Abstract
Gliomas are inherently difficult to treat by radiotherapy because glioma cells become radioresistant over time. However, combining radiotherapy with a radiosensitizer could be an effective strategy to mitigate the radioresistance of glioma cells. Gold nanoparticles (AuNPs) have emerged as a promising nanomaterial for cancer therapy, but little is known about whether AuNPs and X-ray radiation have cytotoxic synergistic effects against tumors. In this study, we found that the combination of AuNPs and X-ray irradiation significantly reduced the viabilities, as well as the migration and invasion, of glioma cells. Mechanistically, we observed that the AuNPs inhibited radiation-induced CCL2 expression by inhibiting the TRAF6/NF-κB pathway, which likely manifested the synergistic therapeutic effect between the AuNPs and X-ray radiation. The AuNPs also re-sensitized radioresistant glioma cells by inhibiting CCL2 expression. These results were also observed in another tumor cell line with a different molecular pattern, indicating that the underlying mechanism may be ubiquitous through cancer cells. Lastly, using the glioma mouse model, we observed that AuNPs significantly reduced tumor growth in the presence of X-ray radiation compared to radiotherapy alone.
Keywords: Glioma, Gold nanoparticles, Migration and invasion radio-resistance, Synergistic effect
Highlights
-
•
Finding the potent anti-tumor effect of AuNPs against glioma cells..
-
•
Finding that AuNPs can enhance the radiosensitivity in glioma cells.
-
•
Finding that AuNPs can function by regulating restricting TRAF6/NF-κB induced CCL2 production.
1. Introduction
Gliomas are the most commonly occurring brain tumors and are inherently aggressive and difficult to treat, with just 3% of patients surviving after 5 years, largely due to the poor efficacy of the radiotherapy used to treat the tumors [1,2]. Some studies have identified that the poor efficacy of radiotherapy is attributed to the radioresistance of glioma cells [3]. Furthermore, conventional radiotherapy is poorly selective for tumor cells and causes significant damage to surrounding healthy tissue. However, the utilization of radiosensitizers to enhance the sensitivity of cancer cells to radiotherapy can become a promising strategy for increasing the effectiveness of radiotherapy [4].
Several types of metal and metal oxide nanomaterials have been developed as anti-cancer agents. Among these nanomaterials, silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) have attracted particular attention because of their excellent radiosensitizing properties in addition to their ability to suppress inflammation [5,6]. However, the relationship between radiotherapy and inflammation is complex and poorly understood. On the one hand, inflammation that is induced after radiotherapy promotes the development of anti-tumor immune responses [7]; on the other hand, radiotherapy-induced inflammation in cancer cells is a risk factor for the development of radioresistance [8]. While AuNPs have demonstrated the ability to suppress inflammation and sensitize cancer cells to radiotherapy, it is unclear whether and how the combination of AuNPs and radiotherapy exert a synergistic anti-tumor effect in glioma cells.
Several anti-inflammatory agents have demonstrated radiosensitizing functions both in vitro and in vivo [8,9]. For example, our group recently reported that AuNPs inhibited the secretion of inflammatory factors, including MMP9 and CCL2 [10], that mediate inflammatory signals between cells [11]. It was previously reported that CCL2 promoted tumor growth in glioma cells [12], and several studies revealed that CCL2 plays a key role in enabling the radioresistance of certain cancer cells [13,14].
Herein, we investigated how AuNPs affected the treatment response of human glioma cell lines to single or combined treatments with AuNPs and X-ray irradiation, and we elucidated the potential mechanism underlying the anti-tumor synergism between AuNPs and X-ray radiotherapy.
2. Materials and methods
2.1. AuNPs
The AuNPs were synthesized based on a procedure reported in a publication by our group as well as by another group [10,15]. Transmission electron microscopy (TEM) was used to observe the morphologies of AuNPs, and the chemical states of the AuNPs were characterized by X-ray photoelectron spectroscopy (XPS), the results of which were reported previously [10]. The average size of the AuNPs was 62.2 ± 6 nm based on TEM. The high-resolution Au4f spectrum featured a peak at 83.37 eV, which accounted for 49.84% of the total material and a peak at 87.03 eV, which accounted for 50.16% of the total material [10].
2.2. Cells, reagents, and irradiation (IR)
U251 human glioma cells and mice luciferase-positive glioma GL261 (GL261-luc) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Normal human astrocytes (NHAs) were purchased from Procell (Cat NO: CP-H122; Wuhan, China); a detailed description of the NHAs was previously reported [16]. IR-resistant U251 cells (U251-R) were established according to previously reported methods [3]. Radiation-sensitive oral squamous cell carcinoma cells (OSCC, SCC15–S) and the radiation-resistant variants (SCC15-R) were gifted by Dr. Di Wang at the Eighth Medical Center (PLA General Hospital, Beijing, China), and detailed descriptions of these cells were previously reported [17].
A CCL2-overexpressing plasmid was constructed from the pcDNA3.1 vector (pcDNA3.1-CCL2), and the U251, U251-R, SCC15–S, and SCC15-R cells were all transfected with the pcDNA3.1-CCL2 vector using Lipofectamine 2000, and the transfected cells that were resistant to the aminoglycoside antibiotic G418 were selected and named CCL2-OV. All of the cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum in a humidified cell incubator with an atmosphere of 10% CO2 at 37 °C. The X-RAD 320 cabinet irradiator (Precision X-Ray) was used to irradiate the cells, and the radiation energy was set to 0, 1, 2, or 4 Gy, at a rate of 250 MU/min.
