieri F; Scolaro A; Papeo G A Submarine Journey: The Pyrrole-Imidazole Alkaloids. Mar. Drugs 2009, 7, 705–753. DOI: 10.3390/md7040705. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Al-Mourabit A; Zancanella MA; Tilvi S; Romo D Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. DOI: 10.1039/c0np00013b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Beniddir MA; Evanno L; Joseph D; Skiredj A; Poupon E Emergence of diversity and stereochemical outcomes in the biosynthetic pathways of cyclobutane-centered marine alkaloid dimers. Nat. Prod. Rep. 2016, 33, 820–842. DOI: 10.1039/c5np00159e. [DOI] [PubMed] [Google Scholar]; (e) Seipp K; Geske L; Opatz T Marine Pyrrole Alkaloids. Mar. Drugs 2021, 19, 514. DOI: 10.3390/md19090514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Herath AK; Lovely CJ Pyrrole carboxamide introduction in the total synthesis of pyrrole–imidazole alkaloids. Org. Biomol. Chem. 2021, 19, 2603–2621. DOI: 10.1039/d0ob02052d. [DOI] [PubMed] [Google Scholar]
- (2).Kobayashi J; Ohizumi Y; Nakamura H; Hirata Y A novel antagonist of serotonergic receptors, hymenidin, isolated from the Okinawan marine sponge Hymeniacidon sp. Experientia 1986, 42, 1176–1177. DOI: 10.1007/BF01941300. [DOI] [PubMed] [Google Scholar]
- (3).(a) Burkholder PR; Sharma GM Antimicrobial agents from the sea. Lloydia 1969, 32, 466–483. [PubMed] [Google Scholar]; (b) Sharma GM; Burkholder PR Structure of Dibromophakellin, a New Bromine-containing Alkaloid from the Marine Sponge Phakellia flabellata. J. Chem. Soc. Chem. D 1971, 151–152, 10.1039/C29710000151. DOI: 10.1039/C29710000151. [DOI] [Google Scholar]; (c) Sharma GM; Magdoff-Fairchild B Natural products of marine sponges. 7. The constitution of weakly basic guanidine compounds, dibromophakellin and monobromophakellin. J. Org. Chem. 1977, 42, 4118–4124. DOI: 10.1021/jo00445a028. [DOI] [Google Scholar]
- (4).(a) Kinnel RB; Gehrken H-P; Swali R; Skoropowski G; Scheuer PJ Palau’amine and Its Congeners: A Family of Bioactive Bisguanidines from the Marine Sponge Stylotella aurantium1. J. Org. Chem. 1998, 63, 3281–3286. DOI: 10.1021/jo971987z. [DOI] [Google Scholar]; (b) Kinnel RB; Gehrken HP; Scheuer PJ Palau’amine: a cytotoxic and immunosuppressive hexacyclic bisguanidine antibiotic from the sponge Stylotella agminata. J. Am. Chem. Soc. 2002, 115, 3376–3377. DOI: 10.1021/ja00061a065. [DOI] [Google Scholar]; (c) Buchanan MS; Carroll AR; Quinn RJ Revised structure of palau’amine. Tetrahedron Lett. 2007, 48, 4573–4574. DOI: 10.1016/j.tetlet.2007.04.128. [DOI] [Google Scholar]
- (5).(a) Grube A; Kock M Stylissadines A and B: the first tetrameric pyrrole-imidazole alkaloids. Org. Lett. 2006, 8, 4675–4678. DOI: 10.1021/ol061317s. [DOI] [PubMed] [Google Scholar]; (b) Buchanan MS; Carroll AR; Addepalli R; Avery VM; Hooper JN; Quinn RJ Natural products, stylissadines A and B, specific antagonists of the P2X7 receptor, an important inflammatory target. J. Org. Chem. 2007, 72, 2309–2317. DOI: 10.1021/jo062007q. [DOI] [PubMed] [Google Scholar]
