Abstract
Introduction
In the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes (VERTIS CV) trial (NCT01986881), patients with type 2 diabetes mellitus (T2DM) and atherosclerotic cardiovascular disease (ASCVD) were randomized (1:1:1) to placebo, ertugliflozin 5 mg or 15 mg (doses pooled for analyses as prospectively planned). In this post hoc analysis, the effects of ertugliflozin on kidney outcomes were assessed in analyses stratified by baseline heart failure (HF).
Methods
Baseline HF was defined as a history of HF or prerandomization left ventricular ejection fraction ≤45%. Outcomes included estimated glomerular filtration rate (eGFR) over time, total 5-year eGFR slopes and time to first event of a prespecified exploratory kidney composite outcome of sustained ≥40% decrease from baseline eGFR, chronic kidney replacement therapy, or kidney death. All analyses were stratified by baseline HF status.
Results
Compared with no-HF at baseline (n = 5807; 70.4%), patients with HF (n = 2439; 29.6%) had a notably faster rate of eGFR decline, which is unlikely to be explained by the slightly lower baseline eGFR in that group. Ertugliflozin treatment resulted in a slower rate of eGFR decline in both subgroups; total placebo-adjusted 5-year eGFR slopes (ml/min per 1.73 m2 per year [95% confidence intervals; CI]) were 0.96 (0.67–1.24) and 0.95 (0.76–1.14) for HF and no-HF subgroups, respectively. The placebo HF (vs. placebo no-HF) subgroup had a higher incidence of the composite kidney outcome (35/834 [4.20%] vs. 50/1913 [2.61%]). Hazard ratios (95% CI) for the effect of ertugliflozin on the composite kidney outcome did not differ significantly between HF and no-HF subgroups: 0.53 (0.33–0.84) and 0.76 (0.53–1.08), respectively (Pinteraction = 0.22).
Conclusion
Although patients with HF at baseline had a faster rate of eGFR decline in VERTIS CV, the beneficial effects of ertugliflozin on kidney outcomes did not differ when stratified by baseline HF.
Keywords: albuminuria, diabetic kidney disease, ertugliflozin, heart failure, sodium–glucose cotransporter 2 inhibitor, type 2 diabetes mellitus
Graphical abstract
Beyond their established glucose lowering effects, several sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce the risk for various cardiovascular (CV) and kidney outcomes in patients with type 2 diabetes mellitus (T2DM) across a spectrum of cardiorenal risk.1 For CV outcomes, the most consistent benefit across the SGLT2 inhibitor class is a reduction in the risk of hospitalization for heart failure (HF).1, 2, 3 Beyond CV protection, some SGLT2 inhibitors reduce the risk of chronic kidney disease progression in patients with or without T2DM, including those at high risk of atherosclerotic cardiovascular disease (ASCVD).4,5 Dedicated HF trials have similarly shown consistent SGLT2 inhibitor benefits in those with and without T2DM, and attenuation of kidney function decline.6, 7, 8
The eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes (VERTIS CV) trial (NCT01986881) assessed the effects of the SGLT2 inhibitor, ertugliflozin, on CV and kidney outcomes in patients with T2DM and established ASCVD.9,10 Primary results from the VERTIS CV trial have been reported elsewhere.10 The effects of ertugliflozin on the composite kidney outcome, which includes a sustained 40% decline from baseline in estimated glomerular filtration rate (eGFR), are aligned with those of other SGLT2 inhibitors.11,12 Although SGLT2 inhibitors have protective effects on both kidney and hospitalization for HF outcomes,13 little is known about their relative impact on kidney outcomes according to HF status. The present post hoc analysis was performed to explore the effects of ertugliflozin on kidney outcomes in patients with T2DM with and without HF at baseline.
Methods
The VERTIS CV trial (protocol MK-8835-004-01; NCT01986881) was a prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-group, event-driven trial in patients with T2DM and established ASCVD, comparing placebo with 2 doses of ertugliflozin (5 mg and 15 mg). The VERTIS CV trial design, eligibility, primary results, kidney endpoints, and full study protocol have been published previously.3,9,10,12,14
Study Population
The trial recruited patients with T2DM and established ASCVD with a baseline screening eGFR ≥30 ml/min per 1.73 m2. The trial was approved by the appropriate institutional review boards and regulatory agencies, with all participants providing written informed consent.10
Classification of Baseline HF Status
For this post hoc analysis, patients were categorized as baseline HF status, which included patients with the following: (i) HF identified using the narrow HF standardized Medical Dictionary for Regulatory Activities query (version 22.1) at baseline15 and (ii) patients not captured in the Medical Dictionary for Regulatory Activities query who had a documented ejection fraction ≤45% before trial entry.