2.3. Secretory protein quantification and cell viability
Protein CCL2 levels in the cells were quantified using the enzyme-linked immunosorbent assay (ELISA) kit from the R&D Systems (Minneapolis, Minnesota, USA). Furthermore, MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) assays were performed to assess the viabilities of the cells after exposure to the AuNPs and/or X-ray radiation using a kit obtained from R&D Systems (Minneapolis, Minnesota, USA). The experimental protocols and data analysis were conducted in accordance with the manufacturer's instructions.
2.4. mRNA levels determination
We used TRIzol reagent (Invitrogen, Grand Island, NY, USA) to obtain the total RNA from cells. Subsequently, reverse transcription-quantitative polymerase chain reaction (qRT-PCR) assay was performed using the PrimeScript RT-PCR kit (Takara, Bio, Inc., Shiga, Japan). The IQ5 fluorescence quantitative PCR detector (Bio-Rad, Hercules, CA, USA) was used to perform qRT-PCR. Primer sequences for all the targets were described as following:
GAPDH: 5’ATCAAGAAGGTGGTGAAGCA’3(forward) and 5’GTCGCTGTTGAAGTCAGAGGA’3(reverse); CCL2: 5’AGCAGCAAGTGTCCCAAAGA’3(forward) and 5′GTGTCTGGGGAAAGCTAGGG(reverse); TGCATATTTGTTTGGGGCAGG’3(reverse); TRAF6: 5’ATGCGGCCATAGGTTCTGC’3(forward) and 5′ TCCTCAAGATGTCTCAGTTCCAT’3(reverse); NF-κB p65: 5′ ATGCGCTTCCGCTACAAGTG’3(forward) and 5′ ACAATGGCCACTTGTCGGTG’3 (reverse).
2.5. Protein levels determination
Protein extracts were prepared in protein lysis buffer (Pierce, Thermo Fisher Scientific). The protein was separated by SDS-PAGE and transferred to PVDF membrane. After blocking, the membrane was incubated with the primary antibody overnight at 4 °C and then with the secondary antibody. Protein bands were observed by enhanced chemiluminescence and film exposure.
2.6. Luciferase assay
The NF-κB luciferase reporter were kindly provided by Dr Zhen Liu from PLA 309 hospital. After certain treatment, luciferase activity of NF-κB was determined by using the dual luciferase reporter assay system (Promega), with the Renilla luciferase activity as internal reference.
2.7. Migration and invasion assays
We added cells to the upper chamber of a 24 well plate with 8-μ m holes. The plate would be coated with Matrigel if invasion analysis was performed. After 48 h of culture, the non-migrating or non-invading cells were removed from the upper well, and the migrating or invading cells were fixed with 95% methanol and stained with 0.1% crystal violet (Kagan Biotechnology Co., Ltd.). The stained cells would be photographed and counted in each well. The cells counting of one control sample was randomly selected as “100%", and the normalized migration and invasion rate of all other samples was set as the “fold change” relative to that of control sample.
2.8. Cell apoptosis analysis and caspase-3 activity determination
We used Annexin V-fluos Staining Kit (Roche Boehringer) to detect apoptotic cells. After certain treatment, the cells were harvested and incubated with Annexin V and PI for 15 min. Then the cells were analyzed on flow cytometer with 488 nm excitation and 515 nm for Annexin V detection and a filter with the wavelengths above 600 nm for PI detection. We used fluorometric method to determine the caspase-3 activity. Caspase-3 substrates (Ac-DEVD-AMC) were used to determine the caspase activity by measuring the fluorescence intensity at an excitation wavelength of 380 nm and 460 nm as an emission wavelength.
2.9. In vivo brain tumor models
A glioma mouse model was established using six-to-ten-week-old C57BL/6 mice. In total, 75,000 GL261-luc glioma cells were injected into the brains of C57BL/6 mice. On day 5 after the injection, a non-invasive in vivo imaging system (IVIS) was utilized to ensure that the tumor had successfully developed. The mice were then categorized into subgroups A: PBS group (control); subgroups B: single AuNPs treatment group; subgroups C: PBS + X-ray treatment group; subgroups D: AuNPs + X-ray treatment group. The mice that were administered the AuNPs or PBS were treated with 1.0 mg kg−1 AuNPs or an equal volume of PBS intravenously. The mice that underwent X-ray radiation therapy were irradiated with 4 Gy radiation 30 min after intravenous injection of the AuNPs, which was consistent with a method previously reported [18]. The glioma size was measured based on total flux (p/sec/cm2/sr) using the Living Image software for each region of interest (ROI) drawn around the tumor signal, the details of which are described in another report [19]. Blood was collected by cardiac puncturing into heparinized propylene tubes to measure the routine haematology analysis, so as to determine the hematological toxicity of the indicated treatment. The animals were weighed throughout the experiment, and the number of mice that died during the experiments was recorded; these data were also used as indicators of systemic toxicity.
