Purpose of review
Although lymph node dissection (LND) during radical nephroureterectomy (RNU) is recommended for high-risk nonmetastatic upper tract urothelial carcinoma (UTUC), adherence to guidelines remains insufficient in clinical practice. Therefore, this review aims to comprehensively summarize the current evidence regarding the diagnostic, prognostic, and therapeutic impact of LND during RNU in UTUC patients.
Recent findings
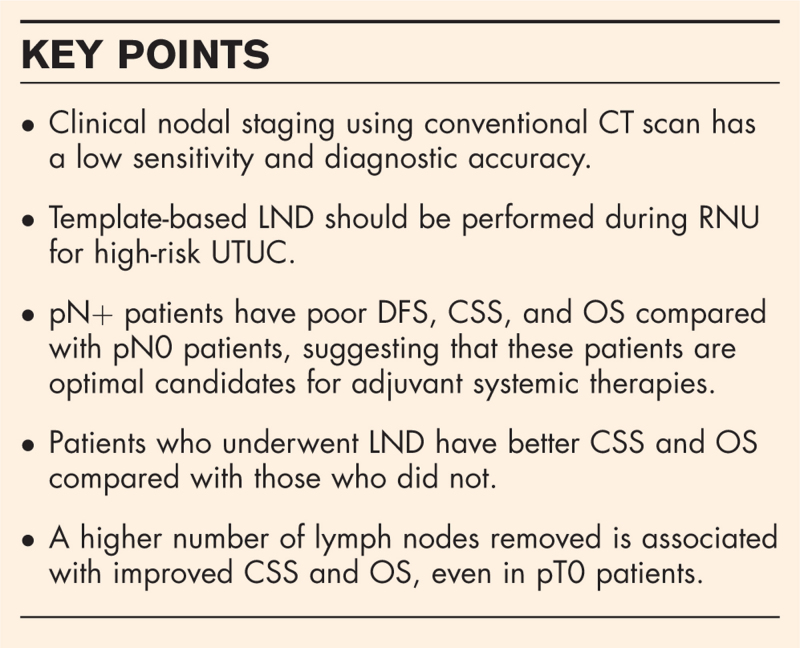
Clinical nodal staging using conventional CT scan has low sensitivity (25%) and diagnostic accuracy [area under the curve (AUC): 0.58] in UTUC, suggesting the importance of LND for obtaining accurate N-staging. Patients with pathological node-positive (pN+) disease have poor disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS) compared with those with pN0. In addition, population-based studies showed that patients who underwent LND improved CSS and OS than those who did not, even in patients who received adjuvant systemic therapy. The number of lymph nodes removed has also been shown to be associated with improved CSS and OS, even in pT0 patients. Template-based LND should be performed as the extent of lymph node is more important than the number of lymph nodes. Robot-assisted RNU may facilitate performing a meticulous LND compared with a laparoscopic approach. Postoperative complications such as lymphatic and/or chylous leakage are increased but adequately manageable. However, the current evidence is not supported by high-quality studies.
Summary
Based on the published data, LND during RNU is a standard procedure for high-risk nonmetastatic UTUC, owing to its diagnostic, staging, prognostic, and, potentially, therapeutic benefits. Template-based LND should be offered to all patients who are planned for RNU for high-risk nonmetastatic UTUC. Patients with pN+ disease are optimal candidates for adjuvant systemic therapy. Robot-assisted RNU may facilitate meticulous LND compared with laparoscopic RNU.
Keywords: lymph node dissection, lymphadenectomy, upper tract urothelial carcinoma
INTRODUCTION
Nonmetastatic upper tract urothelial carcinoma (UTUC) is managed with minimally invasive therapies, such as kidney-sparing endoscopic surgery for low-risk tumors (i.e. single, tumor size <20 mm, low-grade, and ≤cT2) [1–5]. For high-risk UTUC, however, radical nephroureterectomy (RNU) is still the guideline-recommended standard treatment [6,7]. Based on continuously growing evidence, template-based lymph node dissection (LND) during RNU is recommended for high-risk UTUC patients, to improve lymph node staging and prognostication in order to ensure adjuvant treatment when needed [6,8]. Indeed, pathologically confirmed lymph node metastasis is an indication for adjuvant systemic therapies [9,10].
However, UTUC is a relatively rare urological malignancy and potentially heterogeneous disease, making the establishment of reliable and evidence-based recommendations challenging because of the lack of high-level evidence [6]. Indeed, adherence to guideline recommendations is limited in the management of UTUC in real-world practice, including diagnostic tests as well as LND [11–14]. Therefore, this review aims to comprehensively summarize the current evidence regarding the diagnostic, staging, prognostic, and therapeutic impact of LND during RNU in UTUC patients with focus on the recent literature.
Box 1.
no caption available
EVIDENCE ACQUISITION
A literature search on PubMed/Medline databases was performed in December 2022 to identify studies investigating the oncologic impact of LND, including nodal status and extent of LND, during RNU for UTUC. The detailed search strategy was ‘(urothelial) AND (cancer or carcinoma) AND (upper tract) AND (lymph node dissection OR lymphadenectomy)’. Studies lacking original patient data, letters, editorial comments, replies from authors, case reports, and articles not written in English were excluded. After the full-text investigation, two authors independently extracted the relevant data, such as the first author's name, publication year, study design, number of patients, surgical approach, extent of LND, pathologic outcomes, additional systemic therapies, follow-up duration, and oncologic outcomes.
To compare oncologic outcomes across lymph node status, we extracted 5-year disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS). Subsequently, hazard ratios and 95% confidence intervals (CIs) for DFS, CSS, and OS were extracted from Cox regression multivariable analysis. In addition, we only relied on studies with study populations of more than 250 patients for evaluating oncologic outcomes across lymph node status, owing to the limited number of patients who underwent LND and those with pathological node-positive disease.
EVIDENCE SYNTHESIS
Incidence and predictive factors of lymph node metastases in upper tract urothelial carcinoma patients
Up to 25% of UTUC patients harbor regional lymph node metastases at diagnosis, depending on clinical tumor stage and/or tumor grade [15]. For example, Kondo et al.[16] reported that the incidence of lymph node involvement (either clinical or pathological) increased with higher pathological T stages. In this study, they found 5% of pT2, 24% of pT3, and 84% of pT4 tumors to harbor lymph node metastases, whereas patients with pTa/is/1 had no lymph node involvement [16]. T-stage, tumor grade, tumor necrosis, presence of carcinoma in situ (CIS), lymphovascular invasion, and sessile tumor architecture have been reported to be predictive of lymph node metastases [16–18]. Conversely, tumor location (i.e. renal pelvis vs. ureter) was not associated with the presence of lymph node metastases [16,19]. Therefore, experts recommend LND (i.e. template-based LND) in all patients with high-risk UTUC during RNU [20,21].
