Abstract
Background
Umbilical cord blood gases are routinely used by midwives and obstetricians for quality assurance of birth management and in clinical research. They can form the basis for solving medicolegal issues in the identification of severe intrapartum hypoxia at birth. However, the scientific value of veno-arterial differences in cord blood pH, also known as ΔpH, is largely unknown. By tradition, the Apgar score is frequently used to predict perinatal morbidity and mortality, however significant inter-observer and regional variations decrease its reliability and there is a need to identify more accurate markers of perinatal asphyxia. The aim of our study was to investigate the association of small and large veno-arterial differences in umbilical cord pH, ΔpH, with adverse neonatal outcome.
Methods
This retrospective, population-based study collected obstetric and neonatal data from women giving birth in nine maternity units from Southern Sweden from 1995 to 2015. Data was extracted from the Perinatal South Revision Register, a quality regional health database. Newborns at ≥37 gestational weeks with a complete and validated set of umbilical cord blood samples from both cord artery and vein were included. Outcome measures included: ΔpH percentiles, ‘Small ΔpH’ (10th percentile), ‘Large ΔpH’ (90th percentile), Apgar score (0–6), need for continuous positive airway pressure (CPAP) and admission to neonatal intensive care unit (NICU). Relative risks (RR) were calculated with modified Poisson regression model.
Results
The study population comprised of 108,629 newborns with complete and validated data. Mean and median ΔpH was 0.08 ± 0.05. Analyses of RR showed that ‘Large ΔpH’ was associated with a decreased RR of adverse perinatal outcome with increasing UApH (at UApH ≥7.20: RR for low Apgar 0.29, P = 0.01; CPAP 0.55, P = 0.02; NICU admission 0.81, P = 0.01). ‘Small ΔpH’ was associated with an increased RR for low Apgar score and NICU admission only at higher UApH values (at UApH 7.15–7.199: RR for low Apgar 1.96, P = 0.01; at UApH ≥7.20: RR for low Apgar 1.65, P = 0.00, RR for NICU admission 1.13, P = 0.01).
Conclusion
Large differences between cord venous and arterial pH (ΔpH) at birth were associated with a lower risk for perinatal morbidity including low 5-minute Apgar Score, the need for continuous positive airway pressure and NICU admission when UApH was above 7.15. Clinically, ΔpH may be a useful tool in the assessment of the newborn’s metabolic condition at birth. Our findings may stem from the ability of the placenta to adequately replenish acid-base balance in fetal blood. ‘Large ΔpH’ may therefore be a marker of effective gas exchange in the placenta during birth.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-023-05487-8.
Keywords: Birth, Birth asphyxia, Perinatal asphyxia, Umbilical cord blood gases, Umbilical cord pH, Umbilical artery pH, Adverse neonatal outcome, Neonatal morbidity, Apgar score, Continuous positive airway pressure, Neonatal intensive care unit
Introduction
Umbilical cord blood gases are the most objective measure of the newborn’s metabolic condition at birth [1–3]. Sustained intrapartum hypoxia may lead to metabolic acidosis in the fetus and increase the risk of hypoxic brain injury [1, 4]. Previous studies have shown that analysis of cord blood gases, especially cord arterial pH (UApH), can identify newborns at risk of adverse neurological outcomes when most clinical and radiological signs are still absent [3–5]. Cord blood gases are also routinely used in quality assurance of obstetric care by midwives and clinicians, in research and form the basis for solving medicolegal issues in identification of severe intrapartum hypoxia [6].
For the identification of an accurate UApH value, it is important to obtain a correct cord venous pH (UVpH) [7], and although cord arterial blood gases best represent the newborn’s metabolic condition, it is often the umbilical vein that is more easily sampled due to its larger size. Studies have demonstrated the usefulness of cord venous pH as a proxy for cord arterial pH [1, 8]. However, the scientific value of veno-arterial differences in cord pH, also known as ΔpH, are largely undetermined.
Delta pH is widely recognized in the clinical setting, but only a handful of studies have attempted to demonstrate its association with fetal outcomes. A small study evaluating the relationship of ΔpH to cardiotocography patterns found no relation between large ΔpH and acute onset acidemia [9]. Belai and colleagues [10] were able to show a weak correlation of ΔpH to Apgar score, seizures, and hypoxic ischemic encephalopathy. Clinicians, on the other hand, have often relied on the use of the Apgar score to predict adverse neonatal outcome in hypoxic newborns [11–15], but there is need to identify more accurate markers for perinatal asphyxia due to large inter-observer and regional variations with the Apgar score [16]. Determination of ΔpH can therefore be a potential tool for the rapid identification of newborns suffering from perinatal asphyxia.
Using a large, population-based cohort, we aimed to explore the association of veno-arterial differences in cord pH (i.e. the difference between UVpH and UApH), namely ΔpH, with adverse neonatal outcome.
