Abstract
Echinostomatid digeneans belonging to the genus Rhopalias are intestinal trematodes found mainly in opossums in the New World. The genus comprises seven species, but their life cycles and intermediate hosts have been unknown until now. During our long-term study carried out in freshwater habitats within the state of Minas Gerais, Southeast Brazil, echinostomatid cercariae lacking collar spines were found in planorbid snails Biomphalaria glabrata, Biomphalaria straminea, Drepanotrema lucidum and Gundlachia ticaga in six different batches of snail samples collected between 2010 and 2019. Morphologically, the larvae reported herein are morphologically consistent with each other and characterized by the presence of 2–3 large ovoid or spherical corpuscles in each main duct of the excretory system, resembling to Cercaria macrogranulosa previously described from the same region of Brazil. Partial sequences of the ITS (ITS1-5.8S-ITS2) region and 28S gene of the nuclear ribosomal RNA operon, and partial sequences of mitochondrial nad1 and cox1 genes were obtained and compared with the data available for members of the family Echinostomatidae. Nuclear markers indicate that all samples of cercariae evaluated in the present study can be assigned to Rhopalias, but distinct from North American isolates of Rhopalias macracanthus, Rhopalias coronatus and Rhopalias oochi (divergence 0.2–1.2% in 28S and 0.8–4.7% in ITS). The lack of differences verified in both 28S and ITS in 5 out 6 studied samples suggested that they belong to the same species. However, nad1 sequences revealed that our cercariae correspond to three distinct species of Rhopalias (interspecific divergence: 7.7–9.9%), named here as Rhopalias sp. 1, found in B. straminea and G. ticaga, Rhopalias sp. 2 found in B. glabrata and D. lucidum, and Rhopalias sp. 3 also found in D. lucidum. They also differ by 10.8–17.2% from a North American isolate of R. macracanthus sequenced in this study. The cox1 sequences obtained for Rhopalias sp. 1 and Rhopalias sp. 2 (but not Rhopalias sp. 3) reveal that they are distinct from North American isolates of R. macracanthus (genetic divergence 16.3–16.5% and 15.6–15.7%, respectively), R. coronatus (9.2–9.3% and 9.3–9.5%) and Rhopalias oochi (9.0% and 9.5–10.1%). Encysted metacercariae with general morphology similar to that of the body of cercariae were found in tadpoles of Rhinella sp. from the same stream where snails harbored Rhopalias sp. 2, suggesting that the amphibians could act as second intermediate hosts of species of Rhopalias. Data obtained provide the first insights into the life cycle of this unusual echinostomatid genus.
Introduction
Echinostomatid digeneans belonging to the genus Rhopalias Stiles and Hassall, 1898 are intestinal flukes found mainly in didelphid opossums (occasionally reported in bats, rodents, and birds) in the Americas [1–4]. Species of the genus Rhopalias are unique among the echinostomatoideans due to the presence of two eversible proboscises armed with spines situated symmetrically on either side of the oral sucker [1, 3–5]. In the past, Rhopalias was included in the family Rhopaliidae Looss, 1899, as the type and only genus. However, this family was recently synonymized with the Echinostomatidae Looss, 1899 by Tkach et al. [6] based on molecular phylogenetic analyses.
Currently, seven species of Rhopalias are considered valid [4, 5]. The type-species of the genus, Rhopalias coronatus (Rudolphi, 1819), was originally described from marsupials in Brazil [3, 7, 8]. Although reports of species of Rhopalias in definitive hosts are not uncommon, the intermediate hosts and life cycle of these trematodes remain unknown. As with most digenean groups, this deficiency in understanding basic life-history can partially be explained by the methodological difficulties associated with classical experimental approach used to study helminth life cycles [9]. Probably the apparent specificity to marsupials as definitive hosts makes experimental studies involving Rhopalias more complicated compared with other echinostomes, given the difficulties of maintaining such types of wild vertebrates under laboratory conditions.
The introduction of DNA sequencing has accelerated the elucidation of life cycles of trematodes [9, 10], including representative species of the superfamily Echinostomatoidea Looss, 1902 [11–17]. However, no sequence data from Rhopalias spp. were available. Recently, sequence data for adult stages of three species of Rhopalias in North America have been made available [5, 6, 18], enabling molecular matching with other life cycle stages.
Herein, we present the results of morphological and molecular studies of echinostomatid larvae found in planorbid snails from Brazil that enabled us to identify for the first time cercariae of Rhopalias spp. Moreover, we obtained evidence of the probable involvement of anurans as the second intermediate hosts of these digeneans. We also examined phylogenetic relationships within Rhopalias using new and previously published sequence data.
Materials and methods
Sample collection
The present study is part of long-term malacological and helminthological surveys carried out in freshwater habitats across the state of Minas Gerais, southeast Brazil, between 2010 and 2019. Snails were collected with a dip net and transported to the laboratory, where they were individually placed in polystyrene plates and exposed to light for about 2 hrs. After this period, the water was examined with a stereomicroscope to detect the shedding of cercariae. The plates were examined again the next day, before and after a new period of photostimulation. Some of the infected snails were crushed between glass plates and examined for the presence of rediae. The taxonomic identifications of the snail species were based on morphological traits (shell and internal anatomy) [19–22]. The species, localities and date of collection of the snails infected with larval trematodes used for this study are provided in Table 1.
Table 1. Data on the studied cercarial samples collected from planorbid snails in the state of Minas Gerais, Brazil.
| HOST | LOCALITY | GCS | DATE | GENBANK ACCESSION NUMBERS | |||
|---|---|---|---|---|---|---|---|
| 28S | ITS | cox1 | nad1 | ||||
| Biomphalaria straminea | Belo Horizonte | 19°50’18"S, | 06-06-2012 | OP972553 | OP972548 | OP971532 | OP980993 |
| 43°59’40"W | |||||||
| Gundlachia ticaga | Belo Horizonte | 19°50′17"S, | 05-11-2017 | — | OP972549 | — | OP980994 |
| 43°57′32"W | |||||||
| Biomphalaria glabrata | Januária | 15°29’41"S, | 06-07-2010 | OP972554 | — | OP971533 | OP980995 |
| 45°08’59"W | |||||||
| Biomphalaria glabrata | Januária | 15°29’41"S, | 08-29-2019 | OP972555 | OP972550 | — | OP980996 |
| 45°08’59"W | |||||||
| Drepanotrema lucidum | Patos de Minas | 18°42’33"S, | 07-19-2019 | OP972556 | OP972551 | OP971534 | OP980997 |
| 46°35’04"W | |||||||
| Drepanotrema lucidum | Dores do Indaiá | 19°29’41"S, | 12-25-2013 | OP972557 | OP972552 | — | OP980998 |
| 45°36’46”W | |||||||
Molecular study
Aiming to identify and distinguish the species found, as well as to place these within an echinostomatid phylogeny, six samples of echinostome cercariae lacking collar spines were subjected to molecular analysis. Each sample evaluated corresponds to larvae shed by a same infected snail. About 30 ethanol-fixed cercariae from each sample were used for molecular study. DNA was extracted from the pooled cercariae using the QIAamp DNA micro kit (Qiagen Ltd., Crawley, United Kingdom), according to the manufacturer’s instructions. The concentration of the extracted DNA was determined using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Wilmington, USA). Attempts were made to amplify partial fragments of 28S (primers digl2/1500R; [23]) and internal transcribed spacer region (primers BD1/BD2; [24]) regions of the nuclear ribosomal operon, and of mitochondrial genes nad1 (NDJ11 and NDJ2a; [25] or JB11 and JB12; [26]) and cox1 (Dice-1 and Dice-11 [27]; Dice-1 and BarCox-R [5]). DNA amplifications were performed by polymerase chain reaction (PCR) following the PCR conditions described by the authors listed above. PCR reactions were done using Platinum™ Hot Start PCR Master Mix (2X) (Thermo Fisher Scientific) according to the manufacturer’s instructions, 10 μM of each primer and about 50 ng of DNA. Positive PCR products were purified using polyethylene glycol 8000 (20%) (Promega, Madison, WI), according to [28].
