Abstract
Background and Aim:
Pasteurella multocida is considered as a main factor mediating pneumonic pasteurellosis in ruminants, including sheep. It is also a current threat to Phan Rang sheep in Vietnam. This study aimed to characterize P. multocida isolated from Phan Rang sheep, their antibiotic resistance profile, and the prevalence of some virulence-associated genes of these strains.
Materials and Methods:
Bacteria were isolated on brain heart infusion, 10% sheep blood agar plates, and screened by biochemical tests. The polymerase chain reaction technique was used with specific primers to identify P. multocida, the presence of virulence-associated genes, and serotypes of isolates. Antimicrobial susceptibility and biofilm formation of isolates were examined using the disk diffusion method and crystal violet-based method, respectively.
Results:
A total of 41 P. multocida strains were isolated from 485 samples from clinically sick and healthy sheep. Of the isolates, 58.53% were serotype A, 9.75% were serotype B, and 31.71% were serotype D. Healthy animals were infected with serotype D only. All 15 virulence genes were identified in all strains isolated from clinically sick sheep, while strains isolated from healthy sheep carried 11/15 virulence genes tested. Among virulence-associated genes exbB, exbD, tonB, ompA, oma87, fimA, hgbA, and nanB were detected in over 90% of isolates, whereas hgbB, nanH, tbpA and pfhA were less frequent. Interestingly, pmHAS and tadD were highly prevalent in capsular type A strains, whereas the toxA gene was detected in capsular type D strains only. All of the isolated strains were fully susceptible to enrofloxacin, ciprofloxacin, neomycin, and ofloxacin. About 92.68% were susceptible to chloramphenicol and 90.24% to amikacin, but there was high resistance to erythromycin, tetracycline, and amoxicillin. Our results reveal that 53.65% of 41 isolates could produce biofilm, whereas 46.34% could not.
Conclusion:
Pasteurella multocida from Phan Rang sheep possess many virulence genes and resistance to several common antibiotics such as erythromycin, tetracycline, and amoxicillin. The results are an important warning regarding antibiotic resistance of P. multocida.
Keywords: antimicrobial resistance, biofilm formation, capsular type, Pasteurella multocida, Phan Rang sheep
Introduction
Vietnam has thermotolerant sheep that have been reared in Ninh Thuan province for over a century, named Phan Rang sheep [1]. The sheep are well adapted to tropical weather, although their genetic origin has not been identified yet. The sheep were also experimentally reared in different regions of Vietnam other than Ninh Thuan and were considered a local candidate for adapting to global warming. However, research on this breed is still limited, especially in disease-related data. Pasteurella multocida is an important pathogen that causes pasteurellosis, one of the most frequent and dangerous diseases in sheep rearing worldwide [2–4]. In Vietnam, P. multocida, from avians and pigs, has been isolated and characterized [5–7], but no related information has been reported in ruminants, particularly in Phan Rang sheep.
Pasteurella multocida, in normal conditions, can live quietly in the respiratory tract of healthy animals without causing disease-related symptoms [8]. However, the bacteria become threatening in stress conditions, such as extreme weather, water shortages, or cramped barns, which are proposed to compromise the immune system of animals [9]. At least 5 serotypes of P. multocida have been reported based on cellar capsules, including A, B, C, D, E, and F [10, 11]. In sheep, serotype A, particularly sub-serotype A1 and A3, is more predominant than D or B serotypes [11, 12]. Besides cellular capsules, some virulence factors required for in vivo reproduction and disease development (lipopolysaccharide) or cellular survival (iron-regulated or iron-acquisition proteins) have been identified in P. multocida as well as other virulence-associated factors such as toxins, fimbriae, adhesins, sialic acid metabolism, hyaluronidase, or outer membrane proteins [13–15].
Antimicrobial agents are widely used to control and cure pasteurellosis disease in poultry and livestock [16]. However, their use has to be carefully considered since correct dosage and usage are commonly not followed, particularly in developing countries leading to the onset of antibiotic resistance, a global problem in animal husbandry and human health [17–19]. To date, antibiotic-resistant P. multocida has been reported in different food-producing animals, in particular, a high prevalence of antibiotic resistance was observed in pigs [5, 18], in chickens [20], and small ruminants like sheep [21, 22]. Hence, host origin may be a critical factor in the evolution of antibiotic resistance in P. multocida.
Therefore, this study aimed to investigate, for the first time, serotypes of P. multocida isolated from Phan Rang sheep and their antibiotic resistance profile, as well as to estimate the prevalence of some virulence factors in these strains.
Materials and Methods
Ethical approval
Ethical approval from the Institute of Biotechnology, Hue University, to conduct this study was not required. However, the sample collection was conducted as per the standard sample collection procedure without any harm to animals.
Study period and location
The study was conducted from April 2021 to October 2022 at the Institute of Biotechnology, Hue University, Vietnam.
Sample collection and bacterial isolation
Samples were collected from Bac Ai (11°42’06.2”N 108°54’38.0”E), Ninh Phuoc (11°30’09.1”N 108°52’02.1”E), Ninh Hai (11°36’09.6”N 109°07’36.9”E), and Thuan Bac (11°40’53.5”N 109°05’13.8”E) of Ninh Thuan, Province, Vietnam. This province is the site of the greatest number of live sheep and the greatest sheep production in Vietnam. A total of 485 Nasal swab samples were collected from 185 healthy sheep and 300 clinically sick sheep (coughing, breathing discomfort, and nasal discharge). The swab sample was transported ina cool box (4°C) to the Laboratory of the Cell, Institute of Biotechnology, Hue University for further processing.
For preliminary isolation, all samples were plated on brain heart infusion (BHI) (HiMedia, India), 10% sheep blood agar plates and incubated at 37°C for 48 h. After that, bacteria cells from suspected colonies were examined by testing their ability to grow on Macconkey agar (HiMedia), gram’s staining, and microscopy at 100× magnification. The strains that were unable to grow on MacConkey agar and that were Gram-negative were further characterized by biochemical tests using Microgen™ GnA + B-ID System (Microgen, UK) to identify P. multocida.
