Abstract
The Drosophila tko25t point mutation in the gene encoding mitoribosomal protein S12 produces a complex phenotype of multiple respiratory chain deficiency, developmental delay, bang-sensitivity, impaired hearing, sugar and antibiotic sensitivity, and impaired male courtship. Its phenotypic severity was previously shown to be alleviated by inbreeding and to vary with mitochondrial genetic background. Here, we show similarly profound effects conferred by nuclear genetic background. We backcrossed tko25t into each of 2 standard nuclear backgrounds, Oregon R and w1118, the latter used as recipient line in many transgenic applications requiring selection for the white minigene marker. In the w1118 background, tko25t flies showed a moderate developmental delay and modest bang-sensitivity. In the Oregon R background, males showed longer developmental delay and more severe bang-sensitivity, and we were initially unable to produce homozygous tko25t females in sufficient numbers to conduct a meaningful analysis. When maintained as a balanced stock over 2 years, tko25t flies in the Oregon R background showed clear phenotypic improvement though were still more severely affected than in the w1118 background. Phenotypic severity did not correlate with the expression level of the tko gene. Analysis of tko25t hybrids between the 2 backgrounds indicated that phenotypic severity was conferred by autosomal, X-chromosomal, and parent-of-origin-dependent determinants. Although some of these effects may be tko25t specific, we recommend that, in order to minimize genetic drift and confounding background effects, the genetic background of nonlethal mutants should be controlled by regular backcrossing, even if stocks are usually maintained over a balancer chromosome.
Keywords: mitochondria, ribosome, semilethality, nuclear background
Jacobs, Tuomela, and Lillsunde backcross tko25t, a mutation in Drosophila affecting mitochondria, into two commonly used wild-type nuclear backgrounds. Despite the presence of balancer chromosomes—and without deliberate selection—the backcrosses gave systematically different phenotypes that evolved over two years in the laboratory. Their results underscore the potential effects genetic background and drift can have on mutant phenotypes, especially when studying the genetics of multicomponent structures, and they recommend regular backcrossing to control for confounding background effects.
Introduction
The Drosophila bang-sensitive mutant tko25t, harboring a point mutation in the gene encoding mitoribosomal protein S12 (Royden et al. 1987; Shah et al. 1997), was originally classified as semilethal (Judd et al. 1972). It was subsequently maintained by ourselves and others, both as an inbred stock and over an FM7 balancer chromosome. The major features of the organismal phenotype, in addition to bang-sensitivity, are delayed larval development, deafness (non-responsiveness to sound), impaired male courtship, and hypersensitivity to antibiotics targeting the mitochondrial translation system (Toivonen et al. 2001). In an earlier study, we reported that the inbred, laboratory-maintained tko25t stock manifested a milder phenotype than originally described, but that a more severe phenotype could be restored by backcrossing the mutation into 2 different standard nuclear backgrounds (Canton S and Oregon R) to create a hybrid (Jacobs et al. 2004). Subsequently, we have explored various genetic and environmental manipulations that further modified the tko25t phenotype. Individual lines established from the original tko25t Canton S/Oregon R hybrid, and selected at each generation for the fastest development, showed varying degrees of suppression of the mutant phenotype. The 3 most pronounced suppressors were found to harbor segmental duplications of the region of the X chromosome encompassing the mutated tko gene (Kemppainen et al. 2009).
The mitochondrial genetic background was also shown to be a key determinant of tko25t phenotype: in some mtDNA backgrounds, notably those previously infected with the endosymbiont Wolbachia, partial suppression was found (Chen et al. 2012), while another mtDNA haplotype isolated from the wild was synthetically lethal with tko25t (Salminen et al. 2019). Here, we attempted a similar analysis, but of the effect of nuclear background, keeping mtDNA haplotype and the tko25t mutation itself unchanged. Without imposing any kind of deliberate selection, we obtained divergent outcomes, pointing to the genetic complexity of nuclear–mitochondrial interactions in a metazoan.
Materials and methods
Drosophila culture and crosses
Flies were cultured in standard high-sugar medium (Fernandez-Ayala et al. 2009): 1% agar, 3% glucose, 1.5% sucrose, 3% treacle (Tate & Lyle, UK), 3.5% dried yeast, 1% soy flour, 1.5% maize flour, plus 0.1% Nipagin M (Sigma), and 0.5% (v/v) propionic acid (JT Baker) as antimicrobials, on a 12-hour/12-hour light-dark cycle at the temperatures indicated in figures and legends (25°C where not otherwise stated). For long-term maintenance, fly stocks were kept at 18°C. The tko25t mutation was maintained over an FM7 balancer chromosome. tko25t flies were generated for experimental analysis by crossing tko25t/FM7 females with tko25t/Y males, with an initial cross of tko25t/FM7 females with FM7/Y males to generate the mutant males, where needed.
