Abstract
Background:
Diffuse large B cell lymphoma (DLBCL) is the most commonly diagnosed subtype of non-Hodgkin’s lymphoma (NHL). R-CHOP has significantly improved clinical outcomes in patients with DLBCL, however, its indication in the prevention of CNS relapse and recurrence is still inconsistent. Moreover, prophylactic methotrexate and/or cytarabine have been used prophylactically for DLBCL patients is at high risk of CNS relapse and to treat CNS DLBCL, however, their efficacy remains unclear.
Methods:
The aim of our retrospective study was to determine the incidence of CNS in-volvement in patients with DLBCL and to describe its risk factors and survival outcomes.
Results:
A total of 406 patients with DLBCL were identified, and 17 (4.2%) of DLBCL patients had CNS involvement i.e. 9 (2.2 %) at diagnosis and 8 (~2%) at relapse. The patients were younger, had advanced stage, high CNS-IPI, and had extra nodal involvement. Seven out of the 17 patients who survived received chemotherapy and a prophylactic methotrexate. Considering the CNS-IPI, of the 146 patients with high CNS-IPI at presentation, 18 received the prophylactic HDMTX and 3 (16.7%) of them had CNS relapse. Two (1.6%) out of 128 who did not receive the prophylactic HDMTX had CNS relapse. On the other hand, of the 223 patients with intermediate CNS-IPI, 25 received the prophylactic HDMTX and 2 (8%) of them had CNS relapse and in 198 patients who did not receive the prophylactic HDMTX, 2 (1.01%) had CNS relapse. The 5-year progression-free survival and overall survival rates for the entire cohort were 73% and 84%, respectively. The median OS for those who had CNS involvement was 17 months and the 2-year OS was 40%.
Conclusion:
CNS involvement in DLBCL has a poor prognosis, thus, aggressive CNS-directed therapy should be considered, especially in young patients.
Key Words: diffuse large B-cell lymphoma, central nervous system involvement, synchronous, relapse, methotrexate
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most established subtype of non-Hodgkin lymphoma (NHL) that accounts 25-40% of all lymphoma cases worldwide (Swerdlow et al., 2016). In Saudi Arabia, NHL has the estimated incidence of 5.9 per 100,000 and approximately 51% of NHL cases are adults afflicted with DLBCL (WHO, 2021; SCR, 2021). DLBCL is a heterogeneous and aggressive cancer that is often curable with chemotherapy or with a combination of chemotherapy and immunotherapy. Overall, the current standard treatment for DLBCL is the combination of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) which has improved disease outcomes (Sehn et al., 2005; Coiffier et al., 2002; Habermann et al., 2006; Coiffier et al., 2010).
Central nervous system (CNS) involvement is uncommon in DLBCL and is associated with poor prognosis (Kridel et al., 2011). Although R-CHOP has significantly improved clinical outcomes in patients with DLBCL, however, its indication for the prevention of CNS relapse and recurrence remains inconsistent (Schmitz et al., 2012; Ghose et al., 2014; Boehme et al., 2009; Yamamoto et al., 2010; Arkenau et al., 2007; Tomita et al., 2015). Intrathecal methotrexate (ITMTX) or high-dose intravenous methotrexate (HDMTX) and cytarabine have been used prophylactically for DLBCL patients categorized as being a high risk of CNS relapse and to treat CNS DLBCL; however, their efficacy is still in question, and both are associated with significant adverse effects (Kridel et al., 2011; Kwong et al., 2008; Schmiegelow K, 2009).
Evaluating the efficacy of methotrexate presents a substantial clinical challenge due to several prognostic models used to select DLBCL patients at increased risk of CNS disease while others were found to be unreliable in predicting the risk of CNS relapse; however, no difference in the risk of CNS relapse between different ethnic groups exists in literature (Schmitz et al., 2012; Ghose et al., 2014; Hollender et al., 2002; Schmitz et al., 2013; Savage et al., 2014; Gleeson et al., 2014). Nevertheless, several factors have been consistently identified in multiple studies to be associated with an increased risk of CNS relapse and recurrence including elevated levels of lactate dehydrogenase (LDH), presence of B symptoms and extra-nodal site involvement (i.e., renal, adrenal, testicular, bone marrow, and nasal sinuses) (Kridel et al., 2011; Ghose et al., 2014; Boehme et al., 2009; Haioun et al., 2000; Fletcher et al., 2014; Zhang et al., 2014).
IPI defined as International Prognostic Index is risk model developed in diffuse large B-cell lymphoma (DLBCL) patients for their identification and management (Ruppert et al., 2020) and a recent model of CNS-IPI was developed to better characterize a high-risk group de-veloping secondary CNS disease thereby enabling quick intervention with novel treatment for the management and better prognosis (Klanova et al., 2019). Recently a study using cell of origin based on Hans’ algorithm in DLBCL patients failed to establish it as prognostic factor (Probowati et al., 2022).