Polymerase chain reaction (PCR) detection of P. multocida
Cells from a single colony on a blood agar plate were inoculated in BHI broth at 37°C for 12 h in a shaking incubator. Bacterial culture was then collected by centrifugation at 18000× g for 1 min. Genomic DNA was isolated using the DNA isolation kit (Qiagen, Germany) according to the manufacturer’s instructions. A specific PCR assay was carried out to confirm the presumptive isolates of P. multocida by amplifying a fragment of the kmt1 gene as described by Townsend et al. [23] using specific primers as shown in Table-1 [23–25]. The PCR reaction was performed in PCR thermal cyclers (PTC200, MJ, USA) using PCR standard master mix (Canvax Biotech, Spain) in a total volume of 25 μL containing 0.5 µL of each primer (10 pM) and 1 μL of template DNA. The PCR conditions consisted of an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 1 min, 1 min for primer annealing at 52°C extension at 68°C for 1 min, and a final extension at 68°C of 5 min. About 5 μL of PCR products were electrophoresed on a 1.5% agarose gel with safe red (Intron, Korea) for visualization under the MUPID® One LED Illuminator (Nippon genetics, EU). DNA of P . multocida strains was used as a positive control, while PCR water was used as a negative control, as published by Vu-Khac et al. [5].
Table-1.
Primers used for the identification of P. multocida isolates and for the detection of capsular types and virulence-associated genes in P. multocida.
| Target gene | Description | Sequence (5′ – 3′) | Annealing temp (°C) | size (bp) | References |
|---|---|---|---|---|---|
| kmt1 | Detection of all P. multocida | CCTCCGACTAACACCCAAGT TGGGCTTGTCGGTAGTCTTT | 56 | 633 | This study |
| hyaD-hyaC | Serogroup A cap gene | TGCCAAAATCGCAGTGAG TTGCCATCATTGTCAGTG | 50 | 1044 | [24] |
| bcbD | Serogroup B cap gene | CATTTATCCAAGCTCCACC GCCCGAGAGTTTCAATCC | 51 | 760 | [24] |
| dcbF | Serogroup D cap gene | TTACAAAAGAAAGACTAGGAGCCC CATCTACCCACTCAACCATATCAG | 54 | 657 | [24] |
| ecbJ | Serogroup E cap gene | TCCGCAGAAAATTATTGACTC GCTTGCTGCTTGATTTTGTC | 50 | 511 | [24] |
| fcbD | Serogroup F cap gene | AATCGGAGAACGCAGAAATCAG TTCCGCCGTCAATTACTCTG | 54 | 851 | [24] |
| ompA | Outer membrane protein A | CGCATAGCACTCAAGTTTCTCC CATAAACAGATTGACCGAAACG | 55 | 201 | [23] |
| oma87 | Outer membrane protein 87 | GGCAGCGAGCAACAGATAACG TGTTCGTCAAATGTCGGGTGA | 55 | 838 | [23] |
| fimA | Fimbriae | CCATCGGATCTAAACGACCTA AGTATTAGTTCCTGCGGGTG | 55 | 866 | [23] |
| pfhA | Filamentous hemagglutinin | TTCAGAGGGATCAATCTTCG AACTCCAGT TGGTTTGTCG | 55 | 286 | [23] |
| nanB | Neuraminidases | CATTGCACCTAACACCTCT GGACACTGATTGCCCTGAA | 55 | 555 | [23] |
| nanH | Neuraminidases | GTGGGAACGGGAATTGTGA ACATGCCAAGTTTGCCCTA | 55 | 287 | [23] |
| exbB | Iron regulated and acquisition factors | TTGGCTTGTGATTGAACGC TGCAGGAATGGCGACTAAA | 55 | 283 | [23] |
| exbD | Iron regulated and acquisition factors | CGTTCTGATTACAGCCTCTT AACGAAATCTTGGAAACTGG | 55 | 247 | [23] |
| tonB | Iron acquisition related factors | CGACGGTGAAACCTGAGCCA CCGAGCGATAAGCATTGACT | 55 | 261 | [23] |
| tbpA | Iron acquisition related factor | TGGTTGGAAACGGTAAAGC TAACGTGTACGGAAAAGCC | 54 | 728 | [23] |
| hgbA | Hemoglobin binding protein | TGGCGGATAGTCATCAAG CCAAAGAACCACTACCCA | 51 | 419 | [25] |
| hgbB | Hemoglobin binding protein | ACCGCGTTGGAATTATGATTG CATTGAGTACGGCTTGACAT | 55 | 788 | [25] |
| pmHAS | Hyaluronidase | TCAATGTTTGCGATAGTCCGTTAG TGGCGAATGATCGGTGATAGA | 51 | 430 | [23] |
| toxA | Dermonecrotic toxin | CTTAGATGAGCGACAAGG GAATGCCACACCTCTATAG | 55 | 864 | [23] |
| tadD | Adhesin | TCTACCCATTCTCAGCAAGGC ATCATTTCGGGCATTCACC | 55 | 416 | [23] |
P. multocida=Pasteurella multocida
Capsular typing and virulence-associated gene detection
The capsular types of P. multocida isolates were determined by a multiplex PCR using specific primers targeting capsule biosynthesis genes (cap A, B, D, E, and F) as described previously [24].
The detection of virulence-associated genes was investigated by PCR with specific primers. The details of all primer pairs and annealing temperature are listed in Table-1 [23–25]. The PCR conditions were carried out as previously described [24, 25].
Antibiotic susceptibility
An antimicrobial susceptibility test was carried Polymerase chain reaction
out by the disk diffusion method in accordance with the guidelines of Clinical and Laboratory Standards Institute (CLSI) [26]. In the pipeline for testing, all P. multocida isolates were grown in BHI broth until OD600 reached 0.5 and then were spread over Mueller-Hinton plates (HiMedia). After a few minutes of surface drying, 12 antibiotic discs (Mast group, England) with concentrations as follows: Amikacin (30 μg), amoxicillin (10 μg), ampicillin (10 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), erythromycin (15 μg), gentamicin (10 μg), kanamycin (30 μg), neomycin (10 μg), ofloxacin (5 μg), enrofloxacin (5 μg), and tetracycline (30 μg) were placed equally-spaced on the surface of the plates and incubated at 37°C for 24 h. The antibiotic susceptibility of isolates was based on the inhibition zone diameter standards provided by the CLSI [26]. Escherichia coli ATCC 35218 and Staphylococcus aureus ATCC 25923 were used as the quality control strains.
