Abstract
Background and Aims
Poor sleep may be prospectively associated with worse disease course in inflammatory bowel disease (IBD). Chronic insomnia is the most common cause of poor sleep complaints in IBD and is theorized to be maintained by dysfunctional thoughts and behavioral patterns. However, data characterizing patterns specific to insomnia in IBD are lacking. Understanding the nuances of insomnia and patients’ preferences for treatment is critical for addressing this significant comorbidity in IBD.
Methods
We conducted an anonymous, mixed-method online survey of people with IBD and asked questions about sleep patterns, thoughts, and behaviors related to sleep, treatment preferences, and barriers to treatment.
Results
312 participants (60.9% Crohn’s, 66.3% women, mean age of 48.62 years) were included in this study. Participants with insomnia were significantly more concerned about the consequences of sleep loss, felt more helpless about their sleep, and were more likely to engage in behaviors known to perpetuate insomnia (e.g., spending time in bed in pain; ps ≤ 0.001) than those without insomnia. 70.3% of participants were interested in discussing sleep as part of IBD care, 63.5% were interested in receiving sleep recommendations from their gastroenterologist, and 84.6% of those with insomnia were interested in participating in sleep treatments.
Conclusion
Participants with IBD and insomnia are interested in treatment and reported patterns that can be targeted in Cognitive Behavioral Therapy for Insomnia, as opposed to traditional sleep hygiene guidelines. Additionally, people with insomnia engaged in several sleep-interfering behaviors related to pain. Clinical trials that target insomnia in people with IBD should include pain management in the intervention.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Sleep, Pain, Psychogastroenterology
Introduction
Poor sleep is common in inflammatory bowel disease (IBD) and emerging data indicate that it may be prospectively associated with increased risk for disease flare, surgery, and hospitalization [1–5]. We and other authors have demonstrated that while poor sleep is reflective of a variety of different sleep disorders, it is most often indicative of insomnia—difficulty falling asleep, staying asleep, or early morning awakening combined with daytime impairment [6, 7]. Sleep problems are also uniquely associated with poor quality of life in IBD and people with IBD believe that sleep impacts and/or worsens gastrointestinal (GI) symptoms [3, 8, 9]. Thus, addressing insomnia in people with IBD is of importance for improving quality of life, disease severity, and disease course.
Cognitive Behavioral Therapy for Insomnia (CBT-I) is the recommended first-line treatment for insomnia by the American College of Physicians [10] and following treatment, remission rates are typically around 60% [11]. However, as we have demonstrated previously, IBD-related sleep disruptions contribute significantly to insomnia symptoms. In particular, bowel movements at night, IBD-specific pain, other kinds of pain, and worries about IBD symptoms are common disruptions [7]. Due to these disease-specific nighttime symptoms, we cannot assume that the types of cognitive and behavioral factors thought to drive insomnia in the general population are present in IBD. Specific information on the types of thoughts and behaviors associated with insomnia in IBD is needed to appropriately tailor CBT-I treatment to patients with IBD. Thus, herein we draw upon the same dataset [7] using both qualitative and quantitative data to more deeply understand patients’ experiences with insomnia and how providers can best support them. Specifically, we sought to identify (1) thought and behavior patterns that characterize insomnia in IBD, (2) participants’ perceptions of the relationship between sleep and IBD, (3) insomnia treatment areas of interest, and (4) possible barriers and facilitators of treatment. These results will be of critical importance as we adapt CBT-I to an IBD population and/or develop other treatments for sleep disorders co-morbid with IBD.
Materials and Methods
Participants
This cross-sectional mixed-method study included people with IBD who were recruited from an academic medical center in New Hampshire, United States.
Procedure
Procedures have been described in detail elsewhere [7] and thus, will be presented here in brief. Patients with IBD at Dartmouth–Hitchcock Medical Center were invited to participate in a survey on “sleep and pain in IBD” via a secure online patient messaging portal. They were offered the chance to win one of ten $100 Amazon gift cards if they participated. All data were collected anonymously, and survey records were not linked to medical records.
Measures
Demographics and Diagnostic History
Participants were queried on their demographic and health history information (age, sex, marital status, race, and current/past medical conditions), including age of onset of IBD symptoms and age of IBD diagnosis.
IBD Disease Activity
Patient-Reported Outcomes-3 [12] items were used to categorize active versus inactive disease for people with CD and UC. Participants rated their average stool frequency, abdominal pain, rectal bleeding, and general well-being for the previous 7 days. Responses from participants with UC were classified as active or inactive disease based on previously established scoring procedures [13]. Disease activity was classified based on the two items from the 6-point Mayo score and a third item on general well-being from the simple clinical colitis activity index. Standardized rules were applied to define Crohn’s disease status; those in remission were termed with “inactive” disease and anyone with current symptoms were “active” disease. Participants with “active” disease included those who endorsed a combination of significant levels of pain, loose stools, and/or generally feeling unwell. Specifics on how participants were categorized can be found in the initial paper from this dataset [7].
