Abstract
Circulating tumor cells (CTCs) are a major cause of tumor metastasis and resistance to anticancer therapies. To date, no effective low-toxicity chemotherapeutic agents or antibodies have exhibited significant clinical activity against CTCs. Macrophages are important mediators of antitumor immunity. Tuftsin (TF), a tetrapeptide located at residues 289–292 of the CH2 domain of the Fc region of the IgG heavy chain, binds to Nrp-1, a receptor on the surface of macrophages that promotes phagocytosis and induces nonspecific activation of the immune system against tumors. Lidamycin (LDM) is an antitumor chemotherapy agent that is strongly cytotoxic to tumors and can dissociate into an apoprotein (LDP) and active enediyne (AE) in vitro. We previously constructed the fusion protein LDP-TF through genetic engineering and inserted the chromophore AE to produce LDM-TF, which can target macrophages to promote their phagocytic and cytotoxic activity against tumor cells. Preliminary experiments confirmed the anti-tumor activity of LDM-TFs. In this study, we found that LDM-TF effectively inhibited the growth of CTCs of gastric cancer origin and enhanced macrophage phagocytosis both in vivo and in vitro. Tumor cell expression of CD47, which helps to evade phagocytosis by macrophages, was substantially downregulated by LDM-TF. Notably, our in vitro experiments demonstrated that the combination of LDM-TF and anti-CD47 antibodies promoted phagocytosis more than either component alone. Our findings demonstrate the significant inhibitory effect of LDM-TF on the growth of CTCs of gastric cancer origin and suggest that the combination of LDM-TF and anti-CD47 antibodies may exhibit synergistic effects, thereby providing a new option for the clinical treatment of patients with advanced tumors that have metastasized.
Keywords: Tuftsin, Lidamycin, Fusion protein, CD47, Circulating tumor cells, Macrophage
Highlights
-
•
Tuftsin-tailored fusion protein inhibits circulating gastric tumor cells.
-
•
Tuftsin-tailored fusion proteins enhance macrophage phagocytosis.
-
•
Tuftsin-tailored fusion proteins work synergistically with anti-CD47 antibodies.
Abbreviations
- AE
Active enediyne from lidamycin
- TF
Tuftsin
- BSA
Bovine serum albumin
- CTCs
Circulating tumor cells
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- FITC
Fluorescein isothiocyanate
- HPLC
High-performance liquid chromatography
- IFN
Interferon
- TNF
Tumor necrosis factor
- IL-2
Interleukin-2
- IL-12
Interleukin-12
- IgG
Immunoglobulin
- LDM
Lidamycin
- LDM-TF
The enediyne-energized fusion protein composed of LDP, TF, and AE
- LDP
Lidamycin apoprotein
- LDP-TF
The fusion protein composed of LDP and TF
- EMT FOL-FOX
Calcium folinate-fluorouracil-oxaliplatin
- SOX
Tegafur-oxaliplatin
1. Introduction
Tuftsin (TF) is a naturally occurring human tetrapeptide (Thr-Lys-Pro-Arg) that promotes macrophage phagocytosis. TF binds to neuropilin-1 (Nrp1), a specific receptor on the membranes of immune cells, such as macrophages and monocytes, to promote phagocytosis and exert antibacterial and antitumor effects [1]. The immunomodulatory effects exerted following binding occur through the TGF-β signaling pathway [2]. TF does not exert direct cytotoxic effects on tumor cells and has no significant inhibitory effect on the growth of solid tumors; however, it can prolong patient survival [3]. Compared to etoposide alone, the combination of etoposide and TF was more effective in the treatment of fibrosarcoma. Although the TF-containing polypeptide ([TKPR]4-K2--G-OH) does not show potent cytotoxicity against tumor cells in vitro, it can promote the proliferation of macrophages, induce their differentiation to M1 macrophages, and promote the production of anti-tumor cytokines tumor necrosis factor-α (TNF-α) and NO by macrophages. These anti-tumor effects have been validated via animal experiments [[4], [5], [6]]. However, the anti-tumor effects of TF-associated antibodies and drugs on circulating tumor cells (CTCs) have yet to be reported.