The obtained amplicons were sequenced in both directions using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Inc., Foster City, CA) and the same primers as used in PCR reactions. Sequencing reactions were cleaned using a BigDye Sequencing Clean Up Kit (MCLAB, California, USA.) and run on an ABI 3130 automated capillary sequencer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). An adult specimen of Rhopalias macracanthus Chandler, 1932 previously collected from a specimen of the Virginia opossum, Didelphis virginiana Kerr, 1792 from North Carolina, USA, by one of the authors (VVT) was sequenced using the same primers and PCR conditions as described above.
The obtained sequences were visualized and assembled in ChromasPro (Technelysium Pty Ltd, Australia). The resulting contiguous sequences were aligned with sequences of selected echinostomatid taxa available in GenBank using MEGA X [29]. The alignments were each trimmed to the shortest sequence. Unreliable positions (ambiguous homology) in the alignments were identified and excluded using the Gblocks web server (http://phylogeny.lirmm.fr/) [30]. Six new 28S sequences (5 for the cercariae from Brazil and 1 for adult from USA) were obtained (1052–1186 bp) and final alignment consisted of 31 sequences and was 1077 bp long (4 ambiguous positions excluded). For ITS, we analyzed the whole fragment (ITS1-5.8S-ITS2) as well as the ITS2, because the whole region is not available for several taxa of the Echinostomatoidea. For this molecular marker, five new ITS sequences (994 bp) were obtained and a dataset containing 19 sequences was evaluated (954 bp long; 12 ambiguous positions removed). In the case of ITS-2, the analysis was based on 17 sequences and a trimmed alignment of 378 bp (7 ambiguous positions excluded). We obtained nad1 sequences (332–413) bp from the six samples of cercariae and the final dataset consisted of 42 sequences and 398 bp. For cox1, three sequences were generated (612 bp, 643bp and 675bp), and the final dataset consisted of 33 sequences with a trimmed alignment of 612 bp. Phylogenetic reconstructions were performed by Bayesian inference (BI) and maximum likelihood (ML) methods using the programs MrBayes v.3.2.6 [31] and MEGA X [29], respectively. The best nucleotide substitution models were determined according to the Bayesian Information Criterion in MEGA X. The best fitting models were: GTR+G+I for 28S data; K2+G for ITS and ITS2 data; GTR+G+I for nad1, and HKY+G+I for cox1. The selection of outgroups was based on the phylogeny of the Echinostomatoidea by Tkach et al. [6]. The ML trees were generated via MEGA X and the nodal support was estimated using the bootstrap method with 1,000 pseudoreplicates. BI analyzes were performed using Markov chain Monte Carlo (MCMC) in two simultaneous runs of four chains for 1,000,000 generations and sampling every 100 generations. The first 25% of the sampled BI trees were discarded as ’burn-in’. Phylogenetic trees and data files were visualized in FigTree version 1.4.3 [32]. The new sequences obtained in this work were deposited in GenBank (Table 1).
Morphological study
Cercariae that emerged from naturally infected snails were stained with vital dyes (aqueous solution of 0.05% neutral red or Nile blue sulfate), wet mounted and examined under a light microscope. Cercariae for the morphometric study were killed in water at 70°C and fixed in 10% formalin. Subsequently, they were temporarily mounted between slide and coverslip and measured using an ocular micrometer. Some of the fixed cercariae were stained in alum acetocarmine, dehydrated in graded ethanol series, cleared in beechwood creosote, and mounted on permanent slides. Rediae were studied alive on temporary wet mounts. Photographs were taken with a Leica ICC50 HD digital camera coupled to a light microscope. Morphological characterization and preliminary identification of the cercariae were based on descriptions of digenean larvae reported in South America [33–36]. All measurements in morphological descriptions are shown in micrometers as mean ± standard deviation and range in parentheses.
Experimental infection
In order to obtain metacercariae experimentally, we attempted to infect laboratory-reared snails [Biomphalaria glabrata (Say, 1818)] and fish (Poecilia reticulata Peters, 1859). These species were used as experimental hosts due to their availability in the laboratory and previous knowledge on the involvement of snails and fish as second intermediate hosts of echinostomes. The behavior of cercariae in the presence of these potential hosts was observed under a stereomicroscope. After 24hs of exposure to cercariae, the snails and fish were necropsied. We also searched for metacercariae in samples of insects, fishes, snails, and tadpoles collected in the same water bodies where snails were found infected.
Metacercariae found in tadpoles collected in the stream where snails were found infected were used for an experimental infection study. We suspected they could be of the same species based on the number of excretory corpuscles. Aiming to obtain adult parasites for taxonomic identification, a sub-sample of 50 metacercariae was orally administered to one specimen of a dexamethasone-immunosuppressed (50 mg/kg) male Swiss mouse. The infected mouse was maintained on a 12/12h light–dark cycle and allowed access to food and water ad libitum. Coproparasitological examinations by the sedimentation technique were conducted daily, starting from seven days post-infection. The mouse was euthanized via barbituric overdose (sodium pentobarbital, injected intraperitoneally) and necropsied for the search of adult parasites 14 days post-infection.
Ethics statement
The snails were collected under authorization by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) (SISBIO 52870–1). Experimental studies were approved by the local Ethics Committee on Animal Use of the Universidade Federal de Minas Gerais (CEUA -UFMG, protocol 20/2016).
Results
Molecular study
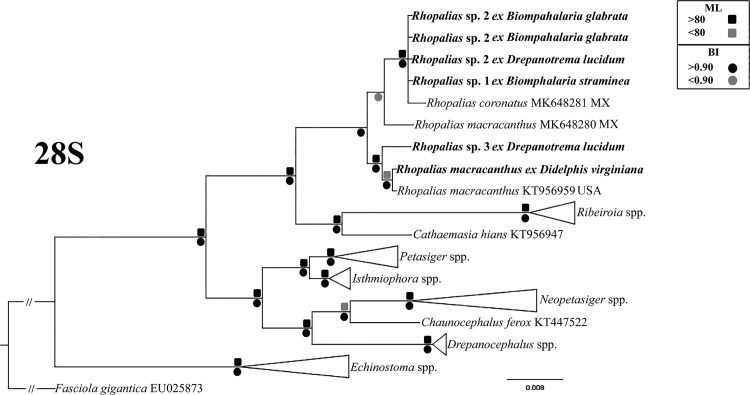
For this study, six samples of morphologically similar unspined echinostome cercariae were used in the molecular study (Table 1). Analyses of novel molecular data from these infections are consistent with the presence of three species, and suggest that all three represent species of the genus Rhopalias, referred to here as Rhopalias sp. 1–3. Our phylogenetic analyses based on the partial ITS and 28S revealed that sequences of all larval samples obtained in this study appeared in well-supported clades with sequences of the two isolates of adult R. coronatus and R. macracanthus from marsupials (Fig 1). This finding provides clear evidence that the cercariae found in this study in planorbid snails from Brazil belong to the genus Rhopalias. Most of new 28S sequences generated from cercariae evaluated in this study were identical each other, except for the cercariae found in D. lucidum from Dores do Indaiá, which differed in 1%. The genetic divergence found for this same molecular marker between the Brazilian cercariae and previously and new adult-based sequences of Rhopalias spp. ranged from 0.23 to 1.34%. The intergeneric differences between species of Rhopalias and those of the sister genera Ribeiroia [Ribeiroia ondatrae (Price, 1931) from USA, Ribeiroia sp. 2 and Ribeiroia sp. 3 from Kenya] and Cathaemasia [Cathaemasia hians (Rudolphi, 1809) from Czech Republic] were 4.1–4.9% and 2.7–3.1%, respectively.