Biofilm formation assay
The biofilm formation capacity of P. multocida isolates was examined according to Sager et al. [27] with some modifications. Briefly, bacterial cells of P. multocida isolates were grown in BHI broth overnight at 37°C. Cultures were harvested by centrifugation at 14000× g for 5 min; cells were then resuspended in tryptic soy broth without glucose (Merck, Germany), and were adjusted to OD600 = 1 (equal to approximately 3 × 108 CFU/mL). Subsequently, 150 μL of the diluted culture was inoculated in triplicate to 96-well plates (SPL Life Science, Korea) and incubated at 37°C in static conditions. After 24 h of incubation, non-adhesive cells and culture medium were removed, subsequently, adhered cells of biofilms in the well were washed 3 times with distilled water and stained by 150 μL of 1% crystal violet (Merck) for 15 min. After that, the plates were rinsed 3 times, before adding 150 μL of 70% ethanol and incubated for 15 min. Finally, 100 μL of soluble crystal violet was transferred to a new 96-well plate, and absorbance was determined by optical density (OD) at wavelength 540 nm. The experiments were carried out with three replicates. The biofilm formation ability of isolates was calculated according to the method described by Stepanović et al. [28]. In brief, the strain is classed as a non-biofilm producer when the average OD is less than the control value (ODc), weak biofilm producer means ODc < OD ≤ 2 × ODc, moderate biofilm producer as the following formation: 2 × ODc < OD ≤ 4 × ODc, and strong biofilm producer when OD > 4 × ODc. In all experiments, ODc (control measurement) was carried out in a microtiter plate without cells.
Results and Discussion
Pasteurella multocida isolation and capsular detection
The suspected P. multocida isolates were obtained from blood agar and biochemical tests, with typical characteristics bacterial cells were small, Gram-negative and coccobacillus with bipolar staining features; the colonies were gray, viscous with odor and non-hemolytic. They were further verified by PCR. Specific PCR assay using Gram-negative KMT1 primers identified 41 isolates as P. multocida (The representative ten isolates of P. multocida are shown in Figure-1) with an incidence of 8.45% (41/485 samples), of which 38 isolates were successfully isolated from clinically sick sheep (38/300) and three isolates from healthy sheep (3/185). This average percentage result is higher than that of Fernandez et al. [13], in which the incidence was 6.2% (37/598 samples), but lower than the reports of Shayegh et al. [29], which was 9.07% (47/518 samples). Our results also indicated that P. multocida was significantly higher in clinically sick sheep (12.66%) compared to healthy ones (1.62%). These results are consistent with a previous study, in which 18.62% P. multocida was isolated from clinically sick lambs compared to healthy ones 0.49% [13]. However, there was not much difference from the study of Shayegh et al. [29], in which prevalence was 8.14% and 9.27% for healthy sheep and diseased sheep, respectively. The difference observed here may be from the size of the sample investigation and the characteristics of the local animal studied.
Figure-1.

Pasteurella multocida-specific polymerase chain reaction (PCR) assay by kmt1 primers and multiplex PCR for capsular typing of P. multocida isolates. (a) Verification of P. multocida by PCR. Lane M, 1 kb DNA ladder marker (ThermoScientific, USA); lanes 1–10: detection of P. multocida from isolates (1–10); lane P: Positive control (633 bp), lane N: Negative control. (b) Capsular identification in isolated P. multocida by multiplex PCR assay. Lane M, 1 kb DNA ladder marker (ThermoScientific, USA); lane N, negative control; lanes 1–10 isolates (1–10); A positive control of capA (1044 bp); B, positive control of capB (760 bp); and D, positive control of capD (657 bp).
Based on PCR serotyping of 41 P. multocida isolates, the capA, capB, and capD genes were detected in 24 (58.53%), 4 (9.75%), and 13 (31.71%) isolates, respectively. However, the capE, and capF genes were not detected in all of the isolates (Table-2 and Figure-1). A compatible result was also observed in a study conducted by Aski and Tabatabaei [30], in which the prevalence of capA, capB, and capD genes in P. multocida isolates from sheep was 45.45, 13.63, and 34.09%, respectively. However, in some cases, the capB gene was absent in P. multocida isolates from sheep [13, 15]. Nevertheless, data in the literature suggest that capsular types A and D are common among P. multocida strains isolated from sheep and goats [13, 15, 29, 30].
Table-2.
The prevalence of virulence genes associated with capsule serotypes among 41 isolates of P. multocida.
| P. multocida isolation | Capsular type | Number of isolates (%) | Virulence gene (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| fimA | pfhA | pmHAS | toxA | hgbA | hgbB | tbpA | exbB | exbD | tonB | nanB | nanH | tadD | ompA | oma87 | |||
| Healthy (185) | A | 0 (0) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| B | 0 (0) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| D | 3 (7.32) | 3 (7.32) | 0 (0) | 0 (0) | 3 (7.32) | 3 (7.32) | 0 (0) | 1 (2.44) | 3 (7.32) | 3 (7.32) | 3 (7.32) | 2 (4.88) | 0 (0)) | 1 (2.44) | 3 (7.32) | 3 (7.32) | |
| Sick (300) | A | 24 (58.53) | 22 (53.65) | 6 (14.63) | 24 (58.53) | 0 (0) | 23 (56.09) | 17 (41.46) | 20 (48.78) | 24 (58.53) | 24 (58.53) | 24 (58.53) | 23 (56.09) | 14 (34.14) | 17 (41.46) | 24 (58.53) | 24 (58.53) |
| B | 4 (9.75) | 3 (7.32) | 1 (2.44) | 1 (2.44) | 0 (0) | 3 (7.32) | 2 (4.88) | 3 (7.32) | 4 (9.75) | 4 (9.75) | 4 (9.75) | 2 (4.88) | 3 (7.32) | 2 (4.88) | 4 (9.75) | 4 (9.75) | |
| D | 10 (24.39) | 10 (24.39) | 1 (2.44) | 0 (0) | 10 (24.39) | 10 (24.39) | 6 (14.63) | 9 (21.95) | 10 (24.39) | 10 (24.39) | 10 (24.39) | 10 (24.39) | 7 (17.07) | 1 (2.44) | 10 (24.39) | 10 (24.39) | |
| Total (485) | 38 (92.68) | 8 (19.51) | 25 (60.97) | 13 (31.71) | 39 (95.12) | 25 (60.97) | 33 (80.48) | 41 (100) | 41 (100) | 41 (100) | 37 (90.24) | 24 (58.53) | 21 (51.22) | 41 (100) | 41 (100) | ||
P. multocida=Pasteurella multocida
Interestingly, only three P. multocida strains were isolated from healthy sheep and all of them (3/3) were detected as capsular type D. On the other hand, among 38 P. multocida strains that were isolated from clinically sick sheep, serotype D made up 26.31% (10/38). These findings are in line with the previous study [13], but completely different from those reported by Shayegh et al. [29], which indicated that capsular type D was isolated only from diseased sheep, whereas capsular type A presented in both diseased and healthy sheep. Similarly, Khamesipour et al. [19] reported that capsular type A was detected in both pneumonic lung and healthy lung isolates. In agreement with the literature, no strong correlation between serotype and the prevalence of P. multocida was identified. The distribution and prevalence of P. multocida serotypes have been reported in other studies and vary from region to region, host to host. Serotype D was found most commonly in cattle (82.6%) [31], and varied in sheep (6.3 to 57.1%) [13, 29]. Meanwhile, serotype A was predominantly found in sheep (83.3%) [29], and swine (about 50%) [5, 32]. In addition, serotype B was absent in a previous study [13, 29], but it was detected in the present study. Similarly, P. multocida type B was also detected in cattle in Vietnam [33].
Detection of virulence-associated genes
The frequency of 15 virulence genes of P. multocida isolates was detected by PCR (Table-2) and the representative gel pictures for the distribution of four virulence genes in 10 isolates are shown in Figure-2. The results showed that all isolates carried iron-acquisition encoding genes (exbB, exbD and tonB), and outer membrane protein genes (ompA, oma87). The fimA gene encoding an adhesin was detected in 92.68% of isolates, whereas another gene in the same functional group (pfhA) was detected as low as 19.51%. The incidence of toxA, tadD, pmHAS, and tbpA genes was 31.71, 51.22, 60.97, and 80.48% in all the isolates, respectively. Among the heme receptors (hgbA and hgbB) studied, hgbA was detected in 95.12% of isolates, particularly in all type D isolates, while 60.97% of isolates carried the hgbB gene. Similarly, nanB was present in 90.24% of isolates, compared to a lower frequency of nanH (58.53%).
Figure-2.

Polymerase chain reaction assay checking the prevalence of virulence genes of Pasteurella multocida isolated from Phan Rang sheep. Lane M, 1 kb DNA ladder marker (ThermoScientific, USA); lanes 1–10: Samples, lane N: Negative control, lane P: Positive control. (a) Detection of toxA gene; (b) detection of tadD gene; (c) detection of ompA gene; (d) detection of pmHAS gene.
It is known that P. multocida has a number of virulence factor encoding genes that are essential for bacterial infection [34]. Among these genes, fimA and pfhA encode factors that mediate bacterial cell adherence to host cells, one of the first steps of bacterial infection [21]. The previous studies reported that the pfhA gene has a very high prevalence and irrespective of its capsule type [21, 31, 35]. However, in this study, we observed a relatively low occurrence of the pfhA gene in isolates from sheep. These results are consistent with other studies [25, 29, 30] that reported the low distribution of this gene with great variance among the strains of P. multocida. Another factor gene fimA which has not been well characterized, exhibited high prevalence in this study; similar results were also observed in the studies of Aski et al. [30], Tang et al. [18] and Ewers et al. [25], shown that more than 80% isolates harbored this gene. These findings suggest that the fimA gene may play an important role in bacterial adhesion.
Meanwhile, the tadD gene encoding a non-specific adhesion factor was identified here in more than 50% of isolates, of which 70.8% (17/24 isolates) of capsular A carry the tadD gene, whereas 15.3% (2/13) isolates of capsular D carry the tadD gene (Figure-2a). Comparison of the findings with those of the previous studies supports the suggestion that the distribution of tadD in capsular A is more frequent than it is in capsular D [18, 25, 30, 36]. Other adhesion-related genes, nanB and nanH (sialidases) play a significant role in the colonization of epithelial surfaces [37]. In agreement with the previous studies [25, 30], the nanB gene was present in most isolates (>90%), whereas the nanH gene was detected in <60% of isolates. Conversely, nanH was detected in 100% of Indian bovine isolates, while nanB was absent in the study of Verma et al. [31]. These again suggest that the varying prevalence of nanH depends on the host or host origins and geographical location as previous studies [21, 25].
Iron is an essential nutrient for nearly all bacterial pathogens [38], P. multocida may turn on the expression of hgbA, hgbB, and tbpA genes, and TonB complex genes (exbB, exbD, and tonB) for acquiring iron from different heme iron sources [39]. In this study, the prevalence of three genes in the TonB complex was 100% in all isolates. These results are consistent with the previous studies [18, 25] and reasonable since iron uptake depends on the energy generated from the TonB complex [34, 39]. In contrast, the regular distribution of two genes (hgbA and hgbB) mediating iron-acquisition directly from the heme component was very different. About 95.12% of isolates carried the hgbA gene, whereas hgbB was present in 60.97% of isolates. These findings broadly supported those of other studies [21, 25, 30] in which the hgbA gene is found to be highly prevalent (>95%) among P. multocida isolates. In contrast, hgbB gene distribution varied and depended on strains of different host origins. Notably, Shayegh et al. [29] reported that the regular distribution of the hgbB gene is 36.36% among sheep isolates, our study revealed a higher prevalence (60.97%) of this gene.
The TbpA protein has been described as an important virulence factor, and the tbpA gene is present in small ruminant strains [21, 25, 35, 40]. Our results revealed that a high frequency (80.48%) of the tbpA gene was detected among isolates from sheep; similar results were found in the studies of Katsuda et al. [36] and Sarangi et al. [21]. However, the frequency was lower compared to the study of Fernandez et al. [13], who found that the tbpA gene was present in all P. multocida strains from sheep.
Interestingly, pmHAS and toxA exhibited distinctive relations to serotypes (Figures-2b-d); the pmHAS was detected in all the capsular type A isolates with a prevalence of 58.53% (24/41 isolates), whereas the toxA gene was correlated to capsular type D only with 31.71% of the distribution of 13/41 isolates. Similar results were also observed, in which several virulence genes, including pmHAS and toxA have been reported as factors linked to specific capsular types [13, 21, 25, 29, 41, 42].