Macrophages are among the most abundant immune cells in the human body. They differentiate into different cell types in various tissues and organs with high specificity; however, they generally have similar functions, including phagocytosis, cytotoxic activity, antigen presentation (T-cell activation), and immunomodulation [7]. Macrophages also play an important role in antitumor immune response, which is driven by the binding of Fc receptors to antibodies [8]. Phagocytosis of tumor cells by macrophages is controlled by a balance of pro- and anti-phagocytic inputs, known respectively as “eat me” and “don't eat me” signals [9]. CD47 is overexpressed on tumor cells and inhibits macrophage activity by interacting with its receptor, SIRPα [10]. CD47 antagonists have shown promising clinical activity in early human trials [11,12]. In particular, a combination of rituximab, a CD20-targeted monoclonal antibody, and anti-CD47 is effective in patients with rituximab-resistant lymphoma [12,13]. It is unclear which anticancer drugs can effectively block CD47.
In the present study, we investigated the efficacy of previously developed fusion proteins, LDP-TF and LDM-TF, against CTCs of gastric cancer origin. We demonstrated the antitumor effects of LDP-TF and LDM-TF, as well as their ability to recruit tumor-associated macrophages (TAMs). In addition, these responses were associated with the altered expression of cytokines that readjusted the balance of macrophage activity toward phagocytosis of tumor cells, including a reduction in CD47 expression. These results suggest that LDP-TF and LDM-TF have significant anti-CTC effects and that combining them with anti-CD47 antibodies may result in unique synergistic effects with the potential to improve the prognosis of patients with advanced tumor metastases significantly.
2. Materials and methods
2.1. Cell culture
The HGC, MGC, and J774.1 cell lines were purchased from the Chinese Academy of Medical Sciences Cancer Cell Bank (Beijing, China). The human gastric circulating tumor cells (CTC-105 and CTC-141) used in this study were previously established by CD44+/CD45-isolation [14]. Macrophages J774A.1 was routinely grown in RPMI-1640 (GIBCO) supplemented with 10% fetal bovine serum (GIBCO), penicillin (100 IU/ml), and streptomycin (100μg/ml). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Beijing, USA). All cell lines were maintained in a humidified atmosphere containing 5% CO2.
2.2. In vitro phagocytosis assay
The phagocytic specificity of the LDP-TF fusion protein was analyzed in J774A.1, using fluorescence microscopy, as previously described [2]. As previously reported, the indicated LDP-TF or LDP protein (10 μM) was added and incubated [2]. To quantitatively compare the effect on the phagocytosis affinity of macrophages by LDP-TF or LDP, FITC-labeled BSA fluorescence was then determined by flow cytometry.
2.3. Live cell phagocytosis assay
Mouse macrophages (5 × 104 cells/well) were plated overnight in 24-well plates. After a single PBS wash, 141 and 105 cells were labeled by incubation with 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) for 2 h. Cells were washed twice with complete media before adding 2 × 105 target cells to each well of the 24-well plate. Where indicated, 10 μM of LDP-TF, 10 μg/ml of anti-CD47, or the combination of both protein and antibodies was added. Live cell imaging was performed using a live-cell station (AxioObserver 7, Zeiss, Oberkochen, Germany). Phagocytosis was quantified as the area under the curve (AUC) of the integrated green fluorescence intensity between 0 and 24 h.
2.4. Western blot analysis
Western blotting was performed as described previously [15]. After incubation with LDP-TF or LDM–TF for 24 h, whole-cell lysates were prepared and analyzed by western blotting. Total protein from 141 to 105 cells was separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, ISEQ00010). The PVDF membrane was then incubated with antibodies against CD47 (Epitomics, Abcam Company, City, USA), ZEB1 (Epitomics, Abcam Company, City, USA), AKT (Cell Signaling Technology, City, USA), p-AKT (473) (Epitomics, Abcam Company, City, USA), GAPDH or β-ACTIN overnight at 4 °C. Proteins were visualized using ECL Western Blotting Substrate (Pierce, 32,106).
