Abstract
Direct acting antivirals and monoclonal antibodies reduce morbidity and mortality associated with severe acute respiratory syndrome coronavirus 2 infection. Persons at higher risk for disease progression and hospitalized patients with coronavirus disease-2019 (COVID-19) benefit most from available therapies. Following an emphasis on inpatient treatment of COVID-19 during the early pandemic, several therapeutic options were developed for outpatients with COVID-19. Additional clinical trials and real-world studies are needed to keep pace with the evolving pandemic.
Keywords: COVID-19, SARS-CoV-2, Remdesivir, Molnupiravir, Nirmatrelvir, Bebtelovimab, Tixagevimab/cilgavimab
Key points
-
•
Coronavirus disease-2019 (COVID-19) therapies are most effective when administered early in the disease course.
-
•
Standard of care for hospitalized persons with moderate-to-severe COVID-19 remains remdesivir with immunomodulatory therapy.
-
•
Oral antiviral agents and monoclonal antibodies are cornerstones of outpatient COVID-19 treatment.
Introduction
With 614 million cumulative cases and over 6.3 million deaths worldwide as of September 30, 2022, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic persists.1 The periodic emergence of novel variants produces a changing landscape, making the development of novel mitigation strategies to reduce coronavirus disease-2019 (COVID-19) associated morbidity and mortality increasingly important. Vaccination and other public health measures (eg, masking and social distancing) are vital for reducing infection rates, but disparities related to distribution and uptake of vaccines remain.2 , 3
For persons who develop SARS-CoV-2 infection, clinical manifestations are highly variable, and illness severity can range from asymptomatic to life-threatening.4 , 5 In addition to supportive care, treatment is indicated for outpatient persons at risk for disease progression or inpatient persons with moderate-to-severe disease. A diverse assemblage of COVID-19 management strategies has been evaluated, and several evidence-based treatment options are now available (Fig. 1 , Table 1 ).
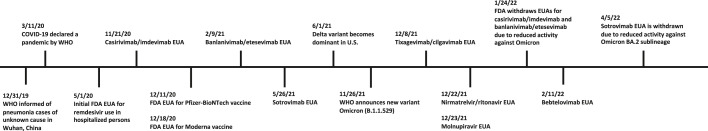
Fig. 1.
Timeline for SARS-CoV-2 antiviral therapies in the United States. FDA, US Food and Drug Administration; EUA, emergency use authorization; WHO, World Health Organization.
Table 1.
Direct acting antivirals and monoclonal antibodies for SARS-COV-2
| Therapeutic Agent | Mechanism of Action | Indication(s) for Use | Drug-Drug Interaction(s) | Contraindication(s) | Adverse Event(s) |
|---|---|---|---|---|---|
| Remdesivir | Adenosine analog whose active form interferes with RNA-dependent RNA polymerase | 5-day course for inpatient persons with moderate-to-severe COVID-19; 3-day course for outpatient persons with COVID-19 at-risk for disease progression | No significant interactions | Advanced hepatic or renal impairment | Constipation, nausea, possible transaminitis or AKI |
| Nirmatrelvir/ritonavir | Nirmatrelvir is an inhibitor of SARS-CoV-2 3CL protease; ritonavir is an HIV protease inhibitor co-administered to inhibit CYP3A4 and achieve therapeutic nirmatrelvir levels | 5-day course for outpatient persons with COVID-19 at-risk for disease progression | Co-administered medications with CYP3A4 metabolism (eg, phenytoin, tacrolimus, and warfarin) may impair antiviral activity or cause toxicity | Avoid co-administered medications with significant CYP3A4 interactions; avoid use in pregnant individuals | Diarrhea, dysgeusia |
| Molnupiravir | Ribonucleoside analog of N-hydroxycytidine whose active form interferes with RNA-dependent RNA polymerase | 5-day course for outpatient persons with COVID-19 at-risk for disease progression | No significant interactions | Risk-benefit discussion advised for pregnant individuals | Diarrhea, nausea, dizziness |
| Casirivimab/imdevimab | Monoclonal antibody which binds SARS-CoV-2 Spike protein | Single-dose infusion for outpatient persons with COVID-19 at-risk for disease progression (EUA withdrawn January 24, 2022) | No significant interactions | No absolute contraindications | Infusion-related reaction |
| Bamlanivimab/etesevimab | Monoclonal antibody which binds SARS-CoV-2 Spike protein | Single-dose infusion for outpatient persons with COVID-19 at-risk for disease progression (EUA withdrawn January 24, 2022) | No significant interactions | No absolute contraindications | Infusion-related reaction |
| Sotrovimab | Monoclonal antibody which binds SARS-CoV-2 Spike protein | Single-dose infusion for outpatient persons with COVID-19 at-risk for disease progression (EUA withdrawn April 5, 2022) | No significant interactions | No absolute contraindications | Infusion-related reaction |
| Bebtelovimab | Monoclonal antibody which binds SARS-CoV-2 Spike protein | Single-dose infusion for outpatient persons with COVID-19 at-risk for disease progression | No significant interactions | No absolute contraindications | Infusion-related reaction |
| Tixagevimab/cilgavimab | Monoclonal antibody which binds SARS-CoV-2 Spike protein | Preexposure prophylaxis (two 600 mg doses) for persons who are not anticipated to respond to vaccine series and/or unable to complete vaccine series due to hypersensitivity reaction | No significant interactions | No absolute contraindications | Injection site reaction |
Abbreviations: AKI, acute kidney injury; EUA, emergency use authorization; HIV, human immunodeficiency virus; RNA, ribonucleic acid.
In light of the pathophysiology of COVID-19, treatment options aim to reduce viral replication, block viral entry into host cells, and/or modulate the host immune response. In this review, we focus on the body of evidence supporting the use of direct antiviral agents and monoclonal antibodies for the treatment of COVID-19. We aim to enumerate treatment options and summarize management approaches in relevant patient populations.
Direct antiviral agents
Remdesivir
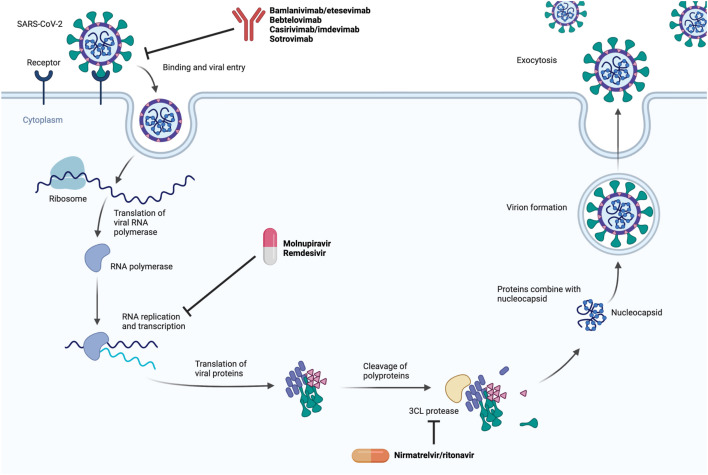
Remdesivir is an adenosine analog with in vitro activity against SARS-CoV-2.6 Its spectrum of antiviral activity extends to other RNA viruses (eg, Ebola virus).7 After intracellular processing, remdesivir’s active triphosphate form interferes with RNA-dependent RNA polymerase and causes termination of RNA transcripts.7 Fig. 2 displays an overview of the SARS-CoV-2 replication cycle and the stage at which remdesivir exerts its antiviral activity. Remdesivir, administered intravenously, is US Food and Drug Administration (FDA) approved for the treatment of moderate-to-severe SARS-CoV-2 infection; oral formulations are not available due to poor bioavailability in humans.8
Fig. 2.
