Abstract
Changes in the brain and spinal cord microvasculature during normal aging contribute to the “sensitive” nature of aged central nervous system tissue to ischemic insults. In this review, we will examine alterations in the central nervous system microvasculature during normal aging, which we define as aging without a dominant pathology such as neurodegenerative processes, vascular injury or disease, or trauma. We will also discuss newer technologies to improve the study of central nervous system microvascular structure and function. Microvasculature within the brain and spinal cord will be discussed separately as anatomy and physiology differ between these compartments. Lastly, we will identify critical areas for future studies as well as key unanswered questions.
Keywords: Brain, Microvessels, Normal aging, Spinal cord
The Brain and Spinal Microvasculature in Aging
The microvasculature (MV), typically defined as vasculature <100 µm in diameter that includes capillaries and adjacent vascular regions, of the central nervous system (CNS) regulates nutrient delivery and waste removal. Studies of changes in MV have focused primarily on alterations in the presence of disease including Alzheimer’s/neurodegenerative processes, ischemia/infarctions, and traumatic injuries. However, normal aging itself confers structural and functional differences that result in deficits in the microvascular responses to CNS stress such as ischemia, infection, and inflammation. The impairments, which ultimately predispose to inadequate CNS perfusion, reflect changes in vascular density, tortuous vessels, and rarefaction (microvascular drop-out) (1–3) at the same time there is decreased angiogenic potential and physiological reactivity (4,5). In this review, we will detail changes in the CNS MV during normal aging, which we define as aging without a dominant pathology such as neurodegenerative processes, vascular injury or disease, or trauma. Attention to whether changes are progressive or occur stepwise will be discussed when known. We will briefly comment on other perivascular structures that are intricately related to the MV and the glymphatic system, a glial-dependent clearance system in the perivascular and interstitial spaces that is highly regulated by sleep (6–8). When possible, we will highlight studies in humans but acknowledge that much of what is known is based on rodent models. Brain and spinal cord will be discussed separately, as these 2 regions serve different functions in the CNS and there is a significant disparity in our knowledge of the microvascular changes in these 2 compartments during normal aging.
Brain Microvasculature
General Morphology and Structure
The effects of aging on microvascular morphology and density in the brain include alterations in shape and decreases in density, with a magnitude of change that is regionally dependent (9–11). Notably, the impact of age-related decreases in the volume of the brain parenchyma (due to atrophy) on these calculations is not well-studied (12) despite the fact that regional changes in atrophy might disproportionately affect the neocortex and white matter over other structures such as the hippocampus (13). With these caveats in mind, several studies note that normal aging can result in as much as a 30% reduction of microvascular density in the cerebral cortex (9,11,14,15). Similar levels of microvascular loss have not been consistently detected in the hippocampus or white matter (1,16). Decreases in cortical capillary density, in conjunction with alterations in microvascular function as discussed below, contribute to the vulnerability of the aging brain to injuries such as ischemia, infarction, neurodegeneration, or trauma (17).
The concept of an increase in tortuous vessels is well described in larger vessels and is prevalent in descriptions of the MV in several aged tissues (18,19). This commonly seen alteration can be relatively mild and the functional implications are unclear (11). The term tortuous is best defined in retinal MV to indicate deviation from a straight path and likely reflects age-related remodeling of vascular extracellular matrix composition and stiffness that determines shape (3,20). Rarefaction is another common, but rarely defined, change in aged tissues that refers to a loss of vessels (drop-out) where they were previously present (1,2). This drop-out appears to be more prevalent in gray and white matter regions of the brain where microvascular density is already lower (3), and is thought to reflect changes in perfusion that results in microvascular shrinkage and loss (15). Vascular aging is also accompanied by an increase in the well-defined processes of apoptosis and the senescence of cellular components (21,22). At the same time, rarefaction, apoptosis, and senescence increase, angiogenesis, the development of new vessels from existing MV, is decreased with aging. This change is due, in part, to reductions in the number and function of endothelial progenitor cells with age (4,5). Nonetheless, de novo formation of blood vessels is rarely desired in the normal aging brain, making the loss of angiogenic capacity less impactful to subsequent tissue function than rarefaction. Interventions to decrease the amount of rarefaction and increase the angiogenic potential of aged brain vasculature range from studies of exercise (23) to nicotinamide mononucleotide. The latter has been shown to rescue angiogenic capacity in aged cerebromicrovascular endothelial cells (24), suggesting that brain MV retains some plasticity with aging.