2.5. ELISA
Blood samples were collected from the orbital blood capillaries of the mice 24 h after fusion protein treatment. The TNF-α, IFN-γ, IL-10, and IL-12 production following stimulation was measured using an ELISA KIT (BioLegend) [2] (BioLegend, San Diego, CA).
2.6. Mouse xenograft tumor models
To evaluate the in vivo role of LDP-TF or LDM-TF, we subcutaneously inoculated 5 × 106 CTC-141 cells into the fossa axillaries of four-to six-week-old female nude BALB/c mice (Hufukang, Beijing, China) (n = 5 per group). Tumor growth was measured every two days after the tumor volume reached 100 mm3. When the tumor size reached approximately 100 mm3, mice were divided into groups (n = 5) and treated with different doses of fusion proteins or energized fusion proteins in a 200 μL volume of sterile saline by intravenous (i.v.) injection into the tail vein. One group of mice was administered i. v. saline as a control. Tumor volumes were measured as described previously [16]. All procedures involving mice were approved by the Animal Care Committee of the Shandong Cancer Hospital and Institute.
2.7. Cell viability assay
Cells were plated in 96-well plates at 2 × 103 cells/well for the CCK-8 assay. The cells were serum-starved for 8 h and further incubated with various concentrations of LDP-TF, trastuzumab, or cetuximab for 24 h at 37 °C. To quantify cell growth, CCK-8 assays were performed as previously described [2].
Briefly, each cancer cell line was seeded in 96-well plates (4000 cells/well). After incubating overnight, cells were treated with LDM-TF, LDM, oxaliplatin, or 5-FU at varying concentrations (1 × 10−6, 1 × 10−7, 1 × 10−8, 1 × 10−9, 1 × 10−10, 1 × 10−11, 1 × 10−12 M, respectively) and continuously incubated for 48 h at 37 °C. To quantify cell growth, MTT assays were performed as described [13,17]. Results were derived from three independent experiments.
2.8. Histopathological examination
Circulating gastric cancer cells were monitored by histopathological examination. When the tumor volume was more than 1000 mm3, athymic mice were euthanized, and specimens were taken from the tumors of the group treated with 20 mg/kg LDP-TF and the control group. After fixation with 10% formalin and paraffin embedding, 5 μm thick histological sections were prepared and stained with hematoxylin and eosin (H&E). Histopathological changes were observed using a Leica microscope (manufacturer, City, Country).
2.9. Immunohistochemical analysis
Tumors were collected as previously described. Formalin immobilizes tumor tissues. Immunohistochemical analysis of the tumor tissue sections was performed using an F4/80 specific antibody (Cell Signaling Technology, D2S9R) [18]. In short, tissue sections were incubated in a 5 mM citric acid buffer (F4/80), incubated at 100 °C for 25 min (F4/80) to obtain antigenicity, and then incubated with F4/80 antibody for 1 h. The stained sections were observed under a microscope, and the images were systematically analyzed using Leica software.
2.10. Statistical analysis
All analyses were performed using SPSS software (version 22.0; SPSS Inc.) or GraphPad Prism 5.0 (GraphPad Software, Inc.). The 2-tailed unpaired Student's t-test or paired Student's t-test was used to perform statistical comparisons between the two groups. The level of significance was set at p < 0.05.
3. Results
3.1. Tuftsin-based fusion proteins promote phagocytosis of macrophages and downregulate CD47 expression in CTCs
The TF-based fusion protein, LDP-TF, was produced by genetic engineering (Supplemental figure 1). LDP-TFs are formed by linking the C-terminus of LDP to the N-terminus of a tetrapeptide (TF) via a linker peptide (Fig. 1A). The AE moieties of LDM were integrated into LDP-TF separately to obtain the enediyne fusion protein LDM-TF (Fig. 1B). Fluorescence microscopy (Fig. 1C) revealed that LDP-TF treatment promoted phagocytosis of bovine serum albumin (BSA) by mouse macrophages. The findings of FACS analysis showed that, compared to the LDP and control groups, the LDP-TF-treated group exhibited significantly increased phagocytosis in macrophages (Fig. 1D). Compared to the BSA and LDP controls, CFSE-labeled CTC-141 and CTC-105 cells exhibited increased phagocytosis by LDP-TF-activated mouse macrophages (Fig. 1E). CD47 expression was significantly correlated with macrophage phagocytic activity. Notably, CD47 protein levels were significantly reduced in LDM-TF-treated CTC-141 and CTC-105 cells (p < 0.01; Fig. 1F). Thus, LDM-TF may downregulate CD47 expression by enhancing the phagocytic capacity of macrophages.
