Abstract
Because of the potential for high aerosol transmission during pulmonary function testing and pulmonary procedures, performing these tests and procedures must be considered carefully during the coronavirus disease-2019 (COVID-19) pandemic. Much has been learned about the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by aerosols and the potential for such transmission through pulmonary function tests and pulmonary procedures, and subsequently preventative practices have been enhanced and developed to reduce the risk of transmission of virus to patients and personnel. This article reviews what is known about the potential for transmission of SARS-CoV-2 during pulmonary function testing and pulmonary procedures and the recommended mitigation steps to prevent the spread of COVID-19.
Keywords: COVID-19, SARS-CoV-2, Aerosol transmission, Pulmonary function testing, Bronchoscopy, Tracheostomy, Pleural procedures
Key points
-
•
Pulmonary function testing and procedures represent important challenges during the coronavirus disease-2019 (COVID-19) pandemic because they have the potential for high aerosol generation and transmission of severe acute respiratory syndrome coronavirus 2.
-
•
Important considerations in operating the pulmonary function laboratory and conducting pulmonary procedures are local prevalence and risk of COVID-19, clinical importance of the test, relative risk of the test for aerosol generation, and availability of resources to enhance mitigation of viral transmission to patients and staff.
-
•
Lessons learned during the COVID-19 pandemic will be helpful in managing pulmonary function testing and pulmonary procedures during any similar circumstances in the future.
Introduction
One of the unique features of pulmonary function testing is the effort dependence of testing and the reliance of spirometry on a forced exhalation. By necessity this creates the potential for high aerosol generation, which is of primary concern for transmission of infectious agents. This concern quickly resulted in the abandonment of pulmonary function testing (PFT) at the beginning of the coronavirus disease-2019 (COVID-19) pandemic. Subsequently, many authorities issued summary statements, recommendations, and guidelines on how to best proceed with pulmonary function testing and other pulmonary procedures during the pandemic, especially in cases that were felt to be necessary for proper and appropriate patient care.1, 2, 3, 4, 5, 6, 7, 8, 9 This article reviews the key principles involved in the development of such statements and guidelines as well as review the guidelines themselves. A detailed international document has just been issued that likewise reviews these topics.10
Basics of Aerosol Science
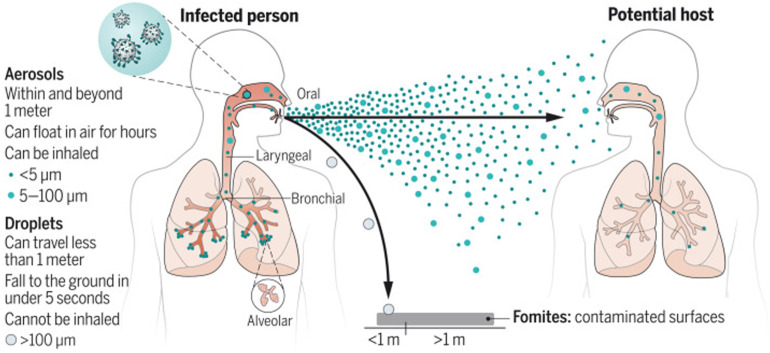
Aerosols are collections of particles that settle out very slowly by gravity because of their small size (Fig. 1 ). Aerosols are characterized by their mass median aerodynamic diameter (MMAD), which can by described by the diameter at which 50% of the particles are larger, and 50% are smaller. Particles of MMAD = 0.5 to 5 μ are considered the respirable fraction because they are most likely to be inhaled and settle into the lung. Larger particles are considered droplets and are typically > 5 to 10 μ in size. Aerosol particles are thought to be generated during inhalation from the reopening of small, collapsed airways during quiet breathing, but additional mechanisms including shear stress and vibration of airway walls contribute to particle generation during coughing or sneezing.11 These aerosols are then emitted during exhalation together with droplets in the range of 0.1 to 1000 μ in size.12 Humans emit aerosols through not only forced exhalation but also quiet breathing and speaking. The factors that determine how droplets and aerosols behave in the air are their size, inertia, gravity, and evaporation.12 If they undergo gravitational settling before evaporation, they can contaminate surfaces on contact. If they undergo evaporation faster than they settle, they remain buoyant and can be transported across distances in the air. Small particles on the order of 0.5 μ in size settle out slowly, whereas larger particles of 20 μ size settle out much more quickly.13
Fig. 1.
Overview of aerosol generation and transport. Aerosols and droplets are emitted during exhalation, including breathing, speaking, and coughing/sneezing. Larger droplets will settle out quickly to surfaces, whereas smaller aerosols may travel significant distances.
(From Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558):eabd9149.)
Evidence that Severe Acute Respiratory Syndrome Coronavirus 2 is Transmitted by Aerosols
Most studies of viral transmission by aerosols have involved influenza. Influenza A, as well as rhinovirus, respiratory syncytial virus (RSV), influenza B, parainfluenza 1, 2, and 3, and human metapneumovirus, have been detected mainly in smaller particles < 5 μ in size. Like these other viruses, severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2), which is 80 to 160 nm in size,14 is thought to transmit primarily by aerosols.13 , 15
Production of Aerosols by Spirometry
Previous work has shown that microbiological contamination of spirometers by aerosol is quite uncommon, with minimal risk of transmission after 5 min of time between tests.16 However, this work was performed using a volume spirometer and did not specifically investigate aerosol transmission of virus, only transmission of non-pathogenic bacteria. To our knowledge, there has never been a reported outbreak of viral infection associated with a PFT laboratory. The first study to show aerosol transmission during spirometry was published based on data from five volunteers at Mayo Clinic.17 Spirometry resulted in an increase in respirable 0.3 μ particles when measured near the exhalation port of the spirometer, but none were detected at 1.5 or 3 feet away. In a subsequent study of 28 patients conducted in 3 PFT laboratories, there was a small increase in ambient small particles (<0.5 μ) with return to pretest baseline within 25 to 30 min.18 Larger particles were also detected. As expected, particles took longer to return to baseline in smaller rooms or those with less ventilation. With the use of bacterial filters, particles were not detected during testing, only after when patients removed the mouthpiece and started talking or breathing without a face mask.19 Another study found small particle emission increases during simple breathing maneuvers that might be used during lung function testing, as well as during cough.20
Production of Aerosols by Other Pulmonary Function Procedures
One study has shown that aerosol generation increases not only during spirometry, but also with measurement of lung volumes and diffusing capacity (DLCO) while the patient sat in a body plethysmograph. There were no differences in particle emissions between tests, although the sample size was small (n = 25).15 Aerosol transmission is increased during peak flow testing, as expected, but the concentration is small.21 Also as expected, methacholine challenge testing, which involves repeated spirometry, results in high emission of ultrafine particles (0.02–1 μ), but particle generation was significantly reduced by using a breath-actuated dosimeter with viral filter.21 Importantly, this study showed significant variability between patients, with most particles detected only a short distance from the mouth despite likely inadequate room ventilation. Of note, the estimated number of viral gene copies emitted was about one order of magnitude higher than would be expected from quiet breathing and the same order of magnitude as expected from normal speaking.