Fig 1. Phylogenetic relationships between Rhopalias spp. found in planorbids from Brazil and members of the family Echinostomatidae inferred from partial 28S sequence data (1077 bp).
The trees were generated by Bayesian Inference (BI) and Maximum Likelihood (ML) methods. New sequences from the present study are in bold. Scale bar represents the number of nucleotide substitutions per site.
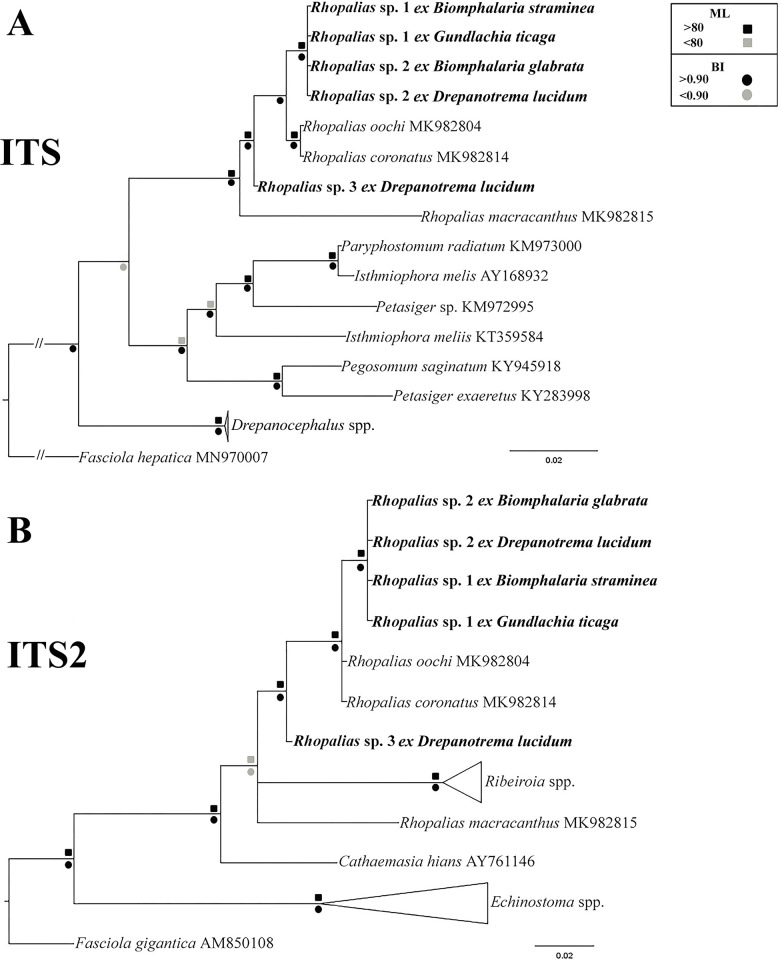
Similar to 28S data, the phylogenetic analyses based on ITS also revealed a clade formed by our samples and North American isolates of R. macracanthus, Rhopalias oochi López-Caballero, Mata-López, Pérez-Ponce de León, 2019 and R. coronatus (Fig 2A). The genetic divergence between the Brazilian samples was 0–1.2%; they differed from North American isolates of R. macracanthus, R. oochi and R. coronatus by 0.6–4.7%. Only ITS-2 data were available for comparison with the species of the sister genera Ribeiroia and Cathaemasia. also revealed that the species of both genera form a clade with Rhopalias spp. (Fig 2B), although in this tree the clade of Ribeiroia spp. was nested among lineages of Rhopalias. The intergeneric differences between Rhopalias spp. and Ribeiroia spp. and C. hians were 7.7–8.6% and 5.6–7.2%, respectively.
Fig 2.
Phylogenetic relationships between Rhopalias spp. found in planorbids from Brazil and members of the family Echinostomatidae inferred from (A) ITS1-5.8S-ITS-2 (954 bp) and (B) ITS2 (378 bp) sequence data. The trees were generated by Bayesian Inference (BI) and Maximum Likelihood (ML) methods. New sequences from the present study are in bold. Scale bar represents the number of nucleotide substitutions per site.
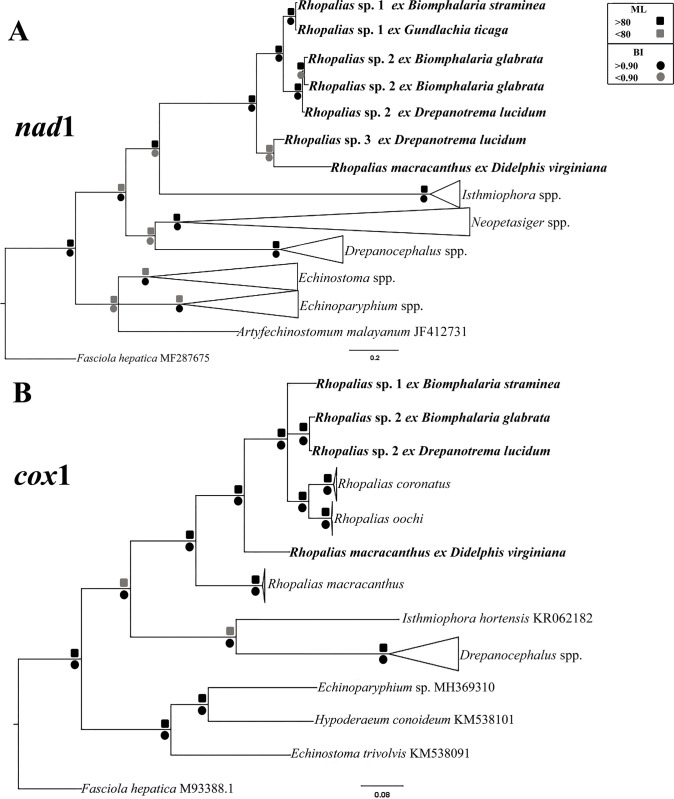
The tree resulting from molecular phylogenetic analyses based on nad1 sequences showed 6 cercarial samples sequenced in this study, in three different clades, interpreted here as representing three different species of Rhopalias (Fig 3A). The pairwise comparison between the nad1 sequences obtained for the six samples evaluated in this study is presented in Table 2. Sequences from cercariae found in Biomphalaria straminea (Dunker, 1848) and Gundlachia ticaga (Marcus & Marcus, 1962) collected in two urban lakes from Belo Horizonte, were identical and named herein Rhopalias sp. 1. Cercariae found in B. glabrata from Januária and Drepanotrema lucidum (Pfeiffer, 1839) from Patos de Minas differ by merely 2.1–2.4%, suggesting these parasites belong to the same species, named here Rhopalias sp. 2. It differs from Rhopalias sp. 1 in 7.7–8.4%. The cercariae found in D. lucidum from Dores do Indaiá differs by 9.9% and 9.8–9.9% from Rhopalias sp. 1 and Rhopalias sp. 2, suggesting it is a distinct species, Rhopalias sp. 3. Moreover, nad1 sequence data for samples of these three species formed a clade, and samples of each species grouped in the respective subclades. The nad1 sequence of the North American isolate that morphologically corresponds to R. macracanthus, showed 10.8–17.2% divergence from 3 Rhopalias spp. from Brazil collected in this study as cercariae. Moreover, in the phylogenetic analyses based on nad1, this isolate of R. macracanthus grouped in a distinct subclade with Rhopalias sp. 3, but the nodal support was low. Genetic divergences between Rhopalias spp. and selected species from 6 genera of the Echinostomatidae that were included in the analyses ranged from 20.7 to 36.1%.