Consistent with the literature [25, 43], in the present study, ompA and oma87 genes mediate the biosynthesis and integrity of the outer membrane of P. multocida were identified in all isolates (Figure-2c). The high prevalence of these genes demonstrates their importance to infection by P. multocida and survival in the host.
Antimicrobial susceptibility of P. multocida isolates
Antimicrobials are still widely used to treat infections caused by P. multocida. However, today the problem of antibiotic resistance is becoming more and more serious not only in Vietnam [44], but throughout the world [45]. Bacterial cells can become resistant to antibiotics and drugs due to drug misuse in many aspects of life, such as antibiotic use in livestock, in disease treatment, and the use of unsuitable drugs, or drugs at the wrong dose. The antimicrobial susceptibility of Pasteurella species should be carefully monitored to detect resistance development. In this study, we found that the antibiotics enrofloxacin, ciprofloxacin, neomycin, and ofloxacin were the most effective in the treatment of P. multocida isolates as all strains were susceptible (100%) to these drugs (Table-3 and Figure-S1), followed by chloramphenicol (92.68%) and amikacin (90.24%). These results are in accordance with those obtained by Kumar et al. [46] and Sarangi et al. [21], who also found enrofloxacin, chloramphenicol and ofloxacin were the most effective against P. multocida isolated from sheep.
Table-3.
The antimicrobial susceptibility profiles of Pasteurella multocida strains by disk diffusion test.
| Antimicrobial agents | Sensitivity of isolates to antimicrobial agents | ||
|---|---|---|---|
|
| |||
| Sensitive (%) | Intermediate (%) | Resistant (%) | |
| Amikacin | 37 (90.24) | 1 (2.44) | 3 (7.32) |
| Amoxicillin | 12 (29.27) | 9 (21.95) | 20 (48.78) |
| Ampicillin | 11 (26.83) | 8 (19.51) | 22 (53.66) |
| Ciprofloxacin | 41 (100) | 0 | 0 |
| Chloramphenicol | 38 (92.68) | 0 | 3 (7.32) |
| Erythromycin | 8 (19.51) | 6 (14.63) | 27 (65.85) |
| Gentamicin | 33 (80.48) | 2 (4.88) | 6 (14.63) |
| Kanamycin | 35 (85.36) | 1 (2.44) | 5 (12.19) |
| Neomycin | 41 (100) | 0 | 0 |
| Ofloxacin | 41 (100) | 0 | 0 |
| Enrofloxacin | 41 (100) | 0 | 0 |
| Tetracycline | 12 (29.27) | 8 (19.51) | 21 (51.22) |
P. multocida=Pasteurella multocida
Conversely, the results shown in Table-3 indicate that erythromycin, tetracycline, and amoxicillin are less effective against P. multocida since the resistance rates are 65.85, 51.22, and 48.78%, respectively. On the other hand, Singh et al. [47] found the full susceptibility (100%) of amoxicillin and tetracycline against 12 P. multocida isolated from sheep. Similarly, Berge et al. [48] reported that all 19 P. multocida isolates were susceptible to amoxicillin. The difference may be due to grazing conditions in Vietnam, sheep are often raised with some other livestock and poultry such as cattle, chickens, pigs, and buffalo, which can lead to the transmission of resistant bacteria. Moreover, a recent report conducted on P. multocida isolated from pigs in Vietnam demonstrated higher resistance of isolates to amoxicillin and tetracycline (75.9 and 59%, respectively) compared to our results (48.78 and 51.22%, respectively) [5].
In our study, ampicillin was an inefficient antibiotic for treatment since 53.66% of P. multocida strains were resistant. This finding is consistent with that of Marru et al. [2], who found 53.1% of P. multocida strains were resistant to ampicillin and supported evidence from a previous Spanish study by Cid et al. [49], who reported that all 87 P. multocida strains isolated were resistant to ampicillin. In contrast, ampicillin has been reported as the most effective against P. multocida in other studies, of which all 28 P. multocida isolates were fully susceptible to ampicillin [17], of which ampicillin was significantly active against 177/186 P. multocida isolates [50].
Based on the antibiotic susceptibility test results (Table-3 and Figure-S1), 12.19 and 14.63% of isolates were resistant to kanamycin and gentamicin, respectively. Similar findings were also observed by Sarangi et al. [21] and the study of Singh et al. [47], who showed that more than 80% of P. multocida isolates were susceptible to these antibiotics. This finding is contrary to previous studies [46, 51] in which more than 60% of P. multocida isolates were resistant to kanamycin. Marru et al. [2] reported that all Pasteurella isolates were completely resistant to gentamicin. But the findings in the present study were very close to the result of Vu-Khac et al. [5], who found that the rate of resistance to gentamicin and kanamycin of porcine Pasteurella isolates in Vietnam were 14.5 and 15.7%, respectively.
Biofilm is a characteristic phenotype of many pathogenic bacteria; it helps them to survive in harsh environmental conditions and enhances their virulence and resistance to antibiotics [52]. Several pathogenic members of the Pasteurellaceae, including P. multocida have the ability to form biofilms [27, 53, 54] and such biofilms are often difficult to handle due to their limited antimicrobial activity [27]. In this study, as the results shown in Figure-3, 22 isolates (53.65%) were able to adhere to polystyrene and form biofilm, of which only 1 isolate (NT7) exhibited strong adherence, four isolates (NT17, NT24, NT29, and NT39) adhered moderately, and many of them, 18 isolates poorly adhered to polystyrene. On the other hand, 19 isolates (46.34%) could not produce biofilm at all. This finding is consistent with that of Emery et al. [53], who reported that 53.13% of P. multocida strains exhibited weak adherence to and 40.42% no adherence to polystyrene. However, the result was lower than those of Saha et al. [54], who found 81.82% of P. multocida strains could produce biofilm. Interestingly, the tadD gene is known to play an important role in adhesion and biofilm formation [34, 53], but in this study, there was no correlation between the prevalence of tadD and biofilm formation. 8/17 capsular A, 2/4 capsular B, and 1/4 capsular D isolates carrying tadD were able to form biofilm. Thus, the biofilm formation of P. multocida may be related to many complex genetic factors, in which the tadD and other genes may be tightly controlled by an unknown mechanism and need further research.
Figure-3.