Diagnostic issues of clinical staging of the nodes
For clinical staging of UTUC, computed tomography (CT) remains a standard radiographic tool with a high pooled overall sensitivity (92%) and specificity (95%) [22]. For the detection of lymph node involvement, Millán-Rodríguez et al.[23] reported, in 1999, that CT had a good diagnostic ability with a sensitivity of 87.5% and specificity of 98% using 93 tumors from 82 patients. Since then, traditionally, clinical lymph node staging has relied on cross-sectional imaging, specifically on CT. However, recently, Pallauf et al. conducted a multicenter observational study with 865 UTUC patients treated with RNU and LND and reported that conventional cross-sectional imaging yielded a good specificity with 91% but with a very low sensitivity (i.e. 25%), resulting in a poor diagnostic accuracy [area under the curve (AUC): 0.58] [24▪▪]. The authors suggested to use conventional cross-sectional imaging as a rule-in but not a rule-out tool for predicting lymph node metastases.
Voskuilen et al.[25] conducted a retrospective multicenter study to assess the diagnostic value of 18F-Fluorodeoxglucose PET/CT (FDG-PET/CT) for the detection of lymph node metastasis in 117 surgically treated UTUC patients. The authors reported that FDG-PET/CT yielded a promising diagnostic performance with a sensitivity of 82% and a specificity of 84% for predicting lymph node metastasis [25]. Despite improvements of preoperative lymph node staging thanks to modern imaging modalities [26], LND remains the standard to ensure true N-staging. Furthermore, the POUT trial, a phase III randomized controlled trial, showed the significant DFS benefit from adjuvant chemotherapy in UTUC patients with pathologically advanced features in the RNU specimen, including positive lymph node [10]. Therefore, accurate N-staging is, indeed, essential for selecting optimal candidates for adjuvant therapy.
Oncologic impact of pN+ versus pN0
Tables 1 and 2 summarize the patient demographics and oncologic outcomes of eligible studies included in the following sections, ‘Oncologic impact of pN+ versus pN0’ and ‘Therapeutic impact of LND’. Several retrospective cohort studies evaluated the differential oncologic outcomes in cN0 UTUC patients who underwent RNU with pathological node-positive (pN+) versus negative (pN0) disease. Among the patients who underwent LND in these studies, the pooled pN+ rate was 21.5% (range: 6.2–34%).
Table 1.
Patient demographics of eligible studies assessing oncologic outcomes stratified lymph node status with large cohort (more than 250 patients included)
| Author | Year | Periods | Institution | Pts. | Surgical approach [n (%)] | Median number of LNs removed | Decision and extent of LND | Additional systemic therapies [n (%)] | Follow-up periods (months) | pN stage [n (%)] |
| Retrospective cohort study | ||||||||||
| Roscigno | 2009 | 1987– 2007 | Multi (International) | 1130 | Open: 924 (82) Lap: 206 (18) | NR | Surgeon's discretion (template) | AC: 187 (17) | Median (range) 45 (1–250) | pN0: 412 (36) pN+: 140 (13) pNx: 578 (51) |
| Burger | 2011 | 1987– 2008 | Multi (International) | 785 | Open: 715 (91) Lap: 70 (9) | 3 (IQR: 2–6) | Surgeon's discretion (Hilar/regional) | AC: 69 (9) | Median (IQR) 34 (15–65) | pN0: 136 (17) pN+: 54 (7) pNx: 595 (76) |
| Mason | 2012 | 1990– 2010 | Multi (Canada) | 1029 | Open: 583 (57) Lap: 446 (43) | 4.3 (mean) | Surgeon's discretion | AC: 112 (11) | Median (IQR) 20 (7.2–54) | pN0: 199 (20) pN+: 77 (7) pNx: 753 (73) |
| Yoo | 2017 | 1998– 2012 | Single (Korea) | 418 | Open: 184 (44) Lapa: 234 (56) | 7 (IQR: 3–10) | Surgeon's discretion (template) | NR | Median 69 | pN0: 116 (29) pN+: 16 (3) pNx: 286 (68) |
| Ikeda | 2017 | 1985–2013 | Multi (Japan) | 399 | Open: 296 (74) Lap: 103 (26) | 6 (IQR: 3–10) | Surgeon's discretion | AC: 74 (19) | Median (IQR) 43 (7.2–54) | pN0: 182 (46) pN+: 40 (10) pNx: 177 (44) |
| Inokuchi | 2017 | 1995–2009 | Multi (Japan) | 2037 | Open: 1234 (61) Lap: 787 (39) | 6 (IQR: 3–11) | Surgeon's discretion | NAC: 71 (3) AC: 329 (16) | Median (IQR) 46 (22–76) | pN0: 955 (47) pN+: 223 (11) pNx: 859 (42) |
| Li | 2021 | 2001–2021 | Multi (Taiwan) | 1340 | Hand-assisted:741 (55) Lap: 458 (34) Robotic: 141 (11) | NR | Surgeon's discretion | NR | NR | pN0: 278 (21) pN+: 58 (4) pNx: 1004 (75) |
| Hsieh | 2022 | 2000–2015 | Single (Taiwan) | 520 | NR | NR | Surgeon's discretion | AC: 107 (21) NAC was excluded | Mean ± SD 48 ± 29 | pN0: 303 (58) pN+: 20 (3.8) pNx: 197 (38) |
| Leeb | 2022 | 2001–2021 | Multi (Taiwan) | 658 | NR | 4 | Surgeon's discretion | NR | Median LND (-): 33 LND (+): 24 | pN0: 159 (24) pN+: 36 (5.4) pNx: 463 (70) |
| Hakimi | 2022 | 2006–2019 | Multi (ROBUUST) | 877 | All robotic | Mean ± SD pN0: 9.8 ± 9.5 pN+: 10.2 ± 9.5 | Surgeon's discretion | NAC: 77 (8.8) AC: 89 (10) | Mean ± SD pN0 : 15.6 ± 18 pN+:10.9 ± 13.6 pNx:13.7 ± 16.7 | pN0: 285 (32) pN+: 73 (8.3) pNx: 519 (59) |
| Population-based study | ||||||||||
| Lughezzani | 2010 | 1988–2004 | SEER database | 2842 | NR | NR | Surgeon's discretion | NR | Median (range) 43 (1–203) | pN0: 1835 (64) pN+: 242 (9) pNx: 747 (26) |
| Chappidi | 2016 | 2004–2012 | SEER database | 2862 | NR | 2 (IQR: 1–5) | Surgeon's discretion | NR | Median (IQR) 28 (11–53) | pN0: 466 (16) pN+: 255 (8.9) pNx: 2141 (75) |
| Lenis | 2018 | 2010–2013 | NCDB | 3116 | Open: 969 (32) Lap: 1385 (44) Robotic: 762 (24) | 3 (IQR: 1–7) | Surgeon's discretion | NAC: 60 (1.9) AC: 400 (13) | NR | pN0: 765 (25) pN+: 257 (8.3) pNx: 2094 (67) |
| Dongc | 2019 | 2004–2014 | SEER database | 2731 | NR | 2 (IQR: 1–5) | Surgeon's discretion | AC: 345 (13) | Median 31 | pN0: 491 (18) pNx: 2240 (82) |
| Zhai | 2019 | 2004–2015 | SEER database | 7278 | NR | NR | Surgeon's discretion | NR | NR | pN0: 1296 (18) pN+: 665 (9.1) pNx: 5317 (73) |
| Lec (1) | 2021 | 2006–2013 | NCDB | 1421 (RNU) | NR | 3 (IQR: 1–6) | Surgeon's discretion | NAC: 23 (1.6) AC: 167 (12) | Median (IQR) 33 (20–56) | pN0: 258 (18) pN+: 20 (1.4) pNx: 1143 (80) |
| Lec (2) | 2021 | 2006–2013 | NCDB | 541 (SU) | NR | 5 (IQR: 2–8) | Surgeon's discretion | NAC: 7 (1.3) AC: 74 (14) | Median (IQR) 32 (18–52) | pN0: 144 (27) pN+: 15 (2.8) pNx: 382 (71) |
AC, adjuvant chemotherapy; IQR, interquartile range; Lap: laparoscopy; LN, lymph node; NAC, neoadjuvant chemotherapy; NCDB, National Cancer Database; NR, not reported; Pts. patients; RNU, radical nephroureterectomy; SD, standard deviation; SEER: Surveillance, Epidemiology, and End Results; SU, segmental ureterectomy.