In addition to alterations in density, shape, and survival, aged brain MV demonstrate increases in mediators of oxidative stress and inflammation that is thought to reflect, in part, glial cell activation (25–28). These changes are part of the commonly accepted concept of inflammaging whereby a combination of accumulated stimuli ranging from non-self-pathogens, cellular debris, excess nutrients, and the microbiome sustain a chronic state of immune activation (29). Taken together these pathways also promote neuroinflammation and increase vulnerability to additional stressors, such as changes in blood flow. The effect of these changes on MV function will be discussed further under vascular reactivity (see section Physiology and Functional Changes).
Cellular and Biochemical Components
Cells of the microvasculature
The brain MV is similar to peripheral MV in being comprised primarily of endothelial cells and associated pericytes, but brain endothelial cells (BEC) are unique in forming a tight monolayer via a series of cell–cell contacts and regulatory proteins that include tight, adherens, and gap junctions (30,31). BEC and pericytes, in conjunction with contiguous astrocyte foot processes and other cellular components of the brain parenchyma act in concert as a neurovascular unit (NVU) to maintain the integrity of the physiological interface known as the blood–brain barrier (BBB; see Figure 1) (32). BEC in the adult brain do not typically proliferate, making them less prone to senescence, and many of the other changes (such as telomere shortening) noted in cells that undergo numerous replicative events. Nonetheless, cells of the NVU in the aged brain demonstrate some features of senescence including a decline in sirtuins, a family of proteins involved in metabolic regulation, and association with senescence-associated secretory proteins(33). Once injured or stimulated, BEC from aged brains demonstrate a longer proliferative time relative to BEC derived from young brains (34).
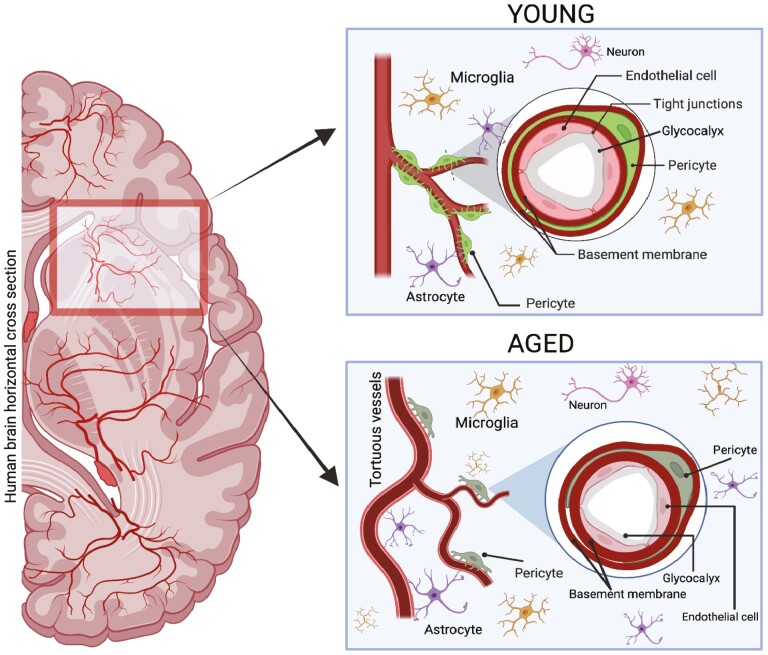
Figure 1.
Schematic drawing depicting the differences in brain microvasculature and surrounding extracellular matrix (ECM) in young (18–44 y, top right box) versus aged humans (65 y and older, bottom right box). Younger microvasculature is composed of less tortuous vessels, a higher degree of pericyte coverage and a thicker glycocalyx. Aged microvasculature (MV) has an increase in tortuosity in vessels, increased astrocyte and microglia count as well as a thicker basement membrane (BM) and thinner glycocalyx with less pericyte coverage. The darker colors in aged are not representative of any physical change but rather a subtle visual to represent the aged group.