Eclosion timing and bang-sensitivity
Eclosion timing was measured, as previously (Kemppainen et al. 2016), using replicate sets of vials (n ≥ 3). Note that mean eclosion times shown in figures for each sex and genotype are the mean of the replicate vials in a given experiment. Numbers of adult progeny are indicated in figures and/or legends. Bang-sensitivity was measured as previously (Toivonen et al. 2001): individual flies were transferred under CO2 anesthesia from a food vial into a single empty vial with a bung. After collection, flies were left for a minimum of 1 hour to recover from CO2 exposure. Each fly was subjected to 10 seconds of vortexing on a laboratory vortex mixer at maximum speed. The vial was then tapped so that the paralyzed fly fell to the bottom of the vial on its back. Each fly was continuously observed for up to 300 seconds until it recovered a normal standing posture, with the elapsed time scored as the recovery time. Recovery time was arbitrarily scored as 300 seconds for any fly that did not resume a normal standing posture during the experiment, and as 0 seconds for any fly that did not visibly become paralyzed at all.
Single-fly genomic DNA extraction
Individual flies in a 0.5-mL tube were mashed for 5–10 seconds with a plastic pipette tip in 50 μL of SB (10 mM Tris-HCl, 1 mM EDTA, 25 mM NaCl, 200 μg/mL Proteinase K, pH 8.2). After incubation at room temperature for 20–30 min, the Proteinase K was inactivated by heating to 95°C for 1–2 min and the mix was used immediately for PCR.
Genotyping
The presence of the tko25t mutation was verified by PCR amplification of a 1.49-kb fragment of the tko gene from single-fly genomic DNA, using primer Tko-51 (5′- GAACAAAAAACTACTGAACAAAACTCC-3′) and Tko-31 (5′-CATTTGAACAACGTGATTAGGAAGT-3′), followed by Sanger sequencing of the agarose gel-purified product using primers tko_TT_L1 (5′-GTGCTTTATTGATTTCGAGCGATCT-3′) and tko_TT_R1 (5′-ACTATTGGCTCTTCTTGACGACGTG-3′).
Expression analysis by qRT-PCR
Total RNA was extracted and tko RNA levels relative to RpL32 were measured by quantitative RT-PCR as previously, using the SYBR Green method (Bahhir et al. 2019) with the following oligonucleotide primers (all shown 5′ to 3′): for tko, tko_L1_qPCR—CGACGGCTGGTACTACAAAC and tko_R1_qPCR—GGTCTCATAGCTGCACTGGA and for RpL32: RpL32_F—AGGCCCAAGATCGTGAAGAA and Rpl32_R—TGTGCACCAGGAACTTCTTGAA.
Courtship analysis
Courtship was analyzed essentially as previously (Toivonen et al. 2001), in batches of 6 pairs of adult male and virgin female 3-day-old flies of a given genotype. For experiments at different temperatures, virgin flies were kept at the experiment temperature until the experiment was started. Flies were transferred into individual mating chambers by drawing them from culture vials one by one using a depressurized plastic bottle and releasing them into the chamber. With the female and male initially separated, mating chambers were placed in an incubator at the specified temperature. Flies were allowed to recover from the transfer for 1 hour before allowed to interact with the opposite sex and then inspected under a stereo microscope at 15-minute intervals during each hour-long experiment to determine how many pairs copulated. Mean copulation frequency on a per batch basis was derived from n > 5 batches of each genotype studied.
Statistical analysis
Data were analyzed, where indicated, either by Student's t-test or by 1-way ANOVA using a standard online tool (https://astatsa.com/OneWay_Anova_with_TukeyHSD/). Recovery times in the bang-sensitivity assay were not normally distributed; therefore, raw data only are presented in the figures.
Results and discussion
Backcrossing of tko25t into standard backgrounds
The tko25t mutation was backcrossed over ≥10 generations according to the scheme outlined in Fig. 1 into each of 2 standard nuclear backgrounds, Oregon R, used in laboratories worldwide since 1925—see Lindsley and Grell (1968), and w1118 (Hazelrigg et al. 1984), widely used for selection of transgenic lines using the mini-white marker, which confers colored eyes in a white-eyed background. After back-crossing, several lines in each background were maintained over a standard X-chromosomal balancer, FM7 (Merriam 1968). For each background, we established multiple independent back-crossed lines.
Fig. 1.
Backcrossing scheme for tko25t. At each backcross generation, heterozygous (tko25t/+) females were identified by single-organism genomic DNA extraction, PCR, and sequencing, after pre-mating. Progeny from wild-type females was discarded. Several parallel lines were established in each backcross, both to the w1118 and to the Oregon R backgrounds. The FM7-balanced, backcrossed lines were established in parallel.
