Abstract
Purpose
We aimed to investigate the prevalences of obesity, abdominal obesity, and non-alcoholic fatty liver disease (NAFLD) among children and adolescents during the coronavirus disease 2019 (COVID-19) outbreak.
Materials and Methods
This population-based study investigated the prevalences of obesity, abdominal obesity, and NAFLD among 1428 children and adolescents between 2018–2019 and 2020. We assessed the prevalences of obesity, abdominal obesity, and NAFLD according to body mass index, age, sex, and residential district. Logistic regression analyses were performed to determine the relationships among obesity, abdominal obesity, and NAFLD.
Results
In the obese group, the prevalence of abdominal obesity increased from 75.55% to 92.68%, and that of NAFLD increased from 40.68% to 57.82%. In age-specific analysis, the prevalence of abdominal obesity increased from 8.25% to 14.11% among participants aged 10–12 years and from 11.70% to 19.88% among children aged 13–15 years. In residential district-specific analysis, the prevalence of both abdominal obesity and NAFLD increased from 6.96% to 15.74% in rural areas. In logistic regression analysis, the odds ratio of abdominal obesity for NAFLD was 11.82.
Conclusion
Our results demonstrated that the prevalences of abdominal obesity and NAFLD increased among obese Korean children and adolescents and in rural areas during the COVID-19 outbreak. Additionally, the prevalence of abdominal obesity increased among young children. These findings suggest the importance of closely monitoring abdominal obesity and NAFLD among children during COVID-19, focusing particularly on obese young children and individuals in rural areas.
Keywords: Obesity, abdominal obesity, non-alcoholic fatty liver disease, child, adolescent, COVID-19
INTRODUCTION
The World Health Organization declared the coronavirus disease 2019 (COVID-19) disease outbreak to be a pandemic on March 11, 2020. To mitigate the spread of the disease, social distancing measures, including school closures and lockdowns, were implemented.1 In South Korea, the first case of COVID-19 was confirmed on January 20, 2020, and by March, schools were closed.2 This unprecedented social scenario resulted in decreased physical activity and alterations in dietary patterns. These lifestyle changes were followed by increased prevalences of obesity and obesity-related comorbidities.3 A meta-analysis reported that globally, the body weight of people increased during the COVID-19 outbreak.1 Similarly, Auriemma, et al.4 reported that the prevalences of obesity and diabetes mellitus increased among adults. Meanwhile, Milic, et al.5 reported that metabolic-associated fatty liver disease became highly prevalent among patients with post-acute COVID-19 syndrome. Non-alcoholic fatty liver disease (NAFLD) comprises the entire spectrum of liver disease induced by excessive fat accumulation in the liver.6 Among obesity-associated comorbidities, NAFLD is emerging as a leading cause of chronic liver diseases, such as liver fibrosis and cirrhosis, with an increasing prevalence globally.6 Moreover, NAFLD is associated with all-cause mortalities, including liver-related deaths, cardiovascular diseases, and diabetes mellitus.7 Our previous study demonstrated that the prevalences of generalized obesity, abdominal obesity, and NAFLD increased among Korean children and adolescents before the pandemic.8 Kim, et al.2 reported that levels of fasting glucose, total cholesterol, and triglycerides increased among Korean children during the COVID-19 outbreak. However, there are limited investigations on changes in the prevalences of obesity and NAFLD during the COVID-19 outbreak among Korean children and adolescents.
The public health impact of COVID-19 may differ between rural and urban areas.9,10,11 Compared to urban areas, rural areas have healthcare limitations and inadequate access to telemedicine owing to inadequate internet facilities.9,11 Additionally, the proportion of rural residents with major vulnerabilities, such as old age and disabilities, is higher.9 Conversely, having a dense population and being a transportation hub are risk factors for viral spread in urban areas. Additionally, the impact of social distancing differs between urban and rural areas.11 Park, et al.11 reported that reductions in mobility were greater in urban areas than in rural areas in Western Pacific countries, including South Korea, owing to poor adherence to social distancing in rural areas.
Therefore, this study aimed to investigate changes in the prevalences of obesity, abdominal obesity, and NAFLD among Korean children and adolescents during the COVID-19 outbreak using data from the Korea National Health and Nutrition Examination Survey (KNHANES). Our objectives were 1) to investigate changes in the prevalences of obesity, abdominal obesity, and NAFLD during the COVID-19 outbreak among Korean children and adolescents and 2) to compare differences between urban and rural areas in relation to the effects of COVID-19 on obesity, abdominal obesity, and NAFLD among Korean children and adolescents.
MATERIALS AND METHODS
Ethical considerations
This study was performed as per the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (IRB, 3-2022-0115). Informed consent was obtained from all the participants.
Study design and participants
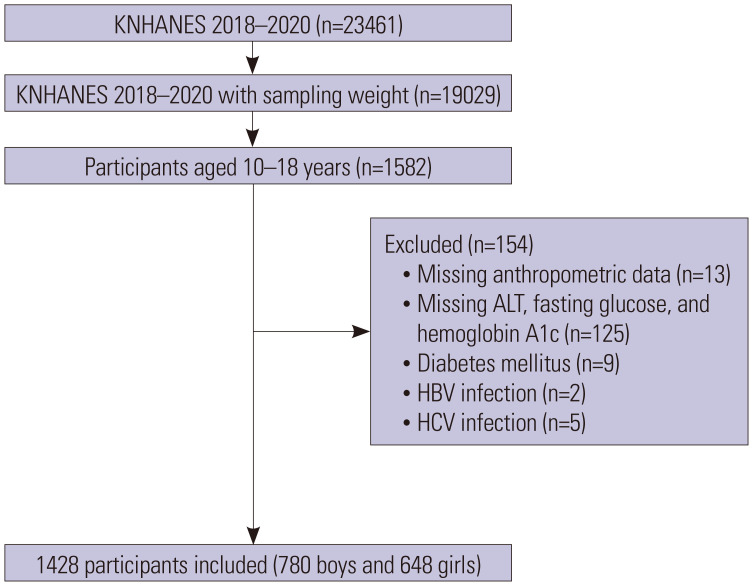
This cross-sectional study involved the investigation of data procured from 2018 to 2020 with the objective of comparing patterns before and during COVID-19. Among the 23461 participants included in the KNHANES 2018–2020, 1582 participants were 10–18 years of age (Fig. 1). After excluding those with missing data on anthropometry, alanine aminotransferase (ALT) levels, fasting blood glucose levels, hemoglobin A1c levels, diabetes mellitus status, and/or hepatitis B/C virus infection status, 1428 participants were eligible for study enrollment. Participants were considered to be diabetic if they had a known history of diabetes, fasting glucose level ≥126 mg/dL, and/or hemoglobin A1c level ≥6.5%.