2.10. In vivo xenograft model
Female BALB/c nu/nu (athymic nude) mice (5–6 weeks of age) were used to establish a xenograft model. U251- or CCL2-overexpressed U251 cells (2 × 106 cells in 150 μL PBS) were inoculated subcutaneously into the right flank of the mice, and the tumors were allowed to grow to 4–8 mm in diameter. The mice were then categorized into subgroups A: PBS group (control); subgroups B: single AuNPs treatment group; subgroups C: PBS + X-ray treatment group; subgroups D: AuNPs + X-ray treatment group. The mice administered the AuNPs or PBS were treated with 1.0 mg kg−1 AuNPs or an equal amount of PBS intravenously. The mice that underwent X-ray radiation were irradiated with 4 Gy radiation 30 min after injection of the nanoparticles [18]. Tumor size was determined by measuring two perpendicular diameters of the tumor once every four days after treatment using a caliper.
2.11. Statistical analysis
The data reported herein represent the mean ± standard deviation from ≥3 separate experiments performed in parallel. The differences between two groups were determined using two-tailed Student's t-test. All statistical analyses were performed with the SPSS 16.0 software package (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Verification of the developed radiation-resistant glioma cells (U251-R)
IR exposure for 24 h decreased U251 cell viability in a dose-dependent manner (Fig. 1A). Meanwhile, 4 Gy IR exposure for 24 h resulted in a significant potentiation of apoptosis of the U251 cells (Fig. 1B) and a reduction in migration and invasion of the cells (Fig. 1C). By contrast, IR exposure had little effect on the viability of the U251-R cells (Fig. 1A–C), which demonstrated that a IR-resistant glioma cell line (U251-R) was successfully developed.
Fig. 1.
Identification of constructed IR resistant glioma cells U251-R. (A) Cell viabilities (%) expressed by the IR against U251 and U251-R cells at dose ranges from 0 to 4 Gy for 24 h (student t-test for differences determination, n = 3 for each group). (B) Cell apoptosis rates (%) expressed by the IR against U251 and U251-R cell lines at dose 4 Gy for 24 h (student t-test for differences determination, n = 3 for each group). (C) Migration rates (%) and invasion rates (%) expressed by the IR against U251 and U251-R cell lines at dose 4 Gy for 24 h (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group.
CTL: control group; IR: X-ray irradiation. *: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
3.2. AuNPs and X-ray radiation had a synergistic effect on glioma cells viability, migration, and invasion
Cells viabilities, migration, and invasion of cancer cells are important indicators of tumor malignancy. In this study, we conducted MTT assays to assess the viabilities of U251 and U251-R after exposure to the AuNPs. After 24 h following exposure to AuNPs, the viabilities of both U251 and U251-R cells decreased in a dose-dependent manner, with a 50% inhibitory concentration (IC50) of approximately 150 mg/L. Furthermore, there was no significant difference in the effect of AuNP exposure on cell viability between the U251 and U251-R cell lines (Fig. 2A). In addition, treatment of the U251 and U251-R cells with AuNPs (150 mg/L) reduced the migration and invasion to similar extents (Fig. 2B).
Fig. 2.
AuNPs and IR had a synergistic anti-tumor effect against glioma cells. (A) Cell viabilities (%) expressed by the AuNPs against U251, U251-R and NHA cells at dose ranges from 0 to 150 mg/L (student t-test for differences determination, n = 3 for each group). (B) Migration rates (%) and invasion rates (%) expressed by the AuNPs at 150 mg/L against U251 and U251-R cell lines (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group. (C) Cell viabilities (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against U251 (student t-test for differences determination, n = 3 for each group). (D) Migration rates (%) and invasion rates (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against U251 (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group.
CTL: control group; IR: X-ray irradiation; Comb: defined as AuNPs at 75 mg/L (half concentration) + IR at 2 Gy (half concentration) treatment. *: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
After assessing the effects of the AuNPs on cell viability, we determined whether there was synergistic anti-tumor effect between the AuNPs and IR in the glioma cells. We treated the U251 cells individually with either AuNPs (150 mg/L) or IR (4 Gy), as well as together at half the dose of AuNPs (75 mg/L) and IR intensity (2 Gy) (defined as “Comb”). Both of the individual treatments (i.e. AuNPs (150 mg/L) and IR (4 Gy)) had obvious anti-tumor efficacy against U251 cells, while the combination of the two was more efficacious than the individual treatments based on the results of cell viabilities (Fig. 2C) as well as migration and invasion (Fig. 2D). These results demonstrated that the AuNPs and X-ray radiation had a synergistic anti-tumor effect against the glioma cells.
3.3. Synergism between AuNPs and X-ray radiation involves inhibiting the radiation-induced CCL2 production
We previously found that treatment of AuNPs regulated the expression of several secretory cytokines, including MMP9 and CCL2 [10]. In this study, we studied whether the AuNPs with and without IR could regulate CCL2 secretion in U251 cells because IR-induced CCL2 secretion has been reported as the key factor in preventing radiotherapy effectiveness [[20], [21], [22]].
While exposure of U251 cells to IR induced CCL2 expression, co-treatment of the cells with AuNPs significantly inhibited CC12 expression (Fig. 3A), and the overexpression of CCL2 (CCL2-OV) led to a reversal of the anti-tumor effect that was observed after treatment of the cells with the individual or combination of treatments based on the results of cell viabilities (Fig. 3B), migration, and invasion (Fig. 3C). To better understand the underlying mechanism of the synergistic effect between AuNPs and IR, we assessed the effects of the individual and combination treatments in another tumor cell SCC15–S (OSCC cells). Similar to the U251 cells, IR upregulated CCL2 expression in the SCC15–S cells, while AuNPs co-treatment significantly mitigated this upregulation (Supplementary Fig. 3A). Meanwhile, individual treatments of the SCC15–S cells with AuNPs (150 mg/L) and X-ray radiation (4 Gy) had obvious anti-tumor efficacy against the cells, while the combination was more efficacious than the individual treatments, as indicated by the results in cell viabilities (Supplementary Fig. 3B) as well as migration and invasion of the cells (Supplementary Fig. 3C). These synergistic effects could be reversed by CCL2 overexpression in the SCC15–S cells (Supplementary Figs. 3B–C).
