Abstract
Obesity is a common complex trait that elevates the risk for various diseases, including type 2 diabetes and cardiovascular disease. A combination of environmental and genetic factors influences the pathogenesis of obesity. Advances in genomic technologies have driven the identification of multiple genetic loci associated with this disease, ranging from studying severe onset cases to investigating common multifactorial polygenic forms. Additionally, findings from epigenetic analyses of modifications to the genome that do not involve changes to the underlying DNA sequence have emerged as key signatures in the development of obesity. Such modifications can mediate the effects of environmental factors, including diet and lifestyle, on gene expression and clinical presentation. This review outlines what is known about the genetic and epigenetic contributors to obesity susceptibility, along with the albeit limited therapeutic options currently available. Furthermore, we delineate the potential mechanisms of actions through which epigenetic changes can mediate environmental influences and the related opportunities they present for future interventions in the management of obesity.
Keywords: Genetics, Genomics, Obesity, Monogenic, Polygenic, GWAS, Epigenetics
Introduction
Obesity is a phenotype in which the percentage of body fat is increased to the point where health and well-being are impaired. The World Health Organization has declared obesity a “global epidemic” given its alarming prevalence both in developed and developing countries. In the US, the prevalence of obesity between 2017 and 2020 was 41.9% for adults over 20 years old, with 19.7% being approximately 14.7 million children and adolescents aged 2–19 years [1]. Obesity is a driver of a wide range of chronic cardio-metabolic diseases, including type 2 diabetes and cardiovascular disease, along with numerous non-metabolic co-morbidities such as several types of cancer [2]. The mechanical issues resulting from increased body weight can drive risk for osteoarthritis [3] and sleep apnea [4]. The recent COVID-19 pandemic revealed that individuals living with obesity were at increased risk of severe illness and hospitalization [5–7], highlighting its impact on communicable diseases, particularly viral infection [8]. Altogether, obesity represents a significant health burden on society, shortening life expectancy and reducing life quality.
While there is clear evidence that environmental factors contribute substantially to obesity risk, including a sedentary lifestyle, high-calorie/nutrient-poor food intake and reduced energy expenditure, it is also widely known that genetics contribute substantially to determining an individual’s response to an ‘obesogenic environment’ [9]. Early evidence from family [10–12], twin [13–15], and adoption [16] studies has estimated the heritability of obesity/BMI at 70–80%. It is now feasible to characterize underlying genetic mechanisms that influence variation in BMI.
The genetics community typically places obesity into two broad categories: monogenic and polygenic. The monogenic form is generally inherited through Mendelian inheritance; the related rare traits present as relatively severe and early age of onset, and caused by genomic deletions or deleterious variants in specific genes. On the other hand, the common polygenic form of obesity results from hundreds of independent variants across the genome, each conferring a small effect. Since the first 2007 report, genome wide association studies (GWAS) have revealed multiple new insights into obesity and BMI genetics. However, they have fallen short of defining the entire repertoire of genetic contributors to date; meta-analysis studies of multiple GWAS datasets have shown that the identified variants to date collectively only explain less than 6% of the observed variability in BMI [17, 18], indicating that much of the “missing heritability” [19] still needs to be found. Indeed, a recent GWAS of height that reached saturation for discovered loci revealed more than 12,000 signals [20]; as such, one would expect that many additional BMI loci remain to be uncovered. Even if one could account for the missing heritability, there is a substantial proportion of variability between individuals driven by gene-environment interactions contributing to the etiology of obesity. Non-genetic/behavioral factors, such as diet and exercise, can alter epigenetic signatures, and consequently influence gene expression. Clinical variables relevant to obesity strongly correlate with epigenetic changes in cell types, such as those from skeletal muscle, liver, and adipose [21–24]. Moreover, such epigenetic modifications can be reversed, making them amenable for perturbation via therapeutics. Indeed, a broad range of study designs, ranging from cell-based systems, rodent models to human systems, have revealed multiple factors that correlate with the etiology of human obesity. And with these expanding biometric indicators of obesity, the phenotyping and subtyping of obesity has become even more complex.
To achieve precision medicine for obesity treatment, it is crucial to identify risk profiles for individuals through assessing multiple contributing factors. This will not only help predict obesity risk and related diseases for a given individual, but will also aid in determining treatment response. This review summarizes the current understanding of genetic factors, gene-environment interactions and epigenetic alterations that lead to the derailed metabolism observed for obesity, which in turn can be harnessed to aid precision therapies for this disease in the future.
Anthropometric parameters of obesity
BMI – a classic with limitations
Body mass index (BMI) is commonly used to measure excess body weight and obesity. Adult BMI between 18.5 and 25 kg/m2 is considered average weight, 25–30 kg/m2 overweight, and over 30 kg/m2 is defined as obese. But despite its widespread use, BMI as a measure of adiposity has limitations. Most notably BMI does not differentiate fat-free and fat mass nor consider body fat distribution, which can lead to misleading interpretations about an individual’s health risks. Indeed, extensive research has shown that fat distribution has a greater correlation with certain health risks, including cardiovascular disease and cancer [25, 26].
Other measurements
To establish the presence of obesity and its relation to potential associated diseases, other indices – waist and neck circumference, waist-to-hip ratio (WHR) and waist-to-height ratio – have shown to independently serve as better indicators of central obesity, predictors of cardiometabolic disease [26, 27] and more accurately associated with overall mortality [28–30]. However, differences between individuals in the same apparent categories persist with respect to the percentage of fat and lean body mass observed across different ancestral groups [31]; specifically, differences in gynoid subcutaneous adipose tissue between age groups in females [32]. Stratifying anthropometric measurements by BMI, sex and ethnicity have improved health risk assessment accuracy [33, 34].
Measuring methods
Advances in technology have enabled assessment of an individual’s anthropometric classification based on body fat using more accurate measurement methods, including magnetic dual-energy X-ray absorptiometry (DXA), air-displacement plethysmography (BodPod), bioimpedance analysis (BIA), computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound (US). MRI, CT, DXA, and ultrasounds were previously used as reference standards in decade-apart meta-analysis studies, which consistently rendered low sensitivities and relatively high specificities for anthropometric measures. Despite the cost, invasiveness and sparse accessibility, these imaging techniques triumphed over traditional anthropometric measurements such as BMI, WC, and WHR in predicting obesity-related health risks.
Genetic determinants of obesity
Monogenic obesity is the consequence of a mutation in a given gene and can present as either syndromic or non-syndromic; indeed, this setting has blazed the trail with respect to the first obesity genes discovered. The most common form of obesity is the polygenic version, driven by hundreds to possibly thousands of independent single nucleotide polymorphisms (SNPs) distributed across the human genome and therefore has a complex mode of inheritance typical of common traits. The expression of mutations driving the pathogenesis of monogenic obesity can be partly impacted by polygenic obesity susceptibility in a given subject [35].
Syndromic and monogenic obesity
This rare form of obesity typically presents with various co-morbidities, such as cognitive delay [36]. Currently, of the almost eighty obesity syndromes that have been identified to date, only a minority have been either fully or partially defined, with the remainder just mapped to an approximate genomic location or not characterized at all [36]. The best-known syndromes include Prader-Willi syndrome (PWS) caused by an imprinting change on chromosome 15, the related Prader-Willi-like syndrome driven by deletion events on chromosome 16 impacting genes such as SIM1 (which encodes a crucial transcription factor for hypothalamus paraventricular and supraoptic nuclei development) [37], Fragile X syndrome, Bardet-Biedl syndrome (BBS, caused by multiple different genes), Albright’s hereditary osteodystrophy caused by mutations in GNAS, and Wilms-Tumor-Aniridia-Syndrome (WAGR) driven by deletion events on chromosome 11) [38]. Given how rare these presentations are, they remain challenging to be distinguished from conventional obesity [39].
Heterogeneity of clinical features
Twenty three obesity syndromes display wide phenotypic heterogeneity [36]. Studying such heterogeneity in syndromic obesity is challenging due to limited cases worldwide. Some contributing factors include genetic or allelic heterogeneity, the impact of the environment, including diet and medication, ancestral differences, gene-gene interactions and gene-environment interactions affecting epigenetic patterning.
Genetic heterogeneity includes structural variants like deletions, insertions, inversions and complex rearrangements. Bardet–Biedl syndrome (BBS) is one such example, with in excess of twenty genes implicated to date [40–42] but with the clinical presentation being relatively homogeneous. Likewise, Kallmann syndrome is due to mutations in PROK2, KAL1 and FGFR1, and similarly presents homogeneously. In contrast, Cornelia de Lange syndrome (CdLS) behaves quite the opposite; for instance, an NIPBL c.2827delA mosaic can present with either severe or milder forms [43]. The ciliopathy, Alström syndrome, is driven by a range of missense and frameshift causal mutations in ALMS1, differing almost at the individual level [44].