Aerosol generation is also documented after exercise. A study of 8 healthy volunteers showed significant aerosol generation with exercise, although there was considerable heterogeneity between participants.22 Similarly, exercise involved in cardiac rehabilitation resulted in aerosol generation that peaked at 35 to 40 min after the start of each class, even though patients wore procedural masks.23 However, lower levels of exercise did not generate excess particles while participants in a different study wore procedural masks.24 Wearing a surgical mask has been shown to have negative consequences on exercise performance during maximal cardiopulmonary exercise testing (CPET) in young healthy people, including increased dyspnea, lower peak oxygen consumption, and lower anaerobic threshold.25 Aerosol generation during exercise is significantly reduced by use of an high efficiency particulate air (HEPA) filter with fume hood.26
To date, no studies have documented the extent to which aerosols are generated during other pulmonary function tests such as inert gas washout, measurement of fractional excretion of nitric oxide (FeNO), or oscillometry, although oscillometry, in particular, has been suggested as a relatively safe, low aerosol-generating procedure to use during lung function testing.27
Mitigation of Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 by Aerosols
The primary way to reduce COVID transmission is through masking and social distancing.28 Masks are of critical importance to protect people from viral transmission through droplets and aerosols.28 The WHO recommendation for social distancing of 6 feet is based on studies of respiratory droplets performed in the 1930s.28 We now know 100 μ droplets will settle to the ground as far away as 8 feet and a 1 μ aerosol may take up to 12 hours to settle in still air. Coughs and sneezes can result in particles traveling at 8 m/s that can propel particles more than 20 feet.11 Transmission of virus is much less likely outdoors due to air movement, dilution and the effects of UV radiation and concomitant air pollution.28
Recommendations for Operation of the Pulmonary Function Testing Laboratory
Initial guidelines for operation of the PFT laboratory during the pandemic were issued by major medical societies such as American Thoracic Society (ATS), European Respiratory Society (ERS), Occupational Safety and Health Administration (OSHA), and others.1, 2, 3, 4, 5 , 7 , 9 , 14 A comprehensive literature review of available statements at the time was published by Crimi and colleagues.29 An international guideline statement has now been published and is based on the consensus opinion of 23 experts.10 The first section focuses on transmission, environmental and equipment considerations, with specific recommendations for the type of inline filter and personal protective equipment (PPE) to be used during different PFTs. For common procedures such as spirometry, lung volumes and DLCO, these recommendations include use of an inline filter, N95 or equivalent mask, apron/gown and goggles/shield. For high aerosol potential procedures, such as bronchial challenge testing or bronchodilator testing with a nebulizer and CPET, an N99 mask is recommended, and such procedures should ideally be conducted with a filter on the expiratory port and in a negative pressure room, if available. An important point that is emphasized in the document is the proper consideration of room ventilation, as discussed below.
The second section focuses on referral, triage, and PCR testing. The need for the PFT must be weighed against the potential risks of exposing test personnel and other patients to the virus. Low-priority need might include routine screening or monitoring of stable patients, whereas high-priority need might include monitoring of patients with heart or lung transplant, those on potentially pulmonary toxic drugs, or those with severe symptoms for whom diagnostic testing is important to help with diagnosis of dyspnea. High aerosol generation procedures should be avoided, such as bronchial challenge testing and CPET. If a bronchodilator is administered, it is thought better to use a metered dose inhaler than a nebulizer to minimize exogenous aerosol emission from the device, although evidence for this is lacking.19 , 30 Using biofilters on the exhalation port of a nebulizer will effectively reduce escape of aerosols from the device.19 Doing testing in a body plethysmograph could theoretically reduce environmental aerosol exposure. A protocol must be in place for assessing symptoms and need for COVID testing before pulmonary function testing. The timing of testing should consider local prevalence of COVID-19 and the most recent evidence for infectivity, with current recommendations suggesting performing PFTs no earlier than 10 days after onset of illness in mild-moderate COVID if there are 2 negative PCR tests after disease available, no earlier than 20 days in severe patients who have one negative PCR, and no earlier than 30 days with no PCR needed. The latter guideline seems the most sensible since the CDC recommends against repeat PCR testing up to 90 days after acute illness due to persistent positivity that may last that long even though a patient is no longer infectious.31
The third section focuses on operational issues, including staffing and waiting area. Patients should wear a mask in the waiting area after passing the pre-screening procedure. Patients should be distanced at least 6 feet apart, although this may be flexible depending on room configuration and ventilation. There should be physical barriers such as plexiglass shields and equipment covers to prevent cross-contamination and protect both patients and staff.
The fourth section focuses on testing room precautions including air conditioning and ventilation. It is recommended there be at least 6 air changes per hour but 12 to 15 is better,14 , 32 and testing in a negative pressure room is preferable. Recently, the value of portable HEPA filters in reducing aerosol contamination has been shown.33 Reducing viral transmission by UV light also holds promise.34
The fifth section focuses on lung function testing procedures, with detailed instructions for spirometry, bronchodilator testing, lung volume measurement, DLCO, FeNO, oscillometry, capnography, muscle pressures, 6-min walk test, CPET, and bronchial challenge tests. Of particular importance is the proper use of in-line filters, which should be of sufficient efficacy in screening out viral-sized particles smaller than SARS Co-V-2 (<0.8 μ) while maintaining low enough airway resistance to allow high peak expiratory flows of up to 700 L/min. Manufacturers should provide testing data to show that their filters have at least 99.9% efficiency.