Fig. 1. Design and flowchart of the study population. KNHANES, Korea National Health and Nutrition Examination Survey; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus.
The KNHANES, which began in 1998, is a national-level, cross-sectional, representative survey involving a multistage, stratified, systematic sampling design. It is conducted by the Korea Centers for Disease Control and Prevention over 17 districts in both urban and rural areas with the objective of identifying health behaviors, nutrition status, and chronic disease prevalence. The survey has collected data on health behavior and nutritional information using physical examinations and interviews. The survey has adopted a two-stage stratified sampling method with sampling units as the primary and households as the secondary sampling units, respectively.
Study variables
Data on age, sex, anthropometric measurements, and residential district were collected. The height of the participants was measured (±0.1 cm) using a portable stadiometer (range, 850–2060 mm; Seriter, Holtain Ltd., Crymych, UK), and weight was measured (±0.1 kg) in an upright position using a calibrated balance beam scale (Giant 150N; HANA, Seoul, South Korea). Body mass index (BMI) was calculated as weight divided by height in meters squared. Waist circumference (WC) was measured at the narrowest point between the iliac crest and the lower borders of the rib cage at the end of expiration, and waist-to-height ratio (WHtR) was calculated as WC (cm)/height (cm). Height, weight, and BMI are presented as standard deviation score (SDS) values based on the 2017 Korean National Growth Charts.12 Children were classified as normal (<85th percentile), overweight (85th to <95th percentile), or obese (≥95th percentile) according to BMI. Abdominal obesity was defined as WC >90th percentile using Korean waist reference data.13 Additionally, we investigated the proportion of participants with WHtR >0.5.14
Residential districts were classified into urban and rural areas based on administrative districts and populations. In Korea, district levels are named Dong, Eup, and Myeon. Dong was considered to be an urban area owing to its dense population; Eup and Myeon were considered to be rural areas as they are more sparsely populated.
Laboratory analysis
Blood samples were drawn from an antecubital vein following 8 hours of fasting. The serum levels of fasting glucose, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), triglycerides, aspartate aminotransferase, and ALT were measured using Labospect 008AS (Hitachi, Tokyo, Japan). The Friedewald formula {low-density lipoprotein-cholesterol (LDL-C)=total cholesterol-[HDL-C+ (triglycerides/5)]} was applied to determine LDL-C values for serum samples with triglyceride values ≤400 mg/dL. LDL-C was considered to be missing for samples with triglyceride values >400 mg/dL. This is owing to a reduction in the accuracy of the formula when triglyceride levels are above 400 mg/dL.15 NAFLD was defined as elevated ALT levels (>26 U/L for boys and >22 U/L for girls) without hepatitis B or hepatitis C viral infection.16 Impaired fasting glucose was defined as fasting glucose levels between 100–125 mg/dL.
Statistical analysis
Sampling weights were considered in all analyses; sampling weights were considered to report representative estimates for the Korean population. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA) for the complex survey design, with clustering, stratification, and unequal weighting of the KNHANES sample being accounted for. All continuous variables are expressed as weighted means with standard errors, whereas categorical variables are expressed as weighted percentages with standard errors. We divided the participants into subgroups according to BMI, age, sex, and residential district, and then analyzed the changes in the proportion of participants with obesity, abdominal obesity, and NAFLD between 2018–2019 and 2020. The participants were divided into subgroups according to age: 10–12 years (elementary school), 13–15 years (middle school), and 16–18 years (high school). The differences between groups were tested using an independent two-sample t-test and analysis of variance (ANOVA) for continuous variables and the Rao-Scott chi-square test for categorical variables. To clarify the association between NAFLD and obesity and abdominal obesity, logistic regression analyses were performed with NAFLD as the dependent variable, and the Rao-Scott chi-square test was used to compare the proportion of abdominal obesity between participants with NAFLD and those without. All p-values were calculated using a two-tailed t-test, and statistical significance was set at p<0.05.
RESULTS
Participant characteristics and the prevalence of obesity, abdominal obesity, and NAFLD
Among all participants, mean WC increased from 71.03 cm to 72.88 cm, while mean WHtR increased from 0.44 to 0.45 from 2018–2019 to 2020 (p=0.021 for WC and p=0.008 for WHtR) (Table 1). However, changes in the prevalences of obesity, abdominal obesity, and NAFLD were not statistically significant. In 2020, the prevalences of obesity, abdominal obesity, and NAFLD were 13.75%, 15.47%, and 16.84%, respectively. Changes in the prevalences of obesity, abdominal obesity, and NAFLD were not significant in sex-specific analyses (Supplementary Table 1, only online).
