Abstract
Background:
Cognitive deficit is one of the common impairments that occur post stroke and have a major effect on the quality of life of stroke survivors. However, the intervention and outcome measures used to remediate post-stroke cognitive impairments are diverse and highly heterogeneous. Therefore, a review of intervention and outcome measures for post-stroke cognitive impairments was carried out.
Objectives:
To review all available information on the recent advancements in intervention and outcome measures for post-stroke cognitive impairments.
Methods:
An electronic database search was conducted in PubMed, Medline, Google Scholar, and the Cochrane Library with key search terms between 2001 and 2021. The search results were systematically screened, and data was independently extracted by three reviewers. The data was thematically analyzed and narratively synthesized.
Results:
The search retrieved 2018 records, and we included 12 studies that met the inclusion criteria. Most of the studies targeted global cognitive deficits in ischemic stroke patients in the chronic phase. We categorized data based on the type of cognitive impairment, cognitive- domain targeted, intervention, and available outcome measures for post-stroke cognitive rehabilitation. Attention, memory, executive function, and global cognition were the common cognitive components targeted, managed, and assessed using an outcome measure. We found that technology is replacing conventional approaches to improve cognitive impairment.
Conclusion:
Regardless of many new developments in post-stroke cognitive rehabilitation interventions driven by technology, there is limited data available on actual implementation as a scalable solution. There is an extensive need for future research for evidence-based assessment and management of cognitive impairments in post-stroke rehabilitation.
Keywords: Cognitive impairment, complex interventions, outcome measures, rehabilitation, stroke
BACKGROUND
The incidence and mortality rates of stroke are declining in high-income countries (HIC), whereas in low- and middle-income countries (LMIC) it is increasing.[1] There is a high proportion of hemorrhagic strokes (about 70%) and 87% of stroke-related deaths and disability-adjusted life-years (DALYs) at a younger age in these contexts.[1] Although motor weakness is one of the common visible consequences associated with stroke, close to 53.4% of stroke survivors develop cognitive impairments that are not visible.[2] The incidence of cognitive impairment is six-to-nine times higher in stroke survivors than that in survivors without stroke, with adverse repercussions for themselves, their families, and society at a large.[3]
A variety of factors including the location of lesion, the brain hypoperfusion, the functional deactivation of distant areas in the brain (diaschisis), or excessive pressure on the surrounding brain tissue by the lesion caused by stroke are likely consequences of cognitive deficits.[4] As far as risk factors are concerned, an international cohort study revealed that diabetes mellitus worsened performance in global cognition.[5] Similarly, poorer performance in global cognition, communication, and executive functions can be seen in individuals with previous history of stroke and hypertension.[5]
Additionally, the demographic, clinical, psychological, and physical factors of the affected individuals are also likely to influence cognitive function among stroke survivors. Age is one of the main predictors of cognitive impairment among stroke survivors.[6] Unhealthy lifestyle, for instance, increased alcohol consumption, physical inactivity, poor sleep pattern, irregular health check-up, and inadequate consumption of vegetables, fruits, and milk were identified as potential risk factors of post-stroke cognitive impairment.[6] Clinical factors such as low pre-morbid intellectual ability, pre-existing cognitive impairment, depression and high stroke severity, recurrent stroke or transient ischemic stroke (TIA), post-stroke dysphasia, and urinary incontinence (aconuresis) showed higher risk of cognitive decline post stroke.[7]
Given the substantial impact of post-stroke on cognition, it is inevitable to manage post-stroke disability with an inclusive approach.[8] Incorporating effective interventions that target post-stroke cognitive impairments and capturing the outcomes of those interventions with an evidence-based approach are very pertinent.[9] However, very little importance and lack of awareness exist among stroke rehabilitation experts, and the stroke survivors and their families for effective cognitive rehabilitation, respectively.[10] Cognitive impairments are also so complex in its presentation, implying that not one single interventional approach and its impact on the outcomes are sufficient to enhance functional recovery. Therefore, post-stroke cognitive rehabilitation includes a wide variety of interventions, approaches, and outcome measures to enable functional independence among those affected.
Early post-stroke cognitive functioning is evident to be an independent predictor of long-term functional outcomes of activities of daily living (ADL).[11] Stroke severity is associated with poor performance in tasks and requires higher mental functions like motor control, problem solving, and memory. A few studies revealed that the lower performances in cognitive functions are significantly correlated with dependency on ADL.[12] All these aspects points out to cognition as an important domain to consider and address during stroke rehabilitation, but yet it is very neglected. Thus, this implies the need for extensive research on this aspect for evidence-based practice of assessments and rehabilitation of post-stroke cognitive impairments. Therefore, a review of interventions and outcome measures for post-stroke cognitive impairments was carried out to understand the recent advancements and existing evidence on cognitive rehabilitation for stroke survivors.
OBJECTIVES
To review all available information on the recent advancements in intervention and outcome measures for post-stroke cognitive impairments.
METHODS
Criteria for considering studies to be included in the review.
