Abstract
Osteosarcoma is a malignant bone tumor that commonly occurs in the pediatric population. Despite the use of chemotherapy and surgery, metastasis remains to be the leading cause of death in patients with osteosarcoma. We have previously reported that cytoplasmic mislocalization of p27 is associated with a poor outcome in osteosarcoma. In this study, we further show that lysyl oxidase (LOX) expression was associated with p27 mislocalization. LOX is an enigmatic molecule that acts as a tumor suppressor or a metastatic promoter; however, its role in osteosarcoma is still unclear. Hence, we performed both in vitro and in vivo analyses to dissect the role of LOX in osteosarcoma. The result of our survival analysis indicated that LOX expression significantly correlated with a poor outcome in osteosarcoma with or without controlling for the initial metastasis status (P < 0.05). Functionally, we found that higher LOX expression promoted osteosarcoma cell proliferation, migration, and invasiveness in vitro and produced a higher number of mice with pulmonary metastases in an orthotopic xenograft mouse model. Mechanistically, phospho-FAK was increased in osteosarcoma cells with high LOX expression. Our results further showed that FAK inhibition significantly reduced tumor cell proliferation and migration in vitro as well as LOX-mediated metastasis in mice. Together, our findings suggest that there is a novel link between p27 mislocalization and LOX expression. LOX plays a pivotal role in osteosarcoma metastasis by upregulating FAK phosphorylation. FAK inhibition may constitute a promising therapeutic strategy to reduce the development of metastasis in osteosarcoma with LOX overexpression.
Keywords: Focal adhesion kinase, Lysyl oxidase, Metastasis, Osteosarcoma
Introduction
Osteosarcoma (OS) is the most common malignant primary bone cancer in children and young adults with frequent development of lung metastasis. With the advancement of neo-adjuvant chemotherapy, the 5-year survival rate without initial metastasis is approximately 70%.1 However, the survival rate of patients who develop detectable lung metastasis drops to 30%, which poses a significant clinical challenge.2 Because the underlying metastatic mechanism in OS remains largely unknown, it is difficult to identify actionable molecular targets for the treatment of metastasis in OS patients. In exploring this clinical question, our lab has generated evidence that the tumor suppressor p27 could be exploited as a prognostic biomarker at both tumor and blood levels.3,4 We showed that mislocalization of p27 to the cytoplasm significantly correlated with metastasis and poor survival in OS patients. Our mechanistic study has further indicated that p21-activated protein 1 (PAK1) interacts with and is activated by cytoplasmic p27 in OS cells to promote tumor cell migration in vitro and pulmonary metastasis in a mouse model.3
Lysyl oxidase (LOX) is a secreted enzyme that cross-links collagen and elastin in the extracellular matrix. The two substrates of LOX, collagen and elastin are the major components in bone and lung, suggesting that LOX plays a key role in maintaining the homeostasis in bone and lung morphogenesis.5 In bone tissues, LOX is produced by mature osteoblast cells. Knocking out of the LOX gene in bone leads to mineral nodule formation and markedly decreases osteoblastic differentiation.6 Furthermore, LOX also plays an essential role in normal lung development and function. The dual role of LOX in both bone and lung suggests that the oxidase may play a role in OS tumorigenesis and metastasis.
Similar to p27, LOX is an enigmatic molecule that plays a role in both tumor suppression and metastasis.7,8 LOX is synthesized and secreted as a 50 Kd inactive proenzyme (pro-LOX), which is processed by proteolytic cleavage to a functional 32 Kd enzyme and an 18 Kd propeptide (LOX-PP).9 It has been reported that LOX-PP inhibited oncogene RAS transformation through the AKT/ERK pathways, suggesting that it has a tumor-suppressing function.10,11 On the other hand, secreted LOX has recently been found to prepare the metastatic niche in breast cancer to promote metastasis.12 Furthermore, LOX promotes tumor cell proliferation and invasion in lowly metastatic breast cancer cell lines.13
Fuchs et al previously shown that LOX is expressed in the osteoblastic OS cell line of hexadimethrine bromide were MG63 and is involved in OS tumorigenesis.14 Roman et al similarly reported that LOX is upregulated during metastatic progression in OS cell lines15; however, Xu et al suggested a distinctive tumor suppressor role of LOX by inhibiting proliferation and migration in other OS cell lines.16 These seemingly contradictory results indicate that the function of LOX is poorly understood in OS. Crosslinking of collagens by LOX in the extracellular environment is known to activate various downstream pathways in cancer. One of them is focal adhesion kinase (FAK), which is encoded by the gene PTK2.17,18 FAK is an important kinase involved in many cellular functions, including cell adhesion, invasion, and proliferation.19 Interestingly, Kamolrat et al found that phospho-FAKY397 activation is correlated with a poor prognosis of metastatic OS.20 Ke et al reported that knocking down of FAK expression reduces the migration and invasion of OS cell lines and has distinct effects on proliferation and apoptosis.21 Further examination of the link between LOX and FAK activation in OS may provide therapeutic significance.
In this study, we have employed in vitro assays, orthotopic xenograft mouse models, and expression profiling data from patients to demonstrate that LOX plays a metastatic role in OS. We have also shown a potential novel link between p27 mislocalization and LOX expression and indicated that targeting the LOX–FAK axis may constitute a new and effective therapeutic approach to reducing metastasis in OS.
Materials and methods
Mice and human cell lines
Six-to-eight-week-old NOD. CB17-Prkdc SCID/J mice (Jackson Laboratory) were housed in a pathogen-free facility at Baylor College of Medicine. The luciferase-labeled 143B and NES-p27 cell lines used in this study were previously reported.4,22 The U2OS, MG63, HOS, and SaOS-2 cell lines were purchased from American Type Culture Collection.