Outcomes
Analyses of outcomes by baseline HF status included the following: (i) a clinical composite kidney outcome of a sustained 40% decline from baseline in eGFR, chronic kidney replacement therapy, or kidney death; (ii) eGFR over time (at baseline and at weeks 6, 18, 52, 104, 156, 208, and 260); (iii) eGFR slopes; and (iv) urine albumin-to-creatinine ratio (UACR). Calculation of eGFR was performed using the Modified Diet in Renal Disease formula.
Statistical Analyses
Baseline characteristics were summarized using descriptive statistics. Continuous variables were summarized as mean (standard deviation; SD) or median (interquartile range). Categorical variables were summarized as frequencies and percentages.
The time-to-event outcomes were analyzed using a stratified Cox proportional hazard model, which included treatment, subgroup, and treatment-by-subgroup interaction, with cohort as a stratification factor (cohort 1 [participants randomized before protocol amendment, between December 2013 and July 2015] and cohort 2 [participants randomized after protocol amendment, in 2016 and beyond]). The interaction test (Pinteraction) of treatment by subgroup was performed to determine whether the effect of ertugliflozin versus placebo were different in subgroups. Time-to-event outcome analyses were performed with data from all randomized patients (the intention-to-treat population) for the prespecified exploratory kidney composite end point. Cox proportional hazard models were also used to evaluate the effects of ertugliflozin versus placebo by baseline HF subgroup on time to first event of progression of albuminuria (progression from normoalbuminuria [UACR <30 mg/g] to microalbuminuria or macroalbuminuria [UACR 30–300 mg/g or >300 mg/g, respectively] or progression from microalbuminuria to macroalbuminuria) and on time to first event of regression of albuminuria. Data from all patients as treated were used for the analysis of time to progression of albuminuria and time to regression of albuminuria.
The analyses of eGFR and UACR were performed on the full analysis set (randomized patients who received 1 or more doses of blinded study medication and had 1 or more measurements of the analysis end point). All between-group analyses were performed on the pooled ertugliflozin population (observations from both doses of ertugliflozin were pooled for all analyses) versus the placebo group, as prospectively planned.
As described previously,12 because of the documented rebound effect on eGFR and UACR after the discontinuation of SGLT2 inhibitors,16,17 data obtained >2 days after the last dose of study medication were excluded from the eGFR and UACR outcomes analyses. Least-squares mean changes from baseline over time by subgroup were estimated using the repeated measures analysis of covariance method.18 The models were adjusted for the baseline value of the outcome variable, baseline glycated hemoglobin, treatment, visit, subgroup, treatment-by-subgroup interaction, and treatment-by-subgroup-by-visit interaction. Visit was treated as a categorical variable. An unstructured covariance matrix was used to model the correlation among repeated measurements.
Owing to the non-normal distribution of UACR, UACR data were log-transformed before analysis. The adjusted mean percentage change (derived from exponentiation of adjusted estimates from the repeated measures analysis of covariance model) in UACR with 95% confidence intervals (CI) are presented by treatment and timepoint. The difference between ertugliflozin treatment and placebo in mean percentage change in UACR from baseline was estimated and presented.
The slopes for changes in eGFR per week or per year in placebo, ertugliflozin, and the placebo-adjusted effect of ertugliflozin were analyzed by random coefficient models. The models included the eGFR value as a response variable, with treatment, visit, baseline glycated hemoglobin, baseline eGFR, and treatment-by-visit interaction as linear covariates. Visit was treated as a continuous variable. The model enabled individual participant slopes to vary by random effects of intercept and time. An unstructured covariance matrix was used to model the correlation of random effects. Missing data were not imputed. The random effects model used a likelihood-based estimation, which produced unbiased estimates for data missing at random. Least-square mean differences between ertugliflozin and placebo for the weekly or yearly eGFR slopes were assessed for 5 periods as follows: (i) acute eGFR ‘dip’ period: weekly slope from week 0 (baseline) to week 6; (ii) post-eGFR ‘dip’ readjustment period: yearly slope from week 6 to 52 (year 1); (iii) postadjustment chronic period: yearly slope from week 52 to 260 (year 5); (iv) chronic slope: yearly slope from week 6 to 260; and (v) total yearly slope from week 0 (baseline) to 260.