Fig 3.
Phylogenetic relationships between Rhopalias spp. found in planorbids from Brazil and members of the family Echinostomatidae inferred from (A) nad1 (397 bp) and (B) cox1 (612 bp) sequence data. The trees were generated by Bayesian Inference (BI) and Maximum Likelihood (ML) methods. New sequences from the present study are in bold. Scale bar represents the number of nucleotide substitutions per site.
Table 2. Pairwise distances (uncorrected p-distance) based on nad1 sequences obtained from cercariae of Rhopalias spp. found in planorbid snails from Brazil.
| #1 | #2 | #3 | #4 | #5 | |
|---|---|---|---|---|---|
| Rhopalias sp. 1 –Biomphalaria straminea | |||||
| Rhopalias sp. 1 –Gundlachia ticaga | 0% | ||||
| Rhopalias sp. 2 –Biomphalaria glabrata | 8.28% | 8.88% | |||
| Rhopalias sp. 2 –Biomphalaria glabrata | 7.67% | 8.31% | 1.83% | ||
| Rhopalias sp. 2 –Drepanotrema lucidum | 8.43% | 8.43% | 2.41% | 2.11% | |
| Rhopalias sp. 3 –Drepanotrema lucidum | 9.94% | 9.94% | 9.76% | 9.94% | 9.94% |
Data obtained for cox1 gene confirmed the presence of two species in the samples evaluated for this marker. Rhopalias sp. 1 differs by 7.3–7.9% from Rhopalias sp. 2. The isolates of this last species from Patos de Minas and Januária differed 1.31%. The two sequenced Brazilian species differ by 9.2–9.5% from R. coronatus and 9.0%–10.1% from R. oochi. In the cox1 tree, the Mexican isolates of R. coronatus and R. oochi appeared in a separate sub-clade from Brazilian Rhopalias sp. 1 and Rhopalias sp. 2 (Fig 3B). The two Brazilian isolates differ by 15.2–16.5% from a Mexican isolate of R. macracanthus and by 12–13.1% from the North American sample of the same species sequenced in this study. Notably, the divergence between the two isolates of R. macracanthus from Mexico and the USA was very high (15%) clearly demonstrating that these isolates are not conspecific. The levels of sequence divergence between Rhopalias spp. and other species of echinostomatids included in the cox1 analyses were high at 21.8–25.1%.
Morphological and experimental studies
No apparent morphological differences were found between the cercariae of the three species of Rhopalias found in different species of planorbids from different localities in the state of Minas Gerais, Brazil. Measurements of cercariae from four of the collected samples are shown in Table 3, along with the data available for similar larvae reported by different authors in South America.
Table 3. Morphometric data of larval stages of Rhopalias spp. examined in this study, and other unspined echinostome cercariae described in South America by different authors.
| Rhopalias sp. 1 | Rhopalias sp. 2 | Rhopalias sp. 2 | Rhopalias sp. 3 | Cercaria macrogranulosa | Cercaria echinostoma 2 | Cercaria guaibensis 8 | |||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Present study | Ruiz, 1952 [33] | Ostrowski de Núñez et al., 1990 [36] | Veitenheimer- Mendes, 1982 [34] | Veitenheimer- Mendes et al., 1995 [35] | ||||
| Locality | Belo Horizonte, MG, Brazil | Patos de Minas, MG, Brazil | Januária, MG, Brazil | Dores do Indaiá, MG, Brazil | Belo Horizonte, MG, Brazil | Parana River Basin, Argentina | Camaquã, RS Brazil | Porto Alegre, RS Brazil | |
| Host | Biomphalaria straminea | Drepanotrema lucidum | Biomphalaria glabrata | Drepanotrema lucidum | Biomphalaria glabrata | Drepanotrema depressissimum | Biomphalaria peregrina | Drepanotrema depressissimum | |
| Body | L | 248±12 (232–280) | 220±30 (149–269) | 217±15 (248–177) | 211±14 (186–229) | 215–283 | 242±11 (218–260) | 222–249 | 360 |
| W | 132±8 (123–157) | 118±27 (78–199) | 133±8 (149–121) | 124±14 (107–150) | 91 | 96±8 (84–105) | 111–127 | – | |
| Tail | L | 449±14 (423–478) | 438±24 (383–468) | 411±15 (432–355) | 368±18 (350–400) | 340–493 | 436±19 (420–470) | 360–407 | 420 |
| W | 52±4 (47–62) | 41±6 (36–57) | 39±4 (43–28) | 50±8 (36–57) | – | 46±4 (42–50) | 37–55 | – | |
| Oral | L | 43±1(42–45) | 38±3 (32–45) | 42±3 (45–36) | 45±4 (39–53) | 40–47 | 38±3 (31–42) | 34–41 | 45 |
| sucker | W | 43±1 (42–45) | 37±3 (32–53) | 42±3 (46–36) | 48±4 (44–53) | – | 39±3 (31–42) | – | |
| Ventral | L | 50±3 (45–57) | 47±6 (32–59) | 46±5 (63–36) | 54±11 (35–69) | 54–70 | 57±5 (48–63) | 48–67 | 55 |
| sucker | W | 56±3 (52–58) | 55±7 (35–72) | 63±4 (71–54) | 58±7 (51–70) | – | 60±5 (52–69) | – | – |
| Pharynx | L | 21±2 (18–25) | 18±4 (14–21) | 25±5(36–18) | 21±7 (12–26) | – | 20±7 (21–25) | – | – |
| W | 17±1 (15–18) | 15±2 (13–18) | 17±6 (36–11) | 20±7 (12–23) | – | 18±3 (10–23) | – | – | |
All measurements are in micrometers; mean values are followed by the standard deviation and range in parentheses. Abbreviations: L: length; W: width.
We did not observe any change in the behavior of cercariae of Rhopalias sp. 1 placed in contact with laboratory-reared B. glabrata. No metacercariae were found in these invertebrates 24 hrs. after exposure, indicating that at least this snail species was not a suitable second intermediate host for this digenean. On the other hand, encysted metacercariae and free tails were observed in the wells containing P. reticulata thirty minutes after the exposure to cercariae. Moreover, a few cysts were found in the oral cavity of the fish necropsied 24 hrs after infection. Specimens of experimentally infected fish examined after this period had a few dead metacercariae in the oral cavity. However, beyond the fact that the contact with these fish induced encystment, our data suggest that P. reticulata is likely not a suitable second intermediate host for species of Rhopalias. On the other hand, we found metacercariae morphologically reminiscent of Rhopalias cercariae in the kidneys of Rhinella sp. tadpoles caught in the same stream where Rhopalias sp. 2 from Januária were collected. No adult worm was found in the mouse experimentally orally inoculated with metacercariae obtained from these naturally infected tadpoles.
