Abstract
Background and objectives
The benefits of physical activity (PA) for asthmatic children were increasingly recognized, and as the design of studies on PA and asthma has become more refined in recent years, the latest evidence needed to be updated. We performed this meta-analysis to synthesize the evidence available from the last 10 years to update the effects of PA in asthmatic children.
Methods
A systematic search was conducted in three databases, PubMed, Web of Science, and Cochrane Library. Randomized controlled trials were included, and two reviewers independently conducted the inclusion screening, data extraction, and bias assessment.
Results
A total of 9 studies were included in this review after 3,919 articles screened. PA significantly improved the forced vital capacity (FVC) (MD 7.62; 95% CI: 3.46 to 11.78; p < 0.001), and forced expiratory flow between 25% and 75% of forced vital capacity (FEF25–75) (MD 10.39; 95% CI: 2.96 to 17.82; p = 0.006) in lung function. There was no significant difference in forced expiratory volume in the first second (FEV1) (MD 3.17; 95% CI: −2.82 to 9.15; p = 0.30) and fractional exhaled nitric oxide (FeNO) (MD −1.74; 95% CI: −11.36 to 7.88; p = 0.72). Also, PA significantly improved the quality of life as assessed by the Pediatric Asthma Quality of Life Questionnaire (all items p < 0.05).
Conclusions
This review suggested that PA could improve FVC, FEF25–75, and quality of life in asthmatic children, but there was insufficient evidence of improvement in FEV1 and airway inflammation.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022338984.
Keywords: physical activity, lung function, quality of life, asthma, children
1. Introduction
Asthma is a heterogeneous disease characterized by chronic airway inflammation, and is the most common chronic respiratory disease in children. The Global Initiative for Asthma (GINA) highlights that the prevalence of asthma in children is increasing from year to year, particularly in developing countries and in young children among (1). The guidelines and consensus emphasize that the goals of treatment for asthmatic children are to control symptoms, reduce recurrence, and avoid exacerbations. Currently, the control treatment of asthma is still dominated by medications, including corticosteroids, bronchodilators and biologics. However, long-term use of medications inevitably has adverse effects on children's growth and development (2–4), especially with irregular asthma treatment, and also increases the economic burden. Therefore, scientists have begun to explore the positive role of non-pharmacological treatments in asthma management, including health education (5), exercise training (6), and pulmonary rehabilitation (7, 8), etc.
Exercise had been identified as a common trigger for asthma attacks or exacerbations in children (9, 10). Early on, during the treatment of children with asthma, physicians and caregivers overemphasized exercise-induced asthma and often reduced the duration and intensity of activity in asthmatic children. A recent study found that decreased aerobic fitness and increased sedentary time in children were associated with worsening asthma (11). As the link between exercise and asthma was studied in depth, some of the studies showed that regular exercise could improve the quality of life (QoL) and lung function in asthmatic patients (12, 13). However, the results of different reviews and meta-analyses often led to contradictory conclusions. For example, Joschtel B, et al. reported that exercise significantly improved cardiovascular fitness and QoL in asthmatic children (14), while another systematic review indicated there was insufficient evidence to identify the longitudinal effects of physical activity (PA) on lung function in children (15). Also, GINA only mentioned in its non-pharmacological strategies that regular PA could help to improve the management of asthma and promote children's participation in daily activities, but it remained uncertain what type or intensity of PA was beneficial for children in asthma.
Notably, an earlier meta-analysis summarized the effect of PA on asthmatic children, the authors also noted that the included studies were poorly designed. As a result, little progress was made on the relationship between lung function and PA in asthmatic children (16). Recently, some scholars wanted to study the effect of PA on asthma outcomes in adults, but no meta-analysis was performed due to the high heterogeneity of the included studies. And they concluded that PA improved lung function, QoL, and serum inflammatory markers in patients with asthma (17). Others conducted some meta-analyses of the effects of swimming on asthmatic children, but they did not consider other forms of exercise (18, 19). Therefore, we conducted this systematic review and meta-analysis focusing only on children and synthesized the evidence available for the last 10 years to update the effects of PA in asthmatic children.
2. Methods
This systematic review and meta-analysis was registered in the international prospective register of systematic reviews, and the registration number is CRD42020216469. Following the principles given in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement, a search for randomized controlled trials (RCTs) investigating the effects of PA in asthmatic children was conducted (20).
2.1. Search strategy
The following databases were searched: PubMed, Web of Science and Cochrane Library, and all databases were limited to the search period 2010 to 2020. We described the search strategy of PubMed in Table 1. And we conducted the similar search strategy on the Web of Science and the Cochrane Library based on different specific requirements. Meanwhile, reference lists of relevant studies were also scanned.
Table 1.
The search strategy in PubMed.