Mouse studies
A total of 2 × 106 luciferase-labeled 143B cells were orthotopically injected into the tibia of Six-to-eight-week-old mice with body weights between 25 and 35 g. Ten mice (5 males and 5 females) were injected with OS cells stably transfected with LOX-expressing lentivirus particles (LOXHigh). Nine mice (5 males and 4 females) were injected with parental 143B cells. Mice were sacrificed four weeks after tumor injection for histopathological examination of lung tissues to confirm the development of pulmonary metastases. To investigate the effect of the FAK inhibitor, 12 mice (6 males and 6 females, body weight ranging from 25 to 35 g) were orthotopically injected with the LOXHigh mutant. The tumor-bearing mice were randomly divided into a treatment group and a control group (6 mice per group). The small molecule FAK inhibitor Defactinib (VS-6063) (Selleck Chemicals) was administrated through oral gavage one week after tumor cells injection at 50 μg/kg of body weight twice each day for 3 weeks. An equal volume of the vehicle was administered at the same frequency and over the same duration in the control group. Four weeks after the tumor cells injection, the treated and control mice were sacrificed. The primary tumors were resected and weighed, and the lung tissues with and without metastases were subjected to a histological analysis.
Tissue microarray and immunohistochemistry staining
OS tissue microarrays were purchased from Novus Biologicals.21, 22 The tissue microarray contains 60 OS tumor cores. To perform immunohistochemistry, the tissue microarray was incubated with mouse anti-p27 antibody (1:200, Santa Cruz Biotechnology, sc-56338), or anti-LOX antibody (1:1000, Abcam, #174316) at 4°C overnight. A biotinylated horse anti-mouse IgG antibody (1:200, Vector Laboratory, BA-2000) for detection of p27 or goat anti-rabbit IgG antibody (1:200, Vector Laboratory, BA-1000) for detection of LOX were added sequentially for 60 min using the R.T.U VECTASTAIN Elite Kit (Vector Laboratory). The slides were then dehydrated and mounted. The cellular staining of p27 or LOX was examined and scored under a microscope. The details of the immunostaining and the scoring methods are described in the figure legends.
Lentivirus-mediated LOX overexpression and siRNA-mediated FAK knockdown
Specific MISSION TRC3 Human ORF Lentivirus particles were used to generate a stable OS cell line with high LOX expression following the manufacturer's protocol (clone ID: TRCN0000477950, Sigma–Aldrich). Briefly, the lentivirus (MOI = 0.5) and 8 mg/mL of hexadimethrine bromide were added to the plate with 143B cells and incubated for 4 h before replaced with a fresh culture medium. Positive clones were selected using 1.25 μg/mL of puromycin for 3–5 days after the second day of infection. Immunoblotting was performed to detect the increased levels of LOX expression in the positive clones. For the siRNA-mediated knockdown experiment, two pre-designed and validated siRNA constructs against the human PTK2 gene were used to generate the FAK mutants (siRNA1: SASI_Hs01_00035697 and siRNA2:SASI_Hs01_00035698, Sigma–Aldrich). The MISSION® siRNA Universal Negative Control #1 (Sigma–Aldrich) was used as a negative control. Transfection was performed according to the manufacturer's instructions. Briefly, OS cells were re-plated on the same day of transfection. Serum-free media with two siRNA particles at two different concentrations (10 nM and 25 nM) were mixed with transfection reagents (Sigma–Aldrich, 8 μL and 12 μL, respectively). The mixtures were added to the OS cell lines and incubated at 37°C overnight. Then, the transfection media were replaced with a complete medium and the cells continued incubating for a total of 72 h before Western blotting to confirm FAK knockdown and subsequent functional assays.
Antibodies and Western blotting
Cells were lysed by the RIPA buffer supplemented with the 100X phosphatase/protease inhibitor cocktail (Cell Signaling). Protein concentration was determined by the Bradford method. Primary antibodies used in this study were anti-LOX (1:1000, Abcam, #174316), anti-phospho-FAKY397 (1:1000, Cell Signaling, #3283), anti-phospho-AKTS473 (1:1000, Cell Signaling, #4060), anti-phospho-SMAD2S465/S467/SMAD3S423/S425 (1:1000, Cell Signaling, #8828), anti-Caspase 8 (1:1000, Cell Signaling, #9746), and anti-PCNA (1:200, Santa Cruz, sc-7907). Secondary antibodies used in this study were anti-rabbit IgG, HRP-linked antibody (1:3000, Cell Signaling, #7074), and anti-mouse IgG, HRP-linked antibody (1:3000, Cell Signaling, #7076). An anti-GAPDH antibody (1:200, Santa Cruz, sc-322233) was used as a protein loading control. The Western blotting was performed similarly as previously described.3
Functional assays
The proliferation of OS cell lines was measured by CCK-8 assays (Sigma–Aldrich). QCM™ Collagen Cell Invasion assays (Millipore) were used to quantify the invasion capabilities of OS cell lines through a collagen I-coated membrane. Tumor cell migration was evaluated by a cell comb scratch assay kit according to the manufacturer's instructions (Sigma–Aldrich).