Methods
Study population and setting
Data was retrieved from the prospectively collected Perinatal Revision South Register, a quality database collecting obstetric and neonatal data from nine hospitals in Southern Sweden between 1995 and 2015. The original dataset comprised of 315,174 singleton live births with information regarding maternal characteristics, birth, perinatal outcomes, and neonatal course. Since some records from the Perinatal Revision South Register database did not contain complete sets of UApH and UVpH values, the material was supplemented with blood gas data extracted from the maternity records at Skåne University Hospital in Malmö (21,119 records supplemented), which is the largest maternity unit in Southern Sweden.
Study definitions and validation
Only newborns with a complete set of umbilical cord blood samples from both the artery and vein were included in the study. ΔpH was calculated using the equation:
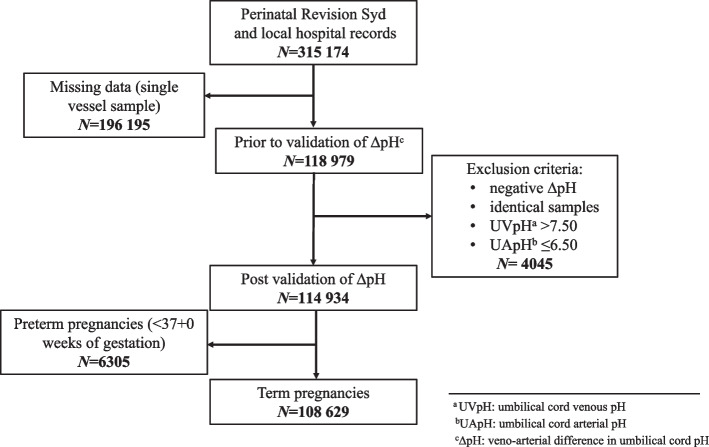
As described by Mokarami et al. [17], both small and negative ΔpH occur physiologically. We therefore validated ΔpH by only removing identical and negative ΔpH values from the dataset to minimize the introduction of selection bias [18]. Similarly, since umbilical cord arterial and venous pH change with advancing gestational age [19, 20], only ‘term’ infants born after 37 completed weeks of gestation were included in the final analyses (N = 108,629) (Fig. 1).
Fig. 1.
Flow chart showing selection of newborns with paired umbilical artery pH (UApH) and venous pH (UVpH) for calculation of delta pH (ΔpH)
Stratification of data were performed as follows: data regarding parity were classified as ‘primipara’, ‘multipara, no previous Cesarean section (CS)’ and ‘multipara, with previous CS’. Similarly, births that were reported to start as CS, but without specified mode of delivery, were regarded as elective CS. Gestational duration was classified term (37 + 0 to 40 + 6 weeks) and (41 + 0 to 41 + 6 weeks) and post-term (≥42 weeks). Birthweight was classified into four groups (< 2500 g, 3000-3999 g, 4000-4499 g, > 4500 g).
In order to study the percentile distribution of ΔpH in relation to changes in cord arterial pH, we divided UApH into six classes (Table 2). Percentiles were for term pregnancies but not parity or fetal sex specific. To calculate the relative risk (RR) for adverse neonatal outcome in association with UApH classes, modified Poisson regression model was chosen. This model was appropriate for large cohorts where independent and rare events occurred at a fixed rate and risk for model misspecification existed [21, 22]. Neonatal outcome parameters included Apgar score (0–6), neonatal intensive care unit (NICU) admission and use of continuous positive airway pressure (CPAP). Finally, to calculate and compare the relative risks of adverse neonatal outcomes, we defined ‘Small ΔpH’ as all ΔpH values less than or equivalent to 0.02 (10th percentile, or ≤ 0.02), and defined ‘Large ΔpH’ as all ΔpH values greater or equivalent to 0.15 (90th percentile, or ≥ 0.15). These cut-off values were selected to elucidate the adverse neonatal outcomes associated with extremes of ΔpH i.e., when ΔpH was assumed to be no longer ‘normal’.
Table 2.