No multiplicity adjustments were made for these post hoc analyses. The analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The baseline demographic and clinical characteristics of patients in the HF and no-HF groups by treatment group are displayed in Table 1. The trial cohort comprised 2439 (29.6%) patients with baseline HF and 5807 (70.4%) without. Patients with HF had a less frequent use of metformin, slightly higher use of antihypertensive medications, especially a higher use of β blockers, diuretics including loop diuretics, and mineralocorticoid receptor antagonists. Within each subgroup, baseline demographic and clinical characteristics were generally balanced between those randomized to ertugliflozin and placebo.
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | Baseline HF (n = 2439) (29.6%) |
Baseline no-HF (n = 5807) (70.4%) |
||
|---|---|---|---|---|
| Placebo n = 834 | Ertugliflozin n = 1605 | Placebo n = 1913 | Ertugliflozin n = 3894 | |
| Female sex, n (%) | 251 (30.1) | 456 (28.4) | 593 (31.0) | 1177 (30.2) |
| Age, yr | 64.6 (7.9) | 64.1 (8.0) | 64.3 (8.0) | 64.5 (8.2) |
| Duration of T2DM, yr | 12.4 (7.9) | 12.0 (8.0) | 13.4 (8.6) | 13.3 (8.4) |
| HbA1c, % | 8.2 (0.9) | 8.3 (0.9) | 8.2 (0.9) | 8.2 (1.0) |
| Body weight, kg | 93.6 (17.4) | 94.0 (18.4) | 91.1 (18.6) | 90.8 (18.5) |
| BMI, kg/m2 | 32.5 (5.4) | 32.5 (5.4) | 31.7 (5.5) | 31.7 (5.3) |
| eGFR, ml/min per 1.73 m2 (MDRD) | 73.5 (20.2) | 74.5 (20.5) | 76.7 (21.0) | 76.8 (21.0) |
| UACR, mg/g (median [IQR]) | 21.0 (6.0, 77.0) | 19.0 (7.0, 67.0) | 18.0 (6.0, 63.0) | 18.0 (6.0, 69.0) |
| SBP, mm Hg | 132.7 (13.6) | 132.9 (13.7) | 133.3 (14.1) | 133.7 (13.7) |
| DBP, mm Hg | 76.7 (8.4) | 77.2 (8.1) | 76.3 (8.8) | 76.6 (8.4) |
| Glucose lowering agents, n (%) | ||||
| Metformin | 616 (73.9) | 1139 (71.0) | 1508 (78.8) | 3029 (77.8) |
| Insulin | 402 (48.2) | 752 (46.9) | 942 (49.2) | 1804 (46.3) |
| Antihypertensive agents, n (%) | 817 (98.0) | 1561 (97.3) | 1815 (94.9) | 3660 (94.0) |
| β blockers | 675 (80.9) | 1284 (80.0) | 1228 (64.2) | 2505 (64.3) |
| RAAS inhibitors | 706 (84.7) | 1343 (83.7) | 1533 (80.1) | 3104 (79.7) |
| Diuretics | 458 (54.9) | 873 (54.4) | 738 (38.6) | 1473 (37.8) |
| Loop diuretics | 233 (27.9) | 435 (27.1) | 193 (10.1) | 391 (10.0) |
| MRAs | 135 (16.2) | 300 (18.7) | 89 (4.7) | 150 (3.9) |
| LDL-C lowering agents, n (%) | 699 (83.8) | 1374 (85.6) | 1614 (84.4) | 3281 (84.3) |
| Anticoagulants, n (%) | 766 (91.8) | 1479 (92.1) | 1680 (87.8) | 3401 (87.3) |
All analyses were performed in subgroups defined by patients with and without baseline HF in the placebo and ertugliflozin groups. Values are mean (standard deviation) unless otherwise stated.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HF, heart failure; IQR, interquartile range; LDL-C, low-density lipoprotein-cholesterol; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonist; RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; UACR, urinary albumin-to-creatinine ratio.
