Abstract
Cutis calcinosis of the hand in the setting of symbrachydactyly is presented in 2 unique patients. Both lesions were treated based on the standard of care protocols with resection of the calcified mass and hand reconstruction, as appropriate. The patients healed uneventfully without recurrence of the calcification at a the 1-year follow-up. The association between symbrachydactyly and calcinosis cutis is discussed along with a hypothesis on the pathophysiologic mechanism that could potentially explain this unusual occurrence and why it might be more common than previously identified.
Key words: Congenital hand disorder, Cutis calcinosis, Symbrachydactyly
Symbrachydactyly represents a variation of embryonic limb development and differentiation resulting in the compromised formation of the proximal-distal axis of the hand plate (isolated hand deformity) or the entire upper extremity (Poland syndrome).1 Typically, it is characterized by a smaller hand than the contralateral side, underdeveloped or absent fingers, and rudimentary nubbins with ectodermal tissue. Multiple different classification systems exist that categorize symbrachydactyly on the basis of the number and morphologic characteristics of the affected digits, finger joint stability, and function of the hand.1 The exact etiology of symbrachydactyly is unknown. However, a held theory suggests an etiology of disruption of the subclavian artery supply distal to the internal thoracic artery before day 42 of gestation.2
Cutis calcinosis is the deposition of calcium phosphate in the skin and subcutaneous tissues. It can be separated into the following 4 different subtypes: idiopathic calcification, dystrophic calcification, metastatic calcification or calciphylaxis, and iatrogenic calcification.3 Cutis calcinosis has been associated with traumatic injuries, infections, and systemic connective tissue disorders, such as systemic lupus erythematosus, scleroderma, and dermatomyositis.3 However, a case of cutis calcinosis in the setting of a congenital hand deformity, such as symbrachydactyly, has not been previously described.
In this report, we present 2 cases of cutis calcinosis of the hand in the setting of symbrachydactyly and its subsequent management. To our knowledge, no report has described the association between the 2 conditions in pediatric patients.
Case report
The first case is a 5-month-old girl who presented for evaluation of an isolated congenital deformity of her left hand. Physical examination revealed a small thumb and rudimentary digits without osseous components. The little finger was missing. Additionally, the patient had a wrist flexion contracture with an inability to extend her wrist past the neutral position. She was able to use her thumb to pinch but had poor stability at the metacarpophalangeal and interphalangeal joints of her left thumb. Poland syndrome was excluded as the pectoralis major and chest wall were intact and no murmur was appreciated. The patient was diagnosed with isolated cleft-type monomelic symbrachydactyly with a reasonably developed thumb. In the following months, the patient developed a soft tissue mass of the left ring finger that progressively enlarged to extend over the dorsal aspect of the fourth metacarpal (Fig. 1). The decision was made to proceed with a surgical reconstruction of her left hand and mass excision. During surgery, the mass was noted to be a 3 × 1–cm cystic lesion with a thin capsule. The finger portion of the cyst contained a yellow–tan pasty material consistent with the typical contents of an inclusion cyst. In contrast, the proximal part of the cyst over the dorsal aspect of the fourth metacarpal contained an opaque, thick yellow fluid. The cyst extended to the dermal layer of the skin with scanty subcutaneous tissue adjacent to the cyst’s walls.
Figure 1.