Elastin staining
Miller's elastin stain with modification was applied to the mouse lung sections.23 Briefly, sections were deparaffinized and then hydrated with water. They were placed into acidified potassium permanganate for 5 min, rinsed in distilled water, and decolorized in 1% oxalic acid for 2 min. Sections were placed into Miller's stain (Electron Microscopy Sciences) for 3 h at room temperature. Then, the sections were rinsed in 95% ethanol, water, and counterstained in 1% neutral red for 1 min. Images of three random fields were captured for each sample. The images were analyzed by Image J color deconvolution plugins to quantify elastin staining.24
Micro CT scan and analysis
The lower extremities of the tumor-bearing mice were removed and fixed in 4% paraformaldehyde solution. All computerized tomography (CT) images were acquired using an Inveon scanner (Siemens AG, Knoxville, TN). Each of the mouse tibia was placed on the 38 mm pallet bed for scanning in a field of view of the transaxial size of 30.26 mm and axial size 38.90 mm with a binning factor of 2. The ex vivo CT scan of mouse tibia was acquired with the following specifications: acquisition mode step and shoot with a total rotation of 360. Each projection was 1440 ms with a settle time of 10 ms and X-ray tube voltage and current set at 70 kV and 500 μA, respectively. The CT scans were reconstructed using Feldkamp (FDK) algorithm. All datasets were in Hounsfield Unit (HU) calibrated for image analysis. The images were analyzed using Osirix software 7 (Pixmeo, Switzerland). A standard tibial mid-shaft cortical volume of interest area was chosen from the tibia-fibula junction point and then scanned distally with a total length of 0.1 mm.
Statistical analysis
The correlation between LOX and p27 scores in TMA was analyzed by Spearman's correlation. Univariate and multivariate survival analyses of LOX gene expression in the TARGET dataset were performed in the coxh package of R using Cox proportional hazard models; P-values were calculated using Wald tests. Kaplan–Meier survival plots were generated using the survminer package in R. Events were defined as disease progression (distant metastasis or local recurrence) or disease-related death. If a patient suffered from more than one event, the earliest event was used in the event-free survival analysis. The statistical significance of each of the phenotypic assays or the number of mice developing metastases in the animal study was calculated by two-sided Student's t-tests or Fisher's exact tests, respectively. P < 0.05 was considered significant in all the analyses. The RNA expression profiling and RNAseq data and the outcome data for the survival analysis were downloaded from the TARGET data portal (https://ocg.cancer.gov/programs/target/data-matrix). All statistical analyses were performed in SPSS (IBM) or R.
Study approval
The mouse study was performed under an animal protocol approved by the Baylor College of Medicine's Institutional Animal Care and Use Committee.
Results
LOX high expression correlates with p27 mislocalization in OS
In a previous study, our lab identified that cytoplasmic mislocalization of the tumor suppressor gene p27 (CDKN1B or Kip1) is associated with metastasis and a poor outcome in OS.4 To identify other p27-associated metastatic players in OS, we explored other genes whose expressions were associated with the cytoplasmic p27 status in OS. One of the candidate genes is the lysyl oxidase (LOX), whose protein expression was higher in a p27-mislocalized OS mutant (NES-p27) relative to the empty vector control that we previously reported (Fig. 1A).4 To examine whether the LOX expression correlated with p27 mislocalization in clinical OS samples, we performed immunohistochemistry of p27 and LOX in an OS tissue microarray (n = 60). Using the intensity and proportion scores, we found that 45% of the tumor cores had high LOX expression (Intensity score ≥ 2) and 62% of the tumor cores had high proportions of LOX-expressing tumor cells (Proportion score ≥ 2). The cytoplasmic p27 and LOX expression in OS tumors were significantly correlated at both intensity (Spearman's rho = 0.382, P = 0.003) and proportion levels (Spearman's rho = 0.522, P < 0.001) (Fig. 1B–D), suggesting the OS cases with high levels of cytoplasmic p27 most likely had high LOX expression.
Figure 1.
LOX expression correlates with cytoplasmic p27 expression in OS cells. (A) Western blotting of LOX in an OS cell line with (NES-p27) and without (Empty) cytoplasmic p27 as previously described. (B) Representative images of high- and low-staining of LOX (upper panel) and p27 (lower panel) of the same tumor cores in the OS tissue array (20X). (C) Pie charts showing the score distribution of cytoplasmic p27 and LOX staining in TMA. The staining scores represent the following, Intensity: 0 - Trace (background, minimal), 1 - Weak, 2 - Moderate, 3 - Strong; Proportion: 0 - <1% of cell population stained, 1 - 1–25% of cell population stained, 2 - >25–50% of cell population stained, 3 - >50–75% of cell population stained, 4 - >75% of cell population stained. (D) Box plots showing the correlation of LOX and p27 staining in the OS tissue microarray. The staining scores of LOX (y-axis) are plotted against the p27 staining score groups (x-axis) of the OS cases (left: intensity; right: proportion). The darker horizontal line within the box denotes the median value of LOX staining scores. The lower and upper limits of the box represent the 1st and 3rd quartiles of the scores. Whiskers indicate the maximum or minimum values of staining scores and the circles denote outlier scores.