Fig. 1.
(A) Diagram of the NdeI/XhoI gene fragments encoding for the protein LDP-TF. (B) The diagram indicates the components of the LDM-TF fusion protein and its reconstituted enediyneintegrated analog LDM-TF. (C) Representative images of mouse macrophages phagocytosing FITC-labled BSA following treatment with fusion proteins. Arrows point to phagocytosed BSA. Cell morphology through microscope (10 × original magnification). (D) Bar graph shows the mean fluorescence intensity of FITC-labeled BSA after being primed by BSA, LDP-TF, LDP or tuftsin for the 2 h time point. Fold change is expressed as a ratio of mean fluorescence intensity level of the different fusion proteins relative to the mean fluorescence intensity level of the BSA protein. *p < 0.05. (E) Graphs show flow-cytometry-based quantification of phagocytosis of CTC cell lines in the presence of LDP-TF, LDP or BSA treatment. Shown are mean ± s.d. of three experimental replicates. (F) CTC-141 (left) cells or CTC-105 (right) cells treated with tested samples (100 μg), including LDP, LDP-TF, LDM (1 nM), and LDM-TF (1 nM), respectively.
3.2. Tuftsin-based fusion proteins display potent cytotoxicity against CTCs
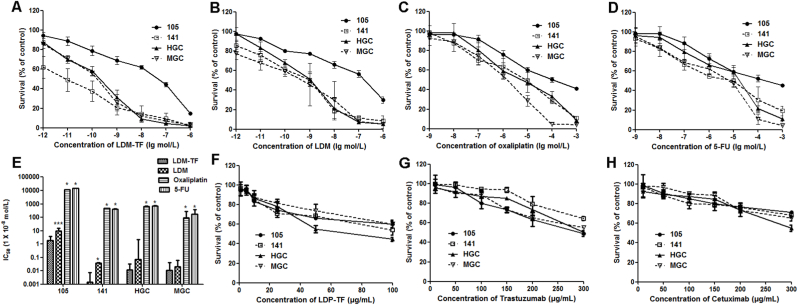
The cytotoxicities of LDP-TF, trastuzumab, cetuximab, oxaliplatin, 5-FU, and LDM-TF were examined in four gastric cancer cell lines and CTC lines 105, 141, HGC, and MGC (Fig. 2). The MTT assay (Fig. 2A–D) revealed that the LDM-DF fusion protein exhibited strong cytotoxicity against gastric cancer cell lines. Against the CTC-105, CTC-141, HGC, and MGC cells, the IC50 values of LDM-DF were 1.84 × 10−8, 1.4 × 10−11, 1.2 × 10−10, and 1.1 × 10−10 M, respectively (Fig. 2H). Furthermore, LDM-TF was more effective than oxaliplatin and 5-FU at the same concentration (p < 0.05). In the CCK-8 assay (Fig. 2E–G), LDP-TF significantly inhibited cancer cell proliferation. In addition, the LDP-TF fusion protein was tested for comparison with trastuzumab and cetuximab; at the same concentration. The LDP-TF was more potent than both.
Fig. 2.
Percentage of CTC cells treated with various concentrations of drugs. (A–D) Percentage of CTC cells treated with various concentrations of LDM, LDM-TF, FU, and oxaliplatin, respectively. € IC50 values of LDM, LDM-TF, FU, and oxaliplatin. One-way ANOVA and Dunnett's multiple comparison tests were used. *p < 0.05, ***p < 0.001 (LDMP-TF vs LDM, FU, or oxaliplatin). (F–H) Percentage of live cells treated with various concentrations of LDP-TF, cetuximab, and trastuzumab, respectively.