Differences in ethnicity can lead to variation in clinical presentation. Phenotypic differences for PWS have been reported in African American patients and can result in underdiagnosis in this population. Treatment can also modify clinical presentation of obesity syndromes; for instance, growth hormone treatment for PWAS can improve symptoms [45]. The role of epigenetics in human diseases, including obesity, is still being actively investigated, e.g., monozygotic twins have shown discordancy for the ROHHAD phenotype (‘Rapid Onset obesity with Hypothalamic dysfunction, Hypoventilation, and Autonomic Dysregulation’), with just one of the twins presenting with the syndrome [46].
Diagnostic challenges due to phenotypic similarities
The clinical presentation of identified obesity syndromes is frequently similar, making diagnosis/phenotyping challenging. For example, the majority of such syndromes present with mental retardation, while microcephaly and macrocephaly are also a common feature [36]. Both clinical differences and commonalities features lead to diagnostic challenges. An eight-year-old patient was first diagnosed with BBS but later correctly diagnosed with Alström syndrome at fourteen years old after updated clinical and genetic analysis. Indeed both these syndromes are ciliopathies, and have similar presentations including obesity and retinal degeneration, but their respective genetic etiologies are distinct [47].
Evolving clinical picture of syndromes
Studies of patients with specific obesity syndromes can help refine diagnosis and treatment options but are limited by small sample sizes and overlap of symptoms between syndromes. For instances, an investigation of seven Kabuki syndrome patients identified ocular anomalies from three cases as novel features for diagnosis and treatment options [48]. Macrosomia was suggested to be excluded from the MOMO syndrome (‘Macrocephaly, Obesity, Mental disability, Ocular abnormalities’) after two additional reported cases [49]. The endocrine manifestations of ROHHADNET syndrome were studied in six patients and varied hypothalamic-pituitary endocrine dysregulation was found, deeming it crucial to be considered during the diagnosis process for all obesity cases with early onset [50]. Such characterization, which does not just entail medical records, is expensive with respect expertise and time required.
Combining and separating syndromes
Advances in genetics have led to the reclassification of syndromes to aid improved understanding and diagnostic approaches. Prior to genetic testing, diagnoses were based principally on physical characteristics. For example, Carpenter, Goodman, and Summit syndromes were proposed to be combined into one due to their similarities in symptoms including obesity features; however, variation in these given symptoms have now be attributed to genetic differences [51]. Recently, genetic evidence has been used to subdivide WAGR syndrome into two separate disorders, WAGR and WAGRO (WARG with Obesity), with the latter characterized by obesity and molecular testing confirmation of BDNF deletions [52, 53].
Advances in genetic elucidation
Genetic elucidation of syndromes is critical for understanding the underlying molecular mechanisms and improving diagnosis, treatment, and care. Techniques such as whole-exome sequencing (WES), linkage mapping, candidate gene assessments and cytogenetics have been leveraged to reveal critical chromosomal regions and genes associated with syndromic obesity. For example, the multiple genes causal for BBS have been determined using various methods [40–42].
Complex patterns of genetic inheritance
With an expanding genetic picture of obesity syndrome drivers, complexities of inheritance are being observed. Kallmann syndrome can be caused by mutations in autosomal genes PROK2, KAL1 and FGFR1, or KAL1 on the X chromosome, each presenting with different heterozygous, homozygous and compound states [54]. Studies have also suggested that BBS may be a complex disorder caused by a combination of three mutant alleles [55, 56], though this is considered a rare phenomenon. Genetic factors that influence the manifestation of a syndrome include mosaicism [57], skewed X inactivation [58] and deletion/duplication of multiple adjacent genes [59].
The current classification of syndromes was developed principally based on cardinal features, which may need to be updated or already is. Leveraging genetic testing to define syndromic obesity should aid efficiency, enhance classification and improve the diagnosis process, management and treatment.
Monogenic (non-syndromic) obesity
Some causal genes for obesity exert substantial effects and are inherited in a Mendelian pattern, whose predominant trait is excess adiposity. Endocrine disorders and hyperphagia typically characterize them. Most genes and pathways causal for monogenic obesity were first discovered in transgenic mice presenting with spontaneous obesity and hyperphagia. ‘Reverse genetics’ could identify causal mutations in the ob (encoding leptin), and db (encoding the leptin receptor) genes [60, 61]. These discoveries in mice were quickly followed with multiple human genes encoding components of the leptin–melanocortin pathway, crucial for control of appetite. Figure 1 summarizes the known genes and factors involved in this key circuit.
Fig. 1.
Leptin-melanocortin pathway. Leptin is an anorexigenic hormone produced by white adipocytes, with its levels driven by the degree of fat mass present, and influences food consumption together with energy balance [112]. When its circulating levels become lower in the fasting state and rise when feeding takes place, leptin influences appetite via the hypothalamus [113, 114]. The arcuate nucleus is a component of the hypothalamus, where a key isoform of leptin receptor resides in two types of neurons, one expressing POMC and the other expressing agouti-related protein (AGRP) [115]. Leptin stimulates neurons expressing POMC, which is subsequently processed to various active melanocortin peptides [116]. The POMC-expressing neurons contact MC4R neurons in the paraventricular nucleus (PVN) where these melanocortin peptides influence a reduction in intake of food [115], whereas AGRP antagonizes MC4R to do the opposite [115, 117]; as such representing a finely tuned balance in the regulation of appetite
LEP
Congenital leptin deficiency is inherited recessively and was initially characterized in two Pakistani cousins presenting with obesity due to a frameshift mutation in LEP [62]. Since then, ten other mutations in LEP have been described [63–73]. Symptoms include rapid weight gain, severe early-onset obesity and intense hyperphagia [74]. Recombinant leptin can be used to improve adiposity and restore related functions [73, 75]. Myalept (metreleptin) is an FDA-approved therapeutic for treatment of congenital leptin deficiency [76].
LEPR
Subjects with leptin receptor (LEPR) mutations present with comparable symptoms to those with leptin deficiency, but lack the signature of serum hormone deficiency [77]. Advances in DNA sequencing have enabled detections of mutations in LEPR, which can affect 2–3% of a given population. Some patients also develop growth hormone and thyroid function deficiency; however, homozygous carriers of LEPR mutations do not respond to recombinant leptin.
POMC
Autosomal recessive inheritance of deficiency in POMC leads to a lack of ACTH, α-MSH and β-endorphins [78]. This can cause red hair and severe obesity via an α-MSH influence on both pigmentation and appetite. A rare deficiency of ACTH causes adrenal insufficiency. Early diagnosis combined with glucocorticoid replacement therapy is vital for efficient treatment. A few studies have found POMC mutations in individuals with obesity, but with no other symptoms [79, 80]. Setmelanotide, which activates the melanocortin-4 receptor, has the potential as a treatment for POMC deficiency [81].
MC4R
Mutations in MC4R, both autosomal dominant [82] and recessive, drive increased appetite and feeding behavior in children, along with additional co-morbidities principally related to growth [83, 84].
MC4R heterozygous mutations are the most frequent drivers of monogenic childhood obesity, being observed in as many as 5% of pediatric patients [83, 85, 86] and caused by an array of nonsynonymous variants across the gene [87–89]. Furthermore, the impact of such mutations can be influenced by polygenic risk scores for common obesity [35]. Researchers are currently exploring ways to perturb MC4R to improve satiety circuits, given that no such treatments are currently available [90–92].
ADCY3
A WES study on consanguineous families from Pakistan identified four children suffered from severe obesity with extremely rare homozygous ADCY3 mutations. The encoded cyclase catalyzes the synthesis of cyclic AMP from ATP. Such loss-of-function mutations are hypothesized to interfere with several anorexigenic signaling cascades [93]. The main clinical features are early onset hyperphagia and obesity.
SIM1
Loss-of-function mutations in the gene encoding the transcription factor ‘Single-minded homolog of drosophila’ (SIM1) lead to changes in feeding behavior and extreme obesity [37, 94]. Furthermore, a novel SIM1 variant, p.D134N, has been recently implicated in monogenic pediatric obesity [95].
NTRK2
Neurotrophins contribute to the development, maintenance and function of nerves in the peripheral and central nervous system. Studies on animals have shown that the tropomyosin receptor kinase B (TrkB, encoded by NTRK2), and its ligand BDNF, play a role in regulating food intake and body weight. A dominantly inherited mutation that results in loss of function of NTRK2 was reported in one subject with severe obesity but no other related symptoms [96].
BDNF
Brain-derived neurotrophic factor (BDNF) exerts its influence in the hypothalamus. It plays a key role in controlling feeding behavior and energy balance, partly due to its influence on leptin signaling [97]. Deletions in BDNF as part of the WAGRO syndrome have been linked to early-onset obesity [53]. Furthermore, multiple missense mutations within BDNF drive the pathogenesis of severe obesity [98–101].
SH2B1
‘Src homology 2 B adapter protein’ (SH2B1) helps regulate sensitivity to leptin [102]. Autosomal dominantly inherited SH2B1 mutations are known to lead to severe childhood obesity [103], along with features of developmental delay. It has been shown that the effects of each mutation can vary [104].