High LOX expression correlates with the metastasis and poor survival of OS patients
Next, we investigated the clinical significance of LOX expression by analyzing the publicly available RNA expression and outcome data of the expression profiling (TARGET-HuEx) and RNA sequencing (TARGET-RNAseq) datasets generated from the TARGET OS project (n = 89) (National Cancer Institute, 2019). Using the Cox Proportional Hazard Model approach, we showed that LOX expression was significantly associated with overall (Hazard Ratio = 2.19, P = 0.0055) and event-free (Hazard Ratio = 1.66, P = 0.0183) survival in the TARGET-HuEx data. Similar associations were also found in the overall (Hazard Ratio = 1.80, P = 0.00457) and event-free (Hazard Ratio = 1.56, P = 0.0078) survival of the TARGET-RNAseq data. To illustrate the survival differences, we performed Kapan-Meier analyses. A higher LOX RNA expression level correlated with worse overall survival in both RNA profiling and RNAseq datasets with P = 0.031 and P = 0.023, respectively (Fig. 2A, B). In event-free survival, high LOX RNA expression correlated with a poor outcome in the RNAseq dataset (Fig. 2C, P = 0.0085) and showed a strong trend in the expression profiling dataset (Fig. 2D, P = 0.074). Using multivariate Cox proportional hazard models, LOX expression remained significant after controlling for the metastatic status at diagnosis in the TARGET-HuEx data, indicating that it is an independent prognostic factor for overall (Hazard Ratio = 2.17, P = 0.0074) and event-free survival (Hazard Ratio = 1.57, P = 0.0344). Similar significant correlations with survival after controlling for initial metastasis were also observed in the TARGET-RNAseq data (Overall survival: Hazard Ratio = 1.80, P = 0.0037; Event-free survival: Hazard Ratio = 1.53, P = 0.0082).
Figure 2.
Kapan-Meier survival analyses in OS patients from the TARGET consortium. (A) The overall survival of OS patients based on the RNAseq data. (B) The overall survival of OS patients based on RNA expression profiling data. (C) The event-free survival of OS patients based on the RNAseq data. (D) The event-free survival of OS patients based on RNA expression profiling data. The survival curves are stratified using the 3rd quartile of LOX expression as a cut-off.
LOX promotes the tumor cell proliferation, migration, and invasion of OS cell lines
To dissect the prognostic role of LOX observed in OS cases, we first measured the expression of the LOX protein in five commonly used OS cell lines. The result showed that LOX protein was expressed in all five OS cell lines (Fig. 3A). Next, we developed LOX high expression (LOXhigh) mutants in U2OS and 143B cell lines, in which higher LOX expression was confirmed by Western blotting (Fig. 3B). It is worth noting that we detected multiple bands between 50 and 60 Kd, probably representing full-length LOX protein with or without glycosylations as previously reported.25 Comparing the growth curves of the two LOXhigh mutants with their respective parental cell lines, we found that higher LOX expression promoted tumor cell proliferation in both cell line backgrounds (Fig. 3C). However, Western blotting of proliferation-related gene PCNA and CASPASE 8 did not show a significant difference between mutants and their parental cell lines (Fig. S1). The abilities of tumor cells to migrate and invade through the matrix are the critical steps of the metastasis process. We employed a scratch assay to evaluate the migratory abilities of the LOX mutant cells. The results showed that high LOX expression decreased the gap created by the scratch in both U2OS and 143B cells more than their parental cell lines, suggesting LOX increased OS cell migration (Fig. 3D). Since a well-known function of LOX is to crosslink collagen I in the extracellular matrix, we measured the invasiveness of OS cells by a chamber assay coated with collagen. The results of the invasion assay demonstrated that the invasiveness of the LOXhigh OS cell lines was higher than the parental cell lines (Fig. 3E).
Figure 3.
LOX expression promotes OS cell proliferation, migration, and invasion. (A) Western blotting of LOX in five commonly used OS cell lines. GAPDH is used as a loading control. (B) Western blotting of LOX and phospho-FAKY397 in two high LOX mutants (LOXHigh) and their parental cell lines (Parental). (C) Growth curves of high LOX mutants and their respective parental cell lines. Five wells per cell line were measured; error bars represent standard deviations of the replicates. Experiments were replicated three times with similar results. (D) Representative images of the cell comb scratch assays of the LOXhigh mutants and their respective parental cell lines at 0 and 24 h after scratches were initiated. Red dash lines indicate the front of migrated cells. Experiments were replicated with similar results. (E) Representative images of the transwell invasion assays of the high LOX mutants and their respective parental cell lines (left) and quantification of the transwell invasion assays (right). Invaded cells were stained, counted, and averaged using ImageJ software in five random and independent microscopic fields (10X). The experiment was replicated three times with similar results. In the quantification, error bars and asterisks represent standard deviations and statistical significance (Student's t-test; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, respectively).