Fig. 3.
AuNPs exerts synergistic effect with IR by reversing the IR induced CCL2 production in glioma cells. (A) Cultured U251 cells were treated with IR at 4 Gy, together with or without AuNPs at 150 mg/L for 24 h, and the CCL2 productions were determined by ELISA assay in the supernatant proteins (student t-test for differences determination, n = 3 for each group). (B) Cell viabilities (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against wide-type U251 (control) and CCL2 stable over-expressed U251 (CCL-OV) (student t-test for differences determination, n = 3 for each group). (C) Migration rates (%) and invasion rates (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against wide-type U251 (control) and CCL2 stable over-expressed U251 (CCL-OV) (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group.
CTL: control group; IR: X-ray irradiation; Comb: defined as AuNPs at 75 mg/L (half concentration) + IR at 2 Gy (half concentration) treatment. *: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
3.4. AuNPs reversed the IR-induced TRAF6/NF-κB signaling activation in glioma cells
It has previously been reported that the radiation-induced secretion of CCL2 is a consequence of the activation of the NF-κB pathway [23,24]. In this study, NF-κB luciferase reporter activities were significantly enhanced after exposure of the U251 cells to IR, whereas this regulation was overcome after exposure of the cells to AuNPs (Fig. 4A). Furthermore, we found that AuNPs significantly decreased NF-κB activity, and this was overcome after overexpressing TRAF6 in the cells; however, AuNPs decreased NF-κB activity was not affected by the presence of TAK1 or IKKβ (Fig. 4B). Meanwhile, both the phosphorylation of p65 and total TRAF6 levels decreased after AuNPs treatment (Fig. 4C). Interestingly, mRNA levels of TRAF6 were not affected significantly by AuNPs (Fig. 4D), indicating that AuNPs affected TRAF6 levels through post-translational regulation.
Fig. 4.
AuNPs inhibits TRAF6/NF-κB signaling activation in glioma cells. (A) Cultured U251 cells were pre-transfected with NF-κB reporter plasmid and phRL-TK plasmid (internal control) for 24 h, and then treated with IR at 4 Gy, together with or without AuNPs at 150 mg/L for another 24 h. The activation of the NF-κB promoter was measured by a dual-luciferase reporter gene assay (student t-test for differences determination, n = 3 for each group). (B) Cultured U251 cells were pre-transfected with NF-κB reporter plasmid and phRL-TK plasmid (internal control), together with TRAF6, TAK1, or IKKβ expression plasmid for 24 h, and then treated with or without AuNPs at 150 mg/L for another 24 h. The activation of the NF-κB promoter was measured by a dual-luciferase reporter gene assay (student t-test for differences determination, n = 3 for each group). (C–D) Cultured U251 cells were treated with or without AuNPs at 150 mg/L for 24 h, and (C) the phosphorylation levels of p65 and TRAF6 total protein levels were determined by western blotting assay; (D) the mRNA levels of p65 and TRAF6 were determined by qRT-PCR assay (student t-test for differences determination, n = 3 for each group). (E) Lysates from certain treated U251 cells transiently co-transfected with Flag-TRAF6 and HA-UB (K48) or HA-UB (K63) plasmids were subjected to immunoprecipitation with anti-Flag antibody followed by Western blot analysis with anti-HA antibody. The full scan of the gel was shown in Supplementary Fig. 5.
CTL: control group; IR: X-ray irradiation; UB: ubiquitin. *: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
It has been established that K48-linked protein polyubiquitination promotes the degradation of the corresponding protein via the 26S proteasome [25]. We observed that, in the presence of MG132, a widely used proteasome inhibitor, the K48-linked polyubiquitination levels of TRAF6 were markedly higher after exposure of the U251 cells to AuNPs, but K63-linked polyubiquitination of TRAF6 was not affected (Fig. 4E). Therefore, the increase in the K48-linked polyubiquitination of TRAF6 indicated that the AuNPs decreased TRAF6/NF-κB signaling activity in the U251 cells. These results were also corroborated in SCC15–S cells (Supplementary Figs. 4A–D).
3.5. The TRAF6/NF-κB/CCL2 pathway plays the key role in promoting glioma cell resistance to IR
We found that CCL2 levels were significant higher in U251-R than in U251 cells. The overexpression of TRAF6 increased the levels of CCL2 in both U251 cells and U251-R cells, which was mitigated by incubation of the cells with an NF-κB inhibitor, indicating that TRAF6/NF-κB is an upstream regulator of CCL2 production (Fig. 5A). Meanwhile, introduction of CCL2 siRNA re-sensitized the radioresistant glioma cells to radiation (4 Gy), which was reflected in the results of cell viabilities (Fig. 5B) as well as migrations and invasions (Fig. 5C). These results demonstrated that the TRAF6/NF-κB/CCL2 pathway plays a key role in promoting the radioresistance of glioma cells.
Fig. 5.
TRAF6/NF-κB/CCL2 pathway play the key role in promoting glioma cell resistant to IR. (A) Cultured U251 cells and U251-R cells were transient transfected with TRAF6 (TRAF6-OV), together with or without 10 mM NF-κB inhibitor (BAY-117082) for 48 h, and the CCL2 productions were determined by ELISA assay in the supernatant proteins (student t-test for differences determination, n = 3 for each group). (B) Cultured U251-R cells were transient transfected with CCL siRNA (CCL-SI) for 24 h, and then were treated with IR at certain doses for another 24 h, and the cell viabilities (%) were determined (student t-test for differences determination, n = 3 for each group). (C) Cultured U251-R cells were transient transfected with CCL siRNA (CCL-SI) for 24 h, and then were treated with IR at 4 Gy for another 24 h, and the migration rates (%) and invasion rates (%) were determined (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group.
*: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
3.6. AuNPs re-sensitizes radio-resistant glioma cells to X-ray radiation by inhibiting CCL2 production
After identifying that the TRAF6/NF-κB/CCL2 pathway plays an important role in enabling the radioresistance of glioma cells, we suspected that AuNPs could re-sensitize the radioresistant glioma cells to IR. To verify our hypothesis, we exposed U251-R cells and SCC15-R cells to AuNPs and determined the viabilities of the cells thereafter. We observed that AuNP treatment significantly decreased U251-R or SCC15-R cell viabilities, while IR alone had no effect, as expected (Fig. 6A, Supplementary Fig. 4A). Interestingly, the viabilities of the cells exposed to both AuNPs and IR were significantly lower than those exposed to only AuNPs, indicating that the AuNPs partially ameliorated the resistance of the U251-R and SCC15-R cells to IR (Fig. 6A, Supplementary Fig. 4A). Meanwhile, the inhibitory functions of AuNPs or combination was reversed when the U251-R or SCC15-R cells were transfected with CCL2-OV, indicating that the AuNPs re-sensitized the radioresistant glioma cells to IR by inhibiting the production of CCL2 (Fig. 6A, Supplementary Fig. 4A). Similar inhibitory functions of AuNPs or combination were also demonstrated by the inhibition of migration and invasion of the U251-R or SCC15-R cells (Fig. 6B, Supplementary Fig. 4B). Collectively, above results indicated that AuNPs could re-sensitize the radio-resistant tumor cells to IR by restricting CCL2 production.
Fig. 6.
AuNPs sensitize the radio-resistant glioma cells to IR by restricting CCL2 productions. (A) Cell viabilities (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against wide-type U251-R (control) and CCL2 stable over-expressed U251-R (CCL-OV) (student t-test for differences determination, n = 3 for each group). (B) Migration rates (%) and invasion rates (%) expressed by the AuNPs at 150 mg/L, IR at 4 Gy and Comb against wide-type U251-R (control) and CCL2 stable over-expressed U251-R (CCL-OV) (student t-test for differences determination, n = 3 for each group). The migration and invasion ability were measured with transwell assay. The representative morphology of migration and invasion cells were shown in Supplementary Fig. 1A in the corresponding group.
CTL: control group; IR: X-ray irradiation; Comb: defined as AuNPs at 25 mg/L (half concentration) + IR at 4Gy (half concentration) treatment.*: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
3.7. Effect of the AuNPs on tumor growth after radiotherapy in vivo
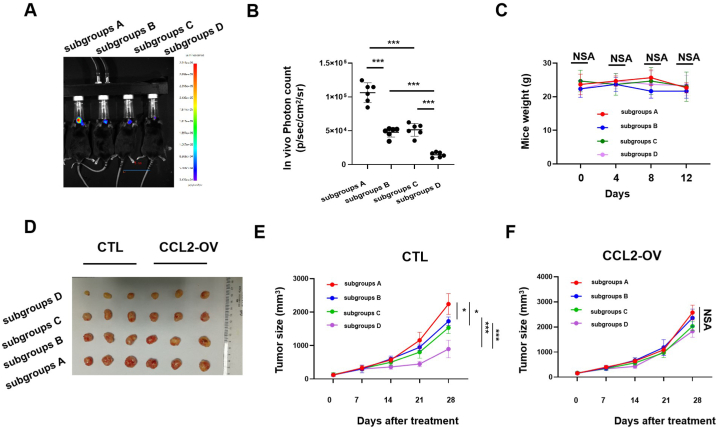
To demonstrate the potential clinical efficacy of the AuNPs in cancer treatment, we assessed the effects of the AuNPs on tumor growth after radiotherapy in vivo. To accomplish this, we developed a GL261-luc brain tumor model, in which we monitored tumor growth in response to each treatment via bioluminescent imaging. Twelve days after intravenous administration of the AuNPs alone (subgroups B) or after exposure of the mice to IR alone (subgroups C), the tumor size was smaller compared to the mice administered PBS (subgroups A); however, while the inhibitory efficacy was significantly improved when AuNPs and IR were used together (subgroups D) (Fig. 7A–B). We measured the weight and the changes in the hematological parameters of the mice to evaluate the systemic toxicity of each treatment. No weight loss was observed in any of the four treatment groups (Fig. 7C). The Monocyte (%) and Platelet count were slightly higher in the subgroups D compared to the subgroups C, but the differences of the two parameters did not reach statistical significance (Supplementary Table 1). Generally, these results indicated that the AuNPs combination had little toxicity against brain tumor mice.
