INTRODUCTION
The use of CD4+FoxP3+ regulatory T cells (Tregs) to inhibit graft vs host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (aHSCT) has been explored for more than a decade [1–10]. Both donor and host Tregs have been demonstrated to possess regulatory potential to ameliorate experimental GVHD [11–12]. Clinical trials infusing donor and cord blood derived Tregs in aHSCT patients have reported their safe application and results from prior and ongoing studies have shown promise [13–15, NCT00529035/NCT01937468/NCT03912064/NCT02991898/NCT01660607 and NCT04013685). Challenges nonetheless remain for their widespread usage as an effective strategy for regulating clinical HSCT [16]. Patient applications necessitate that sufficient Treg numbers (donor or host) will be available at the time of an HSCT. Historically, a similar requirement involving availability and access to sufficient numbers of donor stem / progenitor cells was resolved through development of mobilization procedures in the donor prior to transplant [17–21]. Peripheral blood (PB) from donors for allogeneic as well as autologous HSCT could be obtained following stem / progenitor cell mobilization regimens and successfully transplanted resulting in hematopoietic engraftment and graft versus leukemia (GVL) activity [21,22]. These regimens typically consist of the infusion of filgrastim (G-CSF) and plerixafor, as some individuals respond inadequately to filgrastim failing to mobilize sufficient CD34+ HSC into the peripheral blood to enable harvest of sufficient numbers (~5×106 HSC / kg) for transplant [19, 21, 23].
Notably, with regard to the present studies, G-CSF and plerixafor can also effectively mobilize hematopoietic progenitors in mice [19,24]. Here, we report an experimental aHSCT protocol in which donor mice were prepared using G-CSF alone or with plerixafor regimens to mobilize stem/progenitor cells while enabling concomitant expansion of their peripheral CD4+FoxP3+ Treg compartment. Mobilized animals were administered TNFRSF25 agonists together with low dose IL-2 (rhIL-2LD) to rapidly and markedly expand the CD4+FoxP3+ Treg compartment [5,6,25]. Utilizing multiple donor / recipient strain combinations involving MHC-matched and MHC-mismatched genetic disparities, the findings demonstrated efficient and simultaneous donor HSC / progenitor cell mobilization and Treg expansion. Transplant of PB from such donors resulted in diminished GVHD as assessed by clinical score, histopathology and immune parameters. We conclude HSC/PC mobilization was effective in the presence of Treg expansion and the donor HSC/PC populations exposed to TNFRSF25 agonists and rhIL-2LD were functional post-transplant in recipients. Additionally, donor Tregs could be efficiently expanded in the presence of G-CSF + plerixafor and subsequently were functionally suppressive in recipients. Lastly, using MLL-AF9 leukemia cells, graft vs. leukemia responses (GVL) remained intact in animals transplanted using this “dual” donor stem/progenitor cell and Treg expansion protocol, consistent with prior observations that Treg amelioration of GVHD does not abolish anti-tumor activity [5,9]. In total, these findings support the notion that during the donor stem / progenitor cell mobilization process, targeting and activating Tregs using a two receptor, i.e. TNFRSF25 + CD25 pathway strategy can result in a potential translational approach using PB for allo-HSCT with reduced GVHD severity while maintaining GVL.
MATERIALS AND METHODS
Animals:
C57BL/6J (B6; stock: 000664), B6-CD45.1 breeder (stock: 002014) (H2b), LP/J (H2b), B10.D2 (H2d) and C3H.SW (H2b) mice were purchased from The Jackson laboratory and maintained in University of Miami animal facilities. The FoxP3 reporter mice on a C57BL/6 background (B6-FoxP3RFP= B6-Fir) were originally provided by R. Flavell (Yale University, New Haven, CT). Wild-type BALB/c (H2d) mice were purchased from Taconic Biosciences or The Jackson Laboratory. BALB/c-FoxP3.DTR were obtained from the Fred Hutchinson Cancer Center. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were obtained from the University of Miami animal core. All mice were used at 6–12 weeks of age and were maintained in pathogen-free conditions at the University of Miami (UM) animal facilities. All animal procedures used were performed under protocols approved by the UM IACUC.
Flow cytometry:
Commercial antibodies for use in flow cytometry were purchased from BD Biosciences (San Jose, CA), Biolegend (San Jose, CA), or eBioscience / ThermoFisher (Waltham, MA). All antibodies used in this study are included in Supplemental Table I. Single-cell suspensions were prepared from different organs (spleen, peripheral lymph nodes [pLN]), bone marrow (BM). Peripheral blood (PB) was collected in heparinized tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll density gradient centrifugation. Next, 106 cells were pre-blocked with anti-mouse CD16/CD32 and stained with different antibody combinations. Intracellular staining was performed according to standard procedures. The following mAbs to the indicated molecules and their fluorescent labels were used in this study: CD4, CD8, CD19, CD25, CD44, CD62L, KLRG1, CD39, CD73, I-COS, Nrp-1, PD-1, CTLA-4, Ly-6C, Ki-67, Annexin V, H2Kb, H2Kd, CD45.1 and CD45.2.
Stem/Progenitor cell mobilization:
Donor mice were treated with recombinant murine G-CSF (Biolegend, San Jose, CA) or Neupogen (Filgrastim, AMGEN, Thousand Oaks, CA) for 4 days (2.5 ug/mouse sc) sometimes followed by Plerixafor (AMD3100, (Sigma-Aldrich, St. Louis, MO) (5mg/kg sc) on Days −1 and Day 0. PB was collected and PBMC obtained by density centrifugation (see below) followed by staining with monoclonal antibodies to c-kit (CD117), Ly6A/E (Sca-1), CD11b, Ly6G, CD4, CD8, CD19 and FoxP3 (see Suppl Table I).
Staining was assessed via flow cytometry (see above) and phenotypic analysis to identify populations in the peripheral blood of un-mobilized and mobilized mice for Hematopoietic Stem and Progenitor Cells (HSPCs) including: HSCs (Hematopoietic Stem Cells Lin−Sca-1+c-kit+ = (LSK which contain MPP: multipotent progenitors) CD150+ CD48− cells), CLPs (Common Lymphoid Progenitors Lin− IL-7R+ Sca-1low c-kitlow), GMPs (Granulocyte – Macrophage Progenitors Lin− Sca-1− c-kit+ CD16+CD32+ CD34+), MEPs (Megakaryocyte – Erythroid Progenitors Lin− Sca-1− c-kit+ CD16−CD32−CD34−) and CMPs (Common Myeloid Progenitors Lin− Sca-1− c-kit+ CD16−CD32− CD34+) that reside within Lin-Sca-1−c-Kit+ (LK) population.