Taxonomic summary
Class Trematoda Rudolphi, 1808
Superfamily Echinostomatoidea Looss, 1899
Family Echinostomatidae Looss, 1899
Genus Rhopalias Stiles and Hassall, 1898
Rhopalias sp. 1
Hosts: Biomphalaria straminea and Gundlachia ticaga (Mollusca: Planorbidae)
Locality: Belo Horizonte, Minas Gerais, Brazil
Prevalence: 1/55 (B. straminea) and 3/493 (G. ticaga)
Deposited material: UFMG-TRE 128 and UFMG-TRE 129
Description
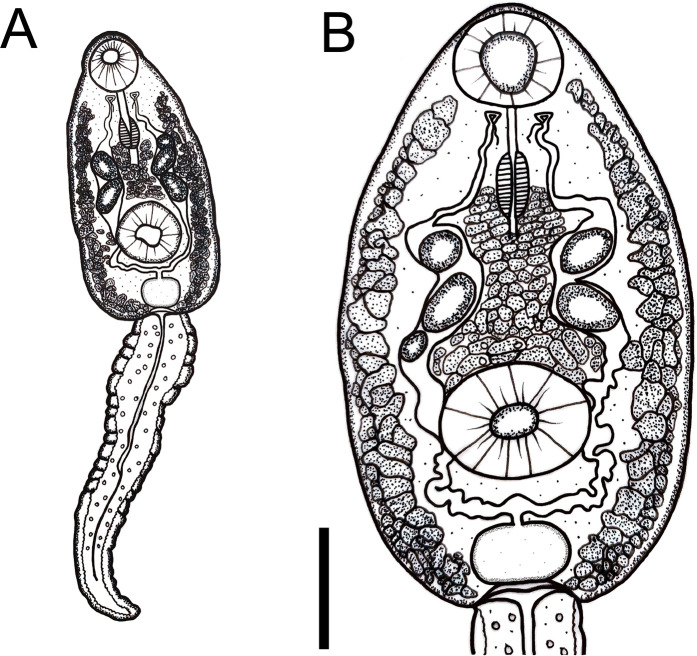
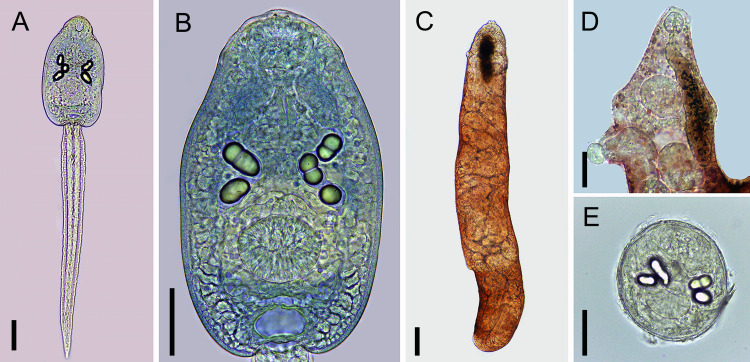
Cercariae (Figs 4A and 4B and 5A and 5B)
Fig 4. Cercaria of Rhopalias from Biomphalaria straminea from Brazil.
(A) Whole view. (B) Detail of body. Cercariae from the same infected snail were genetically identified as Rhopalias sp. 1. Scale bars: 50 μm.
Fig 5. Larval stages of Rhopalias found in Biomphalaria straminea from Brazil.
(A) Whole view of the cercaria stained with Nile blue sulphate. (B) Detail of the cercarial body stained with Nile blue sulphate. (C) Whole view of a redia. (D) Detail of the anterior region of a redia. (E) Metacercaria obtained experimentally in Poecilia reticulata. Cercariae from the same infected snail were molecularly identified as Rhopalias sp. 1. Scale bars: (A, B, D, E) 50 μm, (C) 100 μm.
Body ovoid. Head collar without spines. Oral sucker spherical, subterminal. Prepharynx prominent, pharynx muscular, elongate. Ventral sucker spherical, post-equatorial, larger than oral sucker. Intestinal caeca absent. Cystogenous glands lateral along either side of body. Two or three large ovoid or spherical corpuscles (22–28 by 16–20) present inside each main duct of excretory system. Genital primordium, observed only in carmine-stained larvae, comprised of two masses of cells dorsal to ventral sucker. Excretory vesicle saccular, with a very short stem branching into two lateral arms extending anteriorly to oral sucker, with two to three spherical corpuscles (22–28 by 16–20) within each arm. Tail simple, assuming a figure-eight shape during swimming.
Rediae (Fig 5C and 5D)
Body elongated. Pharynx subterminal. Caecum short. Several developed cercariae as well as developing cercariae and germ balls (not counted) present.
Metacercariae (Fig 5E)
Cyst ovoid to spherical, with thin, transparent cyst wall. Larvae with 2 to 3 large corpuscles inside each main excretory duct. Cyst obtained 24hrs after experimental infection of P. reticulata with cercariae measured 132 ± 7 (120–143) by 109 ± 3 (102–116).
Rhopalias sp. 2
Hosts: Biomphalaria glabrata and Drepanotrema lucidum (Mollusca: Planorbidae);
Rhinella sp. (Amphibia: Bufonidae)
Localities: Januária and Patos de Minas, Minas Gerais, Brazil.
Prevalence of infection: 1/22 and 1/90 of B. glabrata and 8/8 of Rhinella sp. (Januária); 2/35 of D. lucidum (Patos de Minas).
Deposited material: UFMG-TRE 130 and UFMG-TRE 131
Description
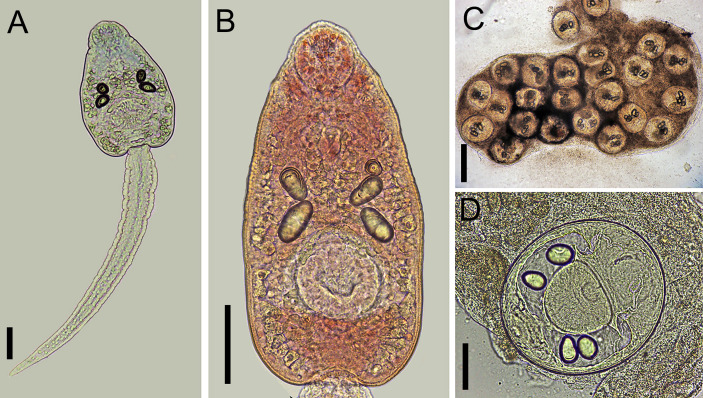
Cercariae (Fig 6A and 6B)
Fig 6. Larval stages of Rhopalias found in Biomphalaria glabrata and Rhinella sp. from Brazil.
(A) Whole view of a cercaria stained with Nile blue sulphate. (B) Detail of the body of a cercaria stained with neutral red. (C) Metacercariae found in a naturally infected tadpole. (D) Detail of a metacercaria. Cercariae from the same infected snail were genetically identified as Rhopalias sp. 2. Scale bars: (A, B, D) 50 μm, (C) 200 μm.
As in Rhopalias sp. 1.
Probable metacercariae (Fig 6C and 6D)
Morphologically similar to that described for Rhopalias sp. 1. They measured 186 ± 4 (183–191) by 153 ± 31 (107–175). Despite the number and size of the excretory corpuscles being similar to those in cercariae, the conspecificity of the two stages requires further molecular study.
Rhopalias sp. 3
Host: Drepanotrema lucidum
Locality: Dores do Indaiá
Prevalence of infection: 1/15.
Deposited material: not available.
Description
Cercariae. As in Rhopalias sp. 1.
Discussion
Although the parasitism of marsupials with Rhopalias spp. has been known since the early 19th century, the life cycles of digeneans in this genus remained unknown until now. In the present study, the occurrence of three species of Rhopalias in planorbid snails from Brazil was revealed by molecular data, representing the first identification of larval stages of species of this genus. The main distinctive characteristic present in cercariae of all three species of Rhopalias is the presence of few large granules in the main ducts of the excretory system, a trait that separates them from other known echinostomatid cercariae, which normally have small, numerous granules (e.g., Drepanocephalus spp., Echinostoma spp., Echinoparyphium spp., Ribeiroia spp.) [1]. The cercariae of Rhopalias spp. found in our study are morphologically indistinguishable from the larvae originally described as Cercaria macrogranulosa by Ruiz (1952) [33]. These cercariae were found in B. glabrata in the same locality (Belo Horizonte, Minas Gerais, Brazil) where our Rhopalias sp. 1 was found. Morphologically similar cercariae, also identified as C. macrogranulosa, were also reported in some species of planorbids from the genus Biomphalaria Preston, 1910 and Drepanotrema Crosse and Fischer, 1880 in Brazil (revised by Pinto and Melo [37]) and Argentina [36]. Likewise, Cercaria echinostoma 2 [34] and Cercaria guaibensis 8 [35], found in planorbids in southern Brazil, are also similar to cercariae described in the present study and thus, probably represent a species of Rhopalias, which requires further study for confirmation.