Other genes
Kinase suppressor of Ras 2 (KSR2) mutations can cause hyperphagia, low heart rate, and insulin resistance, with metformin being used as treatment [105]. Mutations in the genes PCSK1 encoding proprotein convertase-1/3 (PC1/3) result in a range of diabetes-related traits and extreme childhood obesity [106]. The gene products represent attractive therapeutic targets, but no treatments have been developed to date. A homozygous frameshift mutation in TUB was found in a subject with obesity and vision disorders [107]. A truncating mutation in the carboxypeptidase (CPE) gene was found in one subject with severe obesity [108]. A truncating mutation in the retinoic acid induced 1 (RAI1) gene was linked to hypoventilation, developmental disability and severe obesity [109]. Melanocortin receptor accessory protein 2 (MRAP2) variants have been reported to increase obesity risk [110]. And PHIP mutations correlate with developmental delay, intellectual disability and being overweight. The mechanism by which these genes contribute to obesity is principally through repression of POMC expression or interference with leptin-melanocortin signaling [111].
Whole exome sequencing and the future
Newer comprehensive sequencing methods can aid new genetic insights into obesity, and new discoveries are happening nearly every day. For instance, twenty-two GNAS mutations (encoding the Gαs protein, and involved in signaling through G protein-coupled receptors) have been found with WES, resulting in children with severe obesity, reduced growth and developmental delay [118].
And like syndromic, non-syndromic monogenic obesity is approaching the era where diseases are better classified by genetic profiles rather than the underlying cardinal symptoms.
Polygenic obesity
Most individuals with obesity develop the common/multifactorial form, caused by a combination of multiple genetic variations (polygenic), each with modest effects. The discovery of genes contributing to this type of obesity has been a slow process, starting with candidate gene studies in the 1990s, then family-based linkage studies leading up to genome-wide association studies (GWAS).
GWAS
The discovery of genes contributing to common diseases accelerated with the advent of GWAS. The first GWAS for BMI was published in 2007, where it reported the FTO locus as being strongest association signal [119]. To date, GWAS has identified more than a thousand loci associated with BMI/obesity and its related comorbidities. However, despite the identification of a myriad of loci, in combination, these signals only explain approximately 5% of the variance in BMI [18]. Given the limitation of BMI as a proxy for overall adiposity, as discussed above, GWAS has also been performed on more specifically-defined obesity phenotypes, including WHR [120], body fat percentage [121, 122] and circulating leptin and leptin receptor levels [123, 124]. These more specific studies are often smaller in size, and therefore lacking in relative statistical power, but reveal more direct biological relevance underpinning obesity.
GWAS and fat distribution
Genetic components characterize 30–55% of fat distribution. Three major studies have shown that the WHR has a significant genetic component [120, 125, 126]. With their increasing sample sizes, a growing number of significant loci have been reported, with a subset exhibiting sex dimorphism. Furthermore, it has been reported recently that the genetics of sexually dimorphism influence human adipose distribution, where genetic-mediated process were found to underpin adipose distribution specifically in females leading to metabolic dysfunction in women [127].
A study of subjects from five different ancestries found protein-coding variants influencing variations in fat distribution. Fifty-six significant coding variants were identified, with forty-three being common, and twenty-five also associated with BMI. The remaining thirty-one influenced adipose tissue topography. Nineteen had sex-specific effects, where sixteen were more strongly associated with WHR in women [128]. Unlike BMI loci, there was no evidence that genetic variants near genes related to central nervous system regulation had any impact on the distribution of fat [129].
Associations with abdominal visceral adipose tissue (VAT) and WC were found near THNSL2 only in women [130], near BBS9 and CYCSP30 [131]. A more recent study found the UBE2E2 locus associated with the VAT:subcutaneous fat ratio [132], with loss of function mutations in a mouse model impacting differentiation of adipocytes.
The genetics of ectopic fat deposition has also been investigated. A previous study found moderate genetic correlations among six ectopic fat depots, where ENSA, TRIB2, and EBF1 were associated with heart deposition specifically, suggesting common and depot-specific genetic determinants [132]. Another study found several genetic variants associated with liver fat levels, including at UGT1A, SOCS2, RAMP3, PNPLA3 and SUGP1 [133].
Childhood obesity loci
GWAS has been conducted to study the genetics of obesity by integrating demographic factors such as sex and age. The Early Growth Genetics (EGG) consortium and other investigative teams have studied birth weight, pediatric BMI and childhood obesity. Most of the identified loci are also associated with obesity and/or BMI in adults, including at the FTO locus across multiple ancestries, highlighting that the genetics of obesity is relatively consistent over the lifecycle. However, a distinct genetic signature for peak BMI during infancy has also been reported [134].
Sex as confounding factor
A number of loci have been reported for waist-related traits, such as waist-to-hip ratio, that are stronger or exclusive to women [120, 135, 136]. No interaction was found between sex and BMI loci in those studies. By contrast, another study in subjects of Asian ancestry found that four BMI loci were more strongly associated in males [137]. A targeted analysis of BMI, WC, and WHR variants in Chinese subjects found that specific loci like MC4R and LYPLAL1 were associated with female visceral fat area, and ALDH2 in males [138]. Several BMI and waist-hip ratio loci revealed sexual dimorphism in subjects of African ancestry [139]. As such, obesity genetic associations can be impacted by sex in an ethnicity/population-dependent manner.
Ethnicity as cofounding factor
Most GWAS for obesity have been conducted in populations of European ancestry. However, additional loci have been uncovered in other ethnicities [139–142], albeit with much smaller sample sizes. These loci often demonstrate good transferability across other ancestries, but the allele frequencies and effect sizes are often substantially different. Genetic correlation assessments across populations point to yet-to-be-discovered loci for BMI in specific ancestral groups. To address this point, increasing statistical power by including additional subjects in GWAS of specific ancestries have been conducted, along with searching for specific high-impact variants conferring effects specifically in population isolates. Examples include CREBRF discovered in Samoans and ADCY3 first detected in Greenlanders [143–145].
Low frequency and rare variants
Microarray arrays leveraged for GWAS initially provided strong coverage for common variation (MAF > 5%), but as the technology has developed and informed by more fully sequenced human genomes, detection of lower frequency variation (MAF = 1–5%) and even rare variants (MAF < 1%) has become increasingly feasible; indeed, such variants are more likely to reside in coding and regulatory elements and therefore plausibly pathogenic. Furthermore, such genotyping data can be leveraged to detect copy number variants (CNVs) contributing to obesity risk, with a number reported to date. Rare (MAF < 1%) and low frequency (MAF = 1–5%) variants generally impact coding and regulatory elements more frequently [146]. An effort conducted in approximately 700,000 subjects to uncover rare variants contributing to variation in BMI, revealed coding mutations across 13 genes [147]. Despite these successes, the haul of variants at this scale of sample size is considered modest, with more expected to be found as collections increase in number even further.
Target genes and functional annotation
Although GWAS has been very successful in detecting common variants associated with complex traits, understanding the underlying mechanisms of action has remained elusive. GWAS makes no inference of either the causal variant(s) or the corresponding effector gene(s) at a given locus. These variants typically reside in non-coding genomic regions, which are important for gene regulation, and these regulatory elements in turn can provide clues about potential mechanisms via pathway-based analyses. Besides the hypothalamus and pituitary gland being key players within the brain in appetite regulation, the limbic system, hippocampus and substantia nigra likely play a role in the genetic etiology of obesity [17, 140, 148, 149].
Only a relative handful of GWAS-implicated obesity loci have been functionally followed up to date. Figure 2 outlines four different mechanistic examples of how loci can contribute to obesity etiology.
Fig. 2.
Four functional follow-up strategies of different GWAS-implicated obesity loci. (A). A specific variant embedded within the FTO gene is located in an ARID5B regulatory element, which in turn impacts the expression of the neighboring genes, IRX3 and IRX5, which play a role in adipose biology [150]. (B). One of the strongest obesity loci coincides with the TMEM18 gene [151, 152], which encodes a poorly characterized transmembrane protein. Work with a Drosophila melanogaster knock-out model implicates TMEM18 in influencing lipid and carbohydrate levels via disruption of insulin and glucagon signaling [153], while knock-out in a mouse model leads to increased body weight due to elevated food intake, with over-expression of the gene showing an opposite effect [154]. (C). CADM1 and CADM2 encode cell-adhesion proteins in the brain. The associated variants influence the expression of the respective genes in the hypothalamus, leading to increased body weight, insulin sensitivity and energy expenditure. Loss of function of these genes promotes weight loss. Keto diet (“green” food cube) showed lower expression of these genes and promoted weight loss [155, 156]. (D). Deletion variants located just upstream of NEGR1 impacts a binding site for the strong transcriptional repressor NKX6.1. When NKX6.1 binding is lost, increased NEGR1 expression is observed. NEGR1 is expressed in the brain. Studies in mice have found that NEGR1 deficiency lowers body weight via a reduction in lean mass [157], although other studies have found opposite results [158, 159]. High-fat diet seemed to accelerate weight loss.