The sixth section focuses on the management of special populations, such as pediatrics, elderly, lung cancer and surgery patients, immunocompromised patients. Precautions should be taken to prevent infection and cross-contamination in each context, and caregivers of these patients should be adequately protected as well.
The final section focuses on testing outside the hospital, for example, in community and primary care offices. In non-hospital settings, it is important to consider the availability and proper use of in-line filters vs. disposable sensors, PPE, room ventilation, and screening and waiting room policies. In this context, telemedicine or remote, video-coached spirometry is becoming more prevalent. New techniques, such as the use of wearable electromagnetic sensors, have also been described to allow for non-contact, point-of-care monitoring of pulmonary function during the COVID-19 pandemic.35
Outcomes in Pulmonary Function Testing Laboratories So Far
There is no evidence to date that the PFT laboratory has been the source of an outbreak of COVID-19, which is remarkable considering the high-aerosol-generating potential of the procedures involved. Only one study has specifically analyzed the incidence of COVID-19 after lung function testing.36 In a retrospective analysis of 278 patients tested between April and September 2020, the cumulative incidence of COVID-19 within 15 days of testing was 0.36%, with none of the technicians developing symptomatic disease. This finding is very encouraging and speaks to the efficacy of following protocols and guidelines to mitigate spread of COVID-19.
Regarding PFT laboratory performance and adherence to guidelines, a survey of 132 laboratories conducted between August and October of 2020 found that nearly all laboratories required adequate PPE for their technologist, including the use of N95 masks for at least some procedures.37 Likewise, nearly all laboratories used in-line filters, used proper recommended room and equipment cleaning and 83% provided time for air exchange. Although screening for COVID-19 symptoms and temperature checks were nearly universal, PCR testing was variable depending on local COVID prevalence. By the close of the survey, 71% of laboratories were fully operational, demonstrating how PFT laboratories were able to cope with the pandemic in its early phases.
A qualitative review of international practices among pediatric PFT laboratories has also been published.38 A unique feature of this review included the concern of both patients and parents in terms of symptom screening, waiting room conditions, and use of face masks, which is especially challenging for younger children. In addition, special mention was made of the sequence of testing, with the most aerosol-generating procedures such as forced exhalation and exercise testing done last to be able to leave the room as soon as such testing is completed.
Another review of PFT laboratory practices during the pandemic revealed a sharp decline in total number of PFTs performed starting in March 2020, reaching a maximum decline in April 2020, and then variably rebounding since then.39 Bronchoscopies showed the smallest decline, and there were more ambulatory exercise tests and CPETs than compared with before the pandemic.
Pulmonary Procedures Other Than Pulmonary Function Testing
The following section will review pulmonary procedures in the context of COVID-19, including bronchoscopy, tracheostomy, and pleural procedures. The pulmonologist may be in a unique position of risk due to the nature of the procedures performed involving the airway and hence the high aerosol-generating potential of these procedures. In addition, pulmonologists may be involved not only in the care of acutely ill patients with COVID-19, but also of those recovering from the illness who may require post-tracheostomy care or diagnostic bronchoalveolar lavage (BAL) or lung biopsy in the setting of persistent pulmonary fibrosis.40
Bronchoscopy
Bronchoscopy is classified as a high aerosol-generating procedure, which could lead to accidental transmission of droplet and airborne particles to health care professionals.41 In the COVID-19 era, indications for bronchoscopy have not changed apart from following a more cautious approach and careful selection of the procedure if absolutely needed.
Safety of Bronchoscopy in Patients with Coronavirus Disease-2019
In a review published by Saha and colleagues,42 12 cohorts (9 retrospective and 3 prospective) reported on the safety of bronchoscopy and transmission of virus among bronchoscopists. A total of 2245 bronchoscopies were performed among 1345 patients. Eleven of 12 studies (92%) specified the use of PPE. All the health care workers used full PPE, including gown, face shield, eye protector, shoe cover, double gloves, filtering face pieces (FFP2/FFP3), N95 mask, and, sometimes, a powered air-purifying respirator. Only 57% reported the use of negative pressure rooms for all their procedures.42 Only one study reported 1 bronchoscopist who developed COVID-19 during the 3 weeks of study.43
Recommendations for Bronchoscopy from Major Respiratory Societies
American College of Chest Physicians/American Association for Bronchology and Interventional Bronchoscopy (CHEST/AABIP) guidelines suggest avoiding invasive methods like bronchoscopy with BAL to establish diagnosis unless necessary.8 A nasopharyngeal specimen should be obtained first in patients suspected of having COVID-19 infection. In patients with severe respiratory failure who require intubation, specimens from an endotracheal aspirate or bronchoscopy with BAL can be performed.8 In terms of therapeutic bronchoscopy, this guideline-recommended four indications for emergent bronchoscopy regardless of COVID-19 status which included (1) moderate to severe tracheobronchial stenosis; (2) symptomatic central airway obstruction; (3) massive hemoptysis and (4) stent migration. Meanwhile, in patients with confirmed COVID-19 infection and undergoing elective bronchoscopy, the guideline recommended waiting at least 30 days from the resolution of symptoms with two, negative consecutive nasopharyngeal swab PCR specimens collected > 24 hours apart.8 However, local practice varies by the waiting time for negative tests and the number of tests before elective procedures.
The Society for Advanced Bronchoscopy (SAB) provided similar recommendations to CHEST/AABIP,6 with combined recommendations for urgency of bronchoscopy shown in Table 1 . The SAB described the advantages of single-use (disposable) flexible bronchoscopes, which, in addition to avoiding reprocessing equipment, requires less equipment to set up, and only needs a single user to operate, decreasing the number of contact personnel. However, the access to disposable bronchoscopes may vary based on local resources.44 Other procedural recommendations include intubation with general anesthesia rather than conscious sedation, using an endotracheal tube rather than laryngeal mask airway for a tighter airway seal, and consideration of use of paralytics to abolish coughing.8 , 45 , 46 For rigid bronchoscopy, a recommendation is made for a closed-circuit ventilation system rather than jet ventilation.47 Other international expert panels from Europe and Asia also provided recommendations for bronchoscopy which were very similar to other respiratory societies as mentioned above.48
Table 1.