Including laparoscopic, hand-assisted laparoscopic, robot-assisted radical nephroureterectomy.
Only included >pT2 patients.
Only included clinical or pathological node-negative patients.
Table 2.
Oncologic outcomes stratified by lymph node status of studies with large cohort (more than 250 patients included)
| 5-year survival rates | HR (95% CI, or P value) from multivariable analysis | |||||||||||||
| Author | Year | Periods | Institution | Pts. | pN stage [n (%)] | DFS (%) | CSS (%) | OS (%) | DFS | CSS | OS | |||
| Retrospective cohort study | ||||||||||||||
| Roscigno | 2009 | 1987–2007 | Multi (International) | 1130 | pN0: 412 (36) | 71 | 77 | NR | Ref. | |||||
| pN+: 140 (13) | 29 | 35 | NR | 2.40 (P < 0.001) | 2.39 (P < 0.001) | NR | ||||||||
| pNx: 578 (51) | 66 | 69 | NR | 1.16 (P = 0.008) | 1.46 (P = 0.007) | NR | ||||||||
| Burger | 2011 | 1987–2008 | Multi (International) | 785 | pN0: 136 (17) | 72 | 79 | NR | Ref. | |||||
| pN+: 54 (7) | 21 | 27 | NR | 2.0 (1.2–3.4) | 2.1 (1.1–3.8) | NR | ||||||||
| pNx: 595 (76) | 77 | 77 | NR | 1.1 (0.7–1.7) | 1.3 (0.8–2.2) | NR | ||||||||
| Mason | 2012 | 1990–2010 | Multi (Canada) | 1029 | pN0: 199 (20) | 91a | 72 | 66 | Ref. | NR | Ref. | NR | Ref. | NR |
| pN+: 77 (7) | 80a | 30 | 22 | 2.01 (1.18–3.42) | 2.03 (1.31–3.14) | 2.94 (1.32–6.55) | 2.83 (1.54–5.18) | 2.97 (1.47–6.01) | 2.70 (1.56–4.69) | |||||
| pNx: 753 (73) | 71a | 75 | 66 | 1.08 (0.8–1.43) | Ref. | 0.81 (0.51–1.28) | Ref. | 1.04 (0.69–1.56) | Ref. | |||||
| Yoo | 2017 | 1998–2012 | Single (Korea) | 418 | pN0: 116 (29) | 73 | NR | 80 | 1.38 (0.91–2.09) | NR | 1.06 (0.74–1.51) | |||
| pN+: 16 (3) | 13 | NR | 13 | |||||||||||
| pNx: 286 (68) | 76 | NR | 72 | Ref. | ||||||||||
| Ikeda | 2017 | 1985-2013 | Multi (Japan) | 399 | pN0: 182 (46) | 78 | 85 | NR | Ref. | |||||
| pN+: 40 (10) | 33 | 44 | NR | 3.25 (1.90–5.55) | 3.56 (1.90–6.69) | NR | ||||||||
| pNx: 177 (44) | 62 | 73 | NR | 1.91 (1.23–2.96) | 2.28 (1.31–3.95) | NR | ||||||||
| Inokuchi | 2017 | 1995-2009 | Multi (Japan) | 2037 | pN0: 955 (47) | NR | NR | 69 | NR | 1.13 (0.89–1.42) | 1.34 (1.00–1.79) | |||
| pN+: 223 (11) | NR | NR | 30 | NR | ||||||||||
| pNx: 859 (42) | NR | NR | 61 | NR | Ref. | Ref. | ||||||||
| Li | 2021 | 2001-2021 | Multi (Taiwan) | 1340 | pN0: 278 (21) | NR | NR | NR | Ref. | |||||
| pN+: 58 (4) | NR | NR | NR | NR | 3.08 (1.96–4.84) | 4.41 (2.56–7.59) | ||||||||
| pNx: 1004 (75) | NR | NR | NR | NR | 1.10 (0.84–1.44) | 1.14 (0.78–1.67) | ||||||||
| Hsieh | 2022 | 2000-2015 | Single (Taiwan) | 520 | pN0: 303 (58) | 76 | 85 | 77 | Ref. | |||||
| pN+: 20 (3.8) | 29 | 26 | 26 | 1.68 (0.86–3.28) | 2.01 (1.00–4.39) | 1.55 (0.79–3.02) | ||||||||
| pNx: 197 (38) | 65 | 86 | 64 | 1.21 (0.84–1.76) | 0.97 (0.56–1.69) | 1.32 (0.90–1.93) | ||||||||
| Leec | 2022 | 2001-2021 | Multi (Taiwan) | 658 | pN0: 159 (24) | 60 | 75 | 69 | 0.89 (0.64–1.24) | Ref. | 1.07 (0.68–1.67) | Ref. | 1.09 (0.74–1.59) | |
| pN+: 36 (5.4) | P < 0.001 | P = 0.01 | ||||||||||||
| pNx: 463 (70) | 64 | 77 | 68 | Ref. | Ref. | Ref. | ||||||||
| Hakimi | 2022 | 2006-2019 | Multi (ROBUUST) | 877 | pN0: 285 (32) | 61b | 91b | 86b | 1.01 (0.75–1.35) | 1.58 (0.75–3.33) | 1.60 (0.93–2.76) | |||
| pN+: 73 (8.3) | 35b | 45b | 42b | 1.80 (1.20–2.71) | 2.74 (1.34–5.61) | 2.77 (1.59–4.84) | ||||||||
| pNx: 519 (59) | 53b | 90b | 80b | Ref. | ||||||||||
| Population-based study | ||||||||||||||
| Lughezzani | 2010 | 1988–2004 | SEER database | 2842 | pN0: 1835 (64) | NR | 81 | NR | Ref. | |||||
| pN+: 242 (9) | NR | 34 | NR | NR | 2.54 (P < 0.001) | NR | ||||||||
| pNx: 747 (26) | NR | 78 | NR | NR | 0.99 (P = 0.9) | NR | ||||||||
| Chappidi | 2016 | 2004-2012 | SEER database | 2862 | pN0: 466 (16) | NR | 75 | NR | Ref. | |||||
| pN+: 255 (8.9) | NR | 34 | NR | NR | 2.33 (1.50–3.28) | 2.01 (1.48–2.72) | ||||||||
| pNx: 2141 (75) | NR | 72 | NR | NR | 0.97 (0.78–1.42) | 1.12 (0.91–1.39) | ||||||||
| Lenis | 2018 | 2010–2013 | NCDB | 3116 | pN0: 765 (25) | NR | NR | NR | Ref. | |||||
| pN+: 257 (8.3) | NR | NR | NR | NR | NR | 1.87 (1.47–2.37) | ||||||||
| pNx: 2094 (67) | NR | NR | NR | NR | NR | 1.03 (0.85–1.25) | ||||||||
| Dongd | 2019 | 2004–2014 | SEER database | 2731 | pN0: 491 (18) | NR | NR | NR | Ref. | |||||
| pNx: 2240 (82) | NR | NR | NR | NR | 1.27 (1.04–1.55) | 1.28 (1.09–1.51) | ||||||||
| Zhai | 2019 | 2004-2015 | SEER database | 7278 | pN0: 1296 (18) | NR | 77 | 52 | Ref. | |||||
| pN+: 665 (9.1) | NR | 40 | 21 | NR | 2.49 (2.06–3.01) | 2.20 (1.93–2.50) | ||||||||
| pNx: 5317 (73) | NR | 74 | 47 | NR | 1.32 (1.13–1.53) | 1.19 (1.08–1.30) | ||||||||
| Lec (1) | 2021 | 2006-2013 | NCDB | 1421 (RNU) | pN0: 258 (18) | NR | NR | NR | Ref. | |||||
| pN+: 20 (1.4) | NR | NR | NR | NR | NR | 2.41 (1.29–4.47) | ||||||||
| pNx: 1143 (80) | NR | NR | NR | NR | NR | NR | ||||||||
| Lec (2) | 2021 | 2006-2013 | NCDB | 541 (SU) | pN0: 144 (27) | NR | NR | NR | Ref. | |||||
| pN+: 15 (2.8) | NR | NR | NR | NR | NR | 1.43 (0.67–3.07) | ||||||||
| pNx: 382 (71) | NR | NR | NR | NR | NR | NR | ||||||||