- (6).Endo T; Tsuda M; Okada T; Mitsuhashi S; Shima H; Kikuchi K; Mikami Y; Fromont J; Kobayashi J Nagelamides A-H, new dimeric bromopyrrole alkaloids from marine sponge Agelas species. J. Nat. Prod. 2004, 67, 1262–1267. DOI: 10.1021/np034077n. [DOI] [PubMed] [Google Scholar]
- (7).(a) Stout EP; Morinaka BI; Wang YG; Romo D; Molinski TF De Novo Synthesis of Benzosceptrin C and Nagelamide H from 7-15N-Oroidin: Implications for Pyrrole–Aminoimidazole Alkaloid Biosynthesis. J. Nat. Prod. 2012, 75, 527–530. DOI: 10.1021/np300051k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stout EP; Wang YG; Romo D; Molinski TF Pyrrole Aminoimidazole Alkaloid Metabiosynthesis with Marine Sponges Agelas conifera and Stylissa caribica. Angew. Chem. Int. Ed. 2012, 51, 4877–4881. DOI: 10.1002/anie.201108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Andrade P; Willoughby R; Pomponi SA; Kerr RG Biosynthetic Studies of the Alkaloid, Stevensine, in a Cell Culture of the Marine Sponge Teichaxinella morchella. Tetrahedron Lett. 1999, 40, 4775–4778. DOI: Doi 10.1016/S0040-4039(99)00881-3. [DOI] [Google Scholar]
- (9).(a) Mayer AM; Rodriguez AD; Berlinck RG; Fusetani N Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2011, 153, 191–222. DOI: 10.1016/j.cbpc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bian C; Wang J; Zhou X; Wu W; Guo R Recent Advances on Marine Alkaloids from Sponges. Chem Biodivers 2020, 17, e2000186. DOI: 10.1002/cbdv.202000186. [DOI] [PubMed] [Google Scholar]; (c) Souza CRM; Bezerra WP; Souto JT Marine Alkaloids with Anti-Inflammatory Activity: Current Knowledge and Future Perspectives. Mar. Drugs 2020, 18. DOI: 10.3390/md18030147. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chu MJ; Li M; Ma H; Li PL; Li GQ Secondary metabolites from marine sponges of the genus Agelas: a comprehensive update insight on structural diversity and bioactivity. RSC Adv. 2022, 12, 7789–7820. DOI: 10.1039/d1ra08765g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Jin Z Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2005, 22, 196–229. DOI: 10.1039/b316104h. [DOI] [PubMed] [Google Scholar]
- (11).Reyes JC; Romo D Bioinspired total synthesis of agelastatin A. Angew. Chem. Int. Ed. 2012, 51, 6870–6873. DOI: 10.1002/anie.201200959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).de Nanteuil G; Ahond A; Guilhem J; Poupat C; Dau ETH; Potier P; Pusset M; Pusset J; Laboute P Invertebres marins du lagon neo-caledonien-V. Tetrahedron 1985, 41, 6019–6033. DOI: 10.1016/s0040-4020(01)91443-7. [DOI] [Google Scholar]
- (13).Fedoreyev SA; Utkina NK; Ilyin SG; Reshetnyak MV; Maximov OB The structure of dibromoisophakellin from the marine sponge acanthella carteri. Tetrahedron Lett. 1986, 27, 3177–3180. DOI: 10.1016/s0040-4039(00)84747-4. [DOI] [Google Scholar]
- (14).Kuramoto M; Miyake N; Ishimaru Y; Ono N; Uno H Cylindradines A and B: novel bromopyrrole alkaloids from the marine sponge Axinella cylindratus. Org. Lett. 2008, 10, 5465–5468. DOI: 10.1021/ol802263j. [DOI] [PubMed] [Google Scholar]
- (15).Hertiani T; Edrada-Ebel R; Ortlepp S; van Soest RW; de Voogd NJ; Wray V; Hentschel U; Kozytska S; Muller WE; Proksch P From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. DOI: 10.1016/j.bmc.2009.12.028. [DOI] [PubMed] [Google Scholar]
- (16).Fedoreyev SA; Ilyin SG; Utkina NK; Maximov OB; Reshetnyak MV; Antipin MY; Struchkov YT The structure of dibromoagelaspongin - a novel bromine-containing guanidine derivative from the marine sponge agelas sp. Tetrahedron 1989, 45, 3487–3492. DOI: 10.1016/s0040-4020(01)81027-9. [DOI] [Google Scholar]
- (17).Goetz GH; Harrigan GG; Likos J Ugibohlin: a new dibromo-seco-isophakellin from Axinella carteri. J. Nat. Prod. 2001, 64, 1581–1582. DOI: 10.1021/np010202o. [DOI] [PubMed] [Google Scholar]
- (18).Assmann M; Kock M Monobromoisophakellin, a new bromopyrrole alkaloid from the Caribbean sponge Agelas sp. Z. Naturforsch., C. J. Biosci. 2002, 57, 153–156. DOI: 10.1515/znc-2002-1-225. [DOI] [PubMed] [Google Scholar]
- (19).(a) Sun J; Wu J; An B; Voogd NJ; Cheng W; Lin W Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria. Mar. Drugs 2018, 16, 9. DOI: 10.3390/md16010009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) de Souza R; Freire VF; Gubiani JR; Ferreira RO; Trivella DBB; Moraes FC; Paradas WC; Salgado LT; Pereira RC; Amado Filho GM; Ferreira AG; Williams DE; Andersen RJ; Molinski TF; Berlinck RGS Bromopyrrole Alkaloid Inhibitors of the Proteasome Isolated from a Dictyonella sp. Marine Sponge Collected at the Amazon River Mouth. J. Nat. Prod. 2018, 81, 2296–2300. DOI: 10.1021/acs.jnatprod.8b00533. [DOI] [PubMed] [Google Scholar]
- (20).Assmann M; van Soest RW; Kock M New antifeedant bromopyrrole alkaloid from the Caribbean sponge Stylissa caribica. J. Nat. Prod. 2001, 64, 1345–1347. DOI: 10.1021/np000482s. [DOI] [PubMed] [Google Scholar]
- (21).Li T; Li PL; Luo XC; Tang XL; Li GQ Three new dibromopyrrole alkaloids from the South China Sea sponge Agelas nemoechinata. Tetrahedron Lett. 2019, 60, 1996–1998. DOI: 10.1016/j.tetlet.2019.06.049. [DOI] [Google Scholar]
- (22).Foley LH; Buchi G Biomimetic Synthesis of Dibromophakellin. J. Am. Chem. Soc. 1982, 104, 1776–1777. DOI: DOI 10.1021/ja00370a072. [DOI] [Google Scholar]
- (23).(a) Imaoka T; Iwata M; Akimoto T; Nagasawa K Synthetic approaches to tetracyclic pyrrole imidazole marine alkaloids. Nat. Prod. Commun. 2013, 8, 961–964. [PubMed] [Google Scholar]; (b) Iwata M; Kamijoh Y; Nagasawa K Synthetic Studies of Oroidin-derived Pyrrole-imidazole Alkaloids. J. Synth. Org. Chem. Jpn. 2015, 73, 38–44. [Google Scholar]
- (24).Wiese KJ; Yakushijin K; Horne DA Synthesis of dibromophakellstatin and dibromoisophakellin. Tetrahedron Lett. 2002, 43, 5135–5136. DOI: 10.1016/s0040-4039(02)00966-8. [DOI] [Google Scholar]
- (25).Moldovan R-P; Lindel T Improved Conversion of Dihydrooroidin to Oroidin and Ugibohlin. Z. Naturforsch., B: J. Chem. Sci. 2009, 64, 1612–1616. DOI: doi: 10.1515/znb-2009-11-1247 (acccessed 2022-10-19). [DOI] [Google Scholar]
- (26).Wang S; Romo D Enantioselective synthesis of (+)-monobromophakellin and (+)-phakellin: a concise phakellin annulation strategy applicable to Palau’amine. Angew. Chem. Int. Ed. 2008, 47, 1284–1286. DOI: 10.1002/anie.200703998. [DOI] [PubMed] [Google Scholar]
- (27).Poullennec KG; Romo D Enantioselective total synthesis of (+)-dibromophakellstatin. J. Am. Chem. Soc. 2003, 125, 6344–6345. DOI: 10.1021/ja034575i. [DOI] [PubMed] [Google Scholar]
- (28).Lengyel I; Sheehan JC α-Lactams(Aziridinones). Angew. Chem. Int. Ed. 1968, 7, 25–36. DOI: 10.1002/anie.196800251. [DOI] [Google Scholar]
- (29).Acharya A; Anumandla D; Jeffrey CS Dearomative Indole Cycloaddition Reactions of Aza-Oxyallyl Cationic Intermediates: Modular Access to Pyrroloindolines. J. Am. Chem. Soc. 2015, 137, 14858–14860. DOI: 10.1021/jacs.5b10184. [DOI] [PubMed] [Google Scholar]
- (30).DiPoto MC; Hughes RP; Wu J Dearomative Indole (3 + 2) Reactions with Azaoxyallyl Cations--New Method for the Synthesis of Pyrroloindolines. J. Am. Chem. Soc. 2015, 137, 14861–14864. DOI: 10.1021/jacs.5b10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Anumandla D; Acharya A; Jeffrey CS Oxidative (3 + 2) Cycloaddition Reactions of Diaza-Oxyallyl Cationic Intermediates and Indoles for the Synthesis of Imidazoloindolines. Org. Lett. 2016, 18, 476–479. DOI: 10.1021/acs.orglett.5b03527. [DOI] [PubMed] [Google Scholar]
- (32).Rao Kovvuri VR; Xue H; Romo D Generation and Reactivity of 2-Amido-1,3-diaminoallyl Cations: Cyclic Guanidine Annulations via Net (3 + 2) and (4 + 3) Cycloadditions. Org. Lett. 2020, 22, 1407–1413. DOI: 10.1021/acs.orglett.0c00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Travert N; Martin M-T; Bourguet-Kondracki M-L; Al-Mourabit A Regioselective intramolecular N1–C3 cyclizations on pyrrole–proline to ABC tricycles of dibromophakellin and ugibohlin. Tetrahedron Lett. 2005, 46, 249–252. DOI: 10.1016/j.tetlet.2004.11.066. [DOI] [Google Scholar]
- (34).(a) Richards JJ; Ballard TE; Huigens RW 3rd; Melander C Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem 2008, 9, 1267–1279. DOI: 10.1002/cbic.200700774. [DOI] [PubMed] [Google Scholar]; (b) Barker WT; Chandler CE; Melander RJ; Ernst RK; Melander C Tryptamine derivatives disarm colistin resistance in polymyxin-resistant gram-negative bacteria. Bioorg. Med. Chem. 2019, 27, 1776–1788. DOI: 10.1016/j.bmc.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).(a) Dess DB; Martin JC A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. DOI: 10.1021/ja00019a027. [DOI] [Google Scholar]; (b) Ireland RE; Liu L An improved procedure for the preparation of the Dess-Martin periodinane. J. Org. Chem. 1993, 58, 2899–2899. DOI: 10.1021/jo00062a040. [DOI] [Google Scholar]
- (36).(a) Laar C Ueber die Hypothese der wechselnden Bindung. Ber. Dtsch. Chem. Ges. 2006, 19, 730–741. DOI: 10.1002/cber.188601901165. [DOI] [Google Scholar]; (b) Chang CW; Wu CC; Chang YY; Lin CC; Chien TC Synthesis and unexpected oxidization of the tricyclic core of ugibohlin, isophakellin, and styloguanidine. J. Org. Chem. 2013, 78, 10459–10468. DOI: 10.1021/jo401911a. [DOI] [PubMed] [Google Scholar]
- (37).Selected X-ray data for compound 17: Space group P212121, I. R. P., R(int) = 0.0343, R1(all data) = 0.0195, wR2(all data) = 0.0476, GoF = 1.039, abs. struct. para. = 0.037(5).
- (38).Foster R Electron donor-acceptor complexes. J. Phys. Chem. 1980, 84, 2135–2141. DOI: 10.1021/j100454a006. [DOI] [Google Scholar]
- (39).van Loevezijn A; Venhorst J; Iwema Bakker WI; de Korte CG; de Looff W; Verhoog S; van Wees JW; van Hoeve M; van de Woestijne RP; van der Neut MA; Borst AJ; van Dongen MJ; de Bruin NM; Keizer HG; Kruse CG N’-(arylsulfonyl)pyrazoline-1-carboxamidines as novel, neutral 5-hydroxytryptamine 6 receptor (5-HT(6)R) antagonists with unique structural features. J Med Chem 2011, 54, 7030–7054. DOI: 10.1021/jm200466r. [DOI] [PubMed] [Google Scholar]
- (40).(a) Thorn KA; Arterburn JB; Mikita MA 15N and 13C NMR investigation of hydroxylamine-derivatized humic substances. Environ. Sci. Technol. 1992, 26, 107–116. DOI: 10.1021/es00025a011. [DOI] [Google Scholar]; (b) Przychodzen W; Doszczak L; Rachon J Substituent effects on 15N NMR chemical shifts in selected N-alkylthiohydroxamic acids. A comparative study. Magn. Reson. Chem. 2005, 43, 27–30. DOI: 10.1002/mrc.1497. [DOI] [PubMed] [Google Scholar]
- (41).(a) Botto RE; Westerman PW; Roberts JD 15N nuclear magnetic resonance spectroscopy. Natural-abundance15N spectra of aliphatic oximes. Org. Magn. Reson. 1978, 11, 510–515. DOI: 10.1002/mrc.1270111008. [DOI] [Google Scholar]; (b) Clement B; Kämpchen T The Application of 15N-NMR in the Analysis of N-Oxygenated amidines and guanidines. In N-Oxidation of Drugs: Biochemistry, pharmacology, toxicology, Hlavica P, Damani LA Eds.; Springer Netherlands, 1991; pp 19–35. [Google Scholar]; (c) Marthala VR; Jiang Y; Huang J; Wang W; Glaser R; Hunger M Beckmann rearrangement of 15N-cyclohexanone oxime on zeolites silicalite-1, H-ZSM-5, and H-[B]ZSM-5 studied by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 14812–14813. DOI: 10.1021/ja066392c. [DOI] [PubMed] [Google Scholar]; (d) Afonin AV; Ushakov IA; Pavlov DV; Ivanov AV; Mikhaleva AI Study of conformations and hydrogen bonds in the configurational isomers of pyrrole-2-carbaldehyde oxime by 1H, 13C and 15N NMR spectroscopy combined with MP2 and DFT calculations and NBO analysis. Magn. Reson. Chem. 2010, 48, 685–692. DOI: 10.1002/mrc.2650. [DOI] [PubMed] [Google Scholar]; (e) Liu X; Huang XS; Sin N; Venables BL; Roongta V 15N chemical shifts of a series of isatin oxime ethers and their corresponding nitrone isomers. Magn. Reson. Chem. 2010, 48, 873–876. DOI: 10.1002/mrc.2680. [DOI] [PubMed] [Google Scholar]
- (42).Szostak M; Spain M; Procter DJ Preparation of samarium(II) iodide: quantitative evaluation of the effect of water, oxygen, and peroxide content, preparative methods, and the activation of samarium metal. J. Org. Chem. 2012, 77, 3049–3059. DOI: 10.1021/jo300135v. [DOI] [PubMed] [Google Scholar]
- (43).Angle SR; Arnaiz DO Stereoselective Synthesis of Substituted Pipecolic Acids. Tetrahedron Lett. 1989, 30, 515–518. DOI: Doi 10.1016/S0040-4039(00)95241-9. [DOI] [Google Scholar]
- (44).Bergmann M; Zervas L Über ein allgemeines Verfahren der Peptid - Synthese. Berichte der deutschen chemischen Gesellschaft (A and B Series) 1932, 65, 1192–1201. DOI: 10.1002/cber.19320650722. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