Outbred tko25t lines manifest background-dependent phenotypes
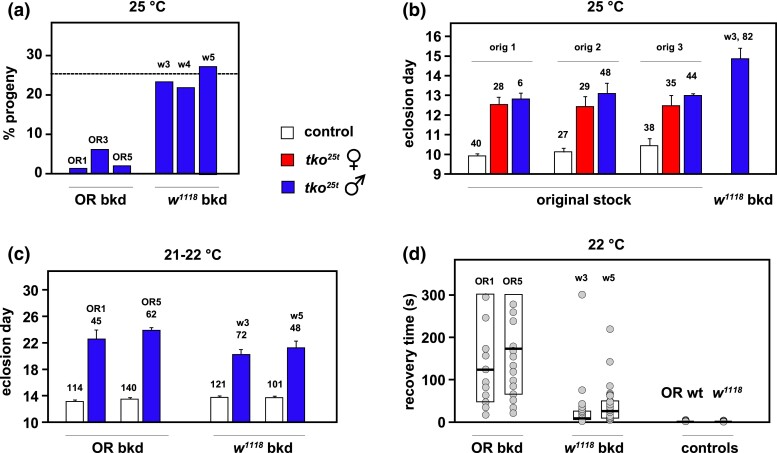
All of the outbred tko25t lines showed a more severe phenotype than the lab-maintained tko25t stock, in terms of both developmental delay and bang-sensitivity. The tko25t phenotypes in the 2 backgrounds were also distinct (Fig. 2), although 3 independent back-crossed lines in the Oregon R background were similar to each other, as were 3 such lines in the w1118 background (Fig. 2). In initial crosses to generate tko25t males from the balanced outbred stocks (see legend to Fig. 2), we observed that very few mutant males eclosed in the Oregon R background at the standard culture temperature of 25°C, resembling the semilethality first reported for the mutation (Fig. 2a), whereas the output of mutant males in the w1118 background was very close to the expected 25% (Fig. 2a). The few tko25t males in the Oregon R background that eclosed were very delayed and extremely bang-sensitive, although there were too few from each line to generate meaningful numbers. For the w1118 background, developmental delay at 25°C was around 5 days, whereas our original tko25t stock showed a developmental delay of only 2–3 days (Fig. 2b). We found that more substantial numbers of tko25t males in the Oregon R background eclosed at room temperature 21–22°C, although they were still very bang-sensitive and did not mate, making it impossible to generate tko25t females in this background. We were, however, able to generate sufficient numbers of mutant males to compare developmental delay (Fig. 2c) and bang-sensitivity (Fig. 2d) in the 2 backgrounds.
Fig. 2.
Phenotype of tko25t in different nuclear backgrounds. a) Proportion of tko25t males among eclosing progeny from crosses of the type tko25t/FM7×FM7/Y, conducted at 25°C, after back-crossing of tko25t into the Oregon R background (OR bkd), to create lines OR1, OR3, and OR5 as indicated, or into the w1118 background, to create lines w3, w4, and w5. The dotted line indicates the expected proportion of tko25t/Y progeny (25%). n = 208 (OR1), 295 (OR3), 314 (OR5), 363 (w3), 330 (w4), and 338 (w5) total eclosed adults, from 4 individual vials in each case. In the original, lab-maintained tko25t stock, the percentage of mutant males in similar crosses without other balancers was always close to the expected 25%, i.e. similar to the findings for tko25t backcrossed into w1118. The output of males in each of the 2 background control strains in equivalent crosses was routinely the expected ∼50%. b) Eclosion times (means ± SD) for tko25t flies generated in multiple crosses using the original tko25t stock. In the 3 experiments shown (orig 1, orig 2, and orig 3), mothers were tko25t/FM7 and carrying different autosomal balancers (respectively, CyO, TM3-Sb, and TM3-Ser) with the scored tko25t progeny free of all balancer markers, some of which are known to interact with tko25t [see Supplementary Fig. 2 of George et al. (2019), which presents some of the same data]. The number of adult flies of each genotype/sex analyzed is indicated above the applicable column. n = 5 replicate vials in all cases except for line w3, where n = 4 replicate vials. All controls were tko25t/FM7 heterozygotes in the same background which, in both back-cross backgrounds, gave indistinguishable eclosion times as wild-type males in the same background (Supplementary Fig. 1a). c) Eclosion times (means ± SD) for similar crosses as in a), but carried out at room temperature (21–22°C). The number of adult flies of each genotype/sex analyzed is indicated above the applicable column. n = 5 replicate vials in all cases. d) Bang-sensitivity assay (recovery times) for progeny flies of the indicated genotypes from the crosses in c), assayed at 22°C. n = 35 (OR1), 38 (OR5), 38 (w3), and 29 (w5) tko25t mutant males, and 119 (OR) and 132 (w1118) control males. Box plots show 25th and 75th percentiles of each distribution and medians (bold lines). Note that the scatter plots are only indicative, since many data points are coincident or almost completely overlapping on this scale: for full details of source data, see Supplementary Table 1.
Outbred tko25t lines maintained as balanced stock show phenotypic alleviation
The tko25t mutation in the outbred lines was maintained for 2 years over an FM7 balancer chromosome with no intentional selection. When retested, the phenotype of the lines in both backgrounds was substantially milder than in the initial tests. In the Oregon R background, the mutation was no longer semilethal, and mutant males were now able to mate and give offspring. We were able to obtain sufficient numbers of mutant flies of both sexes and both backgrounds to reliably measure developmental time (Fig. 3a) and bang-sensitivity (Fig. 3b) at 25°C (Fig. 3). Flies outbred to the w1118 background still showed a milder phenotype than those in the Oregon R background, notably for bang-sensitivity (Fig. 3b), but were also less severely affected than when initially tested, immediately after the completion of the backcrosses. Developmental delay of tko25t flies at 25°C was 3–4 days in the w1118 background and 4–5 days in the Oregon R background (see examples in Fig. 3), with males always more delayed than females.