Therefore, this study aimed to determine the incidence of CNS involvement in patients with Diffuse large B cell lymphoma (DLBCL and describe its risk factors. Moreover, survival outcomes of interest including overall survival (OS) and progression free survival (PFS) of patients with diffuse large B cell lymphoma with CNS involvement was calculated.
Materials and Methods
Study design
This retrospective chart review, of previously treated diffuse large B cell lymphoma, was conducted in the National Guards Health Affairs, King Abdulaziz Medical City (KAMC), a tertiary hospital in Jeddah, specifically chosen because it provides a state-of-the-art practice of medical care services for the Saudi Arabian population in the Western Region. Ethical approval was obtained from the Institutional Review Board (IRB) committee of King Ab-dullah International Medical Research Center (KAIMRC).
Patients were retrospectively identified between January 2005 and December 2019 at the Princes Norah Oncology Center. Patients were included if they were 15 years old or over with biopsy-proven DLBCL, according to the current World Health Organization classification. The CNS was evaluated at diagnosis by clinical examination, brain imaging, cerebrospinal fluid flow cytometry analysis or biopsy if clinically indicated. Patients with human immunodeficiency virus-positive, double or triple hit lymphomas, or Burkitt’s lymphoma were excluded. All patients received at least one cycle of chemotherapy with curative intent. Baseline clinical characteristics including the international prognostic index (IPI), number and type of extra-nodal sites, type of frontline chemotherapy, and type of CNS prophylaxis and treatment were recorded. In patients who relapsed, the site of relapse and the type and response to salvage chemotherapy were documented.
CNS Prophylaxis
After 2010, intravenous high dose methotrexate (3.5g/m2) on day 10-15 post R-CHOP or following completion of chemotherapy for 4-6 cycles was considered for patients with high-risk group (testicular lymphoma, epidural disease, sinus involvement, bone marrow, or renal and adrenal involvement). For patients with synchronous CNS involvement, we consider R-CHOP chemotherapy alternating with intravenous HDMTX (8g/m2) as our frontline regimen. Sometimes, Intrathecal methotrexate (12mg) was considered in synchronous and early CNS relapse based on the physician’s discretion.
Statistical analysis
Descriptive analysis was used for baseline characteristics and categorical variables were compared using chi-square and Fisher-exact tests where appropriate. Overall survival (OS) was calculated from the date of pathological diagnosis to the date of the last follow-up or the date of death of any causes. Progression free survival (PFS) was calculated from the date of diagnosis to the date of disease progression, date of death or the date of the last follow-up. Kaplan-Meier survival method was used to calculate OS and PFS. A multivariate Cox proportional hazard model was also developed using stepwise regression (forward selection) with predictive variables which were significant in the univariate analyses. SPSS statistical package, version 26.0 was used for analysis.
Results
A total of 406 patients with DLBCL were identified. The median age was 58 years. Most patients had stage III and IV disease (68%) and had more than one site of extranodal involvement (65%). About 65% of the patients had intermediate to high IPI and elevated LDH was found in 67% . A large proportion of patients had high CNS-IPI (36%) and received intravenous prophylaxis of high dose methotrexate (11%), intrathecal methotrexate (3%), and both (0.5%). The majority of patients were treated with R-CHOP chemo-therapy (91%) (Table 1).
Table I.
Baseline Characteristics for the Entire Cohort of DLBCL (n=406)
| Variable | Frequency (%) | |
|---|---|---|
| Median age (range), years | 58 (15-87) | |
| Age, years | <60 | 230 (57) |
| ≥ 60 | 176 (43) | |
| Gender | Male | 236 (58) |
| Female | 170 (42) | |
| Stage | Stage I | 40 (10) |
| Stage II | 88 (22) | |
| Stage III | 62 (15) | |
| Stage IV | 216 (53) | |
| Performance status | 0 | 26 (11) |
| 1 | 117 (48) | |
| 2 | 69 (28) | |
| 3 | 27 (11) | |
| 4 | 5 (2) | |
| Extranodal involvement |
Yes | 264 (65) |
| No | 142 (35) | |
| Extranodal sites at presentation | Liver | 46 (11) |
| Bone marrow | 41 (10) | |
| Bone | 42 (10) | |
| Lung | 39 (9) | |
| Gastric | 39 (10) | |
| Kidney and adrenal | 21 (5) | |
| Sinuses | 18 (4) | |
| Thyroid | 24 (6) | |
| Breast | 12 (3) | |
| Pancreatic/colon and intestine | 19 (7) | |
| Skin | 9 (2) | |
| CNS | 9 (2) | |
| Ovarian/uterine and vagina | 5 (1) | |
| Testicular | 2 (0.5) | |
| LDH | Normal | 136 (33) |
| High | 270 (67) | |
| Cell of origin | Germinal center | 139 (34) |
| Non-Germinal center | 148(37) | |
| Not reported | 119 (29) | |
| IPI | Low risk | 37 (9) |
| Low-Intermediate risk | 100 (25) | |
| High-intermediate risk | 125 (31) | |
| High risk | 144 (35) | |
| CNS-IPI | Low | 37 (9) |
| Intermediate | 223(55) | |
| High | 146 (36) | |
| Type of CNS prophylaxis | IV methotrexate | 44 (11) |
| IT methotrexate | 14 (3) | |
| Both IV and IT methotrexate | 2 (0.5%) | |
| Type of chemotherapy |
R-CHOP | 371 (91) |
| R-CVP | 25 (6) | |
| R-EPOCH | 3 (1) | |
| R-CEOP | 5 (1) | |
| R-GDP /R-GEMOX | 2 (1) | |
| Status | Alive | 340 (84) |
| Dead | 66 (16) |
Central nervous system involvement
At a median follow-up of 6 years, In total, 17 (4%) patients had CNS involvement: 9 patients (2.2 %) at diagnosis and 8 (~2%) at relapse (Table II and Table III). All the nine patients who had CNS involvement at diagnosis had advanced-stage disease except one patient (i.e., youngest patient). Six patients had another extra-nodal involvement. Four out of nine patients had a non-germinal center phenotype, and all four patients had parenchymal rather than leptomeningeal involvement. All the patients received R-CHOP chemotherapy alternating with high dose methotrexate except one patient who received palliative/supportive treatment. Five out of nine patients achieved CR and survived (Table 2).