Fig. 7.
In vivo effect of AuNPs on glioma growth after IR therapy. (A–C) C57BL/6 mice were used for brain tumor models construction. Mice were categorized into indicated subgroups. (A) Representative IVIS images showing tumor presence in each group 12 days after treatment. (B) Photon counts obtained from tumors resulting from GL261-luc cells in each group 12 days after treatment (student t-test for differences determination, n = 8 for each group). (C) The weight of mice were measured in each group from 0 to 12 days after treatment (student t-test for differences determination, n = 8 for each group). (D–F) widetype U251 cells (Control) or CCL2 stable overexpressed U251 cells (CCL2-OV) were injected into nude mice to construct xenograft mice model. Mice were categorized into indicated subgroups. (D) Representative images of tumors in nude mice xenografted in each group at the endpoint time (28 days after treatment). (E–F) Tumor volumes in (E) control mice and (F) CCL2-OV mice under certain treatment were measured every 7 days after treatment until endpoint time (28 days after treatment) (student t-test for differences determination, n = 3 for each group).
subgroups A: PBS group (control); subgroups B: single AuNPs treatment group; subgroups C: PBS + X-ray treatment group; subgroups D: AuNPs + X-ray treatment group.
*: P < 0.05; **: P < 0.01; ***: P < 0.001; NS: not significant.
NSA: In the four groups of mice, there was no statistical difference between any two groups.
Next, we used a subcutaneous xenograft mouse model established using the U251 cells to confirm the mechanism underlying the synergistic effect between the AuNPs and IR on tumor cell viability that was observed in vitro. Both administration of the AuNPs alone and IR alone inhibited tumor growth, and tumor growth was inhibited more when AuNPs and radiotherapy were employed together (Fig. 7D–E). However, the efficacy of this synergistic treatment was significantly weaker in the CCL2-OV xenograft mouse model compared to the control xenograft mouse model (Fig. 7D, F), which was consistent with the analogous in vitro results. These results demonstrated that the AuNPs and radiotherapy had a synergistic effect on tumor growth in vivo, and the mechanism underlying the therapeutic synergism involved inhibition of CCL2 secretion.
4. Discussion
In this study, we found that the combination of AuNPs and IR exhibited a strong anti-tumor effect in glioma cells. This result was expected based on previous reports [6,15]. Even when the dosage of the AuNPs and the intensity of the IR were reduced by half, the combination of the two treatments saw a significantly higher anti-tumor effect than AuNPs (150 mg/L) or IR (4 Gy) treatment alone, further corroborating the synergistic effect of the AuNPs and IR on glioma cell viability. AuNPs have a high uptake capacity into tumor cells and accumulate in tissues through the enhanced permeability and retention (EPR) effect [26], making them less toxic to normal cells than to tumor cells [10]. Consistent with this, our in vitro and in vivo results showed that the combination of AuNPs and IR demonstrated higher anti-tumor activity than the individual treatments and control, with little toxicity to the normal host, which is important for ensuring high anti-tumor efficacy while minimizing off-target effects.
It has been established that IR regulates many signaling pathways in cells [26]. Several studies have also reported that AuNPs regulate apoptosis-related signaling pathways. Tian et al. observed that AuNPs activated the expression of numerous apoptosis-related gene in cancer cells [27]. Banu et al. reported that AuNPs induced apoptosis in human breast adenocarcinoma cells through p53 and BCL2 regulation [28]. Saberi et al. reported that AuNPs radiosensitized colon cancer cells through an apoptosis-dependent pathway [29]. These studies indicated that both AuNPs and IR exert their anti-tumor effects by promoting apoptosis. Several reports also confirmed that the synergistic effect of AuNPs and IR was mediated, at least partially, by apoptosis [30].
In addition to corroborating the results published in similar studies, this study highlights that the AuNPs inhibited the side effects induced by IR. As we know, IR can induce significant inflammation, and excessive inflammation is known to diminish the antitumor effects of radiotherapy, eventually leading to radioresistance [20,21]. We found that AuNPs reduced IR-induced inflammation by inhibiting the expression of CCL2. CCL2 is usually expressed at low levels in normal epithelial cells and is expressed in high levels in tumor cells, indicating that CCL2 expression is upregulated during malignant transformation [[31], [32]]. Studies revealed that CCL2 is a tumor-promoting factor and plays a role in inhibiting the apoptosis of cancer cells [12]. Our data showed that IR alone upregulated the expression of CCL2. Furthermore, our results indicated that the AuNPs decrease tumor cell viability mainly by restricting CCL2 expression. This regulation not only has an anti-tumor effect but also eliminates the IR-induced negative feedback factor that diminishes the anti-tumor effectiveness of radiotherapy, which might be the fundamental reason for their synergistic effect. We also observed that AuNPs re-sensitized the radioresistant U251-R cells to radiotherapy by inhibition CCL2 secretion. These results were also observed in another tumor cell line with a different molecular pattern, indicating that the underlying mechanism may be ubiquitous through cancer cells. Therefore, the AuNPs in combination with IR may provide a potential therapeutic strategy for IR-resistant tumors in clinical cases.
