Abstract
SAMHD1 (Sterile alpha motif and histidine/aspartic acid domain‐containing protein 1) is a dNTP triphosphohydrolase crucial in the maintenance of balanced cellular dNTP pools, which support genome integrity. In SAMHD1 deficient fibroblasts isolated from Aicardi‐Goutières Syndrome (AGS) patients, all four DNA precursors are increased and markedly imbalanced with the largest effect on dGTP, a key player in the modulation of telomerase processivity. Here, we present data showing that SAMHD1, by restricting the dGTP pool, contributes to telomere maintenance in hTERT‐immortalized human fibroblasts from AGS patients as well as in telomerase positive cancer cell lines. Only in cells expressing telomerase, the lack of SAMHD1 causes excessive lengthening of telomeres and telomere fragility, whereas primary fibroblasts lacking both SAMHD1 and telomerase enter normally into senescence. Telomere lengthening observed in SAMHD1 deficient but telomerase proficient cells is a gradual process, in accordance with the intrinsic property of telomerase of adding only a few tens of nucleotides for each cycle. Therefore, only a prolonged exposure to high dGTP content causes telomere over‐elongation. hTERT‐immortalized AGS fibroblasts display also high fragility of chromosome ends, a marker of telomere replication stress. These results not only demonstrate the functional importance of dGTP cellular level but also reveal the critical role played by SAMHD1 in restraining telomerase processivity and safeguarding telomere stability.
Keywords: Aicardi‐Goutières syndrome (AGS), deoxynucleotide metabolism, deoxynucleotide pool imbalance, sterile alpha motif and HD domain containing protein 1 (SAMHD1), telomere maintenance
A Model for SAMHD1 as a gatekeeper of telomere stability in telomerase positive cells. In SAMHD1 proficient cells, the dGTP pool represents less than 10% of the total dNTP pool that preserves telomere length and structure. SAMHD1 deficiency in both immortalized fibroblasts and telomerase positive cancer cells expands the dGTP content, which enhances telomerase processivity leading to telomere elongation (open arrows) and fragility (dark arrows).

Abbreviations
- AGS
Aicardi‐Goutières syndrome
- dNTPs
deoxynucleoside triphosphates
- Dox
doxycycline
- mQ‐FISH
metaphase quantitative fluorescence in situ hybridization
- qPCR
quantitative PCR
- qRT‐PCR
quantitative reverse transcription PCR
- RNR
Ribonucleotide Reductase
- SAMHD1
Sterile Alpha Motif and Histidine‐Aspartate Domain containing protein 1
- SDHA
Succinate Dehydrogenase Complex Flavoprotein Subunit A
- TAQ PCR
telomerase activity quantification PCR
1. INTRODUCTION
The Sterile Alpha Motif and Histidine‐Aspartate Domain containing protein 1 (SAMHD1) is a master regulator of dNTP pools in mammalian cells, restricting their sizes in both cycling and resting cells. 1 , 2 SAMHD1 is an allosterically regulated triphosphohydrolase that cleaves deoxynucleoside triphosphates (dNTPs) into deoxyribonucleosides and inorganic triphosphate. 3 , 4 Aside from its dNTPase activity, SAMHD1 exerts additional functions in DNA replication fork progression 5 and double strand break repair. 6 Here, we focus on the enzymatic activity of SAMHD1 and in particular on its ability to fine‐tune the concentration of dGTP, securing its correct proportion in the pool relative to the other three dNTPs.
Genetic defects in SAMHD1 are associated with the rare genetic autoimmune disease Aicardi‐Goutières syndrome (AGS), characterized by aberrant activation of the innate immune system. 7 The main feature of primary skin fibroblasts isolated from AGS patients with mutated SAMHD1 is the large increase of all four dNTPs, especially purine dNTPs, with an impressive expansion of the dGTP pool, normally the smallest. 1 , 8 The concurrent expansion of dATP exerts feedback inihibition of ribonucleotide reductase (RNR) preventing the arrest of cell cycle progression by excessive dNTP concentrations. 9
The dNTP pool imbalances have destabilizing effects during DNA replication, compromising the fidelity of DNA synthesis. Interestingly, SAMHD1 was found mutated or downregulated in solid tumors and chronic lymphatic leukemia, suggesting its contribution to the control of malignancy and preservation of genetic stability. 10 In colon cancer cells with inactivated DNA mismatch repair (MMR), an expansion of dNTP pools dramatically increases mutation rates. 11 Remarkably, SAMHD1 deficiency is per se sufficient to elicit a mutator phenotype in non‐transformed fibroblasts from AGS patients and the effect is exacerbated by MMR downregulation. 8 Besides being present in SAMHD1 deficient fibroblasts, dNTP pools characterized by high dGTP content are also found in some E. coli and S. cerevisiae mutants characterized by extreme hypermutability, 12 , 13 indicating that restriction of dGTP is required for genome stability both in prokaryotic and eukaryotic cells. In addition, high dGTP levels may affect the replication of GC‐rich genomic regions, such as telomeres.
In vertebrates, telomeric DNA consists of TTAGGG tandem repeats that in humans span 5 to 15 Kb. 14 Specific telomeric features, dependent on the presence of heterochromatin, shelterin complexes and DNA repeats, hamper fork progression during DNA replication. The inherent difficulty of chromosome end replication 15 together with the role of SAMHD1 in the processing of stalled replication forks 5 suggests a further connection between SAMHD1 and telomere stability.
Telomere maintenance depends on telomerase activity. Telomerase is a multisubunit complex regulated at multiple levels, expression, post‐transcriptional and post‐translational modifications, complex assembly and recruitment to telomeres. 16 Telomerase expression is highest in germ cells and present in undifferentiated stem or progenitor cells in highly proliferative compartments of normal tissues, but it declines in differentiated cells where the expression of the catalytic subunit hTERT is limited or undetectable. 17
At each cell division, the telomeres shorten due to incomplete DNA replication at the ends of linear DNA. Telomere erosion eventually results in very short and unprotected chromosome ends, leading to cell‐cycle arrest and replicative senescence. 18 , 19 In proliferating cells expressing telomerase, the telomeric repeats extend at the 3′ends of the chromosome strands through repeated rounds of reverse transcription by the telomerase that uses a short stretch of its RNA subunit as a template. The processivity of telomerase, i.e., addition of multiple telomeric repeats after a single substrate binding event, strongly depends on the dGTP pool. In vitro studies with S. cerevisiae, 20 T. thermophila 21 or mammalian cells 22 show that telomerase processivity is markedly reduced when the dGTP concentration is lower than normal, and strongly increased even by modest dGTP expansions. In yeast, a balanced increase of all dNTP levels has no significant effect on the homeostasis of telomeres, but quantitative changes of dGTP alone positively correlate with telomerase processivity and telomere length. 23 Considering its effect on telomerase processivity, dGTP is considered the major pool determinant of telomere maintenance in vivo. 23
Here, we address the question of SAMHD1 involvement in telomere stability by investigating the relation between SAMHD1 activity as regulator of the dNTP pools and the presence of active telomerase in human cells in vitro. We employ long term cultures of hTERT‐immortalized human fibroblasts and telomerase‐positive THP1 and SUIT2 cancer cells either expressing functional SAMHD1 or devoid of SAMHD1 activity. We find that SAMHD1 by strictly regulating dGTP concentration warrants telomere stability in telomerase‐positive cells.