Insomnia Severity Index (ISI) [14]
This questionnaire includes 7 items assessing the severity of insomnia disorder symptoms. Items are rated from 0 to 4 and scores range from 0 to 28. Categories of insomnia include no clinically significant insomnia (0–7), subthreshold insomnia (8–14), moderate clinical insomnia (15–21), and severe clinical insomnia (22–28). Scores ≥ 10 are consistent with an Insomnia Disorder diagnosis with a sensitivity of 86.1% and specificity of 87.7% [15]. Internal consistency was good (α = 0.88).
Dysfunctional Beliefs and Attitudes About Sleep (DBAS) [16]
This 16-item questionnaire assesses the degree to which people hold problematic beliefs about their sleep (e.g., beliefs about sleep need, the detriment of not sleeping well) and/or worry about their sleep patterns. Items are rated from 0 (strongly disagree) to 10 (strongly agree) and can be broken down into four factors—consequences of insomnia, worry/helplessness, expectations, and medication/biological attributions. Items are averaged for a total score ranging from 0 to 10, with higher scores indicating more dysfunctional thought patterns. This questionnaire is reliable and valid in clinical and research samples. Herein, internal consistency for the total scale was good (α = 0.87).
Insomnia-Related Behaviors Questionnaire (IRBQ)
This 13-item questionnaire was designed by authors JSD and MTS based on the Sleep Hygiene Index [17] (6 items included herein), Sleep-Pain Behaviors Survey [18] (all 3 items included herein), and several other items that relate directly to treatment targets in CBT-I (4 items). Items were rated on a scale from 0 (never) to 4 (always). As is expected for a questionnaire on different, not necessarily co-occurring behaviors, internal consistency was low, although acceptable (α = 0.67).
Information and Treatment Preferences
JSD and CAS developed a series of questions on the ways in which participants would be willing to receive sleep information and insomnia treatments that participants would be willing to try. Multiple choice questions included “Do you think your IBD doctor or gastroenterologist should ask about sleep during regular visits?” and “Do you think your IBD doctor or gastroenterologist should give you recommendations on how to improve your sleep?” Participants were also provided with a list of possible sleep treatments and asked to select any they would be willing to do to improve sleep.
Open-ended questions included, “Tell me about how your sleep and IBD impact each other.” “What would make your sleep better?” They were also asked about barriers to seeking treatment: “What gets in the way of you seeking treatment for your sleep problems?” and facilitators, “What would make it easier for you to seek treatment for your sleep problems?”
Data Analytic Strategy
As we have described previously, quantitative data were examined for outliers, skew, kurtosis, and missingness. Analyses were completed using IBM SPSS 27. Person-mean imputation was used for questionnaire data and pairwise deletion was used for inferential statistics. Demographics and DBAS and IRBQ means were initially examined in participants with and without insomnia. We then investigated whether IBD type and disease activity were associated with greater risk of maladaptive beliefs and behaviors specifically in those participants with insomnia. We examined information preferences in all participants and treatment preferences in those with insomnia. For each family of quantitative analyses, we adjusted the p value for significance using a Holm–Bonferroni correction [19].
For qualitative data, we followed procedures specified for thematic analysis [20]. Initial coding was completed by CHJ and then reviewed by JSD. Areas of disagreement were discussed by CHJ and JSD and coded following discussion. Themes were discussed and developed by JSD, CHJ, and CMG. To reduce redundancy, responses regarding barriers and facilitators to treatment were collapsed into one variable.
Results
Sample Characteristics
As is shown in Table 1, participants were 312 adults with IBD, including 60.9% (n = 190) with Crohn’s disease and 39.1% (n = 122) with ulcerative colitis. Exactly 50% (n = 156) of the sample met the clinical threshold for insomnia disorder. Participants were predominantly women (66.3%) and predominantly White (95.8%). Participant age, age of onset of IBD symptoms, age of IBD diagnosis, and IBD type did not differ significantly based on insomnia status (p’s 0.20 to 0.63). However, women were more likely than men to endorse clinical levels of insomnia, X2(1, N = 311) = 8.55, p = 0.003. Participants with active disease were also more likely than participants with inactive/remissive disease to endorse clinical levels of insomnia, X2 (1, 310) = 31.15, p < 0.001.
Table 1.