Percentile distribution of ΔpH in relation to umbilical cord arterial pH (UApH) in term pregnancies (N = 108,629)
| Number of cases | ΔpH percentiles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5% | 10% | 25% | Median | 75% | 90% | 95% | Mean | ||
| Total cases | 108,629 | 0.01 | 0.02 | 0.05 | 0.08 | 0.11 | 0.15 | 0.18 | 0.08 |
| UApHa | |||||||||
| < 7.00 | 799 | 0.02 | 0.03 | 0.06 | 0.10 | 0.18 | 0.29 | 0.35 | 0.13 |
| 7.00–7.049 | 1405 | 0.02 | 0.04 | 0.07 | 0.12 | 0.19 | 0.26 | 0.31 | 0.14 |
| 7.05–7.09 | 3466 | 0.02 | 0.04 | 0.07 | 0.12 | 0.17 | 0.23 | 0.26 | 0.13 |
| 7.10–7.149 | 8345 | 0.02 | 0.04 | 0.07 | 0.11 | 0.16 | 0.20 | 0.23 | 0.12 |
| 7.15–7.19 | 15,920 | 0.02 | 0.04 | 0.07 | 0.10 | 0.14 | 0.18 | 0.20 | 0.11 |
| ≥ 7.20 | 78,694 | 0.01 | 0.02 | 0.04 | 0.07 | 0.10 | 0.13 | 0.15 | 0.07 |
a UApH umbilical cord arterial pH
Data management and analysis
Statistical analyses were performed with SPSS data software (26) (IBM Corp, Armonk, NY). Background characteristics were calculated and distributions between parameters were compared with Pearson Chi-2-test. The distribution of ΔpH across the population was illustrated by histogram to investigate normality and percentile distribution of ΔpH in different UApH groups. Modified Poisson regression model with 95% confidence intervals (CI) was used for RR of adverse neonatal outcomes.
Results
Characteristics of the study population
The selection of newborns for inclusion in the study is illustrated in Fig. 1. From the initial 315,174 newborns, after strict validation and exclusion of all preterm cases, 206,545 were excluded. The final study population comprised of 108,629 newborns with complete and validated data. Background characteristics are displayed in Table 1. The study population was described and cut-offs for ‘Small ΔpH’ (10th percentile, ≤0.02) and ‘Large ΔpH’ (90th percentile, ≥0.15) were also described. Vaginal non-instrumental birth (78.4%) was associated with higher rate of ‘Large ΔpH’ (87.7%) as compared to ‘Small ΔpH’ (75.9%). Neonates with birthweight < 2500 g (0.9%) accounted for 1.5% of all neonates who had a ‘Small ΔpH’. In contrast, birthweight 4000-4499 g (16.2%) and 4500 g + (4.1%) had higher percentage of neonates with ‘Large ΔpH’ (21.6 and 5.8%). Using the Chi-2-test, differences between ‘Small’ and ‘Large ΔpH’ were compared and found to be statistically significant for maternal age, parity, maternal body mass index (BMI), maternal smoking, birth start, birth mode, gestational age, birthweight, and Apgar score at 5 minutes. Infant sex was therefore the only background characteristic that was evenly distributed between the two cut-off groups.
Table 1.
Background characteristics of the study population including Chi-2-test comparing ‘Small ΔpH’ (10th percentile ≤0.02) and ‘Large ΔpH’ (90th percentile ≥0.15)
| N | % | Small ΔpHa ≤ 0.02 | Large ΔpH ≥0.15 | Chi-2-test | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | P-value | |||
| Total N | 108,629 | 100 | 12,015 | 100 | 12,261 | 100 | |
| Maternal age in years | 0.028 | ||||||
| < 20 | 2053 | 1.9 | 229 | 1.9 | 184 | 1.5 | |
| 20–34 | 85,681 | 78.9 | 9543 | 79.4 | 9671 | 78.9 | |
