ABSTRACT
Drug efflux systems have recently been recognized as an important mechanism of multidrug resistance in bacteria. Here, we described the identification and characterization of a novel chromosomally encoded multidrug efflux pump (SA09310) in Staphylococcus aureus. SA09310 is a 43-kDa protein with 12 transmembrane helices. The conserved amino acid sequence motifs of the major facilitator superfamily (MFS) were identified in the protein SA09310, which indicated that SA09310 belonged to the MFS transporters. Expression of the sa09310 gene was induced by different types of antibiotics, including aminoglycoside, tetracycline, macrolides, and chloramphenicol. An sa09310 gene knockout mutant (Δsa09310) was constructed, and its susceptibility to 30 different antibiotics was evaluated. The Δsa09310 mutant exhibited increased sensitivity to tetracycline and doxycycline, with 64-fold- and 8-fold-decreased MICs, respectively. The mechanism of SA09310 mediation of tetracycline resistance was demonstrated by its ability to extrude intracellular tetracycline from within the cells into the environment. The efflux activity of SA09310 was further confirmed by ethidium bromide (EtBr) accumulation and efflux assays. In addition, the efflux activity of SA09310 was observed to be blocked by the known efflux pump inhibitor carbonyl cyanide chlorophenylhydrazone (CCCP), which provided direct evidence that suggested the H+-dependent activity of the SA09310 efflux pump. The conservation of SA09310 homologs in Staphylococcus indicated the universal function of these SA09310-like protein clusters. In conclusion, the function-unknown protein SA09310 has been identified and characterized as a tetracycline efflux pump mediating tetracycline resistance in S. aureus.
KEYWORDS: Staphylococcus aureus, antibiotic resistance
INTRODUCTION
Staphylococcus aureus is a major Gram-positive pathogenic bacterium causing a variety of diseases in humans (1). The success of S. aureus as a leading pathogen is undoubtedly due to its ability to develop resistance to a wide variety of antimicrobial compounds (2). Antimicrobial resistance of S. aureus is mediated by various strategies, including enzymatic modification of the antimicrobial binding site to decrease the affinity of the antibiotic, enzymatic inactivation or degradation of the antimicrobial, decreased permeability of the bacterial cell to antibiotics, and reduction of the intracellular concentration of antibiotics by activation of the expression of multidrug efflux pumps to extrude antimicrobial molecules (3–5). Of these, efflux-mediated resistance has been overshadowed by the other mechanisms known. However, it has been attracting more interest recently, as many bacterial efflux pumps are able to recognize and export a broad spectrum of structurally unrelated substrates from the cell, promoting the appearance of multidrug resistance phenotypes (6–9).
Multidrug efflux pumps are membrane-integrated proteins involved in the extrusion of toxic agents, such as antibiotics, biocides, and toxic metals, from within the bacteria into the environment (10). Based on bioenergetic and structural criteria, the multidrug efflux system is classified into five families: the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the multidrug and toxic compound extrusion (MATE) family, the resistance-nodulation-cell division (RND) superfamily, and the ATP-binding cassette (ABC) superfamily (11). The transporters of the first four families are secondary transporters that use an electrochemical gradient, typically proton motive force, as the driving force for transport, while the transporters of the ABC family are the primary transporters that use ATP to drive the extrusion of their substrates (12). Within these multidrug efflux systems, the MFS has been the most extensively studied among staphylococcal multidrug efflux pumps, which include NorA, NorB, NorC, Tet38, LmrS, SdrM, and MdeA (10). Staphylococcal MFS transporters typically contain 380 to 480 amino acids that are arranged into 12 or 14 transmembrane segments (TMS), with a conserved MFS-specific motif that lies in the cytoplasmic loop between the TMS2 and TMS3 helices (13). In addition to MFS transporters, SMR transporters, including QacD, QacG, QacH, and QacJ, are encoded on the plasmid; ABC transporters, including AbcA, Sav1866, and MepA transporters belonging to the MATE family, are characterized as being involved in antibiotic resistance in S. aureus (10).
Based on in silico analysis, the S. aureus chromosome encodes 31 multidrug efflux pumps that cover all five families (14). However, only one-third (10/31) of them have been studied previously, and most of these efflux pumps are function unknown (14). Efflux-mediated multidrug resistance, particularly in staphylococci, is an urgent clinical problem, rendering many of the current antimicrobials ineffective. Thus, inhibition of bacterial multidrug efflux pumps is a reasonable strategy to combat multidrug-resistant S. aureus. This potential strategy has promoted the study of the identification and development of efflux pump inhibitors for S. aureus (15, 16). Therefore, a more in-depth understanding of efflux pump function, regulatory mechanisms, and association with clinical antibiotic resistance will enable the design of better antibiotics that will be less susceptible to bacterial resistance.
In this report, we describe the identification and characterization of the sa09310 gene, encoding a protein with 12 predicted transmembrane helices belonging to the MFS transporter group. We hypothesized that SA09310 is a multidrug efflux pump and investigated its role in mediating antibiotic resistance in S. aureus.
RESULTS
Gene sa09310 encoded a transmembrane protein belonging to the MFS transporter family.
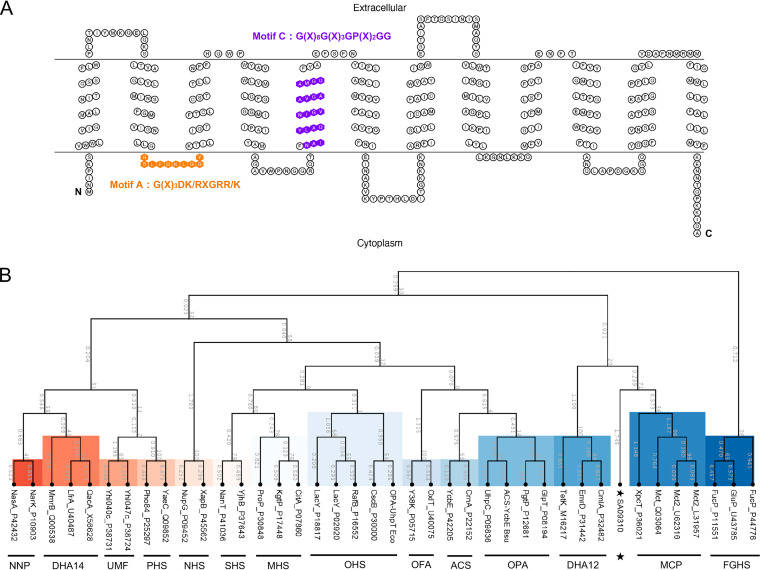
In the genome of S. aureus USA300_FPR3757, the gene sa09310 (gene locus, SAUSA300_09310), with a length of 1,182 bp, was predicted to encode a transmembrane protein with a molecular mass of 43.3 kDa. Similar to most MFS transporters (17), the sa09310 gene-encoded protein (SA09310) exhibited 12 transmembrane segments based on the prediction by TMHMM 2.0 (Fig. 1A). MFS transporters are characterized by a highly conserved amino acid sequence motif, called motif A and consisting of residues G(X)3DK/RXGRR/K, that lies in the cytoplasmic loop between the TMS2 and TMS3 helices (13, 18, 19). As shown by the transmembrane topology of SA09310, motif A was also found between TMS2 and TMS3 of SA09310 (Fig. 1A). In addition, another well-conserved motif, motif C [G(X)8G(X)3GP(X)2GG], harbored by drug-ion antiporters of MFS within their fifth α-helix (TMS5) (19, 20), was identified in the fifth TMS of SA09310 as well (Fig. 1A). All this evidence indicated that protein SA09310 was an MFS transporter.