Mechanism of SARS-CoV-2 antiviral therapies.
(Created with BioRender.com.)
For hospitalized adult patients with COVID-19 who have evidence of lower respiratory tract involvement, the use of remdesivir is supported by ACTT-1, a double-blind, randomized control trial (RCT) comparing remdesivir (n = 541) with placebo (n = 521).9 The median recovery time for patients who received remdesivir was 10 days compared with 15 days in those who received placebo (rate ratio for recovery, 1.29; 95% confidence interval [CI] 1.12–1.49; p < 0.001). In addition, Kaplan–Meier mortality estimates were lower for remdesivir compared with placebo at day 15 (6.7% vs 11.9%; hazard ratio [HR] 0.55, 95% CI 0.36–0.83), but differences in mortality estimates were not statistically significant at day 29 (11.4% vs 15.2%; HR 0.73, 95% CI 0.52–1.03).
Patients in the ACTT-1 trial received remdesivir for up to 10 days,9 with a duration of therapy extrapolated from data on remdesivir’s use in the treatment of other viral infections.10 , 11 A randomized, open-label, phase 3 trial evaluated a 5-day course of remdesivir (n = 200) compared with a 10-day course (n = 197) in hospitalized adult patients with SARS-CoV-2 infection, oxygen saturation ≤94% on room air and evidence of pneumonia on imaging studies.10 The investigators used a 7-point ordinal scale to evaluate clinical status at day 14 and did not find a significant difference between treatment groups after adjusting for differences in baseline clinical status. Results from this RCT are the basis for the 5-day treatment duration currently recommended by National Institutes of Health (NIH) and Infectious Diseases Society of America (IDSA) guidelines.12 , 13
The use of remdesivir in combination with baricitinib, a Janus kinase inhibitor with anti-inflammatory activity, has also been studied in hospitalized adult patients with COVID-19. The ACTT-2 trial was a double-blind, RCT comparing remdesivir and baricitinib (n = 515) with remdesivir and placebo (n = 518).14 The median time to recovery was 7 days in patients receiving remdesivir and baricitinib compared with 8 days in patients receiving remdesivir and placebo (rate ratio for recovery 1.16; 95% CI 1.01–1.32; p = 0.03). The benefit of combination therapy was more pronounced for patients receiving high-flow oxygen or noninvasive ventilation at time of enrollment. Overall, there was no significant difference in 28-day mortality (5.1% remdesivir and baricitinib vs 7.8% remdesivir and placebo; HR 0.65; 95% CI 0.39–1.09).
More recently, the ACTT-4 trial, a double-blind, double-placebo RCT, compared remdesivir plus baricitinib plus placebo (n = 516) with remdesivir plus dexamethasone plus placebo (n = 494) in hospitalized adults with COVID-19 who required supplemental oxygen by low flow, high flow, or noninvasive ventilation.15 The primary outcome of mechanical ventilation-free survival at day 29 was not significantly different between treatment groups (87.0% vs 87.6%); however, more adverse events (p = 0.014), treatment-related adverse events (p = 0.00041), and severe or life-threatening grade 3 or 4 adverse events (p = 0.012) impacted the remdesivir plus dexamethasone plus placebo group. In addition to its combination with dexamethasone or baricitinib, remdesivir’s use has been investigated in combination with tocilizumab,16 and several tocilizumab RCTs included notable percentages of patients who received co-administered remdesivir (33% to 55%).17 , 18 A single RCT has compared remdesivir plus tocilizumab (n = 434) with remdesivir plus placebo (n = 215) in hospitalized patients with severe COVID-19 requiring >6 L/min of supplemental oxygen.16 The primary outcome of time elapsed between randomization and discharge or “ready for discharge” (evaluated using a 7-category ordinal scale) to day 28 was not different between treatment groups (median time 14 days in both groups).16 However, based on other evidence, tocilizumab is recommended as an immunomodulatory alternative to baricitinib in combination with remdesivir and dexamethasone.12 , 13
Remdesivir has also been evaluated as an agent to prevent disease progression in the outpatient setting.19 The PINETREE trial was a double-blind, RCT that compared a 3-day course of remdesivir (n = 279) to placebo (n = 283) for outpatients with COVID-19 who had ≥1 risk factor for disease progression and ≤7 days of symptoms. COVID-19-related hospitalization or all-cause mortality at day 28 was lower for patients who received remdesivir (0.7%) compared with placebo (5.3%) (HR 0.13; 95% CI 0.03–0.59; p = 0.008). Notably, no deaths had occurred in either group by day 28. However, there are logistical challenges to setting up outpatient infusions of remdesivir.
In clinical trials, commonly reported adverse events associated with remdesivir include nausea, constipation, elevated aminotransferase levels, and acute kidney injury.9 , 10 Reports of significant hepatic injury are rare, and most serum aminotransferase elevations associated with remdesivir’s use are self-resolving.20 It is unclear if nephrotoxicity observed in the setting of remdesivir use is a result of the drug or of end-organ damage in the setting of COVID-19 infection. There is concern that different formulations of remdesivir may be more nephrotoxic due to increased amounts of sulfobutylether-β-cyclodextrin, but a retrospective analysis did not support this concern,21 whereas another suggests that remdesivir is not nephrotoxic.22 Nonetheless, it is prudent to participate in shared decision making with patients who qualify for remdesivir and have significant renal impairment.
Overall, the current body of evidence continues to support remdesivir’s use in hospitalized patients with moderate-to-severe COVID-19. Although remdesivir resistance mutations have been reported in immunocompromised patients with delayed viral clearance,23 widespread resistance to remdesivir has not emerged during the SARS-CoV-2 pandemic.24 Newer evidence supports remdesivir’s role in preventing disease progression in the outpatient setting, and further data exploring its real-world effectiveness in outpatient populations at-risk for disease progression are needed. Importantly, logistical challenges to its outpatient administration will require creative solutions, and oral antiviral therapies remain more convenient in the interim.