Statistical significance is represented as italic font. Values are described as rounded up at the first decimal point in survival rates and at the third decimal point in hazard ratio. AC, adjuvant chemotherapy; CI, confidence interval; CSS, cancer-specific survival; DFS, disease-free survival; HR, hazard ratio; IQR, interquartile range; Lap, laparoscopy; LN, lymph node; NAC, neoadjuvant chemotherapy; NCDB, National Cancer Database; NR, not reported; OS, overall survival; Pts. patients; Ref., referent; RNU, radical nephroureterectomy; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results; SU, segmental ureterectomy.
Not adjusting covariates using univariable analysis.
Two-year survival outcomes.
Only included >pT2 patients.
Only included clinical or pathological node-negative patients.
In 2009, Roscigno et al.[27] conducted an international multicenter study with 1130 patients to assess the impact of pN-status as well as LND on oncologic outcomes. The authors showed inferior DFS (hazard ratio 2.40, P < 0.001) and CSS (hazard ratio 2.39, P < 0.001) in pN+ patients compared with pN0 patients using multivariable Cox regression analysis [27]. As shown in Table 1, compared with pN0, the negative prognostic impact of pN+ with regard to DFS and CSS has been supported by several studies, such as Burger et al.[28] in 2011 (hazard ratio for DFS: 2.0, hazard ratio for CSS: 2.1, n = 785), Mason et al.[29] in 2012 (hazard ratio for DFS: 2.01, hazard ratio for CSS: 2.94, n = 1029), Ikeda et al. in 2017 (hazard ratio for DFS: 3.25, hazard ratio for CSS: 3.56, n = 399) [30], Li et al. in 2021 (hazard ratio for CSS: 3.08, hazard ratio for CSS: 4.41, n = 1340) [31], Hsieh et al.[33] in 2022 (hazard ratio for CSS: 2.01, n = 520) [32], Lee et al.[34▪▪] in 2022 (P < 0.001 in DFS and P = 0.01 in CSS, n = 658), and Hakimi et al. in 2022 (HR for DFS: 1.80, HR for CSS: 2.74, n = 877). In addition, two studies showed that pN+ was associated with worse OS in UTUC patients compared to pN0; for example, Mason et al. in 2012 (HR for OS: 2.70, n = 1029) and Hakimi et al. in 2022 (hazard ratio for OS: 2.77, n = 877) [29,31]. The negative prognostic impact of pN+ on CSS and OS has been confirmed in population-based studies with 5-year DFS, CSS, and OS ranging from 13 to 33%, 26 to 44%, and 13 to 30% in pN+ patients and 71 to 78%, 72 to 85%, and 52 to 80% in pN0 patients, respectively [35,36▪,37–39]. Taken together, these data highlight the negative prognostic impact of lymph node metastasis at RNU on survival endpoints.
Therapeutic impact of lymph node dissection
To date, there is no head-to-head randomized controlled trial, assessing the oncologic impact of LND versus no LND. Several retrospective studies have assessed the potential impact of LND on oncologic outcomes, comparing patients who underwent LND (pN0 and/or pN+) with those who did not (pNx). When comparing oncologic outcomes of pN0 with pNx patients, data from retrospective studies are contradictory, with half of them showing worse oncologic outcomes for pNx patients and the other half not [27–32]. For example, Roscigno et al.[27] and Ikeda et al.[30] reported that, compared with pN0 patients, pNx patients had worse DFS and CSS (Table.1). In addition, Dong et al. and Zhai et al. confirmed worse OS in patients with pNx than in those with pN0 using the surveillance, epidemiology, and end results (SEER) database [39,40]. Theoretically, the pNx cohort includes both pN0 and pN+ patients if they had undergone LND; therefore, when compared with pN0 patients, worse oncologic outcomes in pNx patients (no LND) would be expected. On the contrary, Burger et al., Li et al., Hsieh et al., Lughezzani et al., Chappidi et al., and Lenis et al. showed no differences in DFS, CSS, and OS between patients with pNx and pN0, suggesting that survival outcomes are affected by patient selection, as patients harboring advanced disease associated with potential lymph node metastasis being more likely to undergo LND [28,31,32,35,37,38].
Zhai et al.[39] assessed the impact of performing LND during RNU on CSS and OS using 7278 patients from the SEER database. In multivariable Cox regression analyses, performing LND was associated with a higher OS or CSS in the entire cohort; however, LND did not improve OS in patients with pT1 and pT2 disease [39]. In addition, Dong et al. evaluated the survival impact of LND, including only clinical or pathological N0 patients using the SEER database [40]. After propensity score matching, CSS and OS in cNx-pN0 patients were significantly better than those in cN0-pNx patients [40]. When limiting the analysis to patients who received adjuvant treatment, the authors found that performing LND improved CSS and OS (hazard ratio for OS: 0.57, P = 0.013, hazard ratio for CSS: 0.61, P = 0.046) [40]. These findings from studies using SEER database suggest that LND could improve survival outcomes in patients with clinically node-negative UTUC, especially for those with advanced diseases (T3–4 stages) even in patients with pN0 disease likely by removing micrometastasis. Further investigation is needed to establish more robust evidence regarding the potential benefits of LND on survival, specifically taking into consideration the effect of anatomical extent of LND impact on oncologic outcomes.