Inclusion criteria
Studies on rehabilitation of post-stroke cognitive impairment published between August 2001 and September 2021 in the English language
Cognitive rehabilitation in acute, subacute, and chronic phases after stroke
Only randomized, controlled trials
Studies with at least one outcome measuring the cognitive impairment.
Exclusion criteria
Studies not providing any intervention or assessment for cognitive dysfunctions
Non- English articles.
Electronic searches
Search strategies
This review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the electronic databases, namely, PubMed, Medline, Google Scholar, and the Cochrane Library (of systematic reviews) were used to identify articles relevant to the topic. The systematic search was done using key terms with Boolean operators: Cognitive OR Attention OR Dementia OR Executive function OR Visuo-perceptual AND Stroke OR Post Stroke OR Post- Stroke OR Cerebrovascular accident OR Cerebrovascular Disease OR Ischemic Stroke OR intracerebral haemorrhage AND Rehabilitation OR Therapy OR Treatment AND Compensatory OR Remediation [Appendix 1].
Study selection
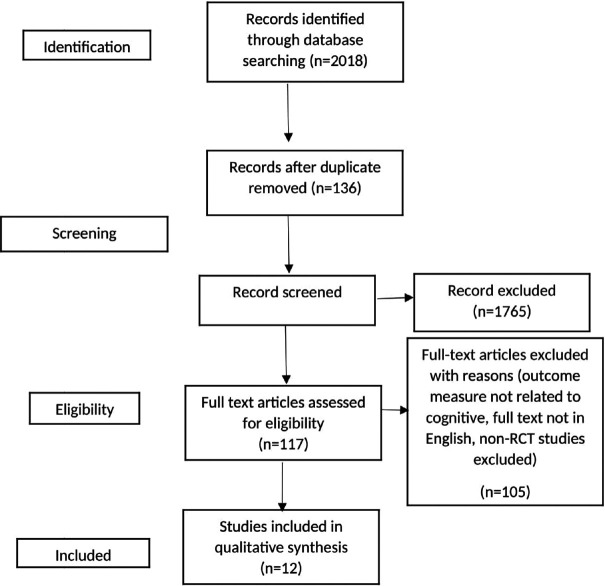
All the studies were screened by three reviewers independently on the basis of their title and abstract. Level two screening and data extraction of full articles fulfilling the inclusion criteria were done by two reviewers independently [Figure 1].
Figure 1.
Prisma flowchart
Data extraction
A data collection form was developed. Relevant data from the included studies on the title, inclusion, and exclusion criteria, type of intervention, study design, outcome measures used, limitations, feasibility, and adherence were extracted. Then the data were classified on the basis of the type of intervention along with the details related to frequency, intensity, duration of intervention, and the domain targeted for rehabilitation. The results were narratively synthesized and summarized under the subheadings: Type of cognitive impairment, evidence of rehabilitative approaches, and outcome measures for cognitive impairment post stroke to assess the existing evidence and gaps to improve further rehabilitation for post-stroke cognitive impairment [Table 1].
Table 1.
Characteristics of included studies
| Author and Year | Domain Targeted | Sample Size | Phase of Stroke | Intervention | Dosage of Intervention |
|---|---|---|---|---|---|
| Jeffrey M. Rogers; 2019[23] | Global cognitive function | 21 | Subacute | Virtual reality+Treatment as usual | 30-40-minute session (4 weeks) |
| Ana Lucia Faria; 2016[22] | Visuospatial abilities, executive functions, memory, attention | 18 | Chronic | Computer-based intervention | 12 sessions of 20 minutes (4-6 weeks) |
| Ana Lucia Faria; 2020[24] | General cognitive functions | 36 | Chronic | Two groups: a) Adaptive VR simulations; b) Task-generated adaptive paper-pencil training |
12 sessions |
| Martina Maier; 2020[26] | Attention, memory, executive function, or spatial abilities | 30 | Chronic | Adaptive conjunctive cognitive training (ACCT) | 30 minutes of daily training (6 weeks) |
| Mingyu Yin; 2020[28] | Executive function, memory, attention, language. and visuospatial function | 34 | Subacute/ Chronic | Repetitive tanscranial magnetic stimualtion (rTMS) treatment | 5 days a week for 4 weeks |
| Suzanne L Barker; 2009[18] | Attention | 78 | Chronic | Attention process training (APT) | Participants received up to 30 hours of APT (1 hour*4 weeks) |
| Catherine M. Haire; 2021[21] | Executive function, cognitive flexibility, short-term memory | 30 | Chronic | a) Therapeutic instrumental music training (TIMP alone) b) TIMP and cued motor imagery (TIMP+cMI) c) TIMP and Motor Imagery (TIMP+ MI) |
Three experiment alarms: (1) 45 minutes of active TIMP, (2) 30 minutes of active TIMP+15 minutes of metronome-cued motor imagery (TIMP+cMI), (3) 30 minutes of active TIMP+15 minutes of motor imagery without cues (TIMP+MI). for 3 times*3 weeks |
| Clara Rozental-Iluz; 2016[31] | Executive function | 39 | Chronic | Interactive video-game group intervention | Two 1-hour group sessions per week for 3 months |
| Attention, executive function | 30 | Chronic | Cognitive-motor dual-task training (CMDT) + Auditory motor synchronization training (AMST) | Intervention session of 30 minutes CMDT * 15 minutes+AMST * 30 minutes. 3 sessions per week for 6 weeks |
|
| Sebastian Koch; 2020[32] | Attention, memory, working memory, processing speed, and executive function | 131 | Chronic | Combined aerobic and resistance training (CARET) + Cognitive training intervention (CTI) | 3 weekly, 40 to 60 minutes of combined aerobic and resistance training (CARET) and 40 minutes of CTI sessions |