Fig. 3.
Phenotype after 2 years in the culture of tko25t backcrossed into different nuclear backgrounds. a) Developmental delay (days, d, means ± SD) and b) bang-sensitivity recovery times (box-plot nomenclature as in Fig. 2) for tko25t flies and controls of the indicated back-crossed lines, after 2 years in culture as balanced stocks. Crosses of the type tko25t/FM7×tko25t/Y were conducted at 25°C, with controls being tko25t/FM7 heterozygotes. In a), a repeat experiment is shown for the w3 line (hatched rectangles), due to the large error bars seen for mutant females in the primary experiment. The number of adult flies of each genotype/sex analyzed is indicated above the applicable column. n = 3 replicate bottles in all cases. In b), the same data for tko25t flies in the w1118 background are shown at 2 different scales, as indicated by the dotted lines, with the 300-seconds data points omitted, for clarity, in the expanded-scale graph (right-hand panel). n = 70 (OR3 females), 46 (OR3 males), 104 (w3 females), and 85 (w3 males). Note that, as for Fig. 2d, the scatter plots are only indicative, since many data points are coincident or almost completely overlapping on both scales: for full details of source data, see Supplementary Table 2. Control flies (see Fig. 2d) were not bang-sensitive.
Tko25t phenotype and tko expression are crudely correlated
In a previous study (Kemppainen et al. 2009), no strict correlation was found between the expression level of the mutant tko gene and the tko25t phenotype, although duplication of the mutant gene in its natural chromosomal milieu did provide a partial phenotypic rescue (Kemppainen et al. 2009). In the present study, it was clear that the phenotype of developmental delay and bang-sensitivity was more pronounced in mutant males than mutant females, in the Oregon R background compared with the w1118 background, and in mutant flies cultured at 25°C than at 21–22°C. To reexamine the influence of tko expression level on phenotype, we used qRT-PCR to analyze tko RNA levels in 2-day-old adults of each sex, background, genotype, and culture temperature (Fig. 4). At 25°C, the expression level of tko was similar in wild-type flies of each sex in the 2 backgrounds (Fig. 4a), being only slightly higher in Oregon R. We thus used RNA from flies of the corresponding sex and background as a control, in all subsequent assays of tko expression in tko25t flies (Fig. 4b–d). In all cases tested, tko expression was higher in tko25t than in control flies, and the difference was more pronounced in males, where it was greatest in the Oregon R background. These findings suggest that increased tko expression might be a compensatory response to (or at least a marker for) a severely deleterious phenotype. However, expression in females was almost identical in the 2 backgrounds, despite the phenotypic differences, and was very similar at the 2 culture temperatures. Thus, tko expression level is not a simple determinant of the tko25t phenotype.
Fig. 4.
Expression level of tko in tko25t flies backcrossed into 2 nuclear backgrounds. tko RNA levels by qRT-PCR (means ± SD), for flies of the indicated sex, background, and culture temperature, normalized a) to values for one isolate of Oregon R wild-type females and b) to mean values for control flies of the same sex and background (Oregon R wild-type or w1118, as appropriate). *, **, *** denote significantly different data classes (pairwise comparisons between genotypes, same sex, and culture temperature, by Student's t-test, P < 0.05, 0.01, or 0.001, respectively). For a), despite experiment-to-experiment variation, tko expression was generally lower in the w1118 background in both sexes and at all temperatures tested, as in the example shown. n = 3 technical replicates of each of 3 biological replicates of batches of 5–7 adults, for all genotypes and sexes.
Autosomal semi-dominance and parent-of-origin effects underlie strain differences in tko25t phenotype
In an attempt to characterize the genetic basis of the strain-background differences, we crossed heterozygous tko25t/FM7 females in each background with tko25t males in the other and assessed the phenotype of the first-generation progeny. Since the exact eclosion timing varies slightly between the background strains, and can also differ between experiments, we plotted the amount of developmental delay for both tko25t females and males in each background, alongside the delay observed for the tko25t background hybrids (Fig. 5). Female tko25t hybrid progeny from tko25t/FM7 mothers in the w1118 background were only slightly more delayed than if both parents were in the w1118 background (Fig. 5a). For males, the delay for hybrid progeny from these mothers was the same as when both parents were in the w1118 background (Fig. 5b). Hybrid tko25t progeny of both sexes showed an intermediate delay when the female parent was from the Oregon R background (Fig. 5, a and b). These findings suggest a mixed contribution from autosomal and maternal determinants. The latter could be mitochondrial, although the mtDNA of the 2 lines was identical at the start of the back-cross. It may also indicate a form of parental imprinting (Lloyd 2000; Anaka et al. 2009), although the existence of imprinting in Drosophila has been questioned (Coolon et al. 2012). Alternatively, it could be a classic maternal effect, e.g. involving noncoding RNAs (Soleimani et al. 2020), localized (Hoch and Jäckle 1993) or pioneer transcription factors (Gaskill et al. 2021) or their mRNAs, or other regulatory proteins (Zhang et al. 2018). A simple role for the X-chromosome in mutant eclosion timing seems to be excluded, since the w1118 background appears to be dominant only when inherited from the mother, and in both sexes. Although clear and mostly statistically significant, the differences in delay between the crosses and sexes are too small to enable a meaningful next-generation cross to be conducted, in order to try to tease out the contributions of autosomal, mitochondrial, and possible X-chromosomal inheritance.