Nirmatrelvir/Ritonavir
Nirmatrelvir is an inhibitor of the SARS-CoV-2 3CL protease (also known as Mpro) and results in disruption of polyprotein cleavage during viral replication.25 It is co-administered with ritonavir, an HIV protease inhibitor that inhibits CYP3A4 metabolism to achieve therapeutic plasma levels.26 In contrast to remdesivir’s intravenous route of administration, nirmatrelvir/ritonavir is orally administered as a twice daily combination of two tablets (300 mg of nirmatrelvir and 150 mg of ritonavir).
Nirmatrelvir/ritonavir is currently available under FDA emergency use authorization (EUA) for early treatment (within 5 days symptom onset) of outpatients with COVID-19 who are at risk for disease progression. In the EPIC-HR double-blind RCT, a 5-day course of nirmatrelvir/ritonavir (n = 1120) was compared with placebo (n = 1126) in symptomatic, non-hospitalized adults with COVID-19 within 5 days of symptom onset who were at risk for disease progression.26 In the final analysis for the modified intention to treat population (n = 1379), the estimated event rate of COVID-19 related hospitalization or all-cause mortality at day 28 was 5.81 percentage points lower for those who received nirmatrelvir/ritonavir (0.72%) compared with placebo (6.53%) (relative risk reduction 88.9%; 95% CI −7.78 to −3.84; p < 0.001). No deaths were reported in the nirmatrelvir/ritonavir group compared with thirteen deaths in the placebo group. Nirmatrelvir/ritonavir has also been evaluated as post-exposure prophylaxis in the Phase 2/3 EPIC-PEP trial, but the differences in risk reduction offered by 5-day and 10-day courses of nirmatrelvir/ritonavir were not significant relative to placebo.27
In the EPIC-HR trial, the incidence of adverse events was similar for patients who received nirmatrelvir/ritonavir (22.6%) compared with placebo (23.9%).26 Dysgeusia and diarrhea were more commonly observed in the nirmatrelvir/ritonavir group compared with placebo. Owing to ritonavir’s CYP3A4 inhibition, nirmatrelvir/ritonavir’s use must account for co-administered medications which interact with the same cytochrome P450 enzyme (eg, phenytoin, cyclosporine, and warfarin).28 Successful implementation of nirmatrelvir/ritonavir requires systematic approaches to dose monitoring in this subpopulation of patients. There are no clinical data to guide the use of nirmatrelvir/ritonavir in pregnancy, but adverse events after exposure to nirmatrelvir were observed in embryo-fetal developmental studies in mammals.29 , 30 The risk of progression to severe COVID-19 should be weighed against potential adverse fetal effects when making a decision on use in pregnancy.
In general, nirmatrelvir/ritonavir is an oral antiviral therapy for preventing disease progression in outpatient persons with COVID-19, and it resulted in an 89% relative risk reduction in hospitalization or all-cause mortality at day 28 in the EPIC-HR trial.26 Its use requires careful monitoring or discontinuation of co-administered medications that interact with CYP3A428; however, this challenge has been overcome in select patient populations (eg, solid organ transplant recipients31). Preliminary reports on “rebound” SARS-CoV-2 infection after nirmatrelvir/ritonavir suggest a mild clinical presentation, but the phenomenon requires further characterization.32 , 33 Patients are advised to follow the Centers for Disease Control and Prevention standard COVID-19 isolation protocol in the event of “rebound.”33
Molnupiravir
Molnupiravir is a ribonucleoside analog of N-hydroxycytidine with antiviral activity against SARS-CoV-2 and related RNA viruses.34 It becomes activated intracellularly and incorporated into RNA by RNA-dependent RNA polymerase, resulting in mutations and eventual loss of viral replication ability. Similar to nirmatrelvir/ritonavir, it is available under FDA EUA as a 5-day oral treatment course (four 200-mg tablets twice daily).35 Molnupiravir is intended for outpatients within 5-days of COVID-19 symptoms onset who are at risk for disease progression.36
Molnupiravir use is supported by the MOVe-OUT trial, a double-blind RCT comparing molnupiravir (n = 716) to placebo (n = 717) for nonvaccinated, non-hospitalized adult patients with COVID-19 at risk for disease progression who were within 5 days of symptom onset.36 For the whole randomized, intention-to-treat population, hospitalization or death at day 29 was lower in the molnupiravir group compared with placebo (6.8% vs 9.7%; 95% CI −5.9 to −0.1). A time-to-event analysis showed a roughly 30% lower rate of hospitalization or death at day 29 for patients who received molnupiravir. Of note, an interim analysis of the same trial showed a 50% decrease in all-cause hospitalization or death at day 29 compared with placebo, suggesting possible computational errors during the trial’s data analysis.37 In contrast to the MOVe-OUT trial,36 a double-blind RCT evaluating molnupiravir at 3 dose levels (200 mg, 400 mg and 800 mg twice daily) for hospitalized adult patients with COVID-19 within 10 days of symptom onset did not show a meaningful benefit for patients receiving molnupiravir compared with placebo.38
In the MOVe-OUT trial, the incidence of adverse events was similar between treatment groups (30.4% in molnupiravir group vs 33% in placebo group).36 Diarrhea, nausea, and dizziness were the most frequent adverse events deemed to be related to the administered regimen (ie, molnupiravir or placebo). Pregnant persons were excluded from the MOVe-OUT trial,36 and molnupiravir is not recommended during pregnancy due to evidence of teratogenicity from animal studies.35 In the absence of alternative therapies, use of molnupiravir in pregnant patients at high risk of progression may be considered beyond 10 weeks of gestation after a documented discussion of risk-benefits between patient and provider.39 Owing to concerns of mutagenicity, patients of reproductive age are also advised to use reliable forms of contraception during and after administration of molnupiravir (4 days after last dose in women, 3 months after last dose in men).35
Similar to nirmatrelvir/ritonavir, molnupiravir is an oral antiviral option for prevention of disease progression in at-risk, outpatient persons with COVID-19. Although molnupiravir increases pill burden (8 pills daily) and did not perform as well as nirmatrelvir/ritonavir in an RCT,26 , 36 its administration does not require meticulous monitoring of co-administered medications that interact with CYP3A4. The safety of molnupiravir use in pregnant patients and patients of reproductive age requires further study, and a risk-benefit discussion ought to inform these patients’ care.35 In addition, the effectiveness of oral antiviral agents is less characterized in high-risk, immunocompromised patients due to RCTs’ focus on general patient populations, and further data are needed.
Monoclonal antibodies
Monoclonal antibodies are sourced from convalescent patients or humanized mice and can serve as antiviral therapies.40 For SARS-CoV-2, monoclonal antibody therapies block fusion and entry of virus by binding the receptor-binding domain (RBD) of the Spike protein on the virion particle’s surface.40 Given the natural history of COVID-19,5 monoclonal antibodies are most effective when administered early after symptom onset, and their use is authorized for outpatient persons with COVID-19. Unvaccinated patients, immunocompromised patients who may not respond to vaccine series, and other at-risk patient populations stand to benefit most from passive immunization with monoclonal therapies.