Relative urgency and timing of indications for bronchoscopy
| Emergent (Same day) | Urgent (1–2 d) | Non-urgent (>2 d) |
|---|---|---|
| Acute foreign body aspiration | Infiltrates in neutropenic or immunocompromised host with fever | Airway inspection for cough or minor hemoptysis |
| Massive hemoptysis (without obvious source for embolization) | Lung mass or mediastinal/hilar adenopathy suspicious for cancer | Mild central airway stenosis |
| Severe symptomatic central airway obstruction or stenosis | Non-massive hemoptysis | Clearance of mucus |
| Migrated stent | Whole lung lavage | Suspected sarcoidosis |
| Acute lobar atelectasis | Detection of chronic infection (eg, mycobacterial, fungal) | |
| Chronic interstitial lung disease | ||
| Bronchoscopic lung volume reduction or bronchial thermoplasty | ||
| Evaluation for tracheobronchomalacia | ||
| Tracheostomy changes | ||
| Surveillance transplant bronchoscopy |
Data from Wahidi MM, Shojaee S, Lamb CR, et al. The Use of Bronchoscopy During the Coronavirus Disease 2019 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest. 2020;158(3):1268-1281 and Pritchett MA, Oberg CL, Belanger A, et al. Society for Advanced Bronchoscopy Consensus Statement and Guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis. 2020;12(5):1781-1798.
Lastly, decontamination of the procedural area is important. Recent literature shows that the SARS-CoV-2 virus can remain aerosolized for up to 3 hours and can be found on surfaces for up to 3 days, depending on the surface type.49 The room turnover time will depend on changing of room air volume. The negative-pressure bronchoscopy rooms require a minimum of 12 total air exchanges per hour to provide dilution and exhaust of contaminated air.31 At this rate, after 23 min 99% of particles will be cleared.6
In conclusion, flexible bronchoscopy must be cautiously performed amid the COVID-19 crisis. Judicious case selection and meticulous contact and airborne precautions are important to minimize infection transmission. Mandatory universal PPE, pre-bronchoscopy PCR tests, dedicated protective barriers and disposable bronchoscopes might be the safest and simplest way to perform bronchoscopy in the setting of COVID-19.
Tracheostomy
Tracheostomy is another high aerosol-generating procedure with risk of infectious transmission for health care workers.50
Optimal Time to Perform Tracheostomy in Coronavirus Disease-2019 Patients
Since the start of the pandemic, the duration of mechanical ventilation for COVID-19-related acute respiratory distress syndrome (ARDS) has been longer compared with non-COVID-19-related ARDS, with many COVID-19 patients remaining intubated for at least one to 2 weeks or longer.51, 52, 53 This observation has been true for patients with asymptomatic COVID-19 infection as well, who have been admitted to intensive care unit (ICU) for trauma-related causes.54 Despite this observation of prolonged ventilation, there is insufficient evidence on optimal timing to perform tracheostomy on COVID patients. The CHEST/AABIP guidelines did not recommend a specific timing of tracheostomy due to insufficient evidence to suggest performing a tracheostomy either early (<10 days) or late (>14 days).55
The early tracheostomy strategy accelerates weaning from the ventilator and may have a critical role in freeing up ventilators, ICU beds, and staff during surges.56 However, there was a concern of COVID-19 transmission for operators and health care workers. The early report of case series for tracheostomy from New York University showed a median of 10.6 days from intubation to tracheostomy. There were no team members testing positive for SARS-CoV-2.57 A multicenter, retrospective study included 118 COVID-19 patients who underwent tracheostomy.58 Early tracheostomy (≤14 days) was associated with decreased ventilator days, decreased ventilator-associated pneumonia, and shorter ICU duration and shorter hospital length of stay (LOS) among patients who were discharged. The median time from intubation to tracheostomy was 22 days with 78% of patients undergoing percutaneous dilatational technique vs. 22% surgical technique. Although there was a concern of COVID-19 infection risk for health care workers, a high rate of infection has not been reported. The systematic review and meta-analysis of 69 studies indicated that enhanced PPE is associated with low rates of SARS-CoV-2 transmission during tracheostomy.59
Meanwhile, delaying tracheostomy may reduce the risk to health care workers because the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be lower, but that reduction in risk must be weighed against prolonged intubation.56 SARS-CoV-2 infectivity peaks 3 to 4 days after infection, and infectivity is diminished by waiting 10 days before performing tracheostomy.56 In addition, SARS-CoV-2 viral loads are highest in upper respiratory tract mucosa, particularly the nasopharynx.60 Thus, aerosolization may be less in patients with mechanical ventilation. A cross-sectional review of institutional protocols and practices from 26 countries showed timing for tracheostomy varied from 3 to > 21 days depending on risk of implied infectivity to personnel performing and handling tracheostomy.61 In this study, over 90% of protocols recommended 14 days of intubation before tracheostomy. Most protocols advocate delaying tracheostomy until COVID-19 testing was negative. A report from a single tertiary care institution reviewed 258 invasive mechanical ventilation patients out of whom 46 (18%) required tracheostomy (Table 2 ). Although tracheostomy placement in patients with COVID did not decrease overall LOS, duration of mechanical ventilation, or ICU LOS, patients with a tracheostomy experienced a significantly lower number of deaths vs. those without tracheostomy.62 A systematic review and meta-analysis of 18 studies exploring 3234 COVID-19 patients showed that only 5.2% of tracheostomies were performed within 7 days (early), and 21.2% were performed between days 8 and 13, whereas most (71.5%) of the tracheostomies were performed 14 days or later post-intubation. The meta-analysis did not reveal the benefit of early tracheostomy in terms of duration of mechanical ventilation or time to decannulation, nor was late tracheostomy associated with increased mortality.63 , 64
Table 2.