| B. Machner; 2014[29] | Visuospatial | 23 | Acute | HEPOKS+usual stroke care (physio, speech, and occupational therapy). | Glasses were worn all day for 7 days+OKS session: Daily (15 minutes each). Seventy colored geometric objects were coherently moving on an 18.4” notebook monitor from right to left at varying speed (8-12°/s). |
| Polly V Peers; 2021[27] | Executive function visuospatial, visual short-term memory | 80 | Subacute/ Chronic | A series of 3-5-minute time-limited games for attention training and memory training to challenge cognitive capacities | 4 weeks |
| G. Zheng; 2020[20] | Global cognitive function, memory, processing speed, execution, attention, and visuospatial ability | 48 | Chronic | Baduanjin exercise (3 days a week and 40 minutes per day) | 24 weeks |
| T. Yeh; 2019[25] | Global cognition, attention, recognition, color and shape identification, calculation, visual perception, visuospatial processing, and executive function | 30 | Chronic | Aerobic exercise training for 30 minutes (3 minutes of warm up, 25 minutes of progressive resisted stationary bicycling, 2 minutes of cool down) followed by 30 minutes of computer-based cognitive training at each session with the BrainHQ program | 12-18 weeks. |
RESULTS
The electronic database search yielded 2018 records. Initial screening retrieved 117 [Figure 1] articles and after full-text screening, we included 12 articles for data extraction and synthesis [Table 1] based on the inclusion criteria. The included studies addressed cognitive impairment after stroke, and out of these most of the studies targeted global cognitive deficits in ischemic stroke patients in chronic phase. The results are summarized into three main categories: prevalence of cognitive impairment, available evidence of intervention and commonly used outcome measures.
Prevalence of cognitive impairment
Many studies showed high prevalence of cognitive impairment poststroke even though it has also been evident that the available data still underestimate the frequency of dementia and cognitive decline in stroke survivors.[13] The cross-sectional study conducted in ten different countries suggested that about 30% of ischemic stroke survivors show a cognitive impairment which is determined by the mini-mental state examination (MMSE) score which was lower than 27.[14]
In the U.K. and Sweden, the prevalence of cognitive impairment three months after stroke ranged from 24% to 39%, according to the MMSE.[15] The studies in Australia showed that according to a series of neuropsychological tests, the prevalence of cognitive deficits three months post stroke was 50% to 58%.[16] A prospective study conducted in India manifested that the prevalence of cognitive impairments was about 20% in total stroke survivors; likewise, in China the prevalence was about 21.8% as measured by the MMSE.[17]
One of the studies revealed that stroke-induced impairment for different domains varied in each hemisphere. Cognitive impairments were found to be significant in the left hemisphere stroke compared to right hemisphere stroke, that is, for global cognition (P = 0.004), memory (P < 0.001), language (P < 0.001), and executive function (P < 0.001).[5]
Brain region of stroke, size of stroke, and educational level
Rogers et al.[23] in 2019 included 21 patients of which 12 had right-sided lesion and 9 patients had left sided lesion of stroke. Mean years of education of the included subjects was 13. Faria et al. in 2016 included 11 patients with left-sided stroke and 7 patients with right-sided stroke with 4–9 years of education. Faria et al.[24] in 2020 included 36 participants. The side of the lesion was not highlighted. The mean years of education for the reh@city group was 8.00 ± 5.32 and 5.50 ± 3.15 for the task generator group. Yin et al.[28] in 2020 included 34 patients with 13 right-sided stroke, 10 left-sided and 11 bilateral stroke patients. The mean years of education was 9–10 years. Barker-Collo et al.[18] in 2009 included 78 patients of which 32 had right-sided stroke, 39 had left sided stroke and 4 had bilateral stroke. The years of education ranged from 8 to 18 years in both the groups. Haire et al.[21] included 30 patients, of which 17 had right-sided stroke and 13 had left-sided stroke. The average years of education was 15 in the control group, 16 in the therapeutic instrumental music performance (TIMP)+ compensatory motor imagery group and 17 in the TIMP + motor imagery group.