Table 1. Baseline Characteristics of the Participants (n=1428).
| Variable | 2018–2019 (n=1017) | 2020 (n=411) | p value | |
|---|---|---|---|---|
| Age, yr | 14.36 (0.10) | 14.18 (0.15) | 0.312 | |
| Sex (male), % | 51.95 (1.83) | 54.37 (2.68) | 0.455 | |
| Residential district (urban), % | 87.94 (2.27) | 90.73 (2.80) | 0.456 | |
| Height SDS | 0.31 (0.04) | 0.32 (0.07) | 0.923 | |
| Weight SDS | 0.20 (0.05) | 0.31 (0.08) | 0.270 | |
| BMI SDS | 0.06 (0.06) | 0.19 (0.08) | 0.186 | |
| BMI percentile | 0.337 | |||
| Normal, % | 78.17 (1.73) | 75.22 (2.39) | ||
| Overweight, % | 8.13 (0.98) | 11.03 (1.65) | ||
| Obesity, % | 13.70 (1.46) | 13.75 (2.00) | ||
| WC, cm | 71.03 (0.45) | 72.88 (0.66) | 0.021 | |
| Abdominal obesity, % | 11.66 (1.23) | 15.47 (2.02) | 0.091 | |
| WHtR | 0.44 (0.00) | 0.45 (0.00) | 0.008 | |
| WHtR >0.5, % | 14.21 (1.45) | 18.86 (2.09) | 0.059 | |
| AST, IU/L | 20.62 (0.30) | 22.05 (0.76) | 0.081 | |
| ALT, IU/L | 17.07 (0.71) | 19.15 (1.11) | 0.116 | |
| NAFLD, % | 13.01 (1.23) | 16.84 (1.87) | 0.076 | |
| Abdominal obesity and NAFLD, % | 5.96 (0.86) | 8.49 (1.52) | 0.122 | |
| Glucose, mg/dL | 91.90 (0.26) | 91.48 (0.56) | 0.495 | |
| Impaired fasting glucose, % | 11.79 (1.06) | 11.46 (1.90) | 0.882 | |
| Hemoglobin A1c, % | 5.36 (0.01) | 5.34 (0.02) | 0.346 | |
| Total cholesterol, mg/dL | 165.37 (1.02) | 164.20 (1.52) | 0.517 | |
| HDL-C, mg/dL | 52.12 (0.38) | 51.31 (0.60) | 0.248 | |
| LDL-C, mg/dL | 96.20 (0.94) | 94.82 (1.30) | 0.385 | |
| Triglycerides, mg/dL | 89.06 (1.84) | 95.03 (3.27) | 0.110 | |
SDS, standard deviation score; BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NAFLD, non-alcoholic fatty liver disease; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol.
Continuous variables are presented as means (standard error) and categorical data as percentages (standard error).
Changes in the prevalences of obesity, abdominal obesity, and NAFLD according to BMI
In BMI-specific analyses, the mean WHtR increased significantly in all groups (Table 2). The proportion of participants with abdominal obesity increased from 75.55% to 92.68% in the obesity group between 2018–2019 and 2020 (p=0.002). Additionally, the proportion of participants with WHtR >0.5 increased from 24.93% to 47.49% (p=0.012) in the overweight group and from 81.66% to 94.22% (p=0.025) in the obese group. In the obese group, the proportion of participants with NAFLD increased from 45.76% to 62.52% (p=0.033), whereas those with both abdominal obesity and NAFLD increased from 40.68% to 57.82% (p=0.032). In the normal BMI group, the proportion of participants with abdominal obesity and NAFLD decreased from 0.07% to 0.00% (p<0.032).
Table 2. Proportions of Abdominal Obesity and NAFLD according to BMI Classification.
| Variable | Normal | Overweight | Obesity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2018–2019 (n=799) | 2020 (n=297) | p value | 2018–2019 (n=86) | 2020 (n=52) | p value | 2018–2019 (n=132) | 2020 (n=62) | p value | |
| Height SDS | 0.24 (0.05) | 0.25 (0.08) | 0.904 | 0.55 (0.12) | 0.28 (0.18) | 0.210 | 0.55 (0.12) | 0.70 (0.15) | 0.442 |
| Weight SDS | -0.29 (0.04) | -0.23 (0.08) | 0.499 | 1.34 (0.06) | 1.21 (0.09) | 0.233 | 2.34 (0.08) | 2.53 (0.10) | 0.139 |
| WC, cm | 66.92 (0.30) | 68.08 (0.57) | 0.075 | 78.69 (0.83) | 80.84 (0.99) | 0.097 | 89.90 (0.98) | 92.74 (1.11) | 0.054 |
| Abdominal obesity, % | 0.25 (0.19) | 0.68 (0.51) | 0.323 | 13.75 (4.58) | 20.08 (6.00) | 0.394 | 75.55 (4.50) | 92.68 (3.13) | 0.002 |
| WHtR | 0.41 (0.00) | 0.42 (0.00) | 0.020 | 0.48 (0.00) | 0.50 (0.00) | 0.002 | 0.54 (0.01) | 0.56 (0.01) | 0.031 |
| WHtR >0.5, % | 1.27 (0.39) | 0.88 (0.53) | 0.586 | 24.93 (5.31) | 47.49 (7.29) | 0.012 | 81.66 (3.81) | 94.22 (3.09) | 0.025 |
| AST, IU/L | 19.83 (0.27) | 20.83 (0.76) | 0.214 | 22.47 (1.29) | 19.85 (0.83) | 0.084 | 24.08 (1.28) | 30.49 (3.20) | 0.063 |
| ALT, IU/L | 13.61 (0.38) | 14.82 (0.72) | 0.141 | 25.60 (5.01) | 18.02 (1.28) | 0.143 | 31.77 (2.73) | 43.74 (5.50) | 0.052 |
| Glucose, mg/dL | 91.56 (0.28) | 91.05 (0.57) | 0.423 | 91.83 (0.91) | 91.72 (1.43) | 0.945 | 93.92 (0.71) | 93.65 (0.93) | 0.820 |
| NAFLD, % | 6.19 (0.95) | 8.51 (1.69) | 0.202 | 23.35 (5.33) | 16.70 (5.83) | 0.413 | 45.76 (4.70) | 62.52 (6.25) | 0.033 |
| Abdominal obesity and NAFLD, % | 0.07 (0.07) | 0.00 (0.00) | <0.001 | 4.07 (2.09) | 4.92 (3.23) | 0.818 | 40.68 (4.64) | 57.82 (6.59) | 0.032 |
NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, Waist-to-height ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Continuous variables are presented as means (standard error) and categorical data as percentages (standard error).