Table 2.
Patients with CNS involvement at the Time of Presentation (N=9)
| No. | Age | Gender | Stage | Cell of Origin | IPI | CNS IPI | Extranodal | Systemic Treatment | Site of Brain | Diagnosis | CNS Treatment | Response | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | Male | IV | Non-GCB | Intermediate | High | Lung | R-CHOP X 6 |
Parenchymal | Brain imaging | HDMTX | CR then | Dead |
| 2 | 22 | Male | II | Non-GCB | Low | Low | No | R-CHOP X 6 | Parenchymal | Brain imaging | HDMTX | CR | Alive |
| 3 | 33 | Female | IV | NR | Low | Low | No | R-CHOP X 6 | Parenchymal | Brain biopsy | Progressed on HDMTX, high dose VP16, and Cytarabine and then RT |
CR | Alive |
| 4 | 58 | Female | IV | GCB | Intermediate | Intermediate | No | R-CHOP X 5 | Leptomeningeal | Brain imaging | HDMTX | PD | Dead |
| 5 | 51 | Female | IV | Non-GCB | High | High | Adrenal | Supportive | Parenchymal | Brain imaging | Supportive | PD | Dead |
| 6 | 71 | Female | IV | GCB | High | High | Parotid + Skull | R-CHOP X 6 | Leptomeningeal | Brain imaging | HDMTX | CR | Alive |
| 7 | 20 | Female | IV | GCB | High | High | Lung+Liver+Kidney+BM | R-CHOP X6 |
Parenchymal | Brain imaging | HDMTX | CR | Alive |
| 8 | 65 | Female | IV | GCB | High | High | Bone | R-CHOP X6 |
Leptomeningeal | Brain imaging | HDMTX | CR | Alive |
| 9 | 47 | Male | IV | Non-GCB | High | High | Lung | R-CHOP X6 |
Parenchymal | Brain imaging + CSF flow cytometry | HDMTX + RT | PD | Dead |
Table 3.
Patients had Relapsed CNS Disease (n=8).
| No. | Age | Gender | Stage | Cell of Origin | IPI | CNS IPI | Extranodal | Systemic Treatment |
CNS prophylaxis |
Site of Brain | Diagnosis | CNS Treatment |
Response | Status | Time to relapse | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Male | IV | GCB | High | High | Liver + BM | R-CVP X 7 | no | Leptomeningeal | Brain imaging + CSF Flow cytometry | No | PD | Dead | 13 | Lymphoma |
| 2 | 75 | Male | IV | Non-GCB | High | High | Sinus + BM | R-CVP X 5 | no | Parenchymal | Brain imaging | palliative RT | PD | Dead | 17 | Lymphoma |
| 3 | 46 | Female | IV | Non-GCB | High | High | BM | R-CHOP X 7 | no | Parenchymal | Brain imaging | R-HDMTX and HDAC | PD | Dead | 11 | Lymphoma |
| 4 | 58 | Female | III | Non-GCB | Intermediate | High | No | R-CHOP X 6 | Radiotherapy | Leptomeningeal | Brain imaging | Supportive | PD | Dead | 13 | Lymphoma |
| 5 | 47 | Male | III | GCB | Intermediate | Intermediate | Gastric | R-CHOP X 6 | No | Leptomeningeal | Brain imaging | HDMTX + R-HDAC X1 + ASCT | CR | Alive | 5 | -------- |
| 6 | 36 | Female | IV | NR | High | High | Lung + Bone | R-CHOP X 6 | No | Leptomeningeal | Brain imaging | HDMTX | CR | Lost to follow up | 10 | --------- |
| 7 | 50 | Female | IV | GCB | High | High | Liver + Ovary | R-CHOP X 6 | HDMTX | Paarenchymal | Brain imaging | Rituximab +Cytarabine +Etoposide +ASCT |
PR | Dead | 19 | Neutropenic fever |
| 8 | 40 | Male | IV | Non-GCB | Intermediate | Intermediate | Gastric | R-CHOP X 6 | HDMTX | Leptomeningeal | CSF Flow cytometry | IT MTX+ R-ESHAP | PR | Alive | 6 | -------- |
For those patients who had CNS relapse (Table 2), the median time to relapse was 11.8 months (range 5 to 19 months), and most of the patients experienced a relapse in the first 5-13 months. All patients had an advanced stage, extra-nodal involvement, intermediate to high CNS-IPI, and 5 out of 8 had leptomeningeal disease. Only 3 patients received prophylactic high dose methotrexate, and one patient received radiotherapy. Only two patients are alive: one patient received high dose methotrexate and high dose Ara C followed by high dose chemotherapy and autologous stem cell transplant. Another patient received salvage R-ESHAP for systemic relapse alternating with intrathecal MTX and waiting for stem cell transplant (Table 3).