Studies involving the use of monoclonal antibodies in hospitalized adults have yielded mixed results.41, 42, 43, 44 Nonetheless, it is interesting to note that casirivimab/imdevimab led to a statistically significant (p = 0.0009) reduction in 28-day mortality for hospitalized patients without detectable antibodies to SARS-CoV-2 (ie, seronegative patients) in the RECOVERY trial.41 These data suggest a potential role for monoclonal antibody administration in seronegative hospitalized patients, especially immunocompromised individuals who may not mount a humoral response to vaccines.45 , 46
In addition to serving as antiviral therapy, monoclonal antibodies have been evaluated as preexposure prophylaxis for SARS-CoV-2.47 Despite previous authorizations, monoclonal antibodies are not currently recommended as postexposure prophylaxis. The following sections summarize monoclonal antibodies with active or prior FDA EUA.
Treatment
Casirivimab/imdevimab
Casirivimab and imdevimab are two monoclonal antibodies identified via high-throughput screening which target the SARS-CoV-2 Spike RBD.48 The use of casirivimab/imdevimab, administered within 72 hours of a positive viral test and 7 days of symptom onset, was evaluated in an adaptive trial that compared 2 different doses [2400 mg (n = 1355) and 1200 mg (n = 736)] with placebo [2400 mg placebo (n = 1341) and 1200 mg placebo (n = 748)] in preventing disease progression in non-hospitalized patients with COVID-19 and ≥ 1 risk for severe disease.48 At day 29, the primary outcome of COVID-19-related hospitalization or death from any cause had occurred in 1.3% of the 2400 mg group compared with 4.6% in the placebo group (relative risk reduction 71.3%; p < 0.001). The 1200 mg group had similar results (relative risk reduction 70.4%; p = 0.002). Owing to efficacy concerns related to the Omicron variant, the FDA EUA was withdrawn for casirivimab/imdevimab on January 24, 2022.49
Bamlanivimab/etesevimab
Originally isolated from plasma of individuals who recovered from COVID-19, bamlanivimab and etesevimab are recombinant monoclonal antibodies with neutralizing activity against the SARS-CoV-2 Spike protein.50 In the BLAZE-1 RCT, the combination of bamlanivimab and etesevimab was evaluated as a means to prevent disease progression in at-risk, non-hospitalized patients with COVID-19 within 3 days of laboratory diagnosis.50 The primary outcome of COVID-19-related hospitalization or all-cause death at day 29 was 4.9 percentage points lower in the bamlanivimab/etesevimab group (n = 518) relative to placebo (n = 517) (absolute risk difference −4.8%; 95% CI −7.4 to −2.3; p < 0.001). Ten deaths occurred in the placebo group versus no deaths in the bamlanivimab/etesevimab group. Owing to concerns of decreased neutralizing activity with the Omicron variant, the EUA for bamlanivimab/etesevimab in the United States was withdrawn on January 24, 2022.51
Sotrovimab
Sotrovimab is a monoclonal antibody which targets a conserved epitope of the RBD of the Spike protein on sarbecoviruses, including SARS-CoV-2.52 Early administration of sotrovimab (within 5 days of symptom onset) was evaluated for prevention of disease progression in non-hospitalized, at-risk adults with COVID-19 in the COMET-ICE trial.53 , 54 At the interim analysis, 1% of the patients who received sotrovimab (n = 291) experienced the primary outcome of hospitalization or death from any cause at day 29 compared with 7% in the placebo group (n = 292) (relative risk reduction 85%; 97.24% CI 44–96; p = 0.002). Rates of adverse were similar in the sotrovimab (17%) and placebo (19%) groups, and infusion-related reactions affected 1% of both groups. Despite its initial promise in treating patients with infections due to the Omicron variant, the sustained rise in the Omicron BA.2 sublineage, against which sotrovimab has significantly reduced neutralizing activity,55 has led to withdrawal of its EUA by the FDA as of April 5, 2022.56
Bebtelovimab
Bebtelovimab is a monoclonal antibody which targets the SARS-CoV-2 Spike RBD, with activity against Omicron subvariants57 and was first authorized for use in the United States on February 11, 2022 under an FDA EUA.58 Bebtelovimab is indicated for prevention of disease progression in high risk outpatients with mild-to-moderate COVID-19. The clinical evidence supporting bebtelovimab’s use stems from the phase 2 BLAZE-4 trial which was notably conducted before the emergence of Omicron.58 , 59 The placebo-controlled arm of the study evaluated low-risk, non-hospitalized patients within 3 days of symptom onset and randomized participants in three groups: bebtelovimab (n = 125), bebtelovimab/bamlanivimab/etesevimab (n = 127), and placebo (n = 128). The primary endpoint of persistently elevated viral load (log viral load >5.27) at day 7 occurred in 21% of the placebo group compared with 13% in the combination group and 14% in the bebtelovimab group. Differences between groups were not statistically significant. Further trial data and real-world datasets are needed.
Preexposure Prophylaxis
Tixagevimab/cilgavimab
The monoclonal antibody combination tixagevimab/cilgavimab, which bind to non-overlapping loci of the SARS-CoV-2 Spike protein,47 received an FDA EUA on December 8, 2021, for the preexposure prophylaxis of COVID-19 for immunocompromised individuals who may not mount an adequate response to available vaccines or individuals with contraindications to current vaccines due to serious adverse events.60 Its use as preexposure prophylaxis is supported by the PROVENT phase 3 trial which compared tixagevmab/cilgavimab administered as two consecutive injections of 300 mg doses (n = 3460) with saline placebo (n = 1737).47 The primary endpoint of symptomatic COVID-19 occurred in 0.2% (8/3441) in the tixagevimab/cilgavimab group versus 1.0% (17/1731) in the placebo group (Relative risk reduction 76.7%; 95% CI 46–90 p < 0.001). Unlike other available monoclonal antibodies for SARS-CoV-2, tixagevimab/cilgavimab is not currently authorized for treatment of COVID-19 or postexposure prophylaxis.60 The combination is expected to have activity against the Omicron BA.2 sublineage, with reduced activity against BA.1 and BA.1.160; however, real-world data are limited. In addition, the PROVENT trial was conducted before the emergence of Omicron, and the dosage of tixagevimab/cilgavimab currently recommended by the FDA (two 600 mg injections) is double the trial’s dose.60 A recent report has highlighted the risk of SARS-CoV-2 infection in the time period following administration and emphasized the need to reinforce masking and social distancing in patients who receive tixagevimab/cilgavimab.61
Other therapies for treatment of coronavirus disease-2019
In addition to the direct acting antivirals and monoclonal antibodies discussed above, numerous therapies have been evaluated as treatment options for COVID-19. Some therapies (eg, convalescent plasma)62 , 63 have yielded mixed results in clinical trials, whereas others have consistently shown no meaningful benefit. Supplement Table 1 summarizes data on select therapeutic options not currently recommended as treatment of COVID-19.