| 35–39 | 17,552 | 16.2 | 1904 | 15.8 | 2050 | 16.7 | |
| 40+ | 3343 | 3.1 | 339 | 2.8 | 356 | 2.9 | |
| Parity | < 0.000 | ||||||
| Primipara | 50,116 | 46.1 | 5867 | 49.2 | 5159 | 42.6 | |
| Multipara | 57,372 | 52,8 | 6054 | 50.8 | 6960 | 57.4 | |
| Multipara with previous CSb | 5464 | 5.0 | – | – | – | – | |
| Missing | 1141 | 1.1 | – | – | – | – | |
| Maternal BMIc | 0.005 | ||||||
| < 18.5 | 149 | 0.1 | 25 | 3.4 | 11 | 1.6 | |
| 18.5–24.9 | 3433 | 3.2 | 384 | 51.7 | 306 | 45.7 | |
| 25–29.9 | 2221 | 2.0 | 247 | 33.2 | 243 | 36.3 | |
| 30+ | 885 | 0.8 | 87 | 11.7 | 109 | 16.3 | |
| Missing data | 101,941 | 93.8 | – | – | – | – | |
| Maternal smoking | < 0.000 | ||||||
| No | 70,714 | 65.1 | 8099 | 91.5 | 7898 | 93.6 | |
| Yes 1–9 cigarettes/day | 4635 | 4.3 | 551 | 6.2 | 400 | 5.5 | |
| Yes, ≥10 cigarettes/day | 1718 | 1.6 | 119 | 2.2 | 139 | 2.0 | |
| Missing data | 31,562 | 29.1 | – | – | – | – | |
| Birth start | < 0.000 | ||||||
| Spontaneous | 91,017 | 83.8 | 10,020 | 83.4 | 10,885 | 88.8 | |
| Induction | 10,379 | 9.6 | 1183 | 9.9 | 1242 | 10.1 | |
| CS | 7214 | 6.6 | 807 | 6.7 | 132 | 1.1 | |
| Missing data | 19 | 0.0 | – | – | – | – | |
| Birth mode | < 0.000 | ||||||
| Vaginal, non-instrumental | 85,196 | 78.4 | 9052 | 75.9 | 10,636 | 87.7 | |
| Elective CS | 6623 | 6.1 | 713 | 6.0 | 118 | 1.0 | |
| Emergency CS | 7785 | 7.2 | 1167 | 9.8 | 112 | 0.9 | |
| Immediate emergency CS | 791 | 0.7 | 155 | 1.3 | 43 | 0.4 | |
| Instrumental (VEd/forceps) | 7407 | 6.8 | 837 | 7.0 | 1215 | 10.0 | |
| Missing | 827 | 0.8 | – | – | – | – | |
| Gestational duration in weeks (days) | < 0.000 | ||||||
| 37 + 0–40 + 6 (259–286) | 80,716 | 74.3 | 9117 | 75.9 | 8780 | 71.6 | |
| 41 + 0–41 + 6 (287–293) | 20,072 | 18.5 | 2038 | 17.0 | 2531 | 20.6 | |
| > 42 + 0 (294+) | 7841 | 7.2 | 860 | 7.2 | 950 | 7.7 | |
| Sex | 0.132 | ||||||
| Male | 56,300 | 51.8 | 6148 | 51.2 | 6432 | 52.5 | |
| Female | 52,244 | 48.1 | 5855 | 48.7 | 5817 | 47.4 | |
| Missing | 85 | 0.1 | 12 | 0.1 | 12 | 0.1 | |
| Birthweight (g) | < 0.000 | ||||||
| < 2500 | 931 | 0.9 | 177 | 1.5 | 26 | 0.2 | |
| 2500–3999 | 85,612 | 78.8 | 9731 | 81.0 | 8880 | 72.4 | |
| 4000–4499 | 17,569 | 16.2 | 1703 | 14.2 | 2647 | 21.6 | |
| 4500+ | 4463 | 4.1 | 397 | 3.3 | 706 | 5.8 | |
| Missing data | 54 | 0.0 | – | – | – | – | |
| Apgar score at 5 minutes | 0.001 | ||||||
| 0–3 | 95 | 0.1 | 9 | 0.1 | 21 | 0.2 | |
| 4–6 | 863 | 0.8 | 109 | 0.9 | 161 | 1.3 | |
| 7–10 | 107,652 | 99.1 | 11,895 | 99.0 | 12,078 | 98.5 | |
| Missing data | 19 | 0.0 | – | – | – | – | |
aΔpH veno-arterial differences in umbilical cord pH, b CS Cesarean section, c BMI body mass index (kg/m2), d VE vacuum extraction
ΔpH distribution
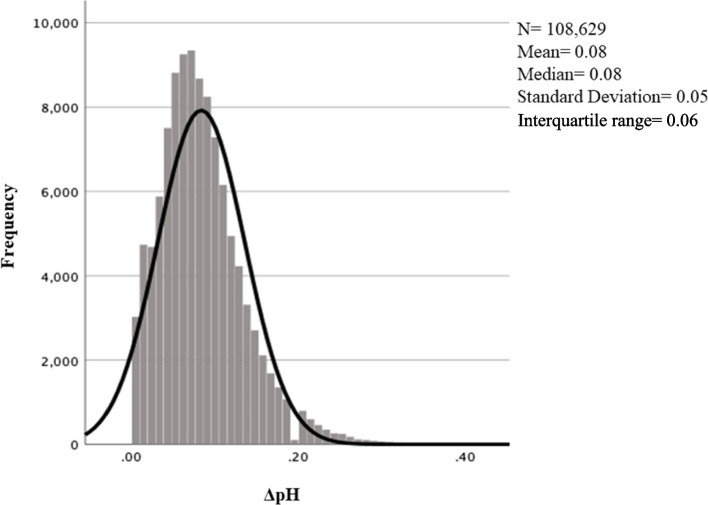
The distribution of ΔpH across the study population is illustrated in Fig. 2. Both the mean and median ΔpH were 0.08, standard deviation 0.05 and interquartile range 0.06. In Table 2, percentile values including median and mean values for ΔpH are reported for the whole study population (N = 108,629) and for UApH classes. The mean ΔpH decreased with rising UApH, from 0.13 in pH < 7.00 to 0.07 in pH ≥7.20 and varied within each percentile.
Fig. 2.