Risk factors for CNS involvement
Table IV shows the baseline characteristic between patients who had CNS involvement and those who didn’t. Those who developed CNS involvement are younger in age, with ad-vanced stage, high CNS-IPI, and extra-nodal involvement compared to the non-CNS in-volvement group (Table 4).
Table 4.
Baseline Characteristic between Patients with and without CNS Involvement
| Variable | No CNS involvement | CNS involvement | Total | p-value | |
|---|---|---|---|---|---|
| Stage of the cancer | I | 40 (100.0) | 0 | 40 | 0.202** |
| II | 87 (98.9) | 1 (1.1) | 88 | ||
| III | 60 (96.8) | 2 (3.2) | 62 | ||
| IV | 202 (93.5) | 14 (6.5) | 216 | ||
| Total | 389 | 17 | 406 | ||
| Extranodal | Yes | 249 (94.3) | 15 (5.7) | 264 | 0.040* |
| No | 140 (98.6) | 2 (1.4) | 142 | ||
| Total | 389 | 17 | 406 | ||
| Age | <60 years | 216 (93.9) | 14 (6.1) | 230 | 0.029* |
| ≥60 years | 173 (98.3) | 3 (1.7) | 176 | ||
| Total | 389 | 17 | 406 | ||
| LDH | Normal value | 130 (95.6) | 6 (4.4) | 136 | 0.535** |
| Abnormal value | 259 (95.9) | 11 (4.1) | 270 | ||
| Total | 389 | 17 | 383 | ||
| IPI | Low Risk | 36 (97.3) | 1 (2.7) | 37 | 0.605** |
| Low-Intermediate Risk | 97 (97.0) | 3 (3.0) | 100 | ||
| High-Intermediate Risk | 121 (96.8) | 4 (3.2) | 125 | ||
| High Risk | 135 (93.8) | 9 (6.2) | 144 | ||
| Total | 389 | 17 | 406 | ||
| CNS IPI | Low | 36 (97.3) | 1 (2.7) | 37 | 0.372* |
| Intermediate | 216 (96.9) | 7 (3.1) | 223 | ||
| High | 137 (93.8) | 9 (6.2) | 146 | ||
| Total | 389 | 17 | 406 | ||
| IT methotrexate | Yes | 14 (100.0) | 0 | 14 | 0.544** |
| No | 375 (95.7) | 17 (4.3) | 392 | ||
| Total | 389 | 17 | 406 | ||
| IV methotrexate | Yes | 34 (77.3) | 10 (22.7) | 44 | <0.001** |
| No | 355 (98.1) | 7 (1.9) | 362 | ||
| Total | 389 | 17 | 406 | ||
| Both (IV and IT methotrexate) |
Yes | 1 (50.0) | 1 (50.0) | 2 | 0.082** |
| No | 388 (96.0) | 16 (4.0) | 404 | ||
| Total | 389 | 17 | 406 |
The risk of CNS relapse among patients with high CNS-IPI at presentation was 3.4 % (n=5/146). Of the 146 patients, 18 received the prophylactic HDMTX and 3 of them had CNS relapse. Unfortunately, 2 out of 128 who did not receive the prophylactic HDMTX had CNS relapse. On the other hand, the risk of CNS relapse in the intermediate CNS-IPI group was 1.8% (n=4/223). The prophylactic HDMTX was given to 25 patients and 2 of them had CNS relapse and in 198 patients who did not receive the prophylactic HDMTX, 2 had CNS relapse (Table 5).
Table 5.