Biofilm formation of 41 Pasteurella multocida strains. The ability of isolates to form biofilm was measured at 540 nm using crystal violet after 24 h of growth in 96-well plates. Error bars indicate standard deviation from three independent experiments. The orange line indicates control measurement.
Interestingly, all four isolates carrying capsular B were able to produce biofilm, of which one formed weakly, one very strong production of biofilm and two formed biofilms moderately. These results support the findings of a great deal of the previous work in which capsular serotype group B of P. multocida isolates could form strong biofilms [55]. Prajapati et al. [55] also reported that 63% of serogroups A, and 100% of serogroups D of P. multocida isolates could not produce biofilm. This differs from the findings presented here: 58.33% (14/24) of P. multocida serotype A were able to form a biofilm, of which 8.33% (2/24) isolates could form moderate biofilms, and 50% (12/24) serotype A isolates were able to produce weak biofilms, whereas, 41.16% (10/24) of P. multocida serotype A isolates were unable to produce biofilm. Meanwhile, 30.76% (4/13) isolates carrying capsular D could form weak biofilms.
Conclusion
This present study showed that P. multocida is frequently isolated from Phan Rang sheep in Vietnam and that isolates carry an abundance of virulence-associated genes. Several particular virulence genes, such as toxA, pfhA, and tbpA may be used as an epidemic marker for P. multocida and are also good candidates for vaccine development against P. multocida. Our findings also provide valuable information on the current susceptibility status and biofilm formation of P. multocida isolates.
Authors’ Contributions
PVN and KCTN: Contributed to the conception of the study and drafted the manuscript. CTL, XHN and TMN: Contributed to sample collection, investigation, and data analysis. All authors have read and approved the final manuscript.
Acknowledgments
The authors thank Dr. Vu-Khac Hung from Institute of Veterinary Research and Development of Central Vietnam for kindly providing us with genomic DNA of P. multocida samples to carry out this study, and Dr. Derek Wilkinson from Charles University in Prague for proofreading the manuscript. This study was supported by Ministry of Education and Training, Vietnam, under the project CT-2021-01-DHH-02 and by Hue University, Vietnam, under the Core Research Program, Grant No. NCM.DHH2020.13.
Supplementary Figure
Figure-S1.
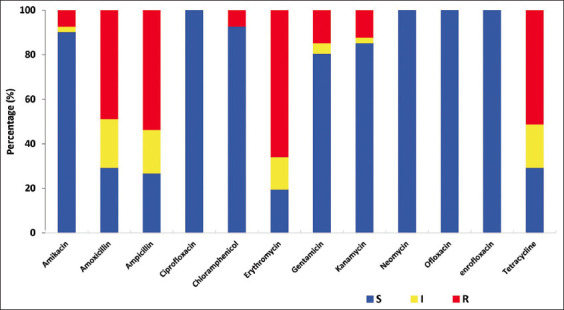
Antibiotic resistance profile of Pasteurella multocida isolates: Resistant (R) in red, intermediate (I) in yellow, and susceptible (S) in green color.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Chon T.T. Phan Rang sheep-a genetic resource in Vietnam. J. Agric. Sci. Technol. (2000);1(2):141–142. [Google Scholar]
- 2.Marru H.D, Anijajo T.T, Hassen A.A. A study on ovine pneumonic pasteurellosis:Isolation and identification of Pasteurellae and their antibiogram susceptibility pattern in Haramaya District, Eastern Hararghe, Ethiopia. BMC Vet. Res. 2013;9:1–8. doi: 10.1186/1746-6148-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odugbo M.O, Odama L.E, Umoh J.U, Lamorde A.G. Pasteurella multocida pneumonic infection in sheep:Prevalence, clinical and pathological studies. Small Rumin. Res. 2006;66((1–3)):273–277. [Google Scholar]
- 4.Cid D, García-Alvarez A, Domínguez L, Fernández-Garayzábal J.F, Vela A.I. Pasteurella multocida isolates associated with ovine pneumonia are toxigenic. Vet. Microbiol. 2019;232:70–73. doi: 10.1016/j.vetmic.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Vu-Khac H, Trinh T.T.H, Nguyen T.T.G, Nguyen X.T, Nguyen T.T. Prevalence of virulence factor, antibiotic resistance, and serotype genes of Pasteurella multocida strains isolated from pigs in Vietnam. Vet. World. 2020;13(5):896–904. doi: 10.14202/vetworld.2020.896-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend K.M, Hanh T.X, O'Boyle D, Wilkie I, Phan T.T, Wijewardana T.G, Trung N.T, Frost A.J. PCR detection and analysis of Pasteurella multocida from the tonsils of slaughtered pigs in Vietnam. Vet. Microbiol. 2000;72((1–2)):69–78. doi: 10.1016/s0378-1135(99)00188-1. [DOI] [PubMed] [Google Scholar]
- 7.Gunawardana G.A, Townsend K.M, Frost A.J. Molecular characterisation of avian Pasteurella multocida isolates from Australia and Vietnam by REP-PCR and PFGE. Vet. Microbiol. 2000;72(1–2):97. doi: 10.1016/s0378-1135(99)00191-1. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty S, Kumar A, Tiwari R, Rahal A, Malik Y, Dhama K, Pal A, Prasad M. Advances in diagnosis of respiratory diseases of small ruminants. Vet. Med. Int., 2014. 2014:508304. doi: 10.1155/2014/508304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legesse A, Abayneh T, Mamo G, Gelaye E, Tesfaw L, Yami M, Belay A. Molecular characterization of Mannheimia haemolytica isolates associated with pneumonic cases of sheep in selected areas of Central Ethiopia. BMC Microbiol. 2018;18:1–10. doi: 10.1186/s12866-018-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper M, Boyce J.D, Adler B. Pasteurella multocida pathogenesis:125 years after Pasteur. FEMS Microbiol. Lett. 2006;265(1):1–10. doi: 10.1111/j.1574-6968.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam N.D, Ajam N, Blackall P.J, Asiah N.M, Ramlan M, Maria J, Yuslan S, Thong K.L. Capsular serotyping of Pasteurella multocida from various animal hosts-a comparison of phenotypic and genotypic methods. Trop. Biomed. 2011;28(1):55–63. [PubMed] [Google Scholar]