The main limitation is that the observation time is short, and therefore it is still unable to evaluate the long-term anti-tumor effect and chronic toxicity of the AuNPs treatment or the combination strategy. Further, even though AuNPs have certain tumor targeting ability, their toxic effects on normal cells have also been reported. Therefore, more experiments need to be carried out to compare the anti-tumor activity and toxicity of different doses of AuNPs in a long period, so as to explore the optimal dosage of AuNPs.
5. Conclusions
In conclusion, AuNPs re-sensitized radio-resistant glioma cells by inhibiting TRAF6/NF-κB-induced CCL2 expression. These results provide a foundation for developing a potential therapeutic strategy for radiation-resistant gliomas in patients.
Statement of ethics
The study was approved by the ethics committee of Qingdao Municipal Hospital.
Declarations
Author contribution statement
Hao Wang; Qian Xu; Xianning Dong; Zhiling Guan; Ziyu Wang; Yuanyuan Hao: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Ruichun Lu; Ling Chen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Shandong medical and health science and technology development plan project (2018WS362, 2014WS0447).
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14362.
Contributor Information
Ruichun Lu, Email: qqtang_lu@163.com.
Ling Chen, Email: chlsea2006@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ostrom Q.T., Cote D.J., Ascha M., Kruchko C., Barnholtz-Sloan J. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4:1254–1262. doi: 10.1001/jamaoncol.2018.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Nifterik K.A., Van Den Berg J., Slotman B.J., Van Rijn J. Anti-tumour effects by a trimodal combination of temozolomide, meloxicam and X-rays in cultures of human glioma cells. Int. J. Radiation Biol. 2011;87:192–201. doi: 10.3109/09553002.2010.519423. [DOI] [PubMed] [Google Scholar]
- 3.Du H.Q., Wang Y., Jiang Y., Wang C.H., Zhou T., Liu H.Y., et al. Silencing of the TPM1 gene induces radioresistance of glioma U251 cells. Oncol. Rep. 2015;33:2807–2814. doi: 10.3892/or.2015.3906. [DOI] [PubMed] [Google Scholar]
- 4.Liu C., Wang L., Qiu H., Dong Q., Feng Y., Li D., et al. Combined strategy of radioactive 125I seeds and salinomycin for enhanced glioma chemo-radiotherapy: evidences for ROS-mediated apoptosis and signaling crosstalk. Neurochem. Res. 2018;43:1317–1327. doi: 10.1007/s11064-018-2547-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J., Liu P., Ma J., Li D., Yang H., Chen W., et al. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int. J. Nanomed. 2019;14:9483–9496. doi: 10.2147/IJN.S224160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar D., Mutreja I., Chitcholtan K., Sykes P. Cytotoxicity and cellular uptake of different sized gold nanoparticles in ovarian cancer cells. Nanotechnology. 2018;28 doi: 10.1088/1361-6528/aa935e. [DOI] [PubMed] [Google Scholar]
- 7.Ali M., Fulci G., Grigalavicius M., Pulli B., Li A., Wojtkiewicz G.R., et al. Myeloperoxidase exerts anti-tumor activity in glioma after radiotherapy. Neoplasia. 2022;26 doi: 10.1016/j.neo.2022.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho P., Silva L., Faria I., Vieria M., Monteiro A., Pinto G., et al. Adipocyte secretome increases radioresistance of malignant melanocytes by improving cell survival and decreasing oxidative status. Radiat. Res. 2017;187:581–588. doi: 10.1667/RR14551.1. [DOI] [PubMed] [Google Scholar]
- 9.Ungvari Z., Podlutsky A., Sosnowska D., Tucsek Z., Toth P., Deak F., et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity, the journals of gerontology. Series A, Biol. Sci. Med. Sci. 2013;68:1443–1457. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao Y., Hu J., Wang H., Wang C. Gold nanoparticles regulate the antitumor secretome and have potent cytotoxic effects against prostate cancer cells. J. Appl. Toxicol.: JAT. 2021;41:1286–1303. doi: 10.1002/jat.4117. [DOI] [PubMed] [Google Scholar]
- 11.Brandi J., Manfredi M., Speziali G., Gosetti F., Marengo E., Cecconi D. Proteomic approaches to decipher cancer cell secretome. Semin. Cell Dev. Biol. 2018;78:93–101. doi: 10.1016/j.semcdb.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Lu B., Zhou Y., Su Z., Yan A., Ding P. Effect of CCL2 siRNA on proliferation and apoptosis in the U251 human glioma cell line. Mol. Med. Rep. 2017;16:3387–3394. doi: 10.3892/mmr.2017.6995. [DOI] [PubMed] [Google Scholar]
- 13.Lee I.Y., Lin Y.Y., Yang Y.H., Lin Y.S., Lin C.L., Lin W.Y., et al. Dihydroisotanshinone I combined with radiation inhibits the migration ability of prostate cancer cells through DNA damage and CCL2 pathway. BMC Pharmacol. Toxicol. 2018;19:5. doi: 10.1186/s40360-018-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesemann A., Ketteler J., Slama A., Wirsdörfer F., Hager T., Röck K., et al. Inhibition of radiation-induced Ccl2 signaling protects lungs from vascular dysfunction and endothelial cell loss. Antioxidants Redox Signal. 2017;30:213–231. doi: 10.1089/ars.2017.7458. [DOI] [PubMed] [Google Scholar]