Unique to the brain is the tight relationship of the BEC with the pericyte, which envelopes the BEC in ratios as high as 1:1 and covers a larger percent area than in other MV. The high prevalence of pericytes is thought to underscore the importance of pericyte–BEC interactions in maintaining the barrier functions of the BBB (35). It is well established that pericyte loss occurs with age, resulting in reductions in blood flow as well as disruptions to optimal BBB function (36). A recent study found that pericyte remodeling is impaired in aged mice, resulting in dysfunctions of capillary structure, and flow in the brain (37). Transcriptomic studies of pericytes have found that ARHGAP42, which regulates blood pressure and is enriched in pericytes, decreases with age in both humans and mice (38). Understanding the activity and physiology of pericytes will be an important area of research for future studies of age-related changes in brain MV function.
Astrocytes are the largest cell population in the brain and the dominant glial cell associated with the brain MV. Astrocytes function as the gatekeepers of the glymphatic system as they mediate the movement of fluid between paravascular spaces and the interstitium via the water channel aquaporin 4 (AQP4) (39). Astrocyte foot processes are accepted as part of the NVU, and astrocyte biosynthesis is a major source of the MV basement membrane (BM). Changes in astrocytes in the MV with normal brain aging are less well studied than BEC and pericytes, but there are anatomical and functional changes in subcellular domains related, in part, to a decrease in AQP4 (40,41). Moreover, rather than significant differences in cell numbers, there are increases in reactivity and biosynthesis in a subset of astrocytes that is regionally dependent (42–44). Other glial cells such as microglia do not have a direct connection to the MV, but function in an indirect role as they contribute to astrocyte reactivity (44). In summary, the cellular components of the aging MV are altered in a manner that increases vulnerability to ischemia and injury. These changes include decreased BEC replicative capacity, increased pericyte loss, and enhanced glial cell reactivity.
Extracellular matrix
The basement membrane (BM) of the BBB is an approximately 40–100 nm thick composite of structural extracellular matrix (ECM) on the abluminal (brain parenchymal) side of the BEC (Figure 1). The BM of the brain MV is typically described as having endothelial and parenchymal compartments that are separated by pericytes, a distinction that reflects the appreciation of pericytes as independent regulators of cell–ECM interactions at the BBB. However, BEC, pericytes, and astrocytes all contribute to the synthesis of BM components that are designed to undergo minimal turnover with age (45,46). This resistance to turnover reflects the stability of the BM backbone, which is largely comprised of the heterotrimer laminin and the sheet-like collagen IV (col IV). The deposition of laminin and col IV are interconnected and further stabilized by cellular fibronectin and heparan sulfate proteoglycans (PGs), such as perlecan and agrin, and glycoproteins such as the nidogens (formerly known as entactins).
The “stiffening” of the vasculature that occurs with age produces increases in systolic blood pressure and widened pulse pressure that stresses the MV (47). In this context, early observations noted general thickening of the BM of tissue beds with normal human aging (48). Studies in animal models, which do not typically have age-related increases in systolic blood pressure and widened pulse pressure, also noted overall higher widths in the cerebral microvascular BM with aging (49,50). The thickening of the BM is increased by the presence of senescent cells and senescence-associated secretory products. Changes that are associated with aging reflect variations in composition that are specific to the ECM components, and each brain region, in both human and animal models (49–51). The consensus is that most aged human MV BM has an increase in col IV throughout the cerebral cortex, with unclear changes in laminin in the absence of disease (1,51,52). Studies of other BM components, such as perlecan and fibronectin are made difficult by the presence of additional isoforms (49,50,53). Examining BM composition with aging is further complicated by an ultrastructural study (54) that found up to 5-fold increases in the deposition of lipid droplets and aggregates on the abluminal side of the BM in aged C57BL/6 mice. As is often the case when there are changes in ECM with aging, it is difficult to determine the relative contributions of synthesis and turnover. In summary, the brain BM tends to thicken with normal aging but does so in an ECM component and region-specific manner that likely affects local microvascular function.