| Search | Query |
|---|---|
| 1 | "asthma"[MeSH Terms] OR “asthma"[All Fields] OR “asthmas"[All Fields] OR “asthma s"[All Fields] OR (“asthma"[MeSH Terms] OR “asthma"[All Fields] OR (“bronchial"[All Fields] AND “asthma"[All Fields]) OR “bronchial asthma"[All Fields]) OR (“asthma"[MeSH Terms] OR “asthma"[All Fields] OR (“asthma"[All Fields] AND “bronchial"[All Fields]) OR “asthma bronchial"[All Fields]) |
| 2 | "child"[MeSH Terms] OR “child"[All Fields] OR “children"[All Fields] OR “child s"[All Fields] OR “children s"[All Fields] OR “childrens"[All Fields] OR “childs"[All Fields] OR (“child, preschool"[MeSH Terms] OR (“child"[All Fields] AND “preschool"[All Fields]) OR “preschool child"[All Fields] OR “preschooler"[All Fields] OR “preschoolers"[All Fields] OR “preschool"[All Fields] OR “preschooler s"[All Fields] OR “preschools"[All Fields]) OR (“infant"[MeSH Terms] OR “infant"[All Fields] OR “infants"[All Fields] OR “infant s"[All Fields]) OR (“infant, newborn"[MeSH Terms] OR (“infant"[All Fields] AND “newborn"[All Fields]) OR “newborn infant"[All Fields] OR “newborn"[All Fields] OR “newborns"[All Fields] OR “newborn s"[All Fields]) OR (“toddler"[All Fields] OR “toddler s"[All Fields] OR “toddlers"[All Fields]) OR (“child"[MeSH Terms] OR “child"[All Fields] OR “children"[All Fields] OR “child s"[All Fields] OR “children s"[All Fields] OR “childrens"[All Fields] OR “childs"[All Fields]) OR (“adolescences"[All Fields] OR “adolescency"[All Fields] OR “adolescent"[MeSH Terms] OR “adolescent"[All Fields] OR “adolescence"[All Fields] OR “adolescents"[All Fields] OR “adolescent s"[All Fields]) OR (“adolescent"[MeSH Terms] OR “adolescent"[All Fields] OR “teen"[All Fields]) OR (“adolescent"[MeSH Terms] OR “adolescent"[All Fields] OR “youth"[All Fields] OR “youths"[All Fields] OR “youth s"[All Fields] |
| 3 | "exercise"[MeSH Terms] OR “exercise"[All Fields] OR “exercises"[All Fields] OR “exercise therapy"[MeSH Terms] OR (“exercise"[All Fields] AND “therapy"[All Fields]) OR “exercise therapy"[All Fields] OR “exercise s"[All Fields] OR “exercised"[All Fields] OR “exerciser"[All Fields] OR “exercisers"[All Fields] OR “exercising"[All Fields] OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“physical"[All Fields] AND “activity"[All Fields]) OR “physical activity"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“activities"[All Fields] AND “physical"[All Fields]) OR “activities physical"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“activity"[All Fields] AND “physical"[All Fields]) OR “activity physical"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“physical"[All Fields] AND “activities"[All Fields]) OR “physical activities"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “physical"[All Fields]) OR “exercise physical"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“physical"[All Fields] AND “exercise"[All Fields]) OR “physical exercise"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“physical"[All Fields] AND “exercises"[All Fields]) OR “physical exercises"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“acute"[All Fields] AND “exercise"[All Fields]) OR “acute exercise"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“acute"[All Fields] AND “exercises"[All Fields]) OR “acute exercises"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “acute"[All Fields]) OR “exercise acute"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercises"[All Fields] AND “acute"[All Fields]) OR “exercises acute"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “isometric"[All Fields]) OR “exercise isometric"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercises"[All Fields] AND “isometric"[All Fields]) OR “exercises isometric"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“isometric"[All Fields] AND “exercises"[All Fields]) OR “isometric exercises"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“isometric"[All Fields] AND “exercise"[All Fields]) OR “isometric exercise"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “aerobic"[All Fields]) OR “exercise aerobic"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“aerobic"[All Fields] AND “exercise"[All Fields]) OR “aerobic exercise"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“aerobic"[All Fields] AND “exercises"[All Fields]) OR “aerobic exercises"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercises"[All Fields] AND “aerobic"[All Fields]) OR “exercises aerobic"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “training"[All Fields]) OR “exercise training"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“exercise"[All Fields] AND “trainings"[All Fields]) OR “exercise trainings"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“training"[All Fields] AND “exercise"[All Fields]) OR “training exercise"[All Fields]) OR (“exercise"[MeSH Terms] OR “exercise"[All Fields] OR (“trainings"[All Fields] AND “exercise"[All Fields])) |
| 4 | #1 and #2 and #3 |
2.2. Selection criteria
Published studies were considered to be eligible for inclusion if they met the following criteria: (a) Studies were RCTs published in English; (b) Participants included had to be children and adolescents (age <18 years) and meet the asthma diagnostic criteria in the GINA guidelines; (c) Exercise intervention should include different forms of PA in the experimental group for at least 4 weeks; (d) The outcomes of studies had to report lung function or QoL at the end.
Studies were excluded if they failed to meet the inclusion criteria. Two authors independently performed the primary literature screening. A third author was consulted for any disagreements between the two authors.
2.3. Data extraction
Two authors extracted data from the full text of the final included studies. Data extracted included author, year, country, participant, age, group, intervention, and outcome. In this regard, interventions and outcomes would be reported in more detail.
2.4. Quality assessment
The methodological quality of each included study was assessed using the Cochrane Collaboration tool by two independent authors, in which included the following seven contents: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. And each item was considered to high risk, low risk, or unclear. All disagreements between the two authors were in consultation with the third author.
2.5. Meta-analysis
In the studies we included, the control group received conventional medication therapy, while the intervention group was treated with PA in addition to the control group. This meant that we compared PA and non-PA. The meta-analysis included studies with one of the following results: the forced expiratory volume in the first second (FEV1), the forced vital capacity (FVC), FEV1/FVC, the forced expiratory flow between 25% and 75% of forced vital capacity (FEF25–75), or the peak expiratory flow (PEF), and the QoL assessed by the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) (21). Data analysis was performed using Review Manager software version 5.3. The mean difference (MD) and the 95% confidence interval (CI) and weight between groups were calculated by the mean and standard deviation (SD) of post-intervention from groups. And due to the diversity of intervention, we used a random-effect model. A p-value below 0.05 was deemed statistically significant. We combined the values when two or more experimental groups were reported.
We judged heterogeneity by χ2 test, and when p-value was less than 0.10, it indicated statistical significance. Meanwhile, when I2 was more than 50% by I2 test, it indicated moderate-to-high heterogeneity. We conducted sensitivity analyses by excluding one study sequentially and comparing the results using a random-effect model and fixed-effect model. Based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, we assessed the quality of evidence in the included studies.
2.6. Summary of findings
We used the GRADEprofiler software to summarize the main findings of studies and to assess the certainty of the evidence, including the lung function, QoL and airway inflammation.
3. Results
We identified 3,919 records through three databases, and removed 1,158 duplicates automatically and manually. There were 2,761 records remained, of which 2,685 records were excluded from screening the titles and abstracts. The remaining 76 full-text articles were assessed for eligibility, and 67 articles were excluded due to inappropriate intervention, study design, outcome, or other reasons. Finally, 9 studies (22–30) were meta-analyzed. The PRISMA flowchart illustrated this search process (Figure 1).
Figure 1.