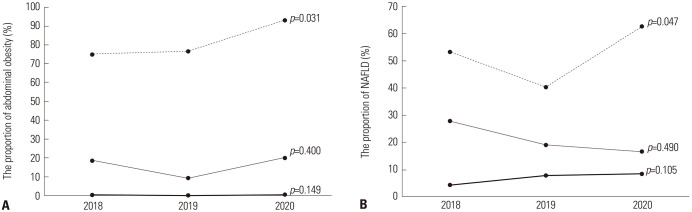
The ANOVA test comparing data from 2018, 2019, and 2020 in the obese group showed that the proportion of participants with abdominal obesity (p=0.031) and those with NAFLD (p=0.047) increased from 2018 to 2020 (Fig. 2).
Fig. 2. Changes in the proportions of abdominal obesity and NAFLD in children and adolescents according to BMI groups during the COVID-19 outbreak. (A) Changes in the proportions of abdominal obesity in children and adolescents according to BMI during the COVID-19 outbreak. (B) Changes in the proportions of NAFLD in children and adolescents according to BMI during the COVID-19 outbreak. The solid line represents the normal group, the narrow line represents the overweight group, and the dashed line represents the obesity group. NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; COVID-19, coronavirus disease 2019.
Changes in the prevalences of obesity, abdominal obesity, and NAFLD according to age
In the age-specific analysis, mean WC increased from 65.59 cm to 67.75 cm in those aged 10–12 years (p=0.026) and from 71.70 cm to 74.72 cm in those aged 13–15 years (p=0.038) (Supplementary Table 2, only online). Mean WHtR increased from 0.44 to 0.45 in those aged 10–12 years (p=0.045) and 16–18 years (p=0.023) and from 0.43 to 0.45 in those aged 13–15 years (p=0.030). The proportion of abdominal obesity increased from 8.25% to 14.11% in participants aged 10–12 years (p=0.034) and from 11.70% to 19.88% in those aged 13–15 years (p=0.037). The proportion of participants with WHtR >0.5 increased from 12.69% to 22.52% in those aged 13–15 years (p=0.014). However, changes in the prevalence of obesity and NAFLD were not statistically significant.
Changes in the prevalences of obesity, abdominal obesity, and NAFLD according to residential district
In the residential district-specific analyses, mean WC increased from 70.90 cm to 72.67 cm (p=0.042), and mean WHtR increased from 0.44 to 0.45 (p=0.017) from 2018–2019 to 2020 for participants in urban areas (Table 3). Meanwhile, in rural areas, the proportion of participants with NAFLD increased from 15.16% to 24.86% (p=0.044), and those with both abdominal obesity and NAFLD increased from 6.96% to 15.74% (p=0.040). The change in obesity prevalence was not statistically significant in either group.
Table 3. Proportions of Obesity, Abdominal Obesity and NAFLD according to Residential District.
| Variable | Urban | p value | Rural | p value | |||
|---|---|---|---|---|---|---|---|
| 2018–2019 (n=866) | 2020 (n=350) | 2018–2019 (n=151) | 2020 (n=61) | ||||
| Height SDS | 0.31 (0.04) | 0.32 (0.07) | 0.901 | 0.31 (0.13) | 0.29 (0.14) | 0.908 | |
| Weight SDS | 0.18 (0.05) | 0.28 (0.09) | 0.311 | 0.37 (0.16) | 0.55 (0.16) | 0.416 | |
| BMI SDS | 0.03 (0.06) | 0.16 (0.09) | 0.206 | 0.30 (0.17) | 0.49 (0.17) | 0.434 | |
| BMI percentile | 0.565 | 0.222 | |||||
| Normal, % | 79.17 (1.85) | 76.49 (2.53) | 70.85 (4.88) | 62.82 (5.90) | |||
| Overweight, % | 8.28 (1.02) | 10.41 (1.71) | 7.07 (3.42) | 17.15 (6.29) | |||
| Obesity, % | 12.55 (1.56) | 13.11 (2.13) | 22.07 (3.89) | 20.04 (4.45) | |||
| WC, cm | 70.90 (0.49) | 72.67 (0.71) | 0.042 | 71.99 (1.14) | 74.93 (1.31) | 0.091 | |
| Abdominal obesity, % | 10.69 (1.30) | 14.28 (2.12) | 0.127 | 18.75 (3.28) | 27.14 (5.37) | 0.160 | |
| WHtR | 0.44 (0.00) | 0.45 (0.00) | 0.017 | 0.45 (0.01) | 0.46 (0.01) | 0.098 | |
| WHtR >0.5, % | 13.10 (1.53) | 17.82 (2.21) | 0.069 | 22.25 (4.15) | 29.04 (4.96) | 0.288 | |
| AST, IU/L | 20.68 (0.32) | 22.07 (0.83) | 0.119 | 20.20 (0.88) | 21.84 (0.94) | 0.200 | |
| ALT, IU/L | 17.16 (0.79) | 19.03 (1.21) | 0.196 | 16.45 (1.15) | 20.29 (1.92) | 0.084 | |
| Glucose, mg/dL | 91.81 (0.27) | 91.40 (0.61) | 0.531 | 92.54 (0.99) | 92.28 (0.75) | 0.837 | |
| NAFLD, % | 12.71 (1.32) | 16.02 (2.00) | 0.152 | 15.16 (3.33) | 24.86 (4.12) | 0.044 | |
| Abdominal obesity and NAFLD, % | 5.82 (0.91) | 7.75 (1.54) | 0.257 | 6.96 (2.29) | 15.74 (4.88) | 0.040 | |
NAFLD, non-alcoholic fatty liver disease; SDS, standard deviation score; BMI, body mass index; WC, waist circumference; WHtR, Waist-to-height ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Continuous variables are presented as means (standard error) and categorical data as percentages (standard error).