Lymph node dissection in clinical node-positive patients
Given the high prevalence of lymphatic spread in UTUC, clinical node-positive (cN+) UTUC patients should be considered for systemic platinum-based combination chemotherapy followed by RNU and LND; however, there is no guideline endorsement of this treatment strategy due to a lack of data to support this strategy that is used in urothelial cancer of the bladder [6,41]. Regarding the optimal timing of chemotherapy for cN+ UTUC patients who were candidates for RNU, Shigeta et al.[42] recently published the results from a retrospective multicenter study comparing oncologic outcomes of systemic induction chemotherapy followed by RNU versus RNU followed by adjuvant chemotherapy. The authors reported that systemic induction chemotherapy followed by RNU was associated with significantly better DFS and CSS compared with RNU followed by adjuvant chemotherapy [42]. However, although this data reflected clinical reality, there was severe selection bias that patients who did not respond to induction chemotherapy received second-line therapy (e.g. immunotherapy) instead of surgical intervention [43]. This is in line with the concept that cN+ is to be considered as systemic disease that requires systemic therapy. Indeed, most studies assessing the impact of LND during RNU excluded cN+ patients, thereby, making the assessment of the natural history of this disease difficult. Recently, Piontkowski et al.[44▪] conducted a retrospective study assessing the impact of LND in 423 patients with cN+ UTUC using the National Cancer Database (NCDB). The authors found that LND does not impact OS (hazard ratio 0.93, 95% CI 0.70–1.24) after adjusting for the effects of covariates in multivariable analysis, irrespective of clinical N stage (i.e. cN1 vs. cN2–3) [44▪]. Therefore, to date, the oncologic benefit of performing LND and its optimal extent for cN+ UTUC remain controversial. Based on the biology of cN+ disease, timely and effective systemic therapy is key to the success of the treatment for patients with cN+; LND may have a limited impact on oncologic outcomes. However, accurate N-staging is also essential for the decision on additional systemic therapy in this clinical setting [9,10].
Optimal extent of lymph node dissection
The therapeutic benefit of LND may depend on the number of lymph node removed. As such, Lec et al.[36▪] reported that LND was associated with improved OS in RNU patients when more than three lymph nodes were removed (hazard ratio 0.58, 95% CI 0.39–0.89). In addition, population-based studies showed that a lower number of lymph nodes removed was associated with worse CSS and OS, even in pN0 patients [35,37]. In 2009, Roscigno et al. assessed the optimal cut-off value of how many lymph node should be removed prognosticating CSS using a multicentric cohort constituting 522 patients [15]. The authors demonstrated that eight lymph nodes were the best cut-off in predicting CSS (hazard ratio 0.42; P = 0.004) [15]. Of note, analyzing in patients with pN0, a higher number of lymph nodes removed remained associated with better disease recurrence (hazard ratio 0.97; P = 0.03) and CSS (hazard ratio 0.96; P = 0.04), whereas there was no association between the number of lymph nodes removed and disease recurrence and CSS in pN+ patients [15]. However, in previous studies, the majority of patients undergoing RNU had less than eight lymph nodes removed (Table 1); therefore, standardized LND templates are needed to ensure that the lymph nodes are adequately removed in the right anatomic area.
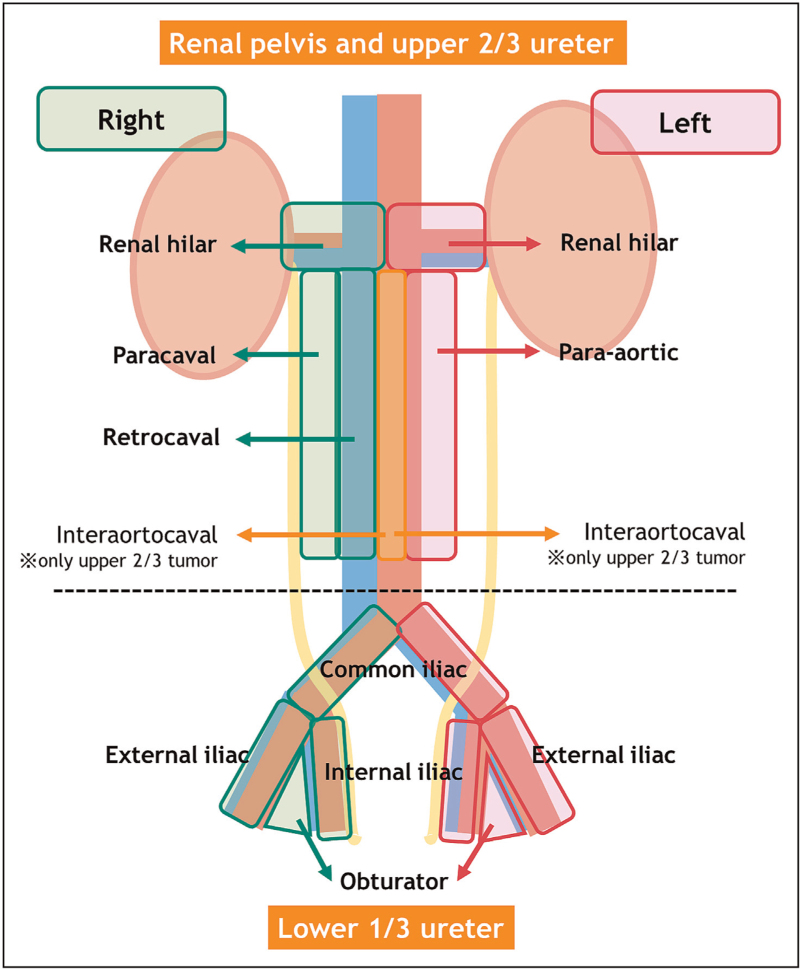
In 2010, Kondo et al.[45] advocated template-based LND depending on the primary tumor site. This template was based on their preliminary study assessing the association between location of lymph node metastasis and the primary tumor site [16]. The authors demonstrated that template-based LND affects survival outcomes and is superior to just assessing the number of removed lymph nodes [45]. Since then, several studies have confirmed that performing an anatomical template-based LND is the best approach/concept from a diagnostic and oncologic perspective [46,47,48▪,49]. A systematic review showed that template-based LND and the level of completeness of LND both improve CSS in patients with muscle-invasive disease and reduce the risk of local recurrence [21]. In addition, Kanno et al.[48▪] recently conducted a multicenter retrospective study to compare oncologic outcomes in patients who underwent template-based LND during RNU and those who did not. After propensity score matching using confounders such as, age, sex, presence of hydronephrosis, and clinical T stage, the authors showed that the estimated 5-year recurrence-free survival was higher in patients who underwent template-based LND (86.8%) compared with those who did not (64.2%) (P = 0.014) [48▪]. Therefore, a template-based LND should be offered to all patients who are planned for RNU for invasive nonmetastatic UTUC. The modified template for LND based on Kondo et al. is described in Fig. 1. This template includes renal hilar, paracaval, and retrocaval lymph nodes for tumors located in right renal pelvis or upper 2/3 of the ureter, and renal hilar and para-aortic lymph nodes for tumors located on the left-side. For tumors located in the lower 1/3 of the ureter, common iliac, external iliac, internal iliac, and obturator lymph nodes are included in the template, irrespective of tumor side. A multicenter study by Matin et al.[47], that included 73 patients, confirmed the validity of this template. In patients with pelvic or upper ureteral tumors, the template without interaortocaval lymph nodes covered 82.9% in right-side tumors and 86.9% in left-side tumors [47]. However, when including interaortocaval lymph nodes, it increased to 95.8% in right-side tumors and 90.2% in left-side tumors [47]. This suggests that, in the case of proximal/upper ureteral tumors, interaortocaval nodes should be removed (Fig. 1).