Participant demographics by insomnia status
| Overall | No insomnia (n = 156) | Insomnia (n = 156) | Significance test for insomnia vs no insomnia | |
|---|---|---|---|---|
| Age in years | M = 48.62 (SD = 16.10) | M = 48.18 (SD = 16.31) | M = 49.07 (SD = 15.93) | t(307) = − 0.49, p = 0.63 |
| Gender | ||||
| Women | 207 (66.3%) | 91 (58.3%) | 116 (74.4%) | X2(1, N = 311) = 8.55, p = 0.003 |
| Men | 104 (33.3%) | 64 (41.0%) | 40 (25.6%) | |
| Other | 1 (0.3%) | 1 (0.6%) | 0 | |
| Race/ethnicity | ||||
| African American/Black | 1 (0.3%) | 0 | 1 (0.6%) | X2(5, 312) = 9.03, p = 0.11 |
| American Indian/Alaska Native | 2 (0.6%) | 2 (0.6%) | 0 | |
| Asian | 2 (0.6%) | 0 | 2 (0.1.3%) | |
| Hispanic | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) | |
| White | 299 (95.8%) | 150 (96.2%) | 149 (95.5%) | |
| Other | 3 (1%) | 3 (1%) | 0 | |
| Inflammatory bowel disease | ||||
| Crohn’s disease | ||||
| Active | 113 (36.5%) | 39 (25.3%) | 74 (47.4%) | X2(1, 310) = 31.15, p < 0.001 for disease activity; |
| Inactive | 75 (24.2%) | 50 (32.5%) | 25 (16.0%) | |
| Ulcerative colitis | ||||
| Active | 57 (18.4%) | 21 (13.6%) | 36 (23.1%) | X2(1, 310) = 1.04, p = 0.31 for Crohn’s vs UC |
| Inactive | 65 (21.0%) | 44 (28.6%) | 21 (13.5%) | |
| Age of IBD symptom onset in years | M = 27.82 (SD = 15.66) | M = 26.67 (SD = 14.89) | M = 28.97 (SD = 16.36) | t(309) = − 1.30, p = 0.20 |
| Age of IBD diagnosis in years | M = 32.34 (SD = 16.01) | M = 31.45 (SD = 15.10) | M = 33.22 (SD = 16.88) | t(309) = − 0.97, p = 0.33 |
IBD inflammatory bowel disease
Insomnia-Related Beliefs and Behaviors Data
We analyzed cognitive and behavioral factors related to insomnia, with results presented in Tables 2 and 3. As is shown in these tables, participants with insomnia thought there were more consequences of insomnia, worried more/felt more helpless about their insomnia, and attributed insomnia more to medication/biological explanations (p’s < 0.001). Additionally, participants with insomnia were significantly more likely to take daytime naps (p = 0.001), go to their bedrooms during the day if pain was bad (p < 0.001), spent 2 or more hours in their bedroom when in pain during the day (p < 0.001), take extra narcotics/opioids at night to help them sleep (p < 0.001), take sleep medication (p < 0.001), and think/worry/plan in bed (p < 0.001). They were also marginally more likely to stay in bed when awake during the night if they could not sleep (p = 0.008).
Table 2.
Insomnia-related maladaptive cognitions in participants with and without insomnia (N = 239)
| DBAS subscale | No insomnia mean (SD) | Insomnia mean (SD) | t test | p value |
|---|---|---|---|---|
| DBAS total | 3.44 (1.40) | 5.08 (1.77) | − 8.02 | < 0.001 |
| Consequences subscale | 3.82 (2.00) | 5.42 (2.14) | − 5.98 | < 0.001 |
| Worry/helplessness subscale | 2.65 (1.59) | 5.10 (2.00) | − 10.52 | < 0.001 |
| Expectations about sleep subscale | 6.06 (2.60) | 5.82 (3.20) | 0.64 | 0.52 |
| Medication/biological explanations subscale | 2.58 (2.20) | 4.00 (2.34) | − 5.02 | < 0.001 |
Bolded values are significant based on a Holm–Bonferroni correction to p < 0.05
DBAS dysfunctional beliefs and attitudes about sleep, SD standard deviation
Table 3.
Insomnia-related behaviors in participants with and without insomnia (N = 280)
| IRBQ Items | No insomnia mean (SD) | Insomnia mean (SD) | t test | p value |
|---|---|---|---|---|
| 1. I took daytime naps lasting longer than 30 min | 0.91 (1.02) | 1.35 (1.26) | − 3.24 | 0.001 |
| 2. I went to bed at different times from one day to the next (more than 30 min difference) | 1.51 (0.96) | 1.74 (0.95) | − 1.95 | 0.05 |
| 3. I get up for the day at different times from one day to the next (more than 30 min) | 1.49 (0.87) | 1.51 (0.94) | − 0.13 | 0.90 |
| 4. When pain was bad during the day, I went to my bed or bedroom to lie down and/or rest | 0.89 (1.077) | 1.63 (1.24) | − 5.32 | < 0.001 |
| 5. When I had a poor nights’ sleep I tried to sleep in the next day | 1.32 (1.08) | 1.49 (1.18) | − 1.30 | 0.19 |
| 6. I drank alcohol before bed or when I woke up in the middle of the night to help me sleep | 0.20 (0.58) | 0.27 (0.68) | − 0.90 | 0.37 |
| 7. I spent 2 or more hours during the day in my bedroom when I was experiencing pain | 0.55 (0.92) | 1.05 (1.17) | − 4.01 | < 0.001 |
| 8. I used my bed/bedroom for things other than sleeping or sex (examples: watch tv, read, eat, talk, or study) | 1.65 (1.38) | 1.75 (1.41) | − 0.57 | 0.57 |
| 9. When I had trouble sleeping in the middle of the night, I lay in bed awake, rather than getting up | 2.29 (1.25) | 2.66 (1.04) | − 2.69 | 0.008* |
| 10. I took extra narcotic (e.g., morphine, codeine, etc.) pain medications at night to help me sleep | 0.03 (0.17) | 0.28 (0.76) | − 3.84 | < 0.001 |
| 11. I took sleep medication (prescription or over the counter) when I had trouble sleeping | 0.62 (1.11) | 1.31 (1.47) | − 4.45 | < 0.001 |
| 12. I thought, planned, or worried when I was in bed | 1.78 (1.00) | 2.58 (1.05) | − 6.54 | < 0.001 |
| 13. I do important work before bedtime (for example: pay bills, schedule, or study) | 1.09 (0.97) | 1.06 (0.97) | 0.23 | 0.81 |
Bolded values are significant based on a Holm–Bonferroni correction to p < 0.05
SD standard deviation
*Value is just above the Holm–Bonferroni cut-off for significance 0.0071 [cut-off] verses 0.0076 [value]
We then analyzed cognitive and behavioral factors related to insomnia only in our participants with insomnia, investigating whether there were significant differences between those with Crohn’s disease and ulcerative colitis. There were no significant differences in DBAS total or subscale scores between the two groups (p’s ranged from 0.22 to 0.81). With regard to the IRBQ items, only one item trended toward significance. Participants with Crohn’s disease scored significantly higher than those with ulcerative colitis on the item “when pain was bad during the day, I went to my bed or bedroom to lie down and/or rest” (mean (SD) = 1.85 (1.25) versus 1.24 (1.12); t(138) = − 2.94, p = 0.004).