Histogram with distribution of veno-arterial differences in umbilical cord pH (ΔpH) in term pregnancies after validation
ΔpH and adverse neonatal outcomes
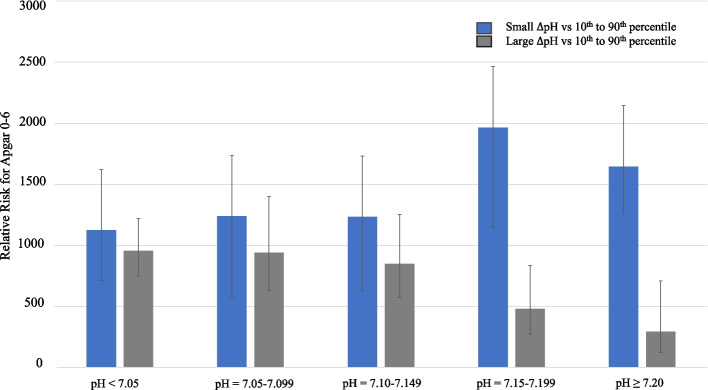
In Table 3, the RR for ‘Small’ and ‘Large ΔpH’ were compared to ΔpH between the 10th and 90th percentiles and parameters associated with adverse neonatal outcomes. For ‘Small ΔpH’, at UApH < 7.15–7.199, the RR for Apgar 0–6 at 5 minutes was RR = 1.96 (95%CI = 1.15–3.37) (P = 0.01); and at UApH ≥7.20, the RR was 1.65 (95%CI = 1.26–2.1) (P < 0.00). In contrast, a lower relative risk was found for ‘Large ΔpH’ for Apgar 0–6 at 5 minutes. At UApH 7.15–7.199, the RR was 0.48 (95%CI = 0.27–0.84) (P = 0.01). This apparent protective effect was enhanced for UApH ≥7.20, where the RR for Apgar 0–6 was 0.29 (95%CI = 0.12–0.71) (P = 0.01). These changes are illustrated in Fig. 3, where the RR for Apgar 0–6 is seen to decrease with enhancing UApH for ‘Large ΔpH’.
Table 3.
Relative risk of adverse neonatal outcome with Small ΔpH (≤0.02; 10th percentile) or Large ΔpH (≥0.15; 90th percentile) as compared to ΔpH between 10th–90th percentiles. Modified Poisson regression model with 95% confidence intervals and P-values were calculated for term pregnancies. N = 108,629
| Total cases | 108,629 | % | Apgar 0–6 at 5 minutes | CPAPa | NICUb admission | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-value | RR c | 95% CId | P-value | RR | 95% CI | P-value | RR | 95% CI | ||||
| UApH < 7.05 | Small ΔpH | 135 | 6.1% | 0.62 | 1.12 | 0.71–1.77 | 0.457 | 0.800 | 0.44–1.44 | 0.89 | 0.98 | 0.74–1.30 |
| Large ΔpH | 813 | 36.9% | 0.71 | 0.95 | 0.75–1.22 | 0.199 | 0.833 | 0.63–1.10 | 0.15 | 0.90 | 0.78–1.04 | |
| Total | 2204 | 100% | ||||||||||
| UApH 7.05–7.099 | Small ΔpH | 178 | 5.1% | 0.58 | 1.24 | 0.58–2.66 | 0.404 | 0.708 | 0.32–1.59 | 0.71 | 0.93 | 0.64–1.36 |
| Large ΔpH | 1208 | 34.9% | 0.76 | 0.94 | 0.63–1.40 | 0.082 | 0.730 | 0.51–1.04 | 0.00 | 0.75 | 0.62–0.91 | |
| Total | 3466 | 100.0% | ||||||||||
| UApH 7.10–7.149 | Small ΔpH | 456 | 5.5% | 0.55 | 1.23 | 0.62–2.44 | 0.380 | 0.728 | 0.36–1.48 | 0.36 | 1.14 | 0.86–1.50 |
| Large ΔpH | 2577 | 30.9% | 0.41 | 0.85 | 0.57–1.25 | 0.924 | 1.015 | 0.75–1.37 | 0.24 | 0.91 | 0.79–1.06 | |
| Total | 8345 | 100.0% | ||||||||||
| UApH 7.15–7.199 | Small ΔpH | 901 | 5.7% | 0.01 | 1.96 | 1.15–3.37 | 0.411 | 0.755 | 0.39–1.47 | 0.99 | 1.00 | 0.78–1.28 |
| Large ΔpH | 3452 | 21.7% | 0.01 | 0.48 | 0.27–0.84 | 0.048 | 0.679 | 0.46–1.00 | 0.01 | 0.82 | 0.70–0.95 | |
| Total | 15,920 | 100.0% | ||||||||||
| UApH ≥ 7.20 | Small ΔpH | 10,345 | 13.1% | 0.00 | 1.65 | 1.26–2.1 | 0.918 | 1.013 | 0.78–1.31 | 0.01 | 1.13 | 1.04–1.24 |
| Large ΔpH | 4211 | 5.4% | 0.01 | 0.29 | 0.12–0.71 | 0.022 | 0.549 | 0.30–0.92 | 0.01 | 0.81 | 0.69–0.95 | |
| Total | 78,694 | 100.0% | ||||||||||
a CPAP continuous positive airway pressure, b NICU neonatal intensive care unit, c RR relative risk, d CI confidence interval
Fig. 3.