Fig. 5.
Eclosion timing in tko25t strain-background crosses. Developmental delay (days, d, means ± SD) at 25°C of a) female and b) male tko25t progeny from the indicated crosses between tko25t/FM7 females and tko25t/Y males in the backgrounds as shown (OR—Oregon R, w—w1118). For ease of comparison, dotted lines indicate values for the non-hybrid crosses, reproduced from Fig. 3a, which were conducted in parallel using the same expanded parental stocks. Data from a) and b) are from the same crosses, but with the sexes shown separately, for clarity. Control flies were tko25t/FM7 heterozygous females, which show an identical eclosion timing as wild-type males (see Supplementary Fig. 1a), with only the SD shown (means were all set to zero, so as to exclude minor, strain-specific differences in eclosion timing from the analysis). The number of adult flies of each genotype/sex analyzed is indicated above the applicable column. n = 3 replicate bottles in all cases.
We next examined bang-sensitivity in the progeny of these crosses (Fig. 6). Although the recovery times are not normally distributed, making standard statistical tests unreliable, it is clear from the cumulative plots for each sex that a rather similar pattern of dominance and parent-of-origin effects is seen as for developmental delay. In the hybrid crosses, the phenotype of tko25t progeny from mothers of the w1118 background but Oregon R fathers (green data plots) is very similar to that of tko25t flies of the pure w1118 background (red data plots). tko25t females with Oregon R mothers but w1118 fathers (blue data plots, Fig. 6a) are intermediate between the 2 pure lines (red vs black data plots), whereas tko25t males with Oregon R mothers but w1118 fathers (blue data plots, Fig. 6b) are almost identical with tko25t males of the pure OR background (blue vs black data plots, Fig. 6b). Thus, the data indicate an overall “dominance” of the milder w1118 cytoplasm or epigenome, as well as a negative influence on recovery from mechanical shock of the Oregon R X-chromosome.
Fig. 6.
Bang-sensitivity in tko25t strain-background crosses. Recovery times (plotted cumulatively, over time) at 25°C for a) female and b) male tko25t progeny from the indicated crosses between tko25t/FM7 females and tko25t/Y males of the indicated parental strain backgrounds (OR—Oregon R background, w—w1118 background). Control progeny from all crosses were not bang-sensitive. n = 60 (OR × OR), 117 (OR × w), 119 (w × OR), and 118 (w × w) females, and 84 (OR × OR), 74 (OR × w), 56 (w × OR), and 53 (w × w) males.
tko25t courtship analysis is compromised by background effects
We attempted to analyze a third phenotypic parameter of tko25t flies, namely defective male courtship (Toivonen et al. 2001). However, this did not prove meaningful for the following reasons. Immediately after the back-cross, males in the Oregon R background were extremely weak and unable to mate. Furthermore, previous investigators have found that mutation of the white locus itself impairs male courtship behavior, although flies do eventually mate in sufficient numbers to maintain white– lines. Working on the assumption that the w1118 mutation itself was the cause of the line's courtship defect, as previous authors have inferred (Xiao et al. 2017), we created a line of Oregon R flies back-crossed into the w1118 background, but retaining red eyes (this was also true of the tko25t back-cross into w1118, since tko and w map very close together on the X-chromosome). Red-eyed tko25t males in the w1118 background were courtship-impaired (Fig. 7) in a similar manner as inbred tko25t males studied previously (Toivonen et al. 2001), but red-eyed wild-type males in the w1118 background were also courtship-impaired (Fig. 7), rendering the main experiment meaningless. Although seemingly at odds with the previous findings on the male-courtship effects of white (Xiao et al. 2017), it should be noted that the males derived from the back-cross undertaken by the previous authors gave only 6 successful copulations out of 16 trials (Xiao et al. 2017). Furthermore, these authors showed that the absence of eye color per se was not the cause of the w1118 courtship defect (Xiao et al. 2017), and that the genotype of females in the assay also influenced the copulation success of w1118 males. In conclusion, although tko25t males in the Oregon R background were unable to mate, the less severe courtship defect in the w1118 background was impossible to quantify reliably. Note that, despite the male courtship defect of w1118 males, they are not bang-sensitive, as confirmed here (Fig. 2d).
Fig. 7.