- 12.Kumar A.A, Shivachandra S.B, Biswas A, Singh V.P, Singh V.P, Srivastava S.K. Prevalent serotypes of Pasteurella multocida isolated from different animal and avian species in India. Vet. Res. Commun. 28(8):657–667. doi: 10.1023/b:verc.0000045959.36513.e9. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez S, Galapero J, Gomez L, Perez C.J, Ramos A, Cid D, Garcia A, Rey J. Identification, capsular typing and virulence factors of Pasteurella multocida isolates from Merino lambs in Extremadura (Southwestern Spain) Vet. Med. (Praha), 2018. 2004;63(3):117–124. [Google Scholar]
- 14.Vougidou C, Sandalakis V, Psaroulaki A, Siarkou V, Petridou E, Ekateriniadou L. Distribution of the OmpA-Types among ruminant and swine pneumonic strains of Pasteurella multocida exhibiting various Cap-Locus and ToxA patterns. Microbiol. Res. 2015;174:1–8. doi: 10.1016/j.micres.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Mombeni E.G, Gharibi D, Ghorbanpoor M, Jabbari A.R, Cid D. Toxigenic and non-toxigenic Pasteurella multocida genotypes, based on capsular, LPS, and virulence profile typing, associated with pneumonic pasteurellosis in Iran. Vet. Microbiol. 2021;257:109077. doi: 10.1016/j.vetmic.2021.109077. [DOI] [PubMed] [Google Scholar]
- 16.Mostaan S, Ghasemzadeh A, Sardari S, Shokrgozar M.A, Brujeni G.N, Abolhassani M, Ehsani P, Karam M.R.A. Pasteurella multocida vaccine candidates:A systematic review. Avicenna J. Med. Biotechnol. 2020;12(3):140–147. [PMC free article] [PubMed] [Google Scholar]
- 17.Sahay S, Natesan K, Prajapati A, Kalleshmurthy T, Shome B.R, Rahman H, Shome R. Prevalence and antibiotic susceptibility of Mannheimia haemolytica and Pasteurella multocida isolated from ovine respiratory infection:A study from Karnataka, Southern India. Vet. World. 2020;13(9):1947–1954. doi: 10.14202/vetworld.2020.1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Zhao Z, Hu J, Wu B, Cai X, He Q, Chen H. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. J. Clin. Microbiol. 2009;47(4):951–958. doi: 10.1128/JCM.02029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khamesipour F, Momtaz H, Mamoreh M.A. Occurrence of virulence factors and antimicrobial resistance in Pasteurella multocida strains isolated from slaughter cattle in Iran. Front. Microbiol. 2014;5:536. doi: 10.3389/fmicb.2014.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T.M, Lin T.L, Wu C.C. Antimicrobial susceptibility and resistance of chicken Escherichia coli, Salmonella spp and Pasteurella multocida isolates. Avian Dis. 2009;53(1):89–93. doi: 10.1637/8268-021608-Reg.1. [DOI] [PubMed] [Google Scholar]
- 21.Sarangi L.N, Thomas P, Gupta S.K, Priyadarshini A, Kumar S, Nagaleekar V.K, Kumar A, Singh V.P. Virulence gene profiling and antibiotic resistance pattern of Indian isolates of Pasteurella multocida of small ruminant origin. Comp. Immunol. Microbiol. Infect. Dis. 2015;38:33–39. doi: 10.1016/j.cimid.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Shayegh J, Mikaili P, Sharaf J.D, Rastgu A. Antimicrobial resistance evaluation of Iranian ovine and bovine Pasteurella multocida. J. Anim. Vet. Adv. 2009;8(8):1753–1756. [Google Scholar]
- 23.Townsend K.M, Frost A.J, Lee C.W, Papadimitriou J.M, Dawkins H.J.S. Development of PCR assays for species-and type-specific identification of Pasteurella multocida isolates. J. Clin. Microbiol. 36(4):1096–1100. doi: 10.1128/jcm.36.4.1096-1100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend K.M, Boyce J.D, Chung J.Y, Frost A.J, Adler B. Genetic organization of Pasteurella multocida cap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol 1998. 2001;39(3):924–929. doi: 10.1128/JCM.39.3.924-929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewers C, Lübke-Becker A, Bethe A, Kießling S, Filter M, Wieler L.H. Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status. Vet. Microbiol. 2006;114((3–4)):304–317. doi: 10.1016/j.vetmic.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 26.CLSI I. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. CLSI Suppl. VET,08. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 27.Sager M, Benten W.P.M, Engelhardt E, Gougoula C, Benga L. Characterization of biofilm formation in [Pasteurella] pneumotropica and [Actinobacillus] muris isolates of mouse origin. PLoS One, 2015;10(10):e0138778. doi: 10.1371/journal.pone.0138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Ćirković I, Ruzicka F. Quantification of biofilm in microtiter plates:Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. Apmis. 2007;115(8):891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 29.Shayegh J, Atashpaz S, Hejazi M.S. Virulence genes profile and typing of ovine Pasteureila multocida. Asian J. Anim. Vet. Adv. 2008;3(4):206–213. [Google Scholar]
- 30.Aski H.S, Tabatabaei M. Occurrence of virulence-associated genes in Pasteurella multocida isolates obtained from different hosts. Microb. Pathog. 96:52–57. doi: 10.1016/j.micpath.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Sharma M, Katoch S, Verma L, Kumar S, Dogra V, Chahota R, Dhar P, Singh G. Profiling of virulence-associated genes of Pasteurella multocida isolated from cattle. Vet. Res. Commun (2016) 2013;37(1):83–89. doi: 10.1007/s11259-012-9539-5. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Zhao Z, Xi X, Xue Q, Long T, Xue Y. Occurrence of Pasteurella multocida among pigs with respiratory disease in China between 2011 and 2015. Ir. Vet. J 2017. (2018);70:1–6. doi: 10.1186/s13620-016-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend K.M, O'Boyle D, Phan T.T, Hanh T.X, Wijewardana T.G, Wilkie I, Trung N.T, Frost A.J. Acute septicaemic pasteurellosis in Vietnamese pigs. Vet. Microbiol. 1998;63((2–4)):205–215. doi: 10.1016/s0378-1135(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 34.Hatfaludi T, Al-Hasani K, Boyce J.D, Adler B. Outer membrane proteins of Pasteurella multocida. Vet. Microbiol. 2010;144((1–2)):1–17. doi: 10.1016/j.vetmic.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Prabhakar P, Thangavelu A, Prabhakar T.G, Kirubaharan J.J, Chandran N.D.J. Rapid virulence typing of Pasteurella multocida in sheep isolates of Tamil Nadu. Indian J. Anim. Sci. 2012;82(4):351–354. [Google Scholar]