Correlation of ΔpH to relative risk of 5-minute Apgar score 0–6 and changes in umbilical cord arterial pH
Of interest, ‘Large ΔpH’ showed the same protective trend at UApH 7.15–7.199 from need of CPAP (P = 0.048) and NICU admission (P = 0.01); and at UApH ≥7.20 for CPAP (P = 0.02) and NICU admission (P = 0.01).
Discussion
We investigated the association of veno-arterial differences in cord pH (ΔpH) with adverse neonatal outcome and found that large differences between umbilical cord venous and arterial pH (‘Large ΔpH’) were strongly associated with a lower relative risk for Apgar < 7 at 5 minutes, need for CPAP and NICU admission. These changes were strongest for infants born without acidemia.
As oxygen, carbon dioxide and hydrogen ions readily diffuse across the placenta to the maternal side for elimination, the fetus is dependent on adequate placental function in order to maintain its acid-base balance [23]. This dependency is especially important during birth, when the intermittent compressions of the umbilical cord, caused by forceful uterine contractions, lead to partial suspension of cord blood flow to and from the fetus leading to considerable metabolic stress in the fetus [24].
Clinically, most cases of fetal acidosis are acute in onset and are accompanied by large veno-arterial differences in pH (i.e. ‘Large ΔpH’). This is because the extracellular placental compartment has not had enough time to become saturated with acids from the fetus, or cord occlusion or fetal bradycardia have prevented fetal acids from being transferred to the placenta [7, 25, 26]. However, where a degree of chronic hypoxia has occurred, both the venous and arterial gases will show metabolic acidosis with a small veno-arterial difference (i.e. ‘Small ΔpH’) [7, 25, 26]. This information explains our finding that ‘Large ΔpH’ were associated with a decreased risk of adverse neonatal outcome because it likely reflects an acute intrapartum insult with a venous pH in the normal range.
Earlier studies have investigated the role of different cord blood gas parameters in predicting perinatal outcomes. Knutzen et al. [27] found that once pH was considered, base deficit was not of additional help in predicting adverse neonatal outcomes. As base deficit is a key component in respiratory acidosis, Low et al. [28] showed that acute acidosis was not linked to adverse outcome in the same way as metabolic acidosis. On the other hand, Wiberg and colleagues [29] found that umbilical cord lactate values were as good as cord pH or base deficit in predicting depressed neonatal condition at birth. Due to methodological confounding involved in calculation of base deficit, lactate may replace base deficit as an acid–base outcome parameter at birth. In essence, these studies highlight the difference between respiratory acidosis, which is considered short standing, as compared to the more longstanding metabolic acidosis that depletes the fetus’s buffer store.
It is well known that umbilical cord pH analysis gives a picture of the metabolic state of the newborn at birth, but previous studies have presented conflicting data. Martin et al. [30] in a small study comparing nuchal cord labors with acidosis (N = 33) to normal labors, found large differences between UVpH and UApH but the Apgar score was not affected. Different outcomes have been observed for acute versus the chronic acidosis, as seen by Bobrow et al. [24], where chronic acidosis was associated with adverse outcome for neonates. Importantly, since pH is based on a logarithmic scale, a unit decrease in pH is much more substantial than a unit decrease in the corresponding hydrogen ion concentration, an effect that becomes ever stronger with decreasing pH [31].
Our data showed that a ‘Large ΔpH’ was associated with decreased risk for adverse neonatal outcome, yet only 1 % of the cases with the lowest Apgar score had a ‘Small’ or ‘Large ΔpH’.
Whilst reviewing the literature, only a handful studies were found to have investigated the prognostic value and clinical significance of veno-arterial differences in cord pH [1, 4, 8–10, 29] with only one study correlating these differences to adverse neonatal outcome. A study evaluating ΔpH in relation to cardiotocography patterns, found no relation between large ΔpH and acute onset acidemia [9]. The sample size, however, was small (N = 83). Similarly, Belai et al. [10], showed a weak correlation of ΔpH to Apgar Score, seizures and hypoxic ischemic encephalopathy. By using a large population-based cohort with validated cord blood gases, this study has comprehensively correlated differences in ΔpH to adverse neonatal outcome.
