Abstract
Three Lactobacillus Plantarum (LP), namely LP28, LP226 and LPC2W, were employed to investigate the effect on the aroma profiles of pasteurized litchi juice using E-nose, GC-IMS, GC-MS, and sensory evaluation. The E-nose results showed that pasteurization weakened the flavor profile of litchi juice, while LP fermentation effectively promoted flavor formation. The GC-MS analysis demonstrated that pasteurization significantly reduced the content of alcohols (28.51%), especially geraniol and citronellol, which give litchi juices a fruity and floral aroma. Different LP fermentation enhances the characteristic aroma and produces some new compounds that give it a strong fruity and citrus-like aroma. Moreover, 37 aroma-active compounds (OAV>1) indicated that the linalool (OAV 7504) was the highest, followed by (Z)-rose oxide (OAV 4265), 1-octen-3-ol (OAV 1055) and geraniol (OAV 764), which jointly form the main characteristic flavor. More esters were identified by GC-IMS, indicating the advantage of the combined approach for a better understanding of the impact of pasteurization and fermentation on the litchi juice. The sensory evaluation confirmed that the aroma attributes of fruity, citrus-like, floral, sweet and litchi-like were stronger for the samples fermented by LP28 than those for the other samples. The combination strategy used in this study would facilitate the awareness of litchi juice aroma and broaden our insight into the deep processing of litchi.
Keywords: Litchi juice, Lactic acid bacteria, Fermentation, Gas chromatography-mass spectrometry, Gas chromatography-ion mobility spectrometry, Odor activity value
Graphical abstract
Highlights
-
•
Pasteurization resulted in a loss of geraniol and citronellol in litchi juices.
-
•
LAB fermentation gives the litchi juice more floral and citrus-like aromas.
-
•
Different LAB in fermented litchi juice showed diversity in the flavor profile.
-
•
Inoculation LP28 strain had a positive effect on the flavor of litchi juice.
1. Introduction
Litchi (Litchi chinensis Sonn.) is a subtropical and tropical fruit belonging to the Sapotaceae family and widely cultivated in more than 20 countries worldwide (L. Zhao et al., 2020). The output of litchi was 2.5 million tons in China, and it is considered the pillar industry of the Guangdong region (D. Wang et al., 2021). Litchi has been broadly appreciated by consumers due to its favorable aroma and delicious flesh. Moreover, the litchi pulp is rich in bioactive components such as polysaccharides, polyphenols and γ-aminobutyric acid (GABA), which have been reported to exhibit antioxidant, anti-obesity, hepatoprotective effects and immunomodulatory activities (Reichel et al., 2011; Su et al., 2016; Xie et al., 2014). Generally, litchi is considered to be highly suitable for deep processing, not only for selling as fresh fruit but also for processing into dried, canned and pulp juice (D. Wang et al., 2021). It is worth noting that fresh litchi is seasonal fruit with the features of being high in sugar and perishable at ambient temperature during transportation and storage, which would result in poor fruit quality and short shelf life (Liu et al., 2022). This will limit litchi processing industries' potential economic benefits and long-term development. Traditional litchi processing products are facing serious challenges due to the presence of high sugars and the severe loss of flavor components upon processing (An et al., 2019). Therefore, it is imperative to explore novel processing technologies to enrich litchi product categories and meet contemporary consumers’ pursuit of healthy food.
Probiotics, defined as “live microorganisms which when administered in adequate amounts, confer health benefits to the host”, impart various health benefits to consumers by modulating the immune system, preventing diarrhea and reducing atopic diseases and food allergies (Mishra et al., 2015). Lactic acid bacteria (LAB) are one of the main probiotic resources, playing a positive role in both maintaining the immune function of the intestinal mucosa and interacting with the functional components of food (Li et al., 2021; Lynch et al., 2018). LAB has been used in the fermentation of fruits or vegetables to yield products with desirable organoleptic qualities and prolonged shelf-life (Gu et al., 2022; G. Wang et al., 2022). With ongoing in-depth research on the health functions of LAB fermentation beverages, it is an established fact that the biotransformation of phytochemical substances in LAB fermented beverages has various physiological activities (Z. Wang et al., 2022a). For commercial distribution, fruit juices need to be sterilized after bottling, and among many sterilization approaches, pasteurization has been found as the best one with the advantages of low cost, quick and safe handling (Zhu et al., 2022). Additionally, heat sterilization has been shown to have a negative impact on the flavor of juices, but this step is essential in conventional juice processing to prevent contamination by foodborne pathogens (Teribia et al., 2021). Our previous research has shown that litchi juice is a suitable substrate for LAB growth due to the presence of high-level sugars and amino acids (D. Wang et al., 2021). Although the flavor compounds in litchi are extremely minor compared to the non-volatile components, it directly determines the customer preferences and economic value of fresh and processed litchi products. Therefore, it is desirable to unravel the effect of processing technology (pasteurization and fermentation) on the flavor of litchi juice. However, to the best of the authors’ knowledge, the effects of Lactobacillus Plantarum fermentation on the flavor profiles of pasteurized litchi juice have not been documented.
Aroma determines the unique sensory characteristics of fermented foods (Sherman et al., 2020). Gas chromatography-mass spectrometry (GC-MS) has been widely used in the analysis of flavor characteristics in fruits, and over one hundred volatile compounds have been detected in litchi fruits (An et al., 2019). As a two-dimensional gas-phase separation technology, gas chromatography-ion mobility spectrometry (GC-IMS) provides a more intuitive ion chromatogram to better elaborate the volatile profiles, and has advantages such as reasonable selectivity and lower detection limits (S. Wang et al., 2020). Moreover, electronic nose (E-nose) is an odor recognition and analysis technology developed by imitating the human olfactory system (Zhang et al., 2021). In order to obtain more comprehensive and scientific information, the use of a combination of various techniques to elucidate food flavor characteristics has become a prevailing trend. For example, Yu et al. demonstrated that the Huangjiu made around Winter Solstice has a more harmonious flavor profile by GC-MS combined with GC-IMS (Yu et al., 2021). Di Cagno et al. elucidated the flavor profile of pomegranate juice by GC-MS (Di Cagno et al., 2017). The flavor profile of litchi and litchi wine has been discussed using GC-MS (Cai et al., 2019; L. Zhao et al., 2021). However, no studies have investigated the flavor profiles of pasteurized and LAB-fermented litchi juices based on combined flavor analysis strategies. Nevertheless, this information is important for litchi processing industry to produce litchi beverages with premium qualities. This study analyzed the volatile compounds in the original, pasteurized and fermented litchi juices using E-nose, GC-IMS, GC-MS and sensory evaluation. The aims in the present study were (I) to investigate the effect of pasteurization on the flavor profiles of litchi juice. (II) to elucidate the benefits of different LAB fermentation on the flavor changes of pasteurized litchi juices. (III) to identify the crucial odors by the odor activity values (OAVs) calculation and the partial least squares discriminant analysis (PLS-DA) model. The findings will broaden our insight into LAB fermented litchi juices and provide new strategies for the manufacturing technology of litchi processing products with a distinctive flavor.
2. Materials and methods
2.1. Strains and reagents
Two commercial strains namely Lactobacillus plantarum 28 (LP28) and Lactobacillus plantarum 226 (LP226) were purchased from Xiannong Biotechnology Company (Shanghai, China). One strain namely Lactobacillus plantarum C2W (LPC2W) was isolated from the traditional Chinese kimchi as reported in our previous study (D. Wang et al., 2021) and preserved by the Guangdong Microbial Strain Conservation Center (Guangzhou, China). Litchi (cv. ‘Feizixiao’) at commercial maturity (°Brix: 18%) were purchased from the local market in Guangdong province in 2021. A mixture of n-alkanes (C7–C30, ≥99%) for linear retention index (LRI) calculation was obtained from Sigma-Aldrich Co., Ltd (St. Louis, MO, USA). Ethanol (≥99.8%), sodium chloride (99.5%), cyclohexanone (internal standard, ≥99.5%), geraniol (99.5%), ethyl acetate (≥95%), ethyl isovalerate (≥97%) and D-limonene (≥95%) were purchased from Aladdin (Shanghai, China).