As such, a full functional appraisal of such GWAS loci can reveal novel understanding of the genetic etiology of obesity; however, the vast majority of BMI/obesity loci remain to be fully characterized with respect to precise mechanism of action. Determining the causal variant(s) at each of these loci, and then connecting them to the causal effector gene(s) remains challenging. Various methods have been used to achieve this, including SNP enrichment analysis, molecular trait profiling, colocalization analysis, transcriptome-wide association studies (TWAS), regulome-wide association studies (RWAS), integrating polygenic risk scores with functional annotations, and the use of different types of quantitative traits (eQTLs, sQTLs, 3′aQTLs) and tissue types. More recently, machine learning and AI networks have also been used to predict effector genes. These findings then require further functional validation, such as with CRISPR-based approaches. Furthermore, using gene ontology to analyze gene overlap across co-morbidities for obesity, including diabetes and hypertension, new insights into key pathways can be revealed [160].
Emerging high-throughput methods to aid mapping of regulatory elements, multi-omics databases and advanced computational techniques are expected to accelerate the process of understanding the biology behind GWAS loci.
The genetic architecture of monogenic versus polygenic obesity
Recent GWAS have identified loci harboring genes that were initially uncovered in studies of extreme and early-onset obesity, including MC4R [161, 162] and POMC [163]. Many of these gene products operate in the BDNF–TrkB and leptin–melanocortin signaling pathways. Severe obesity can present when these pathways are disrupted, while common susceptibility variants colocalized nearby the same genes can influence an individual’s BMI. While many genes were initially identified in studies of extreme obesity, some, like ADCY3, were first found in studies of common obesity [147], and later linked to extreme obesity [143]. Given larger GWAS efforts for BMI and obesity are expected in the future, one would expect this list of converging genes to continue to grow.
Gene-environment interaction: emerging role of epigenetics
Studies suggest that people with genetic susceptibility to obesity are also more susceptible to adverse environments. This is known as gene-by-environment interaction, transmitted via epigenetic processes. The agouti mouse model demonstrates the influence of epigenetics on obesity through an Avy mutation. The mutation leads to the disruption of MC4R and triggers obesity, but can be reversed by feeding the mice with food rich in methyl donors. This establishes a link between obesity and epigenetic alterations driven by environmental factors [164]. DNA methylation, histone modifications and non-coding RNAs represent the most frequent and well-studied epigenetic changes.
Epigenetic and obesity
DNA methylation
DNA methylation is an epigenetic feature that contributes to obesity pathogenesis. This process involves adding a methyl group to a cytosine residue in DNA, specifically at CpG sites. This chemical modification can prevent transcription factors interacting with DNA, thereby interfering with gene transcription.
Studies have found mixed results for global DNA hypomethylation in obesity, but more consistent findings for specific candidate genes methylation. For example, a negative association has been found between body weight in adults with obesity [165] and BMI in subjects with obesity and methylation at the LEP promoter and adiponectin gene [166–168]. Positive association was found for methylation status of members of the insulin signaling pathway, including INS, IRS1 and PIK3R1 [23, 169, 170], with obesity and metabolic disease. Increased POMC and lower NPY methylation has been reported for subjects presenting with resistance to weight loss [171]. TNF, IL6 and TFAM exhibit changes in DNA methylation in subjects presenting with obesity.
Collectively, DNA methylation differences are observed in obesity and may offer new avenues for diagnostic approaches and therapeutic interventions. Imbalances in the activity of enzymes responsible for methylation and demethylation, such as DNA Methyltransferases (DNMTs) and Tet methylcytosine dioxygenases (TETs) may cause these changes. However, more studies are required to fully understand the mechanisms behind such changes.
Histone modifications
Histones help to compact DNA into chromatin. Histone alterations by mechanisms such as methylation and acetylation can impact how compact DNA becomes and subsequently influence gene expression. Enzymes that modify histones include histone methyltransferases, histone demethylases, histone deacetylases (HDACs) and histone acetyltransferases. Changes in the levels of these enzymes have been linked to obesity, and specific enzymes, such as HDACs and Jhdm2a, have been shown to accelerate the progression of obesity in clinical studies [172–174].
Histone modifications have been shown to regulate gene expression related to adipogenesis, including C/EBPB, C/EBPA, Pref-1, aP2 and PPARG, appetite control, including NPY and POMC [175]. Changes in histone acetylation caused by high-fat diets have been linked to obesity.
Non-coding RNAs
Non-coding RNAs do not encode proteins but can have a crucial impact on gene expression. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) have both been implicated in the pathogenesis of obesity.
Several miRNAs involved in adipogenesis have been shown to be expressed both in subjects and mice with obesity that were placed on a high fat diet. Additionally, specific miRNAs have been reported to be expressed at higher levels in visceral fat tissue derived from subjects with obesity [176]. miR21 and miR221 have been reported to have higher expression in the white adipose of subjects with obesity, while upregulation of miR221 has been observed in diet-induced obese mice. Silencing these miRNAs can lead to reductions in adipogenesis, triglyceride accumulation and alterations to BMI. Multiple miRNAs have now been uncovered that show differences in expression in subjects with obesity, including those operating within adipogenesis, insulin signaling and hypoxia [177].
Several lncRNAs, including GYG2P1, lncRNAp21015 and lncRNA-p5549, have reduced expression levels in obesity [178, 179]. RP11-20G13.3, lnc-dPrm16 and MIST are among the lncRNAs that impact metrics of adipogenesis [180, 181]. As such there is increasing evidence that lncRNAs place a specific role in conferring obesity risk [182].
Environment and Lifestyle impact epigenetics
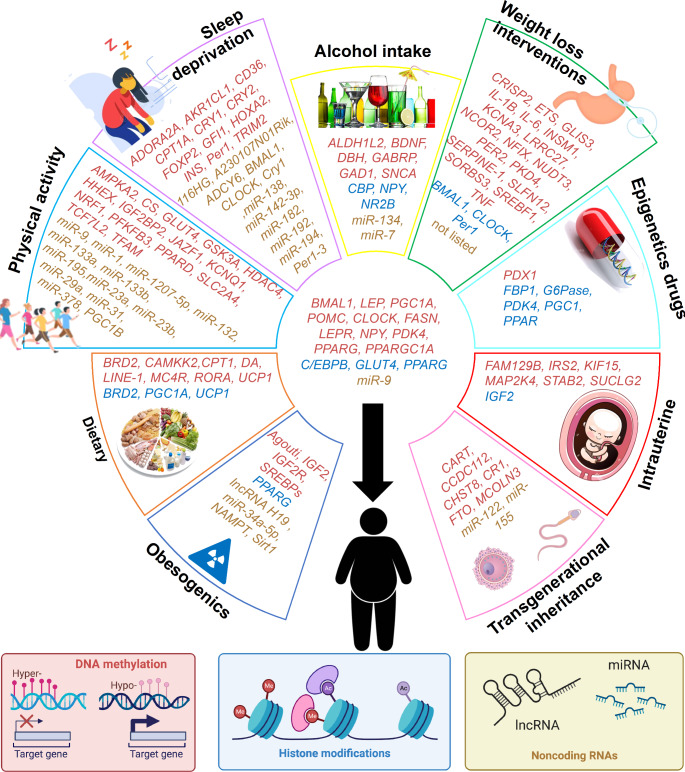
Epigenetic processes are nimble in responding to lifestyle and the environment, enabling a subject to respond to external factors and to then return to the original state once that factor is no longer present. This includes endocrine disrupting chemicals exposures, dietary including high-fat, high/low-carb, sugar/oil-rich and micronutrients, physical activity including short/long-term training, sleep disturbance and deprivation, alcohol intake, weight loss interventions and the use of epigenetic drugs [183]. Figure 3 summarizes the identified genes affected by different environmental factors.
Fig. 3.
Genes affected by environmental factors through epigenetic mechanisms. Genes are color-coded by mechanism. The genes within each section are specific for each factor, and genes placed in the middle space are common between at least two factors
The following section will look at two environmental exposures that take place before an individual is born.
Inheritance of epigenetic susceptibility to obesity
A birth cohort study in the Netherlands reported a relationship between parental nutrition prior to conception and inherited epigenetic patterns [184]. This can be potentially exacerbated by assortative mating, which can increase predisposition to obesity in offspring [185]. Recent studies have shown that obesity can influence modifications of DNA, histones and ncRNAs in both sperm and the oocyte [186]. The negative effect of obesity on oocytes has been studied mainly in model organisms for obesity, where alterations in histone modifications and DNA methylation in this setting are observed [186–188].
Research is growing on how epigenetics can be passed down via the sperm of men and affect the next generation. Studies have shown high-fat, low-protein diets and bariatric surgery can cause changes in the expression of certain types of RNA and DNA methylation patterns in the sperm, leading to insulin resistance and weight changes in the offspring [189, 190].