Male courtship effects of tko25t and w1118. Percentage of successful copulation (mean ± SEM) of multiple samplings of 6 pairs of flies, at the indicated temperatures. In all assays, male and female flies were of the same genotype and background, and all had red eye color. n—number of batches analyzed for each genotype as indicated. Note that, to exclude the possibility of contamination, the tko locus from the red-eyed control flies back-crossed into the w1118 background was sequenced and confirmed to be tko+.
Concluding comments
After its initial isolation in 1972, the tko25t mutant has been maintained in various backgrounds in different laboratories for 50 years, and its exact history is practically impossible to reconstruct. During this time, as a result of inbreeding and inadvertent selection in many different laboratories, the phenotype has become milder (Fig. 2a). A restoration of the original semilethal phenotype was achieved by backcrossing into standard reference strain Oregon R. However, despite being maintained subsequently over a balancer chromosome with no deliberate selection, its phenotype has again become partially alleviated, during 2 years of standard laboratory maintenance. For any mutant strain with a nonlethal phenotype, especially one like tko25t, where reproductive competence and developmental timing are affected, we would recommend that the strain be periodically backcrossed into the original strain background and the phenotype rechecked, before the strain is used in any experimental context.
Given that the phenotype recorded after back-crossing into 2 commonly used and broadly wild-type nuclear backgrounds (apart from the w mutation in w1118) was so different (semilethal vs fully viable, 5- to 10-fold difference in median recovery times from mechanical shock, ∼30% increase in developmental delay, mild male courtship defect vs inability to mate), we would advise to conduct such a back-cross additionally into several other reference strains. Although partial phenotypic suppression of tko25t by functional absence (or heterozygosity) of white cannot be excluded, it seems more likely that other background alleles not knowingly under selection are responsible for the phenotypic differences.
In principle, the novel mutations or background heterogeneity revealed by our study could affect many diverse functions or pathways. The tko gene product is part of a complex structure, the mitoribosome, containing dozens of other polypeptides and RNA moieties. Furthermore, the mitoribosome directly influences the functional expression of the 13 mtDNA-encoded polypeptides and, indirectly, the dozens of nuclear-coded polypeptides with which they interact, both transiently and within stable complexes, to maintain essentially the biological processes of respiration and oxidative phosphorylation (OXPHOS). In turn, these fundamental metabolic functions affect other key processes, including ionic homeostasis, cell death regulation, steroid synthesis, and resistance to oxidative stress, so the instrumental alleles might govern any or many of these. Investigating all of them would be a major task, although some insight might be gained from analyzing the composition, relative abundance, and assembly status of the OXPHOS complexes using complexomics (Heide et al. 2012), and from respirometry (Gaviraghi et al. 2021), to try to pinpoint a specific biochemical target in the OXPHOS system. A more classical, genetic approach would be to conduct a mutational screen in the freshly rederived Oregon R background, to look for a suppressor.
This problem of cryptic mutations in inbred reference strains is a widespread phenomenon. The rapid generation time and use of different culture conditions in different laboratories, not to mention the possible human error in stock passaging, mean that different isolates of the same reference strain are unlikely to be or to remain genetically identical and thus may confer different traits upon a given mutation such as tko25t. The Drosophila melanogaster Genetic Reference Panel DmGRP (Mackay et al. 2012), a collection of outbred/inbred fly lines, is an extremely valuable tool in this regard, with a vast array of applications, both potential and realized (e.g. see Spierer et al. 2021; Havula et al. 2022). It could be more widely used by the community, to identify background polymorphisms that influence the phenotypic expression of mutations such as tko25t, especially given the increasing recognition of the importance and flexibility of Drosophila in modeling human diseases (Verheyen 2022). The same issue plagues mouse genetics, for example, where disease-modeling mutations can have no phenotype in one background, yet are lethal in another. The creation of a reference collection of outbred/inbred mouse strains (the CC resource, equivalent to DmGRP) to model complex traits in a genetically diverse population has yielded hundreds of such lines (Churchill et al. 2004; Abu Toamih Atamni et al. 2018).
Ideally, unexpected phenotypes should be verified by back-crossing to a different isolate of the same background strain. Given our previous observations of profound effects of different mtDNA backgrounds on tko25t (Chen et al. 2012; Salminen et al. 2019), we would recommend that attention should also be paid to mitochondrial genotype, in such back-crossing schemes, regardless of whether the expected phenotype is obviously “mitochondrial.” Note that the phenotypic differences observed in our back-crossed tko25t lines are not the result of genetic heterogeneity within the wild-type lines, even though this may be considerable, as inferred previously for Oregon R (Lints and Bourgois 1987). Back-crossed tko25t lines within each background had relatively uniform phenotypes.
Given the functional pleiotropy of the tko gene product and of the tko25t mutation, it is hardly surprising that the mutation imposes a selective pressure that can be compensated by diverse changes in genotype or gene expression. But in these respects, tko is far from unusual. Therefore, the possible influence of genetic background, of selection operating for a supposedly recessive mutation even in the heterozygous condition, and of parent-of-origin effects, should not be discounted for any mutation under study in Drosophila or in any model organism.