2.2. Litchi juice preparation and fermentation
Litchi juice was prepared according to the method of Wang et al. (2021) with minor modifications. The litchi fruits were peeled, deseeded, squeezed and filtrated with 200 mesh gauze to produce litchi juice. Then, the original litchi juice (150 mL) was pasteurized at 85 °C for 10 min, cooled to 37 °C and inoculated with 0.4% (v/v) of the individual inoculants at 37 °C for 40 h and then the fermented litchi juices were filtered. Lastly, the original, pasteurized and fermented litchi juices were stored at −80 °C before analyze in order to keep the intact properties.
2.3. E-nose analysis
E-nose (PEN3, Airsense Analytics Co. Ltd., Schwerin, Germany), equipped with 10 different metal oxide semiconductors (Table S1), was employed to analyze differences between samples. An accurately quantized litchi juice sample (20 mL) was placed in an E-nose detection vial and then equilibrated for 30 min before analysis. The test conditions for the E-nose were: system cleaning, 120 s; pre-sampling time, 5 s; test time, 120 s; and gas flow rate, 300 mL/min.
2.4. HS-GC-IMS analysis
The GC-IMS was analyzed on an Agilent 490 GC with a capillary column (MXT-WAX, 30 m × 0.53 mm × 1.0 μm) and an auto sampler unit. Samples (1 mL) were packed into 20 mL headspace bottles and stirred at 500 r/min (20 min, 45 °C). Then, the samples (500 μL) were injected by using a pre-heated syringe (85 °C). The column was kept at 60 °C during isothermal processes, and the samples were driven via N2 at a gradient program as follows: initial flow rate 2 mL/min for 2 min, raised to 10 mL/min in 10 min, then to 100 mL/min in next 10 min and kept 100 mL/min until 30 min. The drift gas flow rate was set to 150 mL/min. Volatile compounds were identified by matching retained indices and drift times with GC-IMS data and NIST libraries (G.A.S., Dortmund, Germany).
2.5. GC-MS analysis
The compounds of volatile were identified by headspace solid-phase microextraction (HS-SPME) combined with GC-MS according to Ricci et al. (2018) with slight modifications. DB-WAX column (60 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA) was applied on analyte separation. First, the SPME fiber coated with DVB/CAR/PDMS (100 μm in thickness) was aged in the GC inlet at 240 °C for 1 h. Then, 5 mL of the sample was accurately pipetted into a 50 mL conical flask and 1 g NaCl was added to promote volatile compound extraction. Subsequently, 50 μL of internal standard cyclohexanone (0.5 mg/L) was added. The SPEM was inserted into the conical flask for 1 cm and the fiber head was pushed out. The adsorption was performed for 30 min at 40 °C under a constant temperature. The volatile compounds were then desorbed in the GC inlet at 240 °C for 5 min for GC-MS analysis.
The GC temperature gradient program was initiated at 40 °C held for 3 min and climbed to 230 °C at a rate of 8 °C/min. Helium (99.99% purity) was used as a carrier gas and the flow rate was set as 1 mL/min at the constant flow mode. The MS conditions were set as follows: ionization mode EI, electron bombardment energy 70 eV, ion source temperature 230 °C and scan mass range 35–500 amu. Volatile compounds in litchi juice were identified by the comparison of mass spectra with those recorded in the NIST 14 libraries, linear retention index (LRI) and odor description. Meanwhile, LRIs were analyzed based on the retention times of the external standard n-alkanes (C7–C30). Semi-quantitative analysis was carried out via using cyclohexanone as the internal standard substance. The relative concentration of the released volatile was calculated as Eq. (1):
| (1) |
where C0 is the concentration of internal standard; V0 is the volume of the internal standard; SX is the peak area of aroma compounds; CX is the concentration of aroma compounds; S0 is the peak area of the internal standard; V is the volume of the samples.
2.6. Calculation of odor activity value (OAV)
OAV was calculated using the ratio between the concentration of flavor substances and their odor thresholds. The OAV reflects the contribution of volatile compounds to the overall flavor. The key aroma components were determined by the OVA≥1, suggesting a significant contribution to aroma characteristics (Guo et al., 2021).
2.7. Sensory evaluation
The quantitative descriptive analysis (QDA) was carried out according to Chen et al. (2019) with minor modifications. Sensory evaluations were performed in a sensory panel chamber at 20 ± 1 °C and 40 ± 2% relative humidity. Trained panelists, recruited from the Guangdong Laboratory for Lingnan Modern Agriculture (five males and five females from 20 to 35 years old), had more than 3 years of experience in the sensory evaluation of processed litchi products. All sensory personnel need to characterize as many samples as possible for flavor description. After discussion, six aroma attributes, i.e., floral, fruity, sweet, sour, citrus-like, and litchi-like, were selected based on the description of panelists and the literature. Six properties are defined for the following aroma references: geraniol for "floral" properties, ethyl acetate for “sour” properties, ethyl isovalerate for "fruity" properties, D-limonene for "sweet" properties, "citrus-like" properties of the same smell as lemon and orange, "litchi-like" properties of the Feizixiao litchi juices. Standard solutions were assigned to the panelists to smell in order to familiarize the characteristic odor properties. During sensory evaluation, 10 mL of each litchi juice sample was separately poured into a sensory-tasting cup and randomly labeled by a three-digit number and then assigned to panelists to evaluate the aroma intensity. The panelists scored the samples according to the intensity of the aroma, from 0 to 10, in order of weakness to strength. Each group of samples was scored 3 times at different times by each panelist.
2.8. Statistical analysis
IBM SPSS Statistics 21 (SPSS Inc., Chicago, USA) statistical software was used for statistical analysis. Origin 2021 (Origin Lab Corporation, Northampton, MA, USA) was used to draw the graphs and principal component analysis (PCA). Venn diagram were plotted by Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/). Heatmap clustering, partial least squares discriminate analysis (PLS-DA) and variable importance in projection (VIP) scores were performed using MetaboAnalyst 2.0 (www.metaboanalyst.ca). The data are expressed as means ± standard deviations.
3. Results and discussion
3.1. Volatile compounds identified by E-nose
The E-nose system can evaluate the sample information within the measurable range comprehensively. Samples do not require any pre-treatment for volatile component detection. The slight changes in volatile compounds will be detected by the sensor response. As shown in Fig. 1A, the five sensors (W2S, W1W, W2W, W1S and W5S) had higher responses to the litchi juice samples, while the other five sensors had no obvious responses. The contents of flavor compounds in litchi juice after pasteurization were reduced, but they are closer to the original juice in terms of overall flavor after LP28 and LP226 fermentation. This indicates that LAB fermentation is beneficial to remodel the aroma compounds destroyed by thermal treatment.
Fig. 1.
(A) Radar chart of the electronic nose (E-nose) response data; (B) PCA sample distribution diagram and component distribution plot of E-nose of litchi juices, pasteurized litchi juices and LAB fermented litchi juices. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
PCA is an unsupervised statistical analysis that uses a few significant variables to highlight the differences in multiple variables by linearly transform (Guo et al., 2022). As shown in Fig. 1B, the first two components explained 91.2% of the total variance with the PC1 and PC2 accounting for 53.5% and 37.7%, respectively, which indicated the two principal components represented the main characteristic information of all samples. The spatial regions of these samples showed that F, F-28, and F-226 had relatively close volatile compounds. However, they were significantly different from the F–P. In addition, these results illustrated that the inoculation of LP28 and LP226 produced greater flavor intensity than those inoculated LPC2W which could preserve the characteristic flavor of litchi juice.