Clara Rozental-Iluz et al.[31] did not mention about the lateralization of stroke. The average years of education for the participants of this study was 14.8 years. Park et al.[19] included 30 patients comprising of 11 patients with right-sided stroke and 19 patients with left-sided stroke. No comment was made on the level of education of the included participants. Sebastian Koch et al.[32] included 131 participants with an average of 13 years of education. The stroke lateralization was not documented. Zheng et al.[20] included a sample of 48 patients with 24 patients of left- and right-sided stroke each and an average of 10 ± 2 years of education. Five included studies did not comment on the side of the stroke. The size of the lesion was only documented in two studies, namely, Faria et al.[24] and Machner, et al.[29]
Interventions for post-stroke cognitive impairments
Non-pharmacological/cognitive rehabilitation
Four[18,19,20,21] out of 12 studies identified used cognitive retraining and compensatory approach in rehabilitating domains like attention and global cognition. A study[18] showed attention process training (APT), that is, a theoretically based, hierarchical, multi-level treatment including sustained, selective, alternating, and divided attention as a restorative approach that resulted in a significantly greater improvement on attention than standard care. The duration of training was one hour for 4 weeks. Park et al.[19] identified cognitive-motor dual-task training (CMDT) technique that consisted of performing a cognitive task during the execution of a motor task, and auditory motor synchronization training (AMST) that used rhythmic auditory cues for stimulation. The duration of the session was 30 minutes which included 15 minutes of CMDT + AMST, 3 sessions per week for 6 weeks which exerted a positive effect on attention, memory, and executive function. It was more effective in improving attention capacity and attention.
Zheng, et al.[20] showed that Baduanjin exercise, which consisted of eight separate and smooth movements with a frequency of 3 days a week and 40 minutes per day for 24 weeks, had a significant improvement on the global cognitive function in post-stroke survivors and small-to-moderate beneficial effects on memory, executive function, attention, and ADL after a 24-week treatment. Haire et al.[21] identified that TIMP and motor imagery group used selected and positioned acoustic and electronic instruments for 3 times per week for three weeks resulted in significant improvement in executive functioning.
Neurotechnology as a tool for cognitive rehabilitation
Six[22,23,24,25,26,27] out of 12 studies used neurotechnological tools as rehabilitation training for post-stroke cognitive deficits. Two studies[22,23] showed that the use of virtual reality with conventional treatment compared with usual care for four weeks showed improvement in global cognitive function, attention, memory, visuospatial ability, and executive functions. Faria et al.[24] used adaptive virtual reality (VR) simulations which was compared with task-generated adaptive paper pencil trainings. The results indicated that VR group had showed significant improvement in general cognitive function. Where domain specific improvement is concerned, the VR group showed improvement in visuo-executive functions and attention, whereas the control group showed improvement in orientation domain.
Yeh et al.[25] concluded that aerobic exercise training with computer-based cognitive training using the BrainHQ program for 12–18 weeks resulted in significant improvement in terms of general cognitive functions and visuo-spatial memory. However, adaptive conjunctive cognitive training (ACCT) using rehabilitation gaming systems with 30 minutes of daily training for six weeks showed significant changes in attention, spatial awareness, and generalized cognitive functioning.[26] Similarly, a series of 3–5-minute time-limited games for attention training and memory training to challenge cognitive capacities showed greater levels of improvement in everyday functioning rather than working memory training or waitlist participants.[27]
Neurostimulation-based cognitive rehabilitation
Two out of 12 studies used neurostimulation cognitive training. Repetitive transcranial magnetic stimulation (rTMS) showed improvement in executive function in stroke survivors,[28] whereas another study[29] showed that rehabilitated hemi-spatial neglect via HEPOKS (optokinetic stimulation) in addition to the usual stroke care had no additive therapeutic effect on the spontaneous remission of spatial neglect in acute stroke survivors.
Type of outcome measures
The national clinical guidelines for stroke by the intercollegiate working team of Royal College of Physicians, London, emphasized the importance of neuropsychological assessment for stroke rehabilitation services.[30] In the included studies, the most used outcome measure for global cognition is the Montreal Cognitive Assessment (MOCA) among others like Mini-Mental State Examination and Addenbrooke Cognitive Evaluation. For attention, the commonly used tools are trail making test A and B and digit span forward. Similarly, executive function performance test was used to assess the executive functions. Table 2 shows the commonly used assessment tools in the included studies.
Table 2.
Commonly used standardized assessment tools
| Cognitive domains | Standard assessment scale |
|---|---|
| Attention (visual and auditory) | Trail Making Test |
| Digit Span Forward | |
| Ray Auditory Verbal Learning Test | |
| Star Cancellation Test: Unilateral spatial neglect | |
| Integrated Visual Auditory Continuous | |
| Performance Test | |
| Bells Test | |
| Grooved Pegboard | |
| Catherine Bergego Scale | |
| Executive function | Executive Function Performance Test (EFPT) |
| Executive Function Route-Finding Task (EFRT) | |
| Delis-Kaplan Executive Function System | |
| Learning Memory | Picture arrangement from WAS III |
| Corsi Block Tapping Test Forward | |
| Wechsler Adult Intelligence Scale (WAIS IV) | |
| Ray Auditory Verbal Learning | |
| Test- Immediate Memory | |
| Frontal Assessment Battery | |
| Rivermead Behavior Memory Test | |
| Hopkins Verbal Learning Test Revised | |
| Brief Visuospatial Memory Test-R | |
| Delayed Recall Digit | |
| Span Backwards | |
| Dot Matrix and Spatial Span tasks | |
| Wechsler Memory Scale - Third Edition | |
| Global Cognition | MoCA |
| Cognistat | |
| ACE III | |
| Cognitive Failures Questionnaire | |
| WAIS-IV Coding Digit SymbolS ubstitution Test | |
| OCS-BRIDGE- Hearts Cancellation | |
| European Brain Injury Questionnaire (EBIQ) |
DISCUSSION
This current review revealed that the conventional method of using paper and pencil tasks is still the most widely used method for provision of cognitive rehabilitation and for measuring its impact. However, over the past two decades, computer-based versions have started to become clinically feasible and acceptable. Also evident is the technology-based virtual reality interventions as acceptable solutions to improve post-stroke cognitive impairment. However, there is an absence of specific methodologies that inform the health professionals about the precise approaches to utilize these innovative interventions, especially the intensity of the intervention for different kinds of cognitive impairments among stroke survivors with different severity of these impairments. It has also been observed that there is a variability in cognitive impairment post stroke, and it was also identified that studies exclusively on specific domains, their assessment, and management are scarce. Several limitations contribute toward this, such as limited availability of domain-specific intervention and outcome measures. Although in the included studies a wide range of outcome assessments were used, not all were tested for its validity and reliability on the target population with sufficient power [Table 2].