Association between abdominal obesity and NAFLD
The prevalence of abdominal obesity was 47.6% among participants with NAFLD, while the corresponding value was 7.1% among those without NAFLD (p<0.001) (Supplementary Fig. 1A, only online). The proportion of participants with WHtR >0.5 was 55.3% among the participants with NAFLD, while the corresponding value was 9.1% among those without NAFLD (p<0.001) (Supplementary Fig. 1B, only online).
In logistic regression analyses, odds ratios (ORs) [95% confidence interval (CIs)] of being overweight and obese for NAFLD were 3.52 (2.04–6.05) and 14.10 (9.47–21.00), respectively (Supplementary Table 3, only online). Additionally, the ORs (95% CIs) of abdominal obesity and WHtR >0.5 for NAFLD were 11.82 (8.03–17.40) and 12.30 (8.54–17.73), respectively.
DISCUSSION
Our data highlight increased prevalences of abdominal obesity among obese Korean children and adolescents and in individuals aged 10–15 years. The prevalence of generalized obesity increased among children and adolescents aged 13–15 years. In addition, the prevalence of NAFLD increased among obese children and adolescents and among individuals in rural areas. The prevalences of both abdominal obesity and NAFLD increased in children and adolescents with obesity and in those residing in rural areas. Abdominal obesity was positively related to NAFLD in logistic regression analysis and independent two-sample t-tests.
In our study, WC and WHtR increased among all participants, and the prevalence of abdominal obesity increased among obese participants and those aged 10–15 years; however, BMI SDS and the proportion of obesity did not change significantly. According to our previous study, before the COVID-19 outbreak, the prevalences of obesity and abdominal obesity increased from 6.55% to 11.64% and from 5.97% to 10.51%, respectively, from 2007 to 2018 among Korean children and adolescents.17 Several studies have demonstrated the effects of the COVID-19 outbreak on obesity among children and adolescents. A study conducted in Italy reported that the prevalence of obesity increased from 23.2% to 27.4% among children and adolescents during the COVID-19 outbreak.18 Similarly, another study conducted in Italy reported that the mean BMI SDS increased 0.07, and the mean WC increased by 4.4 cm among obese adolescents during the COVID-19 outbreak.19 In Israel, a cohort study reported that the prevalence of obesity increased from 10.5% to 12.3% during the COVID-19 outbreak among children and adolescents,20 while in China, the prevalence of obesity increased from 10.5% to 12.9%.21
In Korea, a population-based study reported that body weight increased during the COVID-19 outbreak.22 A KNHANES-based study reported that the prevalence of obesity increased among Korean men.23 A Korean study that investigated children who visited outpatient clinics in 2019–2020 reported that BMI SDS increased during the COVID-19 outbreak.2 Another Korean retrospective cohort study reported that BMI SDS increased by 0.219, and the prevalence of overweight or obese children increased from 23.9% to 31.4% during the COVID-19 outbreak.24 In another retrospective study, the proportion of overweight or obese children increased from 24.5% to 38.1% during the COVID-19 outbreak.25 More studies, including those encompassing data from 2021–2022, are required to clarify changes in the prevalence of obesity and abdominal obesity among children and adolescents.
An increase in the prevalence of abdominal obesity and NAFLD in our study might be associated with lifestyle changes during the COVID-19 outbreak. A meta-analysis reported that physical activity decreased among people during the COVID-19 outbreak.26 Clemete-Suárez, et al.27 reported that increased intake of high caloric foods and decreased intake of vegetables could be risk factors for the increase in the prevalence of obesity and chronic disease during the COVID-19 outbreak. A Chinese study reported that intake of fresh vegetables, fruits, and soybean products decreased during the pandemic.28 In Korea, a population-based study reported a decrease in physical activity and sleep time and an increase in unhealthy dietary habits, such as fast-food and sugary soda consumption, among adults during this period.29 Park, et al.22 reported that physical activity decreased among Koreans during the COVID-19 outbreak. However, our previous studies have reported increases in the prevalences of abdominal obesity, NAFLD, and metabolic syndrome with an increase in the intake of calories and fat in Korean children and adolescents even before the COVID-19 outbreak.8,17,30 Therefore, more studies are required to investigate lifestyle changes associated with the prevalences of obesity and NAFLD among children and adolescents to elucidate the effect of the COVID-19 outbreak.
In our study, the adverse trend for abdominal obesity was significant among young children. This result was in contrast to our previous study in which the adverse trend of abdominal obesity was more significant among adolescents aged 16–18 years than among children aged 10–15 years during 2009–2018.8 A US study reported that children aged 2–5 years had a 26% increase in the rate of obesity, compared to those aged 6–12 years, during the COVID-19 outbreak.18 A Chinese study reported that increases in BMI SDS and the prevalence of obesity during the COVID-19 outbreak were more apparent in younger children aged 6–11 years than in adolescents aged 12–17 years.31 In Israel, an elevation of BMI SDS was more obvious among children aged 2–6 years than those aged 6–18 years.20 Adverse changes in the prevalence of obesity among young children might be associated with more prominent lifestyle changes since they may be more vulnerable to environmental changes than adolescents.31 For instance, the effects of decreased physical activity due to school closing might be more apparent in young children, particularly since school is an important avenue for physical education in this age group.31,32
In the current study, adverse trends for NAFLD and abdominal obesity were prominent among obese children and adolescents. In our previous study, an adverse trend of NAFLD was apparent among children and adolescents with normal BMI, while abdominal obesity adverse trends were apparent among obese children and adolescents.8 During the COVID-19 outbreak, the development and aggravation of fatty liver was more prominent during lockdown, compared to the pre-social distancing period, in a Korean multicenter study.33 Before the COVID-19 outbreak, the prevalence of NAFLD increased from 8.2% in 2009 to 12.1% in 2018 among Korean children and adolescents.8 In this study, adverse trends for abdominal obesity and NAFLD were more apparent in children and adolescents with normal BMI than those with obesity. Contrastingly, a longitudinal study in the US reported that BMI increased during the COVID-19 outbreak, which could cause adverse metabolic changes, and was more prominent in children with obesity than in those with normal BMI.32 A cohort study also reported that increases in BMI were more prominent in children with pre-existing obesity than those at a healthy weight.34 Thus, in the current study, the increased prevalence of NAFLD in children and adolescents with obesity may be related to the adverse trends in BMI and abdominal obesity seen in obese children during the COVID-19 outbreak.8 Further studies investigating the differential effects of COVID-19 on the liver of children with varying BMI are still needed.