3.2. Volatile compounds identified by GC-IMS
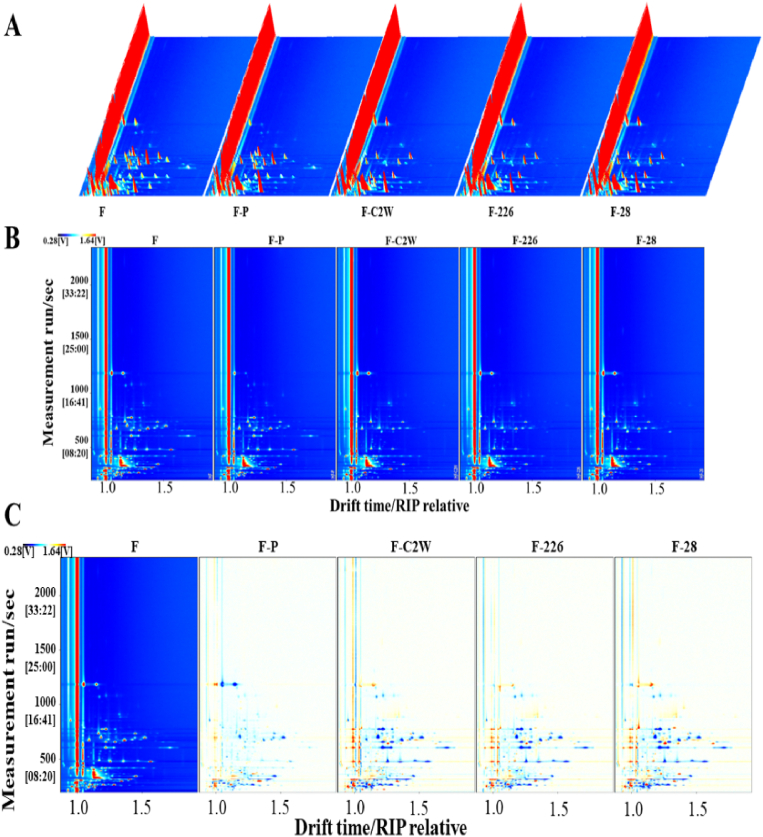
The volatile compounds of original, pasteurized and fermented litchi juices were tested by GC-IMS, which provided an intuitive understanding by the two-dimensional separations. As can be seen from the 3D topographical visualization in Fig. 2A, the volatile compounds between F and F–P are discrepant. Generally, pasteurization is the most economical sterilization technique for extending the shelf life of fruit and vegetable juices in industry (J. Wang et al., 2022). However, high temperatures can accelerate the interaction between different substances in the juice and thus affect its characteristic flavor (Zhu et al., 2022). After pasteurization treatment, the signal intensity of most volatile compounds decreased and even disappeared. This result was in agreement with the E-nose, suggesting that pasteurization has a remarkable effect on the overall flavor of the original litchi juice. Several volatile compounds belonging to F were re-produced by LAB fermentation in the F–C2W, F-226 and F-28. Moreover, the volatile compounds in litchi juice fermented by different LAB are very similar, but the signal intensity is slightly different.
Fig. 2.
The result of original litchi juice, pasteurized litchi juice and LAB fermented litchi juices via GC-IMS. (A) 3D chromatogram; (B) 2D chromatogram; (C) discrepancy image. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
The 2D chromatogram and discrepancy images in the original, pasteurized and fermented litchi juice were shown in Fig. 2B and C. The Y-axis represents the retention time of the gas chromatographic peaks, the X-axis denotes the ion migration time for identification. The red vertical line at the X-axis value of 1.0 is the reaction ion peak and different points at the right of RIP represent different volatile components. As shown in Fig. 2B, the red color denotes that the analyte is present at a high level. It could be seen that most of the signals appeared in the drift time of 1.0–1.6 and the retention time of 100–1400 s. The 2D chromatogram of the original litchi juice was selected as a reference, which was deducted from those of the other samples. The discrepancy image was obtained. If the background was red spots, the contents of volatile compounds were higher than the original litchi juice. The blue spots indicated the contents of the volatile compounds were lower than the original litchi juice and the white background showing the same content compared with the original litchi juice. Fig. 2C shows that the volatile compounds of L-P are not only apparent reduced in the signal intensity, but also in type. Teribia et al. (2021) demonstrated that pasteurization generally resulted in changes in the volatile compounds in strawberry purees. Moreover, previous research indicated that thermal-induced fruit juice might degrade terpenoids with fruity notes (Z. Wang et al., 2022b).
More importantly, thermal processing treatment may lead to denaturation of proteins and accelerate interactions between molecules (carbohydrate, polyphenol and protein) in the juice, resulting in the formation of precipitates (Zhu et al., 2022). Considering the stability of the fermented litchi juice, the final product was filtered, which might result in the loss of some volatile compounds. Therefore, the LAB fermented litchi juices have darker blue spots than the pasteurized juice, but the red spots are correspondingly increased in Fig. 2C. This suggests that these darker blue spots may be related to the volatile compounds in the precipitation. These results indicated that the flavor compounds will be destroyed after pasteurization, while LAB fermentation reshapes the flavor profile and forms some new volatile compounds. Hence, to avoid the impact of pasteurization on litchi juice flavor, LAB fermentation is an optional strategy.
As shown in Table S2, a total of 95 volatile compounds among the five samples were identified by GC-IMS library, including 18 esters, 10 alcohols, 10 aldehydes, 8 ketones, 7 terpenoids, 7 heterocyclics, 2 acetic acid, 4 others and 29 undefined. It should be noted that some single compounds may produce multiple signals, attributing the presence of monomers, dimers and polymers. Twenty chemicals were detected by GC-IMS in dimeric and trimeric forms, which included ethyl acetate, 3-methyl-2-butenal, (E)-2-hexenal, 2-methyl propanal, propanal, (E)-2-pentenal, limonene, beta-myrcene, hexanal, heptanal, 3-methyl-3-buten-1-ol, propyl propanoate, acetic acid, ethanol, 2-methyl-1-propanol, 3-methyl-1-butanol, methyl acetate, heptan-2-one, 3-hydroxy-2-butanone and ethyl hexanoate. In addition, the peak intensity of volatile compounds of F–P decreased by 8.07% compared to the F. This result corresponds to the findings of E-nose analysis. Alcohols dominate aroma compounds in litchi juice and they all increased slightly after LAB fermentation. The intensities of aldehydes were reduced after LAB fermentation. The content of (E)-2-hexenal was decreased by 92.46%, 79.37%, 90.08% in F–C2W, F-226, F-28, respectively. Propanal and 3-methyl-2-butenal were the quantitative major aldehydes, which have dropped dramatically. The main reason for the decline of aldehydes may be attributed to the reduction of aldehydes to alcohols or oxidation to acids under the metabolism of LAB (Di Cagno et al., 2017). Moreover, it can be obtained from Table S2 that three esters and four ketones were produced in the fermented litchi juice. The major esters were ethyl acetate and propyl propanoate, providing fruity odor (Guo et al., 2022). The formation of ketones (1-hydroxy-2-propanone, heptan-2-one, 3-hydroxy-2-butanone and 2-pentanone) was significantly enhanced, especially for F-28. Heptan-2-one was only detected in LAB fermentation litchi juice, endowing fruity and herbal odors (Z. Wang et al., 2022b).
As presented in Fig. 3A, some single volatile compounds in the yellow frame of LAB fermentation samples, including (Z)-2-pentenol, 1-butanol, 1-hexanol, 2-methyl-1-propanol, 3-methyl-1-butanol, 3-methyl-3-buten-1-ol, ethanol, 1-methylethyl acetate, 2-methylbutyl acetate, butyl formate, ethyl propanoate, pentyl acetate, propyl butanoate, propyl propanoate, 2,5-dimethylfuran, 2-butylfuran, 2-pentanone, acetic acid, alpha-terpinolene and tetrahydropyrrole were higher than the F–P, but it is the same volatile compounds that are originally in the F. These results suggested that the flavor compounds produced by the metabolism of LAB facilitate to enhance the overall flavor of fermented litchi juice. In addition, the contents of volatile compounds in F–C2W, including 1-methylethyl acetate, 2-methylbutyl acetate, 2-butylfuran and 1-hexanol, were slightly lower than F-226 and F-28. Those volatile compounds in the green frame are produced by LAB fermentation, such as ethyl heptanoate, ethyl hexanoate, methyl 3-methylbutanoate, methyl acetate, 1-hydroxy-2-propanone, 3-hydroxy-2-butanone, heptan-2-one, 1-penten-3-ol and 2-methyl-3-furanthiol. To better distinguish the differences among the original, pasteurized and fermented litchi juices, the PCA analysis and nearest neighbor analysis were performed with the volatiles identified by GC-IMS. As can be seen in Fig. 3B, the two principal component (PC1 and PC2) accounts for 64% and 21% of the accumulative variance contribution rate, respectively, which represented 85% of the total variability. The distance between the F and F–P indicated that heat sterilization significantly affected the flavor of litchi juice. In addition, the LAB fermentation litchi juices were far away from them, indicating volatile compounds were changed. The nearest neighbor analysis allows for a quick comparison of samples based on the intensity of the compounds in the selected evaluation region by calculating Euclidean distance (Fig. 3B and Table S2). The result showed that volatile compounds and aroma complexity were dramatically changed by LAB fermentation and the volatile fingerprints of original, pasteurized and different LAB fermented litchi juices were established.
Fig. 3.