3.3. LDT-TF exerts higher antitumor efficacy in vivo
The J774A.1 mouse macrophages were treated with LDP-TF, LDP, anti-CD47 (B6H12, mouse IgG1 antibody), or a combination of LDP-TF and anti-CD47 antibodies in vitro, and the phagocytosis of CTC-141 cells by mouse macrophages was measured using phagocytosis assays. The combination of LDP-TF and anti-CD47 resulted in the strongest phagocytosis among all groups tested (Fig. 3A).
Fig. 3.
In vivo efficacy of tuftsin-based fusion proteins. (A) Graphs show flow-cytometry-based quantification of phagocytosis of CTC-141 cell lines in the presence of LDP-TF, anti-CD47, LDP-TF + anti-CD47, or BSA treatment. Shown are mean ± s.d. of three experimental replicates. (B) Antitumor effects of LDP-TF alone on CTC-141 xenograft. Triangles, the day of injection (day 6). (C) Mean body weights of mice in each group are shown. (D) The mean tumor weight after treatment is shown. (E) Histopathological examination of tumors (H&E staining, magnification × 200) of CTC-141 xenograft-bearing mice. (F) The Kaplan–Meyer survival curve (endpoints defined as tumor load of 400 mm3) demonstrated that compared with other groups, the probability of survival (i.e., probability of maintaining a tumor volume <400 mm3) of mice treated with LDP-TF (20 mg/kg) was significantly improved (p < 0.05). (G) Production of cytokine in blood serum treated with fusion proteins. Blood samples were taken 24 h after the injection of drugs. Results are presented in histograms IL-10, IL-12, IFN-γ, and TNF-α per milliliter ± SD. Asterisks (*) indicate a significant difference (p < 0.05) between the untreated tumor group and drugs treated groups.
The anti-tumor effects of LDP-TF in CTC-141 tumor cells were investigated. LDP-TF is more effective than LDP in treating CTC-141 xenografts. Treatment was initiated when the tumor reached approximately 100 mm3 in size. Mice were intravenously injected with the fusion protein on day 6. At a dose of 20 mg/kg, LDP-TF effectively inhibited tumor growth (p < 0.01) (Fig. 3B–D) and delayed tumor growth with better efficacy than LDP (p < 0.05). On day 26 of treatment with LDP-TF, the inhibition rate was 89.1%. Histopathological examination confirmed the therapeutic effect on tumors (Fig. 3E). The survival curve of nude mice showed that LDP-TF significantly increased the likelihood of survival in mice relative to controls (p < 0.05 compared to control) (Fig. 3F).
The antitumor effects of TF-based fusion protein were investigated using TNF-α and IFN-γ as parameters. In the present study, the TNF-α and IFN-γ expression levels were elevated in animals treated with the LDP-TF fusion protein. Compared to levels in the PBS group, TNF-α expression in tumor-bearing mice treated with LDP-TF or LDP increased 8-fold and 7-fold, respectively, and IFN-γ expression increased 10-fold and 2-fold, respectively (Fig. 3G). These results suggest that the LDP-TF fusion protein influences cytokine levels, which may regulate immune responses. A significant decrease in the abundance of IL-10 and a considerable increase in the abundance of IL-12 were also detected in the LDP-TF group compared to those in the control and LDP groups (p < 0.05). Cytokine IL-10 inhibits macrophage function, and IL-12 is primarily secreted by macrophages. Thus, LDP-TFs have the potential to promote macrophage function in cancer immunotherapy.