Risk of CNS Involvement in Terms of CNS IPI and between Patients that Received and didn’t Receive HDMTX
| Cases of CNS Involvement | |||
|---|---|---|---|
| CNS IPI | No. of Cases/ Received HDMTX | No. of Cases/ Not received HDMTX | Risk of CNS involvement |
| Intermediate (n=223) | 2/25 (8%) | 2/198 (1%) | 4/223 (1.8%) |
| High (n=146) | 3/18 (16.7%) | 2/128 (1.6%) | 5/146 (3.4%) |
| Risk of CNS involvement | 5/43 (11.6%) | 4/326 (1.2%) | |
Survival outcome
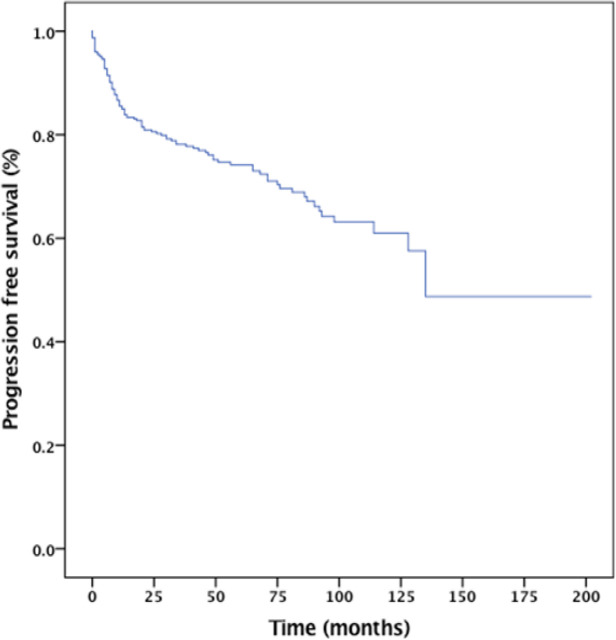
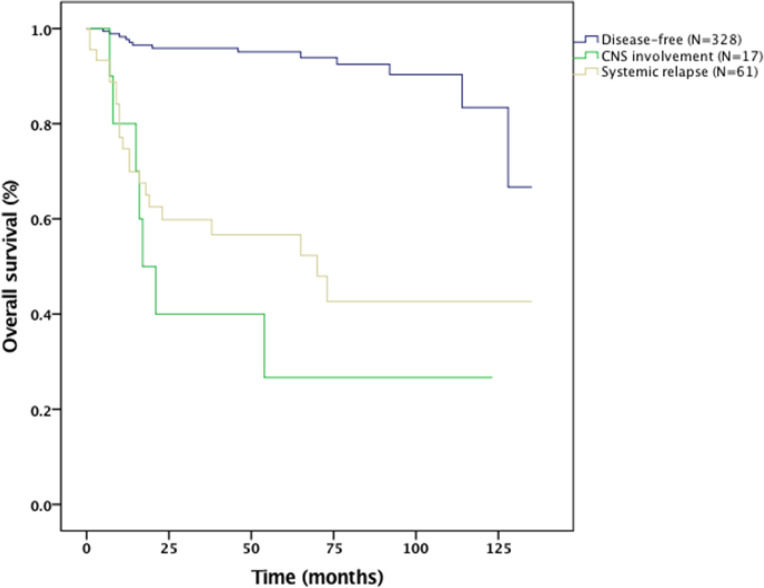
The 5-year progression-free survival and overall survival rates for the entire cohort were 73% and 84%, respectively (Figure 1 and 2). The median OS for those who had CNS involvement was 17 months. The 2-year OS for the CNS involvement group was only 40% (Figure III). The results from the Cox proportional hazard model using the forward stepwise method suggested that IPI, bone marrow involvement, and CNS involvement were poor prognostic factors for survival (Table 6).
Figure 1.
Progression Free Survival the Entire Cohort
Figure 2.
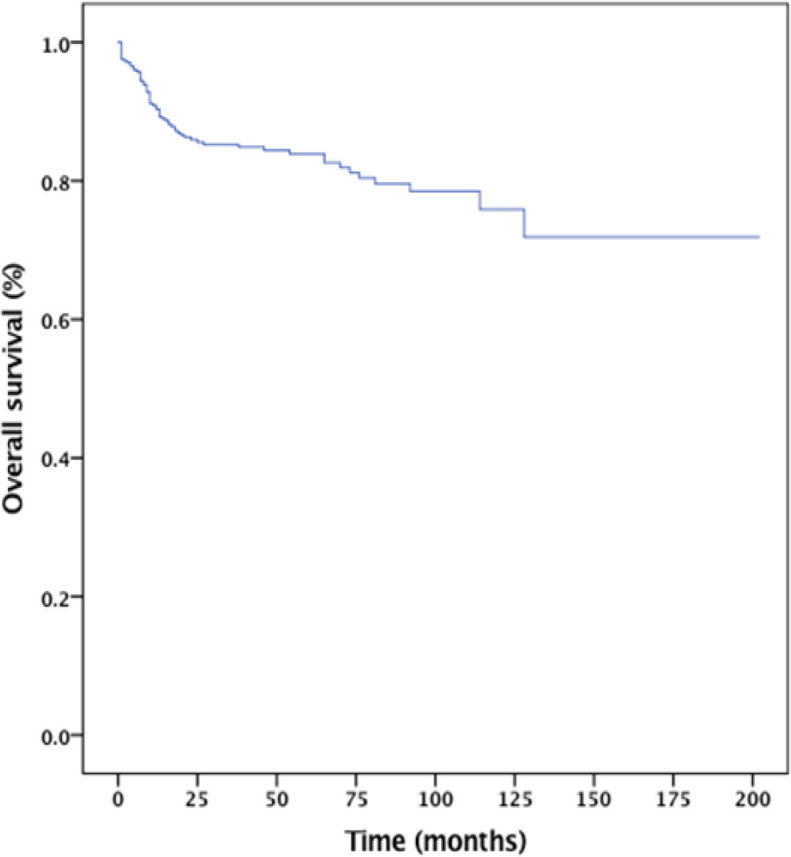
Overall Survival of the EntireCohort
Figure 3.
Overall survival of patients with CNS involvement and CNS relapse
Table 6.
Univariate and Multivariate Analysis of Progression Free Survival Prognostic Factors
| Variable | Univariate Analysis HR (95% CI) |
P value | Multivariate Analysis HR (95% CI) |
P value |
|---|---|---|---|---|
| IPI | 1.82 (1.23-2.68) | 0.003 | 1.72 (1.04-2.83) | 0.033 |
| Extranodal | 0.60 (0.33-1.09) | 0.1 | 1.22 (0.61-2.42) | 0.56 |
| LDH (Normal vs. High) | 2.03 (1.11-3.714) | 0.02 | 1.44 (0.72-2.87) | 0.294 |
| Bone marrow involvement | 0.35 (0.186-0.68) | 0.002 | 0.38 (0.19-0.72) | 0.005 |
| CNS involvement | 0.20 (0.06-0.66) | 0.008 | 0.11 (0.03-0.38) | 0.001 |
Discussion
This study demonstrates that synchronous and early CNS involvement is a rare phenomenon that occurs exclusively in advanced stage DLBCL with intermediate or high IPI, and extra-nodal presentation, which reinforces the importance of CNS evaluation at baseline for patients with known risk factors for CNS relapse. The risk of synchronous CNS involvement in our study was 2.2%, the majority were in advanced stages with extra-nodal involvement. There is no standard of care for the management of DLBCL with synchronous CNS involvement, and most of the data for CNS penetrating chemotherapy were extrapolated from primary CNS lymphoma studies, and R-CHOP chemotherapy alternating with HDMTX +/- high dose Cytarabine become the preferred option. In our study, all patients with synchronous CNS involvement received R-CHOP alternating with HDMTX except one patient, and more than 50% of them achieved complete remission and survived.
The risk of CNS relapse in our study was 2% which is like what was found in other studies (Boehme et al., 2009; Gleeson et al., 2017; El-Galaly et al., 2017; Ferreri AJ, 2014). This could be explained by the effect of chemo-immunotherapy and the use of CNS prophylaxis for a predefined high-risk groups as testicular lymphoma, epidural disease, sinus involvement, bone marrow, or renal and adrenal involvement.
In our study, all the patients with CNS involvement received high-dose methotrexate-based therapy with or without Ara-C and only two patients with early relapse underwent autologous stem cell transplant. The median OS survival for this group was 17 months, and the 2-year OS was 40%. This is consistent with the outcome from other studies (Wight et al., 2019). The Australasian lymphoma alliance reported the outcome of DLBCL with synchronous CNS involvement, the 2-years OS survival was 44% for those who were treated with HDMTX and other CNS conservative strategies (Doolittle et al., 2008).
The role of high-dose chemotherapy and consolidative ASCT in relapsed CNS lymphoma as well as in primary CNS has been previously reported and suggested by multiple studies (Bromberg et al., 2013; Williams et al., 1994; Van Besien et al., 1998; Ferreri et al., 2016; Damaj et al., 2015). However, the role of ASCT in upfront consolidation in a synchronous DLBCL remains controversial and no randomized clinical trials support this approach. In a study from LYSA and the LOC network, Damaj et al. reported on the outcome of 60 patients with concomitant systemic and CNS non-Hodgkin lymphoma, 3-year PSF, and OS for the entire group were 42±7% and 44±7%, respectively. Whereas, in the same study, high-dose chemotherapy and ASCT were performed in 18 patients DLBCL in the first remission, and the 3-year PFS and OS were 75% and 75%, respectively (Zucca et al., 2003). In our study, none of the patients had upfront ASCT consolidation, and only two patients underwent ASCT in the CNS relapsed setting.