Supplementary Material
Acknowledgments
We thank Lotta Kulmala and Eveliina Teeri-Kahelin for their technical assistance. The work was conducted with practical support from the Biocenter Finland-funded Tampere Drosophila Facility.
Contributor Information
Howard T Jacobs, Faculty of Medicine and Health Technology, Tampere University, Tampere FI-33014, Finland; Department of Environment and Genetics, La Trobe University, Melbourne, Victoria 3086, Australia.
Tea Tuomela, Faculty of Medicine and Health Technology, Tampere University, Tampere FI-33014, Finland.
Päivi Lillsunde, Faculty of Medicine and Health Technology, Tampere University, Tampere FI-33014, Finland.
Data availability
Supplementary data files are deposited at Figshare.com as follows: Supplementary Fig. 1 (https://doi.org/10.6084/m9.figshare.22277254) and Supplementary Tables 1 and 2 (https://doi.org/10.6084/m9.figshare.22277287). All data in this paper are freely available on request. Source data files for each numerical figure are available as spreadsheet tables.
.
Funding
HTJ was supported in this work by grants from the Academy of Finland (283157, 307431, and 324730).
Literature cited
- Abu Toamih Atamni H, Nashef A, Iraqi FA. The collaborative cross mouse model for dissecting genetic susceptibility to infectious diseases. Mamm Genome. 2018;29(7-8):471–487. doi: 10.1007/s00335-018-9768-1 [DOI] [PubMed] [Google Scholar]
- Anaka M, Lynn A, McGinn P, Lloyd VK. Genomic imprinting in Drosophila has properties of both mammalian and insect imprinting. Dev Genes Evol. 2009;219(2):59–66. doi: 10.1007/s00427-008-0267-3 [DOI] [PubMed] [Google Scholar]
- Bahhir D, Yalgin C, Ots L, Järvinen S, George J, Naudí A, Krama T, Krams I, Tamm M, Andjelković Aet al. Manipulating mtDNA in vivo reprograms metabolism via novel response mechanisms. PLoS Genet. 2019;15(10):e1008410. doi: 10.1371/journal.pgen.1008410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, MT Oliveira, Sanz A, Kemppainen E, Fukuoh A, Schlicht B, LS Kaguni, HT Jacobs. A cytoplasmic suppressor of a nuclear mutation affecting mitochondrial functions in Drosophila. GENETICS. 2012;192(2):483–493. doi: 10.1534/genetics.112.143719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, DC Airey, Allayee H, JM Angel, AD Attie, Beatty J, WD Beavis, JK Belknap, Bennett B, Berrettini Wet al. The collaborative cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36(11):1133–1137. doi: 10.1038/ng1104-1133 [DOI] [PubMed] [Google Scholar]
- Coolon JD, Stevenson KR, McManus CJ, Graveley BR, Wittkopp PJ. Genomic imprinting absent in Drosophila melanogaster adult females. Cell Rep. 2012;2(1):69–75. doi: 10.1016/j.celrep.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ayala DJ, Sanz A, Vartiainen S, KK Kemppainen, Babusiak M, Mustalahti E, Costa R, Tuomela T, Zeviani M, Chung Jet al. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab. 2009;9(5):449–460. doi: 10.1016/j.cmet.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Gaskill MM, Gibson TJ, Larson ED, Harrison MM. GAF is essential for zygotic genome activation and chromatin accessibility in the early Drosophila embryo. Elife. 2021;10:e66668. doi: 10.7554/eLife.66668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviraghi A, Aveiro Y, Carvalho SS, Rosa RS, Oliveira MP, Oliveira MF. Mechanical permeabilization as a new method for assessment of mitochondrial function in insect tissues. Methods Mol Biol. 2021;2276:67–85. doi: 10.1007/978-1-0716-1266-8_5. [DOI] [PubMed] [Google Scholar]
- George J, Tuomela T, Kemppainen E, Nurminen A, Braun S, Yalgin C, Jacobs HT. Mitochondrial dysfunction generates a growth-restraining signal linked to pyruvate in Drosophila larvae. Fly (Austin). 2019;13(1-4):12–28. doi: 10.1080/19336934.2019.1662266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havula E, Ghazanfar S, Lamichane N, Francis D, Hasygar K, Liu Y, LA Alton, Johnstone J, EJ Needham, Pulpitel Tet al. Genetic variation of macronutrient tolerance in Drosophila melanogaster. Nat Commun. 2022;13(1):1637. doi: 10.1038/s41467-022-29183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T, Lewis R, Rubin GM. Transformation of white locus DNA in Drosophila: dosage compensation, zeste interaction and position effects. Cell. 1984;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x [DOI] [PubMed] [Google Scholar]
- Heide H, Bleier L, Steger M, Ackermann J, Dröse S, Schwamb B, Zörnig M, AS Reichert, Koch I, Wittig Iet al. Complexome profiling identifies TMEM126B as a component of the mitochondrial complex I assembly complex. Cell Metab. 2012;16(4):538–549. doi: 10.1016/j.cmet.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Hoch M, Jäckle H. Transcriptional regulation and spatial patterning in Drosophila. Curr Opin Genet Dev. 1993;3(4):566–573. doi: 10.1016/0959-437x(93)90092-4 [DOI] [PubMed] [Google Scholar]