Kidney Composite Outcomes
Baseline HF status did not modulate the effects of ertugliflozin compared to placebo on the time to first prespecified exploratory composite kidney outcome; the hazard ratio (95% CI) for the time to first sustained ≥40% decline from baseline in eGFR, chronic kidney replacement therapy, or kidney death was 0.53 (0.33, 0.84) and 0.76 (0.53, 1.08) in the HF and no-HF subgroups, respectively (Pinteraction = 0.22; Figure 1).
Figure 1.
Prespecified exploratory kidney composite outcome by baseline HF status. Analyses of the prespecified exploratory kidney composite outcome (sustained ≥40% decrease from baseline in eGFR, chronic kidney replacement therapy, or kidney death) were performed with data from all randomized patients (ITT population). CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; ITT, intention-to-treat.
eGFR Over Time and eGFR Slopes
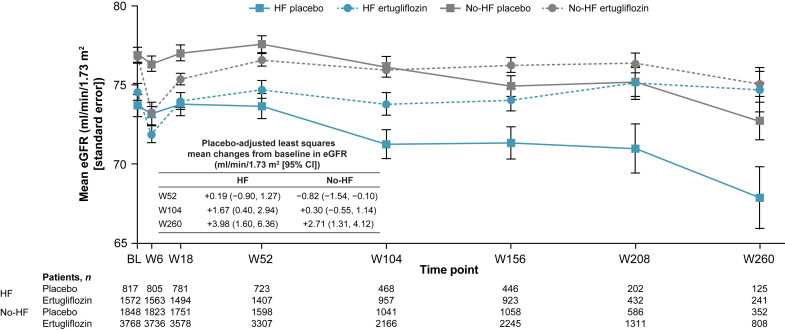
In both HF and no-HF subgroups, compared with placebo, there was an initial decline in the mean eGFR at week 6 in the ertugliflozin group, followed by an increase and higher eGFR over time (Figure 2). The placebo-adjusted mean ertugliflozin impact on change from baseline in eGFR was numerically larger in the HF subgroup than the no-HF subgroup from week 18 to week 260 (Supplementary Table S1). The placebo-adjusted change from baseline in eGFR (95% CI) at week 260 was 3.98 (1.60, 6.36) and 2.71 (1.31, 4.12) ml/min per 1.73 m2 in the HF and no-HF subgroups, respectively.
Figure 2.
eGFR over time by baseline HF status. A RMANCOVA method was used for analysis of mean change from baseline in eGFR (calculated by the MDRD equation). BL, baseline; CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; MDRD, Modification of Diet in Renal Disease; RMANCOVA, repeated measures analysis of covariance; W, week.
In this analysis we found that the long-term rate of eGFR decline was faster (i.e., the yearly decrease in eGFR was larger) in patients with HF at baseline. This was observed within both treatment arms separately (Figure 3 and Table 2), including for the postadjustment (year 1 to year 5), chronic (week 6 to week 260), and total (week 0 to week 260) periods, all of which showed a numerically faster rate of eGFR decline in the HF subgroup than the no-HF subgroup in both placebo and ertugliflozin groups. For example, the total eGFR slope (95% CI) for placebo was −1.70 (−1.94, −1.47) and −1.29 (−1.44, −1.13) ml/min per 1.73 m2 per year in the HF and no-HF subgroups, respectively. However, the placebo-adjusted effect of ertugliflozin on total slope did not differ between the 2 subgroups: 0.96 (0.67, 1.24) and 0.95 (0.76, 1.14) in the HF and no-HF subgroups, respectively (Figure 3).
Figure 3.
Total 5-year eGFR slopes by baseline HF status. Yearly eGFR slope was assessed for the FAS population and analyzed by generalized random coefficient models. CI, confidence interval; eGFR, estimated glomerular filtration rate; FAS, full analysis set; HF, heart failure; LSM, least-squares mean; W, week.
Table 2.