2. MATERIALS AND METHODS
2.1. Cell lines
We used two lines of skin fibroblasts derived from patients affected by AGS with inactivating mutations in SAMHD1 (Prof. Y. Crow collection, University of Manchester, UK). P1 (AGS282) is a compound heterozygote carrying mutations R145X and R164X, 24 P2 (AGS295) carried the homozygous deletion of exons 12–16. 25 Appropriate written informed consent was obtained from the parents of the patients for inclusion in this study, which was approved by the Leeds (East) Multi‐Centre Research Ethics Committee. Two lines of age‐matched control skin fibroblasts were available in our laboratory, WT1 and WT2. 8 Both AGS and WT cell line were isolated from pediatric samples. Human monocytic cells (THP1) knockout for SAMHD1 and matched controls were donated by Prof. T. Gramberg. 26 SUIT2 wild type, SAMHD1 knockout (SUIT2 KO) and TetR‐shSAMHD1 SUIT2 cell lines were donated by Prof C. Radu. 27
2.2. Cell culture and hTERT immortalization
All fibroblast and SUIT2 cell lines were grown in DMEM with 4.5% g/L glucose, 10% (v/v) fetal calf serum (FCS), non‐essential amino acids and antibiotics. THP1 cells were cultured in RPMI with 10% (v/v) FCS. To ensure comparability, we immortalized fibroblasts at early passages (p < 15) after isolation. Fibroblasts were transduced with plasmid CMV‐hTERT/PGK‐Puro kindly provided by Prof. D. Furling (Center of Research in Myology, Sorbonne University, France) in the presence of 4 μg/mL of polybrene (Sigma‐Aldrich) and selected with puromycin (0.2 μg/mL) for 10 days. 28
2.3. Quantitation of dNTP pools
dNTP pools were extracted from cell cultures with 60% ice‐cold methanol. 8 The sizes of the dNTP pools were determined with a DNA polymerase‐based assay 29 with the modifications reported in Ferraro et al. 30
2.4. Telomerase activity quantification by qPCR (TAQ PCR)
Telomerase activities were detected by telomerase activity quantification qPCR assay kit (ScienCell, Carlsbad, CA, USA) which allows to quantitatively compare telomerase activity among cell populations. Briefly, cell lysates were prepared following the manufacturer's protocol starting from 4 million cells. An aliquot of the cell extract was used to set up the telomerase reaction. Then a telomere primer set was used to amplify newly synthesized telomere sequences in the assay. All data were normalized to the value measured in hTERT WT1 fibroblasts when comparing fibroblast cell lines or to corresponding SAMHD1 proficient controls when comparing SAMHD1 KO cancer cell lines.
2.5. RNA extraction and quantitative reverse transcription PCR (qRT‐PCR)
RNA extraction, reverse transcription and real time PCR were done as described in Ref. [1]. TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract RNA from cell cultures according to the manufacturer's protocol. QuantiTect Primer assay kits (Qiagen, Hilden, Germany) were used to detect the relative mRNA expression of human hTERT (QT00073409, Qiagen) and human SAMHD1 (QT00084322, Qiagen) using Succinate Dehydrogenase Complex Flavoprotein Subunit A (SDHA, QT00059486, Qiagen) as reference gene.
2.6. Measurement of telomere length by qPCR
Mean telomere lengths were assessed by absolute human telomere length quantification qPCR Kit (ScienCell, Carlsbad, CA, USA) using genomic DNA extracted by Puregene Core Kit B (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. Briefly, the telomere primer set recognizes and amplifies telomere sequences, while a single copy reference (SCR) primer set recognizes and amplifies a 100 bp‐long region of chromosome 17 used as a reference for data normalization. A reference genomic DNA with known telomere length serves as reference for calculating the mean telomere lengths of target samples.
2.7. Metaphase spreads
Chromosome spreads obtained after exponentially growing cells were exposed for 30‐min to 0.1 μg/mL Colcemid (Sigma‐Aldrich, St. Louis, MO, USA). Chromosome spreads were prepared by a standard procedure consisting of treatment with hypotonic solution (trisodium citrate 0.9%) for 25 min at 37°C followed by fixation with ice‐cold fixative (3:1 v/v ethanol/acetic acid, 3 × 10 min). After metaphase spreading, preparations were dried at 50°C and left at room temperature one week before Q‐FISH.
2.8. Q‐FISH
Chromosome preparations were washed in 2X SSC solution (Saline Sodium Citrate) pH 7 for 5 min and incubated with 0.2 mg/mL RNase (Sigma‐Aldrich, St. Louis, MO, USA) 30 min at 37° C. After dehydration through ethanol series (70% (v/v)–90% (v/v)‐absolute ethanol, 5 min each), the slides were air‐dried, denatured at 70°C for 4 min in 70% formamide, 2X SSC pH 7, and dehydrated again in the ethanol series on ice. The hybridization mix, containing 300 nM of telomeric PNA probe (TelC Alexafluor‐488, Panagene, Daejeon, Republic of Korea) and 100 nM of centromeric PNA probe (CENPB Alexafluor‐647, Panagene Daejeon, Republic of Korea), 50% formamide, 10% dextran‐sulphate and 2X SSC pH 7, was denatured 10 min at 70°C and immediately hybridized to chromosome preparations (10 μL per slide under a 22 × 22 coverslip). After hybridization for 4 h at 37°C, the slides were washed three times with 50% formamide, 2X SSC pH 7, at 42°C, three times with 2X SSC pH 7 and finally twice with PBS, 0.1% Tween‐20. Slides were mounted with antifade solution (Vectashield; Vector Laboratories, Newark, CA, USA) containing 0.2 μg/mL of 4′‐6‐diamidino‐2‐phenylindole (DAPI).
2.9. Image capture
Fluorescence signals were visualized under an epifluorescence microscope (Axio Imager.M1; ZEISS, Germany), equipped with a cooled CCD camera (Photometrix, Coolsnap HQ2). After localization of metaphase spreads, blue (DAPI), green (TelC), and far‐red (CENPB) fluorescence signals were captured at ×100 magnification, using an oil immersion objective with N.A. = 1.30. For capture and image analysis the MetaMorph software (7.1.3.0, Molecular Device) and the MetaVue software (7.8.11.0, Molecular device) were used, respectively. Merged pseudo‐color images were assembled to identify metaphase spreads and quantify fluorescence intensities. Telomere lengths were calculated as the ratio between the telomere fluorescence signal and the fluorescence of CENPB, used as internal reference for each metaphase analyzed. Because of the level of variability in each independent experiment, only metaphases with CENPB fluorescence intensity within a set range were included in the analysis.