Our attempts at experimental infections using cercariae of Rhopalias sp. 1 failed to produce viable metacercariae. The contact of the cercariae with fish induced the encystment, suggesting that a cold-blooded aquatic vertebrate could act as the second intermediate host. Although P. reticulata appears not to be a susceptible host, the possibility of other fish species being infected in natural conditions cannot be ruled out. So far, the only experimental study involving larvae identified as Cercaria macrogranulosa was performed by Ostrowski of Núñez et al. (1990) [36] in Argentina, who reported the penetration and encystment of cercariae in tadpoles, revealing for the first time the possible involvement of amphibians in the transmission of the parasite. In the present study, metacercariae bearing 2–3 large refractile corpuscles in each main collecting duct of the excretory system were found in naturally infected tadpoles of Rhinella sp. collected in the same water body where Rhopalias sp. 2 was found in snails. This finding suggests that anurans play the role of the second intermediate host in the life cycles of Rhopalias spp. On the other hand, the absence of sequences for metacercariae from amphibians prevents us from confidently linking these metacercariae with Rhopalias sp. 2, even though they were found in the same habitat. Morphologically similar metacercariae were reported in the pharyngeal region of tadpoles of Scinax nasicus (Cope, 1862) and Odontophrynus americanus (Duméril and Bibron, 1841) in Argentina [38]. Similar to the previously published phylogeny [6], our phylogenetic analyses (Fig 1) placed Rhopalias spp. from Brazil and North America in a well-supported clade with species of Ribeiroia and Cathaemasia. It is important to note that anurans are also involved in the transmission of species from these two genera phylogenetically related to Rhopalias [6, 39–41]. Amphibians can be part of the diet of didelphid opossums [42–44], which enables the trophic transmission of Rhopalias spp. to the definitive host.
In this study, we attempted experimental infection aiming to obtain adult parasites using the probable metacercariae of Rhopalias sp. 2 found in naturally infected tadpoles. Despite the occasional reports of Rhopalias from non-marsupial hosts, including rodents [3], no adult worm was recovered in the mouse intestine. Similar experimental attempts were reported as successful with other echinostomatids, e. g., Echinostoma spp. [45–47]. New controlled studies are required to evaluate the susceptibility of rodents to Rhopalias spp., which could facilitate new experiments aiming at characterization of other aspects of host-parasite interrelationship involving these parasites (e.g., pathology, immunology, biochemistry).
The only available information on the life cycles of species of the genus Rhopalias is based on an unpublished thesis by Read [48], who obtained data on some aspects of the biology of R. macracanthus in North America. Read [48] was able to infect the physid snail Physella gyrina (Say, 1821) with eggs of R. macracanthus which resulted in the production of echinostome cercariae without a spined collar [48]. Those cercariae were significantly smaller (body: 100–145 by 56–65 μm; tail 70–200μm long) than the cercariae reported in the present study from Brazil. Importantly, the cercariae described by Read [48] are characterized by the presence of 10–15 small refractile granules (measuring 7 μm in diameter) in the main ducts of the excretory system. On the other hand, cercariae in Read [48] had visible caeca, unlike the larvae from Brazil. Read [48] successfully infected tadpoles as experimental second intermediate hosts and adult digeneans successfully developed in young opossums, but not in rats, guinea pigs, cat, and owls.
All cercarial samples obtained in our study were morphologically uniform and similar to previously described C. macrogranulosa. However, molecular data did not confirm the conspecificity among these cercariae. Although sequences of nuclear ribosomal DNA operon demonstrated lack of divergence among 5 out 6 samples from positive snails (the exception was the sample identified as Rhopalias sp. 3, with 0.1% divergence in 28S and 1.15% in ITS), data obtained for nad1 and cox1 unequivocally reveal the presence of different species of Rhopalias in our material. The larvae identified as Rhopalias sp. 2, were found in species from two different genera of planorbids (Biomphalaria and Drepanotrema) in two localities situated about 600 km apart. The divergence in nad1 gene between these isolates (2.1–2.4%) is similar to intraspecific divergence reported for other species of the Echinostomatidae [32, 49, 50]. In fact, higher interspecific divergences in nad1 were reported among species of Echinostoma (4.9–9.1%) [50, 51], Patagifer (6.76–8.55%) [49, 52], and Drepanocephalus (14.3%) [14]. On the other hand, samples of Rhopalias sp. 1 found in two phylogenetically distant genera of planorbids (Biomphalaria and Gundlachia) from the same locality were identical in nad1. Thus, our data indicate low specificity to planorbid first intermediate hosts can be verified in species of Rhopalias, at least for our Rhopalias sp. 1. Interestingly, two samples of Rhopalias sp. 2 collected from the same snail species (B. glabrata) and the same locality (Januária), but at different times, had differences in both cox1 (1.31%) and nad1 (1.83%) genes. Thus, further detailed studies are necessary to understand genetic diversity and population genetic structure of Rhopalias species found in this study.
Our results provide the first identification of planorbid snails as natural intermediate hosts of trematodes of the genus Rhopalias. Although physid snails were shown as potential experimental intermediate hosts of the North American R. macracanthus [48], this host-parasite association was not reported under natural conditions. Our discovery of snail intermediate hosts of species of Rhopalias using molecular tools came 7 decades after the first report of Cercaria macrogranulosa in Brazil. This can be due to the challenges in linking cercariae and adults of digeneans [9]. Therefore, the specific identification of the larvae reported herein requires obtaining DNA sequences from adult specimens of South American Rhopalias spp.
Rhopalias coronatus was the only Rhopalias species known in the state of Minas Gerais. It was found in the white-eared opossum, Didelphis albiventris Lund, 1840 from Belo Horizonte [53], the same area where cercariae of Rhopalias sp. 1 were found. Recently, cox1 sequences were obtained for Mexican isolates of R. coronatus [5], but that species is clearly distinct from our Rhopalias sp. 1 and Rhopalias sp. 2 (9.2–9.7% divergence in cox1). We cannot rule out the possibility of our Rhopalias sp. 3 being conspecific with one of the species sequenced in Mexico. Likewise, the larvae reported herein may belong to some of the other six species of Rhopalias known from marsupials in South America, namely Rhopalias baculifer Braun, 1900, Rhopalias caballeroi Kifune & Uyema, 1982, Rhopalias caucensis Rivillas, Caro, Carvajal & Vélez, 2004, Rhopalias coronatus (Rudolphi, 1819), Rhopalias horridus (Diesing, 1850) and R. macracanthus [8]. Of these, R. baculifer, R. horridus and R. coronatus were reported in Brazil [4, 7], and may correspond to some of the larvae reported herein.
Significant genetic differences between the sample of R. macracanthus from the USA sequenced in the present study and sequences of the same species from Mexico [5] indicate the possibility of these isolates belonging to different species of Rhopalias. It should be noted that the authors pointed out some morphological differences in proboscis spines between their specimens from Mexico and the original description [5]. Considering that R. macracanthus was originally described from the Virginia opossum in the USA [4], it is more likely that our isolate from the USA corresponds to this species. Additional sequencing and detailed morphological studies of specimens from both Mexico and the USA, including hologenophores, are necessary to answer this question.