The result of original, pasteurized and LAB fermented litchi juices via GC-IMS. (A) fingerprints. Each row represents all the signals selected in a sample. Each column represents the signals of the same volatile aroma compounds. M and D denote monomer and dimer, respectively; (B) principal component analysis results; (C) nearest neighbor analysis. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
3.3. Volatile compounds identified by GC-MS
As shown in Table 1 and Fig. S1, a total of 68 of volatile compounds were identified by the GC-MS, which were classified into 7 categories (including 25 alcohols, 10 aldehydes, 9 ketones, 13 terpenoids, 6 ethers, 2 esters and 3 others). Of these, the three component types with the highest relative percentages were alcohols (36.76%), terpenoids (19.12%) and aldehydes (14.71%). The different separation capacities and sensitivities of GC-MS and GC-IMS allow them to be used as complementary techniques for the detection of flavor profiles. Our results suggested that GC-MS was more sensitive to alcohols while GC-IMS measured esters more acutely. Alcohols, terpenoids, aldehydes and esters were the volatile compounds of litchi juice with high content, while ketones and ethers exhibited relatively low contents. The main compound of the original litchi juices was consistent with a previous study on the volatile compounds analysis of litchi juices (Tang et al., 2019). As shown in Fig. 4A, the numbers of volatile compounds in the original, pasteurized and fermented litchi juices were different. The number of alcohols and ketones increased after fermentation, while aldehydes and terpenes showed a decreasing trend. The numbers of other categories of substances did not change obviously.
Table 1.
Compound concentration (mean ± standard deviation) (mg/L), odor description, threshold value of the volatile compounds detected in the litchi juices by using HS-SPME-GC–MS.
| Compounds | CAS | Threshold (mg/L) | Compound concentration (mg/L) |
Odor Description | ||||
|---|---|---|---|---|---|---|---|---|
| F | F–P | F-28 | F-226 | F–C2W | ||||
| 3-Methyl-1-butanol | 763-32-6 | 0.547 | 0.86 ± 0.33b | 1.16 ± 0.05a | 0.71 ± 0.01b | 0.70 ± 0.05b | 0.79 ± 0.03b | sweet fruity |
| Prenol | 556-82-1 | 0.25 | 0.69 ± 0.11bc | 0.55 ± 0.03c | 0.79 ± 0.01ab | 0.96 ± 0.26a | 0.93 ± 0.02a | green, fruity |
| Hexanol | 111-27-3 | 0.8205 | 0.90 ± 0.13b | 0.72 ± 0.02c | 1.48 ± 0.06a | nd | 1.48 ± 0.03a | green, fruity, apple-skin |
| 3-Octanol | 589-98-0 | 0.042 | 0.54 ± 0.13bc | 0.52 ± 0.01c | 0.60 ± 0.02bc | 0.55 ± 0.01b | 0.96 ± 0.03a | mushroom, earthy |
| (E)-2-Hexen-1-ol | 928-95-0 | 5 | 0.18 ± 0.02b | 0.07 ± 0.00d | 0.16 ± 0.00c | 0.16 ± 0.01c | 0.66 ± 0.02a | green leafy, fresh, fatty |
| 1-Octen-3-ol | 3391-86-4 | 0.0015 | 1.58 ± 0.04a | 1.04 ± 0.02d | 1.11 ± 0.05c | 1.14 ± 0.01c | 1.32 ± 0.03b | mushroom, earthy |
| 1-Heptanol | 111-70-6 | 0.0054 | 0.06 ± 0.02c | 0.05 ± 0.00c | Nd | 0.08 ± 0.00b | 0.84 ± 0.02a | musty, violet, herbal, |
| Coriander heptenol | 1569-60-4 | 2 | Nd | nd | Nd | nd | 0.61 ± 0.03 | sweet |
| Linalool | 78-70-6 | 0.00022 | 1.65 ± 0.07d | 1.49 ± 0.12e | 3.34 ± 0.10b | 2.87 ± 0.03c | 3.86 ± 0.09a | orange, lemon, floral |
| 1-Octanol | 111-87-5 | 0.1258 | 0.10 ± 0.01c | 0.07 ± 0.04d | 0.14 ± 0.01b | 0.14 ± 0.00b | 0.32 ± 0.01a | green, citrus, orange |
| Terpinen-4-ol | 562-74-3 | 1.2 | Nd | nd | 0.13 ± 0.01 | nd | nd | pepper, woody, earth |
| alpha-Terpineol | 98-55-5 | 1.2 | 0.39 ± 0.04c | 0.32 ± 0.04d | 0.62 ± 0.05b | 0.62 ± 0.02b | 1.35 ± 0.02a | pine, woody, floral |
| Citronellol | 106-22-9 | 2.2 | 8.49 ± 0.60bc | 6.11 ± 0.61d | 8.10 ± 0.44c | 9.04 ± 0.03b | 9.62 ± 0.12a | floral, rose, sweet |
| L-Citronellol | 13066-51-8 | Nf | 0.44 ± 0.01a | 0.35 ± 0.05c | 0.39 ± 0.04bc | 0.40 ± 0.02b | 0.46 ± 0.01a | lemon, citrus |
| Nerol | 106-25-2 | 0.68 | 5.61 ± 0.08a | 0.91 ± 0.13e | 1.38 ± 0.11d | 1.61 ± 0.00c | 3.94 ± 0.09b | lemon, bitter, green |
| (Z)-3,7-Dimethyl-3,6-octadien-1-ol | 5944-20-7 | nf | 1.89 ± 0.17c | 1.12 ± 0.16d | 1.78 ± 0.15c | 2.09 ± 0.04b | 2.28 ± 0.08a | \ |
| Geraniol | 106-24-1 | 0.0066 | 5.04 ± 0.70b | 2.75 ± 0.38c | 4.47 ± 0.29b | 5.00 ± 0.00b | 5.88 ± 0.13a | floral, rosy, waxy |
| Carveol | 99-48-9 | 0.25b | nd | nd | Nd | 0.18 ± 0.03a | 0.06 ± 0.01b | minty, green, herbal |
| Benzyl alcohol | 100-51-6 | 2.546 | 0.18 ± 0.01 | 0.16 ± 0.02 | Nd | nd | nd | rose, floral, fruity |
| Phenylethyl alcohol | 60-12-8 | 0.56 | 0.51 ± 0.05a | 0.33 ± 0.04c | 0.41 ± 0.04b | 0.45 ± 0.03ab | 0.48 ± 0.03a | floral, sweet, rose |
| Isoamyl alcohol | 123-51-3 | 0.004 | nd | nd | Nd | nd | 0.41 ± 0.05 | fusel, fermented, fruity |
| 2-Nonanol | 628-99-9 | 0.058 | nd | nd | 0.13 ± 0.03b | nd | 0.37 ± 0.01a | waxy, soapy, musty |
| 2-Ethenyl-6-methyl-5-hepten-1-ol | 18479-48-6 | 2d | nd | nd | 0.54 ± 0.05a | 0.47 ± 0.05b | 0.57 ± 0.01a | fresh, grass, herbal |
| 2-Heptanol | 543-49-7 | 0.065 | nd | nd | Nd | 0.05 ± 0.00b | 0.28 ± 0.00a | lemon, grass, herbal |
| 2-Methyl-6-hepten-1-ol | 67133-86-2 | 2000f | nd | nd | 0.35 ± 0.02 | nd | nd | green, melon |
| Hexanal | 66-25-1 | 0.005 | 1.00 ± 0.11a | 0.60 ± 0.04b | Nd | nd | nd | woody, vegetative |