FIG 1.
Transmembrane topology and phylogenetic analysis of the SA09310 protein. (A) The transmembrane topology of the SA09310 protein was created using the TOPO2 online tool based on the predicted transmembrane structure by TMHMM 2.0. The amino acids of SA09310 highlighted in orange are the conserved motif (motif A) of MFS transporters between the TMS2 and TMS3 helices. The amino acids of SA09310 highlighted in purple are another conserved motif of MFS transporters located in the fifth α-helix. (B) The phylogenetic tree of SA09310 was constructed based on the sequence alignment between SA09310 and proteins from 14 groups of classified MFS transporters using ClustalW, as implemented in the CLC Main Workbench. The resulting tree was calculated using the neighbor-joining method and Jukes-Cantor protein distance model. The abbreviations for the classified groups are defined in Materials and Methods, and SA09310 is highlighted with a star.
Currently, the MFS transporters are classified into 17 distinct groups based on their protein sequence similarity and distinct functions (17). An unrooted phylogenetic tree was constructed based on the protein sequence alignment between SA09310 and the classified MFS transporters. SA09310 exhibited a close relationship to the drug-H+ antiporter (12-spanner) drug efflux (DHA12) and monocarboxylate porter (MCP) families (Fig. 1A), which provided us further clues for determining the function of SA09310.
Induction of the sa09310 gene by different types of antibiotics.
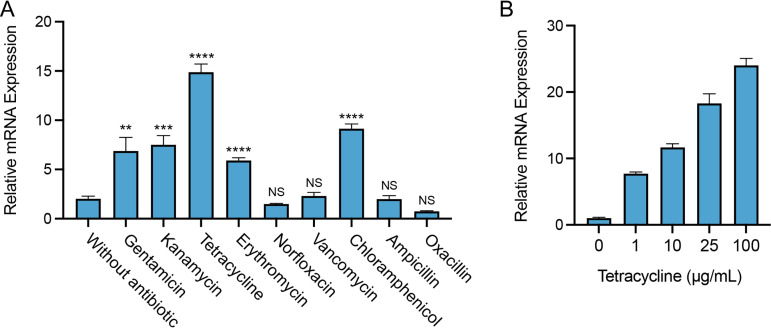
Since SA09310 was predicted to be an MFS transporter that is phylogenetically close to the drug efflux pump, the role of the sa09310 gene in response to different antibiotics in S. aureus was initially investigated. S. aureus cells from the log phase were treated with different types of antibiotics, and the transcriptional level of the sa09310 gene was analyzed by reverse transcription-quantitative PCR (RT-qPCR). Among the tested antibiotics, quinolone (norfloxacin), glycopeptide (vancomycin), and beta-lactam (ampicillin and oxacillin) antibiotics were unable to induce the expression of sa09310 (Fig. 2A). However, sa09310 was significantly induced by treatment of aminoglycoside (gentamicin, kanamycin), tetracycline, macrolide (erythromycin), and chloramphenicol antibiotics (Fig. 2A). Tetracycline stimulated the sa09310 gene with the highest (10-fold) transcription level (Fig. 2A). In addition, transcription of sa0931 induced by tetracycline occurred in a concentration-dependent manner (Fig. 2B), which further confirmed that sa09310 was able to respond to stimulation with tetracycline. The response of sa09310 to different antibiotics suggests that the SA09310 efflux pump might be involved in the extrusion of these antibiotics, which probably mediates the associated antibiotic resistance.
FIG 2.
Expression of the sa09310 gene with the stimulation of different types of antibiotics. (A) Bacterial cells from the mid-exponential phase were treated with 100 μg/mL of different antibiotics. After induction for 1 h, the mRNA levels of the sa09310 gene were determined by RT-qPCR with the 16S rRNA gene as the internal control. ****, P ≤ 0.0001; ***, P ≤ 0.001; **, P ≤ 0.05 relative to the level without antibiotic induction. NS, not significant. (B) The expression of the sa09310 gene was induced by tetracycline in a concentration-dependent manner. S. aureus cells were induced with different concentrations of tetracycline (0, 1, 10, 25, or 100 μg/mL), and the mRNA levels of the sa09310 gene were checked by RT-qPCR.
SA09310 contributed to tetracycline resistance in S. aureus.
Considering that the sa09310 gene was induced by different types of antibiotics, the role of sa09310 in the antimicrobial resistance of S. aureus was subsequently investigated. An sa09310 gene knockout mutant (Δsa09310) and a strain in which Δsa09310 was repaired (Δsa09310_com) were generated. The susceptibilities of the S. aureus USA300 wild type (WT) and Δsa09310 and Δsa09310_com mutants to 32 different antibiotics was tested by disk diffusion assay (see Fig. S1 in the supplemental material). The diameter of the inhibitory zone of most antibiotics presented no difference among the S. aureus WT, Δsa09310, and Δsa09310_com strains (Table S1), even including gentamicin, kanamycin, erythromycin, and chloramphenicol, which significantly induced the expression of the sa09310 gene. However, the Δsa09310 mutant exhibited significantly larger inhibitory zones than the WT when treated with the disks containing tetracycline (27 ± 0.5 mm for the Δsa09310 mutant and 9.5 ± 0.5 mm for the WT) and doxycycline (27 ± 0.5 mm for the Δsa09310 mutant and 14 ± 0.5 mm for the WT) (Table S1). This phenotype of an enlarged inhibitory zone on the Δsa09310 plate was able to be restored by the strain in which Δsa09310 had been repaired, the Δsa09310_com mutant (Table S1). These results suggested that the sa09310 gene was involved in tetracycline and doxycycline resistance in S. aureus.