Management of outpatient coronavirus disease-2019 and preexposure prophylaxis
Outpatient management of COVID-19 in at-risk patient populations is essential in preventing hospitalization and progression of disease. Fig. 3 provides an overview of the Yale New Haven Health System’s (YNHHS) clinical pathway for the management of mild-to-moderate COVID-19 in the outpatient setting. This pathway is largely consistent with guideline statements published by the NIH64 and IDSA.12 When evaluating persons with COVID-19 in an outpatient setting, providers must first identify and appropriately triage patients who may benefit from hospitalization. To qualify for outpatient therapies, patients must also have symptom lengths within acceptable time frames and have predisposing factors that confer a higher risk for disease progression.
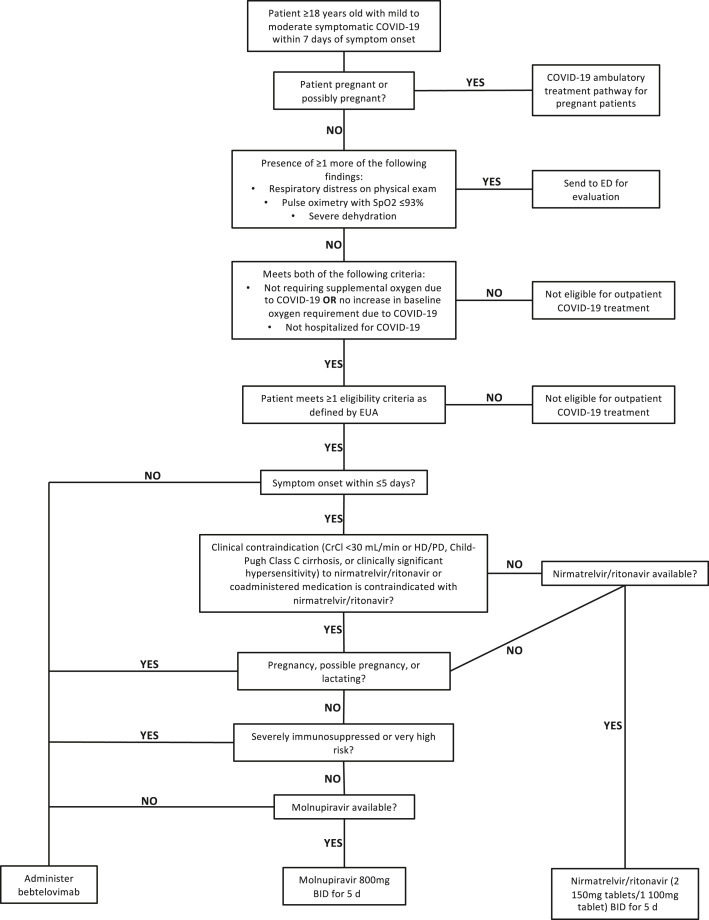
Fig. 3.
Clinical pathway for management of outpatient persons with COVID-19. Clinical pathway as of May 16, 2022. Definitions for “severely immunosuppressed” and “very high risk” provided in Supplement Table 2. CrCl, creatinine clearance; ED, emergency department; EUA, emergency use authorization; HD, hemodialysis; PD, peritoneal dialysis.
After these initial considerations are addressed, patients’ comorbidities and symptom lengths determine which of three treatment options is appropriate. For persons with ≤5 days of symptoms and no contraindications (eg, co-administered medication interacts with CYP3A4), our center prioritizes the use of nirmatrelvir/ritonavir for 5 days. If nirmatrelvir/ritonavir is contraindicated, molnupiravir and bebtelovimab are alternative options. Molnupiravir, administered as a 5-day course, is offered to patients with contraindications to nirmatrelvir/ritonavir who are neither pregnant nor severely immunocompromised. Bebtelovimab is preferred for lactating or pregnant patients, patients who are severely immunocompromised and patients who are beyond 5 days but within 7 days of symptom onset. Our center does not offer remdesivir to outpatient persons with COVID-19 due to logistical constraints; however, remdesivir is a preferred therapy in the NIH guidelines for management of outpatient COVID-19,64 and its use in outpatient settings is supported by the PINETREE trial.19
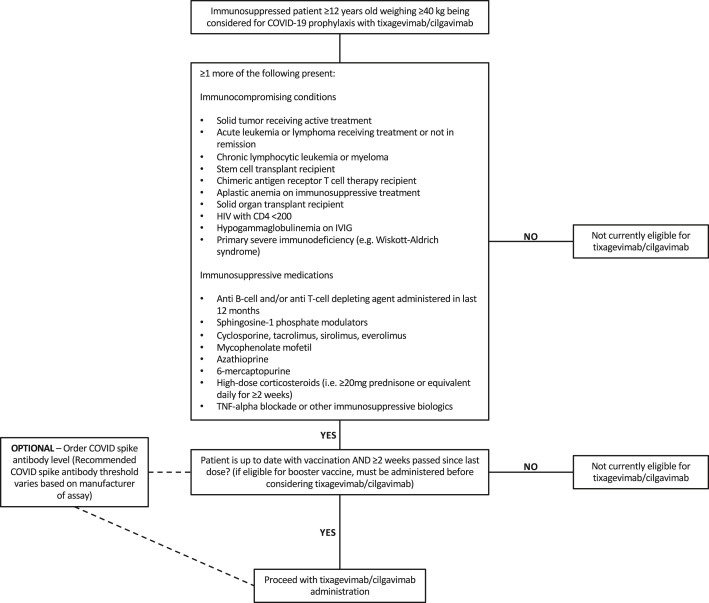
To prevent COVID-19 in immunocompromised patients who may not respond to vaccines and/or patients with severe hypersensitivity reactions to current vaccines, our center offers tixagevimab/cilgavimab as preexposure prophylaxis (Fig. 4 ). In accordance with the FDA’s EUA,60 eligible patients must be ≥ 12 years and weigh ≥40 kilograms with one or more immunocompromising conditions or recent use of immunosuppressive medication(s). Our center’s list of qualifying comorbidities includes conditions with functional or numerical deficits in T cells and/or B cells. Patients eligible for tixagevimab/cilgavimab in the YNHHS are required to have received the most recent vaccine dose >2 weeks beforehand.
Fig. 4.
Clinical pathway for preexposure prophylaxis with tixagevimab/cilgavimab. Clinical pathway as of May 16, 2022. HIV, human immunodeficiency virus; IVIG, intravenous immunoglobulin.
