The concept of compact, ordered sphingolipid–cholesterol membrane domains that function as “rafts” with biologically relevant functions was first put forward 26 y ago (1). Since then, the field of “lipid rafts” has blossomed and matured in several thousand publications. It may therefore come as a bit of a surprise that the recent literature still carefully contemplates fundamental questions about lipid raft characteristics, roles, and even their very existence (2–4). The issue is not with the well-characterized existence of different lipid nanodomains and microdomains in cellular membranes (5, 6) but with nonunified ideas about what constitutes a lipid raft and a raft-dependent, biologically relevant function in living cells (2, 7). What if lipid rafts are not one thing but a host of different, short-lived nanodomains that dynamically and continuously change their lipid composition, protein interactions, and cell biological functions?
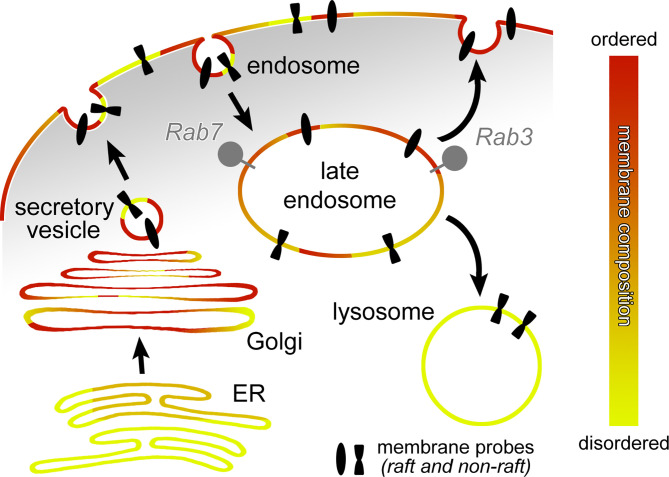
In this issue of PNAS, Diaz-Rohrer et al., used live observation to characterize trafficking of membrane proteins to the plasma membrane (PM) depending on ordered versus disordered lipid association (8). The key observations are based on preparations of giant PM vesicles, membrane blebs derived from cultured cells that retain the lipid diversity, and protein content of living membranes (9). Using the same approach, the group had previously shown that protein palmitoylation, transmembrane domain (TMD) length, and sequence affect both raft association and PM localization (10). In the new study, the authors now propose that raft-mediated trafficking to the PM occurs via a raft-specific recycling pathway that has its checkpoint at the late endosome. Key to the study is the development of single TMD raft and nonraft fluorescently labeled probes that contain no known protein sequence affecting intracellular sorting (Fig. 1). Both raft- and nonraft-associated probes trafficked to the PM through the secretory pathway; however, following constitutive endocytosis and arrival in Rab7-positive late endosomes, nonraft probes were sorted to be degraded in lysosomes, while raft probes were recycled back to the PM (Fig. 1). Based on experimental overexpression of guanosine diphosphate (GDP)- or guanosine triphosphate (GTP)-locked Rab GTPases, this recycling process is indeed dependent on Rab7, but surprisingly not Rab11 or Rab4, the GTPases previously implicated in recycling back to the PM.
Fig. 1.
Proposed roles of ordered and disordered membrane domains in intracellular protein sorting. Gradients from more to less-ordered membrane domains have been proposed for the secretory pathways (ER: more disordered; PM: more ordered) and the recycling pathway (PM: more ordered; lysosome: less ordered). However, lipid microdomains or nanodomains are highly dynamic and heterogeneous to various degrees in all membranes. Diaz-Rohrer et al. now provide evidence for the late endosome as a membrane protein sorting station in the recycling pathway. The authors report that palmitoylated Rab3 functions as a regulator for the recycling of membrane probes in ordered lipid domains, while membrane probes associated with disordered domains traffic to the lysosome. ER: endoplasmic reticulum.
Endomembrane trafficking has long been a prime target in the search for cell biological roles of lipid rafts (11). The study's authors have previously proposed a gradient of ordered membrane domains along the secretory and recycling pathways, where the PM is most enriched, endosomes intermediate, and lysosomes poor in ordered membranes (2) (Fig. 1). Sorting in the secretory and recycling pathways has been extensively studied and is regulated by a host of proteins, from vesicle coats to Rabs and other GTPases, that function in concert to regulate the dynamics of intracellular trafficking. Recycling endosomes are the best-characterized sorting stations for PM recycling, and raft-based sorting has been proposed already in the early days of lipid raft studies (12). However, early tests of the simplified “sorting to the ordered PM” idea with GPI-linked GFP and other raft probes already indicated that intracellular trafficking itineraries do not simply follow a gradient of ordered membrane and that additional sorting signals must be required (13).