| 3-Methyl-2-butenal | 107-86-8 | nf | 0.35 ± 0.03d | 0.93 ± 0.02a | 0.58 ± 0.00b | 0.50 ± 0.01c | 0.30 ± 0.01e | sweet, fruity, green |
| (E)-2-Hexenal | 6728-26-3 | 0.398 | 2.55 ± 0.06a | 0.78 ± 0.01b | Nd | 0.24 ± 0.07c | nd | green, banana |
| Decanal | 112-31-2 | 0.003 | 0.11 ± 0.02c | 0.12 ± 0.04c | 0.31 ± 0.06a | 0.22 ± 0.03b | 0.16 ± 0.01c | waxy, fatty, citrus |
| (Z)-Citral | 106-26-3 | 0.053 | 0.28 ± 0.02a | 0.27 ± 0.03a | 0.07 ± 0.01c | 0.10 ± 0.00b | 0.05 ± 0.00c | lemon |
| (E)-Citral | 141-27-5 | 0.032 | 0.74 ± 0.03a | 0.37 ± 0.06b | Nd | nd | nd | citrus, lemon |
| Nonanal | 124-19-6 | 0.0011 | nd | 1.02 ± 0.04b | Nd | nd | 1.21 ± 0.08a | waxy, rose, orange peel |
| 1,3,5-Trioxane | 110-88-3 | nf | nd | nd | 0.27 ± 0.01a | nd | 0.08 ± 0.00b | \ |
| Octanal | 124-13-0 | 0.000587 | nd | 0.17 ± 0.00a | 0.10 ± 0.03b | nd | 0.06 ± 0.01c | aldehydic, waxy, citrus |
| 2,4-Dimethyl-benzaldehyde | 15764-16-6 | 29.37c | nd | nd | 0.14 ± 0.00a | nd | 0.11 ± 0.00b | cherry, almond |
| 1-Cyclohexyl-1-butanone | 1462-27-7 | nf | nd | nd | Nd | 0.28 ± 0.02b | 0.35 ± 0.01a | \ |
| 3-Octanone | 106-68-3 | 0.0214 | 0.44 ± 0.04a | 0.42 ± 0.00a | 0.29 ± 0.00c | 0.37 ± 0.00b | nd | herbal, sweet |
| Methyl heptenone | 110-93-0 | 0.068 | 0.24 ± 0.00c | 0.31 ± 0.02a | 0.28 ± 0.02b | nd | nd | green, vegetative, musty |
| 2-Heptanone | 110-43-0 | 0.14 | nd | nd | 1.03 ± 0.09a | 0.18 ± 0.12b | nd | fruity, cheese, coconut |
| 2-Nonanone | 821-55-6 | 0.041 | nd | nd | 1.02 ± 0.03a | 0.90 ± 0.02b | nd | green, cheesy, fruity |
| 2-Undecanone | 112-12-9 | 0.0055 | nd | nd | 0.12 ± 0.01a | 0.11 ± 0.01a | 0.04 ± 0.00b | waxy, fruity |
| (E)-Geranyl acetone | 3796-70-1 | 0.06 | nd | nd | 0.28 ± 0.09 | nd | nd | floral, green, fruity |
| 1,4-Dimethyl-4-acetyl-1-cyclohexene | 43219-68-7 | nf | nd | nd | Nd | 0.29 ± 0.18 | nd | fruity |
| 5-Methyl-3-heptanone | 541-85-5 | 0.041 | nd | nd | Nd | nd | 0.13 ± 0.00 | herbal, sweet |
| Myrcene | 123-35-3 | 0.0012 | 1.18 ± 0.18b | 2.45 ± 0.08a | 0.47 ± 0.10c | 0.30 ± 0.04d | 0.37 ± 0.06cd | woody, vegetative |
| D-Limonene | 5989-27-5 | 0.034 | 2.99 ± 0.03c | 3.52 ± 0.08b | Nd | 0.17 ± 0.12d | 0.44 ± 0.00a | citrus, sweet |
| (E)-beta-Ocimene | 3779-61-1 | 0.034 | nd | nd | Nd | nd | 0.23 ± 0.06 | sweet herbal |
| beta-Ocimene | 13877-91-3 | nf | nd | 0.36 ± 0.08 | Nd | nd | 0.41 ± 0.01 | citrus, tropical, woody |
| o-Cymene | 527-84-4 | 11.4e | 0.52 ± 0.01a | nd | Nd | 0.12 ± 0.01c | 0.14 ± 0.00b | aromatic |
| (+)-4-carene | 29050-33-7 | nf | 2.72 ± 0.16b | 3.72 ± 0.08a | Nd | 0.21 ± 0.05c | 0.22 ± 0.01c | \ |
| beta-Copaene | 18252-44-3 | 34 | 0.36 ± 0.00a | 0.17 ± 0.01b | Nd | nd | nd | \ |
| (Z)-beta-Ocimene | 3338-55-4 | 0.034 | 0.33 ± 0.06a | nd | 0.22 ± 0.04b | nd | nd | floral, herb |
| alpha-Himachalene | 3853-83-6 | nf | nd | 0.23 ± 0.01 | Nd | nd | nd | \ |
| γ-Muurolene | 30021-74-0 | nf | 0.22 ± 0.05 | 0.27 ± 0.01 | Nd | nd | nd | herbal, woody, spice |
| 1H-3a,7-methanoazulene, 2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl | 469-61-4 | nf | nd | 0.25 ± 0.09 | Nd | nd | nd | woody, cedar, sweet |
| alpha-Curcumene | 644-30-4 | nf | nd | 0.17 ± 0.00 | Nd | nd | nd | herbal |
| 1,3,8-p-Menthatriene | 18368-95-1 | 0.015 | 0.13 ± 0.02 | nd | Nd | nd | nd | herbal, woody, oily |
| (Z)-Rose oxide | 16409-43-1 | 0.0002a | 0.85 ± 0.03c | 1.41 ± 0.05b | 8.50 ± 0.17a | 8.50 ± 1.01a | nd | rose, green |
| 2-Ethoxyethyl-benzene | 3558-60-9 | nf | 0.20 ± 0.03d | 0.22 ± 0.01cd | 0.24 ± 0.01bc | 0.28 ± 0.02a | 0.25 ± 0.01b | floral |
| trans-Rose oxide | 876-18-6 | nf | nd | 0.71 ± 0.03 | Nd | nd | nd | rose, green |
| Methoxymethyl-benzene | 538-86-3 | nf | nd | nd | Nd | 0.47 ± 0.02 | nd | fruity |
| Geranyl methyl ether | 2565-82-4 | nf | nd | 0.26 ± 0.31 | Nd | nd | nd | rose, sweet |
| 3,6-Dihydro-4-methyl-2-(2-methyl-1-propenyl)-2H-pyran | 1786-08-9 | nf | nd | nd | 1.37 ± 0.05 | 1.42 ± 0.31 | nd | \ |
| Ethyl 3-methyl-2-butenoate | 638-10-8 | 0.025 | 0.31 ± 0.04a | 0.24 ± 0.01b | 0.07 ± 0.00c | nd | nd | \ |
| Perillyl acetate | 15111-96-3 | nf | 0.89 ± 0.07bc | 0.58 ± 0.10d | 0.80 ± 0.08c | 0.94 ± 0.01b | 1.17 ± 0.04a | fruity, phenolic, green |
| 2,4-Di-tert-butylphenol | 96-76-4 | 0.5 | nd | nd | 0.42 ± 0.05b | 0.62 ± 0.06a | 0.58 ± 0.06a | phenolic |
| Acetic acid | 64-19-7 | 99 | nd | nd | 0.37 ± 0.07 | nd | 0.76 ± 0.03 | sour vinegar |
| alpha-Methoxy-benzeneacetic acid | 7021-9-2 | nf | nd | 0.12 ± 0.01 | Nd | nd | 0.08 ± 0.00 | \ |
Odor description found in the “The Good Scents Company Information System (http://www.thegoodscentscompany.com./)”
All odor thresholds were obtained from: Odor & Flavour Detection Thresholds in Water (http://www.leffingwell.com) and the book “compilations of flavor threshold values in water and other media (second enlarged and revised edition)”.
Identification method. MS, identification based on the NIST 2017 mass spectral database (https://chemdata.nist.gov); RI, retention index, which was calculated refereed to the retention time of C7–C30 n-alkanes under the same conditions.
“\”, no odor description information was found in the literature.
‘nf’, data was not found in the literature.
‘nd’, not detect.
Different superscripted letters in the same row of each compound indicated a significant difference.
(Guth, 1997).
Fig. 4.