The endothelial glycocalyx is a gel-like layer that lines the EC on the luminal side throughout the MV of all organs (Figure 1). The glycocalyx has a wide range of thicknesses (0.2–5 μm) depending on the specific vascular bed and is comprised primarily of PGs that have a core protein linked to side chains of sulfated glycosaminoglycans (GAGs), which are named based on their specific carbohydrate moieties. In brain MV, sulfated GAG chains include heparan sulfate (which is the dominant sulfated GAG in MV), dermatan sulfate, keratin sulfate, and chondroitin sulfate (53,54). Proteoglycans (PGs) include endothelial cell membrane-bound syndecans and anchored glypicans. Additional PGs that are linked together in the glycocalyx include the biglycans and the chondroitin sulfate PG versican (55). Another major component of the brain endothelial glycocalyx is the linear nonsulfated GAG, hyaluronan (HA), which can link to any molecules that contain HA binding sites—often termed hyaladherins—that include most PGs and many circulatory components such as TSG-6 (56,57). Brain glycocalyx is similar to that of other organs in composition but differs in having a significantly greater area of coverage and being more resistant to lipopolysaccharide (LPS)-induced injury (58). Glycocalyx composition in normal aging has been recently reviewed (56,59), and it is worth noting that the abundance and make-up of the glycocalyx are dynamic and interact with circulatory factors to regulate MV function. The latter include blood flow, immune cells, and mediators such as growth factors and cytokines (60). Recent advances in imaging have allowed visualization of the glycocalyx in the living rodent brain using 2-photon microscopy (61), but little is known specifically about brain glycocalyx thickness and function in aging. Studies of peripheral MV have demonstrated a thinner glycocalyx in aged mice and humans (62). To summarize, it is probable that aging results in a thinning of the glycocalyx that ultimately results in less barrier protection in both the basal state and in response to injury. Advances in noninvasive imaging, based largely on magnetic resonance imaging (MRI) and ultrasound (US) will be needed to more precisely determine changes in brain glycocalyx structure and function with aging (56).
Physiology and Functional Changes
Blood–brain barrier alterations
The BBB is a physiological interface with multiple cellular (BEC, pericytes, and astrocyte foot processes), protein (tight, gap, and adherens junctions as well as transport proteins), and ECM components (glycocalyx and BM), which work in concert to regulate how and what substances cross from the vasculature into the brain parenchyma. Changes in the BBB with normal aging affect the passage of normal nutrients as well as the myriad number of medications taken by even healthy older adults (see recent reviews (30,63)).
The BBB is limited to capillaries and the immediately adjacent regions, yet the MV is part of a continuous loop structure that includes pre and post capillary segments, venules, arterioles, and major arteries. Consequently, classic studies of BBB function are challenging and generally performed in rodents using dyes and tracers to detect changes in permeability and transport at the capillary level. These studies were the first to indicate that aging is associated with small, but measurable increases in BBB disruption, which has been confirmed by more recent studies (15,31,64). Subsequent experiments in humans, using noninvasive imaging, such as dynamic contrast-enhanced MRI, demonstrated that BBB leakage was significantly associated with older age in the white and gray matter, and in regions of the brain that control higher-level cognitive functions, but was not noted in regions that control basic functions such as movement (65). These changes suggest that BBB breakdown is an early harbinger of cognitive decline (66). A recent review discusses imaging options to examine subtle changes in the BBB in human aging and disease (67). Age-related leaks in the BBB are attributed to alterations in both the cellular and matrix components (as discussed above) that work in concert to increase sensitivity to infection, injury, and ischemia (15,30,63). As expected, there are regional differences in susceptibility to insults, with the hippocampus cited as being a particularly sensitive area for BBB alterations (68). It is also notable that specific BBB functions, such as transcytosis, are affected more than others (69).