eGFR slopes across all investigated periods by baseline HF status
| HF status | Acute dip (W0–W6)a |
Postdip readjustment (W6–W52)b |
Postadjustment (W52–W260)b |
Chronic (W6–W260)b |
Total (W0–W260)b |
|---|---|---|---|---|---|
| LSM (95% CI) | LSM (95% CI) | LSM (95% CI) | LSM (95% CI) | LSM (95% CI) | |
| Placebo | |||||
| HF | –0.07 (–0.23, 0.09) | –0.63 (–1.67, 0.41) | –2.07 (–2.43, –1.71) | –1.80 (–2.07, –1.53) | –1.70 (–1.94, –1.47) |
| No-HF | –0.07 (–0.19, 0.05) | 0.11 (–0.59, 0.81) | –1.73 (–1.95, –1.50) | –1.40 (–1.57, –1.22) | –1.29 (–1.44, –1.13) |
| Ertugliflozin | |||||
| HF | –0.44 (–0.56, –0.32) | 1.71 (0.96, 2.46) | –1.26 (–1.52, –1.01) | –0.80 (–0.98, –0.61) | –0.75 (–0.91, –0.58) |
| No-HF | –0.59 (–0.67, –0.51) | 1.58 (1.09, 2.06) | –0.84 (–0.99, –0.68) | –0.38 (–0.49, –0.26) | –0.34 (–0.44, –0.23) |
| Placebo-adjusted values | |||||
| HF | –0.37 (–0.57, –0.17) | 2.34 (1.05, 3.62) | 0.81 (0.37, 1.25) | 1.00 (0.68, 1.33) | 0.96 (0.67, 1.24) |
| No-HF | –0.52 (–0.66, –0.38) | 1.47 (0.62, 2.32) | 0.89 (0.62, 1.16) | 1.02 (0.81, 1.23) | 0.95 (0.76, 1.14) |
CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; LSM, least-squares mean; W, week.
Weekly eGFR slope (ml/min per 1.73 m2 per week).
Yearly eGFR slope (ml/min per 1.73 m2 per year).
UACR Over Time and Progression/Regression of Albuminuria
The percent change in UACR from baseline was lower in the ertugliflozin group compared with placebo in both the HF and no-HF subgroups (Supplementary Figure S1). In both subgroups, there was a significant reduction in the risk for progression of albuminuria with ertugliflozin; the hazard ratio (95% CI) for time to first progression of albuminuria was 0.81 (0.69, 0.95) and 0.78 (0.70, 0.87) in the HF and no-HF groups, respectively (Pinteraction = 0.75; Supplementary Table S2). The proportion of patients with a regression in albuminuria status was higher with ertugliflozin than placebo, without a significant modulating effect of the baseline HF status, Pinteraction = 0.07 (Supplementary Table S3).
Discussion
The first observation from the present analysis was that patients with T2DM, ASCVD, and prevalent HF exhibited an accelerated rate of eGFR decline, in both placebo and ertugliflozin groups. This finding of accelerated progression of eGFR decline is consistent with results from previous trials reporting a faster rate of eGFR decline in patients with HF. For example, in populations where the baseline prevalence of HF is relatively low, the rate of eGFR decline in the placebo-treated cohorts is likely to be slower than in populations with established HF; In the EMPA-REG OUTCOME trial (10% of participants with HF at baseline1), the chronic eGFR slope in the placebo group was −1.46 ml/min per 1.73 m2 per year,19 and in the CANVAS Program (14% of participants with HF at baseline), chronic long-term eGFR slope in the placebo group was also relatively slow at −0.9 ml/min per 1.73 m2 per year.20 By contrast, in the EMPEROR-Reduced and DAPA-HF trials in patients with established HF with reduced ejection fraction, mean chronic eGFR slopes for the placebo groups were numerically greater at −2.28 ml/min per 1.73 m2 per year7 and −2.87 ml/min per 1.73 m2 per year,21 respectively. Similarly, in the EMPEROR-Preserved trial in individuals with HF and preserved ejection fraction, the mean eGFR slope in the placebo group was –2.62 ml/min per 1.73 m2 per year.8 Although caution is required when comparing rates of eGFR across different trial cohorts, these observations suggest a steeper rate of eGFR decline in patients with HF.
The factors responsible for the faster rate of eGFR decline in the setting of HF are not well understood, but likely involve a complex interaction between neurohormonal activation leading to vasoconstriction, medications such as diuretics that induce a “prerenal” state, and increased activation of proinflammatory pathways leading to kidney fibrosis.22 Importantly, mean baseline eGFR and UACR levels generally did not differ between the HF and no-HF groups, making it unlikely that these clinical parameters contributed significantly to different rates of eGFR decline between the groups.