In our study, the prevalence of NAFLD increased with abdominal obesity in obese children and adolescents and in those residing in rural areas; however, the prevalence of obesity did not increase significantly. Abdominal obesity was found to be strongly associated with NAFLD. Although obesity is typically defined in terms of BMI, considering the metabolically healthy obese and metabolically unhealthy normal weight (MUNW) people, an alternative definition of obesity using WC or body fat percentage is required to assess the risk factors of obesity.35,36,37 MUNW patients have a normal BMI coupled with metabolic abnormalities, such as abdominal obesity, insulin resistance, and/or dyslipidemia, which are associated with NAFLD in children and adolescents.8,38 Dobson, et al.39 reported that MUNW participants had a higher proportion of NAFLD than metabolically healthy obese participants. Junge, et al.40 reported that the association of insulin and hemoglobin A1c levels with WC was stronger than that of body weight and BMI in children and adolescents. Therefore, close monitoring of NAFLD is required in children and adolescents with abdominal obesity, as well as in those with generalized obesity.
In our study, the prevalences of both abdominal obesity and NAFLD in children and adolescents increased in rural areas, while the corresponding values did not change significantly in urban areas. A Chinese study reported that the prevalence of obesity increased more in rural areas than in urban areas between 2020, and the highest value occurred during 2014–2019 among children and adolescents.31 Another Chinese study reported that during the COVID-19 outbreak, the prevalence of obesity among adolescents increased from 13.4% to 15.0% in rural areas, and increased from 15.1% to 15.8% in urban areas.41 The reason why the prevalences of abdominal obesity and NAFLD increased more in rural areas than urban areas may be the associated higher vulnerability for COVID-19 infection in rural areas compared to that in urban areas owing to fewer physicians, poor social health services, and inadequate access to telemedicine.9 This vulnerability might contribute to the increased prevalences of abdominal obesity and NAFLD through decreased physical activity or harmful effects of COVID-19 infection on the liver.9,42 Additionally, an increase in family time during the COVID-19 outbreak owing to the work-from-home set-up of parents in urban areas might have facilitated focused childcare, which may have resulted in a positive effect on obesity and NAFLD in children.43 Meanwhile, a US study reported that the proportion of people with increased body weight during the COVID-19 outbreak was higher in urban areas than in rural areas.44 Further studies are required to clarify the differences in the effects of COVID-19 on obesity and obesity-related comorbidities between rural and urban areas.
This study has some limitations. First, this was a retrospective cross-sectional study investigating only South Koreans. Second, we did not consider lean and fat mass in our definitions of “obese” and “overweight.” Third, imaging studies and liver biopsies were not performed to diagnose NAFLD. Fourth, the factors associated with obesity and NAFLD, such as physical activity and nutritional status, were not considered in this study owing to incomplete data and varying survey methods that changed every year. Fifth, only the year 2020 was considered to be impacted by COVID-19 in this study because KNHANES 2021 and 2022 have not been released yet.
In conclusion, this study demonstrated an increased prevalence of abdominal obesity and NAFLD and a relationship between disease prevalence and the COVID-19 outbreak among Korean youth. This adverse trend was more apparent in young children and obese children and adolescents. Moreover, the trend of abdominal obesity and NAFLD has been worsening steadily in the recent decade, even before the COVID-19 outbreak. Our findings suggest that close monitoring of WC and liver enzymes should continue during the COVID-19 outbreak. Additionally, further studies investigating changes in factors associated with abdominal obesity and NAFLD, as well as other obesity-related comorbidities, including data from 2021 and 2022, are required to clarify the metabolic effect of the COVID-19 outbreak among children and adolescents.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2021-0150).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kyungchul Song and Hyun Wook Chae.
- Data curation: Kyungchul Song, Su Jin Kim, Myeongseob Lee, Junghwan Suh, and Ahreum Kwon.
- Formal analysis: Kyungchul Song and Juyeon Yang.
- Funding acquisition: Kyungchul Song.
- Investigation: Kyungchul Song and Hyun Wook Chae.
- Methodology: Kyungchul Song and Hyun Wook Chae.
- Project administration: Kyungchul Song and Hyun Wook Chae.
- Resources: Kyungchul Song.
- Software: Kyungchul Song and Juyeon Yang.
- Supervision: Ho-Seong Kim and Hyun Wook Chae.
- Validation: Hye Sun Lee.
- Visualization: Kyungchul Song and Juyeon Yang.
- Writing—original draft: Kyungchul Song.