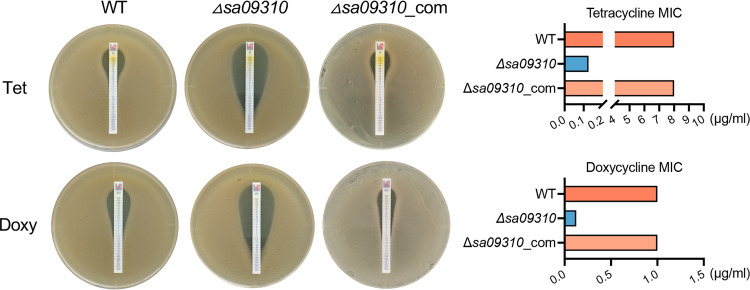
Next, the MICs of tetracycline and doxycycline for the S. aureus WT, Δsa09310, and Δsa09310_com strains were tested and compared by using the Etest method. The Δsa09310 strain displayed a tetracycline MIC of 0.125 μg/mL, which decreased 64-fold compared to that of the WT strain, with a MIC of 8 μg/mL (Fig. 3). The same phenotype was observed for the doxycycline strips as well, with MICs of 0.125 μg/mL for the Δsa09310 mutant and 1 μg/mL for the WT (Fig. 3). Decreased MICs of tetracycline and doxycycline for the Δsa09310 mutant were able to be restored to the WT level in the Δsa09310_com strain (Fig. 3). Taken together, these results confirm that the sa09310 gene is involved in tetracycline and doxycycline resistance in S. aureus.
FIG 3.
Tetracycline (Tet) and doxycycline (Doxy) MIC assay. MICs of Tet and Doxy were tested with S. aureus WT, sa09310 gene knockout mutant (Δsa09310), and Δsa09310 repaired (Δsa09310_com) strains using the Etest strip on TSA plates according to CLSI guidelines (39). The MICs of Tet and Doxy for each strain were read from the plate and presented as histograms on the right side.
The contribution of SA09310 to tetracycline resistance is efflux mediated.
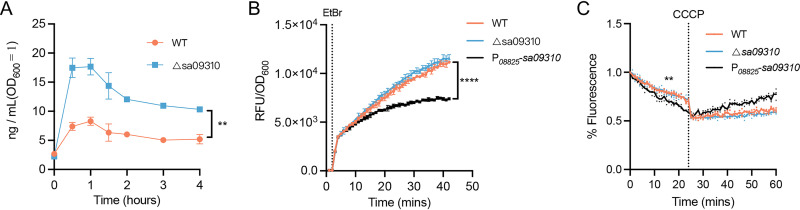
Knockout of the sa09310 gene conferred on S. aureus increased sensitivity to tetracycline, which is known to exert antimicrobial activity by targeting the intracellular ribosome (21–23). We hypothesized that SA09310 might play a role as an efflux pump that extruded tetracycline from within the bacterial cells into the environment. To verify this hypothesis, the S. aureus WT and the Δsa09310 mutant were treated with 5 μg/mL of tetracycline, and the kinetics of the tetracycline concentration within the bacterial cells of each strain were quantified by using enzyme-linked immunosorbent assay (ELISA). The intracellular tetracycline concentrations in the Δsa09310 mutant were 2- to 3-fold higher than those in the WT (Fig. 4A). This result directly supported the notion that SA09310 was an efflux pump that mediated tetracycline resistance of S. aureus by extruding intracellular tetracycline.
FIG 4.
Intracellular tetracycline, EtBr accumulation, and efflux assays. (A) The S. aureus WT and the Δsa09310 mutant were treated with a final concentration of 5 μg/mL of tetracycline. At the indicated time points (0, 0.5, 1, 1.5, 2, 3, and 4 h) after treatment, the bacterial cells were harvested and resuspended in lysis buffer by adjusting the OD600 value to 1.0 (5 × 108 CFU/mL). The tetracycline concentration of the cell lysate was quantified by ELISA. Concentrations from suspensions of the WT and Δsa09310 groups during the indicated period were compared and analyzed. **, P ≤ 0.05 by paired t test. (B) EtBr accumulation assay. S. aureus WT, Δsa09310, and sa09310 overexpression strains were treated with 4 μg/mL of EtBr. The fluorescence of each strain was measured in a 96-well plate by a Bioreader and normalized to the OD600. The dotted line indicates the time point at which EtBr was added. Data are presented as the means of three independent assays. ****, P ≤ 0.0001 by paired t test. (C) EtBr accumulation efflux assay. S. aureus cells were treated with EtBr as described above for 40 min. Then, the extracellular EtBr was removed by centrifugation and resuspension of the cells in fresh PBS, and the fluorescence of each strain was measured. After 20 min, the efflux pump inhibitor carbonyl cyanide chlorophenylhydrazone (CCCP) at a final concentration of 100 μM was added to each well, and the fluorescence was read for an additional 40 min. The dotted line indicates the time point when CCCP was added. All data are presented as the mean of three independent assays. **, P ≤ 0.05 by paired t test.
To further confirm the efflux activity of SA09310, ethidium bromide (EtBr) accumulation and efflux assays were performed on WT, Δsa09310, and sa09310 overexpression strains. Overexpression of sa09310 was achieved by using a strong constitutive promoter and confirmed by RT-qPCR (Fig. S2). In the EtBr accumulation assay, the WT, Δsa09310, and sa09310 overexpression strains were exposed to EtBr, and the fluorescence of each strain was monitored. A time-dependent increase in fluorescence was observed for all strains, with no difference between the WT and Δsa09310 strains (Fig. 4B). However, the sa09310 overexpression strain displayed a significantly lower increase in fluorescence than the WT and Δsa09310 strains (Fig. 4B), which suggested a stronger efflux activity of sa09310 overexpression strain than of the WT and Δsa09310 strains.
In the EtBr efflux assay, the sa09310 overexpression strain showed a slightly lower fluorescence than did the WT and Δsa09310 strains (Fig. 4C). This result indicated that overexpression of the sa09310 gene enhanced the efflux activity of S. aureus. After the addition of the protonophore carbonyl cyanide chlorophenylhydrazone (CCCP), which dissipates the electrochemical potential of H+ across the cytoplasmic membrane, a driving force for the MFS transporters, the fluorescence of all tested strains stopped decreasing due to the collapse of the proton gradient across the membrane (Fig. 4C). This observation provided evidence that suggested H+-dependent activity of SA09310. Taken together, our findings prove SA09310 to be an active efflux pump that mediates tetracycline resistance in S. aureus via extrusion of intracellular tetracycline.
Deletion of sa09310 gene promoted clearance of S. aureus by tetracycline in a Galleria mellonella infection model.