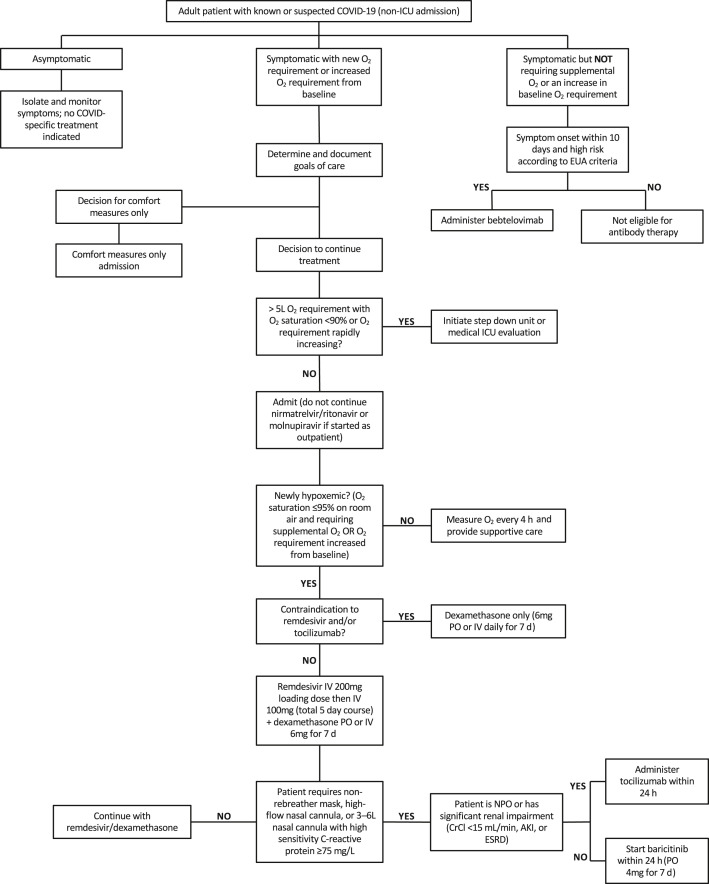
Management of inpatient coronavirus disease-2019
Management of hospitalized adults with COVID-19 varies based on the severity of COVID-19-related symptoms. Fig. 5 summarizes the YNHHS clinical pathway for hospitalized adults with known or suspected COVID-19. The pathway is largely consistent with guidelines published by the NIH13 and IDSA.12 For asymptomatic infections, no COVID-19-specific treatments are administered, and close monitoring with appropriate isolation is recommended.
Fig. 5.
Clinical pathway for management of inpatient persons with COVID-19. Clinical pathway as of May 16, 2022. AKI, acute kidney injury; CrCl, creatinine clearance; ESRD, end stage renal disease; EUA, emergency use authorization; ICU, intensive care unit; IV, intravenous; NPO, nil per os; PO, per os.
For mildly symptomatic patients without a new or increased oxygen requirement, our center recommends bebtelovimab when symptom onset is within 10 days and the patient is considered high risk for disease progression. This subset of patients likely carries COVID-19 as an incidental diagnosis, and the reason for admission is unrelated. Though not a component of published guidelines, our pathway’s recommendation of bebtelovimab is designed to prevent further progression of disease in this subset of patients with COVID-19. Our center’s decision is in line with bebtelovimab’s EUA and motivated by the need to intervene at an earlier timepoint for both immunocompromised persons and patients at higher risk for disease progression.
For symptomatic patients with a new or increased oxygen requirement, management focuses on supportive care (eg, supplemental oxygen) and COVID-19-specific treatments. A 5-day course of remdesivir co-administered with a 7-day course of dexamethasone is recommended for newly hypoxemic patients, and dexamethasone alone is used for patients with contraindications to remdesivir. In addition to these therapies, patients with greater severity of disease and substantial oxygen requirements (eg, high flow nasal cannula) are offered a 7-day course of baricitinib. Owing to tocilizumab shortages, a single dose of tocilizumab is prioritized for patients with significant renal impairment or patients unable to receive orally-administered medications. In each case, it is critical to monitor the illness trajectory of the individual patient, and the level of care must be elevated or deescalated as needed.
Impact of severe acute respiratory syndrome coronavirus 2 variants on treatment
Novel variants of SARS-CoV-2 have emerged at a steady pace since the onset of the pandemic. Detection and characterization of new variants is crucial to determine whether available therapies retain antiviral and neutralizing activity .65 At this time, the dominant variant remains Omicron and its sublineages.66 Importantly, the BA.2, BA.2.12.1, BA.4 and BA.5 variants, which are currently dominant in many areas of the world, contain several mutations in the RBD of the Spike protein which render them less susceptible to neutralization with available monoclonal antibodies (Table 2 ).55 However, bebtelovimab is expected to retain its neutralizing activity against BA.2, BA.2.12.1, BA.4 and BA.5.57 , 67 Small molecule antivirals (ie, remdesivir, molnupiravir, and nirmatrelvir) have been shown to retain activity against the BA.2 sublineage.55 Despite the lower severity of illness observed during the Omicron surge,68 , 69 further data on the effectiveness of monoclonal antibodies and small molecule antivirals are still needed, especially in immunocompromised populations.
Table 2.
Reduction in SARS-CoV-2 lineages’ susceptibility to monoclonal antibodies based on pseudotyped virus-like particle neutralization assay
| SARS-CoV-2 Lineage | World Health Organization Nomenclature | Casirivimab/Imdevimaba Reduction in Susceptibility | Bamlanivimab/Etesevimabb Reduction in Susceptibility | Sotrovimabc Reduction in Susceptibility | Tixagevimab/Cilgavimabd Reduction in Susceptibility | Bebtelovimabe Reduction in Susceptibility |
|---|---|---|---|---|---|---|
| B.1.1.7 | Alpha | No change | No change | No change | 0.5- to 5.2-fold | No change |
| B.1.351 | Beta | No change | 431-fold | No change | No change | No change |
| P.1 | Gamma | No change | 252-fold | No change | No change | No change |
| B.1.617.2/AY.3 | Delta | No change | No change | No change | No change | No change |
| AY.1/AY.2 (B.1.617.2 sublineages) | Delta [+K417 N] | Not determined | 1235-fold | No change | No change | No change |
| B.1.427/B.1.429 | Epsilon | No change | 9-fold | No change | No change | No change |
| B.1.526 | Iota | No change | 30-fold | No change | No change | No change |
| B.1.617.1 | Kappa | No change | 6-fold | No change | No change | No change |
| C.37 | Lambda | Not determined | No change | No change | No change | No change |
| B.1.621 | Mu | No change | 116-fold | No change | 7.5-fold | 5.3-fold |
| B.1.1.529/BA.1 | Omicron [BA.1] | >1013-fold | >2938-fold | No change | 132- to 183-fold | No change |
| BA.1.1 | Omicron [+R346 K] | Not determined | Not determined | No change | 424-fold | No change |
| BA.2 | Omicron [BA.2] | Not determined | Not determined | 16-fold | No change | No change |
| BA.2.12.1 | Omicron [BA.2 + L452Q] | Not determined | Not determined | Not determined | Not determined | No change |
| BA.4/BA.5 | Omicron [BA.4/BA.5] | Not determined | Not determined | Not determined | No determined | No change |
Casirivimab/imdevimab data from Food & Drug Administration (FDA) fact sheet49 updated January 24, 2022.
Bamlanivimab/etesevimab data from FDA fact sheet51 updated January 24, 2022.
Sotrovimab data from FDA fact sheet54 updated March 25, 2022.