Finally, we investigated the impact of disease activity on the within IBD disease findings. For individuals with Crohn’s disease, disease activity (active versus inactive) did not significantly impact the DBAS total score or subscale scores (p’s ranged from 0.16 to 0.88). Further, only one of the IRBQ items trended toward significance: participants with active disease scored higher than those with inactive disease on the item “when pain was bad during the day, I went to my bed or bedroom to lie down and/or rest” (mean (SD) = 1.27 (1.17) versus 0.78 (1.09), t(87) = − 1.76, p = 0.007).
For ulcerative colitis, the DBAS total scale score and consequences of insomnia subscale score both trended toward significance (p’s = 0.013 and 0.011, respectively), with participants with active disease scoring higher in each of these areas. Additionally, only one of the IRBQ items trended toward significance: participants with active disease scored higher than those with inactive disease on the item “I spent 2 or more hours during the day in my bedroom when I was experiencing pain” (mean (SD) = 1.21 (1.27) versus 0.28 (0.58), t(49) = − 2.95, p = 0.005).
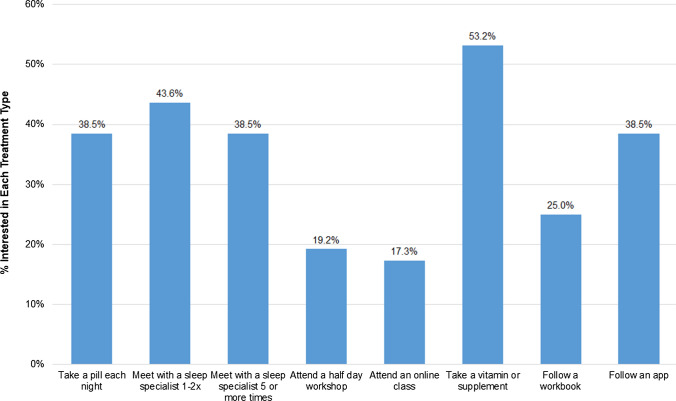
Treatment Preferences
Participants were provided with several different treatment formats (Fig. 1) and asked which of the various options they would be willing to do to improve their sleep. Overall, 84.6% of the participants who had clinically significant insomnia symptoms endorsed at least one treatment option. The most commonly selected treatment was taking a vitamin or supplement (53.2%) followed by meeting with a sleep specialist 1–2 × on telemedicine (43.6%).
Fig. 1.
Endorsement of different treatment formats
Information Preferences
Overall, 70.3% of participants thought that their IBD provider should maybe or definitely ask about sleep during regular visits and 63.4% thought that their IBD provider should give recommendations on improving sleep. Importantly, only a small percentage of participants (5.8%) thought that their IBD providers should maybe or definitely not ask about sleep and 10.4% thought that their IBD provider should not make sleep recommendations. Unsurprisingly, these findings also differed based on insomnia status; participants with insomnia were significantly more likely to want their provider to ask about sleep (78.3% vs 62.4%, X2 (4, N = 279) = 11.15, p = 0.025) and to give sleep recommendations (70.3% vs 56.7%, X2 (4, N = 279) = 12.80, p = 0.01). Information preferences did not differ significantly based on IBD diagnosis.
Qualitative Data Findings
Participants were asked about their beliefs about sleep and IBD through open-ended free-text survey questions. Out of the 312 people who participated in the study, 255 (81.7%) completed open-ended free-text questions and 139 of these participants (55%) had clinically significant insomnia. Participants who did and did not complete open-ended questions did not differ significantly by gender, presence/absence of clinically significant insomnia, IBD type, or IBD disease activity (all ps > 0.27). The results were organized into four themes: (1) participants’ perceptions about the relationship between sleep and IBD, (2) factors associated with co-morbid sleep problems and IBD, (3) sleep treatment areas of interest, and (4) barriers and facilitators to seeking sleep treatment. For themes 3 and 4, these were explored only in participants who met the clinical threshold for insomnia.