Suggested requirements for safe testing in the pulmonary function testing laboratory and selective pulmonary procedures
| Test/Procedure | In-Line Antimicrobial Filter | Mask (N95 or Equivalent) | Gown | Eye Protection | Other |
|---|---|---|---|---|---|
| Spirometry | + | + | + | + | |
| Lung volumes body box gas dilution | + | + | + | + | |
| DLCO | + | + | + | + | |
| Bronchodilator MDI nebulizer |
– + |
+ + |
+ + |
+ + |
Filter on expiratory port |
| Bronchial challenge testing | + | + | + | + | Negative pressure room if available |
| 6 min walk | – | + | + | – | |
| CPET | – | + | + | + | Negative pressure room if available |
| Oscillometry | + | + | + | + | |
| FeNO | + | + | + | + | |
| MIP/MEP | + | + | + | + | |
| Bronchoscopy | NA | + | + | + | Negative pressure room if available |
| Tracheostomy | NA | + | + | + | Negative pressure room if available |
| Pleural procedures | + (in suction tubing) | + | + | + |
+, required; - , not required.
Modified from McGowan A, Laveneziana P, Bayat S, et al. International consensus on lung function testing during the COVID-19 pandemic and beyond. ERJ Open Res. 2022;8(1):00602-2021. Published 2022 Mar 7.
Based on above studies, there is evidence supporting both strategies. Thus, a specific timing of tracheostomy cannot be recommended. When COVID-19 overwhelms capacity in ICUs, early timing of tracheostomy may accelerate ventilator weaning and free up critical care resources. General decisions surrounding optimal timing in the critically ill patient can be complex outside of the pandemic, but given the clinical evidence so far, one may conclude that early tracheostomy may be a better option for these patients given the overall benefits to patients and the health care system. However, more data and research are required.
Tracheostomy Techniques in the Coronavirus Disease Era
As for other high aerosol-generating procedures, patient selection for tracheostomy is important. Contraindications including hemodynamic instability, hypoxemia (Pao 2/Fio 2 <200), high intracranial pressure (>20 mm Hg), multi-organ failure, coagulopathy, platelet dysfunction or anticoagulation, surgical site infection, history of major cervical surgery that alters cervical flexion-extension, and abnormal cervical anatomy apply to COVID patients just as any patient.65 Patients who are in the prone position or likely to be placed prone for respiratory failure should not be considered for tracheostomy due to the increased risk of complications that is, tube displacement, occlusion, or impaired ability to identify complications.56
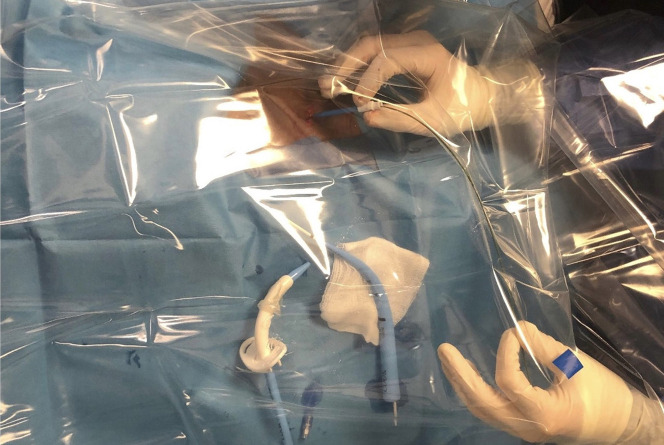
Multiple factors influence the technique for this procedure. The first consideration is the location of the patients. These patients are critically ill and usually admitted to the ICU, hence percutaneous technique is preferred as it minimizes transfer. Ideally aerosolizing procedures should be performed in a negative pressure room but those are not always available. Performing tracheostomy at the bedside offers advantages of time availability as compared with needing to schedule in the operating room. Some disadvantages include less trained ancillary staff, and less equipment to control complications. To minimize aerosolization, Angel and colleagues have developed a modified percutaneous technique where the flexible bronchoscope was passed alongside the endotracheal tube (through vocal cords) and not inside the tube as in conventional percutaneous tracheostomy.57 This modified technique permitted the operator to perform uninterrupted mechanical ventilation after re-positioning the inflated endotracheal tube cuff to the distal trachea. It offered significant mitigation of the risk of virus aerosolization during the procedure. Another modification described is percutaneous tracheostomy using an acrylic box as an aerosol shield (Fig. 2 ).66 This technique had the least aerosolization and contamination but has a long procedure time compared with standard technique with or without a ventilator pause. Another prospective single-system multi-center observational cohort study was conducted examining safety for both patients and health care workers.67 In this series, 29 percutaneous tracheostomies were performed; 19 cases used a conventional technique with intermittent ventilator pause and 10 cases used a modified technique. There was no report of health care worker transmissions resulting from performing the procedure.
Fig. 2.
A clear plastic coverage is used to provide an additional barrier to contamination during percutaneous dilatational tracheostomy. Shown are the positions of the bronchoscopist at the side of a surgical field.
(Adapted from Majid A, Ayala A, Uribe JP, et al. Protective Strategies in a Simulated Model When Performing Percutaneous Tracheostomies in the COVID-19 Era. Ann Am Thorac Soc. 2020;17(11):1486-1488.)
These proposed modifications of standard percutaneous tracheostomy provide minimal aerosolization which may mitigate viral transmission to operator and health care workers. The selection of the technique should depend on the expertise of operator, availability of team and resources, and overall safety of patient and providers. It is imperative that appropriate PPE is always used by every member of the procedural team.68
Pleural Procedures
All pleural procedures, including thoracentesis, chest tube insertion and pleural biopsy by thoracostomy or pleuroscopy, might be considered aerosol-generating as patients may cough, and theoretically virus-containing aerosols may be emitted from a chest drain with an air leak.40 SARS-CoV-2 viral RNA has also been detected in pleural fluid,40 making direct transmission by contact also a potential risk due to fluid splashing and contamination. A statement from the American Association for the Surgery of Trauma (AAST) Acute Care Surgery and Critical Care Committees recommends putting in-line antimicrobial filters on the suction line of a chest tube drainage system while on suction or on water seal.69 Although no other special techniques are described to perform these procedures, the usual precautions in terms of stratifying by current risk of active infection and prioritization of the procedure should apply, with personnel wearing appropriate PPE in all circumstances where a patient has COVID-19 or their COVID-19 status is unknown A summary of suggested requirements for safe testing in the pulmonary function laboratory and selective pulmonary procedures is provided in Table 2.