Tixagevimab/cilgavimab data from FDA fact sheet60 updated May 17, 2022.
Bebtelovimab data from FDA fact sheet58 updated June 16, 2022.
Summary
Direct acting antivirals and monoclonal antibodies reduce morbidity and mortality associated with SARS-CoV-2 infection. Persons at higher risk for disease progression and hospitalized patients with COVID-19 benefit most from available therapies. Following an emphasis on inpatient treatment of COVID-19 during the early pandemic, several therapeutic options were developed for outpatients with COVID-19. Additional clinical trials and real-world studies are needed to both inform the care of immunocompromised patients and keep pace with the evolving pandemic.
Clinics care points
-
•
Remdesivir and dexamethasone with or without additional immunomodulatory therapy are recommended for hospitalized persons with moderate-to-severe coronavirus disease-2019 (COVID-19)
-
•
Bebtelovimab is the only monoclonal antibody authorized for treatment of SARS-CoV-2 infections caused by circulating Omicron sublineages
-
•
Both molnupiravir and nirmatrelvir/ritonavir are oral antiviral options for outpatient persons with COVID-19; relative and absolute contraindications should be taken into account before prescribing these therapies.
Disclosure
The authors report no potential conflicts of interest or funding sources.
Appendix
Supplement Table 1.
Numerous therapeutic agents have been investigated as therapies for COVID-19. The above summaries offer brief synopses of the body of evidence surrounding the use of select agents which are not currently recommended as therapies for COVID-19
|
Supplement Table 2.
The above definitions are used to define select patient populations in the Yale New Haven Health System
|
References
- 1.WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at: Accessed September 30, 2022.
- 2.Nguyen K.H., Anneser E., Toppo A., et al. Disparities in national and state estimates of COVID-19 vaccination receipt and intent to vaccinate by race/ethnicity, income, and age group among adults ≥ 18 years, United States. Vaccine. 2022;40(1):107–113. doi: 10.1016/j.vaccine.2021.11.040. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner C.E., Saad-Roy C.M., Morris S.E., et al. Vaccine nationalism and the dynamics and control of SARS-CoV-2. Science. 2021;373(6562):eabj7364. doi: 10.1126/science.abj7364. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. (In eng) [DOI] [PubMed] [Google Scholar]
- 5.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. (In eng) [DOI] [PubMed] [Google Scholar]
- 6.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren T.K., Jordan R., Lo M.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen S.C.J., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 Days in patients with severe covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulangu S., Dodd L.E., Davey R.T., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IDSA. IDSA Guidelines on the treatment and management of patients with COVID-19. 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Available at: Accessed June 4, 2022.
- 13.Therapeutic N.I.H. Management of hospitalized adults with COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ Available at: Accessed June 4, 2022.
- 14.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe C.R., Tomashek K.M., Patterson T.F., et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. Lancet Respir Med. 2022 doi: 10.1016/S2213-2600(22)00088-1. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas I.O., Diaz G., Gottlieb R.L., et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021;47(11):1258–1270. doi: 10.1007/s00134-021-06507-x. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon A.C., Mouncey P.R., Al-Beidh F., et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb R.L., Vaca C.E., Paredes R., et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NIH. Remdesivir - LiverTox - NCBI Bookshelf 2022. https://www.ncbi.nlm.nih.gov/books/NBK564049/#:∼:text=Hepatotoxicity,other%20evidence%20of%20hepatic%20injury Available at: Accessed June 4, 2022.
- 21.Shah S., Ackley T.W., Topal J.E. Renal and hepatic toxicity analysis of remdesivir formulations: does what is on the inside really count? Antimicrob Agents Chemother. 2021;65(10):e0104521. doi: 10.1128/AAC.01045-21. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackley T.W., McManus D., Topal J.E., et al. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2021;65(2) doi: 10.1128/AAC.02290-20. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi S., Klein J., Robertson A.J., et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat Commun. 2022;13(1):1547. doi: 10.1038/s41467-022-29104-y. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Focosi D., Maggi F., McConnell S., et al. Very low levels of remdesivir resistance in SARS-COV-2 genomes after 18 months of massive usage during the COVID19 pandemic: a GISAID exploratory analysis. Antivir Res. 2022;198:105247. doi: 10.1016/j.antiviral.2022.105247. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen D.R., Allerton C.M.N., Anderson A.S., et al. An oral SARS-CoV-2 M. Science. 2021;374(6575):1586–1593. doi: 10.1126/science.abl4784. (In eng) [DOI] [PubMed] [Google Scholar]
- 26.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022 doi: 10.1056/NEJMoa2118542. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfizer shares top-line results from phase 2/3 EPIC-PEP study of PAXLOVID™ for postexposure prophylactic use. 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study Available at: Accessed June 4, 2022.
- 28.Saravolatz L.D., Depcinski S., Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral COVID antiviral drugs. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac180. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catlin N.R., Bowman C.J., Campion S.N., et al. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 M. Reprod Toxicol. 2022;108:56–61. doi: 10.1016/j.reprotox.2022.01.006. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA. Fact sheet for healthcare providers: emergency use authorization for PaxlovidTM. https://www.fda.gov/media/155050/download Available at: Accessed June 4, 2022.
- 31.Salerno D.M., Jennings D.L., Lange N.W., et al. Early clinical experience with nirmatrelvir/ritonavir for treatment of COVID-19 in solid organ transplant recipients. Am J Transpl. 2022 doi: 10.1111/ajt.17027. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranganath N., O'Horo J.C., Challener D.W., et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac481. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. COVID-19 rebound after paxlovid treatment. 2022. https://emergency.cdc.gov/han/2022/han00467.asp Available at: Accessed June 4, 2022. [DOI] [PMC free article] [PubMed]
- 34.Wahl A., Gralinski L.E., Johnson C.E., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591(7850):451–457. doi: 10.1038/s41586-021-03312-w. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Fact sheet for healthcare providers: emergency use authorization for LagevrioTM (molnupiravir) capsules. https://www.fda.gov/media/155054/download Available at: Accessed June 4, 2022.
- 36.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorlund K., Sheldrick K., Mills E. Molnupiravir for covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(13):e32. doi: 10.1056/NEJMc2201612. (In eng) [DOI] [PubMed] [Google Scholar]
- 38.Arribas J.R., Bhagani S., Lobo S.M., et al. Randomized trial of molnupiravir or placebo in patients hospitalized with covid-19. NEJM Evid. 2022;1(2) doi: 10.1056/EVIDoa2100044. [DOI] [PubMed] [Google Scholar]
- 39.NIH. Molnupiravir. 2022. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/molnupiravir/ Available at: Accessed June 4, 2022.