Epigenetic changes during pregnancy
The placenta regulates nutrients, oxygen and hormonal supply between the mother and fetus. Any abnormalities in this process can lead to issues with fetal growth, causing hepato-metabolic and cardiovascular diseases [191, 192].
Increased LEPR methylation is linked to poor maternal weight gain during pregnancy, affecting newborns’ protein expression in umbilical veins [193]. Humans and animal studies have both found that diets high in fat and low in protein during pregnancy lead to alterations in epigenetics across specific genes and leads to obesity and related co-morbidities in the offspring, lasting into adulthood. Conversely a healthy diet has been shown to have beneficial epigenetic impact [194].
Inadequate diets can cause epigenetic changes in certain genes, including PPARG, IGF2, GLUT4, and C/EBPB, resulting in eating disorders and the presentation of obesity that persists into adulthood [195, 196].
From genetics to the clinic
Advances in genetic technology can lead to better identification of disease genes for forms of obesity that are not yet fully understood. Furthermore, knowing a patient’s genotype could allow for precise diagnosis of obesity type, and inform subsequent precision medicine approaches.
The social burden of obesity is significant, with increased healthcare costs, decreased productivity, and decreased quality of life. This challenge is gradually alleviated by the decreasing cost of advanced genomic technologies, making it increasingly feasible for precision medicine to improve health outcomes. By understanding a patient’s genotype, it is possible to make a more accurate diagnosis of obesity, which in turn leads to more tailored treatment and prevention methods. Additionally, early detection of a subject’s genetic predisposition to obesity has implications for weight control in later life.
Leveraging recombinant human leptin as a treatment serves as a key example of a genetically-informed therapeutic strategy, as described above, which showed responses in some of the cases depending on their genetic makeup. The second treatment for obesity based on patient genotype is setmelanotide, a drug that selectively activates MC4R and has been FDA approved to specifically treat monogenic obesity caused by mutations in LEPR, PCSK1 and POMC [197]. These two treatments demonstrate how understanding the genetic causes of obesity can inform the development of targeted therapies that address specific deficiencies in monogenic forms. However, challenges remain to fully transition to a precision medicine approach for monogenic obesity, such as implementing widespread genetic testing.
Epigenetics is also a rapidly growing field of research, and progress is being made in identifying such biomarkers for obesity. To elucidate how epigenetics impact obesity risk, further research is required to explore the impact of factors such as hypoxia, inflammation, oxidative stress and hormonal imbalances on epigenetics. Additionally, large scale prospective efforts are required to determine the relationship between changes in environmental factors such as diet, physical activity, sleep and alcohol consumption with epigenetic changes. The modifiable nature of epigenetics makes it a promising avenue for obesity prevention and treatment.
Insights from genetic and epigenetic discovery efforts represent exciting advances toward precision medicine, and which should directly affect health outcomes in the decades to follow.
Acknowledgements and funding
The authors are supported by NIH grants R01 HD056465 and UM1 DK126194. SFAG is supported by the Daniel B. Burke Endowed Chair for Diabetes Research.
Author contributions
Drs. Trang and Grant jointly wrote and reviewed the manuscript together.
Declarations
Financial or non-financial interests
The authors declare no competing interests that are relevant to the content of this review article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Khanh Trang, Email: trangk@chop.edu.
Struan F.A. Grant, Email: grants@chop.edu
References
- 1.National Health and Nutrition Examination. 2021. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. [DOI]
- 2.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and Cancer — viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15(7):578–86. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jehan S, Zizi F, Pandi-Perumal SR et al. Obstructive Sleep Apnea and Obesity: Implications for Public Health.Sleep Med Disord, 1(4) (2017). [PMC free article] [PubMed]
- 5.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–70. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X, Gang X, He G, et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front Endocrinol. 2020;11:595109. doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya I, Ghayor C, Pérez Dominguez A, Weber FE. From Influenza Virus to Novel Corona Virus (SARS-CoV-2)-The contribution of obesity. Front Endocrinol (Lausanne) 2020;11:556962. doi: 10.3389/fendo.2020.556962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23(2):120–33. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Pérusse L, Rao DC, Bouchard C. Familial risk of overweight and obesity in the Canadian Population using the WHO/NIH Criteria. Obes Res. 2000;8(2):194–7. doi: 10.1038/oby.2000.21. [DOI] [PubMed] [Google Scholar]
- 11.Pietiläinen KH, Kaprio J, Rissanen A, et al. Distribution and heritability of BMI in finnish adolescents aged 16 y and 17 y: a study of 4884 twins and 2509 singletons. Int J Obes. 1999;23(2):107–15. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- 12.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes. 1996;20(6):501–6. [PubMed] [Google Scholar]
- 13.Feinleib M, Garrison RJ, Fabsitz R, et al. The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results. Am J Epidemiol. 1977;106(4):284–295. doi: 10.1093/oxfordjournals.aje.a112464. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256(1):51–4. doi: 10.1001/jama.1986.03380010055024. [DOI] [PubMed] [Google Scholar]
- 15.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The Body-Mass Index of Twins who have been reared apart. N Engl J Med. 1990;322(21):1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 16.Stunkard AJ, Sørensen TIA, Hanis C, et al. An adoption study of human obesity. N Engl J Med. 1986;314(4):193–8. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- 17.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yengo L, Vedantam S, Marouli E, et al. A saturated map of common genetic variants associated with human height. Nature. 2022;610(7933):704–12. doi: 10.1038/s41586-022-05275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barres R, Kirchner H, Rasmussen M, et al. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013;3(4):1020–7. doi: 10.1016/j.celrep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Keller M, Hopp L, Liu X, et al. Genome-wide DNA promoter methylation and transcriptome analysis in human adipose tissue unravels novel candidate genes for obesity. Mol Metabolism. 2017;6(1):86–100. doi: 10.1016/j.molmet.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson E, Jansson PA, Perfilyev A, et al. Altered DNA methylation and Differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes. 2014;63(9):2962–76. doi: 10.2337/db13-1459. [DOI] [PubMed] [Google Scholar]
- 24.Wahl S, Drong A, Lehne B, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541(7635):81–6. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord. 2004;28(3):402–9. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. The Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Jy, Ge H, Zhu Mf, et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc Diabetol. 2013;12(1):76–76. doi: 10.1186/1475-2840-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashwell M, Gunn P, Gibson S. Waist-to‐height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta‐analysis. Obes Rev. 2012;13(3):275–86. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 29.Carmienke S, Freitag M, Pischon T, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–85. doi: 10.1038/ejcn.2013.61. [DOI] [PubMed] [Google Scholar]
- 30.Correa MM, Thumé E, De Oliveira ERA, Tomasi E. Performance of the waist-to-height ratio in identifying obesity and predicting non-communicable diseases in the elderly population: a systematic literature review. Arch Gerontol Geriatr. 2016;65:174–82. doi: 10.1016/j.archger.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 32.Ponti F, Santoro A, Mercatelli D, et al. Aging and Imaging Assessment of body composition: from Fat to facts. Front Endocrinol (Lausanne) 2019;10:861. doi: 10.3389/fendo.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staiano AE, Bouchard C, Katzmarzyk PT. BMI-specific waist circumference thresholds to discriminate elevated cardiometabolic risk in White and African American adults. Obes Facts. 2013;6(4):317–24. doi: 10.1159/000354712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyle UG, Schutz Y, Dupertuis YM, Pichard C. Body composition interpretation: contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19(7):597–604. doi: 10.1016/S0899-9007(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 35.Chami N, Preuss M, Walker RW, Moscati A, Loos RJF. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 2020;17(7):e1003196. doi: 10.1371/journal.pmed.1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017;18(6):603–634. doi: 10.1111/obr.12531. [DOI] [PubMed] [Google Scholar]
- 37.Bonnefond A, Raimondo A, Stutzmann F, et al. Loss-of-function mutations in SIM1 contribute to obesity and prader-willi–like features. J Clin Investig. 2013;123(7):3037–41. doi: 10.1172/JCI68035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clinical science (London, England: 1979), 130(12), 943–986 (2016). [DOI] [PubMed]
- 39.Blackett PR, Li S, Mulvihill JJ. Ring chromosome 4 in a patient with early onset type 2 diabetes, deafness, and developmental delay. Am J Med Genet: A. 2005;137(2):213–6. doi: 10.1002/ajmg.a.20386. [DOI] [PubMed] [Google Scholar]