2.10. Statistical analysis
Statistical analyses were performed using the OriginPro software (OriginLab Corporation, Northampton, MA, USA). Whole experimental datasets with different samples and culture times were analyzed preliminary by Analysis of Variance. In pairwise comparisons between WT and AGS samples the Student's t test was applied (two‐tailed). When appropriated, the non‐parametric Kolmogorov–Smirnov test was also used.
3. RESULTS
3.1. SAMHD1 deficiency causes telomere lengthening in hTERT‐immortalized fibroblasts derived from Aicardi‐Goutières syndrome patients
The main feature of the dNTP alterations found in SAMHD1 deficient cells is the large accumulation of dGTP, a precursor necessary for the synthesis of the G‐reach telomeric repeats and a modulator of telomerase processivity. To test the effect of SAMHD1 loss on telomere stability in telomerase‐positive cells, human skin fibroblasts derived from two AGS patients and two age‐matched controls were transduced with a lentiviral vector expressing the catalytic subunit of the human telomerase (hTERT). All primary fibroblast lines were previously studied by Franzolin et al. 8 and indicated as P1 and P2 or WT1 and WT2, respectively.
To examine the consequences of chronic alteration of the dGTP pool for telomere stability, we kept the four immortalized fibroblast lines in culture for more than six months. It has been reported that hTERT‐immortalized human fibroblasts behave similarly to primary cells during the first 150 population doublings, but after longer times in culture they tend to become transformed, accelerating their growth rate, losing contact inhibition and acquiring malignant potential. 31 For our experiments, long‐term cultures were passaged for at most 150 population doublings (PDs) without undergoing cell transformation as indicated by the slopes of the growth curves in Figure 1A and the normal morphology (not shown).
FIGURE 1.

hTERT immortalized fibroblasts retain the distinctive dNTP pool content of the corresponding primary cells. (A) Cumulative population doublings (PDs) curves of WT (WT1 and WT2) and AGS (P1 and P2) fibroblasts after immortalization with hTERT. Viable cells were counted every 4 days for about seven months. The daily population doubling numbers (PDN) were calculated by the formula {ln [(number of cells harvested)/number of cell seeded)]/ln2}/4 days and added to the previous PDNs to yield the cumulative PDN value. (B) Relative telomerase activity was measured by TAQ PCR at Mid stage in two biological samples of each immortalized fibroblast line analyzed in triplicate. Telomerase activity data are relative to the activity measured in hTERT WT1 fibroblasts. In all primary cell lines telomerase activity was undetectable or <10−5 relative to WT1. Bars represent means ± SE. (C) Fold change in the dNTP pool concentrations relative to WT immortalized fibroblasts (WT1 + WT2) at Early stage after hTERT immortalization. dNTP pools were measured in at least three biological samples analyzed in duplicate. Bars represent means ± SE. Statistical analysis was carried out by comparing the pooled data from WT (WT1 + WT2) and AGS cells (P1 and P2) using the Student's t test (two‐tailed); ***p < .001. (D) Proportion of each dNTP as a percentage of the total dNTP pool in cycling WT (WT1 and WT2) and AGS fibroblasts P1 and P2.
Cell samples were collected and analyzed after 1 (Early passages), 3 (Mid passages) and 6 (Late passages) months of continuous culture. SAMHD1‐mutated P1 and P2 lines grew like the WT1 and WT2 fibroblasts for all their time in culture (Figure 1A).
Normally, the activity of hTERT is limiting in human cells so that an increase of hTERT expression results in increased telomerase activity and telomere lengthening. 32 , 33 , 34 To exclude differences in hTERT that could bias our analyses, we measured hTERT expression (Figure S1) and telomerase activity (Figure 1B) in the four hTERT‐immortalized fibroblast lines. Early samples of WT and AGS P1 fibroblasts displayed similar hTERT expression while P2 fibroblasts had 60% lower expression (Figure S1). As culture time increased, telomerase expression declined further in both AGS immortalized fibroblasts to about 20% of the WT cells. Additionally, we assessed telomerase activity in Mid samples by an enzymatic assay employing TAQ PCR (Figure 1B). Although PCR‐based telomeric repeat amplification protocol cannot accurately reflect the continuous binding ability of telomerase, it is currently the most commonly accepted method for telomerase activity detection. Whereas P1 fibroblasts displayed a telomerase activity similar to both WT fibroblasts, the activity was almost 50% lower in P2 fibroblasts. The discrepancy between hTERT expression and activity as measured in Figures S1 and 1B can be ascribed to the multiple factors involved in the assembly of active telomerase complexes. Indeed, the activity of telomerase is tightly controlled at multiple levels, from transcriptional regulation of both TERT and telomerase RNA component (TERC) to holoenzyme biogenesis. 35 Nevertheless, the important result of these analyses is that after immortalization neither hTERT expression nor its activity were higher in AGS fibroblasts than in WT fibroblasts.
After immortalization, we determined the dNTP pool size and composition (Figure 1C,D) in cycling cultures of all four immortalized fibroblast lines. In AGS cells we confirmed both the expansion of each of the four dNTPs (Figure 1C, Table S1) and the characteristic dNTP pool imbalance (Figure 1D) observed previously in the parental non‐immortalized P1 and P2 fibroblasts. 8 The largest change was that of the dGTP pool that reached 15%–16% of the total pool, while in WT fibroblasts it represented only 7% (Figure 1D).
To test our hypothesis, we first measured telomere length by qPCR in primary WT and AGS human skin fibroblasts at early passages in culture. Independently of their SAMHD1 status, all fibroblasts exhibited similar telomere lengths of about 2–3 Kb (Figure 2A), suggesting that SAMHD1 deficiency does not affect telomere length when telomerase is undetectable, as is the case in primary cells.
FIGURE 2.

SAMHD1 deficiency promotes telomere over‐elongation in hTERT immortalized AGS fibroblasts. (A) Mean telomere lengths measured by qPCR in primary AGS (P1 and P2) and WT (WT1 and WT2) fibroblasts at early (˂15) passages from isolation and in Early, Mid and Late samples after hTERT immortalization. Genomic DNA sample with known telomere length was used as a reference. Bars represent means ± SE of two biological replicates for each fibroblast line, each analyzed in triplicate. ***p < .001 (two‐tailed Student's t test on pooled data from WT and AGS cells). (B) Telomere length measured using metaphase Q‐FISH in Early, Mid and Late samples of hTERT AGS P1 and P2 and WT fibroblasts (WT1 and WT2). At least 50 metaphases were analyzed for each cell line and for each stage (Early, Mid and Late) indicated in (A). Telomere length is calculated as the ratio of telomere/centromere fluorescence intensity. Bars represent means ± SE. Statistical analyses as described in A, with ***p < .001. No significant difference in the number of telomeric ends lacking a fluorescence signal was observed in any of these cell populations.