- Jacobs HT, Fernández-Ayala DJ, Manjiry S, Kemppainen E, Toivonen JM, O'Dell KM. Mitochondrial disease in flies. Biochim Biophys Acta. 2004;1659(2-3):190–196. doi: 10.1016/j.bbabio.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Judd BH, Shen MW, Kaufman TC. The anatomy and function of a segment of the X chromosome of Drosophila melanogaster. Genetics. 1972;71(1):139–156. doi: 10.1093/genetics/71.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen E, George J, Garipler G, Tuomela T, Kiviranta E, Soga T, CD Dunn, HT Jacobs. Mitochondrial dysfunction plus high-sugar diet provokes a metabolic crisis that inhibits growth. PLoS One. 2016;11(1):e0145836. doi: 10.1371/journal.pone.0145836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen E, Fernández-Ayala DJM, Galbraith LCA, O′Dell KMC, Jacobs HT. Phenotypic suppression of the Drosophila mitochondrial disease-like mutant tko(25t) by duplication of the mutant gene in its natural chromosomal context. Mitochondrion. 2009;9(5):353–363. doi: 10.1016/j.mito.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Grell EH. Genetic variations of Drosophila melanogaster. Publs Carnegie Instn. 1968;627:469. [Google Scholar]
- Lints F, Bourgois M. Phenotypic and genotypic differentiation in cage populations of Drosophila melanogaster. I. Duration of development, thorax size and weight. Genet Sel Evol. (1983). 1987;19(2):155–170. doi: 10.1186/1297-9686-19-2-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd V. Parental imprinting in Drosophila. Genetica. 2000;109(1-2):35–44. doi: 10.1023/a:1026592318341 [DOI] [PubMed] [Google Scholar]
- Mackay TF, Richards S, EA Stone, Barbadilla A, JF Ayroles, Zhu D, Casillas S, Han Y, MM Magwire, JM Cridland, et al. The Drosophila melanogaster genetic reference panel. Nature. 2012;482(7384):173–178. doi: 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam JR. FM7: first multiple seven. Drosoph Inf Serv. 1968;43:64. [Google Scholar]
- Royden CS, Pirrotta V, Jan LY. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987;51(2):165–173. doi: 10.1016/0092-8674(87)90144-9 [DOI] [PubMed] [Google Scholar]
- Salminen TS, Cannino G, Oliveira MT, Lillsunde P, Jacobs HT, Kaguni LS. Lethal interaction of nuclear and mitochondrial genotypes in Drosophila melanogaster. G3 (Bethesda). 2019;9(7):2225–2234. doi: 10.1534/g3.119.400315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZH, O′Dell KM, Miller SC, An X, Jacobs HT. Metazoan nuclear genes for mitoribosomal protein S12. Gene. 1997;204(1-2):55–62. doi: 10.1016/s0378-1119(97)00521-0 [DOI] [PubMed] [Google Scholar]
- Soleimani S, Valizadeh Arshad Z, Moradi S, Ahmadi A, Davarpanah SJ, Azimzadeh Jamalkandi S. Small regulatory noncoding RNAs in Drosophila melanogaster: biogenesis and biological functions. Brief Funct Genomics. 2020;19(4):309–323. doi: 10.1093/bfgp/elaa005 [DOI] [PubMed] [Google Scholar]
- Spierer AN, Mossman JA, Smith SP, Crawford L, Ramachandran S, Rand DM. Natural variation in the regulation of neurodevelopmental genes modifies flight performance in Drosophila. PLoS Genet. 2021;17(3):e1008887. doi: 10.1371/journal.pgen.1008887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen JM, KM O'Dell, Petit N, SC Irvine, GK Knight, Lehtonen M, Longmuir M, Luoto K, Touraille S, Wang Z, et al. Technical knockout, a Drosophila model of mitochondrial deafness. Genetics. 2001;159(1):241–254. doi: 10.1093/genetics/159.1.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen EM. The power of Drosophila in modeling human disease mechanisms. Dis Model Mech. 2022;15(3):dmm049549. doi: 10.1242/dmm.049549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Qiu S, Robertson RM. The white gene controls copulation success in Drosophila melanogaster. Sci Rep. 2017;7(1):7712. doi: 10.1038/s41598-017-08155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Krauchunas AR, Huang S, Wolfner MF. Maternal proteins that are phosphoregulated upon egg activation include crucial factors for oogenesis, egg activation and embryogenesis in Drosophila melanogaster. G3 (Bethesda). 2018;8(9):3005–3018. doi: 10.1534/g3.118.200578 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary data files are deposited at Figshare.com as follows: Supplementary Fig. 1 (https://doi.org/10.6084/m9.figshare.22277254) and Supplementary Tables 1 and 2 (https://doi.org/10.6084/m9.figshare.22277287). All data in this paper are freely available on request. Source data files for each numerical figure are available as spreadsheet tables.
.