The second major observation from the present analyses was that ertugliflozin was associated with kidney effects that were consistent across subgroups of trial participants with and without HF at baseline. Specifically, the impact of ertugliflozin placebo-adjusted change in eGFR slope did not differ in those with and without HF at baseline. The magnitude of mean placebo-adjusted eGFR slope in both the HF and no-HF groups was ≥0.75 ml/min per 1.73 m2, which is associated with a clinically meaningful reduction in the risk of end-stage kidney disease.23 In the present analyses, effects on UACR reduction, as well as rates of UACR progression and regression did not differ between the 2 groups stratified by baseline HF, again reflecting a consistent impact of ertugliflozin on kidney-related parameters captured in the VERTIS CV trial, regardless of baseline HF status. Finally, the prespecified exploratory kidney composite outcome using the sustained ≥40% decline in eGFR-based definition did not vary on the basis of HF status, further emphasizing that kidney clinical effects were consistent across HF status subgroups.
These analyses have limitations. Because patients were enrolled on the basis of ASCVD and not HF, HF status was only present in a limited subgroup of participants. For those with HF, limited data are available regarding systolic function and etiology of HF. No comment can be made about kidney benefits in individuals with established diabetic kidney disease with albuminuria because VERTIS CV patients were generally at low risk of diabetic kidney disease progression24 and the baseline median UACR was in the normal range. This is a post hoc exploratory analysis without adjustments for multiplicity.
To conclude, although patients with HF at baseline had a faster rate of eGFR decline in VERTIS CV, the beneficial effects of ertugliflozin on kidney outcomes did not differ when stratified by baseline HF.
Appendix
List of VERTIS CV Investigators
The list of investigators can be found with the primary publication: Cannon CP, Pratley R, Dagogo-Jack S, et al., for the VERTIS CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. https://doi.org/10.1056/NEJMoa2004967.
Disclosure
DZIC has received consulting fees or speaking honorarium, or both, from AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim-Lilly, Bristol-Myers Squibb, Janssen, JNJ, MAZE, Merck & Co., Inc., Mitsubishi-Tanabe, Novo Nordisk, Otsuka, Prometic, and Sanofi; has received operating funds from AstraZeneca, Boehringer Ingelheim-Lilly, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi; and has served as a scientific advisor or member of AstraZeneca, Boehringer Ingelheim, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi. FC has received fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novo Nordisk, and Pfizer; and research grants from the King Gustav V and Queen Victoria Foundation, the Swedish Heart & Lung Foundation, and the Swedish Research Council. DKM has had leadership roles in clinical trials for AstraZeneca, Boehringer Ingelheim, CSL Behring, Eisai, Esperion, GSK, Janssen, Lexicon, Lilly USA, Merck & Co., Inc., Novo Nordisk, and Sanofi USA; and has received consultancy fees from Afimmune, Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Intercept, Lexicon, Lilly USA, Merck & Co., Inc., Metavant, Novo Nordisk, Pfizer, and Sanofi. AAK has no conflicts of interest to disclose. SD-J has led clinical trials for AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk, Inc.; has received fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Merck & Co., Inc., and Sanofi; and holds equity interests in Jana Care, Inc., and Aerami Therapeutics. REP is an employee of AdventHealth Translational Research Institute; he reports consulting fees from Bayer AG, Corcept Therapeutics Incorporated, Dexcom, Gasherbrum Bio, Inc., Hanmi Pharmaceutical Co., Hengrui (USA) Ltd., Merck, Novo Nordisk, Pfizer, Rivus Pharmaceuticals, Inc., Sanofi, Scohia Pharma Inc., Sun Pharmaceutical Industries and grants/research support from Hanmi Pharmaceutical Co., Janssen, Metavention, Novo Nordisk, Poxel SA, Sanofi. All funds are paid directly to Dr. Pratley’s employer, AdventHealth, a nonprofit organization that supports education and research. RF is an employee of Pfizer Inc. and may own shares/stock options in Pfizer Inc. and may own stock in Bristol-Myers Squibb. MM is an employee of MSD UK. He may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. C-CL is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. CPC reports research grants and consulting fees from Pfizer Inc., and Merck & Co., Inc., and research grants and consulting fees from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, and Janssen, research grants from Better Therapeutics, Daiichi Sankyo, and Novo Nordisk, and consulting fees from Aegerion/Amryt Alnylam, Amarin, Applied Therapeutics, Ascendia, Biogen, Lexicon, Sanofi, Eli Lilly, and Rhoshan, and reports serving on the Data and Safety Monitoring Boards for the Veteran’s Administration, Applied Therapeutics and Novo Nordisk, outside the submitted work.