Next, we evaluated telomere length in the hTERT immortalized fibroblasts at increasing times in culture. The average telomere lengths of AGS and WT fibroblasts were measured by two procedures, i.e., quantitative real‐time PCR (qPCR, Figure 2A) and metaphase quantitative fluorescence in situ hybridization (mQ‐FISH, Figure 2B). Genomic DNA was extracted and metaphase spreads were prepared from Early (≈20 PDs), Mid (≈70 PDs), and Late (120 PDs) passage cells (Figure 2A,B). Data sets obtained by qPCR showed that immortalized WT SAMHD1‐proficient fibroblasts had elongated their telomeres up to a length of 6–8 Kb after 70 PDs, and then remained stable up to 120 PDs (Figure 2A). Conversely, both P1 and P2 SAMHD1‐deficient AGS fibroblasts underwent a continuous elongation of their telomeres, reaching a length of 16–19 Kb after 120 PDs. Considering the original telomere length in non‐immortalized fibroblasts, WT telomeres at most tripled in length, while AGS telomeres never reached a plateau and became roughly 7‐fold longer than before immortalization, with a clear temporal trend (p < .001, Analysis of variance). Results from qPCR analyses were additionally validated by mQ‐FISH, using the TelC PNA probe (CCCTAA)3 that targets telomere repeats in individual chromosomes (Figure 2B). The relative length of telomeres was measured as the ratio between the emitted fluorescence intensity of the TelC PNA probe and the fluorescence of a centromeric CENPB PNA probe. We found that telomere lengths across both WT and AGS fibroblast populations were highly heterogeneous (Figure 2B). This phenomenon was described previously and attributed to variable intercellular expression of hTERT mRNA or to telomerase inhibition by the telomeric shelterin complex in single cells. 36 The microscopic analysis showed that hTERT WT1 and WT2 fibroblasts elongated their telomeres by 30% from Early to Mid‐stage and then remained stable until the Late stage in culture. Elongation of telomeres was stronger in P1 and P2 fibroblasts (80% longer telomeres from Mid stage relative to the WT fibroblasts). Remarkably, with both experimental approaches, telomere lengths were significantly higher in hTERT immortalized AGS fibroblasts than in the WT already at the earliest stage analyzed (p < .001, Figure 2A,B). The nature of the data collected in this study from qPCR and mQ‐FISH differs in that the former method provides average values for all telomeres in multicellular samples, whereas the latter collects values from single cells. Despite this difference, the two sets of results provided a consistent picture of the effects of SAMHD1 deficiency on the regulation of telomeres and suggested that the altered dNTP pools of AGS fibroblasts favor telomere lengthening by telomerase.
3.2. SAMHD1 knock‐out in telomerase‐positive cancer cells increases the dGTP pool leading to telomere lengthening
We studied the role of SAMHD1 as a telomere caretaker in two different types of telomerase‐positive cancer cells, THP1 human promyelocytic leukemia cells and the human pancreatic cancer line SUIT2. For both cancer lines, we had access to SAMHD1‐deficient derivatives obtained by CRISPR/Cas9 knock‐out (THP1‐KO, SUIT2‐KO, see Materials and Methods).
In both KO lines, the lack of SAMHD1 induces dNTP pool expansion, albeit to a different extent. The effect of SAMHD1 loss on the dNTP pools of THP1 cells has been reported by us in earlier publications 2 , 8 showing that in THP1‐KO cells the frequency of dGTP shifted from 3% to 8% of the total dNTP pool. Here, in SUIT2‐KO cells, both purine dNTPs became 10 times higher than in the parental SUIT2 cells, while pyrimidine dNTPs doubled (Figure 3A, Table S1) so that the percentage of dGTP in the total pool raised from 10% to 27% (Figure 3B). Despite the differences between the two cancer cell lines, in both cases, dGTP abundance increased 2.5 fold (Figure 3B, and Ref. [8]).
FIGURE 3.

SAMHD1 deficiency promotes telomere over‐elongation in hTERT‐positive cancer cells. (A) Fold change in the dNTP pool concentrations in SAMHD1‐KO SUIT2 cells relative to SAMHD1‐proficient SUIT2 cells. dNTP pool sizes were measured in cycling cultures of SUIT2 and SAMHD1‐KO SUIT2 cells with similar percentages of S phase cells (30‐35%), by a DNA polymerase‐based assay. Data are means from four biological samples analyzed in duplicate. Bars represent means ± SE. **p < .01 and ***p < .001 (two‐tailed Student's t test). (B) Proportion of each dNTP as a percentage of the total dNTP pool in SUIT2 WT and SAMHD1 KO cells. (C) Telomerase activity in SAMHD1‐KO and parental SAMHD1‐proficient cancer cells. Telomerase activity was measured by TAQ PCR in SAMHD1‐KO THP1 and SAMHD1‐KO SUIT2 and normalized to telomerase activity in SAMHD1 proficient THP1 and SUIT2 cells respectively. Data are means of two biological samples for each cell line, each analyzed in triplicate. Bars represent means ± SE. *p < .05 and ***p < .001 (two‐tailed Student's t test). (D) Mean telomere lengths measured by qPCR analysis in SAMHD1‐KO THP1 and SAMHD1‐KO SUIT2 cells relative to SAMHD1 proficient THP1 and SUIT2 cells, respectively. Bars represent means ± SE. ***p < .001 (two‐tailed Student's t test).
Next, we evaluated telomerase activities in the SAMHD1 KO cancer cell lines and compared them with those measured in the corresponding SAMHD1 proficient cells. In SUIT2‐KO cells, telomerase activity was halved, while in THP1‐KO cells a slight increase (p < .05) was observed (Figure 3C). Then we assessed telomere length in all cell lines by qPCR (Figure 3D). In both SAMHD1 THP1‐KO and SUIT2‐KO cells, telomeres were 3 times longer than in the respective parental SAMHD1 proficient cells, indicating that SAMHD1 restricts telomere lengthening not only in hTERT‐immortalized fibroblasts but also in cancer cells where telomerase is spontaneously activated during oncogenesis.
To reinforce the conclusion that in tumor cells telomere elongation depends on SAMHD1 depletion and prolonged exposure to a high dGTP pool, we used a SUIT2 line with tetracycline‐inducible RNA silencing of SAMHD1 (SUIT2TetR‐sh SAMHD1, see Materials and Methods). In the absence of doxycycline (Dox), SUIT2TetR‐sh SAMHD1 cells exhibited no significant alteration in SAMHD1 expression levels after one, two and three months in culture (Figure 4A). In contrast, exposure to 50 ng/mL doxycycline led to marked downregulation of SAMHD1 to 10%–20% compared to non‐induced cells. SAMHD1 silencing was stable in culture for at least three months (Figure 4A). Downregulation of the total SAMHD1 mRNA by more than 80% in SUIT2TetR‐sh SAMHD1 cells after 2 months of doxycycline exposure induced a much lower dNTP expansion compared with that of the SUIT2‐KO cells where the enzyme was completely turned off (compare Figures 3A and 4B; Table S1). Indeed, in the silenced cells, the dCTP pool did not increase at all, dTTP was less than doubled and purine dNTPs increased 3–4 folds (Figure 4B). Despite the lower pool expansion, the percentage of dGTP in the total pool was twice that observed in non‐silenced cells (Figure 4C). After one, two and three months of culture in the absence of doxycycline, SUIT2 TetRsh‐SAMHD1 cells exhibited the same telomere length as SAMHD1 proficient SUIT2 cells. On the contrary, during one to three months of growth in the presence of 50 ng/mL doxycycline, telomeres elongated continuously, reaching after three months a length similar to that of SUIT2‐KO cells (Figures 3D and 4D). On the whole, data obtained from hTERT AGS fibroblasts and telomerase positive SAMHD1 deficient cancer cells indicate that SAMHD1 deficiency boosts dGTP concentration in the pool and induces a telomere elongation phenotype.