LOX increases the development of pulmonary metastases in mice
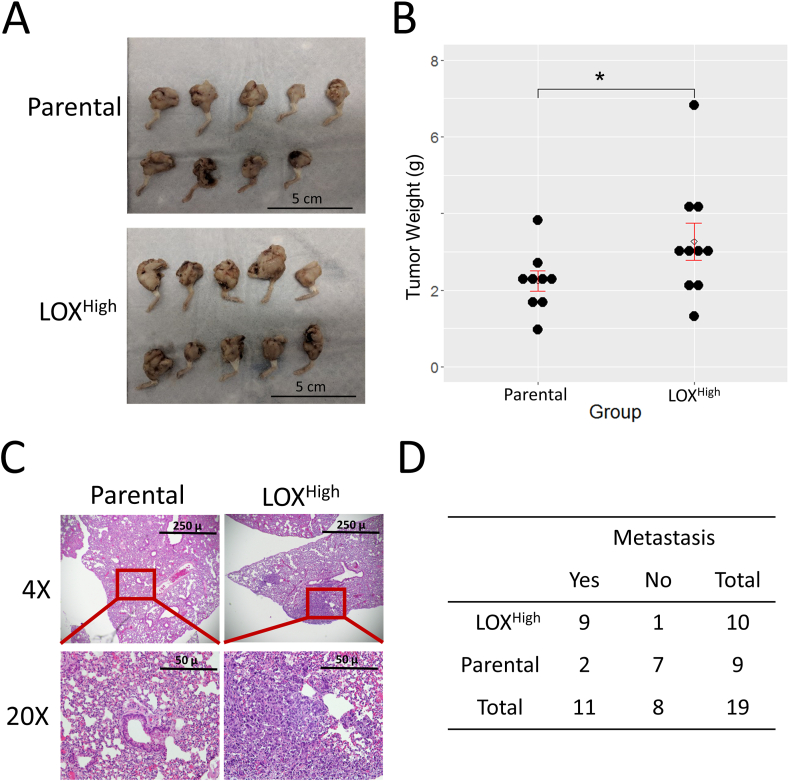
To evaluate the function of LOX in OS in vivo, we orthotopically injected the 143B-LOXHigh or parental 143B into the tibia of immunodeficiency mice (NOD/SCID) to examine the effects of LOX in tumor growth and metastasis development. Consistent with the in vitro study, the growth of primary tumors in the mice injected with the LOXHigh mutant was significantly higher when compared with that of the parental cell line (Fig. 4A, B; P < 0.05). Four weeks after the tumor implantation, mice were sacrificed and lung tissues were analyzed histologically for the development of metastatic nodules. The result showed that nine of 10 mice transplanted with the LOXHigh mutant had detectable lung nodules while only two of nine mice injected with the parental cell line developed lung metastases (Fig. 4C, D; P = 0.0049). To further investigate the role of LOX in osteogenesis and elastin structures, we analyzed the mouse bone and lung tissues by micro-CT imaging and elastin staining. The results indicated higher LOX expression in the tumor cells did not significantly alter the number of elastin fibers in lung mesenchymal tissues (Fig. S2A, B). Similarly, higher tumoral LOX expression had no detectable effects on normal cortical bone structure when comparing the tibial midshaft areas of two groups of mice (Fig. S3A, B).
Figure 4.
LOX expression promotes tumor growth and lung metastasis in vivo. (A) Images of the primary tumors generated from the orthotopic injection of parental 143B (n = 9) and high LOX mutant cells (n = 10). (B) Scatterplot showing the measurement of tumor weights shown in A. The open circle denotes the average, and the upper and lower bars denote the standard errors of the weights. (C) Representative H&E staining (4X) of mouse lung sections from the 143B and high LOX mutant groups (upper panel). High magnification (20×) of the red rectangle areas in low magnification images (lower panel). (D) Table showing the numbers of mice with or without pulmonary metastases in the high LOX and parental groups (Fisher's exact test, P = 0.0049).
Knockdown of FAK reduces cell proliferation and migration but not invasion in the LOXhigh mutants
To determine whether FAK is the downstream effector of LOX-mediated metastatic functions, we created FAK knockdown mutants using two siRNA constructs (siRNA-1 and siRNA-2) in the LOXhigh mutants of the 143B and U2OS cell lines. The results of Western blotting verified a significant reduction of the expression of FAK in the siRNA mutants (Fig. 5A). FAK knockdown significantly inhibited tumor cell proliferation when compared to the negative control (Fig. 5B). Also, both FAK siRNA mutants showed an impaired cell migration ability relative to the negative control cells as indicated by the cell scratch assay (Fig. 5C). However, we did not observe a similar impairment of the invasiveness in the FAK siRNA mutants, suggesting the LOX-FAK axis is specifically involved in promoting cell growth and migration rather than invasion in OS cells with high LOX expression (Fig. 5D).
Figure 5.
FAK knockdown reduces cell proliferation and migration but not invasion in the LOXhigh mutants. (A) Western blotting of phospho-FAKY397 in the FAK knockdown mutants of the 143B-LOXhigh and U2OS-LOXhigh mutants. GAPDH was used as a loading control. Two transfection doses (10 nM and 25 nM) were tested in each of the siRNA constructs. CTL referred to cells transfected with universal negative control particles (10 nM). The 10 nM dose was selected for subsequent functional assays. (B) Growth curve of the two FAK siRNA mutants and the negative controls (CTL) in the 143B-LOXHigh and U2OS-LOXHigh mutants. Five wells per cell line were measured; error bars represented standard deviations of replicates. Experiments were replicated three times with similar results. (C) Representative images of the cell comb scratch assays of the FAK siRNA mutants and their respective control cells (CTL) at 0 and 24 h after scratches were initiated. Red dash lines indicate the front of migrated cells. Experiments were replicated with similar results. (D) Representative images (left) and quantification (right) of the transwell invasion assays of the FAK siRNA mutants and their respective control cells. Invaded cells were stained, counted, and averaged using ImageJ software in five random and independent microscopic fields (10X). The experiment was replicated three times with similar results. In the quantification, error bars and asterisks represent standard deviations and statistical significance (Student's t-test; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, respectively).