Knockout of the sa09310 gene rendered S. aureus more sensitive to tetracycline in an in vitro assay. However, whether the SA09310 efflux pump promotes tetracycline resistance in vivo requires further investigation, as this information will provide direct evidence of its potential as a target for developing an efflux inhibitor for clinical use. Thus, the clearance of the S. aureus WT or the Δsa09310 mutant by tetracycline in a Galleria mellonella larva infection model was evaluated.
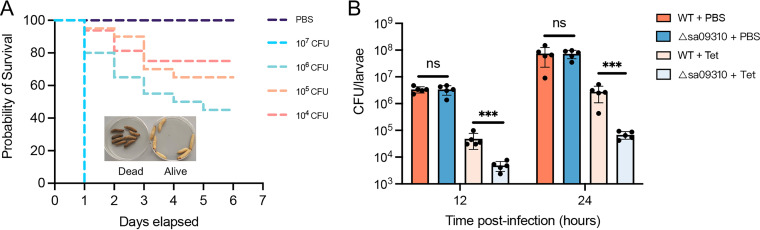
To determine the optimal infection doses for the S. aureus clearance assay, G. mellonella larvae were infected with different doses of S. aureus. Mortality at 2 days was 100% at 107 CFU/larva and was between 60% and 80% at 106 CFU/larvae, while the group infected with 105 CFU/larva showed 90% survival (Fig. 5A). Therefore, the maximum infection dose, 105 CFU/larva, that resulted in a survival rate above 80% was used in the clearance assay.
FIG 5.
(A) Survival curves of G. mellonella larvae infected with the S. aureus USA300 WT at doses ranging from 1 × 104 to 1 × 107 per larva. Larvae were incubated at 37°C, and their viability was assessed over 6 days. (B) Clearance of the S. aureus WT and Δsa09310 mutant by tetracycline in the larval infection model. Larvae were infected with an optimal dose of 1 × 105 CFU/larva of the WT or Δsa09310 mutant. After 2 h of infection, 10 μg of tetracycline was injected into each larva (tetracycline-treated group). PBS was administered as the control for antibiotic treatment. Five live larvae were randomly selected from each group and were homogenized. The bacterial burden of each selected larva at 12 and 24 h after infection was determined by serial dilution and plating assays. ***, P ≤ 0.001. ns, not significant.
The efficiency of S. aureus WT or Δsa09310 mutant clearance by tetracycline in larvae was evaluated by injecting the larvae with tetracycline after 2 h of infection. First, we confirmed that colonization of the S. aureus WT and Δsa09310 strains exhibited no difference in larvae, as larvae from the WT- and Δsa09310 mutant-infected groups possessed the same bacterial burdens after 12 or 24 h of infection (Fig. 5B). When comparing the infected larvae treated with tetracycline and phosphate-buffered saline (PBS), the bacterial burdens in the tetracycline-treated groups were noted to be significantly lower than in the PBS-treated groups in both the WT and Δsa09310 mutant infection groups (Fig. 5B), which suggested that tetracycline is able to eliminate S. aureus efficiently in a larva infection model.
Notably, when comparing the WT- and Δsa09310 mutant-infected groups treated with tetracycline, the bacterial burden in the Δsa09310 mutant-infected group was found to be 10- to 40-fold lower than that in the WT-infected group (Fig. 5B). The WT-infected group possessed a bacterial burden of 4.83 × 104 CFU/larva, while the Δsa09310 mutant-infected group carried 4.86 × 103 CFU/larva after 12 h of infection (Fig. 5B). This difference was observed after 24 h of infection as well, in which the WT-infected group had a bacterial burden of 2.77 × 106 CFU/larva and the Δsa09310 mutant-infected group possessed 6.8 × 104 CFU/larva (Fig. 5B). All these results suggest that deletion of the sa09310 gene renders S. aureus significantly prone to be cleared by tetracycline in vivo.
Conservation of SA09310-like efflux pump in staphylococci.
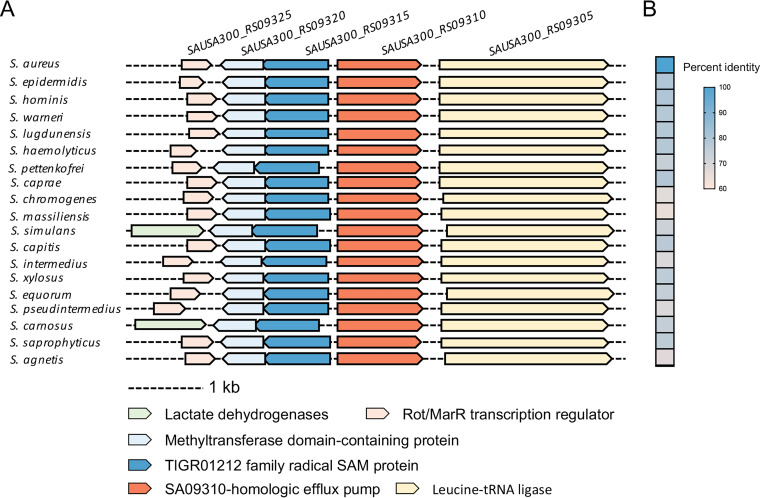
To explore the universality of the SA09310 efflux pump in staphylococci, all 19 proteomes of different S. aureus strains were downloaded from the MicrobesOnline database. Homologs of SA09310 were identified in all of these strains and exhibited high sequence similarity to SA09310, possessing an identity of 99.75% to 100% (Table S3). In addition, the protein sequence of SA09310 was submitted to the STRING database to identify its homologs among members of the Staphylococcus genus. Homologs of SA09310 were identified in all 29 Staphylococcus species. The genomic context of these efflux pump-encoding genes presented a conserved pattern, which included genes encoding a Rot/multiple antibiotic resistance repressor (MarR) transcription regulator, methyltransferase domain-containing protein, and TIGR01212 family radical SAM protein upstream of sa09310 and a leucine-tRNA ligase-encoding gene downstream of sa09310 (Fig. 6A). Moreover, the protein sequence of SA09310 from S. aureus displayed a 64.6% to 79.6% identity to its analogs from other Staphylococcus species (Fig. 6B). These observations imply that SA09310 and its analogs potentially function the same as a tetracycline efflux pump in staphylococci.
FIG 6.
Conservation of SA09310 efflux pump in staphylococci. (A) Conserved genomic context of sa09310 and its homologous genes in different Staphylococcus species. (B) Heat map of percentage identities between the SA09310 protein and its analogous proteins from other Staphylococcus species.