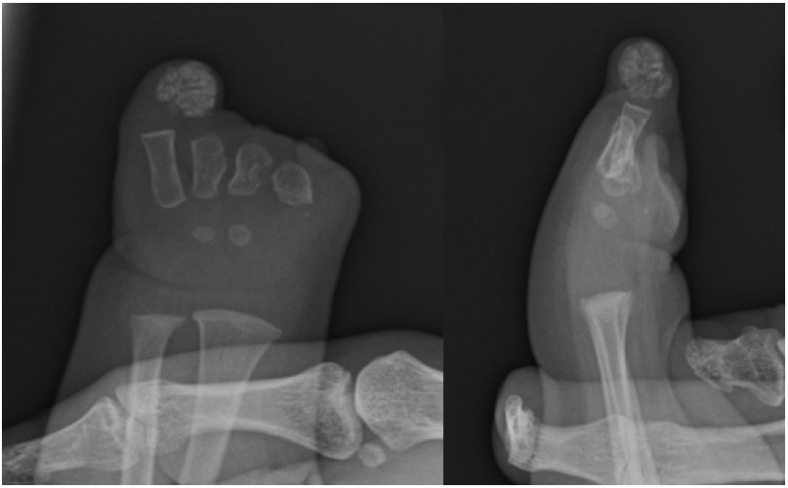
Clinical and radiographic images of the first patient’s (19-month-old girl) left hand. A-C The fingers were comprised of digital skin tubes without phalanges. The thumb and ring fingers were the longest of the skin tubes, with very short underdeveloped skin tubes of the index, middle, and little fingers. The nail plate is absent in all “fingers,” with a hypoplastic nail bed at the dorsal distal tip of the thumb. Additionally, the left ring finger showed a soft tissue mass within the skin tube. Underdeveloped thenar and hypothenar eminences were also noted. D, E Radiographic imaging of the left hand demonstrates a small left hand containing 5 short metacarpals. The first, second, third, and fourth metacarpals are approximately equal in length. Synostosis of the proximal shafts of the second and third metacarpals is also noted. There are no phalanges in any of the skin tubes. A soft tissue mass with evidence of calcification is noted at the distal aspect of the ring finger stump.
Similarly, the second patient is a 5-week-old boy who presented for management of symbrachydactyly of the left hand with no identifiable thumb. No other musculoskeletal deformities were identified, and Poland syndrome was again ruled out. Gradually the patient started developing a mass at the distal aspect of the little finger stump (Fig. 2). At 13 months of age, an infectious process was noted on the skin overlying the aforementioned mass. The patient was taken to the operating room for incision, drainage, and mass excision. During surgery, an encapsulated cyst was noted in the little finger stump, containing a white pasty calcified material. The family elected not to pursue reconstruction of the hand deformity.
Figure 2.
Radiographic images of the second patient’s left hand at 13 months of age. The first metacarpal is absent. The rest of the metacarpals are present, with the second and third metacarpals hypoplastic. All phalanges are absent. A calcified heterogeneous soft tissue mass is noted at the distal aspect of the ring finger stump.
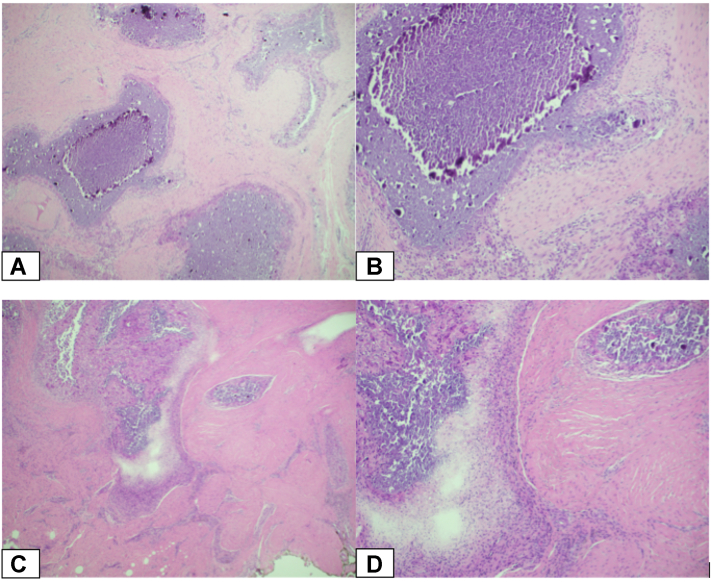
Histologic evaluation of both cases demonstrated soft tissue with a diffuse, intense inflammatory reaction surrounding multiple areas of calcified debris (Fig. 3). The stroma was pink and fibrous, consisting of cells with eosinophilic cytoplasm and small, round nuclei without a visible nucleolus. The inflammatory infiltrate consisted primarily of aggregates of macrophages and multinucleated giant cells surrounding areas of calcific debris. There was no evidence of malignancy, dysplasia, or viral cytopathic effect. In both cases, the masses were identified as cutis calcinosis. No local recurrence of the disease was noted in either patient at the 1-year follow-up (Fig. 4). Written informed consent was obtained from both patient families for publication of this case report.