Similar to observations in some other groups of digeneans, our results demonstrated that despite the usefulness of the nuclear ribosomal RNA sequences for phylogenetic inference at levels of genera and higher taxa, their level of variation can be too low for the specific level. This fact was verified between our Rhopalias sp. 1 and Rhopalias sp. 2. In a study carried out in Mexico, cox1 sequences (barcoding region) were obtained for three species of Rhopalias [5]. In our study, we successfully amplified the same region of cox1 only from one of our samples (Rhopalias sp. 1) using the primers Dice-1 and Dice-11 developed by Van Steenkiste et al. [27]. Instead, we successfully amplified and sequenced Rhopalias sp. 2 using the combination of Dice-1 and BarCox-R primers. Unlike cox1, partial sequences of the fragment of the nad1 gene have been widely used in studies of the Echinostomatoidea [12–17;49–52]. Amplification and sequencing of this fragment in our study of Rhopalias spp. was not problematic; therefore, its use is encouraged in future studies involving these digeneans.
More than 30 species of digeneans, including six species of Rhopalias, have been reported in South American marsupials thus far [8]; remarkably, none of these species had molecular data available prior to our study. This deficiency in the generation of molecular data is particularly obvious in South America [54]. Thus, obtaining sequence data from quality, well-fixed and identified adult specimens of Rhopalias spp. is critical for future progress in our knowledge of the biology and taxonomy of digeneans parasitic in marsupials.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Pro-Reitoria de Pesquisa (PRPq) and Pro-Reitoria de Pós-Graduação (PRPg), Universidade Federal de Minas Gerais (UFMG), with grant from the Edital 01/2022 to HAP. This work was supported by National Council for the Improvement of Higher Education (CAPES) (student scholarships to DLH and MCV), and National Council for Scientific and Technological Development (CNPq) (research scholarships to HAP).
References
- 1.Yamaguti S. Synopsis of Digenetic Trematodes of Vertebrates. vol. 1. Keigaku Publishing Company: Tokyo; 1971. [Google Scholar]
- 2.Marshall ME, Miller GC. Some digenetic trematodes from Ecuadorian bats including five new species and one new genus. J. Parasitol. 1979;65: 909–917. [Google Scholar]
- 3.Radev V, Gardner SL, Kanev I. Family Rhopaliidae Looss, 1899. In: Keys to the Trematoda, Jones A, Bray RA, and Gibson DI (eds). London, U.K.: CAB International and The Natural History Museum; 2005. p. 119–120. [Google Scholar]
- 4.Haverkost TR, Gardner SL. A review of species in the genus Rhopalias (Rudolphi, 1819). J. Parasitol. 2008;94: 716–726. [DOI] [PubMed] [Google Scholar]
- 5.López-Caballero J, Mata-López R, Pérez Ponce de León G. Molecular data reveal a new species of Rhopalias Stiles & Hassall, 1898 (Digenea, Echinostomatidae) in the Common opossum, Didelphis marsupialis L. (Mammalia, Didelphidae) in the Yucatán Peninsula, Mexico. ZooKeys 2019;854: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tkach VV, Kudlai O, Kostadinova A. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int. J. Parasitol. 2016;46: 171–185. doi: 10.1016/j.ijpara.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Gomes DC, Vicente JJ. Estudo do gênero Rhopalias Stiles & Hassall, 1898 (Trematoda, Rhopaliasidae). Mem. Inst. Oswaldo Cruz 1972; 70:115–133. [Google Scholar]
- 8.Fernandes BMM, Justo CN, Cárdenas MQ, Cohen SC. South American trematodes parasites of birds and mammals. Rio de Janeiro: FIOCRUZ; 2015. [Google Scholar]
- 9.Blasco-Costa I, Poulin R. Parasite life-cycle studies: a plea to resurrect an old parasitological tradition. J. Helminthol. 2017;91: 647–656. doi: 10.1017/S0022149X16000924 [DOI] [PubMed] [Google Scholar]
- 10.Blasco-Costa I, Poulin R, Presswell B. Species of Apatemon Szidat, 1928 and Australapatemon Sudarikov, 1959 (Trematoda: Strigeidae) from New Zealand: linking and characterising life cycle stages with morphology and molecules. Par. Res. 2016;115: 271–289. [DOI] [PubMed] [Google Scholar]
- 11.Blasco-Costa I, Locke SA. Life history, systematics, and evolution of the Diplostomoidea Poirier, 1886: progress, promises and challenges emerging from molecular studies. Adv. Parasitol. 2017;98: 167–225. doi: 10.1016/bs.apar.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Prasad PK, Goswami L, Tandon V, Chatterjee A. PCR-based molecular characterization and in silico analysis of food-borne trematode parasites Paragonimus westermani, Fasciolopsis buski and Fasciola gigantica from Northeast India using ITS2 rDNA. Bioinformation 2011;6: 64–68. doi: 10.6026/97320630006064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sereno-Uribe AL, Pinacho-Pinacho CD, Cordero VS, García-Varela M. Morphological and molecular analyses of larval and adult stages of Echinoparyphium recurvatum von Linstow 1873 (Digenea: Echinostomatidae) from central Mexico. J. Helminthol. 2014; 89: 458–464. doi: 10.1017/S0022149X14000297 [DOI] [PubMed] [Google Scholar]
- 14.Pinto HA, Griffin MJ, Quiniou SM, Ware C, Melo AL. Biomphalaria straminea (Mollusca: Planorbidae) as an intermediate host of Drepanocephalus spp. (Trematoda: Echinostomatidae) in Brazil: a morphological and molecular study. Par. Res. 2016;115: 51–62. [DOI] [PubMed] [Google Scholar]
- 15.Cech G, Molnár K, Székely C. Molecular biological studies of adult and metacercarial stages of Petasiger exaeretus Dietz, 1909 (Digenea: Echinostomatidae). Acta Vet. Hung. 2017;65: 198–207. doi: 10.1556/004.2017.020 [DOI] [PubMed] [Google Scholar]
- 16.Assis JCA, López-Hernández D, Favoretto S, Melo AL, Martins NRS, Pinto HA. Identification of a transmission focus of the avian tracheal trematode Typhlocoelum cucumerinum (Trematoda: Cyclocoelidae): diagnosis, life cycle and molecular phylogeny. Parasitology 2021;148: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galaktionov KV, Solovyeva AI, Miroliubov A. Elucidation of Himasthla leptosoma (Creplin, 1829) Dietz, 1909 (Digenea, Himasthlidae) life cycle with insights into species composition of the north Atlantic Himasthla associated with periwinkles Littorina spp. Par. Res. 2021;120: 1649–1668. doi: 10.1007/s00436-021-07117-8 [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Ponce de León G, Hernández-Mena DI. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019;93: 260–276. doi: 10.1017/S0022149X19000191 [DOI] [PubMed] [Google Scholar]
- 19.Paraense WL. Estado atual da sistemática dos planorbídeos brasileiros. Arq. Mus. Nac. 1975;55: 105–128. [Google Scholar]
- 20.Santos SB. Estado atual do conhecimento dos ancilídeos na América do Sul (Mollusca: Gastropoda: Pulmonata: Basommatophora). Rev. Biol. Trop. 2003; 191–224. [Google Scholar]
- 21.Simone LRL, Bunioto TC, Avelar WE, Hayashi C. Morphology and biological aspects of Gundlachia ticaga from SE Brazil (Gastropoda: Basommatophora: Ancylidae). Arch. Molluskenkd. 2012;141: 21–30. [Google Scholar]
- 22.Lacerda L, Lacerda LE, Miyahira I. First record and range extension of the freshwater limpet Gundlachia radiata (Guilding, 1828) (Mollusca: Gastropoda: Planorbidae) from southeast Brazil. Check List 2013;9: 125–128. [Google Scholar]
- 23.Tkach VV, Littlewood DT, Olson PD, Kinsella JM, Swiderski Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003;56: 1–15. doi: 10.1023/a:1025546001611 [DOI] [PubMed] [Google Scholar]
- 24.Luton K, Walker D, Blair D. Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol. Biochem. Parasitol. 1992;56: 323–328. doi: 10.1016/0166-6851(92)90181-i [DOI] [PubMed] [Google Scholar]
- 25.Kostadinova A, Herniou EA, Barrett J, Littlewood DTJ. Phylogenetic relationships of Echinostoma Rudolphi, 1809 (Digenea: Echinostomatidae) and related genera re-assessed via DNA and morphological analyses. Syst. Parasitol. 2003;54: 159–176. doi: 10.1023/a:1022681123340 [DOI] [PubMed] [Google Scholar]
- 26.Bowles J, McManus DP. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 1993;23: 969–972. [DOI] [PubMed] [Google Scholar]
- 27.Van Steenkiste N, Locke SA, Castelin M, Marcogliese DJ, Abbott CL. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Mol. Ecol. Resour. 2015;15: 945–952. doi: 10.1111/1755-0998.12358 [DOI] [PubMed] [Google Scholar]
- 28.Paithankar KR, Prasad KS. Precipitation of DNA by polyethylene glycol and ethanol. Nucleic Acids Res. 1991;19: 1346. doi: 10.1093/nar/19.6.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35: 1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008; 36: W465–W469. doi: 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. Software for systematics and evolution MrBayers 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rambaut A, Drummond AJ. FigTree. 2012; Available from http://tree.bio.ed.ac.uk/software/figtree/. Accessed Apr 2020.
- 33.Ruiz JM. Contribuição ao estudo das formas larvares de trematódeos brasileiros. 3. Fauna de Belo Horizonte e Jaboticatubas, Estado de Minas Gerais. Mem. Inst. Butantan 1952;24: 45–62. [PubMed] [Google Scholar]
- 34.Veitenheimer-Mendes IL. Cercárias em moluscos planorbídeos de Camaquã, Rio Grande do Sul, Brasil. Rev. Bras. Biol. 1982;42: 545–551. [Google Scholar]
- 35.Veitenheimer-Mendes IL, Ohlweiler FP, Blum C. Gastrópodes límnicos (Mollusca), hospedeiros intermediários de trematódeos (Platyhelminthes) em Porto Alegre e Viamão, Rio Grande do Sul. Biociências 1995;3: 73–84. [Google Scholar]
- 36.Ostrowski de Núñez M, Hamann MI, Rumi A. Larval trematodes of Schistosoma mansoni transmiting snail: Biomphalaria spp. in northeastern Argentina. Acta Parasitol. Pol. 1990;35: 85–96. [Google Scholar]
- 37.Pinto HA, Melo AL. A checklist of cercariae (Trematoda: Digenea) in molluscs from Brazil. Zootaxa. 2013;3666: 449–475. doi: 10.11646/zootaxa.3666.4.3 [DOI] [PubMed] [Google Scholar]
- 38.Hamann MI, González CE. Larval digenetic trematodes in tadpoles of six amphibian species from northeastern Argentina. J. Parasitol. 2009;95: 623–628. doi: 10.1645/GE-1738.1 [DOI] [PubMed] [Google Scholar]
- 39.Szidat L. Beitrage zum Aufbau eines naturlichen systems der Trematoden. I. Die entwicklung von Echinocercaria choanophila U. Szidat zu Cathaemasia hians und die Ableitung der Fasciolidae von den Echinostomatidae. Z. Parasitenkd. 1939;11: 239–283. [Google Scholar]
- 40.Yamaguti SA. Synoptical Review of Life Histories of Digenetic Trematodes of Vertebrates with Special Reference to the Morphology of their Larval Forms. Keigaku Publishing Co.: Tokyo; 1975. [Google Scholar]
- 41.Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB. Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history and pathogenesis with special emphasis on the amphibian malformation problem. Adv. Parasitol. 2004;57: 191–253. doi: 10.1016/S0065-308X(04)57003-3 [DOI] [PubMed] [Google Scholar]
- 42.Stiegltiz WO, Klimstra WD. Dietary pattern of the Virginia opossum, Didelphis virginianus Kerr, late summer-winter, southern Illinois. Trans. Ill. State Acad. Sci.1962; 55: 198–208. [Google Scholar]
- 43.Valerio CE. A gran capacidad adaptiva del zorro pelon (Didelphis masrupialis Linn). Rev. Univ. Costa Rica 1969;26: 43–44. [Google Scholar]
- 44.Toledo LF, Ribeiro RS, Haddad CFB. Anurans as prey: an exploratory analysis and size relationships between predators and their prey. J. Zool. 2007; 271: 170–177. [Google Scholar]
- 45.McMaster RP, Huffman JE, Fried B. The effect of dexamethasone on the course of Echinostoma caproni and E. trivolvis infections in the golden hamster (Mesocricetus auratus). Par. Res. 1995;81: 518–521. doi: 10.1007/BF00931795 [DOI] [PubMed] [Google Scholar]
- 46.Fujino T, Ichikawa H, Fried B, Fukuda K. The expulsion of Echinostoma trivolvis: suppressive effects of dexamethasone on goblet cell hyperplasia and worm rejection in C3H/HeN mice. Parasite. 1996;3: 283–289. doi: 10.1051/parasite/1996033283 [DOI] [PubMed] [Google Scholar]
- 47.Fried B, Mueller TJ, Frazer BA. Observations on Echinostoma revolutum and Echinostoma trivolvis in single and concurrent infections in domestic chicks. Int. J. Parasitol. 1997;27: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 48.Read CP. The life history and morphology of Rhopalias macracanthus Chandler (Trematoda) [dissertation]. Houston, Texas: William Marsh Rice Institute 1948.
- 49.Laidemitt MR, Brant SV, Mutuku MW, Mkoji GM, Loker ES. The diverse echinostomes from East Africa: with a focus on species that use Biomphalaria and Bulinus as intermediate hosts. Acta Trop. 2019;193: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgieva S, Selbach C, Faltýnková A, Soldánová M, Sures B, Skírnisson K, et al. New cryptic species of the ‘revolutum’ group of Echinostoma (Digenea: Echinostomatidae) revealed by molecular and morphological data. Parasites Vectors. 2013;6: 64. doi: 10.1186/1756-3305-6-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohanta UK, Watanabe T, Ohari Y, Itagaki T. Characterization of Echinostoma revolutum and Echinostoma robustum from ducks in Bangladesh based on morphology, nuclear ribosomal ITS2 and mitochondrial nad1 sequences. Parasitol. Int. 2019;69: 1–7. [DOI] [PubMed] [Google Scholar]
- 52.Sereno-Uribe AL, González-García MT, Ortega-Olivares MP, López-Jiménez A, García-Varela M, Andrade-Gómez L. First record of Patagifer bilobus (Rudolphi, 1819) Dietz, 1909 (Digenea: Echinostomatidae), with a morphological and molecular characterization from two threskiornithid species in Mexico. Parasitol. Res. 2022;121: 1921–1935. doi: 10.1007/s00436-022-07526-3 [DOI] [PubMed] [Google Scholar]
- 53.Quintão e Silva M, Costa HM. Helminths of white-bellied opossum from Brazil. J. Wildl. Dis. 1999;35: 371–374. doi: 10.7589/0090-3558-35.2.371 [DOI] [PubMed] [Google Scholar]
- 54.Poulin R, Jorge F. The geography of parasite discovery across taxa and over time. Parasitology. 2019;146: 168–175. doi: 10.1017/S003118201800118X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.