Also, there is no set protocol or national and international guidelines that enhance evidence-based practice for cognitive rehabilitation. Apart from varying datasets on dosage, frequency, and modes of intervention, there are no reliable measures in rehabilitating cognitive impairment post stroke from the included studies. This review could not establish any appropriate therapy including its frequency and intensity, as it varied widely in all the included studies. Moreover, the domain-specific intervention is lacking except for attention, on which there is immense literature available. Besides, this review have been relatively successful in addressing the global cognitive deficits using different cognitive rehabilitation approaches. Virtual reality and repetitive transcranial magnetic stimulation are two different techniques used in combination with usual practice that have been proven effective and that manipulate neural activity and drive plasticity.
Although, technology-driven interventions and outcome assessments are increasingly utilized for cognitive rehabilitation, there is paucity of evidence on its systematic development and validity. These innovations are also mostly coming from high-income countries, and implementation of these interventions and outcome measures in LMICs are highly debatable. There is a huge need for rigorous research on the validation of these innovative interventions and outcome measures, especially in the context of LMICs.
This review presented various modalities with varied dosage to address the post-stroke cognitive deficits. However, this study also has some limitations due to heterogeneity and narrow database search. Therefore, it might be difficult to pose any evidence synthesis and also the standard protocol for post stroke cognitive rehabilitation could not be established from the available search. Most of the studies are from developed and upper-middle-income countries. There is very limited number of studies available and large trials are needed to confine the applicability of these findings. However, we have been able to identify the existing gaps in the literature and recommend the following measures to improve the quality of post-stroke cognitive rehabilitation:
Long-term recovery trajectory studies are required
Studies to build recommendations on specific approaches to cognitive rehabilitation
Studies on domain-specific intervention and assessment
Development of clinical care guidelines on cognitive rehabilitation and also inclusion of standardized assessment outcomes for evaluation of cognitive deficits.
Large trials are required to make recommendations on the dosage and frequency of intervention.
CONCLUSION
This review identified the emergence of technology-driven interventions and outcome assessments as a tool for contemporary cognitive rehabilitation. There are a number of promising interventions available. However, the effectiveness and scalability of these innovations for cognitive rehabilitation still needs rigorous research. This could be potentially possible through well-powered rehabilitation trials that can target the different domains in post-stroke cognitive impairments. To accelerate the progress in developing the evidence base and to tackle the multiple gaps in the provision of cognitive rehabilitation poststroke, coordinated efforts are required with synergistic collaborations between HICs and LMICs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix 1: Summary of all the included studies
| Author and Year | Domain Targeted | Phase of Stroke | Intervention | Duration of Intervention | Findings | Limitation |
|---|---|---|---|---|---|---|
| Suzanne L. Barker, 2009 | Attention | Chronic (6 months after stroke) | Attention process training (APT): APT is a theoretically based, hierarchical, multilevel treatment, including sustained, selective, alternating, and divided attention. | Participants received up to 30 hours of APT. APT conducted for 1 hour*4 weeks. |
APT resulted in a significantly greater improvement in attention than standard care. Differences in other measures of attention and broader outcomes were not significant. | Strict inclusion criteria Sample size is too small The number of t tests performed may have led to chance findings Because of the nature of the intervention, it was not possible to blind the treating neuropsychologist or participants and this may have influenced outcomes. |
| B. Machner; 2014 | Visuospatial | Acute | HEPOKS in addition to the usual stroke care (physio-, speech, and occupational therapy). HEP was applied by spectacle frames of which the right half was patched with dark tape. |
Glasses were worn all-day for 7 days and to be removed only for the OKS session. OKS Session: Daily (15 minutes each); seventy colored geometric objects were coherently moving on an 18.4” notebook monitor from right to left at varying speed (8-12°/s). |
There was significant improvement in individual patients, independent of the allocated intervention. The early intervention of combined HEPOKS had no additive therapeutic effect to the spontaneous remission of spatial neglect in acute stroke patients. |
Spontaneous remission from acute spatial neglect was not taken into account. Two subjects dropped out of the sample size. |