- 40.Héon E, Kim G, Qin S, et al. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21) Hum Mol Genet. 2016;25(11):2283–94. doi: 10.1093/hmg/ddw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Bardet–Biedl syndrome: is it only cilia dysfunction? FEBS Lett. 2015;589(22):3479–91. doi: 10.1016/j.febslet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer E, Stoetzel C, Scheidecker S, et al. Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet-Biedl syndrome. J Hum Genet. 2016;61(5):447–450. doi: 10.1038/jhg.2015.162. [DOI] [PubMed] [Google Scholar]
- 43.Castronovo P, Delahaye-Duriez A, Gervasini C, et al. Somatic mosaicism in Cornelia de Lange syndrome: a further contributor to the wide clinical expressivity? Clin Genet. 2010;78(6):560–4. doi: 10.1111/j.1399-0004.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 44.Marshall JD, Muller J, Collin GB, et al. Alström Syndrome: Mutation Spectrum of ALMS1. Hum Mutat. 2015;36(7):660–8. doi: 10.1002/humu.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitman BY, Myers SE, Carrel AL, Allen DB. The behavioral impact of growth hormone treatment for children and adolescents with Prader-Willi Syndrome: a 2-Year, controlled study. Pediatrics. 2002;109(2):E35–e35. doi: 10.1542/peds.109.2.e35. [DOI] [PubMed] [Google Scholar]
- 46.Patwari PP, Rand CM, Berry-Kravis E, Ize-Ludlow D, Weese-Mayer DE. Monozygotic twins discordant for ROHHAD phenotype. Pediatrics. 2011;128(3):e711–715. doi: 10.1542/peds.2011-0155. [DOI] [PubMed] [Google Scholar]
- 47.Milani D, Cerutti M, Pezzani L, Maffei P, Milan G, Esposito S. Syndromic obesity: clinical implications of a correct diagnosis. Ital J Pediatr. 2014;40(1):33–3. doi: 10.1186/1824-7288-40-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner CLS, Lachlan K, Amerasinghe N, et al. Kabuki syndrome: new ocular findings but no evidence of 8p22-p23.1 duplications in a clinically defined cohort. Eur J Hum genetics: EJHG. 2005;13(6):716–20. doi: 10.1038/sj.ejhg.5201377. [DOI] [PubMed] [Google Scholar]
- 49.Di Donato N, Riess A, Hackmann K, et al. Macrocephaly, obesity, mental (intellectual) disability, and ocular abnormalities: alternative definition and further delineation of MOMO syndrome. Am J Med Genet: A. 2012;158(11):2857–62. doi: 10.1002/ajmg.a.35481. [DOI] [PubMed] [Google Scholar]
- 50.Bougnères P, Pantalone L, Linglart A, Rothenbuhler A, Le Stunff C. Endocrine manifestations of the Rapid-Onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neural tumor syndrome in Childhood. J Clin Endocrinol Metab. 2008;93(10):3971–80. doi: 10.1210/jc.2008-0238. [DOI] [PubMed] [Google Scholar]
- 51.Cohen DM, Green JG, Miller J, Gorlin RJ, Reed JA. Acrocephalopolysyndactyly type II–Carpenter syndrome: clinical spectrum and an attempt at unification with Goodman and Summit syndromes. Am J Med Genet. 1987;28(2):311–324. doi: 10.1002/ajmg.1320280208. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-López R, Pérez JMC, Balsera AM, et al. The modifier effect of the BDNF gene in the phenotype of the WAGRO syndrome. Gene. 2012;516(2):285–90. doi: 10.1016/j.gene.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 53.Han JC, Liu Q-R, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–27. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dodé C, Teixeira LA, Levilliers J, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2(10):1648–52. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katsanis N, Ansley SJ, Badano JL, et al. Triallelic inheritance in Bardet-Biedl Syndrome, a mendelian recessive disorder. Sci (New York N Y) 2001;293(5538):2256–9. doi: 10.1126/science.1063525. [DOI] [PubMed] [Google Scholar]
- 56.Katsanis N, Eichers ER, Ansley SJ, et al. BBS4 is a minor contributor to Bardet-Biedl Syndrome and May also participate in triallelic inheritance. Am J Hum Genet. 2002;71(1):22–9. doi: 10.1086/341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rio M, Royer G, Gobin S, et al. Monozygotic twins discordant for submicroscopic chromosomal anomalies in 2p25.3 region detected by array CGH. Clin Genet. 2012;84(1):31–6. doi: 10.1111/cge.12036. [DOI] [PubMed] [Google Scholar]
- 58.Matsuo K, Murano I, Kajii T. Borjeson-Forssman-Lehmann syndrome in a girl. Jinrui idengaku zasshi The Japanese journal of human genetics. 1984;29(2):121–6. doi: 10.1007/BF01873532. [DOI] [PubMed] [Google Scholar]
- 59.Ballabio A. Contiguous deletion syndromes. Curr Opin Genet Dev. 1991;1(1):25–9. doi: 10.1016/0959-437X(91)80036-L. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–5. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 62.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 63.Ozata M, Ozdemir IC, Licinio J. Human Leptin Deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and Immune System Dysfunction Indicate New targets for Leptin Action, Greater Central than Peripheral Resistance to the Effects of Leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 64.Rau H, Reaves BJ, O’Rahilly S, Whitehead JP. Truncated human leptin (delta133) associated with extreme obesity undergoes proteasomal degradation after defective intracellular transport. Endocrinology. 1999;140(4):1718–23. doi: 10.1210/endo.140.4.6670. [DOI] [PubMed] [Google Scholar]
- 65.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 66.Gibson WT, Pissios P, Trombly DJ, et al. Melanin-concentrating hormone receptor mutations and human obesity: functional analysis. Obes Res. 2004;12(5):743–9. doi: 10.1038/oby.2004.89. [DOI] [PubMed] [Google Scholar]
- 67.Mazen I, El-Gammal M, Abdel-Hamid MS, Amr K. A novel homozygous missense mutation of the leptin gene (N103K) in an obese egyptian patient. Mol Genet Metab. 2009;97(4):305–8. doi: 10.1016/j.ymgme.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Fischer-Posovszky P, von Schnurbein J, Moepps B, et al. A new missense mutation in the Leptin Gene causes mild obesity and hypogonadism without affecting T cell responsiveness. J Clin Endocrinol Metab. 2010;95(6):2836–40. doi: 10.1210/jc.2009-2466. [DOI] [PubMed] [Google Scholar]
- 69.Fatima W, Shahid A, Imran M, et al. Leptin deficiency and leptin gene mutations in obese children from Pakistan. Int J Pediatr obesity. 2011;6(5–6):419–27. doi: 10.3109/17477166.2011.608431. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Hong N, Liu X et al. A Novel Mutation in Leptin Gene Is Associated with Severe Obesity in Chinese Individuals. BioMed research international, 2014(NA), 912052–912052 (2014). [DOI] [PMC free article] [PubMed]
- 71.Thakur S, Kumar A, Dubey S, Saxena R, Peters A, Singhal A. A novel mutation of the leptin gene in an indian patient. Clin Genet. 2013;86(4):391–3. doi: 10.1111/cge.12289. [DOI] [PubMed] [Google Scholar]
- 72.Chekhranova MK, Karpova SK, Iatsyshina SB, Pankov IA. A new mutation c.422 C > G (p.S141C) in homo- and heterozygous forms of the human leptin gene. Bioorg Khim. 2008;34(6):854–6. doi: 10.1134/s1068162008060198. [DOI] [PubMed] [Google Scholar]
- 73.Wabitsch M, Funcke J-B, Lennerz B, et al. Biologically inactive leptin and early-onset Extreme obesity. N Engl J Med. 2015;372(1):48–54. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- 74.Funcke J-B, von Schnurbein J, Lennerz B, et al. Monogenic forms of childhood obesity due to mutations in the leptin gene. Mol Cell Pediatr. 2014;1(1):3–3. doi: 10.1186/s40348-014-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 76.FDA approval for Biologic License Application (BLA) 125390: MYALEPT as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. (2014). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125390Orig1s000SumR.pdf
- 77.Farooqi IS, Wangensteen T, Collins SC, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 79.Lee YS, Challis B, Thompson DA, et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metabol. 2006;3(2):135–40. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Challis BG, Pritchard LE, Creemers JWM, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11(17):1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- 81.Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin Deficiency treated with a Melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–6. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 82.Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin North Am. 2009;38(3):525–48. doi: 10.1016/j.ecl.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farooqi IS, Yeo GSH, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Investig. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farooqi IS, Keogh JM, Yeo GSH, Lank E, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 85.Vaisse C, Clément K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Investig. 2000;106(2):253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung WK. An overview of mongenic and syndromic obesities in humans. Pediatr Blood Cancer. 2011;58(1):122–8. doi: 10.1002/pbc.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Berg LH, van Beekum O, Heutink P, et al. Melanocortin-4 receptor gene mutations in a dutch cohort of obese children. Obes (Silver Spring Md) 2010;19(3):604–11. doi: 10.1038/oby.2010.259. [DOI] [PubMed] [Google Scholar]
- 88.Xi B, Chandak GR, Shen Y, Wang Q, Zhou D. Association between Common Polymorphism near the MC4R gene and obesity risk: a systematic review and Meta-analysis. PLoS ONE. 2012;7(9):e45731–NA. doi: 10.1371/journal.pone.0045731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dwivedi OP, Tabassum R, Chauhan G, et al. Strong influence of variants near MC4R on adiposity in children and adults: a cross-sectional study in indian population. J Hum Genet. 2012;58(1):27–32. doi: 10.1038/jhg.2012.129. [DOI] [PubMed] [Google Scholar]
- 90.Fani L, Bak S, Delhanty PJD, van Rossum EFC, van den Akker ELT. The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int J Obes. 2013;38(2):163–9. doi: 10.1038/ijo.2013.80. [DOI] [PubMed] [Google Scholar]
- 91.Skowronski AA, Morabito MV, Mueller BR, et al. Effects of a novel MC4R agonist on maintenance of reduced body weight in diet-induced obese mice. Obes (Silver Spring Md) 2014;22(5):1287–95. doi: 10.1002/oby.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen KY, Muniyappa R, Abel BS, et al. RM-493, a Melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100(4):1639–45. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saeed S, Bonnefond A, Tamanini F, et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat Genet. 2018;50(2):175–9. doi: 10.1038/s41588-017-0023-6. [DOI] [PubMed] [Google Scholar]