The role of CNS prophylaxis with intrathecal or systemic chemotherapy has been analyzed previously with conflicting results (Boehme et al., 2009; Zahid et al., 2016; McMillan et al., 2013; Schmitz et al., 2016; Ollila et al., 2018). In our study, only 11.6 % (43 out of 369 patients) with high or intermediate CNS-IPI received prophylactic HDMTX, thus indicating that the physicians in our center used a risk models other than CNS-IPI to assess the risk of CNS relapse (published in 2016). This risk model relied primarily on the specific extra-nodal involvement as testicular, sinuses, renal or adrenal. The risk associated with exra-nodal involvement and CNS spread may be related to genomic subtypes of these lymphoma and molecular features as (MYD88/CD79B- mutated) (Ayed et al., 2018). It demonstrates the need for a prospective clinical trial to have a standardized risk model when considering CNS prophylaxis. Additionally, those who received HDMTX were at higher risk for CNS relapse than others who did not receive CNS prophylaxis. The role of HDMTX in this group was to lower the risk of relapse but not to eliminate it. Furthermore, Ferreri et al. (2016) reported the risk and type of CNS prophylaxis retrospectively in 200 patients with DLBCL. He found that the risk of CNS relapse was 12% for patients treated with IT or inadequate prophylaxis compared to 0% for patients managed with intravenous HDMTX prophylaxis.
Our study has a few limitations. Firstly, the retrospective nature of the study with different treatment strategies towards synchronous CNS involvement. Secondly, the cell of origin was missing in about one-third of the patients, and molecular features as MYC/BCL2 were not requested routinely for all patients with DLBCL. Thirdly, a small sample of patients with synchronous CNS involvement, makes it difficult to generalize treatment recommendations. There is a variation between institutions on the optimal approach for high-risk CNS involvement prophylaxis. Most of the experts are favoring intravenous HDMTX. However, intrathecal (IT) MTX is still an acceptable option. Moreover, the timing and the number of the cycles are controversial and vary between institutions. We are lacking prospective controlled studies that are directly comparing those approaches. However, retrospective studies show that the risk of CNS relapse may be lower with intravenous HDMTX compared to (IT) MTX alone. Our study includes many patients with longer follow up and confirms prior findings regarding the risk of CNS relapse. In our study, the definition of high-risk CNS was unified and based on a risk model rather than CNS-IPS, and we used only HDMTX as CNS prophylaxis.
Although more than 50% of patients with the Synchronous CNS in our study experience a favorable response to chemotherapy with long-term survival, the overall prognosis remains poor. Ongoing clinical trials investigating different novel agents that cross blood-brain barrier in the management of diffuse large B cell lymphoma, that might reduce further the risk of CNS relapse and improve the outcome of synchronous CNS lymphoma. Lenalidomide data were analyzed from two R2CHOP clinical trials, 136 patients were included with a median follow-up of 48.2 months, only one patient developed a CNS relapse (0.7%) (Soussain et al., 2019). Ibrutinib also showed activity in relapsed and refractory PCNS (Soussain et al., 2019; Yuan et al., 2021).
In conclusions, CNS involvement in diffuse large B cell lymphoma carries a poor prognosis. Patients who developed CNS involvement in this study are younger in age, with advanced stage, high CNS-IPI, and extra-nodal involvement. The median OS for this group was 17 months, and the 2-year OS was 40%. IPI, bone marrow involvement, and CNS involvement were poor prognostic factors for survival. Aggressive CNS-directed therapy should be considered, especially in young fit patients.
Author Contribution Statement
All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript. MM conceptualized the study, designed the study, surveyed the patients charts, searched the existing literature, and wrote the entire manuscript. SSA searched the existing literature, wrote the manuscript and revised it in context. ZA, AA, AA, WA, SH, RA, and AA contributed equally to the data collection, and proofread the final manuscript. MAK statistically analyzed the collected data.
Acknowledgments
Funding
The project pertaining to this publication was scrutinized and approved by the Institutional Ethic and Research Board (IRB) of King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) & King Abdullah International Medical Research Center (KAIMRC). No funding was involved in implementing and running this project.
Ethical approval
A proper ethical approval as per Helsinki protocol was taken prior to carrying out this study via Institutional Research Board. This study was approved by the Institutional Review Board (NRJ21J/032/02; Dated 05th of July, 2021) of King Abdullah International Medical Research Centre (KAIMRC), a research wing of KSAU-HS, Jeddah).
Consent to participate
A due written informed consent was taken from every participant and or their parents/legal guardians of this study.
Availability of data
The raw data is available on request from the corresponding authors.
Conflict of Interest
There is no conflict of interest in between the authors.