| Ana Lucia Faria; 2016 | Visuospatial abilities, executive functions, memory, and attention | Chronic | Virtual reality-based intervention (Reh@ city) | 12 sessions of 20 minutes distributed from 4 to 6 weeks | Within group; experimental group showed improvement in global cognitive function, attention, memory, visuospatial ability, executive functions, emotion AND within control group memory and social participation. Between the groups, VR group showed greater improvement in global cognition, attention, and executive functions. | a) Small sample size; b) Outcome assessor not blinded; c) Heterogeneity between group in terms of time post stroke |
| Clara Rozental-Iluz; 2016 | Executive function | Chronic | Interactive video-game group intervention | Two 1-hour group sessions per week for 3 months | Interactive video games have potential to improve executive functioning because of combined cognitive and motor stimulation | |
| Park MO; 2018 | Attention, executive function | Chronic | Cognitive-motor dual-task training (CMDT) + Auditory motor synchronization training (AMST): CMDT is a training technique of performing a cognitive task during the execution of a motor task. AMST uses rhythmic auditory cues for stimulation. |
Intervention session of 30 minutes in which CMDT is of 15 minutes+AMST is of 30 minutes 3 sessions per week for 6 weeks |
Combined CMDT and AMST using rhythmic auditory cues exerted a positive effect on attention, memory, and executive function. It was more effective in improving attention capacity and attention. | Small number of participants; no follow-up on long-term cognitive changes |
| Jeffrey M. Rogers; 2019 | Global cognitive function | Subacute | Virtual reality+treatment as usual | 30-40 mis session for four weeks | Both groups showed significant improvement with substantially higher magnitude of effect size for intervention group | a) Predominantly done on mild severity; of ischemic stroke patients only; b) Treatment session in terms of time in therapy is not balanced between the groups; c) Outcome assessors are not blinded which can increase the risk of bias; d) Shorter follow-up period of only one month |
| T. Yeh; 2019 | Global cognition, attention, recognition, color and shape identification, calculation, visual perception, visuospatial processing, and executive function | Chronic | Aerobic exercise training for 30 minutes (3 min of warm up, 25 min of progressive resisted stationary bicycling, 2 min of cool down) followed by 30 minutes of computer-based cognitive training at each session with the BrainHQ program. | 12-18 weeks. | In post-stroke patients with cognitive dysfunction, 12-18-week sequential aerobic exercise and computerized cognitive training resulted in significantly improved cognitive abilities in terms of general cognitive functions and visuospatial memory. | a) Lack of follow-up assessment. b) Small sample size. c) The control group had more number of older patients with more chromic stroke. |
| Ana Lucia Faria; 2020 | General cognitive functions | Chronic | Two groups: Adaptive VR simulations; and Tas- generated adaptive paper-pencil training | 12 sessions and 2 months follow-up | Reh@city VR group showed significant improvement in general cognitive function; where domain-specific improvement is concerned, VR group showed improvement in visuo-executive functions and attention, whereas control group showed improvement in orientation domain | Groups differed in motor demand of the training VR group require to use paretic arm while its hard or impossible to use paretic arm during pencil task; b) Small sample size; c) Max lost to follow-up; d) Outcome assessor not blinded |
| Martina Maier; 2020 | Attention, memory, executive function, or spatial abilities | Chronic | Adaptive conjunctive cognitive training (ACCT) using rehabilitation gaming system | 30 minutes of daily training for 6 weeks | Significant changes in attention spatial awareness and generalized cognitive functioning were seen. No significant changes in executive functioning and memory domain |
a) Small sample size b) Number of neuropsychological tests performed were excessive for the sample size tested c) Unable to blind the patients and could only partly blind the outcome assessor d) Lack of attention from therapist on EG e) Different locations might have influenced EG |
| Mingyu Yin; 2020 | Executive function, memory, attention, language, and visuospatial function | Sub-acute and chronic stroke (between 1 and 6 months) | Repetitive tanscranial Magnetic stimulation (rTMS) treatment | Once a day, 5 days per week for 4 weeks | rTMS improved cognitive functions and ADLs; rTMS at DLPFC improved executive function for PSCI patients. The results were in accordance with a previous study reporting that TMS improves executive function for patients of cerebrovascular disease with cognitive impairment | a) Small sample size b) Males more than females c) Usage of one parameter d) Lack of follow-up assessment |
| Sebastian Koch; 2020 | Attention, memory, working memory, processing speed, and executive function | Chronic | Combined aerobic and resistance training (CARET): Stationary treadmill or bicycle ergometer were used for aerobic training. Strength training included core exercises and resistance exercises. Cognitive Training Intervention: The CTI included an adaptive computerized platform which was designed to target auditory and visual attention as well as memory, working memory, processing speed, and executive function. |