- 40.Taylor P.C., Adams A.C., Hufford M.M., et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. doi: 10.1038/s41577-021-00542-x. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Group R.C. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundgren J.D., Grund B., Barkauskas C.E., et al. A neutralizing monoclonal antibody for hospitalized patients with covid-19. N Engl J Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundgren J.D., Grund B., Barkauskas C.E., et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels : a randomized controlled trial. Ann Intern Med. 2022;175(2):234–243. doi: 10.7326/M21-3507. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group A-TfIwC-TS Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22(5):622–635. doi: 10.1016/S1473-3099(21)00751-9. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckerle I., Rosenberger K.D., Zwahlen M., et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. doi: 10.1371/journal.pone.0056974. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhodapkar M.V., Dhodapkar K.M., Ahmed R. Viral immunity and vaccines in hematologic malignancies: implications for COVID-19. Blood Cancer Discov. 2021;2(1):9–12. doi: 10.1158/2643-3230.BCD-20-0177. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin M.J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med. 2022 doi: 10.1056/NEJMoa2116620. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGEN-COV antibody combination and outcomes in outpatients with covid-19. N Engl J Med. 2021;385(23):e81. doi: 10.1056/NEJMoa2108163. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.FDA. Fact sheet for healthcare providers emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab) 2022. https://www.fda.gov/media/145611/download Available at: Accessed June 4, 2022.
- 50.Dougan M., Nirula A., Azizad M., et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N Engl J Med. 2021;385(15):1382–1392. doi: 10.1056/NEJMoa2102685. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.FDA. Fact sheet for healthcare providers emergency use authorization (EUA) of bamlanivimab and etesevimab. 2022. https://www.fda.gov/media/145802/download Available at: Accessed June 4, 2022.
- 52.Pinto D., Park Y.J., Beltramello M., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. (In eng) [DOI] [PubMed] [Google Scholar]
- 53.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. (In eng) [DOI] [PubMed] [Google Scholar]
- 54.FDA. Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab. 2022. https://www.fda.gov/media/149534/download Available at: Accessed June 20, 2022.
- 55.Takashita E., Kinoshita N., Yamayoshi S., et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386(15):1475–1477. doi: 10.1056/NEJMc2201933. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FDA. FDA updates Sotrovimab emergency use authorization. 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization Available at: Accessed June 4, 2022.
- 57.Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022 doi: 10.1038/s41586-022-04594-4. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FDA. Fact sheet for healthcare providers: emergency use authorization for bebtelovimab. 2022. https://www.fda.gov/media/156152/download Available at: Accessed June 20, 2022.
- 59.Dougan M., Azizad M., Chen P., et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv. 2022 doi: 10.1101/2022.03.10.22272100. [DOI] [Google Scholar]
- 60.FDA. Fact sheet for healthcare providers: emergency use authorization for EvusheldTM (tixagevimab co-packaged with cilgavimab) 2022. https://www.fda.gov/media/154701/download Available at: Accessed June 4, 2022.
- 61.Ordaya E.E., Beam E., Yao J.D., et al. Characterization of early-onset SARS-CoV-2 infection in immunocompromised patients who received tixagevimab-cilgavimab prophylaxis. Open Forum Infect Dis. 2022:ofac283. doi: 10.1093/ofid/ofac283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libster R., Pérez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NIH. Therapeutic Management of nonhospitalized adults with COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/ Available at: Accessed June 4, 2022.
- 65.Krause P.R., Fleming T.R., Longini I.M., et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385(2):179–186. doi: 10.1056/NEJMsr2105280. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CDC. CDC COVID data tracker: variant proportions. 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions Available at: Accessed June 5, 2022.
- 67.Wang Q., Guo Y., Iketani S., et al. SARS-CoV-2 Omicron BA.2.12.1, BA.4, and BA.5 subvariants evolved to extend antibody evasion. bioRxiv. 2022 doi: 10.1101/2022.05.26.493517. [DOI] [Google Scholar]
- 68.Maslo C., Friedland R., Toubkin M., et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327(6):583–584. doi: 10.1001/jama.2021.24868. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ison M.G., Scheetz M.H. Understanding the pharmacokinetics of Favipiravir: implications for treatment of influenza and COVID-19. EBioMedicine. 2021;63:103204. doi: 10.1016/j.ebiom.2020.103204. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuzuki S., Hayakawa K., Doi Y., et al. Effectiveness of favipiravir on nonsevere, early-stage COVID-19 in Japan: a large observational study using the COVID-19 registry Japan. Infect Dis Ther. 2022 doi: 10.1007/s40121-022-00617-9. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosaeed M., Alharbi A., Mahmoud E., et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28(4):602–608. doi: 10.1016/j.cmi.2021.12.026. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–534. doi: 10.1093/cid/ciaa1176. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chuah C.H., Chow T.S., Hor C.P., et al. Efficacy of early treatment with favipiravir on disease progression among high risk COVID-19 patients: a randomized, open-label clinical trial. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab962. (In eng) [DOI] [PubMed] [Google Scholar]
- 75.Udwadia Z.F., Singh P., Barkate H., et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holubar M., Subramanian A., Purington N., et al. Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac312. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan H., Peto R., Henao-Restrepo A.M., et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Griensven J., Edwards T., de Lamballerie X., et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz L.M., Little A. Clarity on convalescent plasma for covid-19. N Engl J Med. 2021;384(7):666–668. doi: 10.1056/NEJMe2035678. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korley F.K., Durkalski-Mauldin V., Yeatts S.D., et al. Early convalescent plasma for high-risk outpatients with covid-19. N Engl J Med. 2021;385(21):1951–1960. doi: 10.1056/NEJMoa2103784. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan D.J., Gebo K.A., Shoham S., et al. Early outpatient treatment for covid-19 with convalescent plasma. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119657. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lenze E.J., Mattar C., Zorumski C.F., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2):ofab050. doi: 10.1093/ofid/ofab050. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., et al. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee T.C., Vigod S., Bortolussi-Courval É., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(4):e226269. doi: 10.1001/jamanetworkopen.2022.6269. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhuta S., Khokher W., Kesireddy N., et al. Fluvoxamine in nonhospitalized patients with acute COVID-19 infection and the lack of efficacy in reducing rates of hospitalization, mechanical ventilation, and mortality in placebo-controlled trials: a systematic review and meta-analysis. Am J Ther. 2022;29(3):e298–e304. doi: 10.1097/MJT.0000000000001496. (In eng) [DOI] [PubMed] [Google Scholar]
- 89.Markowski M.C., Tutrone R., Pieczonka C., et al. A phase 1b/2 study of sabizabulin, a novel oral Cytoskeleton disruptor, in men with metastatic castration-resistant prostate cancer with progression on an androgen receptor targeting agent. Clin Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-22-0162. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veru’s novel COVID-19 drug candidate reduces deaths by 55% in hospitalized patients in interim analysis of phase 3 study; independent data monitoring committee halts study early for overwhelming efficacy. https://verupharma.com/news/verus-novel-covid-19-drug-candidate-reduces-deaths-by-55-in-hospitalized-patients-in-interim-analysis-of-phase-3-study-independent-data-monitoring-committee-halts-study-early-for-overwhelmin/ Available at: