Abstract
Objective
Subjective illness perception (IP) can differ from physician's clinical assessment results. Herein, we explored patient's IP during coronavirus disease 2019 (COVID-19) recovery.
Methods
Participants of the prospective observation CovILD study (ClinicalTrials.gov: NCT04416100) with persistent somatic symptoms or cardiopulmonary findings one year after COVID-19 were analyzed (n = 74). Explanatory variables included demographic and comorbidity, COVID-19 course and one-year follow-up data of persistent somatic symptoms, physical performance, lung function testing, chest computed tomography and trans-thoracic echocardiography. Factors affecting IP (Brief Illness Perception Questionnaire) one year after COVID-19 were identified by regularized modeling and unsupervised clustering.
Results
In modeling, 33% of overall IP variance (R2) was attributed to fatigue intensity, reduced physical performance and persistent somatic symptom count. Overall IP was largely independent of lung and heart findings revealed by imaging and function testing. In clustering, persistent somatic symptom count (Kruskal-Wallis test: η2 = 0.31, p < .001), fatigue (η2 = 0.34, p < .001), diminished physical performance (χ2 test, Cramer V effect size statistic: V = 0.51, p < .001), dyspnea (V = 0.37, p = .006), hair loss (V = 0.57, p < .001) and sleep problems (V = 0.36, p = .008) were strongly associated with the concern, emotional representation, complaints, disease timeline and consequences IP dimensions.
Conclusion
Persistent somatic symptoms rather than abnormalities in cardiopulmonary testing influence IP one year after COVID-19. Modifying IP represents a promising innovative approach to treatment of post-COVID-19 condition. Besides COVID-19 severity, individual IP should guide rehabilitation and psychological therapy decisions.
Keywords: COVID-19, Clustering, Illness perception, Persistent somatic symptoms, Post-COVID-19 condition, Regularized regression
1. Introduction
A subset of coronavirus disease 2019 (COVID-19) patients is affected by protracted somatic symptoms, cardiopulmonary pathology and mental health disorders [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]]. Persistent COVID-19-related symptoms have been initially described by patient initiatives [12] and subsequently recognized as ‘post-COVID-19 condition’ by the clinical community [1,2,[6], [7], [8],10]. Most patients recover completely from acute COVID-19 symptoms within four weeks. The clearance rate is particularly fast for non-specific infection complaints [2,10,13]. In a recent meta-analysis of 1.7 million patients, the pooled prevalence estimate of post-COVID-19 condition, i.e. symptoms present for 3 months or longer, was 43% [8,11]. Yet, in a rigorously controlled study, approximately 12.7% of SARS-CoV-2-positive individuals suffered from persistent somatic symptoms directly associated with COVID-19 [14]. Fatigue, concentration and memory problems, sleep disorders, physical performance deficits, smell/taste disorders and hair loss, dyspnea and pain are the most common, frequently overlapping and relapsing manifestations [2,4,7,9,11,[13], [14], [15]].
Yet, patients' and clinicians' perceptions of post-COVID-19 condition and its impact on patients´ lives are not always consistent, a phenomenon that has also been observed in other conditions such as chronic obstructive pulmonary disease [16,17] or functional disorders [18]. More specifically, persistent symptoms in many COVID-19 patients were not accompanied by lung, heart, biochemical, inflammatory and immunity abnormalities [4,19]. Persistent somatic symptoms (PSS) are defined as any subjectively distressing somatic complaints, including medically explained, unexplained and functional symptoms [20]. Manifestations of post-COVID-19 condition like fatigue or neuro-cognitive disorders were also associated with depression, anxiety, mental stress and other signs of mental health impairment [[5], [6], [19], [21], [22], [23], [71]]. Intriguingly, somatic complaints comparable to post-COVID-19 condition symptoms have also been observed in COVID-19-free individuals [[24], [25], [26]] suggestive of effects of socioeconomic background or altered social behavior during the SARS-CoV-2 pandemic [27,28]. Fatigue, sleep problems and dyspnea were also common symptoms in the general pre-pandemic population [29].
Illness perception (IP) consists of cognitive and emotional dimensions [30]. The cognitive dimensions comprise self-perceived consequences, expected duration, personal control, expected treatment effect, symptom perception and understanding of the disease. The emotional dimensions encompass disease-associated concerns and emotions e.g. fear, anger or distress [30,31]. According to the common-sense model of self regulation, aiming to explain how individuals cope with with health threats, IP is influenced by situational stimuli such as symptoms, health information and patient's knowledge [27,32,33]. IP was shown to influence adjustment to adverse life events or chronic conditions and compliance with prevention, treatment and rehabilitation [34,35], also in COVID-19 [[36], [37], [38]]. Emotional response, concerns and consequence IP dimensions correlate with anxiety, depression and stress both in the general population and acute COVID-19 patients [11,[39], [40], [41], [42]]. Similar observations were made recently in individuals with post-COVID-19 condition recruited from self-help and social media platforms [21]. However, characterization of IP and its influencing factors in a defined observational COVID-19 cohort is still missing. In particular, it is unclear how PSS, lung and heart pathology [1,3,4] shape IP during long-term COVID-19 recovery.
We aimed to describe IP and IP components, identify demographic and clinical variables affecting IP and explore IP patterns in convalescents suffering from persistent somatic symptoms (PSS), lung or heart abnormalities one year after COVID-19. To this end, we gauged IP in a long-term observatory cross-sectional COVID-19 collective [[1], [2], [3], [4]] with the Brief Illness Perception Questionnaire (BIPQ) [30] and investigated components, influential factors and patterns of IP by factor analysis, regularized multi-parameter modeling and unsupervised clustering.
2. Methods
2.1. Study design and approval
Participants of the longitudinal observation CovILD study (ClinicalTrials.gov: NCT04416100) were recruited during the first wild-type SARS-CoV-2 outbreak between April and June 2020 at the University Hospital of Innsbruck, the St. Vinzenz Hospital in Zams and the Karl-Landsteiner Rehabilitation Facility in Münster (all in Austria) [1,3,4]. The study inclusion criteria were age ≥ 18 years and symptomatic, PCR-confirmed SARS-CoV-2 infection. The follow-ups were scheduled at two, three, six months and one year after COVID-19 diagnosis.
In total, 190 individuals were screened for participation. The screened subset size was limited by the number of COVID-19 cases diagnosed at the study centers, their follow-up availability (residency in the study region) and available resources for the follow-up assessments. No statistical sample size estimation was performed, as the kinetic of COVID-19 recovery was unknown at the pandemic onset. Out of 145 enrolled individuals, 108 completed the one-year follow-up. A subset of participants (n = 74) was analyzed who displayed (1) PSS or (2) any abnormality in chest computed tomography or (3) any lung function testing deficits or (4) any heart abnormality in trans-thoracic echocardiography at the one-year follow-up and had a complete set of BIPQ, demographic and clinical analysis variables (Fig. 1 , Supplementary Tables S1 - S2). We decided to restrict the analysis to incomplete COVID-19 recovery, because BIPQ was initially designed for and validated in somatic conditions [30].
Fig. 1.
Flow diagram of study enrollment and analysis inclusion process.
CT: computed tomography of the chest; LFT: lung function testing, TTE: trans-thoracic echocardiography.
The study was performed in accordance with the Declaration of Helsinki and European Data Policy. All participants gave written informed consent. The study protocol was approved by the ethics committee at the Medical University of Innsbruck (approval number 1103/2020).
2.2. Procedures
For full descriptions of procedures and variables, see Supplementary Methods and Supplementary Tables S1 and S2.
Baseline clinical and acute COVID-19 data were recorded retrospectively at the two-month follow-up [1]. Study participants were classified as ambulatory (outpatient, WHO ordinal scale for clinical improvement 1–2), moderate (hospitalized without oxygen therapy, WHO 3–4) and severe COVID-19 survivors (hospitalized with non-invasive ventilation or high-flow oxygen or mechanical ventilation, WHO 5–7).
Physical performance was rated with the Eastern Cooperative Oncology Group scale. Dyspnea was scored with the Modified Medical British Research Council scale. Fatigue was rated with likert and bimodal Chalder's Fatigue Scales [43,44]. Exertional capacity was assessed by six-minute walking distance [45].
The following PSS were analyzed at the one-year follow-up: reduced physical performance (Eastern Cooperative Oncology Group scale ≥ 1), dyspnea (Modified Medical British Research Council scale ≥ 1), self-reported cough (yes/no item), self-reported sleep problems (yes/no), self-reported night sweating (yes/no), self-reported hyposmia or anosmia (yes/no), self-reported dermatological symptoms (yes/no), self-reported gastrointestinal symptoms (yes/no), self-reported hair loss (yes/no), significant fatigue (bimodal Chalder's Fatigue Scale ≥ 4). Records of reduced physical performance, dyspnea, cough, sleep problems, night sweating, hyposmia/anosmia and gastrointestinal symptoms were available for acute COVID-19 and all follow-ups [4].
Lung function testing abnormality was defined as at least one parameter <80% (forced vital capacity, forced expiratory volume in 1 s, total lung capacity, diffusion lung capacity for carbon monoxide) or < 70% (ratio of forced expiratory volume in 1 s to forced vital capacity) of the reference value [4]. Computed tomography images were evaluated with the Fleischner Society glossary terms [46] and the computed tomography severity score [1,3,4]. Blood biomarkers encompassed hemoglobin and parameters of iron turnover, inflammation and coagulation.
IP was investigated with a German translation of BIPQ [30] with the following 8 items: Q1 consequences (0: no consequences, 10: very severe consequences), Q2 timeline (0: very short, 10: forever), Q3 lacking personal control (negative item, 0: full control, 10: no control at all), Q4 lacking treatment control (negative item, 0: full control, 10: no control at all), Q5 identity (0: no complaints, 10: multiple severe complaints), Q6 concerns (0: not at all, 10: extreme concerns), Q7 lacking coherence (negative item, 0: very good understanding, 10: no understanding at all) and Q8 emotional representation (0: no emotions at all, 10: extreme emotions). Items were rated with 11-point likert scales, with 0 corresponding to neutral or positive and 10 representing severely distorted or negative IP dimension. The Q3, Q4 and Q7 conceptualized as negative items were re-coded prior to analysis. The total IP score was defined as all BIPQ item sum as a measure of overall negative IP alterations (0: neutral - 80: extremely severe) [30]. The emotion/concern/consequences score was defined as the Q1, Q2, Q5, Q6 and Q8 item sum (0: neutral - 50: extremely severe) and the lacking control/coherence score was defined as the Q3, Q4 and Q7 item sum (range: 0: good control/understanding - 30: no control/understanding at all) to measure intensity of the two main IP components identified by factor analysis.