Comparison of volatile compounds in the original, pasteurized and LAB fermented litchi juices by HS-SPME-GC-MS. (A) Numbers of volatile compounds types in the litchi juices. (B) PLS-DA score plot of volatile compounds. (C) VIP scores in PLS-DA. (D) Heat map clustering of volatile compounds. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
Hierarchical cluster analysis intuitively illustrated the effect of LAB fermentation on the flavor of F–P. All the samples were divided into 3 categories in Fig. 4B. The first cluster was presented by F and F–P, with a higher proportion in terpenoids (i.e., alpha-cedrene, alpha-himachalene, D-limonene, alpha-curcumene, myrcene, (Z)-beta-ocimene and o-cymene), aldehydes (i.e., 3-methyl-2-butenal, octanal, (E)-citral, hexanal and (E)-2-hexenal) and alcohols (i.e., 3-methyl-1-butanol, nerol and 1-octen-3-ol). F–C2W is classified into the second cluster, including abundant alcohols (i.e., coriander heptenol, isoamyl alcohol, 1-heptanol, 2-heptanol, 1-octanol, alpha-terpineol, 3-octanol and (E)-2-hexen-1-ol). The third cluster was described by the LP28 and LP226 fermented litchi juices, with a richness in ketones (i.e., methyl heptanone, (E)-geranyl acetone, 2-heptanone and 1,4-dimethyl-4-acetyl-1-cyclohexene) and alcohols (i.e., 2-nonanol, 2-methyl-6-hepten-1-ol, terpinen-4-ol and carveol). As shown in the PLS-DA analysis (Fig. 4C), the original, pasteurized and fermented litchi juices were clearly distinguished according to the first two principal components, accounting for about 90% cumulative contribution rate. The results of multivariate statistics among the different flavor analysis methods consistently showed that pasteurization had a greater effect on litchi juice flavor, while LAB fermentation reshaped the flavor of litchi juice. The variable importance for the projection (VIP) score of volatile compounds evaluating their differentiation contribution was shown in Fig. 4D. A total of 11 volatile compound variables with VIP score greater than 1 demonstrated their potential as markers to determine the significance of the samples. Among these 11 volatile compounds, there were four alcohols (nerol, citronellol, geraniol and (Z)-3,7-dimethyl-3,6-octadien-1-ol), three terpenoids (myrcene, (+)-4-carene and D-limonene), two aldehydes ((E)-2-hexenal and nonanal), one ester (5-methylene-6-isopropenyl-3-cyclohexen-1-ol acetate) and one ether (trans-rose oxide). These volatile compounds could be used to differentiate original, pasteurized and LAB fermented litchi juice, and would show practical applications in product quality control.
As shown in Table 1, alcohols dominate the flavor profile of the original litchi juice, and citronellol was considered to be the major alcohol endowing floral, rose and sweet odors (Z. Wang et al., 2022a). It should be noted that geraniol and nerol were the other two dominant alcohols in the F, endowing floral, lemon and green odors (Guo et al., 2021). However, the alcohol contents of F–P decreased by 28.51%; especially nerol was not detected. We speculate that the alcohols are very sensitive to high temperatures, resulting in a significant loss during thermal processing. This may be one of the main reasons affecting the overall flavor of litchi juice. Similar phenomena were observed for sterilized watermelon juice, in which the contents of some flavor substances were significantly decreased upon heat sterilization, whereas some undesirable flavor compounds such as ethanol, 1-octen-3-ol, and benzyl alcohol were newly present in the final products (Yang et al., 2021). In addition, the geraniol content of litchi juice increased by 38.56%, 45.01% and 53.25% after fermentation with LP28, LP226 and LPC2W, while citronellol increased from 6.11 μg/mL to 8.10 μg/mL, 9.04 μg/mL and 9.62 μg/mL respectively. It was shown that the combination of geraniol, linalool and nerol enhanced the floral aroma in litchi in the flavor chemistry evaluation (An et al., 2019; D. Chen and Liu, 2016). Moreover, new alcohols were produced in fermented litchi juice, including coriander heptenol, terpinen-4-ol, carveol, isoamyl alcohol, 2-nonanol and 2-methyl-6-hepten-1-ol. Briefly, the intensity of alcohols in fermented litchi juices was notably increased, especially for F–C2W increasing by 51.01%. This may be attributed to the synthesis by glucose and branched-chain amino acid metabolism pathways in the presence of different LAB (C. Chen et al., 2019).
Terpenoids are a more abundant class of compounds in the original litchi juice, mainly D-limonene, (+)-4-carene, myrcene, o-cymene and (Z)-beta-ocimene, giving litchi juice a citrus, herbal and sweet aroma. However, the contents of terpenoids showed different changes after fermentation by different LAB. Few terpenoids remained in the F-28, while the F–C2W maintained the abundance of terpenoids. Myrcene and D-limonene possess lower thresholds (0.0012 and 0.034), suggesting that they might play remarkable roles in the flavor profile of litchi juices, which was in agreement with a previous study (An et al., 2019). Except for D-limonene, which was not detected after LP28 fermentation, all other strains were able to retain them to some extent after fermentation.
As for the aldehydes, a total of six compounds (i.e., (E)-2-hexenal, hexanal, (E)-citral, 3-methyl-2-butenal, (Z)-citral and decanal) were detected in the original litchi juice. (E)-2-hexenal and hexanal are the prominent aldehydes in litchi juices and studies have shown that they are also the more abundant aldehydes in many other natural fruits (Sevindik et al., 2022; Z. Wang et al., 2022b; Y. Zhao et al., 2021). Hexanal, imparting green, woody and vegetative notes, was significantly reduced after pasteurization and was not detected in any of the fermentation litchi juices. The large loss of aldehydes in pasteurized litchi juice also indicates that aldehydes are extremely instability (Di Cagno et al., 2017). The higher levels of aldehydes in apple juice have been shown to have a negative effect on flavors (C. Chen et al., 2019). The effect of fermentation by indigenous LAB on the flavors in Baijiu has also been investigated and the results show that LAB fermentation significantly reduced the content of aldehydes in the samples (Pang et al., 2021).
Regarding the ketones, 3-octanone and methyl heptanone, endowing herbal, sweet and green odor, were detected in the F and F–P. Ketones play a crucial role in food flavors, due to their low threshold, it can present strong aromas even at very low concentrations (C. Chen et al., 2019). Thermal treatment had no significant effect on the type and content of ketones in litchi juice, but after LAB fermentation, several new ketones appeared, such as 2-heptanone, 2-nonanone, 2-undecanone, (E)-geranyl acetone, 1,4-dimethyl-4-acetyl-1-cyclohexene and 5-methyl-3-heptanone. 2-nonanone with cheesy and fruity odor was increased in the F-28 and F-226. Higher contents of 2-heptanone, (E)-geranyl acetone and 2-undecanone were also presented in fermented litchi juices, giving fruity and cheesy notes. In addition, the different LAB strains had a greater effect on the aldehyde content of litchi juice. Aldehydes were detected significantly higher in F-28 and F-226 than in F–C2W, with only 5-methyl-3-heptanone being detected in F–C2W.
Total ethers content was higher in LAB fermentation juices than in the original juice sample. The major ethers in the F were (Z)-rose oxide, imparting rose, green and floral odor. It is worth noting that the ethers increased to 10.10 mg/L, 10.66 mg/L and 8.33 mg/L in F-28, F-226 and F–C2W, respectively. (Z)-rose oxide, which accounts for more than 90% of the ethers, is considered the key flavor compound in litchi juice. The (Z)-rose oxide is produced by the acid or enzymatic hydrolysis of its odorless precursor glycoconjugate or glycoside, which is also associated with litchi-like odor in previous reports (Tang et al., 2019).
As for the esters, only two compounds (i.e., ethyl 3-methylbut-2-enoate and perillyl acetate) were detected. We inferred that esters providing floral and fruity notes are mainly produced by the esterification of alcohols (Selli, 2007). Meanwhile, a significant increase of esters was observed in the fermented litchi juice of F-226. In addition, the fermented litchi juice produces three others.
3.4. OAVs analysis
The odor activity value (OAV) was used to assess the contribution of volatile compounds to the overall aroma of litchi juices. Generally, the OAV≥1 is considered to be a key flavor compound. (M. Q. Wang et al., 2020). As shown in Fig. 5, there were 24, 22, 23, 20 and 25 volatile components with OAVs higher than 1 in the F, F–P, F-28, F-226 and F–C2W, respectively (Fig. 5A and B). The overlapping parts of the Wenn diagram, which contained 20 volatile compounds, are the volatile compounds shared by the F and the F–P, determining the basic flavour of the litchi juice. Two new aroma-active compounds, nonanal and octanal, were produced after pasteurization, giving the litchi juice a waxy and chemical aroma. Aldehydes have been shown to exert a negative influence in flavor studies in a variety of matrices. Meanwhiles, the waxy and chemical odors are possibly consistent with their emerging flavor with partial of cooking odor in the factory after the litchi juice was pasteurized. Fig. 5B shows that eleven active aroma compounds of fermented litchi juices were the same compared to pasteurized litchi juice, which indicates that most of the aroma characteristics of litchi juice were retained after LAB fermentation. Some alcohols (i.e., 1-octanol, hexanol, 2-nonanol, nerol, ɑ-terpineol, isoamyl alcohol and 2-heptanol) and aldehydes (i.e., 2-heptanone, 2-nonanone, 2-undecanone, (E)-geranyl acetone and 5-methyl-3-heptanone) were mainly produced to enrich the flavor diversity of LAB fermented litchi juice.