The BBB changes during normal aging can reflect the influence of senescence (70,71). As an example, the development of a senescence-associated secretory milieu damages angiogenic potential that is already compromised and promotes the turnover and degradation of glycocalyx (33,72,73). At the same time, senescence increases BM thickening, a process that is accelerated by disease. A well-studied age-related alteration of ECM is the deposition of advanced glycation end (AGE) products: nonenzymatic, posttranslational modifications of proteins that are augmented in aging and disease processes that enhance carbonyl stress. AGE modifications of proteins in the brain MV interfere with BBB function due to effects on cellular and protein components of the NVU (74–76). Taken together, there is consensus that subtle amounts of BBB disruption occur in normal aging and contribute to age-related deficits and sensitivity to injury and ischemia. However, these changes do not appear to affect basic neurologic functions and are a minor contribution to BBB dysfunction in the basal state. In the future, it will be important to determine the effect of age-related increases in BBB permeability on maintaining cognitive health.
Reactivity
Studies of MV reactivity to vasodilatory and vasoconstrictive stimuli with normal aging are complicated by steady-state differences in circulating mediators that affect microvascular responses. The latter includes higher levels of compounds reflecting oxidative stress and an increase in overall levels of inflammatory molecules (26–28,77). Additional changes in the peripheral MV with normal aging, which alter reactivity, include higher resting tone, and decreased vascular conductance (78,79). Moreover, there are age-related delays in the timing of the initial response to stress and the return to baseline levels, which is thought to reflect basal increases in endothelin expression and signaling (80). There are far fewer functional studies specifically in brain MV, but recent work underscores the importance of nitric oxide synthesis and metabolism, coupled with glutamatergic activation, as a key contributor in age-related changes that contribute to neurodegeneration and injury (81). Indeed, a decrease in overall nitric oxide bioavailability, a key mediator in optimizing vascular tone, is a common theme in the increased sensitivity of the aged brain to intrinsic and environmental stresses (82).
As noted previously, the effect of systemic blood pressure on microvascular function and reactivity must be considered given the high prevalence of isolated systolic hypertension and low diastolic blood pressure in older humans (48). The effect of widened pulse pressure on downstream MV is typically implied rather than directly studied, but there is increasing evidence that increased intravascular stress has a detrimental effect on MV function and negatively affects glymphatic clearance (83–85). The beneficial effect of treating hypertension on cognitive outcomes, may in part, reflect a dampening of subsequent damage to the MV (86,87).
Studies of the vascular responses to a low oxygen or high carbon dioxide environment are thought to have the greatest clinical relevance to the aged brain. Alterations in vascular reactivity (dilatation and contraction) and flow in response to increased metabolic demand, such as that conferred by changes in oxygen and carbon dioxide tension have been measured in both gray and white matter (88–90). Multiple studies have demonstrated that, regardless of microvascular density or morphology, the aged brain MV is less able to adapt to low oxygen tension whether due to occlusion, hypoxic conditions, or an increase in carbon dioxide (16,87,91). The generalizability of any of the above data to normal aging is made difficult by regional differences in the microvascular response. The latter includes gray versus white matter as well as specific brain structures such as the hippocampus (3,68). Variations in the portion of the MV examined, whether limited to capillaries or including pre and post capillary sections, further complicates the interpretation of the findings.
Advances in US imaging provide spatial and temporal resolution of the MV, but many of the imaging techniques used in animal models require implants or invasive approaches (such as cranial windows) that are not feasible in humans (92). Most practical techniques proposed for studying brain MV in humans are based on dynamic contrast MRI and magnetic resonance angiography (MRA), which are noninvasive and do not require exposure to radiation necessitated by computerized tomography (CT) imaging (93,94). Although visualizing the actual MV is below the current spatial resolution of MRI, microvascular perfusion can be measured, thereby providing an indication of underlying functional changes (67,95,96). In summary, brain microvascular reactivity, the ability to dilate or constrict in response to stimuli, is diminished with normal aging. Although there is regional variation, this impairment reflects higher levels of systolic blood pressure, widened pulse pressure, and a general milieu that favors an increase in vascular tone. Compensatory actions, such as the release of nitric oxide are diminished. Specific studies in the human brain MV are increasingly available due largely to technological advances in MRI/MRA, which will provide further details on microvascular structure and function in response to physiological challenges.
Spinal Cord Microvasculature
The aged spinal cord, like the aged brain, is vulnerable to ischemic injuries and responds differently from the young spinal cord to stresses such as ischemia and signals for vasoconstriction and dilation. However, unlike the brain, we know little about spinal cord MV and blood flow changes during normal aging. This gap in understanding of aged spinal MV is clinically relevant. According to estimates from the American Academy of Orthopaedic Surgery, spinal stenosis (ie, narrowing of the spinal canal—usually due to degenerative changes of the vertebrae and intervertebral discs that compress the spinal cord), affects 8%–11% of the population in the United States, with nearly 2 million Americans affected by 2021. Indeed, age is a significant risk factor for spinal stenosis underscored by a study evaluating the spinal cord of approximately 450 asymptomatic patients that showed almost 90% of patients (men and women) over 60 years of age had abnormal features within the spinal cord (97), which could affect blood flow within larger vessels as well as MV.
Acute spinal cord injuries (SCIs), resulting in significant ischemia to the intraspinal vasculature, can also occur at any level along the length of the spinal column. The average age of acute SCI has increased from 29 to 43 years (98) to nearly 60 years of age in some studies, likely reflecting a growing older population that is prone to ground-level falls (99). A better understanding of how normal aging alters the MV within the spinal cord is needed to treat acute injuries and develop protective strategies against chronic changes.
General Morphology and Structure
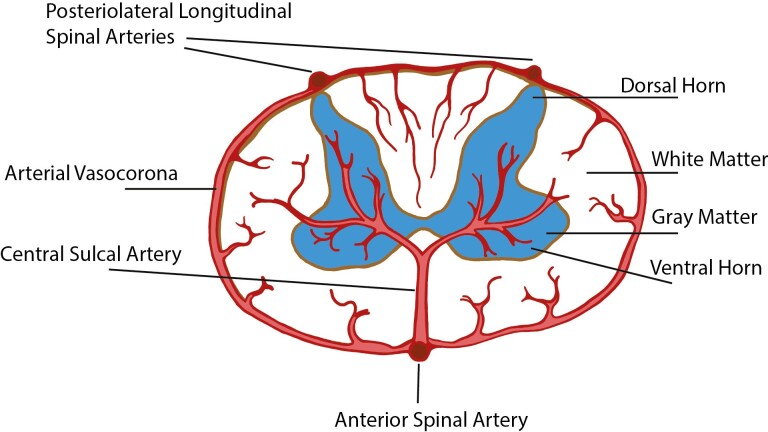
The spinal cord tissue has an anatomic specialization that carries both sensory and motor information to and from the brain. Consequently, disruption of function anywhere along the length of the spinal cord can result in significant neurological impairments. Blood supply to the spinal cord is unique and warrants a short description here. The anterior spinal artery (ASA) runs along the entire length of the anterior surface of the spinal cord and distributes blood to the anterior third of the spinal cord tissue by central and pial branches (Figure 2). The intrinsic arteries of the spinal cord are the central sulcal arteries, which originate from the anterior median longitudinal spinal artery (ie, ASA), and arterioles coming from the pial plexus and the posteriolateral longitudinal artery (Figure 2). Interestingly, the distribution of the central sulcal arteries (ie, intramedullary arteries) varies along the length of the spinal cord; the cervical region has 8–12 arteries per centimeter, while the thoracolumbar region has 2–3 arteries per centimeter (100). There are also “watershed zones” which are regions that do not get a direct blood supply but are dependent on overlapping terminals of vascular fields. These regions may be especially vulnerable to ischemic damage. Although vessel tortuosity has been reported in the penetrating central sulcal arteries in aged rodent spinal cord (101), almost nothing is known about changes in microvascular density and function of the spinal cord with aging. This lack of knowledge is clinically important as aging can affect the response to additional metabolic demands on the spinal cord from chronic pathologies, such as cervical stenosis, as well as acute changes, such as trauma.
Figure 2.
Schematic drawing showing a simplified visual of the general structure and vascular architecture of the spinal cord in cross section. Relevant blood vessels are labeled as such and differences in color between white and gray matter do not depict any physiological differences.