2.3. Analysis endpoints
The primary analysis endpoints were demographic and clinical factors affecting total IP and its emotion/concern/consequences and lacking control/coherence components in COVID-19 convalescents with PSS or cardiopulmonary abnormalities at the one-year follow-up. The secondary analysis endpoints were components of IP, patterns of IP dimensions explored by clustering as well as demographic and clinical factors differentiating between the IP clusters.
2.4. Statistical analysis
Statistical analysis details are provided in Supplementary Methods. Analysis was done with R version 4.2.0. Numeric variables were presented as medians with interquartile ranges. Categorical variables were shown as percentages and counts. Differences in categorical variable distribution were assessed by χ 2 test with Cramer V effect size statistic. Statistical significance for numerical variables was investigated by Mann-Whitney test with r effect size statistic or Kruskal-Wallis test with η 2 effect size statistic. Correlations were investigated by Spearman's test. Differences in symptom frequency over time were investigated by Cochran Q test. Interaction effects were analyzed by two-way ANOVA with η 2 effect size statistic. BIPQ dimensionality and consistency was investigated by factor analysis [47] and McDonald's ω [48].
Multi-parameter modeling was done with regularized regression algorithms: Elastic Net [49,50], LASSO (least absolute shrinkage and selection operator) [50,51] and Bayesian LASSO [52,53]. The choice of regularized linear regression was motivated by multi-dimensional character of the study data set, i.e. comparable number of explanatory variables and observations [54,55]. Ordinary least square models with backward term elimination developed in the study data set had poor reproducibility and over-parameterization as demonstrated by fit error expansion in cross-validation.
Modeling responses: the total IP score, emotion/concern/consequence and lacking control/coherence scores, were square root-transformed to guarantee normality. Both first and second order terms of numeric explanatory variables were included in the models. Numeric explanatory and response variables were Z-score normalized. The optimal λ for Elastic Net and LASSO were obtained by 200-repeats 10-fold cross-validation. The ‘sparsity’ parameter in Bayesian LASSO was found by 10-repeats 10-fold cross-validation [56]. Explained variance (R 2) and model root mean squared error were assessed in the entire data set and 10-repeats 10-fold cross-validation [56]. Elastic Net and LASSO model coefficients were calculated for the optimal λ values. Bayesian LASSO coefficients were calculated as medians over all algorithm iterations [52]. The number of non-zero model coefficients was found to differ substantially between the models as reported for data sets of similar size [55]. Hence, variables with non-zero coefficients in models constructed with all three algorithms were deemed the key factors for the total IP score, emotion/concern/consequences and lacking control/coherence scores.
Clustering by the BIPQ items was accomplished by the partitioning around medoids algorithm with Euclidean distance [57,58]. The clustering algorithm choice was motivated by its optimal stability in 10-fold cross-validation [59] and good explanatory performance measured by the fraction of clustering variance as compared with hierarchical and KMEANS clustering.
3. Results
3.1. Study cohort at baseline and one year after COVID-19
The CovILD cohort was recruited during the first outbreak of SARS-CoV-2 between April and June 2020 [1]. Out of 145 participants enrolled, 74 individuals with PSS or abnormalities in lung computed tomography, lung function testing or cardiological abnormalities, complete BIPQ and a complete set of study variables set at the one-year follow-up were analyzed. The major reasons of patient dropout were missing follow-ups and incomplete BIPQ (Fig. 1). The excluded participants tended towards less severe acute COVID-19, more dermatological and gastrointestinal symptoms at the one-year follow-up, and lower blood hemoglobin and ferritin as compared with the analysis collective; those effects were, however, not significant. The excluded participants had significantly lower six-minute walking distance (Supplementary Fig. S3).
The analyzed participants were predominantly male (65%), the median age at COVID-19 diagnosis was 56 years (interquartile range: 47–68), over one-third were active or ex-smokers (38%). Most participants suffered from comorbidities (74%), with cardiovascular disease, metabolic and respiratory disorders as leading conditions. The participants were classified by COVID-19 severity as ambulatory (20%), moderate (hospitalized, no intensive care, no oxygen therapy, 54%) and severe COVID-19 survivors (hospitalized, oxygen therapy or intensive care, 26%). The ambulatory COVID-19 subset had the lowest median age, smoking and comorbidity rates (Table 1 ).
Table 1.
Baseline demographic and clinical characteristic of the study cohort. Numeric variables are presented as medians with interquartile ranges (IQR). Categorical variables are shown as percentages and counts within the strata.
| Variable | Cohort | Ambulatory COVID-19 | Moderate COVID-19 | Severe COVID-19 | Significancea | Effect sizea |
|---|---|---|---|---|---|---|
| n, participants | 74 | 15 | 40 | 19 | ||
| Male sex | 65% (n = 48) | 33% (n = 5) | 70% (n = 28) | 79% (n = 15) | p = .01 | V = 0.34 |
| Age, years | 56 [IQR: 47–68] range: 19–87 |
45 [IQR: 36–55] range: 19–70 |
62 [IQR: 53–73] range: 27–87 |
54 [IQR: 50–62] range: 44–72 |
p = .001 | η2 = 0.16 |
| Smoking historyb | 38% (n = 28) | 13% (n = 2) | 48% (n = 19) | 37% (n = 7) | ns (p = .07) | V = 0.27 |
| Weight classc | normal: 38% (n = 28) overweight: 43% (n = 32) obesity: 19% (n = 14) |
normal: 60% (n = 9) overweight: 27% (n = 4) obesity: 13% (n = 2) |
normal: 30% (n = 12) overweight: 50% (n = 20) obesity: 20% (n = 8) |
normal: 37% (n = 7) overweight: 42% (n = 8) obesity: 21% (n = 4) |
ns (p = .37) | V = 0.17 |
| Comorbidity present | 74% (n = 55) | 47% (n = 7) | 80% (n = 32) | 84% (n = 16) | p = .02 | V = 0.32 |
| Cardiovascular comorbidity | 43% (n = 32) | 13% (n = 2) | 48% (n = 19) | 58% (n = 11) | p = .02 | V = 0.32 |
| Hypertension, comorbidity | 30% (n = 22) | 13% (n = 2) | 30% (n = 12) | 42% (n = 8) | ns (p = .19) | V = 0.21 |
| Metabolic comorbidity | 38% (n = 28) | 13% (n = 2) | 45% (n = 18) | 42% (n = 8) | ns (p = .09) | V = 0.26 |
| Hypercholesterolemia | 22% (n = 16) | 0% (n = 0) | 32% (n = 13) | 16% (n = 3) | p = .03 | V = 0.31 |
| Type II diabetes, comorbidity | 14% (n = 10) | 6.7% (n = 1) | 7.5% (n = 3) | 32% (n = 6) | p = .03 | V = 0.31 |
| Gastrointestinal comorbidity | 14% (n = 10) | 0% (n = 0) | 20% (n = 8) | 11% (n = 2) | ns (p = .14) | V = 0.23 |
| Malignancy, comorbidity | 12% (n = 9) | 6.7% (n = 1) | 18% (n = 7) | 5.3% (n = 1) | ns (p = .31) | V = 0.18 |
| Respiratory comorbidity | 24% (n = 18) | 13% (n = 2) | 28% (n = 11) | 26% (n = 5) | ns (p = .54) | V = 0.13 |
| Chronic kidney disease, comorbidity | 6.8% (n = 5) | 0% (n = 0) | 7.5% (n = 3) | 11% (n = 2) | ns (p = .46) | V = 0.14 |
| Immune deficiency, comorbidity | 4.1% (n = 3) | 0% (n = 0) | 2.5% (n = 1) | 11% (n = 2) | ns (p = .23) | V = 0.2 |
| WHO COVID-19 severityd | 4 [IQR: 3–4.8] range: 2–7 |
2 [IQR: 2–2] range: 2–2 |
4 [IQR: 3–4] range: 3–4 |
6 [IQR: 6–6] range: 5–7 |
p < .001 | η2 = 0.86 |
COVID-19 severity strata comparison; categorical variables: χ2 test with Cramer V effect size statistic, numeric variables: Kruskal-Wallis test with η2 effect size statistic.
Former or active smoker.
Overweight: body mass index 25–30 kg/m2, obesity: > 30 kg/m2.
WHO ordinal scale for clinical improvement.
Nearly three-quarter of participants (72%) suffered from PSS one year after COVID-19. Significant fatigue (41%), reduced physical performance (35%), sleep disorders (32%) and exertional dyspnea (22%) were the most frequent PSS. Recovery pace for the longitudinally recorded PSS was the fastest within the first 3 months after COVID-19. There was little improvement during later convalescence and percentages of performance deficits, sleep problems and smell disorders were substantially higher at the one-year than at the 6 month follow-up suggestive of relapse. Except for self-reported anosmia, which was absent in severe COVID-19 survivors, PSS frequencies and fatigue rating were comparable between the COVID-19 severity strata (Supplementary Fig. S1, Supplementary Table S4).
Lung function testing abnormalities affected 32% of participants and were substantially enriched in severe COVID-19 survivors. Abnormalities in lung computed tomography were found in 54% individuals and their frequency and scoring was significantly higher in moderate-to-severe COVID-19 than in the ambulatory subset. The leading cardiological finding was low grade diastolic dysfunction (64%), which was significantly enriched in moderate and severe COVID-19 survivors. Concomitant PSS, abnormal lung computed tomography, lung function testing and diastolic dysfunction in echocardiography one year after COVID-19 were observed in 14.9% of participants. In 18.9% of participants, PSS were not accompanied by cardiopulmonary abnormalities (Supplementary Fig. S2). Nearly 80% of severe disease survivors attended COVID-19-specific rehabilitation, the rehabilitation rates in the remaining severity strata were below 20% (Supplementary Table S4). Most laboratory parameters at the one-year follow-up were within their normal values. Mild anemia and improper glycemia control assessed by glycated hemoglobin levels were evident primarily in moderate and severe COVID-19 survivors (Supplementary Table S5).
Females displayed significantly less severe COVID-19 and less chest computed tomography abnormalities but higher PSS rates and counts than males at the one-year follow-up. Differences between the genders in frequencies and scoring of specific PSS were not significant (Supplementary Table S6 and not shown).
3.2. Illness perception one year after COVID-19
Factor analysis of BIPQ [30,47] revealed two IP components one year after COVID-19: (1) an emotional representation, concern, identity, consequences and timeline component (Q1, Q2, Q5, Q6 and Q8, termed further 'emotion/concern/consequens component'), and (2) a lacking treatment/personal control and lacking coherence component (Q3, Q4 and Q7, termed further 'lacking control/coherence component') (Supplementary Table S3). The entire BIPQ (ω = 0.9) and the emotion/concern/consequence component (ω = 0.94) had good internal consistencies as determined by McDonald's omega [48]. The lacking control/coherence component consistency was markedly lower (ω = 0.71), presumably due to weak correlation of the lacking personal control (Q3) with the remaining items (Supplementary Fig. S4).
The median total IP score representing the sum of all BIPQ items was 23 (interquartile range: 15–32) in the study collective, the differences between ambulatory, moderate and severe COVID-19 were not significant. The emotion/concern/consequences IP component rated by the Q1, Q2, Q5, Q6 and Q8 item sum was the lowest in moderate and the highest in severe COVID-19. Moderate COVID-19 convalescents had in turn the highest intensity of the lacking control/coherence IP component scored as the Q3, Q4 and Q7 item sum as compared with the remaining severity subsets; this difference was not significant (Table 2 ).
Table 2.
The total illness perception (IP) score (sum of all BIPQ items), emotion/concern/consequences IP component score (sum of items 1, 2, 5, 6 and 8), lacking control/coherence IP component score (sum of items 3, 4 and 7) and values of single Brief Illness Perception Questionnaire items (BIPQ, Q1 - Q8) at one year after COVID-19. Values are presented as medians with interquartile ranges (IQR).
| Variable | Cohort | Ambulatory COVID-19 | Moderate COVID-19 | Severe COVID-19 | Significancea | Effect sizea |
|---|---|---|---|---|---|---|
| n, participants | 74 | 15 | 40 | 19 | ||
| Total IP score (BIPQ sum) | 23 [IQR: 15–32] range: 0–59 |
25 [IQR: 18–30] range: 2–59 |
22 [IQR: 12–31] range: 3–53 |
30 [IQR: 15–40] range: 0–50 |
ns (p = .67) | η2 = 0.017 |
| Emotion/concern/consequences (BIPQ Q1/2/5/6/8) | 9 [IQR: 4–18] range: 0–47 |
10 [IQR: 3.5–17] range: 0–34 |
5 [IQR: 2–12] range: 0–47 |
15 [IQR: 9.5–30] range: 0–43 |
p = .03 | η2 = 0.071 |
| Lacking control/coherence (BIPQ Q3/4/7) | 10 [IQR: 5–17] range: 0–30 |
8 [IQR: 5.5–22] range: 1–30 |
12 [IQR: 5.8–18] range: 2–30 |
8 [IQR: 3–12] range: 0–18 |
ns (p = .07) | η2 = 0.045 |
| Consequences (BIPQ Q1) | 1 [IQR: 0–3.8] range: 0–10 |
1 [IQR: 1–3.5] range: 0–8 |
1 [IQR: 0–2] range: 0–10 |
3 [IQR: 1–6] range: 0–9 |
p = .01 | η2 = 0.091 |
| Timeline (BIPQ Q2) | 1 [IQR: 0–5] range: 0–10 |
1 [IQR: 0.5–4.5] range: 0–6 |
1 [IQR: 0–3] range: 0–10 |
4 [IQR: 1–6.5] range: 0–9 |
ns (p = .13) | η2 = 0.029 |
| Lacking personal control (BIPQ Q3) | 4.5 [IQR: 1–7] range: 0–10 |
2 [IQR: 1–7] range: 0–10 |
5 [IQR: 2–8.2] range: 0–10 |
3 [IQR: 1–5] range: 0–7 |
ns (p = .07) | η2 = 0.045 |
| Lacking treatment control (BIPQ Q4) | 2 [IQR: 0.25–6.8] range: 0–10 |
2 [IQR: 0.5–9] range: 0–10 |
2 [IQR: 1–8] range: 0–10 |
1 [IQR: 0–4.5] range: 0–10 |
ns (p = .22) | η2 = 0.014 |
| Identity (BIPQ Q5) | 1 [IQR: 1–3.8] range: 0–10 |
1 [IQR: 1–4.5] range: 0–8 |
1 [IQR: 0–3] range: 0–10 |
2 [IQR: 1.5–5.5] range: 0–9 |
ns (p = .10) | η2 = 0.037 |
| Concern (BIPQ Q6) | 1.5 [IQR: 0–3.8] range: 0–9 |
2 [IQR: 0–3] range: 0–8 |
1 [IQR: 0–3] range: 0–9 |
3 [IQR: 2–6] range: 0–9 |
p = .01 | η2 = 0.097 |
| Lacking coherence (BIPQ Q7) | 2 [IQR: 0–5] range: 0–10 |
5 [IQR: 2.5–8.5] range: 0–10 |
2 [IQR: 0–5] range: 0–10 |
2 [IQR: 0–3] range: 0–7 |
p = .02 | η2 = 0.078 |
| Emotional representation (BIPQ Q8) | 2 [IQR: 0–4.8] range: 0–10 |
1 [IQR: 0–3] range: 0–9 |
2 [IQR: 0–3] range: 0–10 |
3 [IQR: 1.5–8] range: 0–10 |
p = .046 | η2 = 0.058 |
COVID-19 severity strata comparison; Kruskal-Wallis test with η2 effect size statistic.
Significant differences between the COVID-19 severity strata were detected for the single BIPQ items of consequences, concern, emotional representation, which peaked in severe COVID-19 convalescents. The maximum scores of the lacking coherence BIPQ item were detected in mild COVID-19 individuals (Table 2). Differences in IP between the genders were not significant (Supplementary Table S6).
3.3. Key factors influencing illness perception
The most important factors among 56 candidate explanatory variables (Supplementary Tables S2) influencing total IP and the emotion/concern/consequences and lacking control/coherence IP components one year after COVID-19, were identified with three regularized multi-parameter regression algorithms: Elastic Net [49], LASSO [51] and Bayesian LASSO [52]. The choice of regularized regression instead of canonical linear regression was motivated by multi-dimensional character of the study data, i.e. comparable number of observations and explanatory variables [54,55].
The final regularized models explained at least 33% and 32% of the total IP score variance in the entire data set and cross-validation, respectively. Explained variances of the emotion/concern/consequences IP component score were substantially higher with at least 57% in the entire collective and 40% in cross-validation. For the lacking control/coherence component, no meaningful models could be established with either of the algorithms (Supplementary Fig. S5). The number of explanatory factors with non-zero coefficients in the total IP models varied between 3 (LASSO) and 6 (Elastic Net) (Fig. 2A, Supplementary Figs. S6–S8). Between 11 (Bayesian LASSO) and 18 (LASSO) explanatory variables were linked to the emotion/concern/consequences rating (Fig. 2B, Supplementary Figs. S9–S11). The key variables affecting total IP identified by all three models were PSS count, reduced physical performance and fatigue scoring at the one-year follow-up. In uni-variable analysis, each of them was significantly associated with total IP (Fig. 3 ). The key explanatory factors for the emotion/concern/consequences IP component identified by all three modeling algorithms were PSS count, reduced physical performance, fatigue scoring, significant fatigue, hair loss, cough, diastolic dysfunction, elevated C-reactive protein at the one-year follow-up as well as rehabilitation during COVID-19 recovery, respiratory comorbidity and body weight class. In uni-variable analysis, PSS number, fatigue scoring and significant fatigue, reduced performance, hair loss, rehabilitation and respiratory comorbidity were significantly associated with increased emotion/concern/consequences scores (Fig. 4 ).
Fig. 2.
Key factors associated with illness perception one year after COVID-19.
The total illness perception (IP) score (A) and the emotion/concern/consequences IP component score (B) at the one-year follow-up were modeled as a function of 56 candidate explanatory variables by Elastic Net, LASSO and Bayesian LASSO. Key factors for the total IP score and emotion/concern/consequences component score were identified as variables with non-zero coefficients in all three models. Key factor numbers identified by each algorithm are presented as quasi-proportional Venn diagrams. The key factors are listed next to the diagrams. CFS: Chalder's fatigue score; reduced performance: Eastern Cooperative Oncology Group (ECOG) ≥ 1; # PSS: number of persistent somatic symptoms at the one-year follow-up; CRP: C-reactive protein.
Fig. 3.
Persistent somatic symptom number, reduced physical performance, fatigue and the total illness perception scoring.
Association of the total illness perception (IP) score with persistent somatic symptom number (PSS), reduced physical performance and fatigue scoring at the one-year follow-up. Statistical significance was determined by Kruskal-Wallis test with η2 effect size statistic (symptom number), Mann-Whitney test with r effect size statistic (reduced performance) and Spearman's correlation (fatigue scoring). The total IP scores in the persistent somatic symptom number or physical performance strata are presented in violin plots; single observations are depicted as points, red diamonds with whiskers represent medians with interquartile ranges. The correlation with fatigue scoring is presented in a point plot; the blue line represents the fitted second-order trend and the gray ribbon depicts the 95% confidence intervals. Effect size statistic and p values are indicated in the plot captions. Numbers of complete observations are displayed in the plot captions or in the plot X axes. # PSS: number of persistent somatic symptoms at the one-year follow-up; CFS: Chalder's fatigue score; ECOG: Eastern Cooperative Oncology Group.
Fig. 4.
Key factors influencing the emotional representation,/concern /consequences component of illness perception.
Association of the total emotion/concern/consequences IP component score at the one-year follow-up with persistent somatic symptoms (A), rehabilitation status, cardiological and inflammatory abnormalities (B), and respiratory comorbidity and body weight (C). Statistical significance was assessed by Kruskal-Wallis test with η2 effect size statistic (number of symptoms), Spearman's correlation (fatigue scoring) and Mann-Whitney test with r effect size statistic (remaining independent variables).The correlation with fatigue scoring is presented in a point plot; the blue line represents the fitted second-order trend and the gray ribbon depicts the 95% confidence intervals. For the remaining independent variables, the score values are presented in violin plots with single observations depicted as points, and red diamonds and whiskers representing medians with interquartile ranges. Effect size statistic and p values are indicated in the plot captions. Numbers of complete observations are displayed in the plot captions or in the plot X axes. # PSS: number of persistent somatic symptoms at the one-year follow-up, CFS: Chalder's fatigue score; ECOG: Eastern Cooperative Oncology Group; CRP: serum C-reactive protein.
Age, gender, or abnormalities in chest computed tomography or lung function testing, or diastolic dysfunction alone had no significant effects on total IP or its components in uni-variable analysis (Supplementary Figs. S12 - S13). However, lung abnormalities in computed tomography at the one-year follow-up amplified the effects of diastolic dysfunction, fatigue and fatigue rating on total IP and the emotion/concern/consequences component, as revealed by significant interactions in two-way ANOVA. In particular, individuals with concomitant diastolic dysfunction plus lung computed tomography abnormalities or with high fatigue scoring plus lung computed tomography abnormalities displayed significantly higher emotion/concern/consequences scores than participants without lung computed tomography findings. Similar interaction effects of lung function testing deficits, fatigue and symptom numbers and of diastolic dysfunction and fatigue were discerned as well (Supplementary Figs. S14 - S16). Participants with respiratory comorbidities had significantly elevated fatigue scores, lung function testing abnormality rates, total IP and emotion/concern/consequences ratings as compared with the remaining collective. This may suggest additional interaction effects of respiratory conditions, lung function testing deficits and persistent fatigue on IP (Supplementary Table S7).
3.4. Illness perception clusters
IP patterns were investigated by unsupervised clustering of the study participants in respect to the BIPQ items. Among several algorithms tested, partition aroud medoids with Euclidean distance [57,58] demonstrated the best reproducibility [59] and good explanatory performance and was hence used for definition of three IP clusters (Supplementary Fig. S17). Roughly half of participants assigned to the cluster #1 displayed low scores for each BIPQ item. This translated to low total IP severity, and low scoring of the emotion/concern/consequences and lacking control/coherence IP components. Another 27% of participants assigned to the cluster #2 were characterized by low self-perceived personal or treatment control but also by low rating of the concern, identity, timeline, consequences and emotional representation items. As a result, the maximum scores of the lacking control/consequence components and low emotion/concern/consequences IP component scores were observed in the cluster #2. Cluster #3 individuals (22% of participants) had highly elevated rating of the concern, identity, timeline, consequences and emotional representation items as compared with the clusters #1 and #2. As a result, the cluster #3 displayed the highest total IP severity, and the highest emotion/concern/consequence ratings. In turn, the scoring of the lacking control/coherence IP component was low, suggestive of good understanding of the disease and trust in therapy or personal control of COVID-19 sequelae (Fig. 5 ). Effect sizes of differences in total IP and its components between the IP clusters were large (Kruskal-Wallis test, total IP: η2 = 0.56, p < .001, emotion/concern/consequences: η2 = 0.56, p < .001, lacking control/coherence: η2 = 0.49, p < .001).
Fig. 5.
Clusters of illness perception.
Three subsets of study participants (illness perception [IP] clusters) were identified by clustering in respect to the Brief Illness Perception Questionnaire items (BIPQ, Q1 - Q8) with the partitioning around medoids algorithm and Euclidean distance metric. Numbers of observations assigned to the clusters are displayed next to the plots or in the plot X axes.
(A) Mean BIPQ item scores at one year after COVID-19 in the IP clusters. Statistical significance was determined by Kruskal-Wallis test. P values are indicated below the item names. Lines represent mean values, tinted ribbons depict 2 × SEM (standard error of the mean) intervals.
(B) The total IP score, emotion/concern/consequences and the lacking control/coherence IP component scores in the IP clusters. Statistical significance was determined by Kruskal-Wallis test with η2 effect size statistic. Response values are presented in violin plots. Points represent single observations. Red diamonds and whiskers depict medians and interquartile ranges. Effect size statistic and p values are indicated in the plot captions.
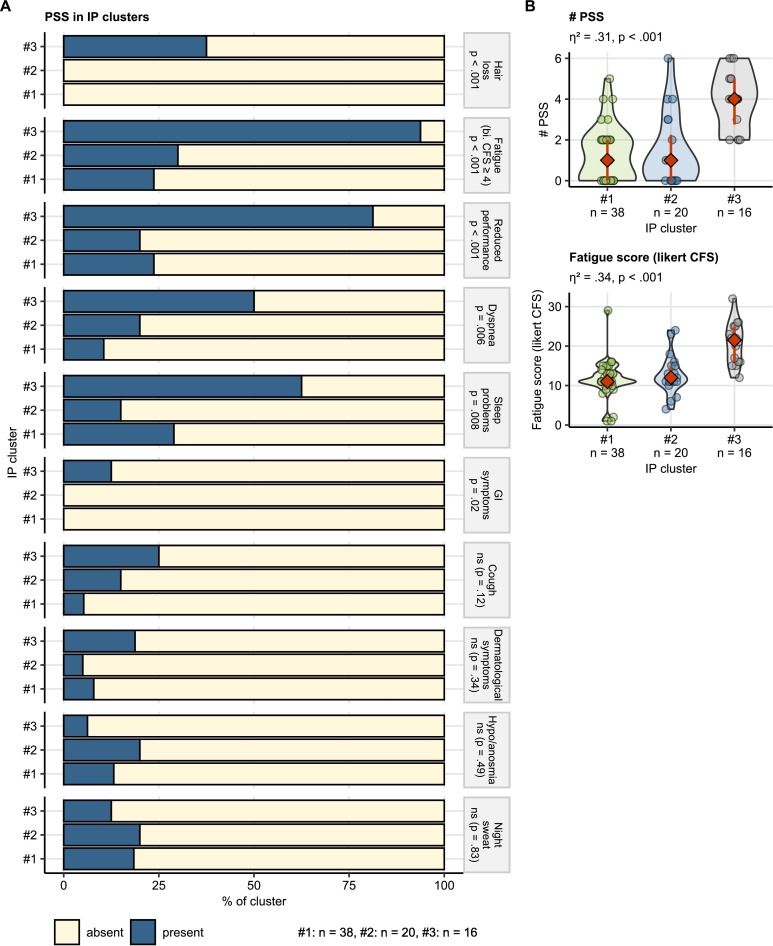
The cluster #1 individuals tended towards the lowest rates of smoking, metabolic and respiratory comorbidities (Supplementary Table S8). In the cluster #2 the lowest acute COVID-19 severity, rates of rehabilitation and lung computed tomography abnormality grades were observed. The cluster #2 tended also towards the lowest frequency of diastolic dysfunction. By contrast, in the cluster #3 affected by disease-related concerns, the frequency of severe COVID-19, COVID-19-specific rehabilitation rate, chest computed tomography finding severity and PSS frequency at the one-year follow-up were the highest (Fig. 6 ). In particular, cluster #3 individuals suffered from protracted fatigue, reduced physical performance, dyspnea and sleep problems to the highest extend, and hair loss and gastrointestinal complaints were observed solely in the cluster #3. Consequently, the PSS number and fatigue scoring peaked in the cluster #3. PSS frequencies and intensity were comparable in the clusters #1 and #2 (Fig. 7 ).
Fig. 6.
Course of COVID-19, rehabilitation and one-year follow-up sequelae of COVID-19 in the illness perception clusters.
COVID-19 severity and post-COVID-19 rehabilitation status (A), persistent somatic symptom and cardiopulmonary abnormality rates (B) and chest computed tomography abnormality severity (C) in the illness perception (IP) clusters. Statistical significance was assessed by Kruskal-Wallis test with η2 effect size statistic (numeric variables) or by χ2 test with Cramer V effect size statistic (categorical variables). Numeric variable values are presented in violin plots with single observations depicted as points, and red diamonds and whiskers representing medians with interquartile ranges. Categorical variable strata frequencies expressed as percentages within the IP cluster are presented in stack plots. Effect size statistic and p values are indicated in the plot captions or plot facets. Numbers of observations assigned to the clusters are displayed below the plots or in the plot X axes. A, HM, HS: ambulatory, hospitalized moderate and hospitalized severe acute COVID-19, PSS: persistent somatic symptoms, WHO COVID-19 severity: WHO ordinal scale for clinical improvement; LFT: lung function testing; CT: chest computed tomography.
Fig. 7.
Persistent somatic symptoms at the one-year follow-up in the illness perception clusters.
(A) Frequencies of persistent somatic symptoms (PSS) in the illness perception (IP) clusters. Statistical significance was determined by χ2 test. Percentages of individuals with and without the specific PSS within the IP cluster are presented in a stack plot. Significance is indicated next in the plot facets. Numbers of complete observations are displayed next to the plot. bi. CFS: bimodal Chalder's fatigue score; reduced performance: ECOG (Eastern Cooperative Oncology Group) ≥ 1.
(B) Numbers of PSS and fatigue scoring in the IP clusters. Statistical significance was determined by Kruskal-Wallis test with η2 effect size statistic. Response values are presented in violin plots. Points represent single observations. Red diamonds and whiskers depict medians and interquartile ranges. Effect size statistic and p values are indicated in the plot captions. CFS: Chalder's fatigue score; # PSS: number of persistent somatic symptoms.
Generally, differences between the IP clusters in PSS readouts like PSS number (Kruskal-Wallis test: η2 = 0.31, p < .001), fatigue rating (η2 = 0.34, p < .001), significant fatigue (χ 2 test: V = 0.57, p < .001), sleep problems (V = 0.36, p = .008) or hair loss (V = 0.57, p < .001) and differences in rehabilitation rates (V = 0.35, p = .01) were of moderate-to-large effect size. Effects of differences in lung computed tomography abnormality (V = 0.25, ns (p = .10)), chest computed tomography abnormality scoring (η2 = 0.09, p = .02), lung function testing deficits (V = 0.25, ns (p = .10)) or diastolic dysfunction (V = 0.24, ns (p = .12)) were substantially weaker (Supplementary Table S8).
4. Discussion
In our observational collective affected by PSS or abnormalities in cardiopulmonary tests one year after COVID-19, two IP facets were identified: an emotion/concern/consequences component, and a lacking control/coherence component. By multi-parameter modeling, we established signatures of total IP and the emotion/concern/consequences IP component. They included reduced physical performance, fatigue intensity and PSS number. The emotions/concerns/consequences IP component correlated additionally with significant fatigue, hair loss, COVID-19-specific rehabilitation, respiratory comorbidity, and, to a lesser degree, with diastolic dysfunction, elevated levels of the inflammatory marker C-reactive protein and obesity. Three IP clusters of study participants were identified based on the BIPQ items. Differences between the IP clusters in PPS readouts such as PSS number, fatigue rating or sleep problems were much more pronounced than differing rates of lung and heart abnormalities in computed tomography, lung function testing or echocardiography.