Fig. 5.
The Venn diagram and the common aroma-active compounds with >1.0 OAVs in the litchi juices and fermented litchi juices. (A): The Venn diagram of aroma-active compounds in the original litchi juice and pasteurized litchi juice; (B): The Venn diagram of aroma-active compounds in the pasteurized litchi juice and fermented litchi juices; (C): The common aroma-active compounds with >1.0 OAVs in all litchi juice samples. Note: Compound name, scent description, and OAVs were listed in detail. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
Volatiles with similar odor descriptions was grouped to establish the characteristic aroma properties of litchi juice (Guo et al., 2021). The aroma properties of litchi juice were classified into six categories, including fruity, citrus-like, floral, sweet, green and waxy (Fig. 5C). There is a consensus that a higher OAV value indicates a greater contribution to the overall flavor of the sample. As can be seen from Fig. 5C, there are seven compounds in the F with OAV values greater than 50 (i.e., linalool, D-limonene, geraniol, (Z)-rose oxide, hexanal, 1-octen-3-ol and myrcene), indicating that floral and citrus-like are the main characteristic flavor substances of litchi juices. They were all found in litchi in a previous study (An et al., 2019; L. Zhao et al., 2021). The compounds that increase the OAVs value of pasteurized litchi juice are 3-methyl-1-butanol, D-limonene, methy heptanone, octanal, nonanal and myrcene, mainly imparting waxy and mushroom odor. Furthermore, LAB fermented litchi juice produces a variety of substances with fruit flavor, such as isoamyl alcohol, 2-heptanone, 2-nonanone, 3-undecanone and (E)-geranyl acetone, while retaining the sweet and green aroma belonging to the F.
Based on the comprehensive analysis results of volatile compositions and odor profiles, pasteurization leads, to some extent, to the formation of negative flavor compounds. Moreover, different LAB fermentation will not only retain the original characteristic flavors of litchi juice, but also give it strong fruity and citrus-like aromas.
3.5. Sensory evaluation of litchi juices
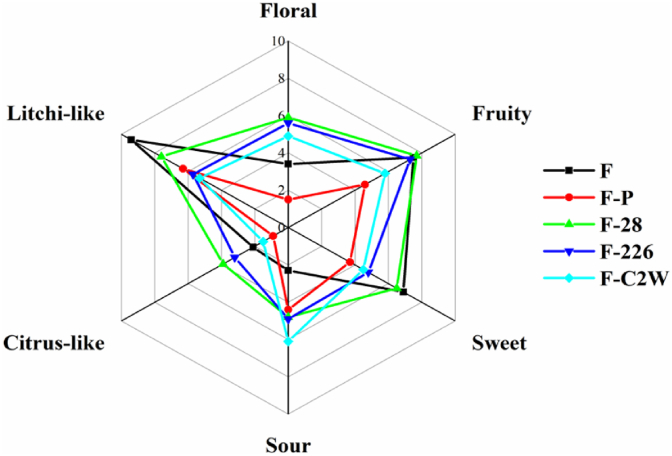
As can be seen from Fig. 6, the attributes of litchi-like, fruity and sweet were strongly perceived in the original and LP28-fermented litchi juices, whereas, the latter two fall sharply in pasteurized litchi juice. The five samples also showed significant differences in citrus aroma attributes, with their aroma strengths ranging from F-28, F-226, F, F–C2W and F–P in descending order. In terms of sourness, litchi juice is significantly increased by LAB fermentation and our previous studies have confirmed that litchi juice fermented by LPC2W effectively degrades its sugar and enhances its total acid content (D. Wang et al., 2021), which promotes the production of acidic flavors. Compared to the original litchi juice flavour, the fermentation samples effectively enrich the aroma profile giving a strong floral odor. This might be due to the high concentrations of geraniol, (Z)-Rose oxide or linalool. In addition, these results exhibited that LAB fermentation might partially re-initiate the transformation of flavour compounds that were altered by thermal sterilization. Moreover, different LAB fermentation led to obvious differences in the aroma of Feizixiao litchi juice. Although the typical flavor profiles in litchi juice were derived from sensory evaluation analysis by standardized protocols (Yu et al., 2021), aroma recombination and omission tests are to be conducted in the future to further demonstrate the characteristic flavor compounds in LAB fermented litchi juice.
Fig. 6.
The radar profile of sensory evaluation of the samples. (F: original litchi juices, F–P: pasteurized litchi juices, F–C2W: F–P fermented by Lactobacillus plantarum C2W, F-226: F–P fermented by Lactobacillus plantarum 226, F-28: F–P fermented by Lactobacillus plantarum 28).
4. Conclusions
The impact of different LP fermentation on the aroma profiles of pasteurized litchi juices was analyzed by E-nose, GC-IMS, GC-MS, OAV and sensory evaluation. The unavoidable thermal sterilization process in industrial production could lead to a serious loss of aroma compounds of litchi juice, especially some alcohols that resemble the floral and green aromas. However, the litchi juices fermented by LAB had more affluent and harmonious aroma characteristics, and those volatile substances that were degraded in pasteurization are reshaped by LAB fermentation, which included prenol, citronellol, geraniol, phenylethyl alcohol and L-citronellol. Furthermore, LAB fermentation plays a positive impact on the aroma profiles, and facilitated the formation of alcohols and ethers which commonly account for the biotransformation of glycogen and the metabolism of amino acids by LAB strains. These findings provide new ideas to alleviate the pressure on the litchi processing industry and enrich the diversity of novel processed litchi products.
CRediT authorship contribution statement
Dongwei Wang: Methodology, Investigation, Formal analysis, Writing – original draft. Yani Deng: Resources, Software. Xiao Chen: Software, Resources, Writing – review & editing. Kai Wang: Formal analysis, Data curation, Software. Lei Zhao: Formal analysis, Conceptualization. Zichen Wang: Data curation, Validation, Formal analysis. Xuwei Liu: Formal analysis, Methodology, Supervision. Zhuoyan Hu: Conceptualization, Methodology, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was financially supported by the Science and Technology Planning Project of Guangzhou City of China (202103000054) and the National Natural Science Foundation of China (32202022).
Handling Editor: Dr. Maria Corradini
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100481.
Contributor Information
Xuwei Liu, Email: xuwei.liu@scau.edu.cn.