During normal aging, spinal cord tissue undergoes several physiologic, cellular, and molecular alterations including a reduction in alpha motor neurons (α-MNs). In a study measuring human neuronal loss, being over 60 years of age correlated with a 50% loss of motor neurons (102). Similarly, aged mice had a 41% decrease of α-MNs in the spinal cord compared to 3–6-month-old mice (103). Interestingly, another study done in rhesus monkeys and mice showed that during aging alpha motor neurons are spared in both species, but their synaptic connectivity was compromised resulting in impaired motor function (104). Therefore, the age-related motor function may not be simply the result of the reduction in alpha motor neuron numbers but rather alterations in their synaptic connectivity. It is likely that there are species and sex differences in how the spinal motor neuron function during normal aging and additional studies are needed to address these discrepancies.
There is also evidence of age-related loss of myelin and mild inflammation, including astrocyte and microglia activation in mice (103). Another study, measuring microglia cell number in close approximation with motor neurons of the lumbar spinal cord of dogs, found that older dogs have more motor neurons with closely activated microglia compared to young adult dogs (74% vs 18%) (105). Thus, age-related spinal cord alterations in motor neurons are associated with increases in inflammation, supporting the idea of inflammaging (age-related increases in proinflammatory markers in blood and tissues) that has been discussed in the context of brain aging (77). Although the deterioration of motor neurons and their function in the spinal cord are noted, the relationship between neuronal loss/death and the aged spinal cord MV is unknown. Additional studies are needed to examine whether alterations in microvascular density and/or function contribute to neuronal loss and increased inflammation during normal aging.
Spinal Microvascular Changes during Normal Aging
Cellular and ECM components that comprise the spinal cord MV, and their age-related alterations, are thought to be similar to that of MVs in the brain; however, experiments to validate these assumptions are lacking. To our knowledge, there is only 1 published study that examined age-related morphological and density changes in the spinal cord MV. A study by Qiu et al. examined the larger penetrating arteries that feed the spinal cord parenchyma and the density of MV using classic techniques (101). The authors found that the number of central arteries that feed the spinal cord decreased significantly with age and that the ventral spinal arteries as well as the central arteries became torturous in aged rats. Interestingly, the drop in the number of central arteries and increased tortuosity occurred in a stepwise manner with age and was present along the entire length of the spinal cord. These data suggest an overall reduction in blood volume and supply to the spinal cord during normal aging.
A study by Buchman et al. examined microinfarcts, cerebral amyloid angiopathy, and arteriolosclerosis in brain and spine specimens from 165 deceased patients with a mean age of 92 of which 73% were female (106). The authors found that although microinfarcts and amyloid angiopathy were not detected in the spinal cord parenchyma, arteriolosclerosis was observed in all levels of the spinal cord and was more severe in the posterior than in the anterior spinal cord. These changes were associated with Parkinsonism, a syndrome that is characterized by motor deficits similar to those of patients with Parkinson’s disease. Interestingly, spinal arteriolosclerosis was especially prevalent in the white matter and was more severe in the spinal cord than in the brain. These data underscore that specific changes in the MV of the CNS (brain vs spinal cord) vary by region.
Like the brain, there are also microvascular differences between the white and gray matter. Qui et al. found that the gray matter of the spinal cord had 3–4 times more capillary density than the white matter (101). The authors noted that capillary length density as well as capillary surface density decreased significantly with age in both gray and white matter, but the decreases were more significant in the gray matter. These findings suggest that gray matter tissue may be more sensitive to injury and ischemia than white matter tissue in the aged spinal cord. In contrast to these findings, in our studies using Brown Norway-Fisher rats from the National Institute of Aging (NIA) colony, we found that MV density counts per area in aged (516.67 ± 33.5 count/mm2 in gray matter and 101.7 ± 9.4 count/mm2 in white matter; males 22–24 months old; N = 4) and young rats (461.8 ± 6.6 count/mm2 in gray matter and 101.6 ± 7.3 count/mm2 in white matter; males 2–3 months old; N = 6) did not differ significantly in either gray or white matter (Figure 3). It is important to note that Qui et al. used Wister rats of both sexes while we used males from the NIA colony, confirming that animal strain, sex, or source could account for discrepant results in studies of aging MV. Additional studies examining the function of the microvascular in response to increased metabolic demand are needed to identify a comprehensive set of changes in the structure, physiology, and function of the spinal MV during normal aging.