Phospho-FAK inhibitor reduces the LOX-mediated metastasis in OS
Previous reports have shown that phospho-FAK is upregulated in LOX-expressing cell lines and phospho-FAK is known to be involved in metastasis.17 In addition, high expression of the FAK or phospho-FAK protein is associated with a poor prognosis in OS.20 We examined whether PTK2 gene expression (FAK) was also associated with a poor outcome in two publicly available OS expression datasets. OS cases with high FAK expression showed a trend toward a poor overall survival in both TARGET (P = 0.068) and Kuijjer (P = 0.177) datasets, but not in event-free or metastasis-free survival26 (Fig. S4). Experimentally, we found that high expression of LOX noticeably increased and slightly increased FAK phosphorylation (phospho-FAKY397) in 143B and U2OS, respectively (Fig. 3B). Conversely, the other two known LOX downstream pathways, AKT and TGFB-SMAD3, were downregulated in LOXhigh cells relative to the parental cell lines (Fig. S1). Since small-molecule phospho-FAK inhibitors are available, we tested whether therapeutic inhibition of FAK phosphorylation could reduce LOX-mediated metastasis in OS. We treated the mice orthotopically injected with LOXHigh cells with a specific inhibitor of phospho-FAK, Defactinib (VS-6063), or vehicle control.27 The ability of FAK phosphorylation inhibition for Defactinib was verified in 143B-LOXhigh cells in vitro (Fig. S5). Three weeks after oral administration of the FAK inhibitor, the growth of primary tumors and the development of pulmonary metastases in the mice were evaluated. Our results showed that the weights of the primary tumors between the treatment and the control groups were not significantly different (Fig. 6A, B; P = 0.108). However, two of six mice (33%) in the treatment group developed microscopic lung metastatic nodules, while all six mice from the control group (100%) developed lung metastases (Fig. 6C, D; P = 0.03). These results indicated that FAK inhibition mitigated the development of OS metastases in a mouse model.
Figure 6.
Small molecule FAK inhibitor reduces lung metastasis in the mice injected with LOXHigh cells. (A) Images of the primary tumors generated from orthotopically injected high LOX mutant cells treated with or without the FAK inhibitor. (B) Scatterplot showing the measurement of tumor weights shown in A. Open circle denotes the average, and the upper and lower bars denote the standard errors of the weights. (C) Representative H&E staining (4X) of mouse lung sections from the FAK inhibitor-treated and -untreated groups (upper panel). High magnification (20×) of the red rectangle areas in low magnification images (lower panel). (D) Table showing the numbers of mice with or without pulmonary metastases in the FAK inhibitor-treated and untreated groups (Fisher's exact test, P = 0.03). (E) A proposed model of the p27-LOX-FAK axis in OS metastasis.
Discussion
The major cause of death in children who suffer from OS is the development of metastasis. Despite the advent of cancer genomics and high-throughput drug screening, all OS patients continue to be treated with surgery and multi-agent chemotherapy despite their differences in clinical risk of metastasis. The lack of clinically useful biomarkers for risk-stratification and guidance for targeted therapies hampers the development of more effective OS treatment. Our lab and others have identified a new role of p27 in promoting metastasis when it is mislocalized into the cytoplasm.4 Further, we have shown that the autoantibody against p27 was elevated in poor prognostic OS patients.4 Ectopic expression of cytoplasmic p27 (NES-p27) can increase OS cell migration and invasion as well as the development of metastasis in mice. In a recent study, we further generated evidence that p27 is frequently mislocalized in the cytoplasm of OS tumors and this mislocalization is associated with poor prognosis and metastasis in OS patients.3 Cytoplasmic p27 interacts and phosphorylates PAK1 and, hence, promotes tumor cell migration and metastasis via increasing actin cytoskeleton remodeling.3 Through an expression profiling analysis, we identified the LOX gene as one of the most significantly upregulated genes in OS cell lines expressing a cytoplasmic p27 construct relative to the vector control (NES-p27 vs. empty vector control, unpublished data). In the current study, we confirmed that LOX protein expression was higher in a p27-mislocalized OS cell line and correlated with cytoplasmic p27 levels in a large number of clinical samples. These results suggest a novel link between cytoplasmic mislocalization of p27 and LOX, where cytoplasmic p27 may upregulate LOX expression to promote OS metastasis in addition to interacting and activating PAK1. Since metastasis is a complex and multi-stage process, it is plausible that p27 mislocalization leads to the activation of multiple downstream pathways, such as LOX and PAK1, to drive the development of metastasis in OS. Further genetic perturbations of LOX expression in p27-mislocalized OS cells can provide evidence if LOX is another downstream effector of p27 mislocalization in OS.
LOX is a secreted enzyme that cross-links collagen and elastin in the extracellular space.5 However, recent studies have revealed a more complex role of LOX in tumorigenesis. The precursor protein of LOX (LOX-PP) can inhibit oncogene RAS and its downstream pathways, such as AKT and ERK in breast cancer.10,11 Thus, LOX has been regarded as a tumor suppressor. On the other hand, other studies of breast cancer recently unraveled a novel pro-metastatic role of LOX when secreted into the circulation from the primary tumor site, facilitating the development of a pro-metastatic niche in secondary organs, such as bone. This pro-metastatic function of LOX is partially through regulating the osteoblast/osteoclast ratio in bone.12 Nonetheless, little is known about the effect of LOX in primary bone tumors. At the expression level, our current study shows that LOX was expressed in all the OS cell lines tested, which is consistent with a previous report.15 Further, we found that the LOX protein was detectable with higher intensity in 45% of OS tumor samples in a tissue microarray (intensity score ≥ 2, Fig. 1C). Functionally, our orthotopic xenograft mouse study showed that high LOX expression increased the development of pulmonary metastases of OS in mice. Consistently, elevated LOX RNA expression was associated with a poorer prognosis in the TARGET OS cohort. Together, these results suggest that LOX functions as a metastatic promoter in OS. This could explain why its expression is associated with cytoplasmic p27, which is another factor we have previously discovered that is important for OS metastasis.3
Mechanistically, we have demonstrated that high LOX expression promoted OS cell proliferation and collagen-associated invasive abilities in vitro. Although apoptosis-related activities measured by Caspase 8 and proliferation gene PCNA did not change in LOXHigh cells (Fig. S1), these cells generated larger primary tumors than the parental cell line, which is corroborated with the in vitro data. Furthermore, the enzymatic function of LOX is to cross-link collagen and elastin, which are the major components of bone and lung. This led us to test if LOX can modify the bone or lung structure to promote metastasis. However, our results did not show any significant differences in elastin staining in the lungs (Fig. S2) and the densities of the normal cortical bones between the mice injected with LOXHigh or parental cells (Fig. S3). A limitation of our current analyses is that we focused on the quantification of the bone density or the number of elastin fibers rather than structure variability of the elastin in the lungs. Given the cross-linking function of LOX, further structural analyses are needed to dissect the function of LOX in local and metastatic microenvironments.