FIGURE 1.
Modified anatomical lymph node templates depending on tumor location based on the studies by Kondo et al. and Matin et al. Data from [45,47].
Lymph node density
Other than the number of lymph nodes removed, lymph node density, defined as the number of positive lymph nodes divided by the number of lymph node removed, has been considered a prognostic factor for oncologic outcomes [29,50]. In 2009, Bolenz et al.[50] assessed the impact of lymph node density on oncologic outcomes in 432 patients with a median of four lymph nodes removed. The authors showed that a lymph node density of more than 30% was significantly associated with worse recurrence and CSS [50]. Mason et al.[29] assessed the prognostic utility of lymph node density in 276 patients with a mean of 4.3 lymph nodes removed. The authors showed that a lymph node density of more than 20% was associated with worse DFS and OS, despite the number of positive lymph nodes; the number of lymph nodes removed was not associated with any survival outcomes [29]. Lymph node density seems a promising prognostic marker for oncologic outcomes after LND; further investigation with template-based lymph node density is needed to understand the complete prognostic value of LND.
Surgical perspective of lymph node dissection
RNU can be performed by an open, laparoscopic, or robotic approach [51]. Laparoscopic RNU has been suggested to result in worse oncologic outcomes in the treatment of aggressive tumors (i.e. pT3-4) compared with open approach [52], while subsequent retrospective studies showed comparable oncologic outcomes between open and laparoscopic procedures [53,54]. To date, the utilization of robot-assisted RNU has increased based on comparative studies showing noninferiority with regard to perioperative and oncologic outcomes [51,55,56]. However, laparoscopic LND is inferior to other approached for LND because of the limited surgical field. Indeed, Peyronnet et al.[52] showed significantly lower LND rates for laparoscopic compared with open approaches. When performing template-based LND using a laparoscopic retroperitoneal approach, an improved technique without patient repositioning has been advocated [57]. In the context of the number of lymph node removed, Hattori et al.[58] initially reported that an open approach was associated with an increased number of lymph node removed compared with a laparoscopic approach, while two other studies showed no differences between the procedures [15,53]. When comparing robotic and laparoscopic approaches, Melquist et al. reported that a robotic approach was associated with improved lymph node yield (median 21 lymph nodes for robotic and 11 lymph nodes for laparoscopic approaches, P < 0.001). Yet, severe selection biases because of retrospective study design should be considered when interpreting these contradictory results. However, several population-based studies reported increased proportion of performing LND in the era of robotic approach, suggesting superior technical feasibility of LND through a robotic approach [37,51,56].
For the assessment of postoperative complications due to LND, Pearce et al.[59] demonstrated that performing LND increased by 30% the risk of postoperative complications compared with not performing LND. Additionally, several studies aimed to assess the safety of extended LND (ipsilateral renal hilar, para-aortic, pelvic lymph node) during RNU. A prospective study by Rao et al.[60], which evaluated 20 patients (open: n = 10, laparoscopic: n = 4, robot-assisted: n = 6), showed that one patient experienced a severe complication (Clavien–Dindo classification: IIIb), such as postoperative lymphatic leakage requiring surgical procedure, while the other eight suffered from minor complications. Another prospective study by Huang et al.[61] assessed the safety of extended LND during laparoscopic extraperitoneal RNU in 39 patients, reporting no severe complications. A recent published retrospective study led by Kanno et al.[62▪] assessed the perioperative complication and its management after template-based LND during laparoscopic extraperitoneal RNU. The authors reported a high rate of postoperative chylous leakage (14/88), which was conservatively managed and prevented by meticulously clipping each lymphatic vessel [62▪].
CONCLUSION
LND during RNU is the standard treatment for invasive nonmetastatic UTUC, owing to its diagnostic, staging, prognostic, as well as therapeutic potential. Although a higher number of lymph node removed is associated with better oncologic outcomes, the template-based LND concept is superior to the number of lymph nodes removed. Robot-assisted RNU seems to facilitate performing a meticulous LND compared with the laparoscopic approach. Surgeons should be aware and prevent postoperative complications associated with LND, such as lymphatic and/or chylous leakage.
Acknowledgements
None.
Authors’ contributions: T.Y. contributed to protocol/project development, data collection and management, and manuscript writing/editing. T.K., M.v.D., and E.L. contributed to manuscript writing/editing. T.K. contributed to supervision and manuscript editing. S.F.S. contributed to supervision, protocol/project development/management, and manuscript editing.
Financial support and sponsorship
None.