Zhuoyan Hu, Email: zyhu@scau.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- An K., Liu H., Fu M., Qian M.C., Yu Y., Wu J., Xiao G., Xu Y. Identification of the cooked off-flavor in heat-sterilized lychee (Litchi chinensis Sonn.) juice by means of molecular sensory science. Food Chem. 2019;301 doi: 10.1016/j.foodchem.2019.125282. [DOI] [PubMed] [Google Scholar]
- Buttery R.G., Teranishi R., Ling L.C., Turnbaugh J.G. 1990. Tomato Paste Volatiles; pp. 336–340. [Google Scholar]
- Cai W., Tang F., Zhao X., Guo Z., Zhang Z., Dong Y., Shan C. Different lactic acid bacteria strains affecting the flavor profile of fermented jujube juice. J. Food Process. Preserv. 2019;43(9) doi: 10.1111/jfpp.14095. [DOI] [Google Scholar]
- Chen C., Lu Y., Yu H., Chen Z., Tian H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019;27:30–36. doi: 10.1016/j.fbio.2018.11.006. [DOI] [Google Scholar]
- Chen D., Liu S.Q. Transformation of chemical constituents of lychee wine by simultaneous alcoholic and malolactic fermentations. Food Chem. 2016;196:988–995. doi: 10.1016/j.foodchem.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Di Cagno R., Filannino P., Gobbetti M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017;248:56–62. doi: 10.1016/j.ijfoodmicro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Gu Z., Jin Z., Schwarz P., Rao J., Chen B. Uncovering aroma boundary compositions of barley malts by untargeted and targeted flavoromics with HS-SPME-GC-MS/olfactometry. Food Chem. 2022;394 doi: 10.1016/j.foodchem.2022.133541. [DOI] [PubMed] [Google Scholar]
- Guo X., Ho C.T., Wan X., Zhu H., Liu Q., Wen Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021;341 doi: 10.1016/j.foodchem.2020.128230. [DOI] [PubMed] [Google Scholar]
- Guo X., Schwab W., Ho C.T., Song C., Wan X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022;376 doi: 10.1016/j.foodchem.2021.131933. [DOI] [PubMed] [Google Scholar]
- Guth H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997;45(8):3027–3032. doi: 10.1021/jf970280a. [DOI] [Google Scholar]
- Li C., Chen X., Jin Z., Gu Z., Rao J., Chen B. Physicochemical property changes and aroma differences of fermented yellow pea flours: role of: lactobacilli and fermentation time. Food Funct. 2021;12(15):6950–6963. doi: 10.1039/d1fo00608h. [DOI] [PubMed] [Google Scholar]
- Liu X., Le Bourvellec C., Yu J., Zhao L., Wang K., Tao Y., Renard C.M.G.C., Hu Z. Trends and challenges on fruit and vegetable processing: insights into sustainable, traceable, precise, healthy, intelligent, personalized and local innovative food products. Trends Food Sci. Technol. 2022;125:12–25. doi: 10.1016/j.tifs.2022.04.016. [DOI] [Google Scholar]
- Lynch K.M., Zannini E., Coffey A., Arendt E.K. Lactic acid bacteria exopolysaccharides in foods and beverages: isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018;9:155–176. doi: 10.1146/annurev-food-030117-012537. [DOI] [PubMed] [Google Scholar]
- Mishra V., Shah C., Mokashe N., Chavan R., Yadav H., Prajapati J. Probiotics as potential antioxidants: a systematic review. J. Agric. Food Chem. 2015;63(14):3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- Ni R., Wang P., Zhan P., Tian H., Li T. Effects of different frying temperatures on the aroma profiles of fried mountain pepper (Litsea cubeba (Lour.) Pers.) oils and characterization of their key odorants. Food Chem. 2021;357 doi: 10.1016/j.foodchem.2021.129786. [DOI] [PubMed] [Google Scholar]
- Pang X.N., Chen C., Huang X.N., Yan Y.Z., Chen J.Y., Han B.Z. Influence of indigenous lactic acid bacteria on the volatile flavor profile of light-flavor Baijiu. LWT (Lebensm.-Wiss. & Technol.) 2021;147 doi: 10.1016/j.lwt.2021.111540. [DOI] [Google Scholar]
- Reichel M., Carle R., Sruamsiri P., Neidhart S. Changes in flavonoids and nonphenolic pigments during on-tree maturation and postharvest pericarp browning of litchi (Litchi chinensis Sonn.) as shown by HPLC-MSn. J. Agric. Food Chem. 2011;59(8):3924–3939. doi: 10.1021/jf104432r. [DOI] [PubMed] [Google Scholar]
- Ricci A., Cirlini M., Levante A., Dall'Asta C., Galaverna G., Lazzi C. Volatile profile of elderberry juice: effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018;105:412–422. doi: 10.1016/j.foodres.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Selli S. Volatile constituents of orange wine obtained from moro oranges (citrus sinensis [L.] osbeck) J. Food Qual. 2007;30(3):330–341. doi: 10.1111/j.1745-4557.2007.00124.x. [DOI] [Google Scholar]
- Sevindik O., Guclu G., Agirman B., Selli S., Kadiroglu P., Bordiga M., Capanoglu E., Kelebek H. Impacts of selected lactic acid bacteria strains on the aroma and bioactive compositions of fermented gilaburu (Viburnum opulus) juices. Food Chem. 2022;378 doi: 10.1016/j.foodchem.2022.132079. [DOI] [PubMed] [Google Scholar]
- Sherman E., Coe M., Grose C., Martin D., Greenwood D.R. Metabolomics approach to assess the relative contributions of the volatile and non-volatile composition to expert quality ratings of pinot noir wine quality. J. Agric. Food Chem. 2020;68(47):13380–13396. doi: 10.1021/acs.jafc.0c04095. [DOI] [PubMed] [Google Scholar]
- Su D., Zhang R., Zhang C., Huang F., Xiao J., Deng Y., Wei Z., Zhang Y., Chi J., Zhang M. Phenolic-rich lychee (Litchi chinensis Sonn.) pulp extracts offer hepatoprotection against restraint stress-induced liver injury in mice by modulating mitochondrial dysfunction. Food Funct. 2016;7(1):508–515. doi: 10.1039/c5fo00975h. [DOI] [PubMed] [Google Scholar]
- Tang Z.S., Zeng X.A., Brennan M.A., Han Z., Niu D., Huo Y. Characterization of aroma profile and characteristic aromas during lychee wine fermentation. J. Food Process. Preserv. 2019;43(8) doi: 10.1111/jfpp.14003. [DOI] [Google Scholar]
- Teribia N., Buvé C., Bonerz D., Aschoff J., Hendrickx M., Loey A. Van. Effect of cultivar, pasteurization and storage on the volatile and taste compounds of strawberry puree. LWT (Lebensm.-Wiss. & Technol.) 2021;150 doi: 10.1016/j.lwt.2021.112007. [DOI] [Google Scholar]
- Wang D., Wang Y., Lan H., Wang K., Zhao L., Hu Z. Enhanced production of γ-aminobutyric acid in litchi juice fermented by Lactobacillus plantarum HU-C2W. Food Biosci. 2021;42 doi: 10.1016/j.fbio.2021.101155. [DOI] [Google Scholar]
- Wang G., Jing S., Song X., Zhu L., Zheng F., Sun B. Reconstitution of the flavor signature of laobaigan-type Baijiu based on the natural concentrations of its odor-active compounds and nonvolatile organic acids. J. Agric. Food Chem. 2022;70(3):837–846. doi: 10.1021/acs.jafc.1c06791. [DOI] [PubMed] [Google Scholar]
- Wang J., Shi J., Zhu Y., Ma W., Yan H., Shao C., Wang M., Zhang Y., Peng Q., Chen Y., Lin Z. Insights into crucial odourants dominating the characteristic flavour of citrus-white teas prepared from citrus reticulata Blanco ‘Chachiensis’ and Camellia sinensis ‘Fudingdabai. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2022.132048. [DOI] [PubMed] [Google Scholar]
- Wang M.Q., Ma W.J., Shi J., Zhu Y., Lin Z., Lv H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108908. [DOI] [PubMed] [Google Scholar]
- Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS) Food Chem. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Wang Z., Feng Y., Yang N., Jiang T., Xu H., Lei H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131455. [DOI] [PubMed] [Google Scholar]
- Wang Z., Feng Y., Yang N., Jiang T., Xu H., Lei H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131455. [DOI] [PubMed] [Google Scholar]
- Xie Z., Xia S., Le G.W. Gamma-aminobutyric acid improves oxidative stress and function of the thyroid in high-fat diet fed mice. J. Funct.Foods. 2014;8(1):76–86. doi: 10.1016/j.jff.2014.03.003. [DOI] [Google Scholar]
- Yang F., Liu Y., Wang B., Song H., Zou T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT (Lebensm.-Wiss. & Technol.) 2021;137(11) doi: 10.1016/j.lwt.2020.110478. [DOI] [Google Scholar]
- Yu H., Guo W., Xie T., Ai L., Tian H., Chen C. Aroma characteristics of traditional Huangjiu produced around Winter Solstice revealed by sensory evaluation, gas chromatography–mass spectrometry and gas chromatography–ion mobility spectrometry. Food Res. Int. 2021;145 doi: 10.1016/j.foodres.2021.110421. [DOI] [PubMed] [Google Scholar]
- Zhang W., Lao F., Bi S., Pan X., Pang X., Hu X., Liao X., Wu J. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021;336(17) doi: 10.1016/j.foodchem.2020.127721. [DOI] [PubMed] [Google Scholar]
- Zhao L., Ruan S., Yang X., Chen Q., Shi K., Lu K., He L., Liu S., Song Y. Characterization of volatile aroma compounds in litchi (Heiye) wine and distilled spirit. Food Sci. Nutr. 2021;9(11):5914–5927. doi: 10.1002/fsn3.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang K., Wang K., Zhu J., Hu Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): a review. Compr. Rev. Food Sci. Food Saf. 2020;19(4):2139–2163. doi: 10.1111/1541-4337.12590. [DOI] [PubMed] [Google Scholar]
- Zhu D., Zhang Y., Kou C., Xi P., Liu H. Ultrasonic and other sterilization methods on nutrition and flavor of cloudy apple juice. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.