3.4. The anticancer effects of the LDP-TF fusion protein are macrophage-dependent
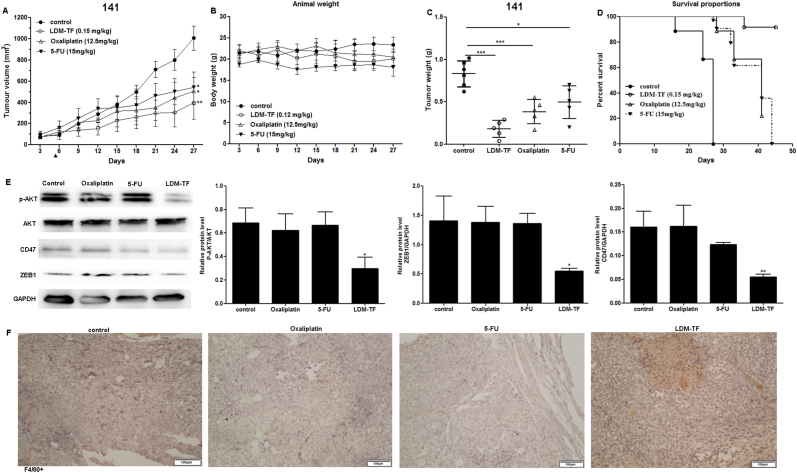
In vivo experiments were performed using nude mice with CTC-141 xenografts. Compared to oxaliplatin and 5-FU, commonly used to treat patients with advanced gastric cancer, the fusion protein LDM-TF exhibited higher anti-tumor efficacy (Fig. 4A). In comparison, the inhibition rates of oxaliplatin, 5-FU, and LDM-TF on CTC-141 growth were 54.1%, 40.6%, and 78.4%, respectively (Fig. 4C). Fig. 4B shows that the weight loss in animals treated with different doses did not exceed 10% of their pre-treatment body weight. Thus, oxaliplatin, 5-FU, and LDM-TF were well-tolerated. Survival curves of the nude mice showed that, compared with the control, mice treated with LDM-TF exhibited a significantly increased likelihood of survival (p < 0.05) (Fig. 4D). Compared to normal gastric cancer cells, CTC-141 and CTC-105 cells exhibited higher expression of the AKT signaling pathway and the EMT-associated protein ZEB1. Therefore, we examined the phosphorylation and total expression of AKT in xenograft tumor tissues in tumor samples from drug-treated nude mice using western blotting. The results showed that Compared with control mice, mice treated with LDM-TF (0.15 mg/kg) exhibited significantly reduced AKT phosphorylation and ZEB1 expression in tumors. Immunohistochemical evaluation of tumors in mice treated with LDM-TF revealed that treatment was associated with increased macrophage infiltration (Fig. 4F).
Fig. 4.
In vivo efficacy of enediyne-energized tuftsin-based fusion proteins. (A) Antitumor effects of LDM-TF on CTC-141 xenograft. Triangles, the day of injection (day 5). (B) Mean body weights of mice in each group are shown. (C) The mean tumor weight after treatment is shown. (D) The Kaplan–Meyer survival curve (endpoints defined as tumor load of 400 mm3) demonstrated that compared with other groups, the probability of survival (i.e., probability of maintaining a tumor volume <400 mm3) of mice treated with LDM-TF (0.15 mg/kg) was significantly improved (p < 0.05). (E) Expression of CD47, ZEB-1, AKT, and p-AKT in CTC-141 cells detected by Western blot. (F) Representative IHC images showing the detection of macrophages (anti-F4/80).
4. Discussion
In our previous study, CTCs from four patients with gastric cancer were found to be unresponsive to oxaliplatin, cetuximab, and trastuzumab. In addition, four patients underwent many cycles of FOL-FOX and SOX chemotherapy before CTC isolation and showed disease progression [14]. Western blotting revealed moderate-to-low expression of HER2 and no expression of HER1 in CTCs, which may explain the resistance of CTCs to cetuximab and trastuzumab in vitro [14]. A phase-III clinical trial demonstrated no benefit of cetuximab in patients with advanced gastric cancer [17]. In radiotherapy studies, CTCs exhibit relative tolerance to X-rays compared to other gastric cancer cell lines [19]. To date, the need for an effective clinical treatment for CTCs has not been addressed; therefore, novel therapeutic options are urgently required.