- 15.Dutta P.P., Bordoloi M., Gogoi K., Roy S., Narzary B., Bhattacharyya D.R., et al. Antimalarial silver and gold nanoparticles: green synthesis, characterization and in vitro study. Biomed. Pharmacother. 2017;91:567–580. doi: 10.1016/j.biopha.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Zheng D., Li Y., Zhang Y., Sui R., Chen Y., et al. Circular RNA circ_0001588 sponges miR-211-5p to facilitate the progression of glioblastoma via up-regulating YY1 expression. J. Gene Med. 2021;23 doi: 10.1002/jgm.3371. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., An W., Zhao Q., Liu Z., Jiang Y., Li H., et al. Hyperbaric oxygen enhances X-ray induced ferroptosis in oral squamous cell carcinoma cells. Oral Dis. 2022;10 doi: 10.1111/odi.14461. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha S., Wu J., Sah B., Vanasse A., Cooper L.N., Ma L., et al. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proc. Natl. Acad. Sci. U. S. A. 2019;116:16823–16828. doi: 10.1073/pnas.1900502116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook R.L., Householder K.T., Chung E.P., Prakapenka A.V., DiPerna D.M., Sirianni R.W. A critical evaluation of drug delivery from ligand modified nanoparticles: confounding small molecule distribution and efficacy in the central nervous system. J. Contr. Release : Official J. Controlled Release Soc. 2015;220:89–97. doi: 10.1016/j.jconrel.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D.L., Wei L., Wen X.M., Song H., Li Q., Lv J.W., et al. The effects of X-ray irradiation on the proliferation and apoptosis of MCF-7 breast cancer cells. Ultrastruct. Pathol. 2014;38:211–216. doi: 10.3109/01913123.2013.861569. [DOI] [PubMed] [Google Scholar]
- 21.Errico A. Radiotherapy: a double-edged sword for NSCLC? Nature reviews. Clin. Oncol. 2014;11:66. doi: 10.1038/nrclinonc.2013.247. [DOI] [PubMed] [Google Scholar]
- 22.Guo S.S., Liu R., Wen Y.F., Liu L.T., Yuan L., Li Y.X., et al. Endogenous production of C-C motif chemokine ligand 2 by nasopharyngeal carcinoma cells drives radioresistance-associated metastasis. Cancer Lett. 2019;468:27–40. doi: 10.1016/j.canlet.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Chishti A.A., Baumstark-Khan C., Koch K., Kolanus W., Feles S., Konda B., et al. Linear energy transfer modulates radiation-induced NF-kappa B activation and expression of its downstream target genes. Radiat. Res. 2018;189:354–370. doi: 10.1667/RR14905.1. [DOI] [PubMed] [Google Scholar]
- 24.Du S., Li Z., Xie X., Xu C., Shen X., Wang N., et al. IL-17 stimulates the expression of CCL2 in cardiac myocytes via Act1/TRAF6/p38MAPK-dependent AP-1 activation. Scand. J. Immunol. 2020;91 doi: 10.1111/sji.12840. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Huang A., Cui X., Han K., Hou X., Wang Q., et al. Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics. 2019;9:4208–4220. doi: 10.7150/thno.33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X., Kaneda-Nakashima K., Kadonaga Y., Kabayama K., Shimoyama A., Ooe K., et al. Astatine-211-Labeled gold nanoparticles for targeted alpha-particle therapy via intravenous injection. Pharmaceutics. 2022;14:2705. doi: 10.3390/pharmaceutics14122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hein A.L., Ouellette M.M., Yan Y. Radiation-induced signaling pathways that promote cancer cell survival (review) Int. J. Oncol. 2014;45:1813–1819. doi: 10.3892/ijo.2014.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian B., Li J., Pang R., Dai S., Li T., Weng Y., et al. Gold nanoparticles biosynthesized and functionalized using a hydroxylated tetraterpenoid trigger gene expression changes and apoptosis in cancer cells. ACS Appl. Mater. Interfaces. 2018;10:37353–37363. doi: 10.1021/acsami.8b09206. [DOI] [PubMed] [Google Scholar]
- 29.Banu H., Renuka N., Faheem S.M., Ismail R., Singh V., Saadatmand Z., et al. Gold and silver nanoparticles biomimetically synthesized using date palm pollen extract-induce apoptosis and regulate p53 and bcl-2 expression in human breast adenocarcinoma cells. Biol. Trace Elem. Res. 2018;186:122–134. doi: 10.1007/s12011-018-1287-0. [DOI] [PubMed] [Google Scholar]
- 30.Saberi A., Shahbazi-Gahrouei D., Abbasian M., Fesharaki M., Baharlouei A., Arab-Bafrani Z. Gold nanoparticles in combination with megavoltage radiation energy increased radiosensitization and apoptosis in colon cancer HT-29 cells. Int. J. Radiation Biol. 2017;93:315–323. doi: 10.1080/09553002.2017.1242816. [DOI] [PubMed] [Google Scholar]
- 31.Nakhla S., Rahawy A., Salam M.A.E., Shalaby T., Zaghloul M., El-Abd E. Radiosensitizing and phototherapeutic effects of AuNPs are mediated by differential noxa and bim gene expression in MCF-7 breast cancer cell line. IEEE Trans. NanoBioscience. 2021;20:20–27. doi: 10.1109/TNB.2020.3028562. [DOI] [PubMed] [Google Scholar]
- 32.Lim S.Y., Yuzhalin A.E., Gordon-Weeks A.N., Muschel R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.