The major strengths of the current study stem from the large sample size and the meticulous validation of paired umbilical cord blood pH values. As small ΔpH may occur physiologically [17], cut-offs were set at identical and negative ΔpH to minimize the introduction of selection bias. There are several pitfalls in cord blood sampling and analyses [17], but the procedure has been routinely used in Sweden for many years and a high-quality dataset was therefore obtained [32]. On the other hand, discarding all single pH samples could have introduced a systematic selection bias in the study. Possible causes for single vessel samples, at 62% of all cases can be explained in several clinical situations. Dual vessel samples are generally drawn from the cord at birth but may not be analyzed when the newborn is healthy. In contrast, with an emergency birth or a suspected asphyxiated newborn, umbilical cord blood gases may not be prioritized due to the urgency in the care of the infant and may lead to a single sample taken to save valuable time. Regardless, the sample size of the current study was large enough to account for this step in the data cleaning process which was essential to obtain values from both cord artery and vein pH. Data was obtained from an established regional database which was used by nine different maternity centers all following the same general routines and procedures. The study population was therefore representative of the whole population [32].
Conclusion
Large ΔpH was associated with a lower risk for neonatal morbidity including low 5-minute Apgar score, the need for CPAP and NICU admission when UApH was above 7.15. Reference values for ∆pH, including stratification of percentile values according to UApH, were reported. Our findings may have important clinical implications in the assessment of the metabolic state of the newborn at birth. They may stem from the positive effects of maintained placenta function during birth and the ability of the placenta to adequately replenish acid-base balance in fetal blood leading to a large veno-arterial differences in cord blood pH. A ‘Large ΔpH’ may therefore be a marker of effective gas exchange in the placenta during birth. Further studies are warranted to investigate changes in ΔpH in correlation to carbon dioxide and oxygen levels in umbilical cord blood gases to verify this hypothesis.
Supplementary Information
Additional file 1. Correlation of ΔpH decentiles to relative risk of 5-minute Apgar score 0–6.
Acknowledgements
The authors would like to thank Professor Per Olofsson for his help in planning the study.
Abbreviations
- ΔpH
delta pH
- UVpH
umbilical cord venous pH
- UApH
umbilical cord arterial pH
- CPAP
continuous positive airway pressure
- NICU
neonatal intensive care unit
- RR
Relative risk
Authors’ contributions
TS, KK and MZ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: KK, MZ. Acquisition, analysis, and interpretation of data: KK, TS, NW, MZ. Drafting of the manuscript: TS, MZ. Critical revision of the manuscript for important intellectual content: KK, TS, NW, MZ. Statistical analysis: KK, TS, MZ. Administrative, technical, or material support: KK, MZ. Supervision: MZ. The authors read and approved the final manuscript.
Funding
Open access funding provided by Lund University. The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (YF0054). The funding organization had no role in the design of the study; collection, management, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to agreements signed with the Regional Ethics Committee in Lund under the Ethical Approval of the study but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations or Declaration of Helsinki. The study was approved by the Regional Ethics Committee in Lund, Dnrs 2009/222 and 2012/5 and the requirement for informed consent was waived by Regional Ethics Committee in Lund due to the retrospective and population-based design of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong L, Stenson BJ. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F430–F434. doi: 10.1136/adc.2006.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JW, Richards DS. The etiology of fetal acidosis as determined by umbilical cord acid-base studies. Am J Obstet Gynecol. 1997;177(2):274–280. doi: 10.1016/S0002-9378(97)70187-X. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee opinion no. 348, November 2006: umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108(5):1319–22. [DOI] [PubMed]
- 4.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeomans ER, Hauth JC, Gilstrap LC, 3rd, Strickland DM. Umbilical cord pH, PCO2, and bicarbonate following uncomplicated term vaginal deliveries. Am J Obstet Gynecol. 1985;151(6):798–800. doi: 10.1016/0002-9378(85)90523-X. [DOI] [PubMed] [Google Scholar]
- 6.Thorp JA, Dildy GA, Yeomans ER, Meyer BA, Parisi VM. Umbilical cord blood gas analysis at delivery. Am J Obstet Gynecol. 1996;175(3 Pt 1):517–522. doi: 10.1053/ob.1996.v175.a74401. [DOI] [PubMed] [Google Scholar]
- 7.Westgate J, Garibaldi JM, Greene KR. Umbilical cord blood gas analysis at delivery: a time for quality data. Br J Obstet Gynaecol. 1994;101(12):1054–1063. doi: 10.1111/j.1471-0528.1994.tb13581.x. [DOI] [PubMed] [Google Scholar]