3 weekly 40 to 60 minutes combined aerobic and resistance training (CARET) and 40 minutes cognitive training intervention (CTI) sessions | Only a modest gain in MoCA scoring, which do not differ from control group, that is, no significant changes in cognitive function after intervention | Intervention period too short to show significant differences between the groups, particularly regarding cognitive outcomes. No blinded assessment of outcomes |
| G.Zheng; 2020 | Global cognitive function, memory, processing speed, execution, attention, and visuospatial ability | Chronic | Baduanjin exercise consisted of eight separate and smooth movements, with a frequency of 3 days a week and 40 minutes per day | 24 weeks | Baduanjin exercise training had a significant improvement on the global cognitive function in post-stroke patients and small-to-moderate beneficial effects on memory, executive function, attention and ADL after a 24-week treatment. | Performance bias due to lack of blinding of the coaches. Small sample size. Results not generalisable to all stroke patients because majority of participants were high NIHSS scores and mild cog impairment |
| Catherine M. Haire, 2021 | Executive function, cognitive flexibility, short-term memory | Chronic | Therapeutic instrumental music training (TIMP) + motor imagery: TIMP is a music therapy which uses selected and positioned acoustic and electronic instruments. |
Three experimental arms: (1) 45 minutes of acti TIMP, (2) 30 minutes of active TIMP followed by 15 minutes of metronome-cued motor imagery (TIMP+cMI), (3) 30 minutes of active TIMP followed by 15 minutes of motor imagery without cues (TIMP+MI). for 3 times*3 weeks |
Executive functioning appears to be enhanced by therapeutic instrumental music training+motor imagery | Small clinical trial, heterogeneous group compositions, and less intervention sessions |
| Polly V. Peers; 2021 | Executive function, visuospatial, visual short-term memory | Subacute | A series of 3-5-minute, time-limited games for attention training and memory training to challenge cognitive capacities | 4 weeks | Attention training participants showed greater levels of improvement in everyday functioning than working memory training or waitlist participants. | Dropouts from WMT group, variability in time since stroke between participants. |
REFERENCES
- 1.Lanas F, Seron P. Facing the stroke burden worldwide. Lancet Glob Health. 2021;9:e235–6. doi: 10.1016/S2214-109X(20)30520-9. [DOI] [PubMed] [Google Scholar]
- 2.Danovska M, Peychinska D. Post-stroke cognitive impairment–phenomenology and prognostic factors. Journal of IMAB–Annual Proceeding Scientific Papers. 2012;18:290–7. [Google Scholar]
- 3.Xuefang L, Guihua W, Fengru M. The effect of early cognitive training and rehabilitation for patients with cognitive dysfunction in stroke. Int J Methods Psychiatr Res. 2021;30:e1882. doi: 10.1002/mpr.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu XD, Ren HY, Prakash R, Vijayadas, Kumar R. Outcomes of neuropsychological interventions of stroke. Ann Indian Acad Neurol. 2013;16:319–28. doi: 10.4103/0972-2327.116909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo JW, Crawford JD, Desmond DW, Godefroy O, Jokinen H, Mahinrad S, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethno-regional groups. Neurology. 2019;93:e2257–71. doi: 10.1212/WNL.0000000000008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohd Zulkifly MF, Ghazali SE, Che Din N, Singh DK, Subramaniam P. A review of risk factors for cognitive impairment in stroke survivors. Scientific World Journal. 2016;2016:3456943. doi: 10.1155/2016/3456943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Shereef HK, Shawky OA, Mohamed KA, Awad EM, et al. Cognitive impairment after cerebrovascular stroke: Relationship to vascular risk factors. Neuropsychiatr Dis Treat. 2009;5:103–16. doi: 10.2147/ndt.s4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamalakannan S, Gudlavalleti Venkata M, Prost A, Natarajan S, Pant H, Chitalurri N, et al. Rehabilitation needs of stroke survivors after discharge from hospital in India. Arch Phys Med Rehabil. 2016;97:1526–32.e9. doi: 10.1016/j.apmr.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar KS, Samuelkamaleshkumar S, Viswanathan A, Macaden AS. Cognitive rehabilitation for adults with traumatic brain injury to improve occupational outcomes. Cochrane Database Syst Rev. 2017;6:CD007935. doi: 10.1002/14651858.CD007935.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhti MIH, Ibrahim MI, Ismail TAT, Nadal IP, Kamalakannan S, Kinra S, et al. Family caregivers' experiences and coping strategies in managing stroke patients during the COVID-19 pandemic: A qualitative exploration study. Int J Environ Res Public Health. 2022;19:942. doi: 10.3390/ijerph19020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinney M, Blake H, Treece KA, Lincoln NB, Playford ED, Gladman JRF. Evaluation of cognitive assessment in stroke rehabilitation. Clin Rehabil. 2002;16:129–36. doi: 10.1191/0269215502cr479oa. [DOI] [PubMed] [Google Scholar]