Acknowledgments
Funding for this study and analysis was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, in collaboration with Pfizer Inc., New York, NY, USA. The authors would like to thank the patients, their families, and all investigators involved in the VERTIS CV study (registered at ClinicalTrials.gov NCT01986881). Some of the results from these analyses were presented at the annual meeting of the American Heart Association 2021 and the 2022 American College of Clinical Pharmacy Virtual Poster Symposium. Medical writing and/or editorial assistance was provided by Moamen Hammad, PhD, Fiona Van, PhD and Ian Norton, PhD, all of Scion, London, UK according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and Pfizer Inc., New York, NY, USA. AAK was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL125247. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
The Sponsors were involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Data Sharing
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Author Contributions
All authors were involved in at least 1 of the following: conception, design of work, or acquisition, analysis, and/or interpretation of data. All authors were involved with drafting of the manuscript and/or revising or reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Figure S1. Percent change from baseline in UACR over time by baseline heart failure status.
Table S1. Least-squares mean (95% CI) change from baseline in eGFR ml/min per 1.73 m2 over time by HF and treatment group.
Table S2. Cox proportional hazard model for time to first progression of albuminuria by baseline heart failure status.
Table S3. Cox proportional hazard model for time to first regression of albuminuria by baseline heart failure status.
Supplementary Material
Figure S1. Percent change from baseline in UACR over time by baseline heart failure status.
Table S1. Least-squares mean (95% CI) change from baseline in eGFR ml/min per 1.73 m2 over time by HF and treatment group.
Table S2. Cox proportional hazard model for time to first progression of albuminuria by baseline heart failure status.
Table S3. Cox proportional hazard model for time to first regression of albuminuria by baseline heart failure status.
References
- 1.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitchett D., Zinman B., Wanner C., et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosentino F., Cannon C.P., Cherney D.Z.I., et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142:2205–2215. doi: 10.1161/CIRCULATIONAHA.120.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 6.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 7.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 8.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 9.Cannon C.P., McGuire D.K., Pratley R., et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) Am Heart J. 2018;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 11.Cherney D.Z.I., Dagogo-Jack S., McGuire D.K., et al. Kidney outcomes using a sustained ≥40% decline in eGFR: a meta-analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021;44:1139–1143. doi: 10.1002/clc.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherney D.Z.I., Charbonnel B., Cosentino F., et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherney D.Z.I., McGuire D.K., Charbonnel B., et al. Gradient of risk and associations with cardiovascular efficacy of ertugliflozin by measures of kidney function: observations from VERTIS CV. Circulation. 2021;143:602–605. doi: 10.1161/CIRCULATIONAHA.120.051901. [DOI] [PubMed] [Google Scholar]
- 14.Cherney D.Z.I., Cosentino F., Dagogo-Jack S., et al. Ertugliflozin and slope of chronic eGFR: prespecified analyses from the randomized VERTIS CV trial. Clin J Am Soc Nephrol. 2021;16:1345–1354. doi: 10.2215/CJN.01130121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Introductory Guide for Standardised MedDRA Queries (SMQs) Version 22.1. International Council for Harmonisation of Technical Requirements For Registration of Pharmaceuticals For Human Use. MedDRA; Published September 2019. https://admin.meddra.org/sites/default/files/guidance/file/000356_smq_intguide_22_1.pdf Accessed May 20, 2021. [Google Scholar]
- 16.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 17.Cherney D.Z.I., Zinman B., Inzucchi S.E., et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 18.Mallinckrod C.H., Lane P.W., Schnell D., et al. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Inf J. 2008;42:303–319. doi: 10.1177/009286150804200402. [DOI] [Google Scholar]
- 19.Wanner C., Heerspink H.J.L., Zinman B., et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018;29:2755–2769. doi: 10.1681/ASN.2018010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkovic V., de Zeeuw D., Mahaffey K.W., et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 21.Jhund P.S., Solomon S.D., Docherty K.F., et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143:298–309. doi: 10.1161/CIRCULATIONAHA.120.050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangaswami J., Bhalla V., Blair J.E.A., et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 23.Inker L.A., Heerspink H.J.L., Tighiouart H., et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30:1735–1745. doi: 10.1681/ASN.2019010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Raalte D.H., Bjornstad P., Heerspink H.J.L., et al. Importance of standardizing renal outcomes in clinical trials: illustration by recent sodium glucose cotransporter 2 inhibitor studies. Kidney Int. 2021;99:768–770. doi: 10.1016/j.kint.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.