References
- Arkenau HT, Chong G, Cunningham D, et al. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann Oncol. 2007;18:541–5. doi: 10.1093/annonc/mdl434. [DOI] [PubMed] [Google Scholar]
- Ayed AO, Chiappella A, Pederson L, et al. CNS relapse in patients with DLBCL treated with lenalidomide plus R-CHOP (R2CHOP): analysis from two phase 2 studies. Blood Cancer J. 2018;8:63. doi: 10.1038/s41408-018-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113:3896–902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- Bromberg JE, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation--an International Primary Central Nervous System Lymphoma Study Group project. Haematologica. 2013;98:808–13. doi: 10.3324/haematol.2012.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation A 60-case retrospective study from LYSA and the LOC network. Haematologica. 2015;100:1199–206. doi: 10.3324/haematol.2015.126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085–93. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- El-Galaly TC, Villa D, Michaelsen TY, et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: An international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195–203. doi: 10.1016/j.ejca.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–27. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- Fletcher CD, Kahl BS. Central nervous system involvement in diffuse large B-cell lymphoma: an analysis of risks and prevention strategies in the post-rituximab era. Leuk Lymphoma. 2014;55:2228–40. doi: 10.3109/10428194.2013.869326. [DOI] [PubMed] [Google Scholar]
- Ghose A, Kundu R, Latif T. Prophylactic CNS directed therapy in systemic diffuse large B cell lymphoma. Crit Rev Oncol Hematol. 2014;91:292–303. doi: 10.1016/j.critrevonc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Counsell N, Cunningham D, et al. Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol. 2017;28:2511–16. doi: 10.1093/annonc/mdx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Cunningham D, Hawkes EA, et al. Risk of CNS relapse with diffuse large B-cell lymphoma (DLBCL) in the rituximab era: results from the UK NCRI R-CHOP 14 v 21 Trial. Blood. 2014;124:1723. [Google Scholar]
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- Haioun C, Besson C, Lepage E, et al. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Ann Oncol. 2000;11:685–90. doi: 10.1023/a:1008394827806. [DOI] [PubMed] [Google Scholar]
- Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13:1099–107. doi: 10.1093/annonc/mdf175. [DOI] [PubMed] [Google Scholar]
- Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood. 2019;133:919–26. doi: 10.1182/blood-2018-07-862862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridel R, Dietrich PY. Prevention of CNS relapse in diffuse large B-cell lymphoma. Lancet Oncol. 2011;12:1258–66. doi: 10.1016/S1470-2045(11)70140-1. [DOI] [PubMed] [Google Scholar]
- Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol. 2008;88:193–201. doi: 10.1007/s00277-008-0645-y. [DOI] [PubMed] [Google Scholar]
- McMillan A, Ardeshna KM, Cwynarski K, et al. Guideline on the prevention of secondary central nervous system lymphoma: British Committee for Standards in Haematology. Br J Haematol. 2013;163:168–81. doi: 10.1111/bjh.12509. [DOI] [PubMed] [Google Scholar]
- Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol. 2018;19:38. doi: 10.1007/s11864-018-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probowati W, Purwanto I, Anggorowati N, et al. Cell of Origin Based on Hans’ Algorithm as Prognostic Factor in Diffuse Large B-Cell Lymphoma: A Clinicopathologic and Survival Study. Asian Pac J Cancer Care. 2022;7:71–8. [Google Scholar]
- Ruppert AS, Dixon JG, Salles G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. 2020;135:2041–8. doi: 10.1182/blood.2019002729. [DOI] [PubMed] [Google Scholar]
- Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146:489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Nickelsen M, Savage KJ. Central Nervous System Prophylaxis for Aggressive B-cell Lymphoma: Who, What, and When? Hematol Oncol Clin North Am. 2016;30:1277–91. doi: 10.1016/j.hoc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Zeynalova S, Glass B, et al. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann Oncol. 2012;23:1267–73. doi: 10.1093/annonc/mdr440. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Zeynalova S, Nickelsen M, et al. A new prognostic model to assess the risk of CNS disease in patients with aggressive B-Cell lymphoma. Hematol Oncol. 2013;31:111. [Google Scholar]
- Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;223:5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–30. doi: 10.1016/j.ejca.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Pileri S, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita N, Takasaki H, Ishiyama Y, et al. Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2015;56:725–9. doi: 10.3109/10428194.2014.931953. [DOI] [PubMed] [Google Scholar]
- van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91:1178–84. [PubMed] [Google Scholar]
- Wight JC, Yue M, Keane C, et al. Outcomes of synchronous systemic and central nervous system (CNS) involvement of diffuse large B-cell lymphoma are dictated by the CNS disease: a collaborative study of the Australasian Lymphoma Alliance. Br J Haematol. 2019;187:174–84. doi: 10.1111/bjh.16064. [DOI] [PubMed] [Google Scholar]
- Williams CD, Pearce R, Taghipour G, et al. Autologous bone marrow transplantation for patients with non-Hodgkin’s lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome--a report by the European Bone Marrow Transplant Lymphoma Registry. J Clin Oncol. 1994;12:2415–22. doi: 10.1200/JCO.1994.12.11.2415. [DOI] [PubMed] [Google Scholar]
- Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol. 2010;85:6–10. doi: 10.1111/j.1600-0609.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Ding T, Wang S, et al. Current and emerging therapies for primary central nervous system lymphoma. Biomark Res. 2021;9:32. doi: 10.1186/s40364-021-00282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid MF, Khan N, Hashmi SK, Kizilbash SH, Barta SK. Central nervous system prophylaxis in diffuse large B-cell lymphoma. Eur J Haematol. 2016;97:108–20. doi: 10.1111/ejh.12763. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymph. 2014;55:509–14. doi: 10.3109/10428194.2013.811239. [DOI] [PubMed] [Google Scholar]
- Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20–7. doi: 10.1200/JCO.2003.11.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data is available on request from the corresponding authors.