- Writing—review & editing: Hyun Wook Chae.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Proportions of Obesity, Abdominal Obesity, and NAFLD according to Sex
Proportions of Obesity, Abdominal Obesity, and NAFLD according to Age Classification
ORs of NAFLD according to Each Parameter
Prevalence of subjects with abdominal obesity and those with WHtR >0.5 among the participants with NAFLD and non-NAFLD. (A) Prevalence of subjects with abdominal obesity among the participants with NAFLD and non-NAFLD. The black bar is the NAFLD group, the gray bar is the non-NAFLD group. P-value from the Rao-Scott chi-square test. (B) Prevalence of subjects with WHtR >0.5 among the participants with NAFLD and non-NAFLD. The black bar is the NAFLD group; the gray bar is the non-NAFLD group. P-value from the Rao-Scott chi-square test. WHtR, Waist-to-height ratio; NAFLD, non-alcoholic fatty liver disease.
References
- 1.Bakaloudi DR, Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Chourdakis M. Impact of the first COVID-19 lockdown on body weight: a combined systematic review and a meta-analysis. Clin Nutr. 2022;41:3046–3054. doi: 10.1016/j.clnu.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim ES, Kwon Y, Choe YH, Kim MJ. COVID-19-related school closing aggravate obesity and glucose intolerance in pediatric patients with obesity. Sci Rep. 2021;11:5494. doi: 10.1038/s41598-021-84766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stavridou A, Kapsali E, Panagouli E, Thirios A, Polychronis K, Bacopoulou F, et al. Obesity in children and adolescents during COVID-19 pandemic. Children (Basel) 2021;8:135. doi: 10.3390/children8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriemma RS, Pirchio R, Liccardi A, Scairati R, Del Vecchio G, Pivonello R, et al. Metabolic syndrome in the era of COVID-19 outbreak: impact of lockdown on cardiometabolic health. J Endocrinol Invest. 2021;44:2845–2847. doi: 10.1007/s40618-021-01563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milic J, Barbieri S, Gozzi L, Brigo A, Beghé B, Verduri A, et al. Metabolic-associated fatty liver disease is highly prevalent in the postacute COVID syndrome. Open Forum Infect Dis. 2022;9:ofac003. doi: 10.1093/ofid/ofac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 7.Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol. 2016;7:211–217. doi: 10.4291/wjgp.v7.i2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song K, Park G, Lee HS, Lee M, Lee HI, Ahn J, et al. Trends in prediabetes and non-alcoholic fatty liver disease associated with abdominal obesity among Korean children and adolescents: based on the Korea national health and nutrition examination survey between 2009 and 2018. Biomedicines. 2022;10:584. doi: 10.3390/biomedicines10030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters DJ. Community susceptibility and resiliency to COVID-19 across the rural-urban continuum in the United States. J Rural Health. 2020;36:446–456. doi: 10.1111/jrh.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck AM, Gilbert AS, Duncan DD, Wiedenman EM. A cross-sectional comparison of physical activity during COVID-19 in a sample of rural and non-rural participants in the US. Int J Environ Res Public Health. 2021;18:4991. doi: 10.3390/ijerph18094991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park M, Lim JT, Wang L, Cook AR, Dickens BL. Urban-rural disparities for COVID-19: evidence from 10 countries and areas in the Western Pacific. Health Data Sci. 2021;2021:9790275. doi: 10.34133/2021/9790275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean national growth charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018;61:135–149. doi: 10.3345/kjp.2018.61.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon J. Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2007;51:2008 [Google Scholar]
- 14.Chung IH, Park S, Park MJ, Yoo EG. Waist-to-height ratio as an index for cardiometabolic risk in adolescents: results from the 1998-2008 KNHANES. Yonsei Med J. 2016;57:658–663. doi: 10.3349/ymj.2016.57.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol. 1988;62:345–346. doi: 10.1016/0002-9149(88)90248-2. [DOI] [PubMed] [Google Scholar]
- 16.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:1357–1364.E2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song K, Jeon S, Lee HS, Choi HS, Suh J, Kwon A, et al. Trends of dyslipidemia in Korean youth according to sex and body mass index: based on the Korea national health and nutrition examination survey (2007-2018) J Pediatr. 2021;237:71–78.e5. doi: 10.1016/j.jpeds.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Mulugeta W, Hoque L. Impact of the COVID-19 lockdown on weight status and associated factors for obesity among children in Massachusetts. Obes Med. 2021;22:100325. doi: 10.1016/j.obmed.2021.100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltoni G, Zioutas M, Deiana G, Biserni GB, Pession A, Zucchini S. Gender differences in weight gain during lockdown due to COVID-19 pandemic in adolescents with obesity. Nutr Metab Cardiovasc Dis. 2021;31:2181–2185. doi: 10.1016/j.numecd.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Shalitin S, Phillip M, Yackobovitch-Gavan M. Changes in body mass index in children and adolescents in Israel during the COVID-19 pandemic. Int J Obes (Lond) 2022;46:1160–1167. doi: 10.1038/s41366-022-01092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Guo B, Ao L, Yang C, Zhang L, Zhou J, et al. Obesity and activity patterns before and during COVID-19 lockdown among youths in China. Clin Obes. 2020;10:e12416. doi: 10.1111/cob.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Yoo E, Kim Y, Lee JM. What happened pre- and during COVID-19 in South Korea? Comparing physical activity, sleep time, and body weight status. Int J Environ Res Public Health. 2021;18:5863. doi: 10.3390/ijerph18115863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GB, Kim Y, Park S, Kim HC, Oh K. Obesity, hypertension, diabetes mellitus, and hypercholesterolemia in Korean adults before and during the COVID-19 pandemic: a special report of the 2020 Korea national health and nutrition examination survey. Epidemiol Health. 2022;44:e2022041. doi: 10.4178/epih.e2022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang HM, Jeong DC, Suh BK, Ahn MB. The impact of the coronavirus disease-2019 pandemic on childhood obesity and vitamin D status. J Korean Med Sci. 2021;36:e21. doi: 10.3346/jkms.2021.36.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwag SH, Oh YR, Ha JW, Kang E, Nam HK, Lee Y, et al. Weight changes of children in 1 year during COVID-19 pandemic. J Pediatr Endocrinol Metab. 2022;35:297–302. doi: 10.1515/jpem-2021-0554. [DOI] [PubMed] [Google Scholar]