Figure 3.
Hematoxylin and eosin (H&E) stained sections demonstrating soft tissue containing an intense inflammatory reaction composed primarily of aggregates of macrophages and multinucleated giant cells. The central portion of these aggregates contains calcific debris. The findings were consistent with tumoral calcinosis in both patients. A and B The first patient. C and D The second patient. (Hematoxylin and eosin stains; A and C, magnification × 40, B and D, magnification × 100).
Figure 4.
Clinical (A) and radiographic (B) images of the first patient’s hand immediately after surgery. The patient underwent a release of her left thumb contracture using a standard Z-plasty, followed by the transfer of a nonvascularized middle phalanx from the left second and third toes into the left thumb and ring fingers to improve function and stabilize the skin tubes. Capsulodesis of the metacarpophalangeal joints of the left thumb and ring finger were also performed. Finally, the left ring finger mass was excised. C Radiographic image of the patient seen in the clinic 1 year later showing an excellent functional outcome and no recurrence of the cutis calcinosis in her hand.
Discussion
We present 2 patients presenting with symbrachydactyly with concomitant ipsilateral cutis calcinosis. The association of cutis calcinosis with traumatic, inherited, or autoimmune diseases is well-established in the literature.3 However, the presence of cutis calcinosis in the pediatric hand is rare,4 and an association with symbrachydactyly has not been previously described.
The exact pathophysiology of cutis calcinosis remains unclear. It has been proposed that alterations in elastin, collagen, or subcutaneous fat can stimulate ectopic calcification in the affected subcutaneous tissue. Another hypothesis is that increased regional cell necrosis may contribute to calcium crystal deposition3 either by creating a more acidic environment that blocks local calcification inhibitors or by providing a nidus for dystrophic calcification in the form of released phosphate-bound denatured proteins of necrotic cells.5 Finally, it has been suggested that local ischemia induced by a pressure effect of steroid-induced hypertrophied fat cells can lead to ectopic calcification.6
Both patients originally presented for evaluation of isolated symbrachydactyly, with a soft tissue lesion being noted a few months later. No other abnormalities and pertinent mutations were identified during the genetic workup, making the diagnosis of dystrophic calcification unlikely. Furthermore, all laboratory values, including calcium and phosphate levels, were within normal limits, which helped us rule out metastatic calcinosis. Therefore, we hypothesized that the presence of calcium deposits in the patients were cases of idiopathic cutis calcinosis. We then considered possible mechanisms behind the simultaneous occurrence of hand symbrachydactyly and cutis calcinosis beyond a coincidence.
It has been suggested that symbrachydactyly arises because of the disruption of the apical ectodermal ridge (AER) that directs proximodistal growth7 and its pertinent transcriptional pathways in the developing limb bud.8 The precise etiology is unknown, but the leading hypothesis suggests disruption of the subclavian artery supply distal to the internal thoracic artery before day 42 of gestation.3 This compromised blood flow to the limb bud could subsequently lead to increased apoptosis in the AER zone. Several observations in animal models indicate that increased rates of cell death in the AER lead to fewer active AER cells during upper extremity formation.9 For example, increased apoptosis in the AER in mice results in regression of the central portion of the AER as seen in the dactylaplasia mutation, a phenotype similar to cleft hand and symbrachydactyly in humans.10
Therefore, it would seem that symbrachydactyly and cutis calcinosis of the hand may share some common pathophysiologic mechanisms, both occurring in the setting of local ischemia and increased cell death. However, the exact pathogenesis of hand soft tissue calcification in pediatric patients with symbrachydactyly cannot be definitively determined on the basis of this case report.