- 94.Michaud JL, Rosenquist T, May NR, Fan C-M. Development of neuroendocrine lineages requires the bHLH–PAS transcription factor SIM1. Genes Dev. 1998;12(20):3264–75. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stanikova D, Buzga M, Krumpolec P. Genetic analysis of single-minded 1 gene in early-onset severely obese children and adolescents. PLoS ONE. 2017;12(5):e0177222. doi: 10.1371/journal.pone.0177222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yeo GSH, Connie Hung CC, Rochford JJ, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–9. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 97.Wang P, Loh KH, Wu MKY, et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583(7818):839–44. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- 98.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocr. 2004;25(2):77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Gray J, Yeo GSH, Cox JJ, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity Associated with Functional loss of One Copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55(12):3366–71. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serra-Juhé C, Martos-Moreno G, de Pieri FB, et al. Heterozygous rare genetic variants in non-syndromic early-onset obesity. Int J Obes. 2019;44(4):830–41. doi: 10.1038/s41366-019-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.da Fonseca ACP, de Medeiros Abreu G, Palhinha L, et al. A rare potential pathogenic variant in the BDNF gene is found in a brazilian patient with severe childhood-onset obesity. Diabetes Metab Syndr Obes. 2021;14(NA):11–22. doi: 10.2147/DMSO.S267202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab. 2006;18(1):38–45. doi: 10.1016/j.tem.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 103.Doche ME, Bochukova EG, Su HW, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Investig. 2012;122(12):4732–6. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pearce LR, Joe R, Doche ME, et al. Functional characterization of Obesity-Associated Variants Involving the α and β isoforms of human SH2B1. Endocrinology. 2014;155(9):3219–26. doi: 10.1210/en.2014-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pearce LR, Atanassova N, Banton MC, et al. KSR2 mutations are Associated with obesity, insulin resistance, and impaired Cellular fuel oxidation. Cell. 2013;155(4):765–77. doi: 10.1016/j.cell.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Rahilly S, Gray H, Humphreys PJ, et al. Brief report: impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N Engl J Med. 1995;333(21):1386–90. doi: 10.1056/NEJM199511233332104. [DOI] [PubMed] [Google Scholar]
- 107.Borman AD, Pearce LR, Mackay DS, et al. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat. 2013;35(3):289–93. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alsters SIM, Goldstone AP, Buxton JL, et al. Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes Mellitus, Intellectual disability and Hypogonadotrophic Hypogonadism. PLoS ONE. 2015;10(6):e0131417. doi: 10.1371/journal.pone.0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thaker VV, Esteves KM, Towne MC, et al. Whole exome sequencing identifies RAI1 mutation in a morbidly obese child diagnosed with ROHHAD Syndrome. J Clin Endocrinol Metab. 2015;100(5):1723–30. doi: 10.1210/jc.2014-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baron M, Maillet J, Huyvaert M, et al. Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nat Med. 2019;25(11):1733–8. doi: 10.1038/s41591-019-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marenne G, Hendricks AE, Perdikari A, et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cell Metab. 2020;31(6):1107–1119.e12. doi: 10.1016/j.cmet.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81(9):3419–23. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 114.Kolaczynski JW, Considine RV, Ohannesian JP, et al. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not Ketones themselves. Diabetes. 1996;45(11):1511–5. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 115.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–63. doi: 10.1016/S0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 116.Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am. 1994;23(3):467–85. doi: 10.1016/S0889-8529(18)30079-3. [DOI] [PubMed] [Google Scholar]
- 117.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Sci (New York N Y) 1997;278(5335):135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 118.Mendes de Oliveira E, Keogh JM, Talbot F, et al. Obesity-Associated GNAS mutations and the Melanocortin Pathway. N Engl J Med. 2021;385(17):1581–92. doi: 10.1056/NEJMoa2103329. [DOI] [PubMed] [Google Scholar]
- 119.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shungin D, Winkler TW, Croteau-Chonka DC. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kilpeläinen TO, Zillikens MC, Stančáková A, et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet. 2011;43(8):753–60. doi: 10.1038/ng.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu Y, Day FR, Gustafsson S, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7(1):10495–5. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kilpeläinen TO, Carli JFM, Skowronski AA, et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat Commun. 2016;7(1):10494–4. doi: 10.1038/ncomms10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun Q, Cornelis MC, Kraft P, et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum Mol Genet. 2010;19(9):1846–55. doi: 10.1093/hmg/ddq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–60. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pulit SL, Stoneman C, Morris AP, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of european ancestry. Hum Mol Genet. 2019;28(1):166–74. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hansen GT, Sobreira DR, Weber ZT, et al. Genetics of sexually dimorphic adipose distribution in humans. Nat Genet. 2023;55(3):461–70. doi: 10.1038/s41588-023-01306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Justice AE, Karaderi T, Highland HM. Protein-coding variants implicate novel genes related to lipid homeostasis contributing to body-fat distribution. Nat Genet. 2019;51(3):452–69. doi: 10.1038/s41588-018-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rask-Andersen M, Karlsson T, Ek WE, Johansson Ã. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun. 2019;10(1):339–9. doi: 10.1038/s41467-018-08000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fox CS, Liu Y, White CC, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet. 2012;8(5):e1002695. doi: 10.1371/journal.pgen.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sung YJ, Pérusse L, Sarzynski MA, et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int J Obes. 2015;40(4):662–74. doi: 10.1038/ijo.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Y, Broadaway KA, Raulerson CK, et al. Colocalization of GWAS and eQTL signals at loci with multiple signals identifies additional candidate genes for body fat distribution. Hum Mol Genet. 2019;28(24):4161–72. doi: 10.1093/hmg/ddz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DiStefano JK, Kingsley C, Wood GC, et al. Genome-wide analysis of hepatic lipid content in extreme obesity. Acta Diabetol. 2014;52(2):373–82. doi: 10.1007/s00592-014-0654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Couto Alves A, De Silva NMG, Karhunen V, et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci Adv. 2019;5(9):eaaw3095. doi: 10.1126/sciadv.aaw3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Randall JC, Winkler TW, Kutalik Z, et al. Sex-stratified genome-wide Association Studies Including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9(6):1003500–NA. doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Winkler TW, Justice AE, Graff M, et al. The influence of Age and Sex on Genetic Associations with adult body size and shape: a large-scale genome-wide Interaction Study. PLoS Genet. 2015;11(10):1–42. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wen W, Zheng W, Okada Y, et al. Meta-analysis of genome-wide association studies in east asian-ancestry populations identifies four new loci for body mass index. Hum Mol Genet. 2014;23(20):5492–504. doi: 10.1093/hmg/ddu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang T, Ma X, Peng D, et al. Effects of obesity related genetic variations on visceral and subcutaneous Fat distribution in a Chinese Population. Sci Rep. 2016;6:20691. doi: 10.1038/srep20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ng MCY, Graff M, Lu Y, et al. Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of african ancestry: african ancestry Anthropometry Genetics Consortium. PLoS Genet. 2017;13(4):1006719–NA. doi: 10.1371/journal.pgen.1006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akiyama M, Okada Y, Kanai M, et al. Genome-wide association study identifies 112 new loci for body mass index in the japanese population. Nat Genet. 2017;49(10):1458–67. doi: 10.1038/ng.3951. [DOI] [PubMed] [Google Scholar]