Our study also attempted to identify the downstream metastatic effector of LOX in OS. Several potential downstream effectors of the LOX pathway, e.g., TGFB/SMAD, AKT, and FAK have been previously reported.11,18,25 However, the downstream effector of LOX in OS is still elusive. Our results have shown that only FAK phosphorylation, but not the other two proteins, phospho-AKTS473 and phospho-SMAD3S423/425, was increased in LOXHigh OS cells (Fig. S1). Interestingly, we showed that higher LOX expression decreased phospho-AKT in RAS-mutated 143B cells. This finding supports the tumor suppressor function of LOX in RAS mutated tumor cells by inhibiting the AKT pathway.11 Regarding the TGFB/SMAD pathway, Phimon et al have reported LOX suppresses TGFB1-induced SMAD3 phosphorylation in murine pre-osteoblast cells likely through its amine oxidase activity.25 Consistent with these conclusions, we found that phospho-SMAD3 was decreased in OS cells with high LOX expression. All these findings are consistent with the previous reports of the dual role of LOX.
Our results indicated that LOX plays a role in FAK phosphorylation, which is supported by other studies in different cancer types. In a breast cancer model, hypoxia conditions induced cancer cell expression of phospho-FAK and promoted cell mobility. This effect was eliminated by knocking down LOX or blocking LOX activation, suggesting that LOX is required in FAK phosphorylation.28 The discovery of the link between LOX and FAK is clinically significant as various FAK inhibitors have been developed and tested in clinical trials. It has been reported that multiple FAK inhibitors can induce cell cycle arrest or apoptosis of cancer cells. Some of them have moved into phase II clinical trials as combinatorial treatments in multiple solid tumors, including pancreatic cancer and non-small-cell lung carcinoma.29 In addition, LOX overexpression in colon cancer cells increases tissue stiffness to drive cancer progression by phosphorylating FAK and activating integrin pathways, which can be blocked by small inhibitors of FAK.17 Interestingly, FAK expression and phosphorylation have been associated with a poor prognosis in OS, and inhibition of FAK produces an anti-tumor effect in vivo.20,30 However, the link between LOX and FAK in OS has not been established. Our study found that phospho-FAKY397 was elevated in two LOXhigh OS cell lines. Furthermore, our result demonstrated that the specific FAK inhibitor, Defactinib (VS-6063, PF-04554878), significantly reduced the LOX-associated metastasis in a pre-clinical OS mouse model. This small molecule inhibitor is under a phase II clinical trial for cancers and it could be readily adopted in future OS clinical trials, highlighting the clinical significance of our findings. Our in vitro knockdown studies further indicated that the effects of LOX on tumor cell proliferation and migration, but not the invasion, are probably manifested via FAK. However, it is worth noting that Defactinib effectively decreased the development of pulmonary metastasis in the mouse model, suggesting that partial inhibition of the metastatic functions of LOX via FAK targeting may be of therapeutic importance. One plausible mediator for LOX-mediated FAK activation is that secreted LOX in the extracellular matrix interacts with integrin, which is a strong FAK activator.31 Further mechanistic studies would be needed to examine the role of integrin in the LOX-FAK activation of the metastatic OS.
In this study, we propose a model to describe the metastatic role of LOX in OS (Fig. 6E). We demonstrated that cytoplasmic mislocalization of p27 is associated with LOX expression in OS. High LOX expression leads to a higher occurrence of OS metastasis in an orthotopic xenograft mouse model. More importantly, LOX RNA expression was also associated with poor overall and event-free survival in a large OS patient cohort. These findings suggest that LOX can be used as a prognostic biomarker to stratify high-risk patients for FAK targeted therapy. Furthermore, our data showed that FAK phosphorylation was higher in OS cells with high LOX expression. Genetic knockdown of FAK expression and inhibition of FAK phosphorylation with the small molecule FAK inhibitor reduced the metastatic potential of OS cells with high LOX expression in vitro and the development of pulmonary metastases in vivo, respectively. The results suggest that a LOX-FAK biomarker-based therapeutic strategy for treating OS metastasis has a high translational potential and its further evaluation in future clinical trials is warranted.
Author contributions
XC and MC performed the experiments. JH reviewed and scored the TMA, PS, and MWG assisted on small animal imaging, and TKM conceived the project. XC, MC, and TKM designed the experiments, analyzed and interpreted the results, and drafted and revised the manuscript. All authors reviewed and agreed on the content of the manuscript.
Conflict of interests
The authors declare no conflict of interests.
Funding
This work was partly supported by funding from Sarcoma Alliance for Research through Collaboration (SARC)/National Cancer Institute (No. U54CA168512), and the National Institute of Child Health and Human Development (No. R01 HD074553) to TKM.