FIGURE 4.

Telomere over‐elongation in TetR‐shSAMHD1 SUIT2 cells depends on prolonged SAMHD1 depletion. (A) SAMHD1 expression in tetR‐shSAMHD1 SUIT2 cells during prolonged silencing with doxycycline. SAMHD1 mRNA expression was analyzed in tetR‐shSAMHD1 SUIT2 cells by qRT‐PCR and normalized to Succinate Dehydrogenase Complex Flavoprotein subunit A (SDHA) mRNA expression. The abundance of SAMHD1 was calculated relative to the expression detected in SAMHD1 proficient SUIT2 cells. TetR‐shSAMHD1 SUIT2 cells were cultured for 1, 2 and 3 months in either the presence or absence of 50 ng/mL doxycycline. Data are means of two independent experiments for each stage analyzed in triplicate. Bars represent means ± SE. ***p < .001 (two‐tailed Student's t test). (B) dNTP pool content was measured in TetR‐shSAMHD1 SUIT2 cells kept in culture for two months in either the presence or absence of 50 ng/mL Doxycycline. Data were obtained from two biological samples of each cell line, each analyzed in triplicate. Bars represent means ± SE. ***p < .001 (two‐tailed Student's t test). (C) Proportion of each dNTP as a percentage of the total dNTP pool in tetR‐shSAMHD1 SUIT2 cells cultured in the presence or absence of 50 ng/mL doxycycline. (D) Mean telomere lengths measured by qPCR analysis in tetR‐shSAMHD1 SUIT2 cells relative to SAMHD1 proficient SUIT2 cells. TetR‐shSAMHD1 SUIT2 cells were cultured for 1, 2 and 3 months in either the presence or absence of 50 ng/mL doxycycline. Bars represent means ± SE. ***p < .001 (two‐tailed Student's t test).
3.3. SAMHD1 deficiency increases telomere fragility in immortalized AGS fibroblasts
The specialized structure of telomeres is fundamental for their stability but at the same time makes them difficult to replicate. Indeed, the heterochromatic nature of telomeric sequences as well as their ability to form secondary structures and bind tightly to the shelterin complex makes them prone to fragility during replication. As described above, SAMHD1 deficiency causes excessive telomere elongation that may exacerbate the fragility of chromosome ends. Being an important player in the replication stress response, SAMHD1 may exert an additional role in telomere stability. Telomere fragility is detected by mQ‐FISH as a distinctive phenotype characterized by multiple or aberrant telomeric signals in metaphase chromosomes (Figure 5A,B). To examine the impact of SAMHD1 deficiency on telomere fragility we verified telomere integrity in hTERT WT and AGS skin fibroblasts by analyzing metaphase chromosomes at Early, Mid and Late stages after immortalization (Figure 5C). Telomeres were tagged as fragile when two or, more rarely, three dots were detected at the same chromosome end and their frequency was normalized by the total number of visible fluorescent telomeres per metaphase. Chromosome analyses revealed that SAMHD1 deficiency is associated with increased incidence of fragile telomeres. The number of fragile telomeres was significantly higher in AGS P1 and P2 compared to both WT fibroblasts in all the culture stages analyzed (p < .001). We observed a 50% increase at the Early stage, and 70 and 100% increase at Mid and Late stages respectively. Strikingly, the proportion of fragile telomeres follows the same trend as the elongation of the telomeres evaluated by mQ‐FISH in AGS fibroblasts from Early to Late stage after immortalization (Figures 5C and 2B). These results indicate that SAMHD1 is required for telomere stability in telomerase positive cells.
FIGURE 5.
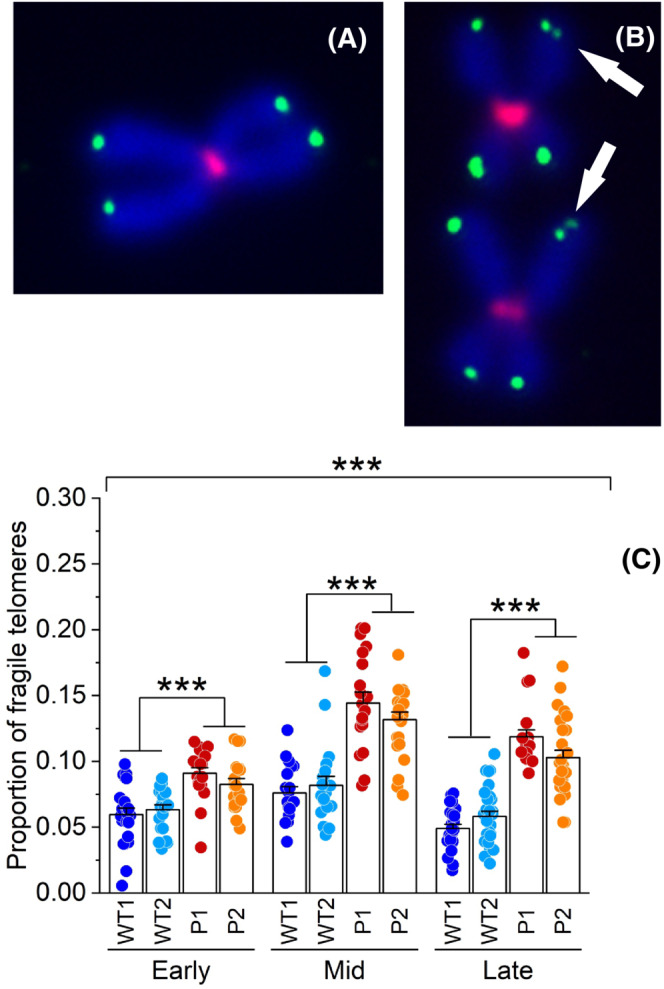
SAMHD1 is required to suppress telomere fragility in hTERT immortalized skin fibroblasts. Representative images of a normal chromosome (A) and two chromosomes with fragile telomeres indicated by white arrows in (B). Telomeres and centromeres were detected respectively with Alexafluor‐488 and Alexafluor‐647, chromosomes were counterstained with DAPI. (C) Quantification of fragile telomeres per metaphase in Early, Mid and Late samples of hTERT AGS P1 and P2 and WT fibroblasts (WT1 and WT2) analyzed by metaphase Q‐FISH. Proportions were calculated from at least 20 metaphase spreads for each cell line and stage. Bars represent means ± SE. ***p < .001 (two‐tailed Student's t test, as applied for mean comparisons, or Analysis of variance as applied on the whole set of data).
