Abstract
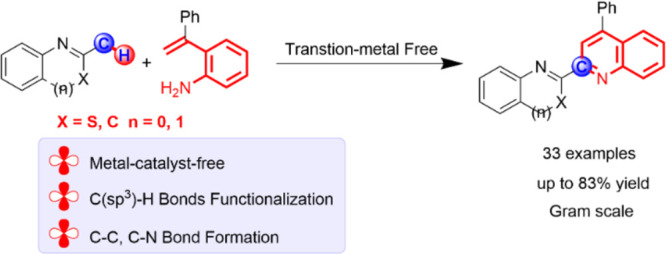
A facile functionalization of C(sp3)–H bonds and tandem cyclization strategy to synthesize quinoline derivatives from 2-methylbenzothiazoles or 2-methylquinolines and 2-styrylanilines has been developed. This work avoids the requirement for transition metals, offering a mild approach to activation of C(sp3)–H bonds and formation of new C–C and C–N bonds. This strategy features excellent functional group tolerance and scaled-up synthetic capability, thus providing an efficient and environmentally friendly access to medicinally valuable quinolines.
1. Introduction
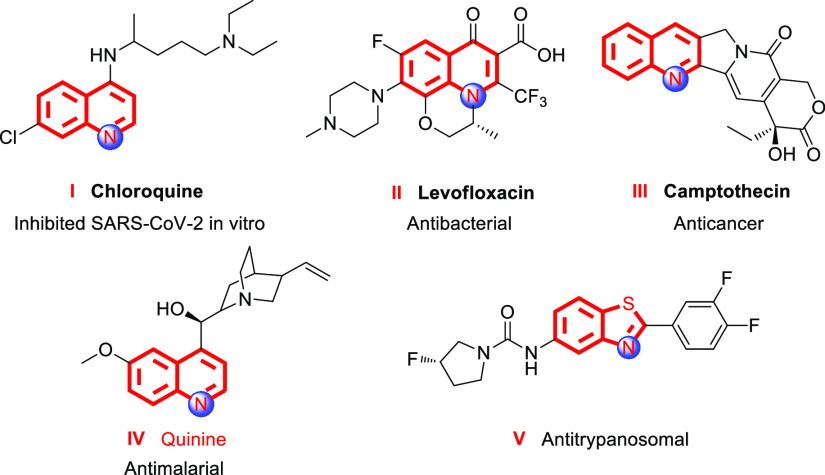
Nitrogen-functionalized heterocycles are ubiquitous in pharmaceuticals, natural alkaloids, and functional materials;1 particularly, functionalized quinoline and benzothiazole motifs possess a wide spectrum of biological activities and are considered as a privileged class of biologically active chemicals in medicinal chemistry.2Figure 1 shows some biologically active quinoline and benzothiazole derivatives. Compound I was proved to show a positive result against SARS-CoV-2, indicating a potential antiviral activity.3 Compound II levofloxacin was synthesized at Daiichi Seiyaku Co., Ltd., Tokyo, Japan. As of now, levofloxacin has become one of the most broad-spectrum antibiotics in pharmaceuticals and is being used to treat or prevent bacterial infections.4 Camptothecin (compound III) is a natural plant alkaloid which shows superior anticancer efficacy.5 Quinine (compound IV) has been commonly prescribed for the treatment of malaria.6 Benzothiazole derivative V is a new potential structure for treating human African trypanosomiasis.7
Figure 1.
Selected quinoline- and benzothiazole-based structures.
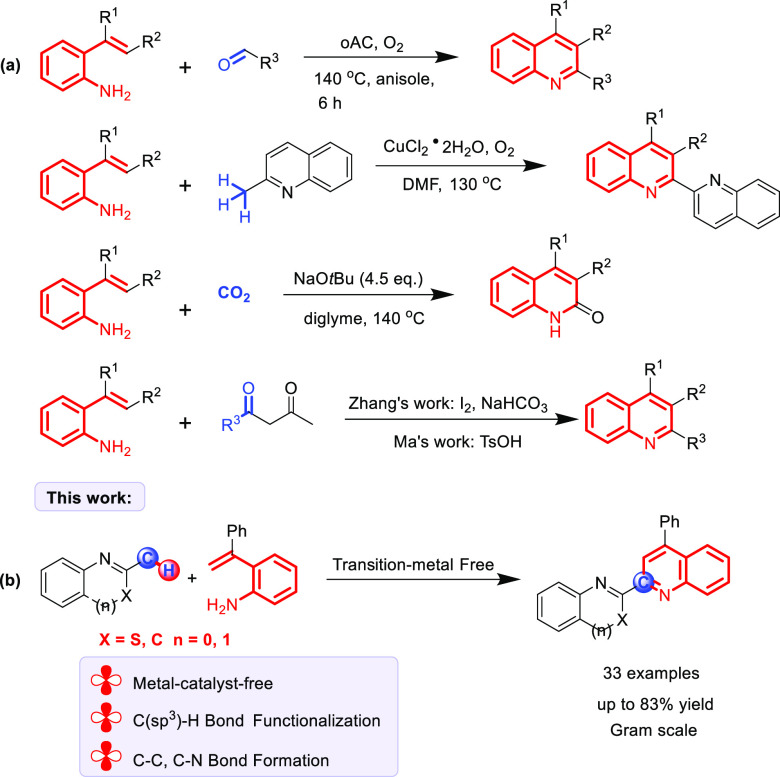
Since their first isolation from coal tar by Friedlieb Ferdinard Runge in 1834, increasing efforts have been devoted to the synthesis of substituted quinoline derivatives. Classical methods involve the condensation of aniline derivatives with ketones or aldehydes.8 These classical methods are still frequently used for preparation of quinoline motifs. However, some of these useful synthesis methods suffer from several drawbacks, for instance, expensive catalysts, limited substrate scope, multiple reaction steps, poor site selectivity, and so on. Recently, some new methods using 2-styrylanilines as starting materials have been disclosed (Scheme 1a). The group of Helaja reported the formation of polysubstituted quinolines by condensation of 2-styrylanilines with aldehydes.9 Chen’s group reported CuCl2·2H2O-catalyzed oxidative cyclization to generate quinolines.10 These methods are step-efficient and atom-economic. However, transition-metal or preactivated catalysts limit the industrial applications. Lyu’s group developed lactamization of the C(sp2)–H bond to synthesize 2-quinolinones, with CO2 as the carbonyl source.11 The groups of Zhang and co-workers12 and Ma and co-workers13 separately reported the condensation of 2-styrylanilines with β-keto esters using I2 or TsOH as the catalyst. Although there have been a number of synthesis strategies using 2-styrylanilines as the starting material, the development of different methods using new substrates for construction of functionalized quinolines remains of great importance.
Scheme 1. (a and b) Synthesis of Quinoline Derivatives from 2-Styrylanilines.