Transitioning Back to Normal Operations
To date, there are no formal guidelines about how to make the transition back to normal operations of the PFT laboratory or pulmonary procedures, other than some societies recommending "return to pre-COVID standards" in the post-pandemic phase.5 , 70 However, the Canadian Thoracic Society has explicitly stated that "return to pre-pandemic infection control practices in the PFT testing will not provide acceptable risk mitigation", and lessons learned and additional precautions taken during COVID-19 should result in updated PFT laboratory testing protocols to protect against SARS CoV-2 as well as other emerging pathogens.71 It makes sense that any transition back to normal operations will need to include ongoing consideration of local prevalence of COVID-19, immune or vaccination status of the community, and importance of testing or procedure for patient relative to risk to PFT and proceduralist staff. Screening procedures will, and already have, become less involved, with many institutions no longer requiring PCR testing and only relying on symptom screening. A selective rather than universal approach to PCR testing before elective surgery has been shown to be safe.72 The role of antigen testing is unclear although has been proposed.73 Individual institutional policy will dictate local PPE and infection control procedures, such as wearing N95 or procedural masks, eye protection, contact precautions, cleaning and disinfection, and procedure room ventilation.
Summary
The COVID-19 pandemic has caused us to carefully reconsider all that we do in the PFT laboratory, from the importance of ordering the test, to the screening of patients, waiting room conditions, testing environment, personnel protection, and decontamination procedures. Although like many aspects of health care the PFT laboratory initially had to stop operations for all but the most urgent conditions, laboratories around the world have adapted to COVID-19, and are most are back in full operation. It is a tribute to the testing personnel and the careful attention and care to infection prevention that the PFT laboratory has not been a source of community spread during the COVID-19 pandemic despite the high aerosol-generating potential of its many procedures. Similarly, regarding pulmonary procedures, it is mandatory that contact precautions and proper training on donning and doffing of PPE be provided to all health care workers. Another key element is advanced planning and keeping each procedure unit well-organized. Although the reduction in the number of elective procedures represents one of the central strategies to improve safety, it is crucial that patients not suffer unnecessary delays in diagnostic or therapeutic procedures. The lessons learned so far regarding the impact of the COVID-19 pandemic on PFTs and pulmonary procedures will certainly apply to any potential future outbreaks.
Clinics care points
-
•
Whether and how to perform pulmonary function testing during the COVID-19 pandemic depends on many factors related to importance of test, risk of aerosol spread, prevalence of local disease, and adequacy of safe testing environment.
-
•
Environmental safety features related to pulmonary function testing include adequate room ventilation, proper PPE for testing personnel, in-line mouth filters, cleaning and disinfecting of equipment, and use of meter dose inhalers rather than nebulizers for administration of aerosolized bronchodilators.
-
•
Whether and how to perform pulmonary procedures also relies on balancing risk vs. benefit with careful attention to protection of health care workers.
-
•
Special protection of health care workers during pulmonary procedures includes adequate PPE and room ventilation, minimizing exposure to staff, intubation rather than concious sedation, use of closed-circuit ventilation rather than jet ventilation during rigid bronchoscopy, and performance of percutaneous tracheostomy with physical shielding from aerosol exposure.
Disclosure
Dr D.A. Kaminsky is a speaker for MGC Diagnostics, Inc. and a contributor to UptoDate, Inc. Dr S.M. Husnain has no conflicts of interest and any funding sources Dr D. Khemasuwan has no conflicts of interest and any funding sources.
Funding sources
Dr D.A. Kaminsky is funded, in part, by the Vermont Lung Center.
References
- 1.Harber P., MC T., Levine M. Occupational spirometry and fit testing in the COVID-19 era: 2021 interim recommendations from the american college of occupational and environmental medicine. https://acoem.org/Guidance-and-Position-Statements/Guidance-and-Position-Statements/Occupational-Spirometry-and-Fit-Testing-in-the-COVID-19-Era-2021-Interim-Recommendations-from-the-American-Collegeof-Occupational-and-Environmental-Medicine Available from:
- 2.American thoracic society. May 18, 2022. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php Available from:
- 3.Association for respiratory technology and physiology and british thoracic society. org.uk/write/MediaUploads/Standards/COVID19/Respiratory_Function_Testing_During_Endemic_COVID_V1.5.pdf May 18, 2022. Available from:
- 4.Canadian society of respiratory therapists. May 18, 2022. https://www.csrt.com/csrt-novel-coronavirus-resources/ Available from:
- 5.European respiratory society. May 18, 2022. https://ers.app.box.com/s/zs1uu88wy51monr0ewd990itoz4tsn2h Available from:
- 6.Pritchett M.A., Oberg C.L., Belanger A., et al. Society for advanced bronchoscopy consensus statement and guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis. 2020;12:1781–1798. doi: 10.21037/jtd.2020.04.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoracic society of Australia and New Zealand. May 18, 2022. https://www.thoracic.org.au/information-public/tsanz-update Available from:
- 8.Wahidi M.M., Shojaee S., Lamb C.R., et al. The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP guideline and expert panel report. Chest. 2020;158:1268–1281. doi: 10.1016/j.chest.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson K.C., Kaminsky D.A., Michaud G., et al. Restoring pulmonary and sleep services as the covid-19 pandemic lessens. from an association of pulmonary, critical care, and sleep division directors and american thoracic society-coordinated task force. Ann Am Thorac Soc. 2020;17:1343–1351. doi: 10.1513/AnnalsATS.202005-514ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan A., Laveneziana P., Bayat S., et al. International consensus on lung function testing during the COVID-19 pandemic and beyond. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00602-2021. 00602-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C.C., Prather K.A., Sznitman J., et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558):eabd9149. doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheuch G. Breathing is enough: for the spread of influenza virus and SARS-CoV-2 by breathing only. J Aerosol Med Pulm Drug Deliv. 2020;33:230–234. doi: 10.1089/jamp.2020.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federation of european heating ventilation and air conditioning associations. 2022. https://www.rehva.eu/activities/covid-19-guidance/rehva-covid-19-guidance Available from:
- 15.Tomisa G., Horváth A., Farkas Á., et al. Real-life measurement of size-fractionated aerosol concentration in a plethysmography box during the COVID-19 pandemic and estimation of the associated viral load. J Hosp Infect. 2021;118:7–14. doi: 10.1016/j.jhin.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiebert T., Miles J., Okeson G.C. Contaminated aerosol recovery from pulmonary function testing equipment. Am J Respir Crit Care Med. 1999;159:610–612. doi: 10.1164/ajrccm.159.2.9803116. [DOI] [PubMed] [Google Scholar]
- 17.Helgeson S.A., Lim K.G., Lee A.S., et al. Aerosol generation during spirometry. Ann Am Thorac Soc. 2020;17:1637–1639. doi: 10.1513/AnnalsATS.202005-569RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Jing G., Fink J.B., et al. Airborne particulate concentrations during and after pulmonary function testing. Chest. 2021;159:1570–1574. doi: 10.1016/j.chest.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink J.B., Ehrmann S., Li J., et al. Reducing aerosol-related risk of transmission in the era of COVID-19: an interim guidance endorsed by the international society of aerosols in medicine. J Aerosol Med Pulm Drug Deliv. 2020;33:300–304. doi: 10.1089/jamp.2020.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greening N.J., Larsson P., Ljungström E., et al. Small droplet emission in exhaled breath during different breathing manoeuvres: implications for clinical lung function testing during COVID-19. Allergy. 2021;76:915–917. doi: 10.1111/all.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subat Y.W., Guntupalli S.K., Sajgalik P., et al. Aerosol generation during peak flow testing: clinical implications for COVID-19. Respir Care. 2021;66:1291–1298. doi: 10.4187/respcare.08731. [DOI] [PubMed] [Google Scholar]
- 22.Sajgalik P., Garzona-Navas A., Csécs I., et al. Characterization of aerosol generation during various intensities of exercise. Chest. 2021;160:1377–1387. doi: 10.1016/j.chest.2021.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helgeson S.A., Taylor B.J., Lim K.G., et al. Characterizing particulate generation during cardiopulmonary rehabilitation classes with patients wearing procedural masks. Chest. 2021;160:633–641. doi: 10.1016/j.chest.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 24.Helgeson S.A., Lee A.S., Patel N.M., et al. Cardiopulmonary exercise and the risk of aerosol generation while wearing a surgical mask. Chest. 2021;159:1567–1569. doi: 10.1016/j.chest.2020.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G., Li M., Zheng M., et al. Effect of surgical masks on cardiopulmonary function in healthy young subjects: a crossover study. Front Physiol. 2021;12:710573. doi: 10.3389/fphys.2021.710573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzona-Navas A., Sajgalik P., Csécs I., et al. Mitigation of aerosols generated during exercise testing with a portable high-efficiency particulate air filter with fume hood. Chest. 2021;160:1388–1396. doi: 10.1016/j.chest.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta N., Sachdev A., Gupta D. Oscillometry-A reasonable option to monitor lung functions in the era of COVID-19 pandemic. Pediatr Pulmonol. 2021;56:14–15. doi: 10.1002/ppul.25121. [DOI] [PubMed] [Google Scholar]
- 28.Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368:1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- 29.Crimi C., Impellizzeri P., Campisi R., et al. Practical considerations for spirometry during the COVID-19 outbreak: literature review and insights. Pulmonology. 2021;27:438–447. doi: 10.1016/j.pulmoe.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein K.M., Ghadimi K., Mystakelis H., et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155–170. doi: 10.1089/jamp.2020.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention 2022. https://www.cdc.gov/media/releases/2021/s1227-isolation-quarantine-guidance.html Available from:
- 32.Centers for Disease Control and Prevention https://acoem.org/Guidance-and-Position-Statements/Guidance-and-Position-Statements/Occupational-Spirometry-and-Fit-Testing-in-the-COVID-19-Era-2021-Interim-Recommendations-from-the-A May 18, 2022. Available from:
- 33.Lindsley W.G., Derk R.C., Coyle J.P., et al. Efficacy of portable air cleaners and masking for reducing indoor exposure to simulated exhaled SARS-CoV-2 Aerosols - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:972–976. doi: 10.15585/mmwr.mm7027e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiappa F., Frascella B., Vigezzi G.P., et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: a systematic review. J Hosp Infect. 2021;114:63–78. doi: 10.1016/j.jhin.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahrestani S., Chou T.C., Shang K.M., et al. A wearable eddy current based pulmonary function sensor for continuous non-contact point-of-care monitoring during the COVID-19 pandemic. Sci Rep. 2021;11:20144. doi: 10.1038/s41598-021-99682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainstein E.J., Peroni H.J., Ferreyro B.L., et al. Incidence of COVID-19 after pulmonary function tests: a retrospective cohort study. Rev Fac Cien Med Univ Nac Cordoba. 2021;78:367–370. doi: 10.31053/1853.0605.v78.n4.34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders M.J., Haynes J.M., McCormack M.C., et al. How local SARS-CoV-2 Prevalence shapes pulmonary function testing laboratory protocols and practices during the COVID-19 pandemic. Chest. 2021;160:1241–1244. doi: 10.1016/j.chest.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beydon N., Gochicoa L., Jones M.J., et al. Pediatric lung function testing during a pandemic: an international perspective. Paediatr Respir Rev. 2020;36:106–108. doi: 10.1016/j.prrv.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Call J.T., Lee A.S., Haendel M.A., et al. The effect of the COVID-19 pandemic on pulmonary diagnostic procedures. Ann Am Thorac Soc. 2022;19:695–697. doi: 10.1513/AnnalsATS.202108-943RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piro R., Casalini E., Livrieri F., et al. Interventional pulmonology during COVID-19 pandemic: current evidence and future perspectives. J Thorac Dis. 2021;13:2495–2509. doi: 10.21037/jtd-20-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zietsman M., Phan L.T., Jones R.M. Potential for occupational exposures to pathogens during bronchoscopy procedures. J Occup Environ Hyg. 2019;16:707–716. doi: 10.1080/15459624.2019.1649414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha B.K., Saha S., Chong W.H., et al. Indications, clinical utility, and safety of bronchoscopy in COVID-19. Respir Care. 2022;67:241–251. doi: 10.4187/respcare.09405. [DOI] [PubMed] [Google Scholar]