The late endosome has been investigated repeatedly as a hub for raft-associated protein sorting and shown to contain raft-type membrane domains of various compositions (14–16). The heterogeneity of membrane domains in compartments like the late endosome, but also the PM, principally suggests that at least some regions of these membranes could provide the proposed sorting signals but also argues against a simple homogenous gradient of ordered membranes. Lipid segregation and ordered versus disordered membrane dynamics offer the attractive perspective of self-organization to a system where protein functions are often discussed as “address labels” and “trafficking codes.” Yet, protein interactions that contribute to the trafficking itineraries of specific proteins (including raft probes) in a context-dependent and likely cell-specific manner must play their part.
Diaz-Rohrer et al., used live observation to characterize trafficking of membrane proteins to the plasma membrane (PM) depending on ordered vs disordered lipid association.
Dias-Rohrer et al. made an inroad into the identification of such regulators by screening a panel of 156 preselected siRNAs to knock down proteins involved in membrane trafficking. One in ten of these yielded significantly reduced raft-TMD recycling to the PM. One prominent hit was Arf6, which had previously been implicated in raft-associated trafficking (17). Their surprise hit was Rab3. This small GTPase has been intensively studied in neurons, where it plays modulatory roles in synaptic vesicle exocytosis and presynaptic active zone assembly (18, 19). While Rab3 is largely specific to neurons in some species like the fruit fly Drosophila, in mice, there are four rab3 genes, and at least one of these is expressed in most cells (19). In a polarized epithelial cell type, Rab3 was shown to localize to the apical PM, and functional analyses revealed defective epithelial polarization (20). On the other hand, the original proposal that lipid segregation is responsible for the polarity of epithelial cells has long met with resistance, for example, by experiments indicating that the more ordered nature of the apical membrane may be due to extensive microvilli formation (21). Dias-Rohrer et al. also discuss a possible role of Rab3 in the biogenesis of secretory vesicles, which could also link their findings to the role of Rab3 in neurons. However, this is not straightforwardly reconciled with their own finding that delivery to the PM via the secretory pathway occurs equally for both raft and nonraft probes, and the role of Rab3 is proposed to be specific to recycling at the late endosome. It also remains unclear how the proposed raft-dependent recycling pathway may work in nonneuronal cells of species where rab3 is not (or only at very low levels) expressed outside the nervous system (19).
Diaz-Rohrer et al. propose palmitoylation of Rab3 (and, to a smaller extent, of Rab7) as a mechanism for its reversible association with rafts and tested this idea with a point mutation of a putative palmitoylation site. The association of these Rab proteins with ordered membranes is consistent with previous proteomic analyses of detergent-resistant membrane fractions that identified several Rab GTPases, including Rab3D and Rab7 (22). On the other hand, Rab GTPases have been shown to preferentially insert in disordered or curved membranes (e.g., highly curved transport vesicles) through their prenyl groups, including Rab1, Rab5, and Rab6 (23). Rab GTPases are known to dynamically shuttle between different membrane compartments, and the dependence of reversible membrane interactions on membrane lipid composition remains largely unknown.
The key findings based on (non)raft probes were validated by the authors for native PM proteins, including the Epidermal Growth Factor (EGF) receptor and the protein “linker for activation of T-cells” (LAT). Using LAT and a reporter for T cell activation, the authors further test their model in Jurkat cells, a human T lymphocyte cell line. Together, these findings make a case for a recycling pathway for which lipid rafts are described as “sufficient” for PM localization of at least some transmembrane proteins. However, any claim for sufficiency should come with a cautionary note on context dependency. A given lipid composition may be sufficient to cause protein trafficking between membrane compartments only if several other factors are in place, including proteins that contribute to the generation and shuttling of vesicles, the localization of lipid-modifying enzymes that alter lipid domain lifetimes and conversions, etc. Temporally and spatially emerging domains of differential lipid composition do not execute organelle-level functions, like intercompartment trafficking, by themselves. They are part of a composite of factors that include proteinaceous mechanisms, lipid biophysics, and spatiotemporal interaction dynamics. Cells may tune any of these parameters in a spatiotemporally controlled manner differently among cell types and even within different compartments or at different times and metabolic states of the same cell.