- 8.Cantu J, Szychowski JM, Li X, Biggio J, Edwards RK, Andrews W, et al. Predicting fetal acidemia using umbilical venous cord gas parameters. Obstet Gynecol. 2014;124(5):926–932. doi: 10.1097/AOG.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 9.Ghi T, Chandraharan E, Fieni S, Dall'Asta A, Galli L, Ferretti A, et al. Correlation between umbilical vein-to-artery delta pH and type of intrapartum hypoxia in a cohort of acidemic neonates: a retrospective analysis of CTG findings. Eur J Obstet Gynecol Reprod Biol. 2018;231:25–29. doi: 10.1016/j.ejogrb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Belai Y, Goodwin TM, Durand M, Greenspoon JS, Paul RH, Walther FJ. Umbilical arteriovenous PO2 and PCO2 differences and neonatal morbidity in term infants with severe acidosis. Am J Obstet Gynecol. 1998;178(1 Pt 1):13–19. doi: 10.1016/S0002-9378(98)70619-2. [DOI] [PubMed] [Google Scholar]
- 11.Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32(4):260–267. [PubMed] [Google Scholar]
- 12.Cnattingius S, Norman M, Granath F, Petersson G, Stephansson O, Frisell T. Apgar score components at 5 minutes: risks and prediction of neonatal mortality. Paediatr Perinat Epidemiol. 2017;31(4):328–337. doi: 10.1111/ppe.12360. [DOI] [PubMed] [Google Scholar]
- 13.Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344(7):467–471. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 14.Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med. 2020;383(1):49–57. doi: 10.1056/NEJMoa1915075. [DOI] [PubMed] [Google Scholar]
- 15.Zaigham M, Marsal K. Apgar score in premature infants associated with neonatal death prediction. J Pediatr X. 2020;226:P309–P313. doi: 10.1016/j.jpeds.2020.08.055. [DOI] [PubMed] [Google Scholar]
- 16.Jepson HA, Talashek ML, Tichy AM. The Apgar score: evolution, limitations, and scoring guidelines. Birth. 1991;18(2):83–92. doi: 10.1111/j.1523-536X.1991.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 17.Mokarami P, Wiberg N, Källén K, Olofsson P. Arterio-venous blood gas Δvalues for validation of umbilical cord blood samples at birth are not only biased by sample mix ups but also affected by clinical factors. Acta Obstet Gynecol Scand. 2019;98(2):167–175. doi: 10.1111/aogs.13471. [DOI] [PubMed] [Google Scholar]
- 18.White CR, Doherty DA, Kohan R, Newnham JP, Pennell CE. Evaluation of selection criteria for validating paired umbilical cord blood gas samples: an observational study. BJOG. 2012;119(7):857–865. doi: 10.1111/j.1471-0528.2012.03308.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaigham M, Källén K, Olofsson P. Gestational age-related reference values for Apgar score and umbilical cord arterial and venous pH in preterm and term newborns. Acta Obstet Gynecol Scand. 2019;98(12):1618–1623. doi: 10.1111/aogs.13689. [DOI] [PubMed] [Google Scholar]
- 20.Kitlinski ML, Källén K, Marsál K, Olofsson P. Gestational age-dependent reference values for pH in umbilical cord arterial blood at term. Obstet Gynecol. 2003;102(2):338–345. doi: 10.1016/s0029-7844(03)00512-x. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18(1):63. doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 23.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2s):S745–Ss61. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 24.Bobrow CS, Soothill PW. Causes and consequences of fetal acidosis. Arch Dis Child Fetal Neonatal Ed. 1999;80(3):F246–F249. doi: 10.1136/fn.80.3.F246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomerance J. Umbilical cord blood gas casebook: interpreting umbilical cord gases part II. J Perinat Med. 1998;18:160–161. [PubMed] [Google Scholar]
- 26.Yeomans, et al. Umbilical cord blood pH, pCo2 and bicarbonate following uncomplicated term vaginal deliveries. Am J Obstet Gynecol. 1985;151:798–800. doi: 10.1016/0002-9378(85)90523-X. [DOI] [PubMed] [Google Scholar]
- 27.Knutzen L, Svirko E, Impey L. The significance of base deficit in acidemic term neonates. Am J Obstet Gynecol. 2015;213(3):373 e1–373 e7. doi: 10.1016/j.ajog.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177(6):1391–1394. doi: 10.1016/S0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]
- 29.Wiberg N, Källén K, Herbst A, Olofsson P. Relation between umbilical cord blood pH, base deficit, lactate, 5-minute Apgar score and development of hypoxic ischemic encephalopathy. Acta Obstet Gynecol Scand. 2010;89(10):1263–1269. doi: 10.3109/00016349.2010.513426. [DOI] [PubMed] [Google Scholar]
- 30.Martin GC, Green RS, Holzman IR. Acidosis in newborns with nuchal cords and Normal Apgar scores. J Perinatol. 2004;25(3):162–165. doi: 10.1038/sj.jp.7211238. [DOI] [PubMed] [Google Scholar]
- 31.Zaigham M. Informative fetal blood: umbilical cord blood Analytes to predict neonatal problems and diseases occurring later in life [dissertation on the internet] Lund: Lund University; 2019. [Google Scholar]
- 32.Socialstyrelsen . Statistik om graviditeter, förlossningar och nyfödda [internet] Stockholm: Socialstyrelsen; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Correlation of ΔpH decentiles to relative risk of 5-minute Apgar score 0–6.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to agreements signed with the Regional Ethics Committee in Lund under the Ethical Approval of the study but are available from the corresponding author on reasonable request.