4. DISCUSSION
In mammalian cells the dNTP pool composition is regulated by a sophisticated network of synthetic and catabolic enzymes where RNR and SAMHD1 are the two major players. The substrate specificity and the allosteric regulation of both enzymes adjust dNTP pool sizes according to the cell needs. In‐depth studies of dNTP metabolism show that among the four dNTPs, dGTP is the one under special surveillance for its concentration. 37 The restriction of the dGTP pool depends on various enzymes, on their substrate specificity and their cellular compartmentalization. 38
The enzymatic network restricting the dGTP pool in mammalian cells has been fully elucidated, but what are the reasons for such a tight control? The possible reasons are numerous: (i) high dGTP stimulates ADP reduction by RNR increasing the concentration of dATP that functions as a negative effector of RNR and thus inhibits cell proliferation, 39 , 40 (ii) dGTP is prone to oxidative damage and its oxidized form can be incorporated into DNA decreasing the fidelity of DNA replication, 41 (iii) dNTP pool imbalances with exceptionally high dGTP content favor replication errors that escape DNA polymerase proofreading and DNA mismatch repair 13 and finally (iv) dGTP levels modulate telomerase processivity affecting telomere length. 22 It was this last aspect that prompted us to investigate a putative role of SAMHD1 in telomere stability.
Telomeric DNA consists of simple repeats of short G‐C rich sequences that are essential for genome stability but difficult to replicate. 16 Telomere length is maintained by telomerase, a large ribonucleoprotein complex containing a reverse transcriptase subunit whose processivity is a major determinant of telomere length in vivo. 23 Under physiological conditions telomerase processivity is limited by the incorporation of the first deoxynucleotide in each repeat and enhanced by high concentrations of dGTP. 42 Interestingly, in yeast, where telomerase is normally expressed, telomere length correlates with the percentage of dGTP in the cellular dNTP pool and not with the absolute size of the pool. 23 In a wild type yeast strain an amount of dGTP close to ten percent of the entire dNTP pool is sufficient to preserve a physiological telomere length, while deviations below or above this value are associated with telomere shortening or lengthening, respectively. 23 In budding yeast defects in the R1 subunit of ribonucleotide reductase cause dGTP depletion and telomere shortening. 43
It is interesting to note that a comparable fraction of dGTP in the total dNTP pool is observed here in both human skin fibroblasts and SUIT2 cancer cells (Figures 1D and 3B) while a dGTP fraction of only 3% was detected in monocytic THP1 cells where a particularly high expression of SAMHD1 may suggests a tighter restriction of dGTP content. 1 The absence of SAMHD1 in THP1 and SUIT2 cancer cells where telomerase is expressed, and in hTERT immortalized AGS skin fibroblasts, boosts the percentage of dGTP that doubles or even triples compared to the control cells (Figures 1D and 3B; Ref. [8]). We postulated that SAMHD1, by setting the concentration and balance of the four DNA precursors and restricting dGTP, not only contributes to the fidelity of DNA synthesis but also restrains telomerase processivity and secures telomere homeostasis.
In non‐immortalized fibroblasts, we found no differences in telomere length between WT and P1 and P2 AGS fibroblasts (Figure 2A). Indeed, non‐immortalized P1 and P2 fibroblasts grew like WT fibroblasts reaching senescence after 50 PDs. 8 The ectopic expression of hTERT reconstitutes telomerase activity in normal human telomerase‐negative fibroblasts and immortalizes these primary cells.
Here WT hTERT‐fibroblasts elongate their telomeres from 2–3 Kb up to a maximum of 6–8 Kb already after one month from immortalization and then remain stable during the 6 months analyzed (Figure 2A). Strikingly, after immortalization, both AGS P1 and P2 progressively lengthen their telomeres, reaching 16–18 Kb after 6 months in culture (Figure 2A). Telomere lengths measured by mQ‐FISH and quantitative real time PCR suggest that, in hTERT immortalized skin fibroblasts, SAMHD1 activity modulates telomerase processivity and restrains telomere length. This effect is not distinctive of immortalized primary cells but takes place also in telomerase‐positive cancer cells. Indeed, both THP1 and SUIT2 cells with complete SAMHD1 knock out by CRISPR/Cas9 have telomeres three times longer than those of SAMHD1 proficient cells (Figure 3D). We suggest that this novel role of SAMHD1 in restricting telomerase processivity and telomere lengths derives from its dNTP triphosphohydrolase activity rather than its other functions in double‐strand break repair or stalled replication fork processing.
In our experiments, telomere lengthening was a gradual process that became most apparent after three months of continued passaging both in immortalized fibroblasts and cancer cell lines (Figures 2, 3, and 4D). Telomerase adds only 50–60 nucleotides for each cycle 44 and most likely only a prolonged exposure to high dGTP content can promote measurable telomere over‐elongation.
We cannot exclude that in addition to the control of telomerase processivity by increased dGTP other mechanisms may affect telomerase regulation in AGS patients. Telomere and telomerase biology are dysfunctional in chronic conditions characterized by persistent inflammation 45 and the same may apply to AGS syndrome which is characterized by the inappropriate induction of a type I interferon‐mediated immune response. 7
From mQ‐FISH data, we noticed that, despite the continued exposure to high dGTP pool, telomere length did not consistently increase moving from Mid to Late stage. We speculate that longer telomeres could not elongate further because they challenge the replication machinery by the formation of secondary structures such as T‐loops and G quadruplexes. Notably, under prolonged growth in culture hTERT, immortalized AGS fibroblasts display high fragility of chromosome ends that increases with telomere lengths (Figure 5C). Indeed, cells expressing a human telomerase variant characterized by increased processivity display heterogenous telomere lengths, telomere elongation and multiple telomeric signals indicative of fragile sites and replicative stress. 46
Telomere fragility may also be ascribed to the role of SAMHD1 in the resolution of the stalled replication forks which naturally occur at chromosome ends. It has been suggested that SAMHD1 preserves genome integrity by preventing R‐loop formation at regions of transcription‐replication conflict. 47 A similar conflict may occur also at the chromosome ends where R loop structures result from direct base‐pairing of long non‐coding telomeric repeat containing RNA (TERRA) with telomeric DNA. 48 Taken together these data suggest that SAMHD1 partakes in the control of telomere maintenance and indicate that SAMHD1 deficiency is detrimental for the maintenance of telomere length and structure in cells where telomerase is expressed. We propose that when SAMHD1 activity restricts dGTP pool content, telomerase processivity is normal, when SAMHD1 is missing the expansion of the dGTP pool enhances telomerase processivity, leading to telomere lengthening and fragility (Figure 6). This model agrees with studies run in transformed cells deprived of the shelterin component TRF1 where short‐term silencing of SAMHD1 leads to telomere breakage events. 49
FIGURE 6.

A Model for SAMHD1 as a gatekeeper of telomere stability in telomerase positive cells. In SAMHD1 proficient cells the dGTP pool represents less than 10% of the total dNTP pool which preserves telomere length and structure. SAMHD1 deficiency both in immortalized fibroblasts and telomerase positive cancer cells expands the dGTP content which enhances telomerase processivity leading to telomere elongation (open arrows) and fragility (dark arrows).