Immunotherapy has received extensive attention in recent years, and multifunctional drugs proposed in some preclinical studies, such as antibody-drug conjugates (ADCs), or similar drug delivery systems, such as protein-drug conjugates, have great potential for clinical applications [20,21]. Liu et al. constructed a novel fusion protein, Ec-LDM-TF, which contains an EGF ligand oligopeptide that specifically recognizes receptors highly expressed in tumor cells [22]. Recombinant LDM is composed of an apoprotein LDP and an enediyne AE and is highly cytotoxic against tumors [23]. TF are endogenous small-molecule peptides that are important components of the natural immune system [1]. In recent years, TF has been found to possess good anti-tumor activity, making it a novel therapeutic agent that treats multiple conditions [24]. We conducted a comprehensive evaluation of its biological activity in vitro, a partial study of its mechanism of action, and a preliminary study of its anti-tumor activity in a mouse tumor transplant model [16]. The results showed that this multifunctional drug has excellent potential for clinical applications. This study involved CTCs without EGFR expression; therefore, the fusion protein LDM-TF was used, and its efficacy in CTCs was observed. In this study, we demonstrated the antitumor effects of LDM-TF on two different types of CTCs, providing a potential approach for treating advanced metastatic tumors. We plan to perform extensive nude mouse tumor transplantation experiments to confirm the anti-CTC effects of LDM-TF, and if validated, we expect to proceed to clinical trials. The significant antitumor activity exhibited in our model was derived from LDM, which is directly cytotoxic to tumors, whereas TF drive phagocytosis by macrophages.
In the CTC-141 model, the antitumor effect of LDP-TF was significantly stronger than that of LDP alone. At equal doses, the inhibition of CTC-141 tumor cell growth by 76.3% and 84.2% for LDP-TF and LDP, respectively (Fig. 3). By testing several factors that may activate macrophages, it was found that TNF-α, IFN-γ, and IL-12 expression levels were significantly upregulated (Fig. 3). In addition, compared to the corresponding non-primed fusion protein, the enediyne-primed LDM-TF showed improved antitumor efficiency. Furthermore, the required dose of enediyne-primed LDM-TF was 5% of the dose of the non-primed fusion protein (Fig. 4). Moreover, LDM-TF treatment did not result in significant weight loss in treated animals. The immunohistochemical results showed increased macrophage infiltration in the tumor sections of the LDM-TF-treated group (Fig. 4). These results suggested that LDP-TF and LDM-TF promoted macrophage function and that their antitumor effects were macrophage-dependent.
In our previous study, the enediyne-primed fusion protein Ec-LDM-TF significantly downregulated CD47 expression in A431 cells, similar to the fusion protein LDM-TF [16]. Coincidentally, LDM-TF significantly down-regulated CD47 expression in CTC-141 cells (Fig. 4E). These results suggested that the antitumor effects of TF fusion proteins are widespread and may be at least partially associated with the downregulation of CD47. Various solid tumors in humans have been reported to require CD47 expression to prevent phagocytosis through surveillance and elimination by the innate immune system [25]. In the present study, we combined LDP-TF or LDM-TF with anti-CD47 antibodies to investigate macrophage function in tumor cells and observed enhanced macrophage phagocytosis. In conclusion, we demonstrated the antitumor effect of LDM-TF on CTCs and demonstrated that this approach is effective. Additionally, this study provides an avenue for combining the activity of LDM-TFs with that of anti-CD47 antibodies. This mechanism of action may be associated with the presence of macrophages in cancer cells. Our findings suggest a potential role of TF-based fusion proteins in the treatment of cancers involving CTCs.