DISCUSSION
In this study, we investigated the role of the sa09310 gene in mediating antimicrobial resistance of S. aureus USA300. The sa09310 gene was predicted to encode an MFS transporter with 12 TMS, which exhibited a close relationship to the multidrug efflux pump DHA12 according to the phylogenetic analysis. Deletion of the sa09310 gene rendered S. aureus more sensitive to tetracycline both in vitro and in vivo. In the presence of tetracycline, the Δsa09310 mutant exhibited a higher concentration of intracellular tetracycline than the WT. Additionally, an EtBr accumulation assay was performed to prove that SA09310 displayed efflux pump activity. All this evidence indicated that SA09310 is a tetracycline efflux pump that mediates tetracycline resistance in S. aureus.
Currently, the multidrug efflux pumps are classified into five families, among which members of the MFS family have been the most extensively studied among staphylococci, including NorA, NorB, NorC, Tet38, LmrS, SdrM, and MdeA (14). The characteristics of MFS transporters are their possession of 12 or 14 TMS (17) and two highly conserved motifs, called motif A and motif C, consisting of residues G(X)3DK/RXGRR/K and G(X)8G(X)3GP(X)2GG, which lie in the cytoplasmic loop between the TMS2 and TMS3 helices and the fifth α-helix (TMS5) (Fig. 1A). The same signatures of MFS were found in SA09310 protein (Fig. 1A). All this evidence strongly suggests that the sa09310 gene encodes an MFS transporter. MFS transporters so far have been classified into 17 distinct groups based on their sequence similarity and their functional relevance (17). To predict the function of the SA09310 transporter, a phylogenetic tree was constructed based on the protein sequence alignment between SA09310 and the classified MFS transporters. SA09310 exhibited a close relationship to the drug-H+ antiporter (12-spanner) drug efflux (DHA12) and monocarboxylate porter (MCP) families (Fig. 1A), which indicated that SA09310 was probably involved in drug or carboxylate efflux.
The expression of most drug efflux pump-encoding genes, such as qarC and abcA in S. aureus, can be induced by their target substrate (24, 25). To identify the substrate transported by SA09310, we first checked the response of the sa09310 gene to the stimulus of different types of antibiotics. Surprisingly, the expression of sa09310 was induced by most of the tested antibiotics (Fig. 2A). This observation implied that the SA09310 efflux pump was most likely involved in the extrusion of and resistance to these antibiotics. Unexpectedly, the susceptibility of the S. aureus WT and Δsa09310 mutant displayed no difference with most of these tested antibiotics except tetracycline and doxycycline. It seems that there is no necessary association between antibiotic induction and efflux pump-mediated corresponding resistance. Typically, the substrate regulates the expression of the efflux pump-encoding gene via specifically impacting a transcriptional regulator, such as the ones from the multiple antibiotic resistance repressor (MarR) family. So far, we have no clue regarding whether the expression of the sa09310 gene can be induced by different types of antibiotics. Probably these antibiotics regulate the expression of sa09310 by affecting distinct regulators whose target is the same gene, sa09310. However, other unknown mechanisms cannot be excluded.
The multidrug efflux systems in bacteria are often regulated by transcription factors that themselves bind the substrates of these export systems, thereby allowing them to activate or repress the expression of their cognate transporter genes (26). One significant family of such transcription factors is the MarR family. MarR-encoding genes are often located in the nearby gene of its controlled transporter-encoding genes, such as the typical genes for MarA and MprA in Escherichia coli (27, 28), MexR in Pseudomonas aeruginosa (29), and MepR in S. aureus (30). Although most of the multidrug efflux genes possess a neighboring gene encoding the MarR regulator, there is no such marR gene nearby sa09310. Instead, a putative MarR-encoding gene with the gene_lucos tag SAUSA300_RS09325 was observed and separated by two genes upstream of sa09310 (Fig. 6A). Whether the expression of the sa09310 gene is regulated by this putative MarR regulator and the regulatory mechanism of tetracycline-induced expression need further investigation.
Tetracyclines are considered antimicrobial agents since they preferentially bind to intracellular ribosomes and interact with a highly conserved 16S rRNA target in the 30S ribosomal subunit, arresting translation by sterically interfering with the docking of aminoacyl-tRNA during elongation (21–23). Tetracycline resistance in bacteria is currently attributed to three well-known mechanisms: active efflux, ribosomal protection, and enzymatic inactivation of tetracycline (31). To date, transporters NorB and Tet38 from the MFS family and MepA from the MATE family have been reported to be involved in tetracycline resistance in S. aureus (30, 32, 33). The mechanism of these transporters mediating resistance was attributed to their ability to export tetracycline (30, 32, 33). The Δsa09310 mutant displayed a significantly lower concentration of intracellular tetracycline (Fig. 4A), suggesting that the mechanism of SA09310-mediated resistance to tetracycline is similar to the mechanism that mediated by Tet38, NorB, and MepA through the extrusion of the substrate (30, 32, 33). This efflux activity of SA09310 was further verified by EtBr accumulation and efflux assays. Although SA09310 promoted S. aureus resistance to tetracycline and doxycycline, the Δsa09310 mutant did not show significantly increased susceptibility to minocycline and tigecycline (Fig. S3), which belong to tetracycline antibiotics. We checked the structures of tetracycline, doxycycline, minocycline, and tigecycline and found that the structure of doxycycline was the most similar to that of tetracycline, followed by minocycline and tigecycline because of their greater modification on the tetracycline backbone. Based on our observation, we speculate that SA09310 recognizes and extrudes tetracycline with high specificity, which means that it is more difficult to be recognized and extruded by SA09310 if the substrate is at more variance with the original structure of tetracycline. Therefore, minocycline and tigecycline evade the recognition and extrusion by SA09310 probably owing to their variant structure compared to that of tetracycline.
Knockout of the sa09310 gene conferred on S. aureus a 64-fold-decreased tetracycline MIC in vitro, suggesting that the SA09310 efflux pump might be utilized as a potential target of an antibiotic adjuvant of tetracycline, thereby overcoming efflux-based resistance. However, the increased susceptibility of the Δsa09310 mutant to tetracycline in vitro might not necessarily predict in vivo outcomes. Bearing that in mind, we investigated the clear efficiency of tetracycline against S. aureus in a G. mellonella larva infection model in vivo. As expected, the Δsa09310 mutant was cleared by tetracycline more easily than the WT. This provided compelling evidence of the feasibility of developing and using an SA09310-targeted inhibitor as an antibiotic adjuvant to overcome efflux-based tetracycline resistance in the clinic.