- 141.Gurdasani D, Carstensen T, Fatumo S, et al. Uganda Genome Resource enables insights into Population History and genomic Discovery in Africa. Cell. 2019;179(4):984–1002.e36. doi: 10.1016/j.cell.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wojcik GL, Graff M, Nishimura KK, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 2019;570(7762):514–8. doi: 10.1038/s41586-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grarup N, Moltke I, Andersen MK, et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat Genet. 2018;50(2):172–4. doi: 10.1038/s41588-017-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Minster RL, Hawley NL, Su C-T, et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat Genet. 2016;48(9):1049–54. doi: 10.1038/ng.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andersen MK, Jørsboe E, Skotte L, et al. The derived allele of a novel intergenic variant at chromosome 11 associates with lower body mass index and a favorable metabolic phenotype in Greenlanders. PLoS Genet. 2020;16(1):e1008544–NA. doi: 10.1371/journal.pgen.1008544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Auton A, Abecasis GR, Altshuler D, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Turcot V, Lu Y, Highland HM, et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet. 2018;50(1):26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ndiaye FK, Huyvaert M, Ortalli A, et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int J Obes. 2019;44(2):539–43. doi: 10.1038/s41366-019-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Finucane HK, Reshef YA, Anttila V, et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat Genet. 2018;50(4):621–9. doi: 10.1038/s41588-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Claussnitzer M, Dankel SN, Kim K-H, et al. FTO obesity variant circuitry and Adipocyte Browning in humans. N Engl J Med. 2015;373(10):895–907. doi: 10.1056/NEJMoa1502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2008;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 152.Willer CJ, Speliotes EK, Loos RJF, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2008;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiemerslage L, Gohel PA, Maestri G, et al. The Drosophila ortholog of TMEM18 regulates insulin and glucagon-like signaling. J Endocrinol. 2016;229(3):233–43. doi: 10.1530/JOE-16-0040. [DOI] [PubMed] [Google Scholar]
- 154.Larder R, Sim MFM, Gulati P, et al. Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc Natl Acad Sci USA. 2017;114(35):9421–6. doi: 10.1073/pnas.1707310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rathjen T, Yan X, Kononenko NL, et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat Neurosci. 2017;20(8):1096–103. doi: 10.1038/nn.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yan X, Wang Z, Schmidt V, et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol metab. 2018;8(NA):180–8. doi: 10.1016/j.molmet.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee AWS, Hengstler H, Schwald K, et al. Functional inactivation of the genome-wide Association study obesity gene neuronal growth Regulator 1 in mice causes a body Mass phenotype. PLoS ONE. 2012;7(7):e41537. doi: 10.1371/journal.pone.0041537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Joo Y, Kim HJ, Lee SJ, Lee S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int J Obes. 2019;43(9):1769–82. doi: 10.1038/s41366-019-0376-2. [DOI] [PubMed] [Google Scholar]
- 159.Boender AJ, van Gestel MA, Garner KM, Luijendijk MCM, Adan RAH. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiological Rep. 2014;2(7):e12083. doi: 10.14814/phy2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Su L, Wang Y-b, Wnag C-g, Wei H. Network analysis identifies common genes associated with obesity in six obesity-related diseases. J Zhejiang Univ Sci B. 2017;18(8):727–32. doi: 10.1631/jzus.B1600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Loos RJ, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–75. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chambers JC, Elliott P, Zabaneh D, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40(6):716–8. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 163.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. 2008;66(8):7–11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sadashiv, Modi A, Khokhar M, et al. Leptin DNA methylation and its association with metabolic risk factors in a Northwest Indian obese population. J Obes Metab Syndr. 2021;30(3):304–11. doi: 10.7570/jomes20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Houshmand-Oeregaard A, Hansen NS, Hjort L, et al. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin epigenetics. 2017;9(1):37–7. doi: 10.1186/s13148-017-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ott R, Stupin JH, Melchior K, et al. Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin epigenetics. 2018;10(1):131–1. doi: 10.1186/s13148-018-0567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim AY, Park YJ, Pan X, et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun. 2015;6(1):7585–5. doi: 10.1038/ncomms8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuroda A, Rauch TA, Todorov I, et al. Insulin gene expression is regulated by DNA methylation. PLoS ONE. 2009;4(9):e6953–NA. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de Souza Pinhel MA, Noronha NY, Nicoletti CF, et al. Changes in DNA methylation and gene expression of insulin and obesity-related gene PIK3R1 after Roux-en-Y gastric bypass. Int J Mol Sci. 2020;21(12):4476–NA. doi: 10.3390/ijms21124476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crujeiras AB, Campión J, Diaz-Lagares A, et al. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept. 2013;186(NA):1–6. doi: 10.1016/j.regpep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 172.Yang H, Yang K, Gu H, Sun C. Dynamic post-translational modifications in obesity. J Cell Mol Med. 2019;24(3):2384–7. doi: 10.1111/jcmm.14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Funato H, Oda S, Yokofujita J, Igarashi H, Kuroda M. Fasting and High-Fat Diet alter histone deacetylase expression in the Medial Hypothalamus. PLoS ONE. 2011;6(4):e18950–NA. doi: 10.1371/journal.pone.0018950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458(7239):757–61. doi: 10.1038/nature07777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mikula M, Majewska A, Ledwon JK, Dzwonek A, Ostrowski J. Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int J Mol Med. 2014;34(6):1647–54. doi: 10.3892/ijmm.2014.1958. [DOI] [PubMed] [Google Scholar]
- 176.Landrier J-F, Derghal A, Mounien L. MicroRNAs in obesity and related metabolic Disorders. Cells. 2019;8(8):859–NA. doi: 10.3390/cells8080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Butler MG, Wang K, Marshall JD et al. Coding and noncoding expression patterns associated with rare obesity-related disorders: Prader-Willi and Alström syndromes. Adv Genomics Genet, 2015(5), 53–75 (2015). [DOI] [PMC free article] [PubMed]
- 178.Liu Y, Ji Y, Li M, et al. Integrated analysis of long noncoding RNA and mRNA expression profile in children with obesity by microarray analysis. Sci Rep. 2018;8(1):8750–0. doi: 10.1038/s41598-018-27113-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun J, Ruan Y, Wang M-m, et al. Differentially expressed circulating LncRNAs and mRNA identified by microarray analysis in obese patients. Sci Rep. 2016;6(1):35421–1. doi: 10.1038/srep35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ding C, Lim YC, Chia SY, et al. De novo reconstruction of human adipose transcriptome reveals conserved lncRNAs as regulators of brown adipogenesis. Nat Commun. 2018;9(1):1329–9. doi: 10.1038/s41467-018-03754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stapleton K, Das S, Reddy MA, et al. Novel long noncoding RNA, macrophage inflammation-suppressing transcript (MIST), regulates macrophage activation during obesity. Arterioscler Thromb Vasc Biol. 2020;40(4):914–28. doi: 10.1161/ATVBAHA.119.313359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Squillaro T, Peluso G, Galderisi U, Di Bernardo G. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. eLife. 2020;9:e59053 [DOI] [PMC free article] [PubMed]
- 183.Catalán V, Avilés-Olmos I, Rodríguez A, et al. Time to Consider the “Exposome Hypothesis” in the Development of the Obesity Pandemic. Nutrients. 2022;14(8):1597. [DOI] [PMC free article] [PubMed]
- 184.Veenendaal MVE, Painter RC, de Rooij SR, et al. Transgenerational effects of prenatal exposure to the 1944-45 dutch famine. BJOG. 2013;120(5):548–54. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- 185.Ajslev TA, Angquist L, Silventoinen K, et al. Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Front Genet. 2012;3:125. doi: 10.3389/fgene.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ou X-H, Zhu C-C, Sun S-C. Effects of obesity and diabetes on the epigenetic modification of mammalian gametes. J Cell Physiol. 2018;234(6):7847–55. doi: 10.1002/jcp.27847. [DOI] [PubMed] [Google Scholar]
- 187.Ge Z-J, Luo S-M, Lin F, et al. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ Health Perspect. 2013;122(2):159–64. doi: 10.1289/ehp.1307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun S-C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6(1):18858–8. doi: 10.1038/srep18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Klastrup LK, Bak ST, Nielsen AL. The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Mol Genet genomics: MGG. 2018;294(1):1–11. doi: 10.1007/s00438-018-1492-8. [DOI] [PubMed] [Google Scholar]
- 190.Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and bariatric surgery drive epigenetic variation of Spermatozoa in humans. Cell Metabol. 2015;23(2):369–78. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 191.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–60. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 192.Amir H, Weintraub AY, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22(9):759–764. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 193.Chavira-Suárez E, Ramírez-Mendieta AJ, Martínez-Gutiérrez S, et al. Influence of pre-pregnancy body mass index (p-BMI) and gestational weight gain (GWG) on DNA methylation and protein expression of obesogenic genes in umbilical vein. PLoS ONE. 2019;14(12):e0226010–NA. doi: 10.1371/journal.pone.0226010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang FF, Morabia A, Carroll JF, et al. Dietary patterns are Associated with levels of global genomic DNA methylation in a Cancer-Free Population. J Nutr. 2011;141(6):1165–71. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng S, Rollet M, Pan YX. Maternal protein restriction during pregnancy induces CCAAT/enhancer-binding protein (C/EBPβ) expression through the regulation of histone modification at its promoter region in female offspring rat skeletal muscle. Epigenetics. 2011;6(2):161–70. doi: 10.4161/epi.6.2.13472. [DOI] [PubMed] [Google Scholar]
- 196.Sohi G, Marchand KC, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7α-Hydroxylase promoter. Mol Endocrinol (Baltimore Md) 2011;25(5):785–98. doi: 10.1210/me.2010-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023;401(10382):1116-1130 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- National Health and Nutrition Examination. 2021. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. [DOI]