Telomeres are implicated in genome integrity control and carcinogenesis. Indeed, 80% of cancer cells become immortalized by telomerase reactivation and contain long telomeres associated with increased sensitivity to DNA damage. 50 Telomerase expression is restricted in humans only to few somatic stem and progenitor cells where telomere length must be maintained just right as too short telomeres lead to senescence and too long telomeres develop instabilities which can lead to cancer initiation. 51 Telomere length may be not relevant at the first transformation step, but it may become a key determinant during the phase of clonal expansion. 52
SAMHD1 is frequently mutated in various tumors and malignancies, suggesting that it is biologically relevant to cancer development. Intriguing is a study in a gastric adenocarcinoma cell line resistant to Adriamycin (MKN/ADR). 53 Those cells displayed extremely long telomeres but reduced telomerase activity compared to the parental MKN‐45 line, which lead the Authors to suggest the existence of another unknown mechanism of telomere length control. Considering that SAMHD1 is downregulated in gastric cancer tissue and cell lines, 54 our findings may provide a possible candidate for the control mechanism postulated in Ref. [53]. Considering the role of SAMHD1 in DNA repair and in limiting the accumulation of R‐loop structures, 47 together with the evidence that alternative lengthening of telomeres (ALT) results from the formation of R –loops, 55 we suggest that SAMHD1 deficiency may also affect telomere maintenance in ALT cancer cells. The nature and extent of such an effect remain to be assessed.
Overall, our study underlines a new role of SAMHD1 in telomere homeostasis in human cells expressing telomerase and provides a further general explanation for the occurrence of SAMHD1 mutations in cancer cells. In addition, this newly identified function may advise against choosing SAMHD1 as a potential target in cancer treatment. 56 , 57
AUTHOR CONTRIBUTIONS
Giulia D'Aronco, Paola Ferraro, Valentina Sassano, Claudia Dagostino, Melissa Biancotto, Ettore Presot performed the experiments and analyzed data. Elisa Palumbo and Antonella Russo contributed to methodology development. Antonella Russo performed statistical analysis. Chiara Rampazzo conceived the project, designed the experiments, and wrote the manuscript. Vera Bianchi and Antonella Russo revised the manuscript. All authors viewed the results and approved the final version of the manuscript.
DISCLOSURES
The authors do not have any conflicts of interest to declare.
Supporting information
Supplemental Figure S1
Supplemental Table S1
ACKNOWLEDGMENTS
We thank Prof. Yanick Crow (Manchester Centre for Genomic Medicine, University of Manchester, United Kingdom) for kindly providing the AGS cell lines, Prof. Thomas Gramberg (Institute of Clinical and Molecular Virology, Friedrich‐Alexander University, Germany) for the THP‐1 cell lines and Prof. Caius Radu and Evan Abt (Department of Molecular and Medical Pharmacology, University of California, USA) for all SUIT2 cell lines used in this study. We thank Prof. Denis Furling (Myology Center of Research, Sorbonne University, France) for providing CMV‐hTERT/PGK‐Puro plasmid for fibroblasts immortalization and Prof. Cristiano Salata for immortalizing skin fibroblast lines used in this study (Department of Molecular Medicine, University of Padova, Italy). Open Access Funding provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement.
D’Aronco G, Ferraro P, Sassano V, et al. SAMHD1 restricts the deoxyguanosine triphosphate pool contributing to telomere stability in telomerase‐positive cells. The FASEB Journal. 2023;37:e22883. doi: 10.1096/fj.202300122R
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the methods and/or supplementary material of this article.
REFERENCES
- 1. Franzolin E, Pontarin G, Rampazzo C, et al. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc Natl Acad Sci U S A. 2013;110:14272‐14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tramentozzi E, Ferraro P, Hossain M, Stillman B, Bianchi V, Pontarin G. The dNTP triphosphohydrolase activity of SAMHD1 persists during S‐phase when the enzyme is phosphorylated at T592. Cell Cycle. 2018;17:1102‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstone DC, Ennis‐Adeniran V, Hedden JJ, et al. HIV‐1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480:379‐382. [DOI] [PubMed] [Google Scholar]
- 4. Miazzi C, Ferraro P, Pontarin G, Rampazzo C, Reichard P, Bianchi V. Allosteric regulation of the human and mouse deoxyribonucleotide triphosphohydrolase sterile α‐motif/histidine‐aspartate domain‐containing protein 1 (SAMHD1). J Biol Chem. 2014;289:18339‐18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coquel F, Silva M‐J, Técher H, et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature. 2018;557:57‐61. [DOI] [PubMed] [Google Scholar]
- 6. Daddacha W, Koyen AE, Bastien AJ, et al. SAMHD1 promotes DNA end resection to facilitate DNA repair by homologous recombination. Cell Rep. 2017;20:1921‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crow YJ, Stetson DB. The type I interferonopathies: 10 years on. Nat Rev Immunol. 2022;22:471‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franzolin E, Coletta S, Ferraro P, et al. SAMHD1‐deficient fibroblasts from Aicardi‐Goutières Syndrome patients can escape senescence and accumulate mutations. FASEB J. 2020;34:631‐647. [DOI] [PubMed] [Google Scholar]
- 9. Chabes A, Stillman B. Constitutively high dNTP concentration inhibits cell cycle progression and the DNA damage checkpoint in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007;104:1183‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kohnken R, Kodigepalli KM, Wu L. Regulation of deoxynucleotide metabolism in cancer: novel mechanisms and therapeutic implications. Mol Cancer. 2015;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rentoft M, Lindell K, Tran P, et al. Heterozygous colon cancer‐associated mutations of SAMHD1 have functional significance. Proc Natl Acad Sci. 2016;113:4723‐4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahluwalia D, Schaaper RM. Hypermutability and error catastrophe due to defects in ribonucleotide reductase. Proc Natl Acad Sci U S A. 2013;110:18596‐18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt TT, Sharma S, Reyes GX, et al. A genetic screen pinpoints ribonucleotide reductase residues that sustain dNTP homeostasis and specifies a highly mutagenic type of dNTP imbalance. Nucleic Acids Res. 2019;47:237‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfeiffer V, Lingner J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol. 2013;5:a010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Lange T. Shelterin‐mediated telomere protection. Annu Rev Genet. 2018;52:223‐247. [DOI] [PubMed] [Google Scholar]
- 16. Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193‐1198. [DOI] [PubMed] [Google Scholar]
- 17. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184:306‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maciejowski J, De Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18:175‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart SA, Ben‐Porath I, Carey VJ, O'Connor BF, Hahn WC, Weinberg RA. Erosion of the telomeric single‐strand overhang at replicative senescence. Nat Genet. 2003;33:492‐496. [DOI] [PubMed] [Google Scholar]
- 20. Peng Y, Mian IS, Lue NF. Analysis of telomerase processivity: mechanistic similarity to HIV‐1 reverse transcriptase and role in telomere maintenance. Mol Cell. 2001;7:1201‐1211. [DOI] [PubMed] [Google Scholar]