We identified fatigue, a leading persistent COVID-19 manifestation [2,7,10,11,21] as a strong covariate of total IP severity and the emotion/concern/consequences IP component. Similar effects were described in arthritis [17], hematological malignancy [60], and recently in post-COVID-19 condition [21]. Physical performance was found to be another key factor of IP and its emotion/concern/consequences component, in line with findings in hospitalized COVID-19 survivors, where reduced mobility was paralleled by anxiety and depression signs [9]. Objective readouts of good physical fitness in turn correlated with shorter symptom duration, lower PSS count, less fatigue and improved mental health in ambulatory COVID-19 patients [61]. Hair loss belongs to the most frequent post-COVID-19 condition manifestations [2,11,61] and may result from psychological stress and/or inflammation [62]. Hair loss, along with C-reactive protein, was linked by us with the emotion/concern/consequences IP component and may reflect an interplay between protracted inflammation and deteriorated mental health. Post-COVID-19 condition encompasses various respiratory symptoms [1,2,4,7,10,11,21]. In addition, COVID-19 was found to exacerbate symptoms and worsen disease control in asthma [63]. In our collective, respiratory comorbidity was associated with the emotion/concern/consequences IP component, higher fatigue rating and lung function testing abnormalities. Such superimposed subjective fatigue feeling and objective lung function deficits may explain more severe IP in individuals with respiratory comorbidity. We proposed PSS count as a strong covariate of IP severity and the emotion/concern/consequences IP component. In line with literature evidence [2,10,11,19], significantly more PSS at the one-year follow-up were discerned in females than male participants. However, IP rating was comparable between the genders. This may be explained by the less severe COVID-19 and less lung computed tomography abnormalities in females. Interestingly, we observed significant super-additive effects of fatigue, reduced physical performance plus lung computed tomography abnormalities on IP. Since pulmonary computed tomography findings affected nearly 75% of male participants, these interactions may influence IP in a male-specific manner and compensate for the higher PSS burden in females.
No explanatory factors affecting the lacking control/coherence IP component were identified by any of the modeling algorithms. This may reflect two non-exclusive phenomena. First, the internal consistency of the lacking control/coherence component was remarkably lower than consistency of the BIPQ tool or the emotion/concern/consequences component. This low reliability may attenuate observed associations [64] and hence obscure true effects of e.g. persistent somatic symptoms or cardiopulmonary findings on the lacking control/coherence component of IP. Second, the lacking control/coherence component covering cognitive IP aspects [30] may be influenced primarily by COVID-19-independent factors such as socioeconomic background, education level or follow-up care. Low socioeconomic status was proposed as a risk factor of post-COVID-19 condition [65] and can affect IP measured by BIPQ [66].
We identified three clusters of participants differing in key IP components. The cluster #1 encompassing roughly half of the participants displayed low total IP severity, good self-perceived coherence, personal and treatment control paralleled by low PSS rates. Another 27% of participants assigned to the cluster #2 enriched in moderate COVID-19 survivors with the lowest grade of lung computed tomography abnormalities showed a similarly low levels of PSS or fatigue. Yet their IP was hallmarked by poor disease understanding and disbelief in personal or treatment control. By contrast, the cluster #3 individuals suffered from multiple PSS such as significant fatigue, sleep problems, physical performance deficits or hair loss, had the highest rehabilitation rate and tended to have more residual lung lesions and severe acute COVID-19 course. Their IP was characterized by high severity of the emotion/concern/consequences IP component and, paradoxically, good coherence and belief in disease control. Emotional IP dimensions were correlated with shame, guilt, stress, depression and anxiety both in the general population during the pandemic [39] and COVID-19 [21,[40], [41], [42]]. Cluster #3 individuals may be at particular risk of COVID-19-related mental disorders and may profit most from psychological and psychiatric interventions, i.e. aiming at IP improvement [67]. Of note, differences in PSS and rehabilitation frequencies between the IP clusters were way stronger than differences in cardiopulmonary abnormalities, which corroborates our modeling results. Rehabilitation associated by us with high emotion/concern/consequences IP scoring may reflect physical and social disability following COVID-19. However, rehabilitation might have helped cluster #3 individuals to understand the disease and foster trust in treatment and personal control. As inferred from high rates of smoking, metabolic and respiratory comorbidities, the clusters #2 and #3 may include patients with lower socioeconomic status, which likely contributed to the more severe IP in these two subsets [65,66].
Collectively, we demonstrate an important effect of PSS on severity and quality of IP at the one-year follow-up which was found to outweigh the effects of objective lung and heart abnormalities. Yet, our data suggests that particular PSS such as fatigue or physical performance loss may be amplified by cardiopulmonary sequelae at shaping negative IP. Mounting evidence suggests that post-COVID-19 condition occurs independently of COVID-19 severity, cardiopulmonary findings, biochemical and immunity markers [4,19], and proposes demographic, acute disease-related [2,10,11,19], mental health and mental stress [5,6,19,22] as explanatory factors. Hence, a positive feedback between IP, mental health and PSS may contribute to post-COVID-19 syndrome [5,19,21] in analogy to somatic symptom disorders [23,68,69]. Additionally, IP may be affected by comorbidities, SARS-CoV-2-independent somatic symptoms, social and economic consequences of the pandemic [[24], [25], [26],28,29]. The symptom kinetics in our cohort [4,13] showed an early rapid recovery followed by a plateau and/or relapse at 3–12 months after COVID-19. This indicates acute SARS-CoV-2 infection as a likely trigger and reflects pathogen clearance in early convalescence. Yet, it is likely that COVID-19-independent factors contribute to the stalled PSS recovery and recurrence during later convalescence.
Our study bears limitations. The most important one is the low participant number and substantial dropout due to missing follow-up visits and the BIPQ answers. This limitation was partly addressed by the regularized regression modeling methodology described as robust in low power, high dimension datasets [54,55]. Yet the IP clusters require validation in a larger collective. As inferred from the substantially lower severity COVID-19 severity in excluded individuals, the analysis inclusion process may have resulted in a selection bias towards moderate-to-severe COVID-19. Furthermore, longitudinal IP rating or a general population control [19,36,37,39,70] would allow us to assess temporal IP changes during recovery, IP-influencing factors at consecutive time points and disease-independent effects [24,25,29]. The study collective was recruited during the first outbreak of wild-type SARS-CoV-2. We were hence unable to assess the effects of improved treatment, prevention and vaccination [38], and of virus variants differing in pathogenicity and symptom spectrum. The study variable set lacks potentially vital parameters for IP such as family status, education and COVID-19 knowledge [27], media consumption [28], quarantine duration [42] and socioeconomic status [65]. At design of our study initially conceptualized as a cardiopulmonary observation cohort [1], the multi-organ spectrum of COVID-19 symptoms was unknown. For this reason, multiple PSS of relevance for mental health and IP like neuro-cognitive symptoms [5,11,21] were not surveyed.
5. Conclusion
One year after COVID, persistent somatic symptoms such as fatigue or physical performance loss impact on severity and quality of IP to a higher degree than lung and heart abnormalities revealed by a systematic clinical assessment. Hence, besides COVID-19 severity, individual IP should be addressed when allocating rehabilitation, psychological or psychiatric resources. Interventions addressing specifically unhelpful IP are a promising approach in treatment of post-COVID-19 condition.
Author's contribution
KH, SS, RH, BP, Günter Weiss, Gerlig Widmann, TS, IT, BSU and JLR designed the study; KH, SS, AL, AB, AP, CS, SK, KK, MA, BMF, BP, VR, AS, SI, AE, EW collected the study data; KH, PT, SS and JLR analyzed the data and wrote the manuscript, IT and JLR acquired the funding. All authors reviewed and approved the final version of the manuscript.
Data and code availability
An R data (RDa) file with anonymized patient data will be made available upon request to the corresponding author. The study analysis pipeline is available at https://github.com/PiotrTymoszuk/CovILD-IPQ.
Declaration of Competing Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Katharina Hüfner has received research grants from Austria Wirtchaftsservice (AWS) and the State of Tyrol as well as lecturer's honoraria from Forum Medizinische Fortbildung (FOMF), the Anton Proksch Institute and the Hospital of Schwaz. Piotr Tymoszuk owns a data science company, Data Analytics as a Service Tirol, and receives payments from statistical data analysis, bioinformatic and scientific writing services. Other authors declare that no conflict of interest exists.
Acknowledgments
We acknowledge commitment of the study participants and the medical staff during the COVID-19 pandemic. The study was funded by the research fund of the State of Tyrol (Project GZ 71934, to Judith Löffler-Ragg) and an Investigator-Initiated Study grant by Boehringer Ingelheim (IIS 1199-0424 to Ivan Tancevski). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2023.111234.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V., Boehm A., Aichner M., Tymoszuk P., Lener D., Theurl M., Lorsbach-Köhler A., Tancevski A., Schapfl A., Schaber M., Hilbe R., Nairz M., Puchner B., Hüttenberger D., Tschurtschenthaler C., Aßhoff M., Peer A., Hartig F., Bellmann R., Joannidis M., Gollmann-Tepeköylü C., Holfeld J., Feuchtner G., Egger A., Hoermann G., Schroll A., Fritsche G., Wildner S., Bellmann-Weiler R., Kirchmair R., Helbok R., Prosch H., Rieder D., Trajanoski Z., Kronenberg F., Wöll E., Weiss G., Widmann G., Löffler-Ragg J., Tancevski I. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahanic S., Tymoszuk P., Ausserhofer D., Rass V., Pizzini A., Nordmeyer G., Hüfner K., Kurz K., Weber P.M., Sonnweber T., Boehm A., Aichner M., Cima K., Boeckle B., Holzner B., Rumpold G., Puelacher C., Kiechl S., Huber A., Wiedermann C.J., Sperner-Unterweger B., Tancevski I., Bellmann-Weiler R., Bachler H., Piccoliori G., Helbok R., Weiss G., Loeffler-Ragg J. Phenotyping of acute and persistent coronavirus disease 2019 Features in the outpatient setting: exploratory analysis of an international cross-sectional online survey. Clin. Infect. Dis. Off. Pub. Infect. Dis. Soc. Am. 2022;75:e418–e431. doi: 10.1093/cid/ciab978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luger A.K., Sonnweber T., Gruber L., Schwabl C., Cima K., Tymoszuk P., Gerstner A.K., Pizzini A., Sahanic S., Boehm A., Coen M., Strolz C.J., Wöll E., Weiss G., Kirchmair R., Feuchtner G.M., Prosch H., Tancevski I., Löffler-Ragg J., Widmann G. Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology. 2022;304:462–470. doi: 10.1148/radiol.211670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnweber T., Tymoszuk P., Sahanic S., Boehm A., Pizzini A., Luger A., Schwabl C., Nairz M., Grubwieser P., Kurz K., Koppelstätter S., Aichner M., Puchner B., Egger A., Hoermann G., Wöll E., Weiss G., Widmann G., Tancevski I., Löffler-Ragg J. Investigating phenotypes of pulmonary COVID-19 recovery: A longitudinal observational prospective multicenter trial. eLife. 2022;11 doi: 10.7554/ELIFE.72500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hüfner K., Tymoszuk P., Ausserhofer D., Sahanic S., Pizzini A., Rass V., Galffy M., Böhm A., Kurz K., Sonnweber T., Tancevski I., Kiechl S., Huber A., Plagg B., Wiedermann C.J., Bellmann-Weiler R., Bachler H., Weiss G., Piccoliori G., Helbok R., Loeffler-Ragg J., Sperner-Unterweger B. Who is at risk of poor mental health following coronavirus disease-19 outpatient management? Front. Med. 2022;9 doi: 10.3389/fmed.2022.792881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 7.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. Vol. 22. 2022. A clinical case definition of post-COVID-19 condition by a Delphi consensus; pp. e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans R.A., McAuley H., Harrison E.M., Shikotra A., Singapuri A., Sereno M., Elneima O., Docherty A.B., Lone N.I., Leavy O.C., Daines L., Baillie J.K., Brown J.S., Chalder T., De Soyza A., Bakerly N. Diar, Easom N., Geddes J.R., Greening N.J., Hart N., Heaney L.G., Heller S., Howard L., Hurst J.R., Jacob J., Jenkins R.G., Jolley C., Kerr S., Kon O.M., Lewis K., Lord J.M., McCann G.P., Neubauer S., Openshaw P.J.M., Parekh D., Pfeffer P., Rahman N.M., Raman B., Richardson M., Rowland M., Semple M.G., Shah A.M., Singh S.J., Sheikh A., Thomas D., Toshner M., Chalmers J.D., Ho L.P., Horsley A., Marks M., Poinasamy K., Wain L.V., Brightling C.E., Abel K., Adamali H., Adeloye D., Adeyemi O., Adeyemi F., Ahmad S., Ahmed R., Ainsworth M., Alamoudi A., Aljaroof M., Allan L., Allen R., Alli A., Al-Sheklly B., Altmann D., Anderson D., Andrews M., Angyal A., Antoniades C., Arbane G., Armour C., Armstrong N., Armstrong L., Arnold H., Arnold D., Ashworth M., Ashworth A., Assefa-Kebede H., Atkin P., Atkins H., Atkins A., Aul R., Avram C., Baggott R., Baguley D., Baillie J.K., Bain S., Bakali M., Bakau M., Baldry E., Baldwin D., Ballard C., Bambrough J., Barker R.E., Barratt S., Barrett F., Basu N., Batterham R., Baxendale H., Bayes H., Bayley M., Beadsworth M., Beirne P., Bell R., Bell D., Berry C., Betts S., Bhui K., Bishop L., Blaikely J., Bloomfield C., Bloss A., Bolger A., Bolton C.E., Bonnington J., Botkai A., Bourne M., Bourne C., Bradley E., Bramham K., Brear L., Breen G., Breeze J., Briggs A., Bright E., Brightling C.E., Brill S., Brindle K., Broad L., Broome M., Brown J.S., Brown M., Brown J., Brown R., Brown V., Brown A., Brugha T., Brunskill N., Buch M., Bularga A., Bullmore E., Burn D., Burns G., Busby J., Buttress A., Byrne S., Cairns P., Calder P.C., Calvelo E., Card B., Carr L., Carson G., Carter P., Cavanagh J., Chalder T., Chalmers J.D., Chambers R.C., Channon K., Chapman K., Charalambou A., Chaudhuri N., Checkley A., Chen J., Chetham L., Chilvers E.R., Chinoy H., Chong-James K., Choudhury N., Choudhury G., Chowdhury P., Chowienczyk P., Christie C., Clark D., Clark C., Clarke J., Clift P., Clohisey S., Coburn Z., Cole J., Coleman C., Connell D., Connolly B., Connor L., Cook A., Cooper B., Coupland C., Craig T., Crisp P., Cristiano D., Crooks M.G., Cross A., Cruz I., Cullinan P., Daines L., Dalton M., Dark P., Dasgin J., David A., David C., Davies M., Davies G., Davies K., Davies F., Davies G.A., Daynes E., De Silva T., De Soyza A., Deakin B., Deans A., Defres S., Dell A., Dempsey K., Dennis J., Dewar A., Dharmagunawardena R., Bakerly N. Diar, Dipper A., Diver S., Diwanji S.N., Dixon M., Djukanovic R., Dobson H., Dobson C., Dobson S.L., Docherty A.B., Donaldson A., Dong T., Dormand N., Dougherty A., Dowling R., Drain S., Dulawan P., Dunn S., Easom N., Echevarria C., Edwards S., Edwardson C., Elliott B., Elliott A., Ellis Y., Elmer A., Elneima O., Evans R.A., Evans J., Evans H., Evans D., Evans R.I., Evans R., Evans T., Fabbri L., Fairbairn S., Fairman A., Fallon K., Faluyi D., Favager C., Felton T., Finch J., Finney S., Fisher H., Fletcher S., Flockton R., Foote D., Ford A., Forton D., Francis R., Francis S., Francis C., Frankel A., Fraser E., Free R., French N., Fuld J., Furniss J., Garner L., Gautam N., Geddes J.R., George P.M., George J., Gibbons M., Gilmour L., Gleeson F., Glossop J., Glover S., Goodman N., Gooptu B., Gorsuch T., Gourlay E., Greenhaff P., Greenhalf W., Greenhalgh A., Greening N.J., Greenwood J., Greenwood S., Gregory R., Grieve D., Gummadi M., Gupta A., Gurram S., Guthrie E., Hadley K., Haggar A., Hainey K., Haldar P., Hall I., Hall L., Halling-Brown M., Hamil R., Hanley N.A., Hardwick H., Hardy E., Hargadon B., Harrington K., Harris V., Harrison E.M., Harrison P., Hart N., Harvey A., Harvey M., Harvie M., Havinden-Williams M., Hawkes J., Hawkings N., Haworth J., Hayday A., Heaney L.G., Heeney J.L., Heightman M., Heller S., Henderson M., Hesselden L., Hillman T., Hingorani A., Hiwot T., Ho L.P., Hoare A., Hoare M., Hogarth P., Holbourn A., Holdsworth L., Holgate D., Holmes K., Holroyd-Hind B., Horsley A., Hosseini A., Hotopf M., Houchen L., Howard L., Howell A., Hufton E., Hughes A., Hughes J., Hughes R., Humphries A., Huneke N., Hurst J.R., Hurst R., Husain M., Hussell T., Ibrahim W., Ient A., Ingram L., Ismail K., Jackson T., Jacob J., James W.Y., Janes S., Jarvis H., Jayaraman B., Jenkins R.G., Jezzard P., Jiwa K., Johnson S., Johnson C., Johnston D., Jolley C., Jolley C.J., Jones I., Jones S., Jones D., Jones H., Jones G., Jones M., Jose S., Kabir T., Kaltsakas G., Kamwa V., Kar P., Kausar Z., Kelly S., Kerr S., Key A.L., Khan F., Khunti K., King C., King B., Kitterick P., Klenerman P., Knibbs L., Knight S., Knighton A., Kon O.M., Kon S., Kon S.S., Korszun A., Kotanidis C., Koychev I., Kurupati P., Kwan J., Laing C., Lamlum H., Landers G., Langenberg C., Lasserson D., Lawrie A., Lea A., Leavy O.C., Lee D., Lee E., Leitch K., Lenagh R., Lewis K., Lewis V., Lewis K.E., Lewis J., Lewis-Burke N., Light T., Lightstone L., Lim L., Linford S., Lingford-Hughes A., Lipman M., Liyanage K., Lloyd A., Logan S., Lomas D., Lone N.I., Loosley R., Lord J.M., Lota H., Lucey A., MacGowan G., Macharia I., Mackay C., Macliver L., Madathil S., Madzamba G., Magee N., Mairs N., Majeed N., Major E., Malim M., Mallison G., Man W., Mandal S., Mangion K., Mansoori P., Marciniak S., Mariveles M., Marks M., Marshall B., Martineau A., Maskell N., Matila D., Matthews L., Mayet J., McAdoo S., McAllister-Williams H., McArdle P., McArdle A., McAulay D., McAuley H., McAuley D.F., McCafferty K., McCann G.P., McCauley H., McCourt P., Mcgarvey L., McGinness J., McGovern A., McGuinness H., McInnes I.B., McIvor K., McIvor E., McMahon A., McMahon M.J., McMorrow L., Mcnally T., McNarry M., McQueen A., McShane H., Megson S., Meiring J., Menzies D., Michael A., Milligan L., Mills N., Mitchell J., Mohamed A., Molyneaux P.L., Monteiro W., Morley A., Morrison L., Morriss R., Morrow A., Moss A., Moss A.J., Moss P., Mukaetova-Ladinska E., Munawar U., Murali E., Murira J., Nassa H., Neill P., Neubauer S., Newby D., Newell H., Cox A. Newton, Nicholson T., Nicoll D., Nolan C.M., Noonan M.J., Novotny P., Nunag J., Nyaboko J., O’Brien L., Odell N., Ogg G., Olaosebikan O., Oliver C., Omar Z., Openshaw P.J.M., Rivera-Ortega P., Osbourne R., Ostermann M., Overton C., Oxton J., Pacpaco E., Paddick S., Papineni P., Paradowski K., Pareek M., Parekh D., Parfrey H., Pariante C., Parker S., Parkes M., Parmar J., Parvin R., Patale S., Patel B., Patel S., Patel M., Pathmanathan B., Pavlides M., Pearl J.E., Peckham D., Pendlebury J., Peng Y., Pennington C., Peralta I., Perkins E., Peto T., Petousi N., Petrie J., Pfeffer P., Phipps J., Pimm J., Hanley K. Piper, Pius R., Plein S., Plekhanova T., Poinasamy K., Polgar O., Poll L., Porter J.C., Portukhay S., Powell N., Price L., Price D., Price A., Price C., Prickett A., Quaid S., Quigley J., Quint J., Qureshi H., Rahman N., Rahman M., Ralser M., Raman B., Ramos A., Rangeley J., Rees T., Regan K., Richards A., Richardson M., Robertson E., Rodgers J., Ross G., Rossdale J., Rostron A., Routen A., Rowland A., Rowland M.J., Rowland J., Rowland-Jones S.L., Roy K., Rudan I., Russell R., Russell E., Sabit R., Sage E.K., Samani N., Samuel R., Sapey E., Saralaya D., Saratzis A., Sargeant J., Sass T., Sattar N., Saunders K., Saunders R., Saxon W., Sayer A., Schwaeble W., Scott J., Scott K., Selby N., Semple M.G., Sereno M., Shah K., Shah A., Shah P., Sharma M., Sharpe M., Sharpe C., Shaw V., Sheikh A., Shevket K., Shikotra A., Short J., Siddiqui S., Sigfrid L., Simons G., Simpson J., Singapuri A., Singh S.J., Singh C., Singh S., Skeemer J., Smith I., Smith J., Smith L., Smith A., Soares M., Southern D., Spears M., Spencer L.G., Speranza F., Stadon L., Stanel S., Steiner M., Stensel D., Stern M., Stewart I., Stockley J., Stone R., Storrie A., Storton K., Stringer E., Subbe C., Sudlow C., Suleiman Z., Summers C., Summersgill C., Sutherland D., Sykes D.L., Sykes R., Talbot N., Tan A.L., Taylor C., Taylor A., Te A., Tedd H., Tee C.J., Tench H., Terry S., Thackray-Nocera S., Thaivalappil F., Thickett D., Thomas D., Thomas D.C., Thomas A.K., Thompson A.A.R., Thompson T., Thornton T., Thwaites R.S., Tobin M., Toingson G.F., Tong C., Toshner M., Touyz R., Tripp K.A., Tunnicliffe E., Turner E., Turtle L., Turton H., Ugwuoke R., Upthegrove R., Valabhji J., Vellore K., Wade E., Wain L.V., Wajero L.O., Walder S., Walker S., Wall E., Wallis T., Walmsley S., Walsh S., Walsh J.A., Watson L., Watson J., Watson E., Welch C., Welch H., Welsh B., Wessely S., West S., Wheeler H., Whitehead V., Whitney J., Whittaker S., Whittam B., Wild J., Wilkins M., Wilkinson D., Williams N., Williams B., Williams J., Williams-Howard S.A., Willicombe M., Willis G., Wilson D., Wilson I., Window N., Witham M., Wolf-Roberts R., Woodhead F., Woods J., Wootton D., Worsley J., Wraith D., Wright L., Wright C., Wright S., Xie C., Yasmin S., Yates T., Yip K.P., Young B., Young S., Young A., Yousuf A.J., Yousuf A., Zawia A., Zhao B., Zongo O. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., Merino J., Tsereteli N., Fall T., Gomez M.F., Duncan E.L., Menni C., Williams F.M.K., Franks P.W., Chan A.T., Wolf J., Ourselin S., Spector T., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27 doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J. Infect. Dis. 2022;226:1593–1607. doi: 10.1093/INFDIS/JIAC136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCorkell L., Assaf G.S., Davis H.E., Wei H., Akrami A. Vol. 6. 2021. Patient-led research collaborative: Embedding patients in the Long COVID narrative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rass V., Tymoszuk P., Sahanic S., Heim B., Ausserhofer D., Lindner A., Kofler M., Mahlknecht P., Boehm A., Hüfner K., Pizzini A., Sonnweber T., Kurz K., Pfeifer B., Kiechl S., Peball M., Kindl P., Putnina L., Fava E., Djamshidian A., Huber A., Wiedermann C.J., Sperner-Unterweger B., Wöll E., Beer R., Schiefecker A.J., Bellmann-Weiler R., Bachler H., Tancevski I., Pfausler B., Piccoliori G., Seppi K., Weiss G., Löffler-Ragg J., Helbok R. Distinct smell and taste disorder phenotype of post-acute COVID-19 sequelae. medRxiv. 2022 doi: 10.1101/2022.06.02.22275932. 2022.06.02.22275932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballering A.V., van Zon S.K.R., Hartman T.C. Olde, Rosmalen J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miravitlles M., Ferrer J., Baró E., Lleonart M., Galera J. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir. Med. 2013;107:1977–1985. doi: 10.1016/j.rmed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M.N., Rimland C.A., Quinn K.A., Ferrada M.A., Gribbons K.B., Rosenblum J.S., Goodspeed W., Novakovich E., Grayson P.C. Utility of the brief illness perception questionnaire to monitor patient beliefs in systemic vasculitis. J. Rheumatol. 2020;47:1785–1792. doi: 10.3899/jrheum.190828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher S., Rief W., Brähler E., Martin A., Glaesmer H., Mewes R. Disagreement in doctor’s and patient’s rating about medically unexplained symptoms and health care use. Int. J. Behav. Med. 2013;20:30–37. doi: 10.1007/s12529-011-9213-2. [DOI] [PubMed] [Google Scholar]
- 19.Sneller M.C., Jason Liang C., Marques A.R., Chung J.Y., Shanbhag S.M., Fontana J.R., Raza H., Okeke O., Dewar R.L., Higgins B.P., Tolstenko K., Kwan R.W., Gittens K.R., Seamon C.A., McCormack G., Shaw J.S., Okpali G.M., Law M., Trihemasava K., Kennedy B.D., Shi V., Shawn Justement J., Buckner C.M., Blazkova J., Moir S., Chun T.W., Clifford Lane H. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann. Intern. Med. 2022;175:969–979. doi: 10.7326/M21-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann M., Pohontsch N.J., Zimmermann T., Scherer M., Löwe B. Diagnostic and treatment barriers to persistent somatic symptoms in primary care – representative survey with physicians. BMC Fam. Pract. 2021;22 doi: 10.1186/s12875-021-01397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierbauer W., Lüscher J., Scholz U. Illness perceptions in long-COVID: a cross-sectional analysis in adults. Cogent Psychol. 2022;9 doi: 10.1080/23311908.2022.2105007. [DOI] [Google Scholar]
- 22.Wang S., Quan L., Chavarro J.E., Slopen N., Kubzansky L.D., Koenen K.C., Kang J.H., Weisskopf M.G., Branch-Elliman W., Roberts A.L. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post–COVID-19 conditions. JAMA Psychiatry. 2022;79:1081–1091. doi: 10.1001/JAMAPSYCHIATRY.2022.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachaner A., Lemogne C., Dave J., Ranque B., De Broucker T., Meppiel E. Somatic symptom disorder in patients with post-COVID-19 neurological symptoms: a preliminary report from the somatic study (somatic symptom disorder triggered by COVID-19) J. Neurol. Neurosurg. Psychiatry. 2022;93:1174–1180. doi: 10.1136/jnnp-2021-327899. [DOI] [PubMed] [Google Scholar]
- 24.Wisk L.E., Gottlieb M.A., Spatz E.S., Yu H., Wang R.C., Slovis B.H., Saydah S., Plumb I.D., O’Laughlin K.N., Montoy J.C.C., McDonald S.A., Lin Z., Lin J.M.S., Koo K., Idris A.H., Huebinger R.M., Hill M.J., Gentile N.L., Chang A.M., Anderson J., Hota B., Venkatesh A.K., Weinstein R.A., Elmore J.G., Nichol G. Association of initial SARS-CoV-2 test positivity with patient-reported well-being 3 months after a symptomatic illness. JAMA Netw. Open. 2022;5 doi: 10.1001/JAMANETWORKOPEN.2022.44486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behnood S.A., Shafran R., Bennett S.D., Zhang A.X.D., O’Mahoney L.L., Stephenson T.J., Ladhani S.N., De Stavola B.L., Viner R.M., Swann O.V. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J. Infect. 2022;84:158–170. doi: 10.1016/J.JINF.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matta J., Wiernik E., Robineau O., Carrat F., Touvier M., Severi G., De Lamballerie X., Blanché H., Deleuze J.F., Gouraud C., Hoertel N., Ranque B., Goldberg M., Zins M., Lemogne C. Association of Self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern. Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man Y., Chen S.H., Li X.T., Jin H., Mei R.R., Qiu T.Y., Li Y.M., Zhang H.L., Chen Q.N., Xie C.Y., Cheng Y.H., Zhou J.W. The effects of disease-related knowledge on illness perception and psychological status of patients with COVID-19 in Hunan, China. Disaster Med. Public Health Preparedness. 2021;16:1415–1422. doi: 10.1017/dmp.2021.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tutzer F., Frajo-Apor B., Pardeller S., Plattner B., Chernova A., Haring C., Holzner B., Kemmler G., Marksteiner J., Miller C., Schmidt M., Sperner-Unterweger B., Hofer A. Psychological distress, loneliness, and boredom among the general population of Tyrol, Austria during the COVID-19 pandemic. Front. Psychiatry. 2021;12:921. doi: 10.3389/fpsyt.2021.691896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz A., Ernst J., Glaesmer H., Brähler E., Rauscher F.G., Petrowski K., Kocalevent R.D. Frequency of somatic symptoms in the general population: normative values for the patient health Questionnaire-15 (PHQ-15) J. Psychosom. Res. 2017;96:27–31. doi: 10.1016/J.JPSYCHORES.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Broadbent E., Petrie K.J., Main J., Weinman J. The Brief Illness Perception Questionnaire. J. Psychosom. Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Weinman J., Petrie K.J., Moss-Morris R., Horne R. The illness perception questionnaire: a new method for assessing the cognitive representation of illness. Psychol. Health. 1996;11:431–445. doi: 10.1080/08870449608400270. [DOI] [Google Scholar]
- 32.Leventhal H., Phillips L.A., Burns E. The common-sense model of self-regulation (CSM): a dynamic framework for understanding illness self-management. J. Behav. Med. 2016;39:935–946. doi: 10.1007/s10865-016-9782-2. [DOI] [PubMed] [Google Scholar]
- 33.Diefenbach M.A. The common-sense model of illness representation: theoretical and practical considerations. J. Soc. Distress Homeless. 1996;5:11–38. doi: 10.1007/BF02090456. [DOI] [Google Scholar]
- 34.Hagger M.S., Orbell S. Vol. 18. 2003. A meta-analytic review of the common-sense model of illness representations; pp. 141–184. [DOI] [Google Scholar]
- 35.Figueiras M.J., Neto D.D. “Tailoring” Adjustment: New Ways of Improving the Response to Chronic Conditions. Vol. 24. 2019. Challenges; pp. 1–6. [DOI] [Google Scholar]
- 36.Dias Neto D., Nunes da Silva A., Roberto M.S., Lubenko J., Constantinou M., Nicolaou C., Lamnisos D., Papacostas S., Höfer S., Presti G., Squatrito V., Vasiliou V.S., McHugh L., Monestès J.L., Baban A., Alvarez-Galvez J., Paez-Blarrina M., Montesinos F., Valdivia-Salas S., Ori D., Lappalainen R., Kleszcz B., Gloster A., Karekla M., Kassianos A.P. Illness perceptions of COVID-19 in Europe: predictors, impacts and temporal evolution. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.640955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lugo-González I.V., Fernández-Vega M., Reynoso-Erazo L., Becerra-Gálvez A.L., Pérez-Bautista Y.Y., Lugo-González I.V., Fernández-Vega M., Reynoso-Erazo L., Becerra-Gálvez A.L., Pérez-Bautista Y.Y. COVID-19 perception and preventive behaviors: a descriptive, comparative study by severity and perceived risk. Salud Mental. 2020;43:285–292. doi: 10.17711/SM.0185-3325.2020.039. [DOI] [Google Scholar]
- 38.Vollmann M., Salewski C. To get vaccinated, or not to get vaccinated, that is the question: illness representations about covid-19 and perceptions about covid-19 vaccination as predictors of covid-19 vaccination willingness among young adults in the Netherlands. Vaccines. 2021;9:941. doi: 10.3390/vaccines9090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierenga K.L., Moore S.E., Pressler S.J., Hacker E.D., Perkins S.M. Associations between COVID-19 perceptions, anxiety, and depressive symptoms among adults living in the United States. Nurs. Outlook. 2021;69:755. doi: 10.1016/J.OUTLOOK.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeghian E., Bashirian S., Soltanian A., Taheri M., Madineshat M. Relashionship between mental health, perception of illness and perceived social support in hospitalized patients with COVID-19. Health Edu. Health Promot. 2022;10:333–340. https://hehp.modares.ac.ir/article-5-55003-en.html [Google Scholar]
- 41.Hamama L., Levin-Dagan N. People who contracted COVID-19: the mediating role of shame and guilt in the link between threatening illness perception and mental health measures. Anxiety Stress Coping. 2022;35:72–85. doi: 10.1080/10615806.2021.1964073. [DOI] [PubMed] [Google Scholar]
- 42.Burke T., Berry A., Taylor L.K., Stafford O., Murphy E., Shevlin M., McHugh L., Carr A. Increased psychological distress during COVID-19 and quarantine in Ireland: a national survey. J. Clin. Med. 2020;9:1–15. doi: 10.3390/JCM9113481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalder T., Berelowitz G., Pawlikowska T., Watts L., Wessely S., Wright D., Wallace E.P. Development of a fatigue scale. J. Psychosom. Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-P. [DOI] [PubMed] [Google Scholar]
- 44.Morriss R.K., Wearden A.J., Mullis R. Exploring the validity of the chalder fatigue scale in chronic fatigue syndrome. J. Psychosom. Res. 1998;45:411–417. doi: 10.1016/S0022-3999(98)00022-1. [DOI] [PubMed] [Google Scholar]
- 45.Crapo R.O., Casaburi R., Coates A.L., Enright P.L., MacIntyre N.R., McKay R.T., Johnson D., Wanger J.S., Zeballos R.J., Bittner V., Mottram C. Vol. 166. 2002. ATS statement: Guidelines for the six-minute walk test; pp. 111–117. [DOI] [Google Scholar]
- 46.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Vol. 246. 2008. Fleischner Society: Glossary of terms for thoracic imaging; pp. 697–722. [DOI] [PubMed] [Google Scholar]
- 47.Bartlett M.S. The statistical conception of mental factors. British J. Psychol. General Section. 1937;28:97–104. doi: 10.1111/j.2044-8295.1937.tb00863.x. [DOI] [Google Scholar]
- 48.McDonald R.P. 1st Editio. Psychology Press; New York: 1999. Test theory: A Unified Treatment. [DOI] [Google Scholar]
- 49.Zou H., Hastie T. Regularization and variable selection via the elastic net. J. Royal Stat. Soc. Series B: Stat. Methodol. 2005;67:301–320. doi: 10.1111/j.1467-9868.2005.00503.x. [DOI] [Google Scholar]
- 50.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tibshirani R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Methodol. 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- 52.Park T., Casella G. The Bayesian lasso. J. Am. Stat. Assoc. 2008;103:681–686. doi: 10.1198/016214508000000337. [DOI] [Google Scholar]
- 53.Gramacy R.B. monomvn: Estimation for MVN and Student-t Data with Monotone Missingness. 2022. https://cran.r-project.org/web/packages/monomvn/index.html
- 54.Kirpich A., Ainsworth E.A., Wedow J.M., Newman J.R.B., Michailidis G., McIntyre L.M. Variable selection in omics data: a practical evaluation of small sample sizes. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morozova O., Levina O., Uusküla A., Heimer R. Comparison of subset selection methods in linear regression in the context of health-related quality of life and substance abuse in Russia. BMC Med. Res. Methodol. 2015;15:1–17. doi: 10.1186/s12874-015-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn M. Building predictive models in R using the caret package. J. Stat. Softw. 2008;28:1–26. doi: 10.18637/jss.v028.i05. [DOI] [Google Scholar]
- 57.Schubert E., Rousseeuw P.J. Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) Springer; 2019. Faster k-medoids clustering: improving the PAM, CLARA, and CLARANS algorithms; pp. 171–187. [DOI] [Google Scholar]
- 58.Drost H.-G. Philentropy: information theory and distance quantification with R. J. Open Source Softw. 2018;3:765. doi: 10.21105/joss.00765. [DOI] [Google Scholar]
- 59.Lange T., Roth V., Braun M.L., Buhmann J.M. Stability-based validation of clustering solutions. Neural Comput. 2004;16:1299–1323. doi: 10.1162/089976604773717621. [DOI] [PubMed] [Google Scholar]
- 60.Schoormans D., Jansen M., Mols F., Oerlemans S. Negative illness perceptions are related to more fatigue among haematological cancer survivors: a PROFILES study. Acta Oncol. 2020;59:959–966. doi: 10.1080/0284186X.2020.1759823. [DOI] [PubMed] [Google Scholar]
- 61.Jimeno-Almazán A., Martínez-Cava A., Buendía-Romero Á., Franco-López F., Sánchez-Agar J.A., Sánchez-Alcaraz B.J., Tufano J.J., Pallarés J.G., Courel-Ibáñez J. Relationship between the severity of persistent symptoms, physical fitness, and cardiopulmonary function in post-COVID-19 condition. A population-based analysis. Intern. Emerg. Med. 2022;17:2199–2208. doi: 10.1007/s11739-022-03039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sattur S.S., Sattur I.S. Vol. 54. 2021. COVID-19 Infection: Impact on Hair; pp. 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philip K.E.J., Buttery S., Williams P., Vijayakumar B., Tonkin J., Cumella A., Renwick L., Ogden L., Quint J.K., Johnston S.L., Polkey M.I., Hopkinson N.S. Impact of COVID-19 on people with asthma: a mixed methods analysis from a UK wide survey. BMJ Open Resp. Res. 2022;9 doi: 10.1136/BMJRESP-2021-001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spearman C. The proof and measurement of association between two things. Am. J. Psychol. 1904;15:72. doi: 10.2307/1412159. [DOI] [PubMed] [Google Scholar]
- 65.Dumont R., Richard V., Lorthe E., Loizeau A., Pennacchio F., Zaballa M.E., Baysson H., Nehme M., Perrin A., L’Huillier A.G., Kaiser L., Barbe R.P., Posfay-Barbe K.M., Stringhini S., Amrein D., Azman A.S., Bal A., Balavoine M., Berthelot J., Bleich P., Boehm L., Bryand G., Bucolli V., Collombet P., Cudet A., Davidovic V., de Mestral Vargas C., D’Ippolito P., Dubos R., Eckerle I., Favier M., El Merjani N., Francioli N., Graindorge C., Harnal S., Hurst S., Kherad O., Lamour J., Lescuyer P., Martinez C., Mermet S., Noël N., Perez-Saez J., Pittet D., Portier J., Poulain G., Pugin C., Pullen N., Rinaldi F., Rizzo J., Rochat D., Sahyoun C., Sakvarelidze I., Samir K., Ramirez H.A.S., Schrempft S., Semaani C., Testini S., Tisserand Y., Rivas D.U., Verolet C., Villers J., Violot G., Vuilleumier N., Yerly S., Zavlanou C., Guessous I. A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. Nat. Commun. 2022;13:1–8. doi: 10.1038/s41467-022-34616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mafla A.C., Villalobos-Galvis F.H., Heft M.W. Illness perceptions amongst individuals with dental caries. Community Dent. Health. 2018;35:16–22. doi: 10.1922/CDH_4098Mafla07. [DOI] [PubMed] [Google Scholar]
- 67.Broadbent E., Ellis C.J., Thomas J., Gamble G., Petrie K.J. Further development of an illness perception intervention for myocardial infarction patients: a randomized controlled trial. J. Psychosom. Res. 2009;67:17–23. doi: 10.1016/j.jpsychores.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Frostholm L., Petrie K.J., Ornbol E., Fink P. Are illness perceptions related to future healthcare expenditure in patients with somatoform disorders? Psychol. Med. 2014;44:2903–2911. doi: 10.1017/S003329171400035X. [DOI] [PubMed] [Google Scholar]
- 69.Ballering A., Olde Hartman T., Rosmalen J. Vol. 75. 2021. Long COVID-19, Persistent Somatic Symptoms and Social Stigmatisation; pp. 603–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiloh S., Peleg S., Nudelman G. Making sense of COVID-19: a longitudinal investigation of the initial stages of developing illness representations. Psychol. Health. 2021 doi: 10.1080/08870446.2021.1925670. [DOI] [PubMed] [Google Scholar]
- 71.Sabina Sahanic, Piotr Tymoszuk, Luger AnnaK. COVID-19 and its continuing burden after 12 months: a longitudinal observational prospective multicentre trial. ERJ Open Research. 2023;(9):00317–02022. doi: 10.1183/23120541.00317-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Data Availability Statement
An R data (RDa) file with anonymized patient data will be made available upon request to the corresponding author. The study analysis pipeline is available at https://github.com/PiotrTymoszuk/CovILD-IPQ.