Flow diagram of studies from search to inclusion.
3.1. Study characteristics and types of PA
The detailed characteristics of studies were described in Table 2. A total of 496 children of 6–18 years of age were included in this meta-analysis, and interventions of all the 9 studies were different types and intensities of PA. Types of PA included high-intensity interval training (HIIT) (22), aerobic exercise on a treadmill (23, 24), riding a bicycle (25), Tai-Chi-Chuan (27), physical exercise portfolio (26, 30), swimming (28, 29), basketball (28) and football (28). The majority of PA were delivered over a time period ranging from 6 to 12 weeks. The duration of PA ranged from 30 to 60 min per section, and training frequency was from one session to three sessions per week. Five studies (22–25, 27) used heart rate (HR) to assess the exercise intensity, with the exception of the HIIT experiment that required more than 90% HR max (22), the other studies were ranging from 50% and 80% HR max (23–25, 27). The studies were conducted in Brazil, China, Denmark, Egypt, Ireland, Portugal, Spain, Turkey, and UK.
Table 2.
Characteristics of include studies.
| Study | Country | Participants | Age | Interventions | Durations | Outcomes |
|---|---|---|---|---|---|---|
| Abdelbasset WK 2018 | Egypt etc. | Moderate persistent asthma IG: n = 19 CG: n = 19 |
8–12 years | Moderate-intensity aerobic exercise program [exercise training at 50%–70% of the maximum heart rate (HR max)] walking on a treadmill | 10 weeks; 3 times per week; each exercise session lasting for 40 min. |
Pulmonary functions (FEV1, FVC); aerobic capacity QoL by PAQLQ |
| Andrade LB 2014 | Brazil | Persistent moderate asthma IG: n = 14 CG: n = 19 |
6–17 years | Aerobic training performed on an electric treadmill at 70%–80% of the maximum heart rate. | 6 weeks; 3 times per week; each exercise session lasting for 40–50 min |
Plasma cytokine; pulmonary functions (FEV1, FVC, FEV1/FVC, PEF); QoL by PAQLQ |
| Carew C 2018 | Ireland | Mild or moderate asthma IG: n = 27 CG: n = 10 |
9–16 years | Swimming, football, and basketball dynamic warm-up, speed work, conditioning games/drills and cool down | 6 weeks; once a week; each exercise session lasting for 40 min. |
Lung function (FVC, FEV1, FEV1/FVC, PEF) |
| Latorre-Román PÁ 2014 | Spain | Stable asthma IG: n = 58 CG: n = 47 |
11.53 ± 1.20 years | Low intensity to high intensity interval training Various types |
12 weeks; 3 times per week; each exercise session lasting for 60 min. |
QoL by PAQLQ Lung function (FEV1, FEV6, PEF) |
| Lin HC 2017 | China | Mild asthma IG: n = 20 CG: n = 9 |
School children | Tai-Chi-Chuan course was specifically designed as a therapy for asthmatic children including moderate-intensity exercise with about ten minutes of higher-intensity activity | 12 weeks; once a week; each exercise class for 60 min. |
QoL by Standardized Pediatric Asthma Quality of Life Questionnaire; lung function (FEV1, FVC, FEV1/FVC, PEF rate); FeNO; |
| Onur E 2011 | Turkey | Stable asthma IG: n = 15 CG: n = 15 |
8–13 years | Bicycle training at 50%–80% of the maximum heart rate. | 8 weeks; twice per week; each exercise session lasting for 60 min. |
Malondialdehyde; glutathione peroxidase; superoxide dismutase; total nitric oxide; lung function (FEV1, FVC) |
| Silva D 2013 | Portugal | Controlled asthma IG: n = 15 CG: n = 15 |
13 ± 3 years | Exercise training include aerobic, strength, balance, and coordination exercises. | 3 months; twice a week; 50 min per session. |
QoL by PAQLQ |
| Wicher IB 2010 | Denmark | Moderate persistent atopic asthma IG: n = 30 CG: n = 31 |
6–18 years | Different swimming session divided by skill level after warming up | 3 months; twice a week; each swimming session lasted 60 min. |
Lung function (FEV1, FVC, FEV1/FVC, FEF25−75); methacholine challenge test. |
| Winn CON 2019 | UK | Stable asthma IG: n = 44 CG: n = 88 |
School children | High-intensity interval training; exercise activities designed to elicit a heart rate of >90% of HR maximum |
6 months; 3 times per week; 30 min per sessions; 1:1 work-to-rest ratio. |
Anthropometrics, Lung function (FEV1, FVC, FEV1/FVC, FEF25−75, PEF), FENO, Asthma control, Asthma-related quality of life, QoL by PAQLQ Cardiorespiratory fitness. |
IG, intervention group; CG, control group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; FEF25−75, forced expiratory flow between 25% and 75% of forced vital capacity; PEF, peak expiratory flow; QoL, quality of life; PAQLQ, the Pediatric Asthma Quality of Life Questionnaire; FEV6, forced expiratory volume in sixth second;. PC20, provocative concentration of methacholine causing a 20% fall in FEV1; FeNO, fractional exhaled nitric oxide.
3.2. Risk of bias in the included studies
We summarized the risk of bias for each included study in Figure 2, and presented each item as a percentage in Figure 3.
Figure 2.
Risk of bias graph.
Figure 3.

Risk of bias summary.
3.2.1. Random sequence generation
Eight of included studies (22–26, 28–30) were randomly allocated, in which only three studies described the details of randomized method and thus were assessed as low risk of bias (22, 24, 28). One study (27) was allocated based on the participants' intention and was judged as high risk of bias. The remaining five studies were classified as unclear risk of bias due to a lack of random sequence generation (23, 25, 26, 29, 30).
3.2.2. Allocation concealment
The allocation list of two studies was opened after the groups were formed so they were assessed as low risk of bias (23, 24). The other seven studies were deemed to be unclear risk of bias for allocation concealment (22, 25–30).
3.2.3. Blinding
Since interventions involved PA and participants were required to perform it, blinding was not possible and all the studies were considered high risk.
3.2.4. Incomplete outcome data
All studies with a withdrawal were assessed as low risk, because the missing data was unlikely to influence the true outcome.