Although we have verified that SA09310 was a tetracycline efflux pump in S. aureus, we found that the SA09310 protein did not primarily mediate tetracycline resistance in all S. aureus strains. For example, the S. aureus Newman and S. aureus 8325 WT strains possess the same SA09310-encoding gene, but these strains were much more sensitive to tetracycline than the S. aureus USA300 WT (Fig. S4A). These results implied that SA09310 functions adequately in S. aureus USA300 but not fully in the S. aureus Newman and S. aureus 8325 WT strains. However, the function of the SA09310 efflux pump in S. aureus Newman and S. aureus 8325 was the same as in S. aureus USA300 (Fig. S4B and C). These different outcomes of the primary resistance to tetracycline of distinct strains probably resulted from diverse expression patterns or regulation loops of the efflux pump-encoding gene in different strains.
Taken together, our findings identified a novel and staphylococcus-conserved MFS transporter and demonstrated its role as a tetracycline efflux pump that mediated tetracycline resistance in S. aureus. Further studies should be carried out to decode the regulatory mechanism of sa09310 expression.
MATERIALS AND METHODS
Bioinformatics analysis.
The transmembrane helices of the SA09310 protein were predicted by using the TMHMM 2.0 online tool (http://www.cbs.dtu.dk/services/TMHMM/). The transmembrane topology was generated by TOPO2 (http://www.sacs.ucsf.edu/cgi-bin/open-topo2.py/) based on the predicted transmembrane structure from TMHMM 2.0. The genomic context of sa09310 and its homologous genes in different Staphylococcus species was generated from the STRING database (https://string-db.org/).
To generate the phylogenetic tree of SA09310 within the MFS transporter family, at least two protein sequences from each of the 14 classified groups of MFS transporters, including nitrate-nitrite porter (NNP), drug-H+ antiporter with 14-spanner efflux (DHA14), phosphate-H+ symporter (PHS), nucleoside-H+ symporter (NHS), sialate-H+ symporter (SHS), metabolite-H+ symporter (MHS), oligosaccharide-H+ symporter (OHS), oxalate-formate antiporter (OFA), anion-cation symporter (ACS), organophosphate-inorganic phosphate antiporter (OPA), drug-H+ antiporter with 12-spanner efflux (DHA12), monocarboxylate porter (MCP), fucose-galactose-glucose-H+ symporter (FGHS), and unknown major facilitator (UMF) (17), were downloaded from UniProt. Multiple-sequence alignments between SA09310 and MFS transporters were performed by using ClustalW (34), and a phylogenetic tree was generated from multiple alignments using the neighbor-joining method and Jukes-Cantor protein distance model, as implemented in CLC Main Workbench (Qiagen).
Strains and growth conditions.
The S. aureus USA300_FPR3757 strain was used to generate all the S. aureus mutants. Plasmids used for S. aureus USA300 transformation were modified by S. aureus RN4220. All S. aureus transformants were obtained through electroporation as described previously (35). S. aureus strains were cultured in tryptic soy broth (TSB) with shaking at 220 rpm or on TSB agar (TSA) plates at 37°C. DH5α was used for plasmid cloning and was grown in Luria-Bertani (LB) broth with constant shaking at 220 rpm or on LB agar plates at 37°C. Antibiotics were added where indicated at the following concentrations: 100 μg/mL for ampicillin, 10 μg/mL for tetracycline, and 25 μg/mL for chloramphenicol.
DNA manipulations.
Genomic isolation and plasmid preparation from E. coli or S. aureus were performed as previously described (36). PCRs were performed using OneTaq 2× master mix or Q5 high-fidelity 2× master mix from New England Biolabs (NEB) according to the manufacturer’s instructions. Cloning was performed by using the ClonExpress II one-step cloning kit (Vazyme, Nanjing, China) based on homologous recombination. All primers used in this study are listed in Table S2.
Generation of sa09310 knockout, repaired, and overexpression strains.
Knockout of the sa09310 gene was achieved by homologous recombination. DNA fragments of 1 kb flanking the sa09310 gene were amplified by PCR using the primer pairs QL1165/QL1166 and QL1167/QL1168. These two DNA fragments were fused by fusion PCR via their overlap sequence. The fused fragment was cloned into the S. aureus-E. coli shuttle vector pBT2 (37) linearized with EcoRI and SalI restriction enzymes. The generated plasmid was modified by RN4220 and subsequently transformed into the S. aureus USA300 WT with the selection of chloramphenicol. Since pBT2 carries a temperature-sensitive replicon, the plasmid was forced to integrate into the chromosomal DNA upstream or downstream of the sa09310 gene by switching the temperature from 37°C to 42°C with chloramphenicol selection. After the correct genotype was confirmed by PCR using primer pair QL1169/QL1170, the resulting strain was cultured in TSB at 25°C to promote the second round of homologous recombination without antibiotics. The double crossovers were counterselected on the basis of chloramphenicol sensitivity. The sa09310 gene deletion mutant (Δsa09310) was confirmed by PCR and sequencing. The strain with repair of Δsa09310 (Δsa09310_com) was generated by complementing the sa09310 gene at its original site on the chromosome in the Δsa09310 mutant using the same method as gene knockout. Overexpression of sa09310 was achieved by using the replicative vector pQLV1025 with the strong constitutive promoter P08825 from S. aureus (36).
Antibiotic stimulation and RT-qPCR.
Cells of S. aureus from the mid-exponential phase were stimulated with 100 μg/mL of different antibiotics, which included quinolone (norfloxacin), glycopeptide (vancomycin), beta-lactam (ampicillin and oxacillin), aminoglycoside (gentamicin and kanamycin), tetracycline, macrolides (erythromycin), and chloramphenicol. After incubation for 1 h, bacterial cells were harvested by centrifugation. Total cellular RNA was isolated by using the RNApure bacterial kit (CwBIO, Jiangsu, China) following the manufacturer’s instructions. Approximately 1 μg of total RNA was used for reverse transcription using the PrimeScript RT reagent kit with genomic DNA (gDNA) eraser (TaKaRa, Beijing, China). After the transcribed cDNAs were 5-fold diluted, 2 μL of the cDNA was used as a DNA template in 15-μL amplification volumes with a 400 nM concentration of each primer and 7.5 μL of SYBR green master mix (TaKaRa) using the following cycling parameters: 95°C for 30 s followed by 40 cycles of 5 s at 95°C, 30 s at 55°C, and 30 s at 72°C. The qPCR was performed in a CFX-96 Touch real-time PCR system (Bio-Rad, Hercules, CA, USA). Primer pairs QL1293/QL1294 and QL0152/QL0153 were used to amplify the sa09310 and 16S rRNA genes, respectively. The expression level of the sa09310 gene under the treatment with different antibiotics was normalized to the expression of the 16S rRNA gene.
Antimicrobial susceptibility assay.
The disk diffusion assay was performed to test the susceptibility of S. aureus to a broad range of antibiotics. Bacterial cells from the mid-exponential phase were harvested and adjusted to an optical density at 600 nm (OD600) of 1.0 in TSB liquid medium. One hundred microliters of the suspension was mixed with 10 mL of soft TSA (5% agarose) and spread on a TSA plate. The antimicrobial-impregnated disks were placed on the surface of the agar. Antibiotic disks with a 6-mm diameter were purchased from Microbial Regent Company (Hangzhou Microbial, Hangzhou, China). The type and amount of the antibiotic for each disk are listed in Table S1 in the supplemental material. After incubation at 37°C for 24 h, the zones of inhibition around the disks were recorded according to previously reported guidelines (38).
The MICs of tetracycline and doxycycline were determined by the Etest method. TSA plates spread with bacteria were prepared as described above. Tetracycline or doxycycline MIC test strips were purchased from Liofilchem (Abruzzi, Italy) with a gradient antibiotic concentration from 0.016 to 256 μg/mL. The strip was placed on the agar surface using forceps. After incubating the plate at 37°C for 24 h, the MIC value was read by viewing the symmetrical inhibition ellipse on the plate according to the guidelines given by the Clinical and Laboratory Standards Institute (CLSI) (39).
Intracellular tetracycline assay.
The day culture of S. aureus was prepared by inoculating 100 μL of the overnight culture into 10 mL of TSB medium. After 2 h of cultivation, a final concentration of 5 μg/mL of tetracycline was added to the culture. One milliliter of bacterial cells was collected after 0.5, 1, 1.5, 2, 3, 4, or 5 h of incubation with tetracycline. Cells were washed 3 times with PBS and resuspended in the lysis buffer (20 mM Tris-Cl, pH 8.0; 2 mM sodium EDTA; 1.2% Triton X-100), with the OD600 value adjusted to 1.0 (5 × 108 CFU/mL). One hundred microliters of the suspension was used and incubated with a final concentration of 50 μg/mL of lysostaphin at 37°C until the bacterial cells were completely lysed. Intracellular tetracycline released into the lysis was measured by using a tetracycline ELISA kit (Ruixin Biotech, Quanzhou, China) according to the manufacturer’s instructions. Briefly, the supernatant of the cell lysate was mixed with horseradish peroxidase (HRP)-labeled anti-tetracycline antibody and then added into the well on a 96-well plate that had been precoated with tetracycline. Tetracycline from lysis or the precoated well will competitively interact with the antibody. After incubation at 37°C for 30 min, each well was washed with 100 μL of PBS 5 times. Next, 100 μL of substrate solution was added to each well and incubated at 37°C for 15 min. The reaction was stopped with 50 μL of stop solution, and the absorbance value of each well at 450 nm was read and recorded. Absorbance values and concentrations of tetracycline standard samples were used to generate the four-parameter logistic standard curve. The concentration of tetracycline from cell lysis was calculated according to the absorbance value based on the equation of the standard curve.
EtBr accumulation and efflux assays.
For the ethidium bromide (EtBr) accumulation assay, S. aureus cells from the mid-exponential phase were harvested through centrifugation at 10,000 × g for 5 min, washed with PBS 3 times, and resuspended at an OD600 of 0.4 in PBS supplied with 10 mM glucose. One hundred microliters of the suspension was added in triplicate to a 96-well black plate with a clear bottom for measurement of the baseline cellular fluorescence for 2 min. Following this, EtBr was added to each well to a final concentration of 4 μg/mL, and fluorescence was measured every 30 s using a Synergy H1 plate reader (Bio-Tek) at emission and excitation wavelengths of 580 nm and 500 nm, respectively. For the EtBr efflux assay, S. aureus cells were treated with EtBr as described above for 40 min. Then, the extracellular EtBr was removed by centrifugation and resuspension of the cells in fresh PBS, and the fluorescence of each strain was measured in a 96-well black plate by the Bioreader. After 20 min, the efflux pump inhibitor carbonyl cyanide chlorophenylhydrazone (CCCP) at a final concentration of 100 μM was added to each well, and the fluorescence was monitored for an additional 40 min.
Clearance assay of S. aureus by tetracycline in G. mellonella larva infection model.
Bacterial inocula were prepared by diluting 100-μL overnight cultures with 10 mL of fresh TSB followed by incubation for 2 h on an orbital shaker at 37°C to obtain bacteria in the exponential growth phase. Bacterial cells were harvested by centrifugation and resuspended in PBS at an OD600 of 1.0 (5 × 108 CFU/mL). G. mellonella larvae were originally obtained from JingmaiBio (Chengdu, China), further bred in our laboratory, and used at a weight between 350 and 400 mg. Twenty larvae in each group were then inoculated with 10 μL of different concentrations of bacterial suspensions (104, 105, 106, or 107 CFU/larva) into the last right proleg using a 25-μL Hamilton syringe (Sangon, Shanghai, China). After injection, larvae were incubated at 37°C for 4 days, and survival was recorded daily.
For the S. aureus clearance assay, 40 larvae in each group were infected with the optimal infection dose of each strain as described above. A total amount of 10 μg of tetracycline was administered in 10 μL into the last left proleg within 2 h after infection. Treatment was given only once, and PBS was administered as a control group for antibiotic treatment. Larvae were incubated in petri dishes at 37°C. Five live larvae were randomly selected from each group and were tested for bacterial burden at 12 and 24 h after infection. Briefly, the larva was externally disinfected with 75% ethanol, dried, and then placed into a 5-mL tube with 2 mL of sterilized PBS. Larvae in the tube were completely homogenized by using a portable homogenizer with a 4-mm tip (PRIMASCI, UK). Larval homogenate (50 μL) was serially diluted in 450 μL of PBS. The dilution series was plated on TSA plates and was incubated at 37°C for 24 h. Colonies were counted after 24 h, and data are expressed as CFU per larva.
ACKNOWLEDGMENTS
This work was supported by the National Science Fund for Distinguished Young Scholars (32000094), Sichuan Natural Science Fund for Distinguished Young Scholars (2022NSFSC1682), the China Postdoctoral Science Foundation (2021M692311), the Post-doctor Research Project, West China Hospital, Sichuan University (20HXBH017), and the 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYXY21004).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, Holland TL, Fowler VG, Jr. 2019. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 17:203–218. 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nataraj BH, Mallappa RH. 2021. Antibiotic resistance crisis: an update on antagonistic interactions between probiotics and methicillin-resistant Staphylococcus aureus (MRSA). Curr Microbiol 78:2194–2211. 10.1007/s00284-021-02442-8. [DOI] [PubMed] [Google Scholar]
- 3.Zapun A, Contreras-Martel C, Vernet T. 2008. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev 32:361–385. 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- 4.Peacock SJ, Paterson GK. 2015. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem 84:577–601. 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 5.Dashtbani-Roozbehani A, Brown MH. 2021. Efflux pump mediated antimicrobial resistance by staphylococci in health-related environments: challenges and the quest for inhibition. Antibiotics (Basel) 10:1502. 10.3390/antibiotics10121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole K. 2002. Mechanisms of bacterial biocide and antibiotic resistance. Symp Ser Soc Appl Microbiol 2002:55S–64S. 10.1046/j.1365-2672.92.5s1.8.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy VK, Vargiu AV, Malloci G, Dreier J, Ruggerone P. 2017. Molecular rationale behind the differential substrate specificity of bacterial RND multi-drug transporters. Sci Rep 7:8075. 10.1038/s41598-017-08747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alekshun MN, Levy SB. 2007. Molecular mechanisms of antibacterial multidrug resistance. Cell 128:1037–1050. 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Guo J, Lu M, Shen N, Du P. 2022. Dissemination of the mobilised RND efflux pump gene cluster tmexCD-toprJ among Klebsiella pneumoniae. Lancet Microbe 10.1016/S2666-5247(22)00325-1. [DOI] [PubMed] [Google Scholar]
- 10.Costa SS, Viveiros M, Amaral L, Couto I. 2013. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J 7:59–71. 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeegan KS, Borges-Walmsley MI, Walmsley AR. 2003. The structure and function of drug pumps: an update. Trends Microbiol 11:21–29. 10.1016/s0966-842x(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 12.Lewis VG, Ween MP, McDevitt CA. 2012. The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma 249:919–942. 10.1007/s00709-011-0360-8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Lekshmi M, Parvathi A, Ojha M, Wenzel N, Varela MF. 2020. Functional and structural roles of the major facilitator superfamily bacterial multidrug efflux pumps. Microorganisms 8:266. 10.3390/microorganisms8020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler BD, Frempong-Manso E, DeMarco CE, Kosmidis C, Matta V, Seo SM, Kaatz GW. 2015. Analyses of multidrug efflux pump-like proteins encoded on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother 59:747–748. 10.1128/AAC.04678-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handzlik J, Matys A, Kiec-Kononowicz K. 2013. Recent advances in multi-drug resistance (MDR) efflux pump inhibitors of Gram-positive bacteria S. aureus. Antibiotics (Basel) 2:28–45. 10.3390/antibiotics2010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindler BD, Jacinto P, Kaatz GW. 2013. Inhibition of drug efflux pumps in Staphylococcus aureus: current status of potentiating existing antibiotics. Future Microbiol 8:491–507. 10.2217/fmb.13.16. [DOI] [PubMed] [Google Scholar]
- 17.Pao SS, Paulsen IT, Saier MH, Jr. 1998. Major facilitator superfamily. Microbiol Mol Biol Rev 62:1–34. 10.1128/MMBR.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith JK, Baker ME, Rouch DA, Page MG, Skurray RA, Paulsen IT, Chater KF, Baldwin SA, Henderson PJ. 1992. Membrane transport proteins: implications of sequence comparisons. Curr Opin Cell Biol 4:684–695. 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 19.Rouch DA, Cram DS, DiBerardino D, Littlejohn TG, Skurray RA. 1990. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol 4:2051–2062. 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 20.Varela MF, Griffith JK. 1993. Nucleotide and deduced protein sequences of the class D tetracycline resistance determinant: relationship to other antimicrobial transport proteins. Antimicrob Agents Chemother 37:1253–1258. 10.1128/AAC.37.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell IH. 1967. Partial removal of bound transfer RNA from polysomes engaged in protein synthesis in vitro after addition of tetracycline. Biochim Biophys Acta 138:337–346. 10.1016/0005-2787(67)90494-7. [DOI] [PubMed] [Google Scholar]
- 22.Brodersen DE, Clemons WM, Jr, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. 2000. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103:1143–1154. 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 23.Pioletti M, Schlunzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J 20:1829–1839. 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grkovic S, Brown MH, Roberts NJ, Paulsen IT, Skurray RA. 1998. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J Biol Chem 273:18665–18673. 10.1074/jbc.273.29.18665. [DOI] [PubMed] [Google Scholar]
- 25.Villet RA, Truong-Bolduc QC, Wang Y, Estabrooks Z, Medeiros H, Hooper DC. 2014. Regulation of expression of abcA and its response to environmental conditions. J Bacteriol 196:1532–1539. 10.1128/JB.01406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher MA, Brennan RG. 2002. Structural mechanisms of multidrug recognition and regulation by bacterial multidrug transcription factors. Mol Microbiol 45:885–893. 10.1046/j.1365-2958.2002.03039.x. [DOI] [PubMed] [Google Scholar]
- 27.Jair KW, Martin RG, Rosner JL, Fujita N, Ishihama A, Wolf RE, Jr. 1995. Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J Bacteriol 177:7100–7104. 10.1128/jb.177.24.7100-7104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong A, Gottman A, Park C, Baetens M, Pandza S, Matin A. 2000. The EmrR protein represses the Escherichia coli emrRAB multidrug resistance operon by directly binding to its promoter region. Antimicrob Agents Chemother 44:2905–2907. 10.1128/AAC.44.10.2905-2907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adewoye L, Sutherland A, Srikumar R, Poole K. 2002. The mexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J Bacteriol 184:4308–4312. 10.1128/JB.184.15.4308-4312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaatz GW, McAleese F, Seo SM. 2005. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob Agents Chemother 49:1857–1864. 10.1128/AAC.49.5.1857-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman TH. 2016. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med 6:a025387. 10.1101/cshperspect.a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol 187:2395–2405. 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAleese F, Petersen P, Ruzin A, Dunman PM, Murphy E, Projan SJ, Bradford PA. 2005. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob Agents Chemother 49:1865–1871. 10.1128/AAC.49.5.1865-1871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk S, Laddaga RA. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73:133–138. 10.1111/j.1574-6968.1992.tb05302.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Li D, Wang N, Guo G, Shi Y, Zou Q, Zhang X. 2022. Identification and application of a panel of constitutive promoters for gene overexpression in Staphylococcus aureus. Front Microbiol 13:818307. 10.3389/fmicb.2022.818307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruckner R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett 151:1–8. 10.1016/S0378-1097(97)00116-X. [DOI] [PubMed] [Google Scholar]
- 38.Barry AL, Coyle MB, Thornsberry C, Gerlach EH, Hawkinson RW. 1979. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J Clin Microbiol 10:885–889. 10.1128/jcm.10.6.885-889.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace RJ, Jr, Warren NG, Witebsky FG. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes, 2nd ed, M24-A2. CLSI, Wayne, PA. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01696-22-s0001.pdf, PDF file, 0.9 MB (902.5KB, pdf)