Finally, the incidence and clinical significance of the simultaneous presence of symbrachydactyly and cutis calcinosis remains unclear, making it somewhat challenging to counsel the parents in such scenarios. It is possible that patients with symbrachydactyly develop cutis calcinosis more often than previously believed as they elect to either not present to clinic for evaluation or proceed with nonsurgical management as long as the mass remains asymptomatic and does not affect hand function. In both of our patients, the families elected to proceed with surgical intervention for the soft tissue mass, each for different reasons, allowing us to make the diagnosis of cutis calcinosis definitively. In the first patient, the mass was resected at the time of surgical reconstruction of the hand for the symbrachydactyly. In the second case, the parents had originally elected to proceed with nonsurgical management of the symbrachydactyly and the little finger mass. However, the patient’s course was complicated by infection of the noted mass, which required surgical intervention. No recurrence of the cutis calcinosis was noted for either patient. Treatment of the cutis calcinosis of the hand in the setting of symbrachydactyly with surgical excision versus expectant management is not yet definitely established. It should be decided in collaboration with the family, based on patient symptomatology and functional goals.
Further studies are needed to understand the features of cutis calcinosis in patients with congenital hand anomalies and any potential pathways that may be shared between these 2 pathologies. Moreover, it would be interesting to elucidate the clinical course of cutis calcinosis if left untreated to determine whether surgical intervention in the setting of symbrachydactyly is absolutely necessary.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
References
- 1.Oberg K.C., Feenstra J.M., Manske P.R., Tonkin M.A. Developmental biology and classification of congenital anomalies of the hand and upper extremity. J Hand Surg Am. 2010;35(12):2066–2076. doi: 10.1016/j.jhsa.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Bavinck J.N., Weaver D.D. Subclavian artery supply disruption sequence: hypothesis of a vascular etiology for Poland, Klippel-Feil, and Möbius anomalies. Am J Med Genet. 1986;23(4):903–918. doi: 10.1002/ajmg.1320230405. [DOI] [PubMed] [Google Scholar]
- 3.Reiter N., El-Shabrawi L., Leinweber B., Berghold A., Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65(1):1–12. doi: 10.1016/j.jaad.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 4.White N., Chester D.L., Khanna A. Congenital bilateral calcinosis cutis of the hands. J Hand Surg Br. 2006;31(5):522–523. doi: 10.1016/j.jhsb.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.Y., Choi H.Y., Myung K.B., Choi Y.W. The expression of molecular mediators in the idiopathic cutaneous calcification and ossification. J Cutan Pathol. 2008;35(9):826–831. doi: 10.1111/j.1600-0560.2007.00904.x. [DOI] [PubMed] [Google Scholar]
- 6.Powell R.J. Systemic lupus erythematosus with widespread subcutaneous fat calcification. Proc R Soc Med. 1974;67(3):215–217. doi: 10.1177/003591577406700319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tickle C. Molecular basis of limb development. Biochem Soc Trans. 1994;22(3):565–569. doi: 10.1042/bst0220565. [DOI] [PubMed] [Google Scholar]
- 8.Goodell P.B., Bauer A.S., Sierra F.J., James M.A. Symbrachydactyly. Hand (N Y) 2016;11(3):262–270. doi: 10.1177/1558944715614857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Teran M., Ros M.A. The apical ectodermal ridge: morphological aspects and signaling pathways. Int J Dev Biol. 2008;52(7):857–871. doi: 10.1387/ijdb.072416mf. [DOI] [PubMed] [Google Scholar]
- 10.Sidow A., Bulotsky M.S., Kerrebrock A.W., et al. A novel member of the F-box/WD40 gene family, encoding dactylin, is disrupted in the mouse dactylaplasia mutant. Nat Genet. 1999;23(1):104–107. doi: 10.1038/12709. [DOI] [PubMed] [Google Scholar]