Conflicts of interest
T.K. is a paid consultant/advisor of Astellas, Bayer, Janssen and Sanofi. S.F.S. received as follows: honoraria – Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, Takeda; consulting or advisory role – Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, Takeda; speakers Bureau – Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, Takeda.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Kawada T, Laukhtina E, Quhal F, et al. Oncologic and safety outcomes for endoscopic surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: an updated systematic review and meta-analysis. Eur Urol Focus 2022; doi: 10.1016/j.euf.2022.11.016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Laukhtina E, Kawada T, Quhal F, et al. Oncologic and safety outcomes for retrograde and antegrade endoscopic surgeries for upper tract urothelial carcinoma: a systematic review and meta-analysis. Eur Urol Focus 2022; doi: 10.1016/j.euf.2022.11.014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Katayama S, Mori K, Schuettfort VM, et al. Accuracy and clinical utility of a tumor grade- and stage-based predictive model in localized upper tract urothelial carcinoma. Eur Urol Focus 2022; 8:761–768. [DOI] [PubMed] [Google Scholar]
- 4.Foerster B, Abufaraj M, Matin SF, et al. Pretreatment risk stratification for endoscopic kidney-sparing surgery in upper tract urothelial carcinoma: an international collaborative study. Eur Urol 2021; 80:507–515. [DOI] [PubMed] [Google Scholar]
- 5.Marcq G, Foerster B, Abufaraj M, et al. Novel classification for upper tract urothelial carcinoma to better risk-stratify patients eligible for kidney-sparing strategies: an international collaborative study. Eur Urol Focus 2022; 8:491–497. [DOI] [PubMed] [Google Scholar]
- 6.Rouprêt M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol 2021; 79:62–79. [DOI] [PubMed] [Google Scholar]
- 7.Lughezzani G, Jeldres C, Isbarn H, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer 2009; 45:3291–3297. [DOI] [PubMed] [Google Scholar]
- 8.Roscigno M, Brausi M, Heidenreich A, et al. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur Urol 2011; 60:776–783. [DOI] [PubMed] [Google Scholar]
- 9.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 2021; 384:2102–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020; 395:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baard J, Shariat SF, Roupret M, et al. Adherence to guideline recommendations in the management of upper tract urothelial carcinoma: an analysis of the CROES-UTUC registry. World J Urol 2022; 40:2755–2763. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Miura N, Babjuk M, et al. Low compliance to guidelines in nonmuscle-invasive bladder carcinoma: a systematic review. Urol Oncol 2020; 38:774–782. [DOI] [PubMed] [Google Scholar]
- 13.Moschini M, Foerster B, Abufaraj M, et al. Trends of lymphadenectomy in upper tract urothelial carcinoma (UTUC) patients treated with radical nephroureterectomy. World J Urol 2017; 35:1541–1547. [DOI] [PubMed] [Google Scholar]
- 14.Soria F, Pradere B, Hurle R, et al. European Association of Urology—Young Academic Urologists EAU-YAU: Urothelial Carcinoma Working Group. Radical nephroureterectomy tetrafecta: a proposal reporting surgical strategy quality at surgery. Eur Urol Open Sci 2022; 42:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roscigno M, Shariat SF, Margulis V, et al. The extent of lymphadenectomy seems to be associated with better survival in patients with nonmetastatic upper-tract urothelial carcinoma: how many lymph nodes should be removed? Eur Urol 2009; 56:512–518. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T, Nakazawa H, Ito F, et al. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology 2007; 69:265–269. [DOI] [PubMed] [Google Scholar]
- 17.Wheat JC, Weizer AZ, Wolf JS, Jr, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ confined urothelial carcinoma following radical nephroureterectomy. Urol Oncol 2012; 30:252–258. [DOI] [PubMed] [Google Scholar]
- 18.Zigeuner R, Shariat SF, Margulis V, et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 2010; 57:575–581. [DOI] [PubMed] [Google Scholar]
- 19.Secin FP, Koppie TM, Salamanca JI, et al. Evaluation of regional lymph node dissection in patients with upper urinary tract urothelial cancer. Int J Urol 2007; 14:26–32. [DOI] [PubMed] [Google Scholar]
- 20.König F, Shariat SF, Karakiewicz PI, et al. Quality indicators for the management of high-risk upper tract urothelial carcinoma requiring radical nephroureterectomy. Curr Opin Urol 2021; 31:291–296. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez-Escrig JL, Peyronnet B, Seisen T, et al. Potential benefit of lymph node dissection during radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the European Association of Urology Guidelines Panel on Nonmuscle-invasive Bladder Cancer. Eur Urol Focus 2019; 5:224–241. [DOI] [PubMed] [Google Scholar]
- 22.Janisch F, Shariat SF, Baltzer P, et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): a systematic review and meta-analysis. World J Urol 2020; 38:1165–1175. [DOI] [PubMed] [Google Scholar]
- 23.Millán-Rodríguez F, Palou J, de la Torre-Holguera P, et al. Conventional CT signs in staging transitional cell tumors of the upper urinary tract. Eur Urol 1999; 35:318–322. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Pallauf M, D’Andrea D, König F, et al. Diagnostic accuracy of clinical lymph node staging for upper tract urothelial cancer patients - a multicenter, retrospective, observational study. J Urol 2023; 209:515–524. [DOI] [PubMed] [Google Scholar]; This study is a multicenter retrospective study assessing the diagnostic performance of conventional cross-sectional imaging using 865 UTUC patients treated with RNU and LND, showing that conventional imaging has a low diagnostic accuracy.
- 25.Voskuilen CS, Schweitzer D, Jensen JB, et al. Diagnostic value of (18)F-fluorodeoxyglucose positron emission tomography with computed tomography for lymph node staging in patients with upper tract urothelial carcinoma. Eur Urol Oncol 2020; 3:73–79. [DOI] [PubMed] [Google Scholar]
- 26.Aydh A, Abufaraj M, Mori K, et al. Performance of fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography imaging for lymph node staging in bladder and upper tract urothelial carcinoma: a systematic review. Arab J Urol 2020; 19:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roscigno M, Shariat SF, Margulis V, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol 2009; 181:2482–2489. [DOI] [PubMed] [Google Scholar]
- 28.Burger M, Shariat SF, Fritsche HM, et al. No overt influence of lymphadenectomy on cancer-specific survival in organ-confined versus locally advanced upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy: a retrospective international, multiinstitutional study. World J Urol 2011; 29:465–472. [DOI] [PubMed] [Google Scholar]
- 29.Mason RJ, Kassouf W, Bell DG, et al. The contemporary role of lymph node dissection during nephroureterectomy in the management of upper urinary tract urothelial carcinoma: the Canadian experience. Urology 2012; 79:840–845. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Matsumoto K, Sakaguchi K, et al. Effect of lymphadenectomy during radical nephroureterectomy in locally advanced upper tract urothelial carcinoma. Clin Genitourin Cancer 2017; 15:556–562. [DOI] [PubMed] [Google Scholar]
- 31.Li CC, Chang CH, Huang CP, et al. Comparing oncological outcomes and surgical complications of hand-assisted, laparoscopic and robotic nephroureterectomy for upper tract urothelial carcinoma. Front Oncol 2021; 11:731460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh HC, Wang CL, Chen CS, et al. The prognostic impact of lymph node dissection for clinically node-negative upper urinary tract urothelial carcinoma in patients who are treated with radical nephroureterectomy. PLoS One 2022; 17:e0278038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HY, Chang CH, Huang CP, et al. Is lymph node dissection necessary during radical nephroureterectomy for clinically node-negative upper tract urothelial carcinoma? a multi-institutional study. Front Oncol 2022; 12:791620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪▪.Hakimi K, Carbonara U, Djaladat H, et al. Outcomes of lymph node dissection in nephroureterectomy in the treatment of upper tract urothelial carcinoma: analysis of the ROBUUST Registry. J Urol 2022; 208:268–276. [DOI] [PubMed] [Google Scholar]; This study assessed the impact of LND in patients who underwent robot-assisted RNU, showing better number of lymph nodes removed and worse oncologic outcomes in patients with pN+.
- 35.Chappidi MR, Kates M, Johnson MH, et al. Lymph node yield and tumor location in patients with upper tract urothelial carcinoma undergoing nephroureterectomy affects survival: a U.S. population-based analysis. Urol Oncol 2016; 34:531.e15–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Lec PM, Venkataramana A, Lenis AT, et al. Trends in management of ureteral urothelial carcinoma and effects on survival: a hospital-based registry study. Urol Oncol 2021; 39:194.e17–194.e24. [DOI] [PubMed] [Google Scholar]; This study showed that LND was associated with improved OS in RNU patients when more than three nodes were removed.