On the other hand, direct C–H functionalization reactions are of great importance in construction of C–C and C–X (X = N, O, S, etc.) bonds due to the omnipresence of C–H bonds in chemical feedstocks, step economy, and atom economy.14 Compared to the C(sp2)–H bonds, C(sp3)–H bonds are relatively inert because of their poor acidity, high bond dissociation energy, and ubiquitous feature.15 The activity and site selectivity of C(sp3)–H bond functionalization remain long-standing challenges. Currently, the transition-metal-catalyzed or metal-free strategies of direct functionalization of C(sp3)–H bonds have attracted tremendous attention in organic synthesis. A transition-metal (such as rhodium, palladium, iron, cobalt, and copper)-catalyzed directing-group-assisted strategy has emerged as a robust and highly efficient way to direct functionalization of C(sp3)–H bonds over the past decades.16 Despite the great achievement of this tactic, using toxic, precious metals and complicated ligands, wasting over stoichiometric amounts of oxidants and harsh reaction temperatures partly hinder the application of this method. Distinct from the above studies, functionalization of C(sp3)–H bonds via the radical process has been reported as a novel method in recent years; the reaction site usually occurs in the neighborhood of the carbonyl group, benzyl group, allyl group, oxygen atom, nitrogen atom, etc.17 These protocols feature metal-free, efficient, and simple operation. Nevertheless, the scope of C(sp3)–H bonds is limited in certain substrate species. Therefore, the development of more general methods for improving the structural diversity is highly desirable. In continuation of our previous efforts on direct C–H bond functionalization,18 we herein report an environment-friendly and efficient functionalization of C(sp3)–H bonds and tandem cyclization strategy from 2-methylbenzothiazoles or 2-methylquinolines and 2-styrylanilines by iodide catalysis to formation of functionalized quinoline derivatives (Scheme 1b). This protocol features metal-free, direct functionalization of C(sp3)–H bonds, broad substrate scope, moderate-to-good yields, and scaled-up synthetic capability. To the best of our knowledge, rare examples about metal-free functionalization of 2-methylbenzothiazole have been documented to date.19
2. Results and Discussion
We started our investigation by studying the reaction of 2-methylbenzo[d]thiazole 1a with 2-styrylaniline 2a. Excitingly, we were delighted to find that molecular iodine (0.2 equiv) catalyst in combination with the oxidant TBHP (3 equiv, 70% aq) in DMSO (1.5 mL) at 120 °C gave the best result, where the desired product 3a was obtained in 78% isolated yield (Table 1, entry 1). The reaction was sensitive to the iodide catalysts. KI and NaI were ineffective (entries 2 and 3). I2, TBAI, and NIS were effective for the reaction, and I2 performed the best, giving 3a in 52% yield (entries 4–6). Catalysis-equivalent screening revealed that decreasing the dosage of catalyst led to poor results, and increasing the dosage of catalyst was less effective (entries 7 and 8). The solvent was crucial for the reaction; DMF, MeCN, and NMP were inferior to DMSO (entries 9–12), owing to DMSO acting as both the solvent and oxidant in the Kornblum-type oxidation process. Expectedly, lowering or increasing the temperature resulted in lower yields (entries 13 and 14). Lowering the oxidant dosage to 0.6 mmol diminished the yield to 52% (entry 15). With a higher oxidant loading of 1.2 mmol, only 60% of 3a was obtained (entry 16). Moreover, the catalyst and oxidant were essential for the reaction, and the target product was not obtained in the absence of either element (entries 17 and 18).
Table 1. Optimization of the Reaction Conditionsa.
| entry | changes from the standard conditions | yield (%)b |
|---|---|---|
| 1 | none | 78 |
| 2 | KI (0.2 equiv) as the catalyst | trace |
| 3 | NaI (0.2 equiv) as the catalyst | trace |
| 4c | I2 (0.2 equiv) as the catalyst | 52 |
| 5 | TBAI (0.2 equiv) as the catalyst | 27 |
| 6 | NIS (0.2 equiv) as the catalyst | 47 |
| 7 | I2 (0.1 equiv) as the catalyst | 49 |
| 8 | I2 (0.3 equiv) as the catalyst | 53 |
| 9 | DMF as the solvent | 15 |
| 10 | MeCN as the solvent | 20 |
| 11 | NMP as the solvent | 43 |
| 12 | toluene as the solvent | trace |
| 13 | temp = 100 °C | 45 |
| 14 | temp = 140 °C | 50 |
| 15 | TBHP (2 equiv) | 52 |
| 16 | TBHP (4 equiv) | 60 |
| 17 | no catalyst | trace |
| 18 | no oxidant | trace |
Reactions were performed on a 0.3 mmol scale, 1a (0.3 mmol) and 2a (0.54 mmol.).
Isolated yield.
2 equiv of TBHP was used.
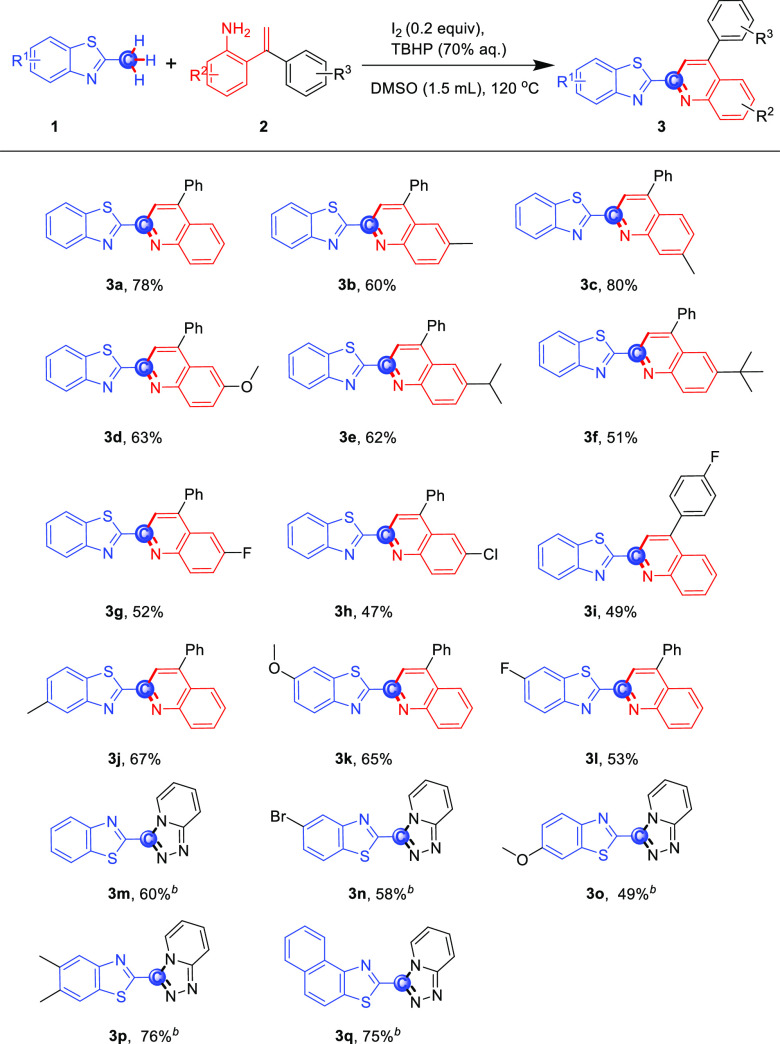
With the optimized reaction conditions in hand, we explored the effect of substituents on 2-styrylanilines and 2-methylbenzothiazoles. As shown in Scheme 2, the reaction between 2-methylbenzothiazole (1a) and 2-styrylanilines 2a–h afforded the corresponding 2-heteroaromatic quinolines 3a–h in fair-to-good yields (47–80%), revealing quite a general compatibility with the electronic nature of substituents on the benzene group of the aniline substrates. Moreover, the 2-styrylaniline substrates with the electron-donating groups showed better reactivity than those with the electron-withdrawing groups. Notably, the substituent on the benzene of the styryl motifs afforded target compounds 3i in moderate yields (49%). In addition, the substrates (2j–l) with the substituents on the benzene of 2-methylbenzothiazole, either electron-donating groups (Me and MeO) or the electron-withdrawing group (F), also afforded the desired products in fair yields (53–67%). For further verifying the generality and flexibility of the direct functionalization of 2-methylbenzothiazole, the reaction was carried out by using 2-hydrazinylpyridines as the substrates. The electron-withdrawing and electron-donating substituents at the benzene of 2-methylbenzothiazole were compatible, affording the corresponding products (3m–p) in good yields (49–76%). It is noteworthy that a strong conjugated aromatic system such as 2-methylnaphtho[1,2-d]thiazole provided the desired product 3q in 75% yield.
Scheme 2. Cyclization of 2-Methylbenzothiazoles with 2-Styrylanilinesa.
Reaction conditions: 1 (0.3 mmol), 2 (0.54 mmol), I2 (0.2 equiv), TBHP (3 equiv), 1.5 mL of DMSO, 120 °C.
Reaction with 2-hydrazinylpyridine.
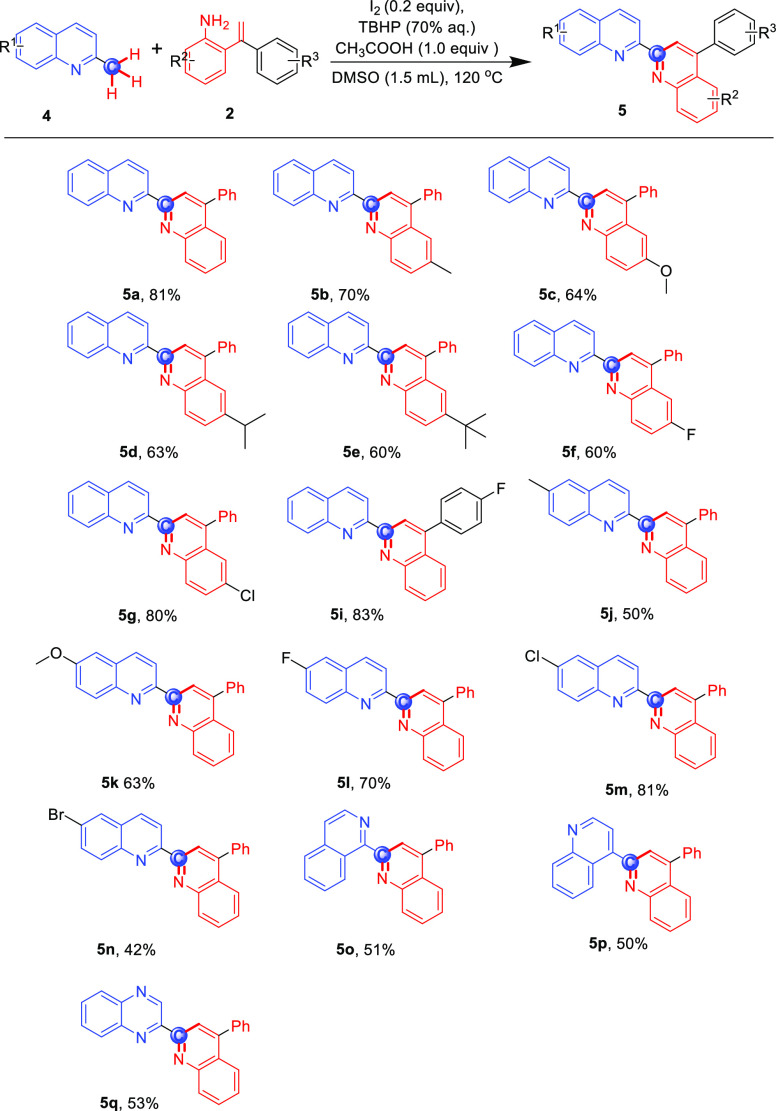
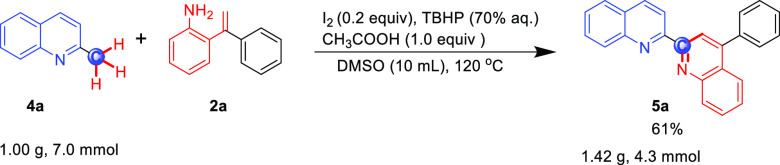
The substrate scope of the reaction for 2-methylquinolines was further investigated by slightly modifying the reaction conditions with 1 equiv of CH3COOH as the promoter, which can activate the methyl group and promote enamine tautomerization,20 and the result is shown in Scheme 3. 2-Styrylanilines, with the aryl group either on the styryl or aniline motifs bearing electron-rich (5a–e) and electron-poor substituents (5f–g and 5i), worked well, and the desired compounds were obtained in moderate-to-good yields (60–83%). The reaction of 2 with various substituted quinoline motifs was also explored. A series of 2-methylquinolines with different electronic properties smoothly finished the reaction; the target compounds were obtained in 42–81% yield (5j–n); generally, electron-withdrawing substituents (F and Cl) on the aromatic ring gave a higher yield than the electron-donating substituents (Me and MeO). 1-Methylisoquinoline, 4-methylquinoline, and 2-methylquinoxaline were all suitable for this reaction, providing the corresponding products in moderate yields (5o–q, 50–53%). Regretfully, 2-methylpyridine, 2-methyl-1H-benzo[d]imidazole, 2-methylbenzo[d]oxazole, 2-methyl-1H-indole, 2-methylimidazo[1,2-a]pyridine, and methylated caffeine were not suitable for this reaction system. Furthermore, we found that the reaction of 4a with 2a is enabled to scale up to gram quantities with good yield and efficiency (Scheme 4).
Scheme 3. Cyclization of 2-Methylquinolines with 2-Styrylanilinesa.
Reaction Conditions: 4 (0.3 mmol), 2 (0.54 mmol), I2 (0.2 equiv), TBHP (3 equiv), CH3COOH (1 equiv), 1.5 mL of DMSO, 120 °C.
Scheme 4. Gram-Scale Synthesis.
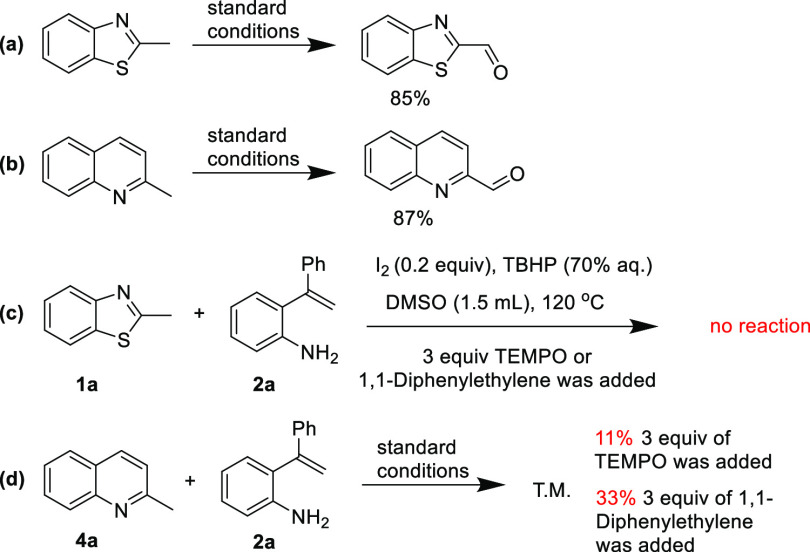
To gain insights into the mechanism of this process, a series of control reactions were investigated. First, 2-methylbenzothiazole and 2-methylquinoline could afford benzothiazole-2-carbaldehyde and quinoline-2-carbaldehyde in 85 and 87% isolated yields, respectively, under the standard conditions (Scheme 5a,b). These results suggest that the reaction may proceed with the oxidation of C(sp3)–H to aldehyde motifs. Second, in the presence of TEMPO or 1,1-diphenylethylene (3 equiv), the reaction of 1a with 2a was totally hindered (Scheme 5c), and the reaction of 4a with 2a was reduced from 81 to 11 and 33%, respectively (Scheme 5d). These results suggest that the reaction might involve radical species in the reaction mechanism.
Scheme 5. (a–d) Control Experiments.
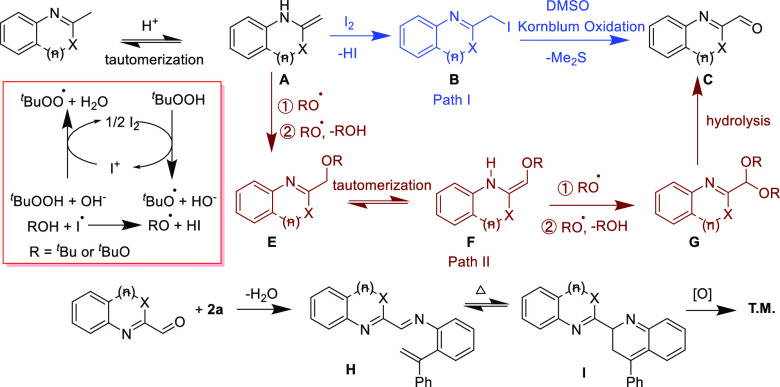
On the basis of these results and previous literatures,18c, 19a, 19e a proposed pathway is described in Scheme 6. The free-radical species tBuO· and tBuOO· were generated by a catalytic cycle of I2 and TBHP. At first, N-heteroaromatic methane undergoes enamine tautomerization to the corresponding enamines A. Then, the addition of iodine to the enamines forms benzylic iodides B, followed by a Kornblum-type oxidation to generate aldehyde motifs C (path I). On the other hand, the free-radical addition and oxidation of enamine with tBuO· or tBuOO· generate an intermediate E, which is further transformed through tautomerization and oxidation to give an intermediate G; hydrolysis of G leads to the formation of aldehyde motifs C (path II). Finally, the reaction of aldehyde motifs C with 2a, followed by thermal electrocyclization and aromatization, forms target molecules.
Scheme 6. Proposed Mechanism.
3. Conclusions
In conclusion, we have developed an environment-friendly and efficient functionalization of C(sp3)–H bonds and tandem cyclization strategy from 2-methylbenzothiazoles or 2-methylquinolines and 2-styrylanilines. Various functionalized quinolines were obtained in moderate-to-excellent yields. This protocol featured avoidance of metal catalysts, broad substrate scope, good functional group compatibility, and scaled-up synthetic capability. These advantages are expected to make this protocol a powerful tool for synthesis of medicinally valuable quinoline structures.
Acknowledgments
This work was supported by the Xinjiang Normal University Research Foundation for Doctoral Program (No. XJNUBS2105). The authors are grateful to the Xinjiang Key Laboratory of Energy Storage and Photoelectrocatalytic Materials, School of Chemistry and Chemical Engineering, Xinjiang Normal University (No. XJCNGDCH-20202) for their financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07736.
Experimental procedure, characterization data, and copies of 1H and 13C NMR spectra for the obtained products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]; b Heravi M. M.; Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020, 10, 44247–44311. 10.1039/D0RA09198G. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Joule J. A. Natural products containing nitrogen heterocycles-some highlights 1990-2015. Adv. Heterocycl. Chem. 2016, 119, 81–106. 10.1016/bs.aihch.2015.10.005. [DOI] [Google Scholar]; d Gao B.; Yang B.; Feng X.; Li C. Recent advances in the biosynthesis strategies of nitrogen heterocyclic natural products. Nat. Prod. Rep. 2022, 39, 139–162. 10.1039/D1NP00017A. [DOI] [PubMed] [Google Scholar]; e Haiduc I. Review Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 1. Five-membered and smaller rings. J. Coord. Chem. 2019, 72, 2127–2159. 10.1080/00958972.2019.1641702. [DOI] [Google Scholar]
- a Shang X. F.; Morris-Natschke S. L.; Liu Y. Q.; Guo X.; Xu X. S.; Goto M.; Li J. C.; Yang G. Z.; Lee K. H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. 10.1002/med.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ajani O. O.; Iyaye K. T.; Ademosun O. T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review. RSC Adv. 2022, 12, 18594–18614. 10.1039/D2RA02896D. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Michael J. P. Quinoline, quinazoline, and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. 10.1039/B612168N. [DOI] [PubMed] [Google Scholar]; d Lihumis H. S.; Alameri A. A.; Zaooli R. H. A review on recent development and biological applications of benzothiazole derivatives. Prog. Chem. Biochem. Res. 2022, 5, 147–164. 10.22034/pcbr.2022.330703.1214. [DOI] [Google Scholar]; e Moor L. F. E.; Vasconcelos T. R. A.; da R Reis R.; Pinto L. S. S.; Da Costa T. M. Quinoline: An Attractive Scaffold in Drug Design. Mini-Rev. Med. Chem. 2021, 21, 2209–2226. 10.2174/1389557521666210210155908. [DOI] [PubMed] [Google Scholar]
- Satarker S.; Ahuja T.; Banerjee M.; Dogra S.; Agarwal T.; Nampoothiri M. Hydroxychloroquine in COVID-19: Potential Mechanism of Action Against SARS-CoV-2. Curr. Pharmacol. Rep. 2020, 6, 203–211. 10.1007/s40495-020-00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P.; Lafredo S. C.; Foleno B.; Isaacson D. M.; Barrett J. F.; Tobia A. J.; Rosenthale M. E. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob. Agents Chemother. 1992, 36, 860–866. 10.1128/AAC.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti M. A.; Knab A. M.; Benedetti P. Yeast Saccharomyces cerevisiae as a model system to study the cytotoxic activity of the antitumor drug camptothecin. Cancer Chemother. Pharmacol. 1994, 34, S1–S5. 10.1007/BF00684856. [DOI] [PubMed] [Google Scholar]
- Schlesinger P. H.; Krogstad D. J.; Herwaldt B. L. Antimalarial agents: Mechanisms of action. Antimicrob. Agents Chemother. 1988, 32, 793–798. 10.1128/AAC.32.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D. A.; Gillespie J. R.; McQueen J.; Hulverson M. A.; Ranade R. M.; Creason S. A.; Herbst Z. M.; Gelb M. H.; Buckner F. S.; Tidwell R. R. Urea Derivatives of 2-Aryl-benzothiazol-5-amines: A New Class of Potential Drugs for Human African Trypanosomiasis. J. Med. Chem. 2017, 60, 957–971. 10.1021/acs.jmedchem.6b01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Manske R. H. F.; Kulka M.. The Skraup synthesis of quinolines. In Organic Reactions; Wiley, 1953; Chapter 7, pp. 59–98. [Google Scholar]; b Bergstrom F. Heterocyclic N compounds. IIA. Hexacyclic compounds: pyridine, quinoline and isoquinoline. Chem. Rev. 1944, 35, 77–277. 10.1021/cr60111a001. [DOI] [Google Scholar]; c Cheng C. C.; Yan S. J.. The Friedlander synthesis of quinolines. In Organic Reactions; Wiley, 1982; Chapter 28, pp. 37–201. [Google Scholar]; d Gould R. G. Jr.; Jacobs W. A. Synthesis of certain substituted quinolines and 5,6-benzoquinolines. J. Am. Chem. Soc. 1939, 61, 2890–2895. 10.1021/ja01265a088. [DOI] [Google Scholar]; e Johnson W. S.; Mathews F. J. Cyclization studies in the benzoquinoline series. J. Am. Chem. Soc. 1944, 66, 210–215. 10.1021/ja01230a016. [DOI] [Google Scholar]
- Makela M. K.; Bulatov E.; Malinen K.; Talvitie J.; Nieger M.; Melchionna M.; Lenarda A.; Hu T.; Wirtanen T.; Helaja J. Carbocatalytic Cascade Synthesis of Polysubstituted Quinolines from Aldehydes and 2-Vinyl Anilines. Adv. Synth. Catal. 2021, 363, 3775–3782. 10.1002/adsc.202100711. [DOI] [Google Scholar]
- Pang X.; Wu M.; Ni J.; Zhang F.; Lan J.; Chen B.; Yan R. Copper-Catalyzed Tandem Aerobic Oxidative Cyclization for the Synthesis of Polysubstituted Quinolines via C(sp3)/C(sp2)-H Bond Functionalization. J. Org. Chem. 2017, 82, 10110–10120. 10.1021/acs.joc.7b01575. [DOI] [PubMed] [Google Scholar]
- Li W.; Li C.; Lyu Y. Lactamization of sp2 C-H bonds with CO2 under transition-metal-free and redox-neutral conditions: a computational mechanistic study. Org. Chem. Front. 2018, 5, 2189–2201. 10.1039/C8QO00394G. [DOI] [Google Scholar]
- Xu H.; Yu F.; Huang R.; Weng M.; Chen H.; Zhang Z. Promoter regulated selective annulation prepn. of polysubstituted quinolines via bond cleavage from styrylanilines and beta ketoesters. Org. Chem. Front 2020, 7, 3368–3373. 10.1039/D0QO00709A. [DOI] [Google Scholar]
- Nan J.; Chen P.; Zhang Y.; Yin Y.; Wang B.; Ma Y. Metal-Free Synthesis of 2-Substituted Quinolines via High Chemoselective Domino Condensation/Aza-Prins Cyclization/Retro-Aldol between 2-Alkenylanilines with β-Ketoesters. J. Org. Chem. 2020, 85, 14042–14054. 10.1021/acs.joc.0c02063. [DOI] [PubMed] [Google Scholar]
- a Godula K.; Sames D. C-H Bond Functionalization in Complex Organic Synthesis. Science 2006, 312, 67–72. 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]; b Zhang Y.; Szostak M. Synthesis of Natural Products by C-H Functionalization of Heterocycles. Chem. – Eur. J. 2022, 28, e202104278 10.1002/chem.202104278. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Davies H. M. L.; Du Bois J.; Yu J. Q. C-H Functionalization in organic synthesis. Chem. Soc. Rev. 2011, 40, 1855–1856. 10.1039/c1cs90010b. [DOI] [PubMed] [Google Scholar]; d Wender P. A.; Verma V. A.; Paxton T. J.; Pillow T. H. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008, 41, 40–49. 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]; e Slathia N.; Gupta A.; Kapoor K. K. I2/TBHP Reagent System: A Modern Paradigm for Organic Transformations. Eur. J. Org. Chem. 2022, 2022, e202200460 10.1002/ejoc.202200460. [DOI] [Google Scholar]; f Trost B. M. The atom economy: a search for synthetic efficiency. Science 1991, 254, 1471–1477. 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- a Seth K. Recent progress in rare-earth metal-catalyzed sp2 and sp3 C-H functionalization to construct C-C and C-Heteroelement bonds. Org. Chem. Front. 2022, 9, 3102–3141. 10.1039/D1QO01859K. [DOI] [Google Scholar]; b Qin Y.; Zhu L.; Luo S. Organocatalysis in Inert C-H Bond Functionalization. Chem. Rev. 2017, 117, 9433–9520. 10.1021/acs.chemrev.6b00657. [DOI] [PubMed] [Google Scholar]; c Wang Z. L.; Wang F. Radical-Mediated Selective Functionalization of Unactivated Primary C-H Bonds. Chin. J. Chem. 2022, 40, 1751–1753. 10.1002/cjoc.202200210. [DOI] [Google Scholar]
- a Dailler D.; Danoun G.; Baudoin O.. Applications of catalytic organometallic C(sp3)-H bond functionalization. In C-H Bond Activation and Catalytic Functionalization II; Topics in Organometallic Chemistry, vol. 56; Springer: Cham, 2016; pp. 133 −153. [Google Scholar]; b Rej S.; Chatani N. Rhodium-Catalyzed C(sp2)- or C(sp3)-H Bond Functionalization Assisted by Removable Directing Groups. Angew. Chem., Int. Ed. 2019, 58, 8304–8329. 10.1002/anie.201808159. [DOI] [PubMed] [Google Scholar]; c Das A.; Maji B. The Emergence of Palladium-Catalyzed C(sp3)-H Functionalization of Free Carboxylic Acids. Chem. – Asian J. 2021, 16, 397–408. 10.1002/asia.202001440. [DOI] [PubMed] [Google Scholar]; d Liu Y.; You T.; Wang H. X.; Tang Z.; Zhou C. Y.; Che C. M. Iron- and cobalt-catalyzed C(sp3)-H bond functionalization reactions and their application in organic synthesis. Chem. Soc. Rev. 2020, 49, 5310–5358. 10.1039/D0CS00340A. [DOI] [PubMed] [Google Scholar]; e Guo S. R.; Kumar P. S.; Yang M. Recent Advances of Oxidative Radical Cross-Coupling Reactions: Direct α-C(sp3)-H Bond Functionalization of Ethers and Alcohols. Adv. Synth. Catal. 2017, 359, 2–25. 10.1002/adsc.201600467. [DOI] [Google Scholar]
- a Golden D. L.; Suh S.-E.; Stahl S. S. Radical C(sp3)-H functionalization and cross-coupling reactions. Nat. Rev. Chem. 2022, 6, 405–427. 10.1038/s41570-022-00388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Campos K. R. Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007, 36, 1069–1084. 10.1039/B607547A. [DOI] [PubMed] [Google Scholar]; c Wang N. X.; Zhang L. Y.; Wu Y. H.; Xing Y. C(sp3)-H Bond Functionalization of Alcohols, Ketones, Nitriles, Ethers and Amides using tert -Butyl Hydroperoxide as a Radical Initiator. Synlett 2021, 32, 23–29. 10.1055/s-0040-1706406. [DOI] [Google Scholar]; d Dong D. Q.; Hao S. H.; Wang Z. L. Functionalization of C(sp3)-H Bonds Adjacent to Oxygen of Alcohols under Metal-free Conditions. Mini-Rev. Org. Chem. 2017, 14, 130–135. 10.2174/1570193X14666170207145101. [DOI] [Google Scholar]; e Ipaktschi J.; Saidi M. R. Transition-metal-catalyzed functionalization of C(sp3)-H bonds of amines. Sci. Synth. 2012, 4, 365–493. [Google Scholar]
- a Chu X.; Wu Y.; Lu H.; Yang B.; Ma C. Copper-Catalyzed Direct Carbamoylation of Quinoxalin-2(1H)-ones with Hydrazinecarboxamides Under Mild Conditions. Eur. J. Org. Chem. 2020, 2020, 1141–1144. 10.1002/ejoc.201901858. [DOI] [Google Scholar]; b Chu X.; Niu Y.; Wang X.; Lin Y.; Li F.; Ma C. Regioselective Oxidative Cross-Coupling Reaction: Synthesis of Imidazo[1,2-a]pyridine Fluorophores. Synthesis 2021, 53, 1619–1628. 10.1055/s-0040-1706000. [DOI] [Google Scholar]; c Chu X.; Duan T.; Liu X.; Feng L.; Jia J.; Ma C. N-Iodosuccinimide involved one-pot metal-free synthesis of 2-heteroaromatic benzothiazole compounds. Org. Biomol. Chem. 2017, 15, 1606–1611. 10.1039/C6OB02731H. [DOI] [PubMed] [Google Scholar]
- a Nagasawa Y.; Tachikawa Y.; Yamaguchi E.; Tada N.; Miura T.; Itoh A. Catalytic Aerobic Photo-oxidation of a Methyl Group on a Heterocycle to Produce an Aldehyde via Homolytic C-I Bond Cleavage caused by Irradiation with Visible Light. Adv. Synth. Catal. 2016, 358, 178–182. 10.1002/adsc.201500811. [DOI] [Google Scholar]; b Kumar R.; Jain H.; Gahlyan P.; Joshi A.; Ramachandran C. N. A highly sensitive pyridine-dicarbohydrazide based chemosensor for colorimetric recognition of Cu2+, AMP2–, F– and AcO– ions. New J. Chem. 2018, 42, 8567–8576. 10.1039/C8NJ00918J. [DOI] [Google Scholar]; c Bai J.; Hou W.; Wu C.; Guo R.; Xie Y.; Li J. Metalloporphyrins-Catalyzed Synthesis of 2-Carboxybenzothiazole from 2-Methylbenzothiazole Using Molecular Oxygen as Oxidant. Curr. Organocatal. 2016, 3, 283–290. 10.2174/2213337202666151016204801. [DOI] [Google Scholar]; d Zheng G.; Liu H.; Wang M. Copper-Catalyzed Aerobic Oxidation of Azinylmethanes for Access to Trifluoromethylazinylols. Chin. J. Chem. 2016, 34, 519–523. 10.1002/cjoc.201500918. [DOI] [Google Scholar]; e Ye R.; Cao Y.; Xi X.; Liu L.; Chen T. Metal- and radical-free aerobic oxidation of heteroaromatic methanes: an efficient synthesis of heteroaromatic aldehydes. Org. Biomol. Chem. 2019, 17, 4220–4224. 10.1039/C9OB00490D. [DOI] [PubMed] [Google Scholar]
- Liu M.; Chen X.; Chen T.; Yin S. A facile and general acid-catalyzed deuteration at methyl groups of N-heteroarylmethanes. Org. Biomol. Chem. 2017, 15, 2507–2511. 10.1039/C7OB00062F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.