- 26.Wunsch K, Kienberger K, Niessner C. Changes in physical activity patterns due to the COVID-19 pandemic: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:2250. doi: 10.3390/ijerph19042250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemente-Suárez VJ, Ramos-Campo DJ, Mielgo-Ayuso J, Dalamitros AA, Nikolaidis PA, Hormeño-Holgado A, et al. Nutrition in the actual COVID-19 pandemic. A narrative review. Nutrients. 2021;13:1924. doi: 10.3390/nu13061924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia P, Liu L, Xie X, Yuan C, Chen H, Guo B, et al. Changes in dietary patterns among youths in China during COVID-19 epidemic: the COVID-19 impact on lifestyle change survey (COINLICS) Appetite. 2021;158:105015. doi: 10.1016/j.appet.2020.105015. [DOI] [PubMed] [Google Scholar]
- 29.Mun H, So ES. Changes in physical activity, healthy diet, and sleeping time during the COVID-19 pandemic in South Korea. Nutrients. 2022;14:960. doi: 10.3390/nu14050960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SI, Suh J, Lee HS, Song K, Choi Y, Oh JS, et al. Ten-year trends of metabolic syndrome prevalence and nutrient intake among Korean children and adolescents: a population-based study. Yonsei Med J. 2021;62:344–351. doi: 10.3349/ymj.2021.62.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J, Liu J, Wang J, Shen M, Ge W, Shen H, et al. Unfavorable progression of obesity in children and adolescents due to COVID-19 pandemic: a school-based survey in China. Obesity (Silver Spring) 2021;29:1907–1915. doi: 10.1002/oby.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange SJ, Kompaniyets L, Freedman DS, Kraus EM, Porter R, Blanck HM, et al. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2-19 years—United States, 2018-2020. MMWR Morb Mortal Wkly Rep. 2021;70:1278–1283. doi: 10.15585/mmwr.mm7037a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haam JH, Hur YI, Kim YS, Kim KK, Kang JH, Ko HJ, et al. Fatty liver change in Korean adults in a systematic social distancing system amid the COVID-19 pandemic: a multicenter analysis. Int J Environ Res Public Health. 2022;19:10444. doi: 10.3390/ijerph191610444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks CG, Spencer JR, Sprafka JM, Roehl KA, Ma J, Londhe AA, et al. Pediatric BMI changes during COVID-19 pandemic: an electronic health record-based retrospective cohort study. EClinicalMedicine. 2021;38:101026. doi: 10.1016/j.eclinm.2021.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65:73–80. doi: 10.1016/j.metabol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Videira-Silva A, Freira S, Fonseca H. Metabolically healthy overweight adolescents: definition and components. Ann Pediatr Endocrinol Metab. 2020;25:256–264. doi: 10.6065/apem.2040052.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, Ahn MB, Choi YJ, Kim SK, Kim SH, Cho WK, et al. Comparison of different criteria for the definition of insulin resistance and its relationship to metabolic risk in children and adolescents. Ann Pediatr Endocrinol Metab. 2020;25:227–233. doi: 10.6065/apem.2040002.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song K, Park G, Lee HS, Lee M, Lee HI, Choi HS, et al. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J Pediatr. 2022;242:79–85.e1. doi: 10.1016/j.jpeds.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Dobson R, Burgess MI, Sprung VS, Irwin A, Hamer M, Jones J, et al. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes (Lond) 2016;40:153–161. doi: 10.1038/ijo.2015.151. [DOI] [PubMed] [Google Scholar]
- 40.Junge J, Engel C, Vogel M, Naumann S, Löffler M, Thiery J, et al. Neck circumference is similarly predicting for impairment of glucose tolerance as classic anthropometric parameters among healthy and obese children and adolescents. J Pediatr Endocrinol Metab. 2017;30:643–650. doi: 10.1515/jpem-2017-0079. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Luo C, Feng X, Qi W, Qu S, Zhou Y, et al. Changes in obesity and lifestyle behaviours during the COVID-19 pandemic in Chinese adolescents: a longitudinal analysis from 2019 to 2020. Pediatr Obes. 2022;17:e12874. doi: 10.1111/ijpo.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JY, Jeon JY. Role of exercise on insulin sensitivity and beta-cell function: is exercise sufficient for the prevention of youth-onset type 2 diabetes? Ann Pediatr Endocrinol Metab. 2020;25:208–216. doi: 10.6065/apem.2040140.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J, Chin M, Sung M. How has COVID-19 changed family life and well-being in Korea? J Comp Fam Stud. 2020;51:301–313. [Google Scholar]
- 44.Greteman BB, Garcia-Auguste CJ, Gryzlak BM, Kahl AR, Lutgendorf SK, Chrischilles EA, et al. Rural and urban differences in perceptions, behaviors, and health care disruptions during the COVID-19 pandemic. J Rural Health. 2022;38:932–944. doi: 10.1111/jrh.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportions of Obesity, Abdominal Obesity, and NAFLD according to Sex
Proportions of Obesity, Abdominal Obesity, and NAFLD according to Age Classification
ORs of NAFLD according to Each Parameter
Prevalence of subjects with abdominal obesity and those with WHtR >0.5 among the participants with NAFLD and non-NAFLD. (A) Prevalence of subjects with abdominal obesity among the participants with NAFLD and non-NAFLD. The black bar is the NAFLD group, the gray bar is the non-NAFLD group. P-value from the Rao-Scott chi-square test. (B) Prevalence of subjects with WHtR >0.5 among the participants with NAFLD and non-NAFLD. The black bar is the NAFLD group; the gray bar is the non-NAFLD group. P-value from the Rao-Scott chi-square test. WHtR, Waist-to-height ratio; NAFLD, non-alcoholic fatty liver disease.