3.2.5. Selective reporting
Since the assessment of QoL was based on subjective judgment, there were risks in reporting. And five studies (22–24, 26, 30) that reported QoL were deemed to be high risk and the remaining were judged to be low risk.
3.2.6. Other bias
Because of the insufficient evidence, all studies were assessed as unclear risk.
3.3. Effects of interventions
Effects of PA intervention on lung function, QoL and airway inflammation were shown in Table 3. We downgraded some of the evidence and justified it.
Table 3.
Summary of findings.
| PA for lung function | ||||||
|---|---|---|---|---|---|---|
|
Patient or population:
children with asthma Settings: outpatient Intervention: PA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect
(95% CI) |
No of Participants
(studies) |
Quality of the evidence
(GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lung function | |||||
| lung function - FEV1 |
The mean lung function - FEV1 in the intervention groups was
3.86 higher (1.15 to 6.58 higher) |
292
(6 studies) |
⊕⊕⊕⊝ moderate1 |
MD 3.17 (−2.82 to 9.15) | ||
| lung function - FVC |
The mean lung function - FVC in the intervention groups was 8.16 higher (5.1 to 11.21 higher) |
292
(6 studies) |
⊕⊕⊕⊝ moderate1 |
MD 7.62 (3.46 to 11.78) | ||
| lung function - FEV1/FVC |
The mean lung function - FEV1/FVC in the intervention groups was
0.84 higher (1.51 lower to 3.2 higher) |
224
(4 studies) |
⊕⊕⊕⊝ moderate1 |
MD 0.73 (−2.76 to 4.22) | ||
| lung function - FEF25 −75 |
The mean lung function - FEF25−75
in the intervention groups was 10.39 higher (2.96 to 17.82 higher) |
158
(2 studies) |
⊕⊕⊕⊝ moderate1 |
MD 10.39 (2.96 to 17.82) | ||
| lung function - PEF |
The mean lung function - PEF in the intervention groups was
0.44 higher (6.71 lower to 7.58 higher) |
134
(2 studies) |
⊕⊕⊕⊝ moderate1 |
MD 0.44 (−6.71 to 7.58) | ||
|
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). PA: Physical activity; CI: Confidence interval; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; FEF25−75: forced expiratory flow between 25% and 75% of forced vital capacity; PEF: peak expiratory flow. | ||||||
|
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1 not fully blind | ||||||
| PA for QoL | ||||||
|
Patient or population:
children with asthma Settings: outpatient Intervention: PA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect
(95% CI) |
No of Participants
(studies) |
Quality of the evidence
(GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | QoL | |||||
| PAQLQ - Overall |
The mean PAQLQ - overall in the intervention groups was
1.38 standard deviations higher (0.26 to 2.5 higher) |
298
(5 studies) |
⊕⊕⊝⊝ low1,2 |
SMD 1.38 (0.26 to 2.5) | ||
| PAQLQ - Symptoms |
The mean PAQLQ - symptoms in the intervention groups was
1.4 standard deviations higher (0.22 to 2.57 higher) |
298
(5 studies) |
⊕⊕⊝⊝ low1,2 |
SMD 1.4 (0.22 to 2.57) | ||
| PAQLQ - Activity limitation |
The mean PAQLQ - activity limitation in the intervention groups was
1.37 standard deviations higher (0.18 to 2.56 higher) |
298
(5 studies) |
⊕⊕⊝⊝ low1,2 |
SMD 1.37 (0.18 to 2.56) | ||
| PAQLQ - Emotions function |
The mean PAQLQ - emotions function in the intervention groups was
1.35 standard deviations higher (0.34 to 2.36 higher) |
298
(5 studies) |
⊕⊕⊝⊝ low1,2 |
SMD 1.35 (0.34 to 2.36) | ||
|
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). PA: Physical activity; QoL: quality of life; CI: Confidence interval; PAQLQ: the Pediatric Asthma Quality of Life Questionnaire | ||||||
|
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
|
1
not fully blind
2 subjective parameter | ||||||
| PA for FeNO | ||||||
|
Patient or population:
children with asthma Settings: outpatient Intervention: PA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect
(95% CI) |
No of Participants
(studies) |
Quality of the evidence
(GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | FeNO | |||||
| FeNO |
The mean FeNO in the intervention groups was
1.74 lower (11.36 lower to 7.88 higher) |
128
(2 studies) |
⊕⊕⊕⊝ moderate1 |
MD −1.74 (−11.36 to 7.88) | ||
|
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). PA: Physical activity; FeNO: Fractional exhaled nitric oxide; CI: Confidence interval; | ||||||
|
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1 not fully blind | ||||||
3.4. Outcome measures and findings
Each included study compared the effects of PA in the experimental group with non-PA in the control group. There were nine studies included in the qualitative synthesis, in which outcomes of eight studies assessed lung functions, including FEV1 (22–29), FVC (22–25, 27–29), FEV1/FVC (22, 24, 27–29), PEF (22, 24, 26, 28), FEF25–75 (22, 29), and outcomes of six studies assessed QoL (22–24, 26, 30).