- 21. Hardy CD, Schultz CS, Collins K. Requirements for the dGTP‐dependent repeat addition processivity of recombinant Tetrahymena telomerase. J Biol Chem. 2001;276:4863‐4871. [DOI] [PubMed] [Google Scholar]
- 22. Maine IP, Chen SF, Windle B. Effect of dGTP concentration on human and CHO telomerase. Biochemistry. 1999;38:15325‐15332. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Sharma S, Reichenbach P, et al. Telomere length homeostasis responds to changes in intracellular dNTP pools. Genetics. 2013;193:1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rice GI, Forte GMA, Szynkiewicz M, et al. Assessment of interferon‐related biomarkers in Aicardi‐Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case‐control study. Lancet Neurol. 2013;12:1159‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramesh V, Bernardi B, Stafa A, et al. Intracerebral large artery disease in Aicardi‐Goutières syndrome implicates SAMHD1 in vascular homeostasis. Dev Med Child Neurol. 2010;52:725‐732. [DOI] [PubMed] [Google Scholar]
- 26. Wittmann S, Behrendt R, Eissmann K, et al. Phosphorylation of murine SAMHD1 regulates its antiretroviral activity. Retrovirology. 2015;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abt ER, Le TM, Dann AM, et al. Reprogramming of nucleotide metabolism by interferon confers dependence on the replication stress response pathway in pancreatic cancer cells. Cell Rep. 2022;38:110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arandel L, Espinoza MP, Matloka M, et al. Immortalized human myotonic dystrophy muscle cell lines to assess therapeutic compounds. Dis Model Mech. 2017;10:487‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sherman PA, Fyfe JA. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem. 1989;180:222‐226. [DOI] [PubMed] [Google Scholar]
- 30. Ferraro P, Franzolin E, Pontarin G, Reichard P, Bianchi V. Quantitation of cellular deoxynucleoside triphosphates. Nucleic Acids Res. 2010;38:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milyavsky M, Shats I, Erez N, et al. Prolonged culture of telomerase‐immortalized human fibroblasts leads to a premalignant phenotype. Cancer Res. 2003;63:7147‐7157. [PubMed] [Google Scholar]
- 32. Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life‐span by introduction of telomerase into normal human cells. Science. 1998;279:349‐352. [DOI] [PubMed] [Google Scholar]
- 33. Counter CM, Meyerson M, Eaton EN, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217‐1222. [DOI] [PubMed] [Google Scholar]
- 34. Lü MH, Liao ZL, Zhao XY, et al. hTERT‐based therapy: a universal anticancer approach (review). Oncol Rep. 2012;28:1945‐1952. [DOI] [PubMed] [Google Scholar]
- 35. Armstrong CA, Tomita K. Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells. Open Biol. 2017;7:160338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravindranathan A, Cimini B, Diolaiti ME, Stohr BA. Preliminary development of an assay for detection of TERT expression, telomere length, and telomere elongation in single cells. PLoS One. 2018;13:e0206525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rampazzo C, Miazzi C, Franzolin E, et al. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat Res Genet Toxicol Environ Mutagen. 2010;703:2‐10. [DOI] [PubMed] [Google Scholar]
- 38. Franzolin E, Salata C, Bianchi V, Rampazzo C. The deoxynucleoside triphosphate triphosphohydrolase activity of SAMHD1 protein contributes to the mitochondrial DNA depletion associated with genetic deficiency of deoxyguanosine kinase. J Biol Chem. 2015;290:25986‐25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen A, Hirschhorn R, Horowitz SD, et al. Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978;75:472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681‐706. [DOI] [PubMed] [Google Scholar]
- 41. Nakabeppu Y. Cellular levels of 8‐oxoguanine in either DNA or the nucleotide pool play pivotal roles in carcinogenesis and survival of cancer cells. Int J Mol Sci. 2014;15:12543‐12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y, Podlevsky JD, Logeswaran D, Chen JJ. A single nucleotide incorporation step limits human telomerase repeat addition activity. EMBO J. 2018;37:e97953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maicher A, Gazy I, Sharma S, et al. Rnr1, but not Rnr3, facilitates the sustained telomerase‐dependent elongation of telomeres. PLoS Genet. 2017;13:e1007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Y, Sfeir AJ, Zou Y, et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill‐in in human cancer cells. Cell. 2009;138:463‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kordinas V, Ioannidis A, Chatzipanagiotou S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes (Basel). 2016;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. D'souza Y, Lauzon C, Chu TW, Autexier C. Regulation of telomere length and homeostasis by telomerase enzyme processivity. J Cell Sci. 2012;126:676‐687. [DOI] [PubMed] [Google Scholar]
- 47. Park K, Ryoo J, Jeong H, et al. Aicardi‐Goutières syndrome‐associated gene SAMHD1 preserves genome integrity by preventing R‐loop formation at transcription–replication conflict regions. PLoS Genet. 2021;17:e1009523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernandes RV, Feretzaki M, Lingner J. The makings of TERRA R‐loops at chromosome ends. Cell Cycle. 2021;20:1745‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Majerska J, Feretzaki M, Glousker G, Lingner J. Transformation‐induced stress at telomeres is counteracted through changes in the telomeric proteome including SAMHD1. Life Sci Alliance. 2018;1:e201800121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fairlie J, Harrington L. Enforced telomere elongation increases the sensitivity of human tumour cells to ionizing radiation. DNA Repair (Amst). 2015;25:54‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rivera T, Haggblom C, Cosconati S, Karlseder J. A balance between elongation and trimming regulates telomere stability in stem cells. Nat Struct Mol Biol. 2017;24:30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aviv A, Anderson JJ, Shay JW. Mutations, cancer and the telomere length paradox. Trends Cancer. 2017;3:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim JH, Lee GE, Kim JC, Lee JH, Chung IK. A novel telomere elongation in an adriamycin‐resistant stomach cancer cell line with decreased telomerase activity. Mol Cells. 2002;13:228‐236. [PubMed] [Google Scholar]
- 54. Chen Z, Jiang Z, Meng L, et al. SAMHD1, positively regulated by KLF4, suppresses the proliferation of gastric cancer cells through MAPK p38 signaling pathway. Cell Cycle. 2022;21:2065‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yadav T, Zhang JM, Ouyang J, Leung W, Simoneau A, Zou L. TERRA and RAD51AP1 promote alternative lengthening of telomeres through an R‐ to D‐loop switch. Mol Cell. 2022;82:3985‐4000.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rampazzo C, Tozzi MG, Dumontet C, Jordheim LP. The druggability of intracellular nucleotide‐degrading enzymes. Cancer Chemother Pharmacol. 2016;77:883‐893. [DOI] [PubMed] [Google Scholar]
- 57. Helleday T, Rudd SG. Targeting the DNA damage response and repair in cancer through nucleotide metabolism. Mol Oncol. 2022;16:3792‐3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1
Supplemental Table S1
Data Availability Statement
The data that support the findings of this study are available in the methods and/or supplementary material of this article.