Treg cell expansion:
TNFRSF25 agonists - (fusion protein: TL1A-Ig, mAbs: 4C12, mPTX-35 (Heat Biologics / Pelican Therapeutics) were administered intraperitoneally. TL1A-Ig+rIL-2 (recombinant IL-2): in vivo treatment with TL1A-Ig (on days 1 to 4) and human rIL-2 (10,000 units / injection) on Days 4,5 and 6. Recombinant mouse IL-2 and α-IL-2 monoclonal antibody, clone JES6–5H4, were purchased from ThermoFisher Scientific, (Waltham, MA.). TL1A-Ig was generated in our laboratory as described previously14.
Xenogeneic human to mouse transplantation with ex-vivo expanded human Tregs
PBMC were isolated from human mobilized (Filgrastim) peripheral blood by ficoll separation and viable T cells counted (all human cells were obtained from consented donors according to IRB approved (20160363). NSG mice were irradiated (2 Gy, total body irradiation) and transplanted the following day with 6×106 PBMC which included 3.6 ×106 T cells and ~2×104 CD34+ cells from the same PBMC donor.
Cryopreserved PBMC from healthy donors mobilized with Neupogen were thawed and phenotyped and Tregs (defined as CD4+CD25hiCD127lo) were obtained by cell sorting (>98.0% Foxp3+). Sorted Tregs (5 × 105/well/ml) were cultured in 24-well plates with anti-CD3/CD28 Dynabeads (4:1 ratio of Dynabeads to cells, ThermoFisher Scientific) and human IL2 (500 unit/mL, Novartis) in OpTmizer CTS™ T-cell expansion medium (designated as SFM) (Life Technologies) on day 0, and then subcultured in SFM with human IL2 (500 unit/mL) for 7–8 days. Post-culture analysis assessed by CD4+FoxP3+CD127lo expression indicated significant Treg expansion with maintenance of high FoxP3 expression (>98%). Tregs were counted and added to the PBMC at a 1:1 ratio. Mice were monitored 3x per week for GVHD clinical score (as above), weight loss, and survival until 6 weeks post-transplantation.
Hematopoietic stem cell transplantation:
Models for HSCTs used were: 1) a major MHC-mismatched model (B6→BALB/c). Female BALB/c mice were conditioned with 7.5–8.0 Gy total body irradiation 1 day prior to transplantation, and 2) an MHC matched minor antigen mismatched model (LP/J or C3H.SW→B6). B6 female mice were conditioned with 10.0 Gy TBI on the day of transplantation. Peripheral blood (see above) cells were obtained from the appropriate donor animals for each experiment. Donor cells were stained for T cells (anti-CD4, clone RM4–5; anti CD8, clone 53-6-7) and adjusted to 0.5×106 to 1.0×106 T cells per mouse. In some experiments, tumor cells (B6-MLL-AF9GFP) previously generated by our laboratory were employed [26]. B6 H2b tumor cells (6.0×103) were added to the PB population prior to infusion into recipients. Recipient mice underwent transplantation (day 0) via i.v. infusion using a 0.2 mL volume via tail vein injection. GVHD was assessed by monitoring recipients for changes in total body weight, clinical signs, and overall survival. Clinical scores for GVHD were recorded for individual mice. Recipients were scored on a scale from 0 to 2 for 5 clinical parameters: weight loss, diarrhea, fur texture, posture, and alopecia according to our previous published studies using a modification of the scoring system previously reported [5, 6,27].
DT depletion of Tregs in vitro.
BALB/c-FoxP3-diptheria toxin knock-in mice were either mobilized only, mobilized + Treg expanded and mobilized + expanded then given (1μg) diphtheria toxin. Cells from PB were then obtained at 24 hrs. after DT was given and plated in round bottom 96 well plate (100,000 cells/well). Anti-CD3 (2C11) hybridoma protein G 1μg/ml was added to the wells. Three and four days later wells were manually counted.
Histologic Analysis
Briefly, GI tissues from animals 5–7 weeks after aHSCT were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin-eosin (H&E) and images were acquired using the Keyence BZ-X700 microscope. Tissue samples were scored following a modified system described by Kaplan D, et al using the multiple parameters hyperplasia, inflammation, submucosal edema and necrosis [28].
Statistical analyses:
Numbers of animals per group are described in the figure legends. All Figure panels include data sets obtained from individual animals. All graphing and statistical analysis were performed using GraphPad Prism 9 (La Jolla, CA). Significance of differences between two experimental groups were determined using two-tailed unpaired t test. For experiments comparing more than two groups, data was analyzed using a one-way ANOVA with a post-hoc Tukey’s multiple comparisons test. For survival analyses, a Kaplan Meyer (Wilcoxin) test was performed. Statistical tests performed are indicated in the figure legends. Significance indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, ns=non-significant. Data shown are means ± SEM.
RESULTS and DISCUSSION:
Development of a regimen to induce concomitant stem / progenitor cell mobilization and highly elevated Treg levels in mouse peripheral blood.
To test a potential strategy whereby Tregs present in transplanted donor blood could be assessed for their ability to ameliorate GVHD, a protocol to induce mobilization of stem / progenitor cells (HSC/PC) together with elevated levels of circulating Tregs was developed. Mice were initially examined for mobilization following the administration of G-CSF alone or together with plerixafor (Fig. S1A).
Neupogen (Filgrastim: rG-CSF) was administered to B6 mice (H2b) over 4 consecutive days and PB analyzed for c-kit expression and several hematopoietic cell markers including CD11b and Ly6G. Significant increases in the overall levels of c-kit+ cells as well as CD11b+ and Ly6G+ populations were noted compared to untreated peripheral blood (Fig. S1B).
In vivo mobilization with G-CSF together with plerixafor concomitantly (simultaneously) resulted in multifold increase of HSCs and MEP, CLP and GMP lineage committed progenitors numbers in the PB of mobilized B6 mice (Fig. 1A–E). Frequencies of HSC and lineage committed progenitors including MEPS, CLPs, GMPs and LSKs were elevated in these mobilized B6 mice (Fig. 1A–E). Additionally, the numbers of progenitor cell populations were increased in peripheral blood from mobilized B6 animals (Supplemental Table 2). Mobilization was confirmed in an independent mouse (LP/J) strain following administration of Filgrastim + plerixafor where elevated levels of c-kit+ populations as well as granulocytes and monocyte cells were readily apparent in mobilized vs untreated mice (Fig. 1F–H). To manipulate the Treg compartment concomitantly with mobilization, some animals also received a fusion protein (TL1A-Ig FP) specific for TNFRSF25 and rhIL-2LD which induces proliferation of CD4+FoxP3+ Tregs [5,29]. Treated animals demonstrating mobilization also exhibited highly elevated Treg (>30% CD4+FoxP3+ / CD4+) levels as assessed by frequency and numbers (Fig. 1I legend).
Fig. 1. Tregs can be expanded concomitantly with HSC and progenitor cell increases in peripheral blood following mobilization with Filgrastim and plerixafor infusion.
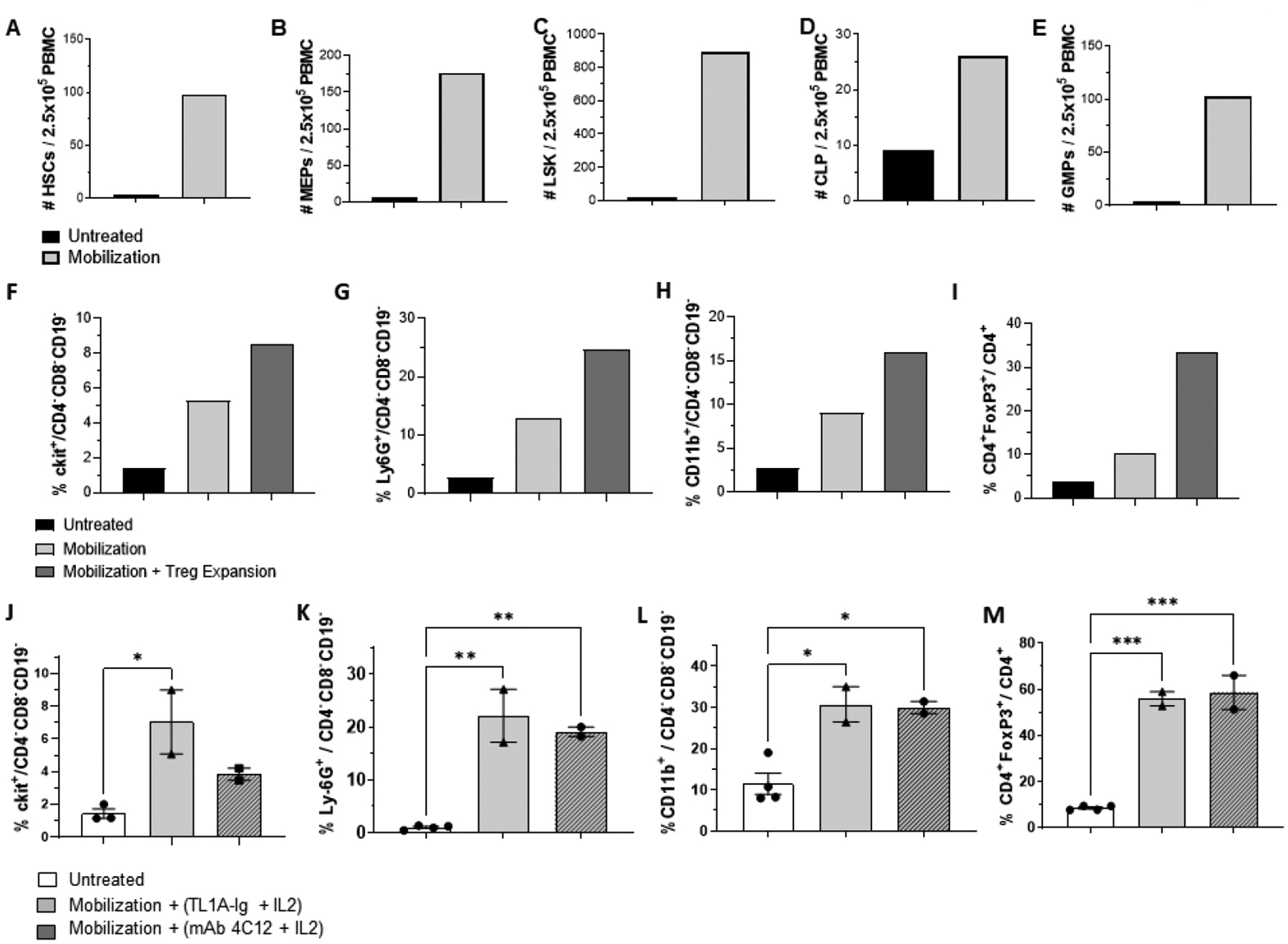
(A-E) B6-Fir,H2b mice were administered rGCSF (2.5ugs / injection) on Days 1–4 daily and plerixafor (5.0mgs/kg) on Days 4 and 5. (Fig. S1). Peripheral blood was collected in the morning of Day 5, 1 hr. following plerixafor injection and PBMC isolated (Methods). Following staining with selected mAbs and analysis via flow cytometry, (A-E) mobilization treatment resulted in 24-, 29-, and 60-fold increases of HSC (A) MEP (B) and LSK (C) populations respectively. CLP (3 fold) (D) and GMP (34 fold) (E) were also elevated compared to control (peripheral blood from non-injected normal mice). Data represents results of pooled peripheral blood from 2 mobilized B6-Fir male mice. (F-I) Mice (LP/J, H2)b were mobilized as above, and administered TL1A-Ig fusion protein and rhIL-2LD. Increased levels of c-kit+ and WBC fractions (F-H populations calculated within the non-lymphoid fraction) together with elevated Treg frequency following mobilization and treatment with TL1a-Ig and rhIL-2LD. Data represents pooled peripheral blood from 5 individual mice in each group (n=5 mice / group). (I) FoxP3+ Tregs within the CD4+ population. Numbers of Tregs were calculated for each group indicated (1.0ml peripheral was collected from each mouse, PBMC collected and pooled (n=5/group). Total PBMC were counted and Treg numbers calculated based on the frequency of CD4+FoxP3+/CD4+ cells. Untreated: 7,502; Mobilized: 102,538; Mobilized plus Treg expansion: 914,514. (J-M) Targeting TNFRS25 with a second agonist (mAb 4C12) also expands Tregs in mobilized peripheral blood. B10.D2 (H2d) mice were mobilized and Treg expanded with either TL1A-Ig (50ug) or mAb 4C12 (100ug) plus rhIL-2LD. (J-L) Heightened levels of c-kit+, myeloid cells and monocytes were detected in mobilized animals receiving eitherTNFRSF25 agonistic reagent. (M) Levels of Tregs were increased in all mobilized B10.D2 animals treated with either agonist (n=2 for mAb 4C12+IL-2LD), n=2 for TL1A-Ig+IL-2LD) vs non-mobilized B10.D2 animals (n=4): Cells per 200ul of peripheral blood = Non-mobilized, 818–984; Mobilized via 4C12, 1640, 2476; mobilized via TL1A-Ig, 1485,3320. (J-M) Data were collected from individual mice and are expressed as mean ± SD and were analyzed by one-way ANOVA with Bonferroni correction for multiple comparisons. *P < .05; **P < .01; ***P < .001; ****P < .0001.
To verify the ability to target Tregs via TNFRSF25 in mice undergoing HSC/PC mobilization, a third strain (B10.D2, H2d) was administered Filgrastim + plerixafor and either TL1A-Ig + IL-2LD or an anti-TNFRSF25 specific agonistic mAb (4C12) plus rhIL-2LD (Fig. 1J–M) [5]. Heightened levels of c-Kit+, myeloid cells and monocytes were detected in mobilized animals receiving either TNFRSF25 agonistic reagent (TL1A-Ig FP or 4C12mAb) (Fig. 1J,K,L). Levels of Tregs were markedly increased in all mobilized animals who also were treated with anti-TNFRSF25 agonists as evidenced by frequency and numbers (Fig. 1M legend).
Phenotypic and functional assessment of Tregs in blood following mobilization with or without TNFRSF25 and CD25 stimulation
Following in vivo stimulation with TLIA-Ig FP+ rhIL-2LD, PB Tregs were analyzed to assess the impact of 2-pathway TNFRS25 and CD25 stimulation on their phenotype. Increased levels of Tregs following administration of the expansion protocol, characterized by diminished frequencies of central Tregs Ly-6C+ and significantly elevated levels of effector Tregs in the 2-pathway stimulated animals compared to unmanipulated PB were detected (Fig. 2A, B). Mobilization alone tended to affect the Treg populations in a similar although less impactive manner. Additionally, Tregs from mobilized and Treg expanded animals compared to animals undergoing mobilization alone expressed elevated levels of ICOS-1 and Nrp-1 (Fig. S1C). Notably, prior findings demonstrated that TL1A-Ig + rhIL-2LD in vivo expanded Tregs exhibited higher suppressive function evidenced by a lower Treg:Teff ratio required to ameliorate GVHD [6].
Fig 2. Tregs Expanded with TL1a-Ig and IL-2(LD) in mobilized donor peripheral blood (PB) exhibited suppressive activity and ameliorated GVHD.
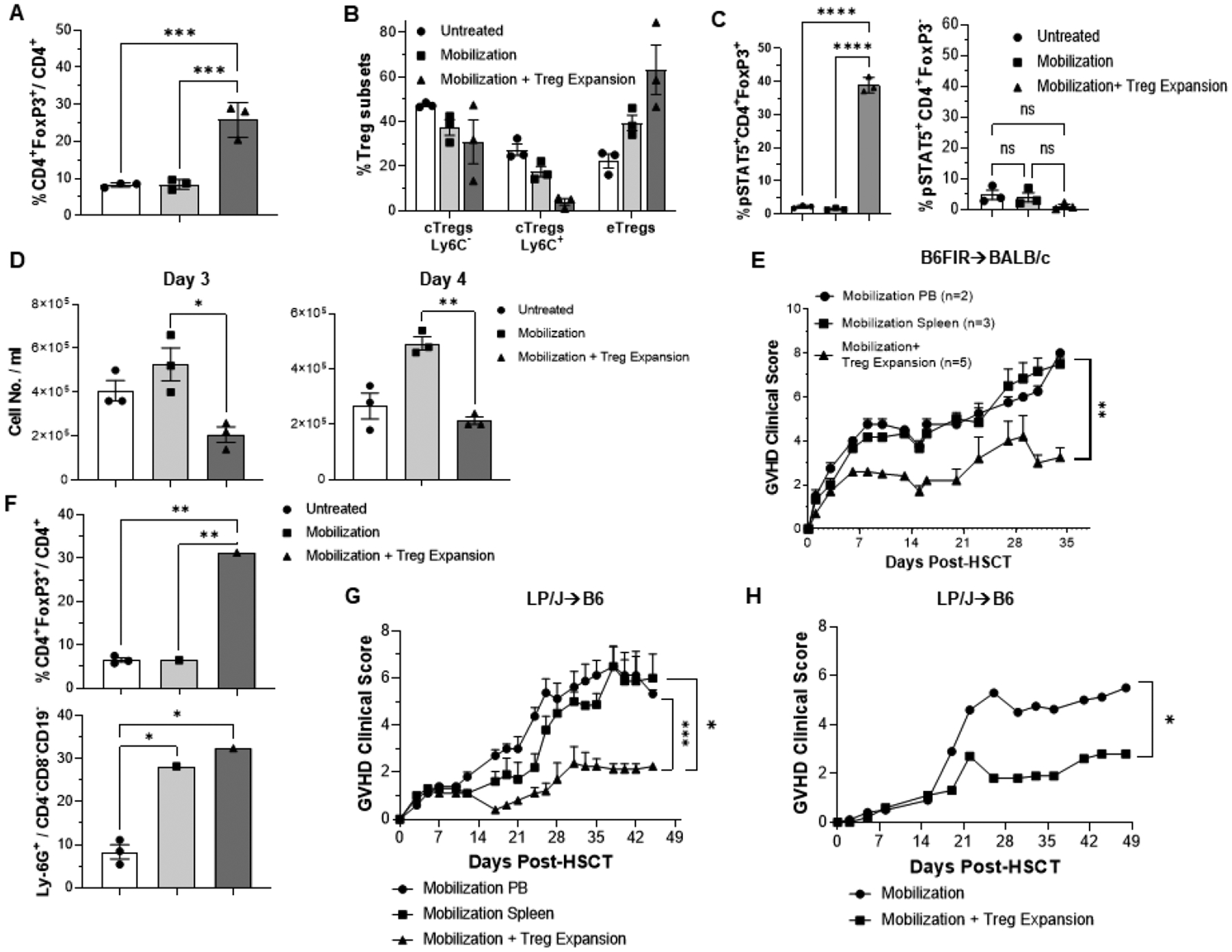
Mice were injected i.p. with TL1A-Ig (50ug) (days 1–4) and IL-2 LD (days 4 to 6). Mice administered TL1A-Ig+IL-2LD showed an increase in overall Treg (CD4+ FoxP3+) frequency in mobilized (rGCSF + Plerixafor) plus Treg expanded PB versus mobilized or untreated mice (A,F). (B) Diminished frequencies of central Tregs and significantly elevated levels of effector Tregs were present in mobilized plus Treg expansion compared to mobilized or untreated mice. (C) One hour after the last IL-2 injection (combined TL1A-Ig+IL-2LD), pSTAT5 staining showed heightened activation of PB Tregs compared to Tcon CD4 T cells. (D) Decreased T cell proliferation in mobilized peripheral blood in animals concomitantly Treg expanded. PBMC were activated with anti-CD3 mab and assessed for proliferation after 72 and 96 h (E) GVHD was diminished in animals receiving mobilized and Treg expanded donor cells. MHC-mismatched HSCT (B6→BALB/c) using T cells from donor B6-Fir mice PB either mobilized or mobilized plus Treg expanded (TL1A-Ig+IL-2LD) adjusted to contain 1.0×106 total T cells. Clinical scoring is presented. (F-H) GVHD was diminished in animals after an MHC-matched minor histocompatibility antigen mismatched HSCT (LP/J -> B6) using T cells from donor PB mobilized (rGCSF + Plerixafor) plus Treg expanded (TL1A-Ig fusion protein + IL-2LD) versus mobilized only. (G) and mPTX mab+IL-2LD (H) adjusted to contain1.0 ×106 total T cells. (F) Treg and Ly6G+ granulocyte levels in PB donors for the transplant results in panel (G) are shown. Values are means ± SEM and were analyzed by multiple variable analysis using ANOVA. A P-value < 0.05 was considered significant. (E,G) a 2-tailed unpaired t-test. was used for comparisons between 2 experimental groups (H) *P < .05; **P < .01; ***P < .001.Significance indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, ns=not significant.
pSTAT5 expression was examined in PB from untreated, mobilized and mobilized + Treg expanded animals. Following the final IL-2LD injection, pSTAT5 levels were markedly elevated on PB Tregs (but not Tconv) only from the mobilized and Treg expanded animals (Fig. 2C, S1D) demonstrating that the downstream IL-2R signaling pathway was functional in these CD4+FoxP3+ cells. To directly assess suppressive capacity, PBMC were obtained from untreated, mobilized and mobilized plus Treg expanded animals and cultured with an anti-TcR (CD3) mAb (Fig. 2D). Stimulation by the anti-TcR mAb in cultures from animals mobilized and concomitantly Treg expanded resulted in substantially reduced cell numbers compared to cultures established from mobilized only or untreated animals (Fig. 2D). Following mobilization and Treg expansion in FoxP3-diptheria toxin knock-in mice, Tregs were depleted in vivo with DT prior to in vitro PBMC culture. DT depletion of Tregs abolished the suppression of the anti-CD3 mAb T cell response (Fig. S2A). These findings illustrate a correlation between the diminished in vitro responses by PB T cells in samples and the presence of elevated levels of Treg cells in PB.
Allogeneic transplantation and analysis of GVHD using peripheral blood from non-mobilized, mobilized or mobilized and Treg expanded donors
Experiments were next performed to compare the GVHD capacity of donor blood from FoxP3RFP knock-in donor B6 mice undergoing either mobilization only or mobilization together with concurrent Treg expansion (Fig. S1A). Based on the levels of CD4+ and CD8+ T cells in the donor PB, cell numbers were adjusted, and all recipient BALB/c mice received 1×106 total T cells. A third group of BALB/c recipients received spleen cells also recovered from mobilized only B6-FIR donors (1×106 total T cells). Recipients receiving either blood or spleen cells from mobilized only donors generated significant GVHD clinical scores (Fig. 2E). In contrast, recipients receiving donor PB from mice concomitantly mobilized and Treg expanded exhibited significantly lower GVHD clinical scores (Fig. 2E) and within the first month post-HSCT contained higher frequency of Tregs in the PB (Fig. S2B). Additionally, 4/5 vs 1/5 mice survived to one month if the mobilized donor PB had been Treg expanded using TL1A-Ig+ IL-2LD (data not shown). Treg expanded donors were alive at 1 month, only 1/5 Transplants were independently performed utilizing MHC-matched minor histocompatibility antigen mismatched LP/J (H2b) donors and B6 (H2b) recipients. Recipients of concurrently mobilized and Treg expanded donor PB (Fig. 2F) and exhibited significantly reduced GVHD clinical scores compared to recipients of PB from either untreated or mobilized only (Fig. 2G).
Our prior studies found that individual treatment with rhIL-2LD alone or TL1A-Ig (or anti-TNFRSF25mAb) alone resulted in the TNFRSF25 agonists inducing a greater frequency of PB (spleen and lymph nodes) Tregs within the CD4 compartment although combining stimulation of both CD25 and TNFRSF25 induced the highest levels in these compartments [5,6,25]. Transplants using mobilized and rhIL-2LD treated donors compared to transplants using mobilized and rhIL-2LD plus TL1A-Ig Treg expanded donors did not show any statistically significant difference in survival benefit (Fig. S2C left panel). However, the recipients of mobilized and rhIL-2LD Treg expanded donors demonstrated little improvement in clinical GVHD scores compared to recipients of mobilized donors receiving rhIL-2LD plus TL1A-Ig to expand Tregs (Fig. S2C right panel). Notably, mobilized donors receiving only the TNFRSF25 agonist, TL1A-Ig also did not result in improved survival compared to recipients of control (mobilized only) donors (Fig. S2D left panel). Again, there was no statistically significant difference in survival benefit between groups transplanted with mobilized donors who were either Treg expanded with one reagent (TL1A-Ig) or the combination of TL1A-Ig+IL-2 (Fig. S2D left panel). However, in contrast to recipients of mobilized and rhIL-2LD treated donors (Fig. S2C, right panel), these mobilized and TL1A-Ig only treated Treg expanded donors exhibited diminished GVHD clinical scores (Fig. S2D right panel). Again, the strongest amelioration of GVHD score were detected in recipients of mobilized together with combination TL1A-Ig+IL-2LD Treg expanded donors (Fig. S2D right panel). Pathology evaluation of GI tissue indicated significantly reduced damage in recipients of mobilized and concomitantly Treg expanded donors compared to recipients of mobilized only or untreated donors (Fig. S2E).
To begin assessing potential clinical application of this strategy, LP/J mice were concomitantly mobilized and treated with an agonistic anti-TNFRSF25 mAb provided to our laboratory by Pelican Therapeutics. This specific mAb is an anti-human TNFRSF25 containing several amino acid differences from the original hamster anti-mouse reagent (4C12mAb) in the complementarity determining regions [30,31]. Treg levels in PB were >30% of CD4+ T cells in stem cell mobilized and mPTX35+IL-2LD treated donor animals (Fig. S3A). PB assessed ~ two weeks post-HSCT indicated that Treg levels were elevated in recipients of mobilized and Treg expanded compared to mobilized only donors (Fig. S3B). Overall weight (not shown) and clinical GVHD scores were decreased in B6 recipients of mobilized and mPTX35 Treg expanded donors compared to recipients of mobilized only (Fig. 2H).
Recipients of mobilized allogeneic peripheral blood containing elevated levels of Tregs mediated GVL against leukemia cells
To assess if animals receiving mobilized PB from donors who were concomitantly Treg expanded could affect graft vs. leukemia (GVL) responses, MLL-AF9 B6 tumor cells were administered at the time of C3H.SW⟶B6 HSCT. Some animals underwent an HSCT using mobilized syngeneic donor PB (B6->B6) and also received MLL-AF9 cells. As anticipated, GVHD was reduced in recipients of tumor + mobilized + Treg expanded donors compared to tumor + mobilized recipients (Fig. 3A). Tumor cells were readily identified in the spleen and marrow of recipients of syngeneic, mobilized PB donors ~1 month post-HSCT, (Fig. 3B, left panels). As morbidity ensued, blood was collected shortly prior to anticipated death. In contrast, recipients of mobilized allogeneic PB (Fig. 3B, middle panels) had very low levels of detectable tumor cell and mobilized allogeneic PB containing elevated levels of expanded Tregs (Fig. 3B, right panels) did not contain detectable tumor at early time points examined (Fig. 3B). At later time-points post-HSCT, low tumor levels could again be detected in some of the allo-HSCT recipients (Fig. 3C). In total, while no apparent difference in the GVL response was observed assessing recipients of mobilized vs. mobilized + Treg expanded donors, the former demonstrated significantly higher levels of GVHD (Fig. 3A). Earlier studies examining the use of Treg cells to ameliorate experimental GVHD in mice did not find ablation of GVL activity (9). Notably within this context, several clinical trials have reported that donor Treg infusion does not increase leukemia relapse in patients (32–36).
Fig 3. Maintaining mouse GVL and suppressing xenogeneic GVHD : Transplants with mobilized mouse and mobilized human peripheral blood.
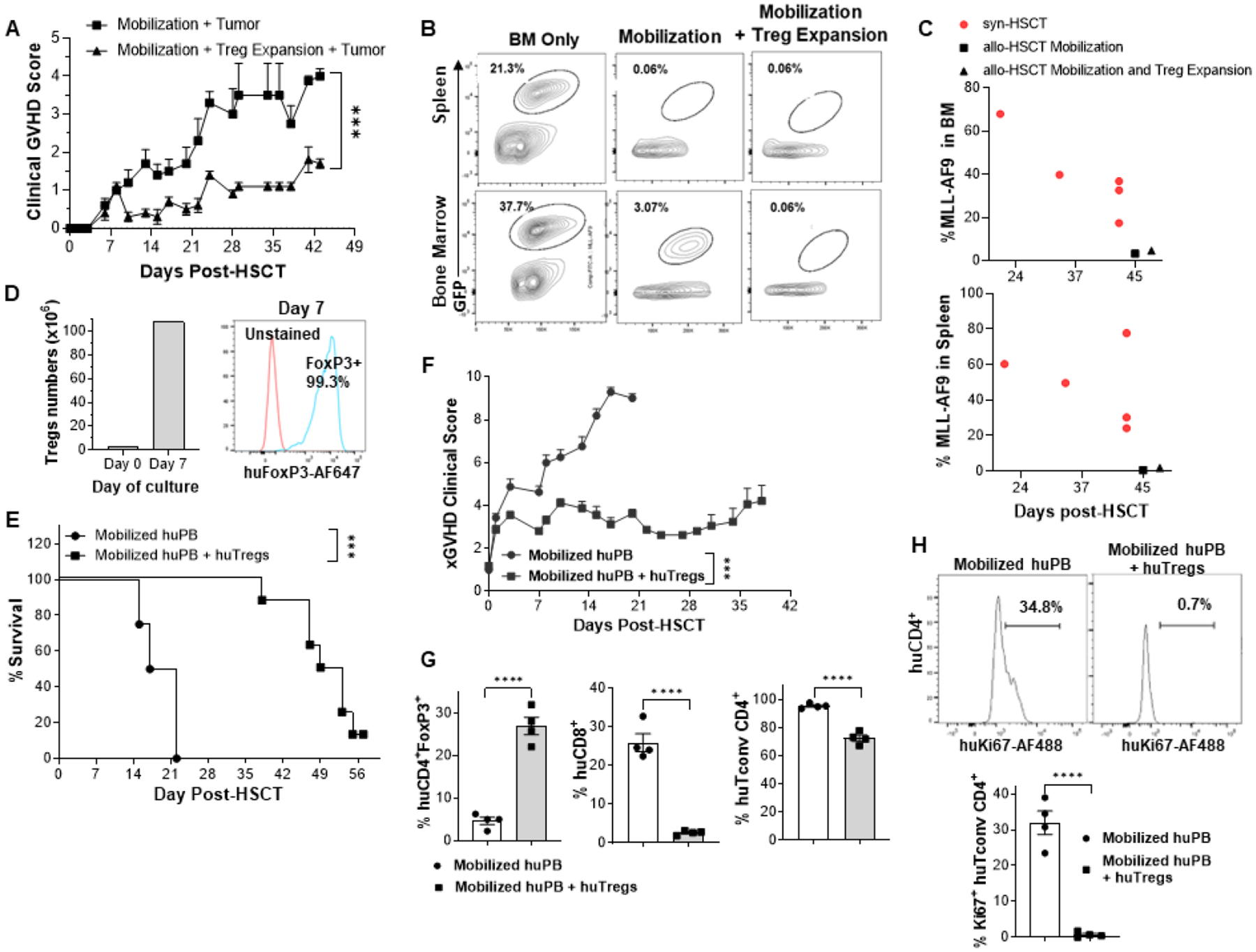
(A-C) An MHC-matched transplant C3H.SW⟶B6 HSCT was performed. Mice received PB T cells from either mobilized only (rGCSF) or mobilized (rGCSF) plus expanded Treg donor C3H.SW mice. MLL-AF9 B6 tumor cells were administered to all mice at the time of transplant. (A) GVHD was reduced in recipients of MLL-AF9+mobilized + Treg expanded donors compared to MLL-AF9 + mobilized only recipients. (B) Representative flow contour plots of spleen and bone marrow cells 28–30 days post-HSCT from individual recipients of BM only (syngeneic, C3H.SW), mobilization only (allogeneic, B6), and mobilization (allogeneic, B6) + Treg expansion (TL1A-Ig + IL-2 LD). (C) GVL is maintained in animals with reduced GVHD. Allo-HSCT recipient groups were examined for MLL-AF9 presence post-HSCT. Tumor cell frequency was always greater in the recipients of syngeneic mobilized PB donors compared to levels in recipients who received allogeneic mobilized PB without or with expanded Tregs. (D-H) Addition of ex-vivo expanded human Tregs to mobilized human peripheral blood suppresses xeno GVHD. Sorted Tregs isolated from huPB were cultured and expanded for 7 days using aCD3/aCD28 beads (Fig. S3C). (D) Treg numbers and FoxP3 expression at Day 7 of culture are shown prior to use in transplant. NSG mice were irradiated on day −1 and injected with mobilized huPBMCs with or without these expanded huTregs on day 0 (n=8 mice/group). (E,F) Mice treated with huTregs exhibited significantly less lethality and better clinical GVHD scores. (G) Mice receiving huTregs with huPB showed persistence of elevated Treg levels in PB, less huCD8+ and conventional CD4+ T cell levels in the blood 13 days post-transplant compared to recipients of huPB without Tregs. (E) Representative histogram and graph of individual mice illustrating huCD4+ Tconv proliferation in blood 13 days post-transplant. *** p<0.001; **** p<0.0001.
Ex-vivo expanded human donor Tregs inhibit xenogeneic GVHD development in animals receiving transplants of mobilized human peripheral blood
The ability of donor human Tregs to inhibit GVHD induced by mobilized human PB cells was also examined. PBMC from healthy donors mobilized with Neupogen were aliquoted and cryopreserved prior to use. Subsequently, a sample was phenotyped for T conventional (CD4+FoxP3−), CD8 (CD8+CD4−FoxP3−) (not shown) and Tregs (CD4+CD8−CD25+FoxP3+CD127lo) (Fig. S3C). CD4+CD25+CD127lo T cells (Treg) were obtained by cell sorting and cultured with anti-CD3/CD28 Dynabeads and IL-2 (Methods) for 7–8 days (Fig. S3C). Post-culture analysis indicated significant Treg expansion (~30x, Fig. S3D) with yields ranging from 0.4 to 1.2×108 (Fig. 3D left panel) and maintenance of high FoxP3 levels (>98%) (Fig. 3D right panel). Expanded donor Tregs were then mixed with PBMC from a freshly thawed aliquot of the same donor and transplanted (1:1) into NSG mice. Recipients receiving PBMC from the mobilized donor without Tregs exhibited severe GVHD and did not survive >21 days post-transplant (Fig. 3E). In contrast, recipients of PBMC containing ex-vivo expanded Tregs had significantly diminished GVHD clinical scores and 100% survival through 5 weeks post-HSCT (Fig. 3E+F) as well as higher levels of hu CD4+FoxP3+ Tregs compared to recipients of mobilized PB without ex-vivo expanded Tregs (Fig 3G, left panel). Human CD45+ CD4 and CD8 T cells were present 1–2 weeks post-transplant in all mice, however, the frequency of CD4 and CD8 T cells was much higher in non-Treg treated recipients (Fig 3G, middle and right panel). Cell proliferation was assessed two weeks post-transplant and Tconv cells (CD4+FoxP3−) in animals receiving PBMC without added in vitro expanded donor Tregs exhibited elevated Ki67 expression compared to Tconv cells in Treg treated recipients which expressed barely detectable Ki67 (Fig. 3H). In total, recipients of allogeneic mobilized human PB without ex-vivo expanded donor Tregs contained higher levels of donor CD4 Tconv and CD8 T cells with increased Ki67 expression compared with recipients of human Tregs.
Concluding remarks
GVHD remains the major immunological complication preventing more wide-spread application of aHSCT. According to the CIBMTR, among adult recipients of matched related donor transplants as well as among adult recipients of unrelated donor transplants, mobilized PB cells is the most common graft type accounting for ~80% of all transplants through 2019 https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx). A number of labs including our own have been exploring potential applications of CD4+FoxP3+ T cells (Tregs) to ameliorate GVHD [1–15,25]. The ability to concomitantly mobilize stem cell donors and effectively augment the peripheral Treg compartment could represent a clinically useful advance. We have previously reported a two pathway Treg expansion strategy developed by our lab to amplify the suppressive effect of the donor inoculum and diminish GVHD in experimental transplant recipients [5,6]. The present studies established a procedure to concurrently induce stem/progenitor cell mobilization and Treg expansion in murine HSCT donors by infusion of TNFRSF25 agonistic fusion protein and IL-2LD into donors receiving G-CSF+/−plerixafor. The findings demonstrated that both the expanded Tregs and progenitor cells were functional as evidenced by survival and suppression of GVHD. It should be noted that at present, an FDA approved human anti-TNFRSF25mab is not available. Nonetheless addition of in vitro expanded human Tregs to mobilized human PB - as did mobilized mouse PB together with in vivo expanded mouse Tregs - suppress GVHD. Importantly, concomitant donor mouse treatment in vivo with rIL-2 and G-CSF did not provide GVHD protection nearly as effective as donors administered anti-TNFRSF25+CD25 agonists. Therefore, the use of IL-2 infusion alone while mobilizing human donors is not likely to be as effective to suppress GVHD. Accordingly, we posit that for translational purposes, co-administration of an FDA approved anti-TNFRSF25 together with a CD25 (IL-2) agonist would be required for donor treatment to produce PB that would optimally ameliorate GVHD. Nonetheless, the xenogeneic GVHD data also supported another potential translational strategy i.e., using ex-vivo expanded donor Tregs (anti-CD3 beads + hulL 2) added to mobilized human PB. Since prior studies did not identify any lingering phenotypic or pathologic changes in blood or tissues following the transient two pathway Treg cell expansion protocol employed here [5], we posit there is potential for administering anti-TNFRSF25 and anti-CD25 reagents in vivo to manipulate the donor – and potentially the host - CD4+FoxP3+ compartment as a novel approach for GVHD prophylaxis [37,38].
Supplementary Material
Highlights.
Donor peripheral blood in mice undergoing stem/progenitor cell mobilization can be concurrently treated in vivo with reagents targeting and expanding their Treg compartment.
Use of this PB for MHC-matched and in MHC mismatched HSCT transplants ameliorated GVHD while maintaining GVT.
Addback of ex-vivo expanded donor human Tregs from mobilized PB to the same mobilized PB donor resulted in marked reduction of xenogeneic GVHD.
ACKNOWLEDGMENTS:
The authors would like to acknowledge the support of the SCCC Flow Cytometry Core and the Flow Cytometry Core Facility at Diabetes Research Institute (Dr. Oliver Umland). We also thank the staff at the Division of Veterinary Resources at the University of Miami Miller School of Medicine for their outstanding animal maintenance and care of the mice used in these studies, we would thank Drs Stephen Nimer and Na Man for providing the construct to induce MLL-AF9 tumor cells in B6 mice used in our GVL experiments. The authors also thank Brandon Kale, MD and Clarisel Lozano for help with injection of the animals in some of these experiments.
Financial disclosure:
This work was supported by funds from the National Institutes of Health (R01 EY024484-06, R01 EY030283-01, R41 AI149916-01, to M.S and R.B.L.; R01 AI31648 to T.R.M.; and R01 CA208634 to R.J. K00CA245728 to C.S.B. and SC is the recipient of a ASTCT New Investigator Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding bodies were not involved in the study design; collection, analysis, and interpretation of data; manuscript preparation; or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: RBL is a compensated consultant/advisory board member for and equity holder in NightHawk Biosciences, Inc. (formally Heat Biologics, Inc.). KVK is an ad hoc consultant for Janssen, Novartis, Genentech/Roche, Kite, Takeda, Iovance, United Healthcare, Avacta Therapeutics and Kiadis. None are directly relevant to the efforts of this study. MMS, RRJ and JW are, or were former, employees of NightHawk Biosciences, Heat Biologics and/or Pelican Therapeutics during the collection of such data that constituted this research report. GRH has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, Neoleukin Therapeutics, Commonwealth Serum Laboratories, Cynata Therapeutics and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, NightHawk Biosciences, Inc. (formally Heat Biologics, Inc.), Laevoroc Oncology and iTeos Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. TRM no conflict. RJ no conflict.
Data availability:
Data reported in this article will be shared under the terms of a Data Use Agreement and may be used only for approved proposals. Requests may be made to hbarreras@med.miami.edu
References
- 1.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood 105 (2005) 1828–36. [DOI] [PubMed] [Google Scholar]
- 2.Shatry A, Levy RB, In situ activation and expansion of host tregs: a new approach to enhance donor chimerism and stable engraftment in major histocompatibility complex-matched allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 15 (2009) 785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, and Wagner JE, Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127 (2016) 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velard Ai, Aversa F, Martelli MF, Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117 (2011) 3921–8. [DOI] [PubMed] [Google Scholar]
- 5.Wolf D, Barreras H, Bader CS, Copsel SN, Lightbourn CO, Pfeiffer BJ, Altman NH, Podack ER, Komanduri KV, and Levy RB. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 23 (2017) 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copsel SN, Wolf D, Kale B, Barreras H, Lightbourn CO, Bader CS, Alperstein W, Altman NH, Komanduri KV, Levy RB. Very Low Numbers of CD4(+) FoxP3(+) Tregs Expanded in Donors via TL1A-Ig and Low-Dose IL-2 Exhibit a Distinct Activation/Functional Profile and Suppress GVHD in a Preclinical Model. Biol Blood Marrow Transplant 24 (2018) 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PA, Lees CJ, Blazar BR, The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99 (2002) 3493–9. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S, Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 196 (2002) 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9 (2003) 1144–50. [DOI] [PubMed] [Google Scholar]
- 10.Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol 4 (2013) 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, and Cohen JL, Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest 112 (2003) 1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, and Shlomchik MJ, Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood 104 (2004) 1565–73. [DOI] [PubMed] [Google Scholar]
- 13.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, and Soiffer RJ, Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365 (2011) 2055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VY, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ, Ritz J. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5 (2013) 179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, Wagner JE, Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127 (2016) 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copsel SN, Wolf D, Komanduri KV, and Levy RB. The promise of CD4(+)FoxP3(+) regulatory T-cell manipulation in vivo: applications for allogeneic hematopoietic stem cell transplantation. Haematologica 104 (2019) 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheridan WP, Begley CG, Juttner CA et al. Effect of peripheral blood progenitor cells mobilized by filgrastim (GCSF) on platelet recovery after high dose chemotherapy. Lancet 1992; 339: 640–644. [DOI] [PubMed] [Google Scholar]
- 18.Hoglund M, Smedmyr B, Simonsson B et al. Dose dependent mobilization of haematopoietic progenitor cells in healthy volunteers receiving glycosylateuG-CSF. Bone Marrow Transplant 1996; 18: 19–27. [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, and Bendall LJ (2003). Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J. Clin. Invest 111, 187–196. doi: 10.1172/JCI15994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashen Amanda, Lopez Sandra, Gao Feng, Calandra Gary, MacFarland Ron, Badel Karin, DiPersio John. Clinical Trial Biol Blood Marrow Transplant 2008. Nov;14(11):1253–61. doi: 10.1016/j.bbmt.2008.08.011. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood, 100 (2002), pp. 761–767. [DOI] [PubMed] [Google Scholar]
- 23.Hopman Rusudan K. and DiPersio John F. Advances in Stem Cell Mobilization Blood Rev. 2014. Jan; 28(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice J Exp Med, 208 (2) (Feb. 14, 2011), pp. 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf D, Bader CS, Barreras H, Copsel S, Pfeiffer BJ, Lightbourn CO, Altman NH, Komanduri KV, Levy RB. JCI Insight. Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models. Journal of Clinical Investigation Insight 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bader CS, Barreras H, Lightbourn CO, Copsel SN, Wolf D, Meng J, Ahn J, Komanduri KV, Blazar BR, Jin L, Barber GN, Roy S, Levy RB. STING differentially regulates experimental GVHD mediated by CD8 versus CD4 T cell subsets Sci Transl Med. 2020. Jul 15;12(552):eaay5006. doi: 10.1126/scitranslmed.aay5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–9. [PubMed] [Google Scholar]
- 28.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft versus-host disease phenotype. J Immunol. 2004;173:5467–75. [DOI] [PubMed] [Google Scholar]
- 29.Khan SK, Tsai MS, Schreiber TH, Wolf D, Deyev VV, and Podack ER, Cloning, expression, and functional characterization of TL1A-Ig. J Immunol 190 (2013) 1540–50 [DOI] [PubMed] [Google Scholar]
- 30.Schreiber TH, Wolf D, Tsai MSi, Chirinos J, Deyev VV, Gonzalez L, Malek TR Levy RB, Podack ER. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. (2010) Oct;120(10):3629–40. doi: 10.1172/JCI42933. Epub 2010 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marfil-Garza BA, Pawlick RL, Szeto J, Kroger C, Tahiliani V, Hefler J, Dadheech N, Seavey MM, Wolf J, Jasuja RJ, Shapiro AMJ, Am J Transplant. Tumor necrosis factor receptor superfamily member 25 (TNFRSF25) agonists in islet transplantation: Endogenous in vivo regulatory T cell expansion promotes prolonged allograft survival 2022. Apr;22(4):1101–1114. doi: 10.1111/ajt.16940. Epub 2022 Jan 12. [DOI] [PubMed] [Google Scholar]
- 32.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs Prevent GVHD and Promote Immune Reconstitution in HLA-haploidentical Transplantation. Blood (2011) 117(14):3921–8. doi: 10.1182/blood-2010-10-311894 [DOI] [PubMed] [Google Scholar]
- 33.Marttelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse Blood (2014) 124(4):638–44. doi: 10.1182/blood-2014-03-564401 [DOI] [PubMed] [Google Scholar]
- 34.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical Cord Blood-Derived T Regulatory Cells to Prevent GVHD: Kinetics, Toxicity Profile, and Clinical Effect. Blood (2016) 127(8):1044–51. doi: 10.1182/blood-2015-06-653667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer EH, Laport G, Xie BJ, MacDonald K, Heydari K, Sahaf B, et al. Transplantation of Donor Grafts With Defined Ratio of Conventional and Regulatory T Cells in HLA-Matched Recipients. JCI Insight (2019) 4(10): e127244. doi: 10.1172/jci.insight.127244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierini A, Ruggeri L, Carotti A, Falzetti F, Saldi S, Terenzi A, et al. Haploidentical Age-Adapted Myeloablative Transplant and Regulatory and Effector T Cells for Acute Myeloid Leukemia. Blood Adv (2021) 5 (5):1199–208. doi: 10.1182/bloodadvances.2020003739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito S, Bollard CM, Carlsten M, et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol Ther. 2014;22(7):1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copsel SN, Wolf D, Pfeiffer B, Barreras H, Perez VL, Levy RB. Recipient Tregs: Can They Be Exploited for Successful Hematopoietic Stem Cell Transplant Outcomes? Front Immunol. 2022. Jun 21;13:932527. doi: 10.3389/fimmu.2022.932527. eCollection 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this article will be shared under the terms of a Data Use Agreement and may be used only for approved proposals. Requests may be made to hbarreras@med.miami.edu