- 36.Katsuda K, Hoshinoo K, Ueno Y, Kohmoto M, Mikami O. Virulence genes and antimicrobial susceptibility in Pasteurella multocida isolates from calves. Vet. Microbiol. 2013;167((3–4)):737–741. doi: 10.1016/j.vetmic.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Tatum F.M, Tabatabai L.B, Briggs R.E. Sialic acid uptake is necessary for virulence of Pasteurella multocida in Turkeys. Microb. Pathog. 2009;46(6):337–344. doi: 10.1016/j.micpath.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Cassat J.E, Skaar E.P. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosch M, Garrido M.E, De Rozas A.M.P, Badiola I, Barbé J, Llagostera M. Pasteurella multocida contains multiple immunogenic haemin-and hemoglobin-binding proteins. Vet. Microbiol. 2004;99(2):103–112. doi: 10.1016/j.vetmic.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Rímac R, Luna L, Hurtado R, Rosadio R, Maturrano L. Detection and genetic characterization of Pasteurella multocida from alpaca (Vicugna pacos) pneumonia cases. Trop. Anim. Health Prod. 2017;49(6):1325–1328. doi: 10.1007/s11250-017-1309-5. [DOI] [PubMed] [Google Scholar]
- 41.Farahani M.F, Esmaelizad M, Jabbari A.R. Investigation of iron uptake and virulence gene factors (Fur, TonB, ExbD, ExbB, HgbA, HgbB1, HgbB2 and TbpA) among isolates of Pasteurella multocida from Iran. Iran. J. Microbiol. 2019;11(3):191–197. [PMC free article] [PubMed] [Google Scholar]
- 42.Prajapati A, Chanda M.M, Yogisharadhya R, Parveen A, Ummer J, Dhayalan A, Mohanty N.N, Shivachandra S.B. Comparative genetic diversity analysis based on virulence and repetitive genes profiling of circulating Pasteurella multocida isolates from animal hosts. Infect. Genet. Evol. 2020;85:104564. doi: 10.1016/j.meegid.2020.104564. [DOI] [PubMed] [Google Scholar]
- 43.Bethe A, Wieler L.H, Selbitz H.J, Ewers C. Genetic diversity of porcine Pasteurella multocida strains from the respiratory tract of healthy and diseased swine. Vet. Microbiol. 2009;139(1–2):97. doi: 10.1016/j.vetmic.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen N.T, Nguyen H.M, Nguyen C.V, Nguyen T.V, Nguyen M.T, Thai H.Q, Ho M.H, Thwaites G, Ngo H.T, Baker S. Use of colistin and other critical antimicrobials on pig and chicken farms in Southern Vietnam and its association with resistance in commensal Escherichia coli bacteria. Appl. Environ. Microbiol. 2016;82(13):3727–3735. doi: 10.1128/AEM.00337-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nhung N.T, Chansiripornchai N, Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens:A review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar P, Singh V.P, Agrawal R.K, Singh S. Identification of Pasteurella multocida isolates of ruminant origin using polymerase chain reaction and their antibiogram study. Trop. Anim. Health Prod. 2009;41(4):573–578. doi: 10.1007/s11250-008-9226-2. [DOI] [PubMed] [Google Scholar]
- 47.Singh F, Sonawane G.G, Meena R.K. Pathology, isolation and characterisation of virulent and diverse Mannheimia haemolytica and Pasteurella multocida associated with fatal pneumonia in sheep, Rajasthan, India. Comp. Clin. Path. 2019;28(2):531–540. [Google Scholar]
- 48.Berge A.C.B, Sischo W.M, Craigmill A.L. Antimicrobial susceptibility patterns of respiratory tract pathogens from sheep and goats. J. Am. Vet. Med. Assoc. 2006;229(8):1279–1281. doi: 10.2460/javma.229.8.1279. [DOI] [PubMed] [Google Scholar]
- 49.Cid D, Fernández-Garayzábal J.F, Pinto C, Domínguez L, Vela A.I. Antimicrobial susceptibility of Pasteurella multocida isolated from sheep and pigs in Spain-short communication. Acta Vet. Hung. 2019;67(4):489–498. doi: 10.1556/004.2019.048. [DOI] [PubMed] [Google Scholar]
- 50.Cucco L, Massacci F.R, Sebastiani C, Mangili P, Bano L, Cocchi M, Luppi A, Ortenzi R, Pezzotti G, Magistrali C.F. Molecular characterization and antimicrobial susceptibility of Pasteurella multocida strains isolated from hosts affected by various diseases in Italy. Vet Ital. 2017;53(1):21–27. doi: 10.12834/VetIt.661.3256.2. [DOI] [PubMed] [Google Scholar]
- 51.Seker E, Kuyucuoglu Y, Konak S. Bacterial examinations in the nasal cavity of apparently healthy and unhealthy Holstein cattle. J. Anim. Vet. Adv. 2009;8(11):2355–2359. [Google Scholar]
- 52.Hall C.W, Mah T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. (2017);41(3):276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 53.Emery B.D, de Furian T.Q, Pilatti R.M, Chitolina G.Z, Borges K.A, Salle C.T.P, Moraes H.L.S. Evaluation of the biofilm formation capacity of Pasteurella multocida strains isolated from cases of fowl cholera and swine lungs and its relationship with pathogenicity. Pesqui. Vet. Bras. 2017;37(10):1041–1048. [Google Scholar]
- 54.Saha O, Islam M.R, Rahman M.S, Hoque M.N, Hossain M.A, Sultana M. First report from Bangladesh on genetic diversity of multidrug-resistant Pasteurella multocida Type B:2 in fowl cholera. Vet. World. 2021;14(9):2527–2542. doi: 10.14202/vetworld.2021.2527-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prajapati A, Chanda M.M, Dhayalan A, Yogisharadhya R, Chaudhary J.K, Mohanty N.N, Shivachandra S.B. Variability in in vitro biofilm production and antimicrobial sensitivity pattern among Pasteurella multocida strains. Biofouling. 2020;36(8):938–950. doi: 10.1080/08927014.2020.1833192. [DOI] [PubMed] [Google Scholar]