- 43.Torrego A., Pajares V., Fernández-Arias C., et al. Bronchoscopy in patients with COVID-19 with invasive mechanical ventilation: a single-center experience. Am J Respir Crit Care Med. 2020;202:284–287. doi: 10.1164/rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barron S.P., Kennedy M.P. Single-use (Disposable) flexible bronchoscopes: the future of bronchoscopy? Adv Ther. 2020;37:4538–4548. doi: 10.1007/s12325-020-01495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lentz R.J., Colt H. Summarizing societal guidelines regarding bronchoscopy during the COVID-19 pandemic. Respirology. 2020;25:574–577. doi: 10.1111/resp.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ost D.E. Bronchoscopy in the age of COVID-19. J Bronchology Interv Pulmonol. 2020;27:160–162. doi: 10.1097/LBR.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozturk A., Sener M.U., Yılmaz A. Bronchoscopic procedures during COVID-19 pandemic: experiences in Turkey. J Surg Oncol. 2020;122:1020–1026. doi: 10.1002/jso.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo F., Darwiche K., Singh S., et al. Performing bronchoscopy in times of the COVID-19 pandemic: practice statement from an international expert panel. Respiration. 2020;99:417–422. doi: 10.1159/000507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tay J.K., Khoo M.L., Loh W.S. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020;146:517–518. doi: 10.1001/jamaoto.2020.0764. [DOI] [PubMed] [Google Scholar]
- 51.Bain W., Yang H., Shah F.A., et al. COVID-19 versus Non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc. 2021;18:1202–1210. doi: 10.1513/AnnalsATS.202008-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. Jama. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad M., Laghi F.A., Jr., Brofman J., et al. Long-term acute care hospital outcomes of mechanically ventilated patients with coronavirus disease 2019. Crit Care Med. 2022;50:256–263. doi: 10.1097/CCM.0000000000005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klutts G.N., Squires A., Bowman S.M., et al. Increased lengths of stay, ICU, and ventilator days in trauma patients with asymptomatic COVID-19 infection. Am Surg. 2022;88(7):1522–1525. doi: 10.1177/00031348221082290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamb C.R., Desai N.R., Angel L., et al. Use of tracheostomy during the COVID-19 pandemic: american college of chest physicians/american association for bronchology and interventional pulmonology/association of interventional pulmonology program directors expert panel report. Chest. 2020;158:1499–1514. doi: 10.1016/j.chest.2020.05.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGrath B.A., Brenner M.J., Warrillow S.J., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angel L., Kon Z.N., Chang S.H., et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg. 2020;110:1006–1011. doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmood K., Cheng G.Z., Van Nostrand K., et al. Tracheostomy for COVID-19 respiratory failure: multidisciplinary, multicenter data on timing, technique, and outcomes. Ann Surg. 2021;274:234–239. doi: 10.1097/SLA.0000000000004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staibano P., Levin M., McHugh T., et al. Association of tracheostomy with outcomes in patients with COVID-19 and SARS-CoV-2 transmission among health care professionals: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147:646–655. doi: 10.1001/jamaoto.2021.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bier-Laning C., Cramer J.D., Roy S., et al. Tracheostomy during the COVID-19 pandemic: comparison of international perioperative care protocols and practices in 26 countries. Otolaryngol Head Neck Surg. 2021;164:1136–1147. doi: 10.1177/0194599820961985. [DOI] [PubMed] [Google Scholar]
- 62.Molin N., Myers K., Soliman A.M.S., et al. Otolaryngol Head Neck Surg; 2022. COVID-19 tracheostomy outcomes. 1945998221075610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benito D.A., Bestourous D.E., Tong J.Y., et al. Tracheotomy in COVID-19 patients: a systematic review and meta-analysis of weaning, decannulation, and survival. Otolaryngol Head Neck Surg. 2021;165:398–405. doi: 10.1177/0194599820984780. [DOI] [PubMed] [Google Scholar]
- 64.Mattioli F., Fermi M., Ghirelli M., et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2133–2135. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith D., Montagne J., Raices M., et al. Tracheostomy in the intensive care unit: guidelines during COVID-19 worldwide pandemic. Am J Otolaryngol. 2020;41:102578. doi: 10.1016/j.amjoto.2020.102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majid A., Ayala A., Uribe J.P., et al. Protective strategies in a simulated model when performing percutaneous tracheostomies in the COVID-19 Era. Ann Am Thorac Soc. 2020;17:1486–1488. doi: 10.1513/AnnalsATS.202004-372RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao T.N., Harbison S.P., Braslow B.M., et al. Outcomes after tracheostomy in COVID-19 patients. Ann Surg. 2020;272:e181–e186. doi: 10.1097/SLA.0000000000004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chee V.W., Khoo M.L., Lee S.F., et al. Infection control measures for operative procedures in severe acute respiratory syndrome-related patients. Anesthesiology. 2004;100:1394–1398. doi: 10.1097/00000542-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Pieracci F.M., Burlew C.C., Spain D., et al. Tube thoracostomy during the COVID-19 pandemic: guidance and recommendations from the AAST acute care surgery and critical care Committees. Trauma Surg Acute Care Open. 2020;5(1):e000498. doi: 10.1136/tsaco-2020-000498. PMID: 32411822; PMCID: PMC7213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodríguez Moncalvo J.J., Brea Folco J.C., Arce S.C., et al. Recommendations for pulmonary function laboratories in the COVID-19 era. Medicina (B Aires) 2021;81:229–240. [PubMed] [Google Scholar]
- 71.Stanojevic S., Beaucage F., Comondore V., et al. Resumption of pulmonary function testing during the post-peak phase of the COVID-19 pandemic. a position statement from the canadian thoracic society and the canadian society of respiratory therapists. Can J Respir Crit Care Sleep. 2020;4 [Google Scholar]
- 72.Moreno-Pérez O., Merino E., Chico-Sánchez P., et al. Effectiveness of a SARS-CoV-2 infection prevention model in elective surgery patients - a prospective study: does universal screening make sense? J Hosp Infect. 2021;115:27–31. doi: 10.1016/j.jhin.2021.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fouzas S., Gidaris D., Karantaglis N., et al. Pediatric pulmonary function testing in COVID-19 pandemic and beyond. a position statement from the hellenic pediatric respiratory society. Front Pediatr. 2021;9:673322. doi: 10.3389/fped.2021.673322. [DOI] [PMC free article] [PubMed] [Google Scholar]