Author contributions
Conceptualization: WL. Methodology: WL, DY, XZ, KZ, GJ. Investigation: DY, XZ, KZ, GJ. Funding acquisition: WL. Project administration: WL. Writing – original draft: WL. Writing – review & editing: VN.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the Natural Science Foundation of Shandong Province, china (ZR2020MH403) and Key Research and Development Program of Shandong Province, China (ZR2019LZL010).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101443.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Fridkin M., Najjar V.A. Tuftsin: its chemistry, biology, and clinical potential. Crit. Rev. Biochem. Mol. Biol. 1989;24:1–40. doi: 10.3109/10409238909082550. [DOI] [PubMed] [Google Scholar]
- 2.Liu W.J., Liu X.J., Li L., et al. Tuftsin-based, EGFR-targeting fusion protein and its enediyne-energized analog show high antitumor efficacy associated with CD47 down-regulation, CANCER IMMUNOL IMMUN. Cancer Immunol. Immunother. 2014;63:1261–1272. doi: 10.1007/s00262-014-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A., Khan A.A., Dwivedi V., et al. Tuftsin augments antitumor efficacy of liposomized etoposide against fibrosarcoma in Swiss albino mice. Mol. Med. 2007;13:266–276. doi: 10.2119/2007–00018. Khan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan M.A. Targeted drug delivery using tuftsin-bearing liposomes: implications in the treatment of infectious diseases and tumors. Curr. Drug Targets. 2021;22:770–778. doi: 10.2174/1389450121999201125200756. [DOI] [PubMed] [Google Scholar]
- 5.Zou B., Xia S., Du X., et al. Treatment effect of tuftsin and antigen peptide combined with immune cells on colorectal cancer. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:5465–5472. doi: 10.12659/MSM.915037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nissen J.C., Tsirka S.E. Tuftsin-driven experimental autoimmune encephalomyelitis recovery requires neuropilin-1. Glia. 2016;64:923–936. doi: 10.1002/glia.22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asgharzadeh S., Salo J.A., Ji L., et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J. Clin. Oncol. 2012;30:3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An Y., Yang Q. Tumor-associated macrophage-targeted therapeutics in ovarian cancer. Int. J. Cancer. 2021;149:21–30. doi: 10.1002/ijc.33408. [DOI] [PubMed] [Google Scholar]
- 9.Chao M.P., Jaiswal S., Weissman-Tsukamoto R., et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiswal S., Jamieson C.H., Pang W.W., et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikic B.I., Lakhani N., Patnaik A., et al. First-in-human, first-in-class Phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Advani R., Flinn I., Popplewell L., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma, N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao M.P., Alizadeh A.A., Tang C., et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan D., Chen L., Li M., et al. Isolation and characterization of circulating tumor cells from human gastric cancer patients. J. Cancer Res. Clin. Oncol. 2015;141:647–660. doi: 10.1007/s00432-014-1814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan D., Xia H., Zhang Y., et al. P-Akt/miR-200 signaling regulates epithelial-mesenchymal transition, migration and invasion in circulating gastric tumor cells. Int. J. Oncol. 2014;45:2430–2438. doi: 10.3892/ijo.2014.2644. [DOI] [PubMed] [Google Scholar]
- 16.Liu W.J., Liu X.J., Xu J., et al. EGFR-targeting, beta-defensin-tailored fusion protein exhibits high therapeutic efficacy against EGFR-expressed human carcinoma via mitochondria-mediated apoptosis. Acta Pharmacol. Sin. 2018;39:1777–1786. doi: 10.1038/s41401-018-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lordick F., Kang Y.K., Chung H.C., et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 18.Theruvath J., Menard M., Smith B.A.H., et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022;28:333–344. doi: 10.1038/s41591-021-01625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Liu Q., Wang T., et al. Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer. 2013;13:202. doi: 10.1186/1471-2407-13-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alas M., Saghaeidehkordi A., Kaur K. Peptide-drug conjugates with different linkers for cancer therapy. J. Med. Chem. 2021;64:216–232. doi: 10.1021/acs.jmedchem.0c01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdollahpour-Alitappeh M., Lotfinia M., Gharibi T., et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J. Cell. Physiol. 2019;234:5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 22.Liu W.J., Zhu K.L., Xu J., Wang J.L., Zhu H. Enediyne-activated, EGFR-targeted human beta-defensin 1 has therapeutic efficacy against non-small cell lung carcinoma. Lab. Invest. 2018;98:1538–1548. doi: 10.1038/s41374-018-0109-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang R., Li L., Duan A., et al. Crizotinib enhances anti-CD30-LDM induced antitumor efficacy in NPM-ALK positive anaplastic large cell lymphoma. Cancer Lett. 2019;448:84–93. doi: 10.1016/j.canlet.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Murugesan K., Srinivasan P., Mahadeva R., Gupta C.M., Haq W. Tuftsin-bearing liposomes co-encapsulated with doxorubicin and curcumin efficiently inhibit EAC tumor growth in mice. Int. J. Nanomed. 2020;15:10547–10559. doi: 10.2147/IJN.S276336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dizman N., Buchbinder E.I. Cancer therapy targeting CD47/SIRPalpha. Cancers. 2021;13 doi: 10.3390/cancers13246229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.