Cumulative evidence, including the new study, clearly supports a contributary role of different membrane domains in modifying the trafficking itineraries of various membrane proteins inside the cell. However, it remains far less clear how membrane domains of varying composition are sorted out among compartments and the PM. Membrane domains and proteins influence each other in a concerted fashion, for example, during endocytosis of ordered membrane domains driven by the coalescence of cargo proteins in such domains (24). Hence, the self-organization of intracellular trafficking more likely depends on feedback of membranes and proteins that affect each other's composition and dynamics, without one being a simple regulator of the other. Correspondingly, not all proteins that preferentially insert into ordered membrane domains traffic alike, and different membrane proteins are subject to divergent sorting signals. The many different types of membrane proteins, particularly in specialized cell types, require many levels of regulation to ensure their function at the right time and place. But maybe they share a raft at least part of the way.
Acknowledgments
Work on membrane trafficking and dynamics in the Hiesinger lab is supported by the German Research Foundation (DFG) Research Units RobustCircuit and Syntophagy as well as the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 101019191).
Author contributions
I.-M.D. and P.R.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
See companion article, “Rab3 mediates a pathway for endocytic sorting and plasma membrane recycling of ordered microdomains,” 10.1073/pnas.2207461120.
References
- 1.Simons K., Ikonen E., Functional rafts in cell membranes. Nature 387, 569–572 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Levental I., Levental K. R., Heberle F. A., Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 30, 341–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinnun J. J., et al. , Biophysical studies of lipid nanodomains using different physical characterization techniques. Biophys. J., 10.1016/j.bpj.2023.01.024 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regen S. L., The origin of lipid rafts. Biochemistry 59, 4617–4621 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Gebhardt C., Gruler H., Sackmann E., On domain structure and local curvature in lipid bilayers and biological membranes. Z. Naturforsch. C Biosci. 32, 581–596 (1977). [DOI] [PubMed] [Google Scholar]
- 6.Mouritsen O. G., Bagatolli L. A., Lipid domains in model membranes: A brief historical perspective. Essays Biochem. 57, 1–19 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Tsai Y. T., Moore W., Kim H., Budin I., Bringing rafts to life: Lessons learned from lipid organization across diverse biological membranes. Chem. Phys. Lipids 233, 104984 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Rohrer B., et al. , Rab3 mediates a pathway for endocytic sorting and plasma membrane recycling of ordered microdomains. Proc. Natl. Acad. Sci. U.S.A. 120, e2207461120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgart T., et al. , Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 104, 3165–3170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Rohrer B. B., Levental K. R., Simons K., Levental I., Membrane raft association is a determinant of plasma membrane localization. Proc. Natl. Acad. Sci. U.S.A. 111, 8500–8505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuck S., Simons K., Polarized sorting in epithelial cells: Raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 117, 5955–5964 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Gagescu R., et al. , The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell 11, 2775–2791 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols B. J., et al. , Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobo K., Chevallier J., Parton R. G., Gruenberg J., van der Goot F. G., Diversity of raft-like domains in late endosomes. PLoS One 2, e391 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fivaz M., et al. , Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 21, 3989–4000 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nada S., et al. , The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 28, 477–489 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramanian N., Scott D. W., Castle J. D., Casanova J. E., Schwartz M. A., Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat. Cell Biol. 9, 1381–1391 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geppert M., et al. , The role of Rab3A in neurotransmitter release. Nature 369, 493–497 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Kohrs F. E., et al. , Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies. Elife 10, e59594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvez-Santisteban M., et al. , Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat. Cell Biol. 14, 838–849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikenouchi J., Roles of membrane lipids in the organization of epithelial cells: Old and new problems. Tissue Barriers 6, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas Filho E. G., et al. , Proteomic analysis of lipid rafts from RBL-2H3 mast cells. Int. J. Mol. Sci. 20, 3904 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulakowski G., et al. , Lipid packing defects and membrane charge control RAB GTPase recruitment. Traffic 19, 536–545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilgemann D. W., Lin M. J., Fine M., Deisl C., On the existence of endocytosis driven by membrane phase separations. Biochim. Biophys. Acta Biomembr. 1862, 183007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]