Theme 1: Participants’ Perceptions About the Relationship Between Sleep and IBD
No Relationship
Participants with and without insomnia perceived no relationship (20%) or a rare or past-only relationship (7%) between sleep and IBD. Those who perceived no relationship wrote “very little,” “no impact,” or “not at all.” The lack of relationship was discussed bidirectionally by participants, with sleep not impacting IBD and IBD not impacting sleep. As evidence of this lack of relationship, some participants described how their IBD symptoms do not seem to change regardless of whether they get a full night of sleep. Others shared other attributions for their insomnia beyond IBD. For example, “they don’t seem to be related. My pain is more arthritis related.” Finally, several participants indicated they were not sure if there was a sleep/IBD relationship but were curious about the idea they could be related, for example, “I have never made a connection between the two. I will pay more attention now.”
Direct Relationship
The remaining 73% of participants made some comment directly or indirectly endorsing a sleep/IBD relationship. Most participants described a relationship between sleep and IBD whether they endorsed insomnia or not. For example, participants made comments, such as “I believe they’re connected and both have a negative impact on the other.” Some participants reflected on the direct impact of sleep on IBD, with reflections on how the relationship can be either positive (good sleep contributes to improved IBD) or negative (poor sleep contributes to worsened IBD). Others described the impact of IBD on sleep, including the impact of IBD medication on sleep.
Participants further discussed the negative impact that IBD has on sleep (worse IBD symptoms contribute to poor sleep). IBD-related pain from flares were the most commonly reported contributor to insomnia (29% of participants), for example, “the more flare-ups I have, the worse my sleep gets. Sometimes due to pain but sometimes due to having to rush to the bathroom in the middle of the night.” Fatigue was often discussed in the context of painful flare-ups; pain can result in fatigue and therefore daytime napping: “I must nap somedays to feel better, which in turn, makes it difficult to sleep at night sometimes.” Nighttime bowel movements and/or accompanying gas or urgency were also described as barriers to obtaining good sleep for many participants (24%). Participants also reported not being able to go to sleep until they felt their bowels were emptied. Finally, some participants reported that medication side effects could worsen sleep, for example “medications used to treat one issue makes other issues worse or causes terrible side effects,” and “prednisone is the worst for inducing insomnia.” Additional quotes exemplifying this theme and others are included in Table 4.
Table 4.
Additional representative quotes for qualitative themes
| Perceptions about the relationship between sleep and IBD | |
| No relationship |
Only in extremely rare instances does my colitis ever interfere with sleep. I’ve only had two flares in the past 13 years I currently have new medication and it helps with former sleep problems—I don’t worry as much about IBD problems No impact—whether I sleep through the night or stay awake most of the night the [IBD] symptoms don’t change IBD is stable, sleep is disturbed by active dreams, overactive mind, fatigue, and unrestful sleep |
| Direct relationship |
General comments: My Crohn’s does not cause sleeplessness, but if I don’t get enough sleep, I can have increase in [Crohn’s] symptoms I need a good night's sleep to have my IBD under control Prior to being prescribed adalimumab, I would sleep constantly during Crohn’s episodes. With the medication I don’t feel like there’s much impact at all IBD has a negative impact on sleep: If I have pain, it prevents restful sleep When I’m flaring, I regularly have trouble falling asleep and staying asleep When I've had flare-ups, stomach cramping and extra trips to the bathroom have made it difficult to get a good night's sleep IBD makes me not get a good night’s sleep due to having to go to the bathroom throughout the night |
| Other factors associated with sleep and IBD | |
| Cognitive/ emotional factors |
If I don’t sleep because I’m having symptoms from Crohn’s, I’ll become anxious, making the symptoms worse and making it more difficult to go to sleep I worry that if I have a flare, I won’t be able to take care of my daughter. That worry keeps me up at night |
| Dietary behaviors |
If it hurts or I eat the wrong thing, I don’t get sleep Less sleep can lead to more caffeine, which can lead to more bathroom trips |
| Ways of improving sleep | |
| IBD Control |
Need for a cure for IBD to stop bloating and pain Learning techniques to reduce bathroom trips and leakage |
| Stress/anxiety management |
Not waking up at night and worrying about all kinds of things Feeling more relaxed at bedtime Something to calm my mind and help my body relax |
| Barriers and facilitators to seeking insomnia treatment | |
| Barriers |
Lack of knowledge about options: Treatment may be worse than the problem; don't want to be on another drug I don’t want to take sleeping medicine because I’m afraid I won’t wake up if I need to go to the bathroom I’ve asked for help just never received it. Melatonin doesn’t work. Physical exercise doesn’t work. Same time doesn’t work. I don’t know what will work If I knew there were something other than drugs to help Didn’t think there was a treatment. Don't want more medicine Downplaying insomnia: I feel like everyone deals with it, and some people have it worse I have many more problems that have a higher priority |
| Facilitators |
Recommendations & streamlined care: If it was streamlined with other IBD treatments and I didn't have to start care with a new department Someone who understand what IBD is and how it affects sleeping habits IBD providers asking about sleep: If it was made more normal to have sleeping issues with gastro issues Talking about it so I know it’s [the insomnia] not normal I f I knew it was definitely connected to my IBD I would probably prioritize it more |
Theme 2. Other Factors Associated with Sleep and IBD
Cognitive/Emotional Factors
Participants discussed the role of how thoughts and feelings impact their sleep and IBD, such as “my IBD has been well under control, but I worry about how to pay for the medication and worry about whether it’s really in remission. The anxiety and uncertainty keep me awake.” Participants also reported both worry about sleep and the resulting impact on IBD and worries about IBD leading to sleeplessness.
Dietary Behaviors
Difficulty with dietary behaviors was also described by participants, particularly the bi-directional relationships among sleep, diet, and IBD symptoms. For example, one participant indicated, “being severely constipated makes me VERY tired during the day. I have to nap somedays to feel better, which in turn make it difficult to sleep at night some times. Also, if I don’t get enough sleep I tend to not eat well during the day, which makes me even more tired.” Content of diet was reported as a concern, with participants noting spicy and processed foods as potential triggers for poor sleep. Relatedly, participants also noted that eating too close to bedtime, going to bed hungry, and evening caffeine intake could impact sleep.
Miscellaneous Factors
Participants also described how their environments impacted their sleep and IBD. Some described how their family (taking care of children), work (getting up in the morning, work stress), financial stressors, and the COVID-19 pandemic (cancelation of doctor’s appointments) impacted their health. Non-IBD-related pain contributed to insomnia, such as arthritis, joint pain (shoulder, knee, ankle), and neck/back pain. Medications not prescribed for IBD were also reported to impact sleep (stopped working or unable to continue taking it due to IBD medication contraindications).
Theme 3. Ways of Improving Sleep
IBD Control
Pain and IBD symptom remission were commonly reported by participants as changes that would result in improve sleep, particularly though pain reduction and/or IBD remission. For example, one participant indicated that her sleep could be improved by “less pain and improved routine with no screens at night” and that she is “too tired to read, but wanting distraction from pain.” Participants also mentioned how knowing about ostomy care would improve their sleep, including having an adequate plan for management of ostomies (positioning, prevention of leakage), improved pouches, and filter system to prevent ballooning. Others wanted to learn ways to reduce nighttime bathroom visits.
Stress/Anxiety Management
Another area to improve insomnia that participants identified is management of their stress, anxiety, or overall arousal. Many listed that “less stress” would improve sleep. Others specifically described the impact of anxiety or worry on their sleep patterns or talked about the need to incorporate relaxation into their lives to improve sleep and reduce arousal.
Other Sleep Disorders Treatment
Use of CPAP was described by many participants to help with sleep, with some suggesting obtaining a working, more effective, or new CPAP device would be helpful. Sleep medications were also found to be an area of interest, with participants wanting to learn about prescription and over-the-counter options and find medications that are more effective, for example, a medication “that will make me fall asleep faster but leaves me well rested when I get up.” Still, others prefer to learn about complementary therapy options for managing sleep, such as medical marijuana and essential oils.
Other Medical Conditions Treatment
Participants also want to treat their non-IBD pain and discomfort to improve their sleep. There were requests for targeting treatment of pain in other body regions besides the stomach. Anxiety reduction was also reported as a target for insomnia treatment with many participants. Reduction of anxiety about medical-related events was endorsed by a few participants including one who reports, “the reason I have trouble sleeping is because I fear I may not wake up. I have kidney disease so I worry I’ll be too dehydrated, and that will spark an emergency.”
Not Sure
Notably, 15% of participants overall and 24% of participants with clinically significant insomnia indicated that they were “not sure” or had “no idea” what could improve their sleep.
Theme 4. Barriers and Facilitators to Seeking Insomnia Treatment
Barriers
Primarily, a number of participants’ responses were reflective of lack of knowledge of available insomnia treatments. Some indicated that they were fearful about medications and their possible side effects or outcomes, while others felt like they had tried everything already or were unaware there were other treatment options. Participants also stated that they might be willing to seek treatment if they knew non-pharmacological treatments existed. Finally, some participants voiced clear disinterest in using a CPAP device, for example, “no way I’m wearing a mask, I’d rather die in my sleep.”
Downplaying of insomnia was another category that became apparent; participants stated they forget that insomnia is a problem or do not see insomnia as a relevant problem to have treated. Yet another barrier identified for insomnia treatment was the time and cost associated with it. Healthcare costs, travel, and constraints placed on participants due to the pandemic were all cited as time/cost obstacles.
Cognitive factors were another barrier for participants when they consider insomnia treatment. Participants report many other sources of stress, including lack of childcare, long work hours, and varied work schedules. Patients also shared emotional reasons for lack of engagement in treatment, citing, lack of motivation, depression, and embarrassment, among others. For example, one participant shared that for her, a barrier to seeking treatment was “embarrassment, as I expect doctors will tell me to lose weight as if I don’t already know that.”
Facilitators
Improved ease of access was reported by participants to be the most important facilitator for seeking insomnia treatment. Specifically, participants listed online resources, telehealth availability, email/phone call options, programs that do not interfere with work schedules, weekend availability, and free or low-cost programs as things that would make it easier to seek treatment.
Participants also wanted guidelines or recommendations on who to seek treatment with, often preferring an embedded provider. Participants also reported wanting to learn about or engage in sleep treatments with a provider who understands how sleep and IBD can affect one another.
Finally, participants want their IBD providers to have open conversations about sleep and IBD; they want to be asked questions about their sleep both to normalize having sleep problems in the context of IBD and to know when poor sleep becomes clinically significant. They also expressed interest in providers talking more about the sleep/IBD connection.
Discussion
Overall, we found that people with IBD and insomnia endorsed both thoughts and behavior patterns that are consistent with the general understanding of how insomnia is maintained [21]. Specifically, people with IBD and insomnia worried more about their sleep, worried more about the consequences of insomnia, and attributed insomnia more to medical explanations. They were also engaging in several behaviors known to maintain insomnia, including taking daytime naps and doing things other than sleeping in their beds. People with insomnia also engaged in pain-related behaviors, including spending time in bed/in the bedroom, while in pain and using pain medications to help them sleep. Importantly, the only items where people with active IBD scored higher than those with inactive IBD were those pertaining to spending time in bed in pain. While some of these behaviors may be adaptive at times, for example, during a severe IBD flare, they can also contribute to the development and maintenance of insomnia. For example, excessive lying in bed as a means of coping with pain may eventually make it harder to fall asleep at night even when not in pain due to the learned association of wakefulness and hyperarousal with being in bed. Given the clear relationship between insomnia and pain in other populations, these behavior patterns are not surprising [22].
As participants reported both cognitive and behavioral patterns that are consistent with the general conceptualization of insomnia disorder [21], it is likely that Cognitive Behavioral Therapy for Insomnia (CBT-I) is appropriate for patients with IBD, as long as they are not in a severe flare of their disease. In our own pilot work, we have demonstrated the feasibility and acceptability of CBT-I in people with mild to moderately active IBD [23]. However, many participants indicated lack of knowledge that sleep treatments outside of medications were an available option. In fact, many patients specified that they did not want to take additional medications or were concerned about how sleep medications would pact their health. Introducing a non-pharmacological option for improving sleep quality may be welcomed information for many patients with insomnia. As many participants downplayed the role importance of sleep, increased education about the role of sleep on IBD, as well as other aspects of physical and mental health may increase willingness to participate in insomnia treatment. Participants also identified a desire for increased access to sleep-related treatment. In addition to the availability of behavioral sleep medicine experts trained in CBT-I and other sleep treatments, patients may also benefit from information about online insomnia tools, such as Sleepio.com [24], Somryst [25], or Insomnia Coach [26].
Clinical Implications
Our quantitative and qualitative data both highlighted individual, provider, and system-level factors that can contribute to lack of insomnia care in IBD. Individually, access to care including affordability, flexible scheduling options (e.g., weekends, weeknights), and appointment delivery (e.g., telehealth, phone call) are important. There is also a lack of understanding about the relationship between sleep and IBD. People are interested in treatment; however, they often do not know what their options are or even how to go about asking the appropriate questions to get help. They want to learn about ways to improve their mental health symptoms (stress and anxiety) so that they can improve their insomnia.
Thus, given both the high rates of poor sleep and insomnia in IBD and the clear patient interest in this topic, IBD providers should include an assessment of sleep quality as part of standard clinical care. Without IBD providers assessing and/or triaging sleep problems during clinical encounters, patients may experience significant difficulty understanding and accessing hospital or community services that could improve their sleep and likely mental and physical health. Questions providers might ask to probe for sleep problems and/or areas of basic recommendations are as follows:
Are you having any problems with sleep or fatigue?
How different are your weekday and weekend schedules? [Recommend same schedule 7 days per week]
When you’re worn out or in pain, where do you go? [Recommend couch, not bed]
More in-depth questions providers can ask patients are included in a prior review on the applicability of CBT-I to people with IBD [27]. Providers can also consider screening using validated tools, such as the Insomnia Severity Index (ISI) [14], Pittsburgh Sleep Quality Index (PSQI) [28], or PROMIS sleep disturbance measure [29]. This investigation can prompt the provider to start a referral to a sleep specialist for further diagnostic testing (e.g., in lab polysomnography, home sleep study) and or treatment recommendations (e.g., CBT-I, medication trials) within the hospital system or external community services.
Limitations and Future Directions
Primarily, due to the location of the medical center where these data were collected, our population is quite homogeneous, which may impact generalizability. Additionally, the topic of the study (sleep and pain in IBD) could have resulted in a biased sample, leaning toward participants who want to report and think about their problems with sleep and/or pain. However, as we have documented elsewhere, prevalence rates of anxiety, depression, insomnia, and pain in our population were consistent with the extant literature. This consistency suggests that our rates of sleep problems were likely not heavily biased by the study topic. Further, because of the anonymous nature of the survey, we were unable to connect participants’ responses with their medical record data, including any objective information on disease severity.
Additionally, qualitative data were obtained through written surveys, whereas verbal communication via individual interviews or focus groups might reveal additional themes. Future research may build upon this characterization of sleep patterns and may include a randomized control trial of CBT-I in people with IBD, examining not only sleep but also IBD outcomes. Optimally, this intervention would also include content directed at pain, as it is a clear driver of insomnia-perpetuating behaviors in this population. Our group has begun this line of work and will continue to examine belief and behavior patterns as they relate to sleep and IBD [23].
Conclusion
People with IBD report cognitive and behavioral patterns that are consistent with the classic understanding of insomnia. In particular, people with both active and inactive IBD are likely to worry about their sleep, spend time in bed awake, and spend time in bed in pain. They also generally recognized the reciprocal relationship between IBD and insomnia. People with IBD are interested in their providers asking about their sleep and discussing the impacts of poor sleep on IBD. They are also interested in treatment, but generally were not aware of the behavioral treatment options for insomnia. Thus, providers should discuss sleep with patients in the context of routine clinical encounters, particularly for patients who report significant worry or pain, and should be prepared to make recommendations or referrals.
Author's contribution
JSD designed the study with critical input from MTS and CAS. JSD and CAS collected the data and JSD, CMG, and CHJ analyzed the data. JSD developed the initial draft of the paper with input from CMG and all authors critically revised the manuscript for important intellectual content. All authors approved the final version of this manuscript, including the authorship list. JSD is the Guarantor of the article.
Funding
This work was supported by JSD’s internal funding from the Departments of Psychiatry and Medicine (Section of Gastroenterology & Hepatology) at Dartmouth–Hitchcock/Geisel School of Medicine at Dartmouth. CMG is a postdoctoral research fellow with the Northern New England Post-Doctoral Primary Care Research Training Program. Health Resources and Services Administration (HRSA): Grant—HRSA T32 HP32520. She completed work on this manuscript as part of her T32.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Conflict of interest
None.
Ethical approval
This research was approved by the Dartmouth–Hitchcock Human Research Protection Program (IRB# 02000126).
Consent to participate
Participants freely agreed to participate in this study.
Financial disclosures
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica K. Salwen-Deremer, Email: Jessica.K.Salwen-Deremer@Hitchcock.org
Cassandra M. Godzik, Email: Cassandra.M.Godzik@Hitchcock.org
Christina H. Jagielski, Email: cjagiels@med.umich.edu
Corey A. Siegel, Email: Corey.A.Siegel@Hitchcock.org
Michael T. Smith, Email: msmith62@jhmi.edu
References
- 1.Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11:965–971. doi: 10.1016/j.cgh.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2440–2443. doi: 10.1097/MIB.0b013e3182a0ea54. [DOI] [PubMed] [Google Scholar]
- 3.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 4.Uemura R, Fujiwara Y, Iwakura N, et al. Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. SpringerPlus. 2016;5:1792. doi: 10.1186/s40064-016-3408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofia MA, Lipowska AM, Zmeter N, Perez E, Kavitt R, Rubin DT. Poor sleep quality in Crohn’s disease is associated with disease activity and risk for hospitalization or surgery. Inflamm Bowel Dis. 2020;26:1251–1259. doi: 10.1093/ibd/izz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott AJ, Flowers O, Rowse G. A comparative study of the nature and magnitude of problems sleeping in inflammatory bowel disease (IBD) compared to healthy controls. Psychol Health Med. 2020;1–11. [DOI] [PubMed]
- 7.Salwen-Deremer JK, Smith MT, Haskell HG, Schreyer C, Siegel CA. Poor sleep in inflammatory bowel disease is reflective of distinct sleep disorders. Dig Dis Sci. 2022;67:3096–3107. doi: 10.1007/s10620-021-07176-y. [DOI] [PubMed] [Google Scholar]
- 8.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2010;17:1882–1889. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- 9.Keefer L, Stepanski EJ, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006;2:409–416. doi: 10.5664/jcsm.26656. [DOI] [PubMed] [Google Scholar]
- 10.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 11.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17:263–298. doi: 10.5664/jcsm.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen ER, Melmed GY. Making a case for patient-reported outcomes in clinical inflammatory bowel disease practice. Clin Gastroenterol Hepatol. 2018;16:603–607. doi: 10.1016/j.cgh.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Bewtra M, Brensinger CM, Tomov VT, et al. An optimized patient-reported ulcerative colitis disease activity measure derived from the Mayo score and the simple clinical colitis activity index. Inflamm Bowel Dis. 2014;20:1070–1078. doi: 10.1097/MIB.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006;29:223–227. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 18.Richards J, Pejsa M, Hand M, et al. (166) Psychometric evaluation and predictive validity of the sleep and pain behaviors survey in knee osteoarthritis patients undergoing total knee replacement. J Pain. 2016;17:S17. doi: 10.1016/j.jpain.2016.01.069. [DOI] [Google Scholar]
- 19.Holm S. A simple sequentially rejective multiple test procedure. Scan J Stat. 1979;65–70.
- 20.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 21.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin N Am. 1987;10:541–553. doi: 10.1016/S0193-953X(18)30532-X. [DOI] [PubMed] [Google Scholar]
- 22.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salwen-Deremer JK, Smith MT, Aschbrenner KA, Haskell HG, Speed BC, Siegel CA. A pilot feasibility trial of cognitive–behavioural therapy for insomnia in people with inflammatory bowel disease. BMJ Open Gastroenterol. 2021;8:e000805. doi: 10.1136/bmjgast-2021-000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvanathan J, Pham C, Nagappa M, et al. Cognitive behavioral therapy for insomnia in patients with chronic pain-a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2021;101460. [DOI] [PubMed]
- 25.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. Jama Psychiatry. 2017;74:68–75. doi: 10.1001/jamapsychiatry.2016.3249. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the insomnia coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53:440–457. doi: 10.1016/j.beth.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Salwen-Deremer JK, Siegel CA, Smith MT. Cognitive Behavioral Therapy for Insomnia: a promising treatment for insomnia, pain, and depression in patients with IBD. Crohn’s Colitis 360. 2020;2:052. doi: 10.1093/crocol/otaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.