- 37.Lenis AT, Donin NM, Faiena I, et al. Role of surgical approach on lymph node dissection yield and survival in patients with upper tract urothelial carcinoma. Urol Oncol 2018; 36:9.e1–9.e9. [DOI] [PubMed] [Google Scholar]
- 38.Lughezzani G, Jeldres C, Isbarn H, et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 2010; 75:118–124. [DOI] [PubMed] [Google Scholar]
- 39.Zhai TS, Jin L, Zhou Z, et al. Effect of lymph node dissection on stage-specific survival in patients with upper urinary tract urothelial carcinoma treated with nephroureterectomy. BMC Cancer 2019; 19:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong F, Xu T, Wang X, et al. Lymph node dissection could bring survival benefits to patients diagnosed with clinically node-negative upper urinary tract urothelial cancer: a population-based, propensity score-matched study. Int J Clin Oncol 2019; 24:296–305. [DOI] [PubMed] [Google Scholar]
- 41.Abufaraj M, Li R, Meeks J, Shariat SF. Cytoreductive surgery in patients with urothelial bladder cancer. Eur Urol Focus 2022. [DOI] [PubMed] [Google Scholar]
- 42.Shigeta K, Matsumoto K, Ogihara K, et al. Does neoadjuvant chemotherapy have therapeutic benefit for node-positive upper tract urothelial carcinoma? Results of a multicenter cohort study. Urol Oncol 2022; 40:105.e19–105.e26. [DOI] [PubMed] [Google Scholar]
- 43.Liedberg F, Abrahamsson J, Bobjer J. Re: Keisuke Shigeta, Kazuhiro Matsumoto, Koichiro Ogihara, et al. Does Neoadjuvant Chemotherapy Have Therapeutic Benefit for Node-positive Upper Tract Urothelial Carcinoma? Results of a Multicenter Cohort Study. Urol Oncol. [in press]. https://doi.org/10.1016/j.urolonc.2021.07.029: A Plea for Uniform Terminology for Patients with Urothelial Carcinoma Treated with Chemotherapy Before Consolidative Surgery with Curative Intent: Induction Versus Neoadjuvant Chemotherapy. Eur Urol 2022; 81:e18–e19. [DOI] [PubMed] [Google Scholar]
- 44▪.Piontkowski AJ, Corsi N, Morisetty S, et al. Benefit of lymph node dissection in cN+ patients in the treatment of upper tract urothelial carcinoma: analysis of NCDB registry. Urol Oncol 2022; 40:409.e9–409.e17. [DOI] [PubMed] [Google Scholar]; This study assessed the impact of LND in 423 patients with cN+ UTUC using the NCDB, showing that LND does not impact OS in multivariable analysis.
- 45.Kondo T, Hashimoto Y, Kobayashi H, et al. Template-based lymphadenectomy in urothelial carcinoma of the upper urinary tract: impact on patient survival. Int J Urol 2010; 17:848–854. [DOI] [PubMed] [Google Scholar]
- 46.Kondo T, Tanabe K. Role of lymphadenectomy in the management of urothelial carcinoma of the bladder and the upper urinary tract. Int J Urol 2012; 19:710–721. [DOI] [PubMed] [Google Scholar]
- 47.Matin SF, Sfakianos JP, Espiritu PN, et al. Patterns of lymphatic metastases in upper tract urothelial carcinoma and proposed dissection templates. J Urol 2015; 194:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Kanno T, Kobori G, Ito K, et al. Oncological outcomes of retroperitoneal lymph node dissection during retroperitoneal laparoscopic radical nephroureterectomy for renal pelvic or upper ureteral tumors: matched-pair analysis. J Endourol 2022; 36:1206–1213. [DOI] [PubMed] [Google Scholar]; This study is a multicenter retrospective study to compare oncologic outcomes in patients who underwent template-based LND during RNU and those who did not, showing better recurrence-free survival in patients who underwent template-based LND compared with those who did not.
- 49.Matsumoto R, Abe T, Takada N, et al. Oncologic outcomes of laparoscopic radical nephroureterectomy in conjunction with template-based lymph node dissection: an extended follow-up study. Urol Oncol 2020; 38:933.e13–933.e18. [DOI] [PubMed] [Google Scholar]
- 50.Bolenz C, Shariat SF, Fernández MI, et al. Risk stratification of patients with nodal involvement in upper tract urothelial carcinoma: value of lymph-node density. BJU Int 2009; 103:302–306. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez JF, Packiam VT, Boysen WR, et al. Utilization and outcomes of nephroureterectomy for upper tract urothelial carcinoma by surgical approach. J Endourol 2017; 31:661–665. [DOI] [PubMed] [Google Scholar]
- 52.Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological outcomes of laparoscopic nephroureterectomy versus open radical nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology Guidelines Systematic Review. Eur Urol Focus 2019; 5:205–223. [DOI] [PubMed] [Google Scholar]
- 53.Abe T, Kondo T, Harabayashi T, et al. Comparative study of lymph node dissection, and oncological outcomes of laparoscopic and open radical nephroureterectomy for patients with urothelial carcinoma of the upper urinary tract undergoing regional lymph node dissection. Jpn J Clin Oncol 2018; 48:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kido K, Hatakeyama S, Fujita N, et al. Oncologic outcomes for open and laparoscopic radical nephroureterectomy in patients with upper tract urothelial carcinoma. Int J Clin Oncol 2018; 23:726–733. [DOI] [PubMed] [Google Scholar]
- 55.Clements MB, Krupski TL, Culp SH. Robotic-assisted surgery for upper tract urothelial carcinoma: a comparative survival analysis. Ann Surg Oncol 2018; 25:2550–2562. [DOI] [PubMed] [Google Scholar]
- 56.Kenigsberg AP, Smith W, Meng X, et al. Robotic nephroureterectomy vs laparoscopic nephroureterectomy: increased utilization, rates of lymphadenectomy, decreased morbidity robotically. J Endourol 2021; 35:312–318. [DOI] [PubMed] [Google Scholar]
- 57.Miki J, Yanagisawa T, Iwatani K, et al. Supine extraperitoneal laparoscopic nephroureterectomy without patient repositioning. Int J Urol 2021; 28:163–168. [DOI] [PubMed] [Google Scholar]
- 58.Hattori R, Yoshino Y, Gotoh M, et al. Laparoscopic nephroureterectomy for transitional cell carcinoma of renal pelvis and ureter: Nagoya experience. Urology 2006; 67:701–705. [DOI] [PubMed] [Google Scholar]
- 59.Pearce SM, Pariser JJ, Patel SG, et al. The effect of surgical approach on performance of lymphadenectomy and perioperative morbidity for radical nephroureterectomy. Urol Oncol 2016; 34:121.e15-21. [DOI] [PubMed] [Google Scholar]
- 60.Rao SR, Correa JJ, Sexton WJ, et al. Prospective clinical trial of the feasibility and safety of modified retroperitoneal lymph node dissection at time of nephroureterectomy for upper tract urothelial carcinoma. BJU Int 2012; 110:E475–E480. [DOI] [PubMed] [Google Scholar]
- 61.Huang J, Qian H, Yuan Y, et al. Prospective clinical trial of the oncologic outcomes and safety of extraperitoneal laparoscopic extended retroperitoneal lymph node dissection at time of nephroureterectomy for upper tract urothelial carcinoma. Front Oncol 2022; 12:791140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Kanno T, Kobori G, Ito K, et al. Complications and their management following retroperitoneal lymph node dissection in conjunction with retroperitoneal laparoscopic radical nephroureterectomy. Int J Urol 2022; 29:455–461. [DOI] [PubMed] [Google Scholar]; This study assessed perioperative complication and its management after template-based LND during laparoscopic extraperitoneal RNU, showing a high rate of postoperative chylous leakage.