Abstract
Due to rapid technology advancement and increasing diagnostic expertise, fetal medicine is rapidly improving. Prenatal diagnostic advancements made it possible to identify structural abnormalities in fetuses as early as the first trimester of pregnancy. However, to validate the echocardiographic diagnosis that led to the pregnancy termination, the termination of pregnancy owing to severe fetal deformities should be audited in accordance with a correct anatomic diagnosis. Following the PRISMA declaration, a systematic literature search was done to find articles on post-mortem first trimester human fetal heart evaluation. Thirteen suitable studies were found using the search method. It is theoretically possible to examine the human fetal heart after death in early pregnancy however these methods are not widely available due the costs associated with the procedure and the equipment, the effects of tissue coloration and distortion brought on by the fixation and contrasting processes (for micro-CT), the current requirement for a skilled operator to acquire, reconstruct, and process the images, and data storage requirements greater than those of conventional clinical scans.
Keywords: Postmortem first trimester fetal heart , virtual autopsy , congenital heart disease
Introduction
The field of obstetrics-gynecology is seeing an increase use of the first-trimester anomaly scans as a result of recent improvements in ultrasound expertise, training, and equipment.
The advantages of early major malformation detection followed by early medical termination of pregnancy are well known because, in contrast to second trimester termination of pregnancy, it not only reduces the likelihood of complications but also has a positive effect on the patient's wellbeing [1, 2].
One of the most common congenital anomalies, heart problems affect about 1% of live newborns and a greater percentage of fetuses [3, 4].
Congenital heart disease (CHD) in utero is often detected between 12 and 84 percent of the time which denotes a moderate sensitivity [5, 6].
Early identification of fetal heart anomalies is preferred and possible [7, 8], since it has been demonstrated that CHDs are associated with genetic abnormalities [9, 10].
A post-mortem examination of the fetal heart is essential when cardiac defects are suspected, in the event of a termination of pregnancy (TOP) or fetal death, for family counselling, as well as for scholarly and research objectives.
However, conventional autopsy is limited by the small size of the heart, the complexity of the cardiac structure, the wide variety of cardiac abnormalities, and can be greatly influenced by the pathologist's experience [11, 12].
Correct fetal heart macroscopic sectioning is crucial to a successful evaluation.
As mentioned by Albu et al. [13], an oblique macroscopic sectioning might result in the under-or over-diagnosis of pathologic lesions and the insufficient visibility of numerous structures.
Imagistic methods of confirmation have been studied as well as other histopathological techniques.
The aim of this paper is to systematically review these methods used for postmortem fetal heart evaluation.
Materials and Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement was followed.
Searches were performed in PubMed, Google Scholar and Medline databases for English language studies until June 2022.
Post-mortem evaluation of first trimester fetal heart studies were searched and the results were manually check by two investigators.
The check phase was performed individually to ensure the inclusion criteria were fulfilled.
If a study did not meet all of the inclusion criteria, it was excluded from the analysis.
Eligibility Criteria
Primary studies, which included any method of first trimester fetal heart evaluation, were selected.
Studies which included first, second and/or third trimester were included, however only first trimester cases were extracted for analysis.
Whole fetal body studies were included only if the heart was examined.
When the investigators encountered multiple publications from the same team with the same study specimens, only one publication was selected.
Reviews, conference abstracts, posters as well as animal studies were excluded.
Data Extraction and Tabulation
The following study characteristics were extrapolated for comparison and tabulated depending on the confirmation method: number of specimens; type of specimen; number of first trimester cases and number of first trimester CHD cases; type of specimen conservation; type of specimen preparation; technical properties for acquisition; availability of comparison with other methods of confirmation.
Data Analysis
No direct comparison (in the form of a meta-analysis) between these approaches is made, as a result of the variety in the methodologies used in the published research.
Descriptive analysis was therefore employed to describe the results of these methods divided by imaging modality.
Studies examining the application of micro-CT, UHF-MRI were presented using the extrapolated and tabulated data mentioned above.
Furthermore, we added a “varia” category for limited studies which used methods other than the ones mentioned above.
Results
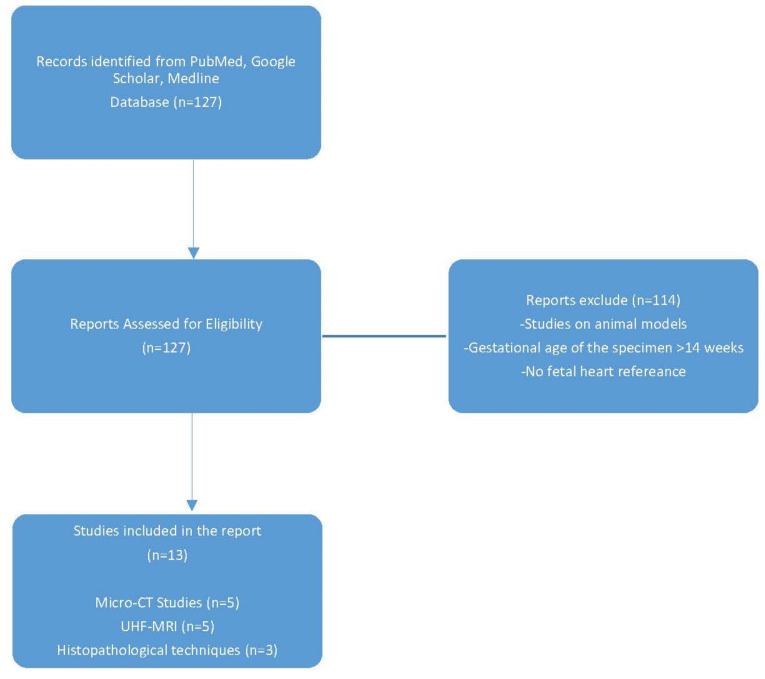
Out of 127 results from the literature search, 114 papers discarded after they were manually examined in accordance with inclusion and exclusion criteria.
Thirteen studies were included divided as following: 5 studies regarding Micro-CT, 5 studies about UHF-RMN and 3 which implied histopathological techniques (Figure 1).
Figure 1.
Study selection flow chart in this systematic review of postmortem evaluation of first trimester fetal heart.
Confirmation through Micro-CT
There were retrieved a total of 5 distinct papers on fetal heart imaging using micro-CT, which included cases with the gestational period from 8 to 35 weeks.
Except the study conducted by Sandaite et al. [14], where it was limited to the first trimester, all of the other studies focused on late first trimester and early second trimester.
Two of the papers discussed whole fetal imaging, and three discussed the fetal heart independently or coupled with the lungs.
Four of the studies compared micro-CT results with autopsy however it was limited or not performed at all for first trimester cases (Table 1).
Table 1.
Micro-CT imaging literature each with the number of cases, first trimester cases and comparison complementary method. NM: not mentioned.
|
Study Ref. |
Number of Cases and Sample Type |
Gestational age (weeks) |
Number of first trimester cases (<14 weeks of gestation) |
CHD cases |
Comparison |
|
Lombardi CM 2014 |
21; 7 entire specimens, 14 isolated hearts |
7w-17w entire specimen, 11w-22w isolated hearts |
7 |
0 |
conventional dissection |
|
Hutchinson JC 2018 |
20; entire specimens |
11w-20w |
11 |
1 |
autopsy |
|
Sandrini C 2019 |
21; 19 hearts; 2 heart+lungs |
12w-20w |
5 |
4 |
autopsy |
|
Shelmerdine S.C 2020 |
268; entire specimens |
11w-24w |
NM |
6 |
autopsy/limited autopsy (when performed) |
|
Sandaite I 2020 |
49; isolated hearts |
8w-12w |
49 |
6 |
Not performed |
Staining is required when examining a soft tissue organ.
Three variables primarily affect staining success: specimen size, staining solution concentration, and staining time.
To reach the fetal body's core in larger specimens, the staining solution must penetrate deeper.
Time and staining concentration are related variables.
Shorter staining exposure durations are made possible by faster diffusion of the staining solution due to higher concentrations' increased osmotic pressure.
Extended exposure times, on the other hand, might cause overstaining and tissue shrinkage or loss of tissue differentiation (to ensure complete and even staining).
The method for staining preparation varied between authors and involved submerging the entire body or the fetal organ in the staining solution.
All of the studies included in this paper used Lugol as a staining agent.
According to the weight of the samples, studies described and applied a protocol with three distinct types of staining preparation (Lugol 25, 50, 50 percent; staining time 48, 48, 72h for samples of weight 1g, 1-2g, and>2g, respectively) [15, 16].
Staining period varied from 24h up to 7d and voxel size was similar throughout the studies, both factors being influenced by the weight and size of the specimen (Table 2).
Table 2.
Micro-CT imaging literature and data regarding fixation, staining protocol and image voxel size NM: not mentioned
|
Study Ref. |
Fixation protocol |
Staining protocol |
Voxel dimension µm |
||
|
Fixation agent |
Fixation duration |
Staining agent |
Staining duration |
||
|
Lombardi CM 2014 |
4% PFA |
4-7 days |
Lugol |
2-7d |
9, 18, 35 |
|
Hutchinson JC 2018 |
10% formalin |
72h |
Lugol |
72h |
7.4-51 |
|
Sandrini C 2019 |
10% formalin |
16-260 days |
Lugol |
72h |
9-18 |
|
Shelmerdine S.C 2020 |
10% formalin |
NM |
Lugol |
>96h |
18.6-121.7 |
|
Sandaite I 2020 |
10% formalin |
NM |
Lugol |
24h |
9 |
Confirmation through MRI
A total of 5 distinct papers on fetal heart imaging using MR, covering cases from 9 to 20 weeks of gestation.
The majority of the studies used a 9,4T magnet MR, while Staicu et al. [17] conducted their study with a 7T magnet MR.
Two studies assessed whole fetal imaging, two discussed the fetal heart independently and one study covered the head, thorax and the abdomen.
Four of the studies compared micro-CT results with autopsy however it was limited or not performed at all for first trimester cases.
Confirmation was performed with either stereomicroscopy [18, 19], conventional autopsy [20, 21] or a combination of these methods [17].
However, conventional autopsy in one study was employed in cases with a gestational age over 13 weeks and 6 days [20].
This resulted in the scanning and analyzing of first trimester cases without a complementary confirmation method (Table 3).
Table 3.
MR imaging literature each with the number of cases, first trimester cases and comparison complementary method. PFA: phosphotungstic acid; NM: not mentioned
|
Study Ref and field strength |
Number of cases and Sample Type |
Gestational age (weeks) |
Number of first trimester specimens |
Number of first trimester specimens with CHD |
Confirmation |
|
Tang 2021; 9,4T |
19 hearts |
12w+6d-19w |
11 |
11 |
conventional autopsy (>13+6 weeks) |
|
Staicu 2019; 7T |
9 Full body |
9w-13w |
9 |
9 |
stereomicroscopy and conventional histology |
|
Verhoye 2013; 9,4T |
9 Head, thorax, abdomen |
12w-20w |
5 |
1 |
conventional autopsy |
|
Votino 2012; 9,4T |
24 Heart |
11w-20w |
6 |
3 |
stereomicroscopy |
|
Thayyil 2009; 9,4T |
17 Full body |
11w-20w |
at least 1 |
0 |
conventional autopsy |
Fixation of the specimen was approached differently between the studies. Votino et al. [18] stored the specimens in-20°C.
According to their study, red blood cells were destroyed during the freeze-thaw procedure, resulting in a coating of hemosiderin along the walls of the great vessels; this provided good hypointense tissue contrast on T2-weighted images, as opposed to the hyperintense lumen filled with serum, which is a natural contrast agent.
This was helpful in the study of heart anatomy, in particular the outflow tracts.
Verhoye et al. [21] reported similar findings in their study.
Formalin fixation before scanning reported by Staicu et al. allowed acquisition of images of appropriate quality for clinical interpretation.
Furthermore, formalin fixation did not significantly alter the acquisition or relaxation time T2, which allowed scanning of the same samples at various times with the same conditions and relaxation times.
Imaging resolution is crucial for the detection and characterization of the smallest anatomical features in addition to picture contrast.
Full body datasets with a respectable resolution of 200-m isotropic voxel size were accomplished by Thayyil et al. [22] in 70min.
Several researchers were able to scan organs partially with fewer slices and higher resolution in shorter scan times, allowing them to use small, sensitive RF-coils [20, 21] (Table 4).
Table 4.
MR imaging literature and data regarding fixation, staining protocol and image voxel size NM: not mentioned
|
Study Ref and field strength |
Fixation protocol |
Scanning protocol |
||||
|
Fixation agent |
Fixation duration |
T(ms) |
TE(ms) |
FOV(mm) |
Matrix size (mm3) |
|
|
Tang 2021; 9,4T |
10% formalin and storage in 4°C |
>2 weeks |
6 |
2.9 |
32x 32mm |
256x256x(80-120) |
|
Staicu 2019; 7T |
10% formalin |
<1 week |
12.7-3441.8 |
6.4-36 |
5×3.5 |
384×384 |
|
Verhoye 2013; 9,4T |
Storage in-20 °C |
1 month |
2500-7632 |
33-44 |
20-50 |
256×256 |
|
256×128×96 | ||||||
|
256×256 | ||||||
|
Votino 2012; 9,4T |
Storage in-20 °C |
2-4 months |
2500 |
33, 42.5 |
20×13×13, 33×33×33 |
256×128×80 |
|
Thayyil 2009; 9,4T |
Storage in 4 °C |
NM |
500 |
120 |
100×50×50 |
512×256×256 |
One should be aware of the negative effects on signal-to-noise ratio (SNR) and consequently image quality as well as the rise in scan time needed with decreasing voxel size.
Confirmation through other techniques
Other techniques aimed at examining the early gestation fetal heart have been studied, however due to the small number of research papers, we will include them in this category.
These are mainly histopathological techniques which use different methods of staining, embedding, slicing and scanning of each slice before reconstructing them in 3D.
Episcopic fluorescence image capture (EFIC) is a histopathological technique where the block face is imaged using tissue autofluorescence before cutting each segment.
The acquired 2D resolution is comparable to that of histology, and such 2D picture stacks are easily reconstructed in 3D.
Dhanantwari et al. [23] stored the embryos in 10% phosphate-buffered formalin before dehydration and embedding in a combination of paraffin wax (70.4 percent), Vybar (24.9 percent), stearic acid (4.4 percent), and red aniline dye Sudan IV (0.4 percent).
After that, slices between 5 and 8 microns thick were cut from the implanted embryos while the block face was progressively photographed under epifluorescent illumination.
The study included 52 embryos with a gestation age of 6w-9w and the aim was to evaluate the cardiac development in the first trimester.
High-resolution episcopic microscopy (HREM), unlike EFIC, requires the specimen to be stained with a fluorescent dye before whole specimen slicing and scanning.
Gindes el al. [24] used HREM to evaluate 30 isolated normal hearts from fetuses with a gestational age between 9w-14w+6 days, without mentioning how many cases were <14weeks of gestation.
Finally, three-dimensional reconstruction from histological slides has been performed. Ruican et al. [25] described a protocol where after specimen embedding, the paraphin block was sliced and all of the slices were stained using classical Hematoxilin-Eozin (HE) stain.
The slides were scanned and all of the images were process and reconstructed using a 3D software.
The study included 5 normal first trimester fetal heart.
Discussions
A number of problems need to be resolved before micro-CT and UHF-MR can be used for large scale scanning.
These include, but are not limited to, the costs associated with the procedure and the equipment, the effects of tissue coloration and distortion brought on by the fixation and contrasting processes (for micro-CT), the current requirement for a skilled operator to acquire, reconstruct, and process the images, and data storage requirements orders of magnitude greater than those of conventional clinical scans.
Furthermore, since a 9.4 T magnet MRI is a research device and not suited for routine medical imaging, it is not accessible at many centers.
This restricts its use and accessibility as a result.
One choice would be to preserve the fetus until the access is possible so that it can be scanned.
However, this is only feasible for research purposes and not for clinical practice to improve couple counselling.
Osmium tetroxide and phosphotungstic acid have both been investigated as potential substitutes for micro-CT in the imaging of soft tissues [26].
Excellent soft-tissue contrast is provided by osmium tetroxide, but it is costly, necessitates a lengthy diffusion period in fetal specimens, and is highly poisonous, requiring specialized disposal of waste osmium [27].
Although more expensive, phosphotungstic acid takes up to 12 days to completely stain adult mouse hearts, has reduced tissue shrinkage during staining over popular iodine-based methods [28].
Furthermore, a water-based solution determines the least amount of shrinkage in bone, muscle and brain tissue [29].
I2KI is easily accessible, stable, and has a low cost.
Furthermore, it also has a low level of toxicity and can diffuse through soft tissue samples with a thickness of up to a few centimeters.
When utilizing an iodine-based dye, further standard autopsy and histological investigation of fetal material is still possible after staining (e.g., Lugol).
However, since the staining solution gives the specimen a reddish-brown appearance, de-staining is required.
Future studies should look at the application of various staining agents for imaging human fetal tissue.
Although UHF-MRI does not need exogenous contrast agents, it has the disadvantage of taking a long 20-78h to complete a scan in order to attain 35-55m resolution.
Micro-CT has the drawback of requiring iodine contrast staining for soft-tissue distinction, which could take up to 14 days for patients weighing 300g, but it also has the benefit of requiring only 45 minutes of scanning time to reach 15 to 90m resolution.
Using a gadolinium-based contrast agent may be beneficial for MR exploration because the contrast agent decreases the T1 relaxation which in terms, decreases TR and, as a result, more signal averages within the same scanning time [30].
There are, however, no studies regarding the use of gadolinium-based contrast agent in fetal heart MR examination.
To avoid tissue autolysis and sample motion in ex vivo MRI investigations with lengthy acquisition durations, the sample is often chemically fixed and embedded in either agarose or Fomblin.
As shown by Hales et al. [31], this might change tissue structure and MRI characteristics.
Therefore, while evaluating changes in cardiac microstructure caused by CVDs, it is important to take into account the possibility that the use of particular chemicals for tissue fixation and embedding may modify tissue characteristics.
Further histological examination of the specimen may be compromised by this.
Staining is required when HREM is used for confirmation, however the procedure is easier to perform.
The specimens are harvested and prepared according to classical histology.
They are stained with eosin red mixes during the dehydration process, and eosin-or eosin/acridine-orange-dyed resin (JB4) is utilized for infiltration and as an embedding medium [32, 33].
Three-dimensional reconstruction from histological slides requires staining with classic HE [25] while episcopic fluorescence image capture require special embedding materials [23].
Given that maintaining the integrity of the fetal body is essential to certain parents, it is obvious that the imaging techniques have the benefit of not changing the look of the fetal body [19].
Consequently, unlike the histopathological techniques, MRI and micro-CT preserve fetal integrity while enabling a reliable description of fetal anatomical defects.
Regarding imaging quality, there is no study which directly compares Micro-CT with UHF-MR.
As mentioned above, Micro-CT has some advantages over UHF-MRI in terms of resolution and scanning time, however micro-CT has the disadvantage that it frequently needs days of staining to create soft-tissue contrast while MRI is performed without any preparation.
While using UHF-MRI, a voxel size of 35-55µm requires 20 to 78 hours of scanning time, doing so with micro-CT takes less than an hour.
Normal first trimester fetal hearts anatomy has been confirmed with the visualization of all anatomical elements using the histological techniques [23, 24, 25].
However, these techniques are invasive methods which slice the entire fetal heart.
Conclusions
It is necessary to examine the fetal heart post-mortem in order to validate prenatal results and provide family counselling.
In addition, 3D reconstruction of datasets and their application enable numerous picture assessments at various periods, multidisciplinary comparison on challenging instances, and research possibilities in the biological and bioengineering domains.
Due to the small number and diversity of methodologies utilized in published studies, a clear statistical interpretation of data from the literature regarding the diagnostic accuracy of micro-CT and UHF-MRI compared to autopsy is not feasible.
Both micro-CT and UHF-MRI are great imaging methods for capturing precise pictures of the human fetus's heart in the first trimester when non-invasive.
Histological techniques have proven their accuracy regarding normal fetal heart anatomy.
However, accurate interpretation of these high-resolution pictures requires the coordinated efforts of skilled radiologists, pathologists, anatomists, and other clinicians.
Conflict of interests
The authors have no conflict of interest to declare.
References
- 1.Garofalo G, Garofalo A, Sochirca O, Alemanno MG, Pilloni E, Biolcati M, Muccinelli E, Viora E, Todros T. Maternal outcomes in first and second trimester termination of pregnancy: which are the risk factors. J Perinat Med. 2018;46(4):373–378. doi: 10.1515/jpm-2017-0106. [DOI] [PubMed] [Google Scholar]
- 2.Zhu R, Gan L, Wang S, Duan H. A cohort study comparing the severity and outcome of intrauterine adhesiolysis for Asherman syndrome after first-or second-trimester termination of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2019;238:49–53. doi: 10.1016/j.ejogrb.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Hunter LE, Simpson JM. Prenatal screening for structural congenital heart disease. Nat Rev Cardiol. 2014;11(6):323–334. doi: 10.1038/nrcardio.2014.34. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 5.Hafner E, Schuchter K, Liebhart E, Philipp K. Results of routine fetal nuchal translucency measurement at weeks 10-13 in 4233 unselected pregnant women. Prenatal Diagnosis. 1998;18(1):29–34. [PubMed] [Google Scholar]
- 6.Becker R, Wegner RD. Detailed screening for fetal anomalies and cardiac defects at the 11-13-week scan. Ultrasound Obstet Gynecol. 2006;27(6):613–618. doi: 10.1002/uog.2709. [DOI] [PubMed] [Google Scholar]
- 7.Iliescu D, Tudorache S, Comanescu A, Antsaklis P, Cotarcea S, Novac L, Cernea N, Antsaklis A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound in Obstetrics&Gynecology. 2013;42(3):300–309. doi: 10.1002/uog.12489. [DOI] [PubMed] [Google Scholar]
- 8.Syngelaki A, Hammami A, Bower S, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11-13 weeks' gestation. Ultrasound Obstet Gynecol. 2019;54(4):468–476. doi: 10.1002/uog.20844. [DOI] [PubMed] [Google Scholar]
- 9.Simmons MA, Brueckner M. The genetics of congenital heart disease understanding and improving long-term outcomes in congenital heart disease: a review for the general cardiologist and primary care physician. Curr Opin Pediatr. 2017;29(5):520–528. doi: 10.1097/MOP.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit, Srebniak MI, Govaerts LC, Van Opstal, Galjaard RJ, Go AT. Additional value of prenatal genomic array testing in fetuses with isolated structural ultrasound abnormalities and a normal karyotype: a systematic review of the literature. Ultrasound Obstet Gynecol. 2014;43(2):139–146. doi: 10.1002/uog.12575. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugasundaram S, Venkataswamy C, Gurusamy U. Pathologist's role in identifying cardiac defects-a fetal autopsy series. Cardiovascular Pathology. 2021;51:107312–107312. doi: 10.1016/j.carpath.2020.107312. [DOI] [PubMed] [Google Scholar]
- 12. Ludwig J. Handbook of Autopsy Practice. 2002 . [Google Scholar]
- 13.Albu C, Staicu A, Popa-Stanilă R, Bondor C, Pop B, Chiriac L, Gheban D, Micu R, Turcu RVF, Simon S, Crișan D, Stamatian F. The Evaluation of the Four-Chamber Cardiac Dissection Method of the Fetal Heart as an Alternative to Conventional Inflow-Outflow Dissection in Small Gestational-Age Fetuses. Diagnostics. 2022;12(1):223–223. doi: 10.3390/diagnostics12010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandaite I, Lombardi C, Cook AC, Fabietti I, Deprest J, Boito S. Micro-computed tomography of isolated fetal hearts following termination of pregnancy: A feasibility study at 8 to 12 weeks' gestation. Prenatal Diagnosis. 2020;40(8):984–990. doi: 10.1002/pd.5719. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi CM, Zambelli V, Botta G, Moltrasio F, Cattoretti G, Lucchini V, Fesslova V, Cuttin MS. Postmortem microcomputed tomography (micro-CT) of small fetuses and hearts. Ultrasound Obstet Gynecol. 2014;44(5):600–609. doi: 10.1002/uog.13330. [DOI] [PubMed] [Google Scholar]
- 16.Sandrini C, Boito S, Lombardi CM, Lombardi S. Postmortem Micro-CT of Human Fetal Heart-A Systematic Literature Review. Journal of Clinical Medicine. 2021;10(20):4726–4726. doi: 10.3390/jcm10204726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staicu A, Albu C, Popa-Stanila R, Chiriac L, Boitor-Borza D, Bondor C, Kovacs T, Caracostea G, Rotar I, Turcu R, Simon S, Muresan D, Stamatian F. Potential clinical benefits and limitations of fetal virtopsy using high-field MRI at 7 Tesla versus stereomicroscopic autopsy to assess first trimester fetuses. Prenatal Diagnosis. 2019;39(7):505–518. doi: 10.1002/pd.5457. [DOI] [PubMed] [Google Scholar]
- 18.Votino C, Jani J, Verhoye M, Bessieres B, Fierens Y, Segers V, Vorsselmans A, Kang X, Cos T, Foulon W, De Mey, Cannie M. Postmortem examination of human fetal hearts at or below 20 weeks' gestation: a comparison of high-field MRI at 9.4 T with lower-field MRI magnets and stereomicroscopic autopsy. Ultrasound Obstet Gynecol. 2012;40(4):437–444. doi: 10.1002/uog.11191. [DOI] [PubMed] [Google Scholar]
- 19.Thayyil S, Sebire NJ, Chitty LS, Wade A, Olsen O, Gunny RS, Offiah A, Saunders DE, Owens CM, Chong WKK, Robertson NJ, Taylor AM. Post mortem magnetic resonance imaging in the fetus, infant and child: A comparative study with conventional autopsy (MaRIAS Protocol) BMC Pediatrics. 2011;11(1):120–120. doi: 10.1186/1471-2431-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang H, Zhang Y, Dai C, Ru T, Li J, Chen J, Zhang B, Zhou K, Lv P, Liu R, Zhou Q, Zheng M. Postmortem 9.4-T MRI for Fetuses with Congenital Heart Defects Diagnosed in the First Trimester. Frontiers in Cardiovascular Medicine. 2022;8 doi: 10.3389/fcvm.2021.764587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhoye M, Votino C, Cannie MM, Segers V, Mabiglia C, Cos T, Lipombi D, Jani JC. Post-mortem high-field magnetic resonance imaging: effect or various factors. The Journal of Maternal-Fetal&Neonatal Medicine. 2013;26(11):1060–1065. doi: 10.3109/14767058.2013.767891. [DOI] [PubMed] [Google Scholar]
- 22.Thayyil S, Cleary JO, Sebire NJ, Scott RJ, Chong K, Gunny R, Owens CM, Olsen OE, Offiah AC, Parks HG, Chitty LS, Price AN, Yousry TA, Robertson NJ, Lythgoe MF, Taylor AM. Post-mortem examination of human fetuses: a comparison of whole-body high-field MRI at 9.4 T with conventional MRI and invasive autopsy. Lancet. 2009;374(9688):467–475. doi: 10.1016/S0140-6736(09)60913-2. [DOI] [PubMed] [Google Scholar]
- 23.Dhanantwari P, Lee E, Krishnan A, Samtani R, Yamada S, Anderson S, Lockett E, Donofrio M, Shiota K, Leatherbury L, Lo CW. Human Cardiac Development in the First Trimester. Circulation. 2009;120(4):343–351. doi: 10.1161/CIRCULATIONAHA.108.796698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gindes L, Matsui H, Achiron R, Mohun T, Ho SY, Gardiner H. Comparison of ex-vivo high-resolution episcopic microscopy with in-vivo four-dimensional high-resolution transvaginal sonography of the first-trimester fetal heart. Ultrasound in Obstetrics&Gynecology. 2012;39(2):196–202. doi: 10.1002/uog.9068. [DOI] [PubMed] [Google Scholar]
- 25.Ruican D, Petrescu AM, Ungureanu AL, Marinaş MC, Pirici D, Istrate-Ofiţeru AM, Roşu GC, Badiu AM, Simionescu CE, Şerbănescu MS, Zorilă GL, Belciug S, Iliescu DG. Virtual autopsy and confirmation of normal fetal heart anatomy in the first trimester using three-dimensional (3D) reconstruction of histological sections. Rom J Morphol Embryol. 2021;62(1):101–108. doi: 10.47162/RJME.62.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JT, Hansen MS, Wu I, Healy LJ, Johnson CR, Jones GM, Capecchi MR, Keller C. Virtual histology of transgenic mouse embryos for high-throughput phenotyping. PLoS Genet. 2006;2(4):e61–e61. doi: 10.1371/journal.pgen.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvard Environmental Health&Safety, Lab Safety Guidelines&SOPs. Available from: https://www.ehs.harvard.edu/programs/lab-safety-guidelines-sops .
- 28.Lesciotto KM, Perrine SMM, Kawasaki M, Stecko T, Ryan TM, Kawasaki K, Richtsmeier JT. Phosphotungstic acid enhanced microCT: optimized protocols for embryonic and early postnatal mice. Dev Dyn. 2020;249(4):573–585. doi: 10.1002/dvdy.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buytaert J, Goyens J, De Greef, Aerts P, Dirckx J. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-CT and light sheet fluorescence microscopy (LSFM) Microsc Microanal. 2014;20(4):1208–1217. doi: 10.1017/S1431927614001329. [DOI] [PubMed] [Google Scholar]
- 30.Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. NeuroImage. 2012;62(3):1848–1856. doi: 10.1016/j.neuroimage.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales PW, Schneider JE, Burton RAB, Wright BJ, Bollensdorff C, Kohl P. Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI. Prog Biophys Mol Biol. 2012;110(2-3):319–330. doi: 10.1016/j.pbiomolbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geyer SH, Weninger WJ. High-Resolution Episcopic Microscopy (HREM): Looking Back on 13 Years of Successful Generation of Digital Volume Data of Organic Material for 3D Visualisation and 3D Display. Applied Sciences. 2019;9(18):3826–3826. [Google Scholar]
- 33.Weninger WJ, Geyer SH, Mohun TJ, Rasskin-Gutman D, Matsui T, Ribeiro I, Costa LdF, Izpisúa-Belmonte JC, Müller GB. High-resolution episcopic microscopy: a rapid technique for high detailed 3D analysis of gene activity in the context of tissue architecture and morphology. Anat Embryol (Berl) 2006;211(3):213–221. doi: 10.1007/s00429-005-0073-x. [DOI] [PubMed] [Google Scholar]