- 12.Chodosh J, Miller-Martinez D, Aneshensel CS, Wight RG, Karlamangla AS. Depressive symptoms, chronic diseases, and physical disabilities as predictors of cognitive functioning trajectories in older Americans. J Am Geriatr Soc. 2010;58:2350–7. doi: 10.1111/j.1532-5415.2010.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann Transl Med. 2014;2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rist PM, Chalmers J, Arima H, Anderson C, Macmahon S, Woodward M, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke. 2013;44:1790–5. doi: 10.1161/STROKEAHA.111.680728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London stroke register 1995-2010. Stroke. 2013;44:138–45. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Berman K, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: The Sydney stroke study. Dement Geriatr Cogn Disord. 2006;21:275–83. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 17.Das S, Paul N, Hazra A, Ghosal M, Ray BK, Banerjee TK, et al. Cognitive dysfunction in stroke survivors: A community-based prospective study from Kolkata, India. J Stroke Cerebrovasc Dis. 2013;22:1233–42. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Barker-Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A. Reducing attention deficits after stroke using attention process training: A randomized controlled trial. Stroke. 2009;40:3293–8. doi: 10.1161/STROKEAHA.109.558239. [DOI] [PubMed] [Google Scholar]
- 19.Park MO, Lee SH. Effects of cognitive-motor dual-task training combined with auditory motor synchronization training on cognitive functioning in individuals with chronic stroke: A pilot randomized controlled trial. Medicine. 2018;97:e10910. doi: 10.1097/MD.0000000000010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng G, Zheng Y, Xiong Z, Ye B. Effect of Baduanjin exercise on cognitive function in patients with post-stroke cognitive impairment: A randomized controlled trial. Clin Rehabil. 2020;34:1028–39. doi: 10.1177/0269215520930256. [DOI] [PubMed] [Google Scholar]
- 21.Haire CM, Vuong V, Tremblay L, Patterson KK, Chen JL, Thaut MH. Effects of therapeutic instrumental music performance and motor imagery on chronic post-stroke cognition and affect: A randomized controlled trial. Neuro Rehabilitation. 2021;48:195–208. doi: 10.3233/NRE-208014. [DOI] [PubMed] [Google Scholar]
- 22.Faria AL, Andrade A, Soares L, Badia SBI. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: A randomized controlled trial with stroke patients. J Neuroeng Rehabil. 2016;13:96. doi: 10.1186/s12984-016-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers JM, Duckworth J, Middleton S, Steenbergen B, Wilson PH. Elements virtual rehabilitation improves motor, cognitive, and functional outcomes in adult stroke: Evidence from a randomized controlled pilot study. J Neuroeng Rehabil. 2019;16:56. doi: 10.1186/s12984-019-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faria AL, Pinho MS, Badia SB. A comparison of two personalization and adaptive cognitive rehabilitation approaches: A randomized controlled trial with chronic stroke patients. J Neuroeng Rehabil. 2020;17:78. doi: 10.1186/s12984-020-00691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh TT, Chang KC, Wu CY. The active ingredient of cognitive restoration: A multicenter randomized controlled trial of sequential combination of aerobic exercise and computer-based cognitive training in stroke survivors with cognitive decline. Arch Phys Med Rehabil. 2019;100:821–7. doi: 10.1016/j.apmr.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Maier M, Ballester BR, Bañuelos NL, Oller ED, Verschure PF. Adaptive conjunctive cognitive training (ACCT) in virtual reality for chronic stroke patients: A randomized controlled pilot trial. J Neuroeng Rehabil. 2020;17:42. doi: 10.1186/s12984-020-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peers PV, Punton SF, Murphy FC, Watson P, Bateman A, Duncan J, et al. A randomised control trial of the effects of home-based online attention training and working memory training on cognition and everyday function in a community stroke sample. Neuropsychol Rehabil. 2021:1–25. doi: 10.1080/09602011.2021.1972817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin M, Liu Y, Zhang L, Zheng H, Peng L, Ai Y, et al. Effects of rTMS treatment on cognitive impairment and resting-state brain activity in stroke patients: A randomized clinical trial. Front Neural Circuits. 2020;14:563777. doi: 10.3389/fncir.2020.563777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machner B, Könemund I, Zhang L, Zheng H, Peng L, Ai Y, et al. Randomized controlled trial on hemifield eye patching and optokinetic stimulation in acute spatial neglect. Stroke. 2014;45:2465–8. doi: 10.1161/STROKEAHA.114.006059. [DOI] [PubMed] [Google Scholar]
- 30.Blake H, McKinney M, Treece K, Lee E, Lincoln NB. An evaluation of screening measures for cognitive impairment after stroke. Age Ageing. 2002;31:451–6. doi: 10.1093/ageing/31.6.451. [DOI] [PubMed] [Google Scholar]
- 31.Rozental-Iluz C, Zeilig G, Weingarden H, Rand D. Improving executive function deficits by playing interactive video-games: secondary analysis of a randomized controlled trial for individuals with chronic stroke. european Journal of Physical and Rehabilitation Medicine. 2016;52:508–15. [PubMed] [Google Scholar]
- 32.Koch S, Tiozzo E, Simonetto M, Loewenstein D, Wright CB, Dong C, et al. AQ-9: Randomized trial of combined aerobic, resistance, and cognitive training to improve recovery from stroke: feasibility and safety. Journal of the American Heart Association. 2020;9:e015377. doi: 10.1161/JAHA.119.015377. [DOI] [PMC free article] [PubMed] [Google Scholar]