3.4.1. Lung function
3.4.1.1. FEV1
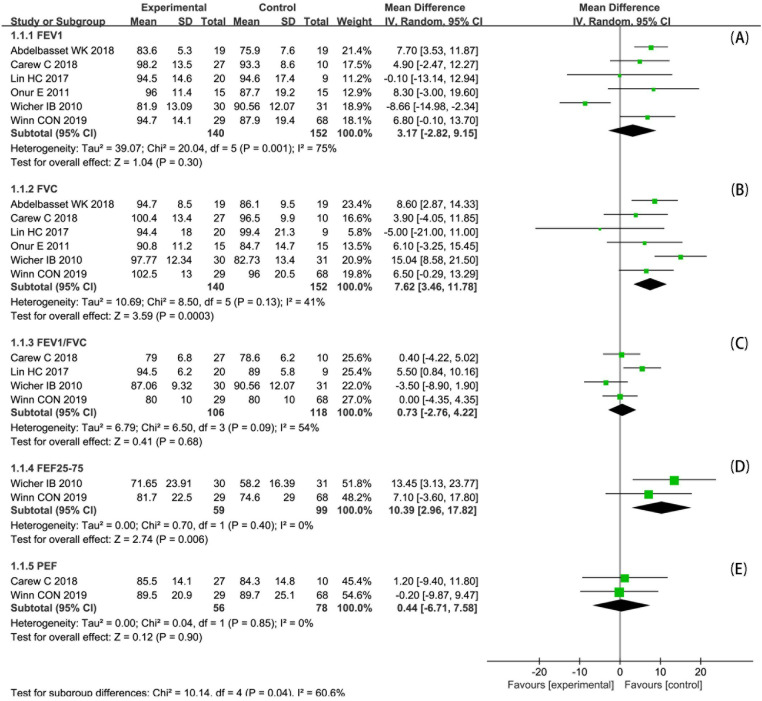
Eight studies (22–29) assessed FEV1, in which significant improvements reported in four of them (25–27, 29). Since two studies (24, 26) reported only changes before and after the intervention, six studies (22, 23, 25, 27–29) were included in the meta-analysis (Figure 4A). The meta-analysis of FEV1%pred (FEV1 in percent predicted values, similarly hereinafter) included 292 participants. There was no statistically significant difference in FEV1%pred of post-intervention between two groups (MD 3.17; 95% CI: −2.82 to 9.15; p = 0.30). And the heterogeneity was high (I2 = 75%; p = 0.001).
Figure 4.
Forest plot: changes in lung function after PA.
3.4.1.2. FVC
FVC was measured in seven studies (22–25, 27–29), in which three studies reported a significant improvement (25, 28, 29). One study (24) reported changes before and after the intervention only, so six studies (22, 23, 25, 27–29) were included in the meta-analysis (Figure 4B). The meta-analysis of FVC%pred included 292 participants. There was a statistically significant difference in FVC%pred of post-intervention between two groups (MD 7.62; 95% CI: 3.46 to 11.78; p < 0.001). And the heterogeneity was acceptable (I2 = 41%; p = 0.13).
3.4.1.3. FEV1/FVC
FEV1/FVC was assessed in five studies (22, 24, 27–29), in which one study reported a significant improvement (27). Since one study (24) reported changes before and after the intervention only, four studies (22, 27–29) were included in the meta-analysis (Figure 4C). The meta-analysis of FEV1/FVC included 224 participants. There was no statistically significant difference in FEV1/FVC of post-intervention between two groups (MD 0.73; 95% CI: −2.76 to 4.22; p = 0.68). And the heterogeneity was moderate (I2 = 54%; p = 0.09).
3.4.1.4. FEF25–75
FEF25–75 was reported in two studies (22, 29), and one (22) of which reported a significant improvement. We included both studies in the meta-analysis (Figure 4D). The meta-analysis of FEF25–75%pred included 158 participants. There was a statistically significant difference in FEF25–75%pred of post-intervention between two groups (MD 10.39; 95% CI: 2.96 to 17.82; p = 0.006). And the heterogeneity was acceptable (I2 = 0%; p = 0.40).
3.4.1.5. PEF
PEF was reported in four studies (22, 24, 26, 28), two (24, 26) of which reported significant improvement. Because two studies (24, 26) reported PEF that did not meet the criteria for pooling, the remaining two studies (22, 28) were included in the meta-analysis (Figure 4E). The meta-analysis of PEF%pred included 134 participants. There was no statistically significant difference in PEF%pred of post-intervention between two groups (MD 0.44; 95% CI: −6.71 to 7.58; p = 0.90). And the heterogeneity was acceptable (I2 = 0%; p = 0.85).
3.4.2. Quality of life
QoL was assessed in six studies (22–24, 26, 27, 30). Five studies used the PAQLQ (22–24, 26, 30), and the remaining one study used different questionnaire (27). Meanwhile, five studies showed statistically significant improvement (22–24, 26, 27, 30), and one study had no significant statistical improvement (22). We conducted a meta-analysis of five studies that used the PAQLQ (Figure 5). The meta-analysis of QoL included 298 participants. And the meta-analysis showed that overall PAQLQ score and the three domains (symptoms, activity limitation, and emotional function) were statistically significant difference between two groups. Separately, they were overall PAQLQ score (MD 1.38; 95% CI: 0.26 to 2.50; p = 0.02), symptoms (MD 1.40; 95% CI: 0.22 to 2.57; p = 0.02), activity limitation (MD 1.37; 95% CI: 0.18 to 2.56; p = 0.02), and emotional function (MD 1.35; 95% CI: 0.34 to 2.36; p = 0.009). And all heterogeneity exceeded acceptable (I2 > 90%).
Figure 5.
Forest plot: changes in QoL after PA.
3.4.3. Airway inflammation
Fractional exhaled nitric oxide (FeNO) is the most commonly used marker of airway inflammation in asthmatic children. Two studies (22, 27) reported changes in FeNO after PA intervention, both of which decreased from baseline levels. We included both studies in the meta-analysis (Figure 6). There was no statistically significant difference in FeNO of post-intervention between two groups (MD −1.74; 95% CI: −11.36 to 7.88; p = 0.72). And the heterogeneity was acceptable (I2 = 0%; p = 0.77).
Figure 6.
Forest plot: changes in FeNO after PA.
4. Discussion
The aim of this systematic review and meta-analysis was to synthesize the available studies investigating the effects of PA in asthmatic children.
Compared with the control groups, our results showed a significant improvement in FVC and FEF25–75 on lung function in experiment groups with PA. Earlier studies found that PA improved lung function in healthy children and adolescents by promoting longitudinal growth in lung volume, increasing FEV1 and FVC (31, 32). An RCT study showed that combined exercise training improved lung function in FEV1, FVC, FEF25–75 (33). However, in another RCT study, which was not included in the meta-analysis because of high heterogeneity, it was concluded that PA did not improve lung function (FEV1, FEV1/FVC) in children with asthma, but it did improve the clinical symptoms and QoL (34). Thus, current studies remained controversial on whether PA improved FEV1, but the evidence was more robust on PA improving FVC. Our study further reinforced this conclusion. Also, we found an increase in FEF25–75 after the intervention of PA, which suggested that the benefits of PA for asthmatic children were not only restricted to central airways but also small airways. This will require our focus in the future. Because asthmatic children who had small airway dysfunction were often poorly controlled and more likely to have frequent exacerbations (35). If PA is shown to improve the small airway dysfunction, this may provide an additional treatment option for these children. But more evidence is needed to support.
However, the specific mechanism by which PA improves lung function is still not well understood. A review concluded that the effect of exercise on lung function may be due to the ability of aerobic exercise to accelerate respiratory rate and strengthen respiratory muscles, thus contributing to the stretching of airway smooth muscle and sustained bronchial dilation (36). Onur E et al. suggested that a potential mechanism for the improvement in lung function in asthmatic children after PA might be associated with an increased oxidative capacity, leading to a reduction in oxidative burden and enhancing the anti-inflammatory effects of steroids (25).
Our review also showed significant improvement in the QoL of children with PA and was consistent with the findings of previous studies (36, 37), but we found high heterogeneity in the meta-analysis of QoL. This might be explained by the fact that the QoL couldn't be evaluated by objective indicators, and subjective judgments were mainly made by means of questionnaires. Participants were susceptible to a variety of factors that led to certain tendencies. A recent review concluded that aerobic exercise could reduce the prevalence and frequency of symptoms in children with nocturnal asthma (38). Therefore, based on the available evidence, we believed that PA could improve the quality of life of asthmatic children.
In this study, we found there was no statistically significant improvement in FeNO. This might be due to the fact that we included too few studies and that the interventions in these studies were not long enough. However, some reviews concluded that exercise could reduce levels of inflammation-related factors and cells, such as FeNO, C-reactive protein, and blood eosinophils (37, 39). Similarly, one study suggested that acute moderate-intensity exercise was associated with decreased exhaled nitric oxide (40). Also, an earlier review described in detail the possible mechanisms of the anti-inflammatory effects of exercise, which believed that anti-inflammatory effects of PA were mainly mediated by the reduction in release of adipokines and the induction of an anti-inflammatory environment through exercise (41). And other studies found that PA also increased the number of regulatory T cells, elevated the expression of anti-inflammatory cytokines, reduced the release of pro-inflammatory cytokines, and decreased the levels of pro-inflammatory cells in the blood (42, 43). These were also demonstrated in animal experiments (44–46). Therefore, we believed that PA could improve airway inflammation in asthmatic children to some extent, but higher quality clinical studies were needed.
The participants included in this study were all mild to moderate asthmatic children. Previous studies discussed the safety of PA in children with mild to moderate asthma and concluded that it was well tolerated and had a low incidence of adverse events (19). It was also concluded that the benefits of regular exercise for asthma patients far outweigh the risks (36). Therefore, we believed that PA was beneficial for children with mild to moderate asthma. In addition, a study suggested an association between poor asthma control and inadequate PA in urban children (47), and another study showed that low levels of PA led to an increased risk of new-onset asthma in children and adolescents (48). Consequently, after assessing the children's condition, physicians should advise them to exercise as much as possible according to an appropriate exercise prescription (49). We noted that some recent studies found improvement in asthma symptoms and quality of life with PA in adults with severe asthma (50, 51), but there were no studies on children yet because of the possible ethical risks.
5. Limitation
Our study also had some limitations. First, the exercise patterns in the study belong to moderate-to-severe intensity exercise, but there was some heterogeneity with frequency, intensity, time and type of exercise. Second, most of the interventions in the studies we included were about 12 weeks in duration, and it was not possible to assess the long-term effects of exercise on patients. Third, we did not make comparisons between different PA, and this prevented us from determining which PA was most beneficial for asthmatic children. One of the studies we included concluded that swimming might have greater benefits for asthmatic children than other forms of exercise (28). Four, although the inclusion of earlier studies might increase heterogeneity, their exclusion would also introduce some bias. Finally, the comprehensiveness of the results in the review might be hindered by the fact that this review only collected three of the most commonly used databases and was limited to studies published in English.
6. Conclusion
In summary, our systematic review and meta-analysis suggests that PA can improve FVC, FEF25–75, and quality of life in asthmatic children, while more evidence is needed for its effect on lung function. And there is insufficient clinical evidence of improvement in airway inflammation. However, we believe PA as an adjunctive therapy should be recommended for asthmatic children, except for severe asthma or exacerbations. In the future, more studies are still needed to explore the specific mechanisms of PA in improving lung function and airway inflammation. Also, it is worthwhile to continue research on what type of PA is more beneficial for asthma.
Funding Statement
This study was supported by the National Natural Science Foundation of China (82170026), Shaanxi Province Technological Innovation Guidance Special Project (2022QFY01-09), Natural Science Foundation of Shaanxi Province (2022JQ-764 and 2021JM-227) and the Discipline Promotion Project of Xijing Hospital (XJZT21L11).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XW and XS conceived and designed the study. XW conducted the literature search. ZJ, PZ, JH and YJ performed data extraction. XW and ZJ conducted the literature quality assessment. ZJ and XW drafted the manuscript with the help of all the other authors. JZ and HW reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.2022 GINA MAIN REPORT. Global Strategy for Asthma Management and Prevention (2022). Available at: https://www.ginasthma.org/gina-reports (Accessed July 26, 2022).
- 2.Haarman MG, van Hunsel F, de Vries TW. Adverse drug reactions of montelukast in children and adults. Pharmacol Res Perspect. (2017) 5(5):e00341. 10.1002/prp2.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. (2018) 11:193–204. 10.2147/jaa.S176026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffler E, Madeira LNG, Ferrando M, Puggioni F, Racca F, Malvezzi L, et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. (2018) 6(3):776–81. 10.1016/j.jaip.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 5.de Benedictis D, Bush A. Asthma in adolescence: is there any news? Pediatr Pulmonol. (2017) 52(1):129–38. 10.1002/ppul.23498 [DOI] [PubMed] [Google Scholar]
- 6.Wanrooij VH, Willeboordse M, Dompeling E, van de Kant KD. Exercise training in children with asthma: a systematic review. Br J Sports Med. (2014) 48(13):1024–31. 10.1136/bjsports-2012-091347 [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Zhang W, Liu L, Yang W, Liu H. Effects of physical therapy on lung function in children with asthma: study protocol for a systematic review and meta-analysis. Medicine (Baltimore). (2019) 98(15):e15226. 10.1097/md.0000000000015226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhao Y, Liu F, Liu L. Effects of physical exercises on pulmonary rehabilitation, exercise capacity, and quality of life in children with asthma: a meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:5104102. 10.1155/2021/5104102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeiger JS, Weiler JM. Special considerations and perspectives for exercise-induced bronchoconstriction (eib) in Olympic and other elite athletes. J Allergy Clin Immunol Pract. (2020) 8(7):2194–201. 10.1016/j.jaip.2020.01.041 [DOI] [PubMed] [Google Scholar]
- 10.Kuti BP, Kuti DK, Teague WG. Determinants of severe exercise-induced bronchoconstriction in Nigerian children with asthma. Pediatr Pulmonol. (2020) 55(Suppl 1):S51–60. 10.1002/ppul.24609 [DOI] [PubMed] [Google Scholar]
- 11.Lu KD, Forno E, Radom-Aizik S, Cooper DM. Low fitness and increased sedentary time are associated with worse asthma-the national youth fitness survey. Pediatr Pulmonol. (2020) 55(5):1116–23. 10.1002/ppul.24678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoodley I, Williams L, Thompson C, Scott H, Wood L. Evidence for lifestyle interventions in asthma. Breathe. (2019) 15(2):e50–61. 10.1183/20734735.0019-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, et al. Exercise as a prescription for patients with Various diseases. J Sport Health Sci. (2019) 8(5):422–41. 10.1016/j.jshs.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joschtel B, Gomersall SR, Tweedy S, Petsky H, Chang AB, Trost SG. Effects of exercise training on physical and psychosocial health in children with chronic respiratory disease: a systematic review and meta-analysis. BMJ Open SEM. (2018) 4(1):e000409. 10.1136/bmjsem-2018-000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassim R, Dharmage SC, Koplin JJ, Milanzi E, Paro FM, Russell MA. Does physical activity strengthen lungs and protect against asthma in childhood? A systematic review. Pediatr Allergy Immunol. (2019) 30(7):739–51. 10.1111/pai.13105 [DOI] [PubMed] [Google Scholar]
- 16.Crosbie A. The effect of physical training in children with asthma on pulmonary function, aerobic capacity and health-related quality of life: a systematic review of randomized control trials. Pediatr Exerc Sci. (2012) 24(3):472–89. 10.1123/pes.24.3.472 [DOI] [PubMed] [Google Scholar]
- 17.Kuder MM, Clark M, Cooley C, Prieto-Centurion V, Danley A, Riley I, et al. A systematic review of the effect of physical activity on asthma outcomes. J Allergy Clin Immunol Pract. (2021) 9(9):3407–21.e8. 10.1016/j.jaip.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran HJ, Jiang Y, Shan CH, Tam WWS, Wang W. A systematic review and meta-analysis on the effectiveness of swimming on lung function and asthma control in children with asthma. Int J Nurs Stud. (2021) 120:103953. 10.1016/j.ijnurstu.2021.103953 [DOI] [PubMed] [Google Scholar]
- 19.Beggs S, Foong YC, Le HC, Noor D, Wood-Baker R, Walters JA. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. (2013) (4):Cd009607. 10.1002/14651858.CD009607.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raat H, Bueving HJ, de Jongste JC, Grol MH, Juniper EF, van der Wouden JC. Responsiveness, longitudinal- and cross-sectional construct validity of the pediatric asthma quality of life questionnaire (paqlq) in Dutch children with asthma. Qual Life Res. (2005) 14(1):265–72. 10.1007/s11136-004-6551-4 [DOI] [PubMed] [Google Scholar]
- 22.Winn CON, Mackintosh KA, Eddolls WTB, Stratton G, Wilson AM, McNarry MA, et al. Effect of high-intensity interval training in adolescents with asthma: the exercise for asthma with commando joe's® (X4acj) trial. J Sport Health Sci. (2021) 10(4):488–98. 10.1016/j.jshs.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelbasset WK, Alsubaie SF, Tantawy SA, Abo Elyazed TI, Kamel DM. Evaluating pulmonary function, aerobic capacity, and pediatric quality of life following a 10-week aerobic exercise training in school-aged asthmatics: a randomized controlled trial. Patient Prefer Adherence. (2018) 12:1015–23. 10.2147/ppa.S159622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade LB, Britto MC, Lucena-Silva N, Gomes RG, Figueroa JN. The efficacy of aerobic training in improving the inflammatory component of asthmatic children. Randomized trial. Respir Med. (2014) 108(10):1438–45. 10.1016/j.rmed.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Onur E, Kabaroğlu C, Günay O, Var A, Yilmaz O, Dündar P, et al. The beneficial effects of physical exercise on antioxidant Status in asthmatic children. Allergol Immunopathol (Madr). (2011) 39(2):90–5. 10.1016/j.aller.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Latorre-Román P, Navarro-Martínez AV, García-Pinillos F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J Asthma. (2014) 51(5):544–51. 10.3109/02770903.2014.888573 [DOI] [PubMed] [Google Scholar]
- 27.Lin HC, Lin HP, Yu HH, Wang LC, Lee JH, Lin YT, et al. Tai-Chi-Chuan exercise improves pulmonary function and decreases exhaled nitric oxide level in both asthmatic and nonasthmatic children and improves quality of life in children with asthma. Evid Based Complement Alternat Med. (2017) 2017:6287642. 10.1155/2017/6287642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carew C, Cox DW. Laps or lengths? The effects of different exercise programs on asthma control in children. J Asthma. (2018) 55(8):877–81. 10.1080/02770903.2017.1373806 [DOI] [PubMed] [Google Scholar]
- 29.Wicher IB, Ribeiro MA, Marmo DB, Santos CI, Toro AA, Mendes RT, et al. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J Pediatr (Rio J). (2010) 86(5):384–90. 10.2223/jped.2022 [DOI] [PubMed] [Google Scholar]
- 30.Silva D, Couto M, Moreira P, Padrão P, Santos P, Delgado L, et al. Physical training improves quality of life both in asthmatic children and their caregivers. Ann Allergy Asthma Immunol. (2013) 111(5):427–8. 10.1016/j.anai.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 31.Hancox RJ, Rasmussen F. Does physical fitness enhance lung function in children and young adults? Eur Respir J. (2018) 51(2):1701374. 10.1183/13993003.01374-2017 [DOI] [PubMed] [Google Scholar]
- 32.Dimitri P, Joshi K, Jones N. Moving more: physical activity and its positive effects on long term conditions in children and young people. Arch Dis Child. (2020) 105(11):1035–40. 10.1136/archdischild-2019-318017 [DOI] [PubMed] [Google Scholar]
- 33.Sanz-Santiago V, Diez-Vega I, Santana-Sosa E, Lopez Nuevo C, Iturriaga Ramirez T, Vendrusculo FM, et al. Effect of a combined exercise program on physical fitness, lung function, and quality of life in patients with controlled asthma and exercise symptoms: a randomized controlled trial. Pediatr Pulmonol. (2020) 55(7):1608–16. 10.1002/ppul.24798 [DOI] [PubMed] [Google Scholar]
- 34.Zhang YF, Yang LD. Exercise training as an adjunctive therapy to montelukast in children with mild asthma: a randomized controlled trial. Medicine (Baltimore). (2019) 98(2):e14046. 10.1097/md.0000000000014046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraft M, Richardson M, Hallmark B, Billheimer D, Van den Berge M, Fabbri LM, et al. The role of small airway dysfunction in asthma control and exacerbations: a longitudinal, observational analysis using data from the Atlantis study. Lancet Respir Med. (2022) 10(7):661–8. 10.1016/s2213-2600(21)00536-1 [DOI] [PubMed] [Google Scholar]
- 36.Lang JE. The impact of exercise on asthma. Curr Opin Allergy Clin Immunol. (2019) 19(2):118–25. 10.1097/aci.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 37.Pitzner-Fabricius A, Toennesen LL, Backer V. Can training induce inflammatory control in asthma, or is it symptom control only? Curr Opin Pulm Med. (2020) 26(1):56–61. 10.1097/mcp.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 38.Francisco CO, Bhatawadekar SA, Babineau J, Reid WD, Yadollahi A. Effects of physical exercise training on nocturnal symptoms in asthma: systematic review. PloS One. (2018) 13(10):e0204953. 10.1371/journal.pone.0204953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakhale S, Luks V, Burkett A, Turner L. Effect of physical training on airway inflammation in bronchial asthma: a systematic review. BMC Pulm Med. (2013) 13:38. 10.1186/1471-2466-13-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott HA, Latham JR, Callister R, Pretto JJ, Baines K, Saltos N, et al. Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma. Ann Allergy Asthma Immunol. (2015) 114(6):470–9. 10.1016/j.anai.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 41.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11(9):607–15. 10.1038/nri3041 [DOI] [PubMed] [Google Scholar]
- 42.de Araújo CC, Marques PS, Silva JD, Samary CS, da Silva AL, Henriques I, et al. Regular and moderate aerobic training before allergic asthma induction reduces lung inflammation and remodeling. Scand J Med Sci Sports. (2016) 26(11):1360–72. 10.1111/sms.12614 [DOI] [PubMed] [Google Scholar]
- 43.Kennedy MD, Davidson WJ, Wong LE, Traves SL, Leigh R, Eves ND. Airway inflammation, cough and athlete quality of life in elite female cross-country skiers: a longitudinal study. Scand J Med Sci Sports. (2016) 26(7):835–42. 10.1111/sms.12527 [DOI] [PubMed] [Google Scholar]
- 44.Alberca-Custódio RW, Greiffo FR, MacKenzie B, Oliveira-Junior MC, Andrade-Sousa AS, Graudenz GS, et al. Aerobic exercise reduces asthma phenotype by modulation of the leukotriene pathway. Front Immunol. (2016) 7:237. 10.3389/fimmu.2016.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almeida-Oliveira AR, Aquino-Junior J, Abbasi A, Santos-Dias A, Oliveira-Junior MC, Alberca-Custodio RW, et al. Effects of aerobic exercise on molecular aspects of asthma: involvement of socs-jak-stat. Exerc Immunol Rev. (2019) 25:50–62. [PubMed] [Google Scholar]
- 46.Camargo Hizume-Kunzler D, Greiffo FR, Fortkamp B, Ribeiro Freitas G, Keller Nascimento J, Regina Bruggemann T, et al. Aerobic exercise decreases lung inflammation by ige decrement in an ova mice model. Int J Sports Med. (2017) 38(6):473–80. 10.1055/s-0042-121638 [DOI] [PubMed] [Google Scholar]
- 47.Koinis-Mitchell D, Kopel SJ, Dunsiger S, McQuaid EL, Miranda LG, Mitchell P, et al. Asthma and physical activity in urban children. J Pediatr Psychol. (2021) 46(8):970–9. 10.1093/jpepsy/jsab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lochte L, Nielsen KG, Petersen PE, Platts-Mills TA. Childhood asthma and physical activity: a systematic review with meta-analysis and graphic appraisal tool for epidemiology assessment. BMC Pediatr. (2016) 16:50. 10.1186/s12887-016-0571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman AT, Staples KJ, Wilkinson TMA. Defining a role for exercise training in the management of asthma. Eur Respir Rev. (2020) 29(156):190106. 10.1183/16000617.0106-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLoughlin RF, Clark VL, Urroz PD, Gibson PG, McDonald VM. Increasing physical activity in severe asthma: a systematic review and meta-analysis. Eur Respir J. (2022) 60(6):2200546. 10.1183/13993003.00546-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evaristo KB, Mendes FAR, Saccomani MG, Cukier A, Carvalho-Pinto RM, Rodrigues MR, et al. Effects of aerobic training versus breathing exercises on asthma control: a randomized trial. J Allergy Clin Immunol Pract. (2020) 8(9):2989–96.e4. 10.1016/j.jaip.2020.06.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.