Acknowledgements
We thank Ricardo Flores, Manjula Nakka, Aaron Kelly for their suggestions and technical assistance in this study. We are also grateful to Ching Lau, Paul Meltzer, and the TARGET OS consortium for generating the expression profiling and RNAseq data.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.12.016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Link M.P., Eilber F. In: Principles and Practice of Pediatric Oncology. 3rd ed. Pizzo M.D., Poplack D.G., Adamson M.D., et al., editors. Lippincott-Raven Publishers; Philadelphia, PA: 1997. Osteosarcoma; pp. 889–920. [Google Scholar]
- 2.Anderson M.E. Update on survival in osteosarcoma. Orthop Clin N Am. 2016;47(1):283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Cates J.M.M., Du Y.C., et al. Mislocalized cytoplasmic p27 activates PAK1-mediated metastasis and is a prognostic factor in osteosarcoma. Mol Oncol. 2020;14(4):846–864. doi: 10.1002/1878-0261.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Nakka M., Kelly A.J., et al. p27 is a candidate prognostic biomarker and metastatic promoter in osteosarcoma. Cancer Res. 2016;76(13):4002–4011. doi: 10.1158/0008-5472.CAN-15-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mäki J.M., Sormunen R., Lippo S., et al. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167(4):927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pischon N., Mäki J.M., Weisshaupt P., et al. Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype. Calcif Tissue Int. 2009;85(2):119–126. doi: 10.1007/s00223-009-9252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartland A., Erler J.T., Cox T.R. The role of lysyl oxidase, the extracellular matrix and the pre-metastatic niche in bone metastasis. J Bone Oncol. 2016;5(3):100–103. doi: 10.1016/j.jbo.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneda A., Wakazono K., Tsukamoto T., et al. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64(18):6410–6415. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- 9.Wang T.H., Hsia S.M., Shieh T.M. Lysyl oxidase and the tumor microenvironment. Int J Mol Sci. 2016;18(1):62. doi: 10.3390/ijms18010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato S., Trackman P.C., et al. The Ras signaling inhibitor LOX-PP interacts with Hsp70 and c-Raf to reduce Erk activation and transformed phenotype of breast cancer cells. Mol Cell Biol. 2011;31(13):2683–2695. doi: 10.1128/MCB.01148-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palamakumbura A.H., Jeay S., Guo Y., et al. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279(39):40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 12.Cox T.R., Rumney R.M.H., Schoof E.M., et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522(7554):106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Payne S.L., Hendrix M.J., Kirschmann D.A. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101(6):1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs B., Zhang K., Bolander M.E., et al. Identification of differentially expressed genes by mutually subtracted RNA fingerprinting. Anal Biochem. 2000;286(1):91–98. doi: 10.1006/abio.2000.4792. [DOI] [PubMed] [Google Scholar]
- 15.Muff R., Ram Kumar R.M., Botter S.M., et al. Genes regulated in metastatic osteosarcoma: evaluation by microarray analysis in four human and two mouse cell line systems. Sarcoma. 2012;2012:937506. doi: 10.1155/2012/937506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X., Wang B., Xu Y. Expression of lysyl oxidase in human osteosarcoma and its clinical significance: a tumor suppressive role of LOX in human osteosarcoma cells. Int J Oncol. 2013;43(5):1578–1586. doi: 10.3892/ijo.2013.2067. [DOI] [PubMed] [Google Scholar]
- 17.Baker A.M., Bird D., Lang G., et al. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32(14):1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 18.Laczko R., Szauter K.M., Jansen M.K., et al. Active lysyl oxidase (LOX) correlates with focal adhesion kinase (FAK)/paxillin activation and migration in invasive astrocytes. Neuropathol Appl Neurobiol. 2007;33(6):631–643. doi: 10.1111/j.1365-2990.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra S.K., Hanson D.A., Schlaepfer D.D. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 20.Thanapprapasr K., Nartthanarung A., Thanapprapasr D., et al. pFAK-Y397 overexpression as both a prognostic and a predictive biomarker for patients with metastatic osteosarcoma. PLoS One. 2017;12(8):e0182989. doi: 10.1371/journal.pone.0182989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren K., Lu X., Yao N., et al. Focal adhesion kinase overexpression and its impact on human osteosarcoma. Oncotarget. 2015;6(31):31085–31103. doi: 10.18632/oncotarget.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed N., Salsman V.S., Yvon E., et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17(10):1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daamen W.F., Hafmans T., Veerkamp J.H., et al. Comparison of five procedures for the purification of insoluble elastin. Biomaterials. 2001;22(14):1997–2005. doi: 10.1016/s0142-9612(00)00383-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y., Yu Q., Xu C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int J Clin Exp Med. 2017;10(10):14904–14910. [Google Scholar]
- 25.Atsawasuwan P., Mochida Y., Katafuchi M., et al. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283(49):34229–34240. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuijjer M.L., Rydbeck H., Kresse S.H., et al. Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes Cancer. 2012;51(7):696–706. doi: 10.1002/gcc.21956. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y., Hu W., Ivan C., et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013;105(19):1485–1495. doi: 10.1093/jnci/djt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erler J.T., Bennewith K.L., Nicolau M., et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 29.Murphy J.M., Rodriguez Y.A.R., Jeong K., et al. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp Mol Med. 2020;52(6):877–886. doi: 10.1038/s12276-020-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu C., Chen X., Wen J., et al. Antitumor effect of focal adhesion kinase inhibitor PF562271 against human osteosarcoma in vitro and in vivo. Cancer Sci. 2017;108(7):1347–1356. doi: 10.1111/cas.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amendola P.G., Reuten R., Erler J.T. Interplay between LOX enzymes and integrins in the tumor microenvironment. Cancers. 2019;11(5):729. doi: 10.3390/cancers11050729. [DOI] [PMC free article] [PubMed] [Google Scholar]