Figure 3.
Microvessel density within the spinal cord does not differ between young and aged rats. Tissue sections were stained with anti-Laminin antibody labeling all vasculature using standard immunohistochemistry techniques. Each whole spinal cord cross-sectional image represents a sample taken from C5 spinal cord of a male Fisher Brown-Norway (F344BN) hybrid rats from the NIA Aged Rodent Colonies (top panel). Young F344BN animals ranged from 7 to 8 months of age while aged animal ranged between 22 and 24 mo. Photomicrographs (bottom panel) depicting the microvessel density within the gray matter of the cross section are shown. The relative similarity in microvessel density in aged and young tissue are noted here. Scale bars = 100 microns. LM = laminin.
Cellular Components of Microvasculature within the Spinal Cord
The endothelial cells and pericytes of the spinal cord MV function like those in the brain with some notable differences. Although less well-studied than BEC, spinal endothelial cells have cell–cell contacts and regulatory proteins that confer barrier properties (107). Pericytes surround the endothelial cells and work in concert to promote vascular structure, responsivity, and integrity. Pericytes are relatively abundant in the spinal cord MV, but do not reach the high pericyte to endothelial cell ratio found in the brain. Interestingly, 1 study showed that pericyte coverage is 39.77% in the ventral horn of the spinal cord while it is only 13.34% in the dorsal horn blood vessels (108). It also appears that multiple subtypes of pericytes exist in the spinal cord MV as determined by distinct labeling with PDGFR-beta, NG2, CD-146, or Desmin (109).
Physiology and Functional Changes in the Microvasculature within the Spinal Cord
Few studies have examined the changes that occur with the blood–spinal cord barrier (BSCB) during normal aging. Like the brain BBB, the BSCB is responsible for regulating nutrient exchange as well as protecting spinal cord parenchyma from toxins within the bloodstream. The BBB and BSCB also differ in their functions, which warrants separate investigation. As an example, it has been shown that the BSCB may have increased baseline permeability than the BBB due to differences in the expression of tight and adherence junction proteins (110). Spinal cord injury (SCI) can exacerbate vascular damage, disrupt the integrity, and increase the permeability of the BSCB thereby compromising the surrounding CNS tissue. Numerous studies have highlighted the impacts to the BSCB in the setting of SCI, but few have examined these changes during normal aging despite the fact that the average age of SCI patients has continuously increased over the past few decades (111). Piekarz et al. found that BSCB permeability increases with age in mice (103). Injury models in both mice and rats have shown that advanced age can exacerbate the resulting damage to the cord tissue (112,113). Microvascular reactivity, such as vasodilation and vasocontraction within the spinal cord during normal aging and after injury, are also unknown. It is important to define changes in the BSCB during aging that are exacerbated by injury and result in worse outcomes after even minor spinal cord trauma in older adults.
Novel Imaging Modalities to Study Spinal Cord Microvasculature
The CT and MR angiograms are the most common ways to visualize vasculature within the spinal cord. Traditionally, both CT and MR angiograms have had limitations in their spatial resolution to be approximately 400 μm for CT and 800–1 000 μm for MR (114,115). This limitation in spatial resolution has not allowed for a detailed morphological or hemodynamic analysis of the intraspinal MV, which are typically <50 μm in diameter. A few technological advances are now producing detailed studies of the MV within the spinal cord. For example, the synchrotron radiation micro-tomography (SRmCT) technique utilizes synchrotron radiation beam light and can detect vessels as small as 7.4 μm (116). Indeed, this method has been used to visualize intraspinal vascular changes associated with a weight-drop injury in an experimental SCI in a rat model (116). Although SRmCT was able to visualize alterations in the MV, a major limitation is that this method does not support in vivo time-lapse imaging and requires euthanizing the rat for imaging. Two-photon (2P) microscopy has been used to examine blood flow dynamics within the spinal cord MV (117–119). These studies have detailed microvascular alterations with time after SCI and have suggested that increases in microvascular angiogenesis may be beneficial to axonal growth and regeneration. However, a major limitation with all 2P methods is the limitation in temporal resolution (in the order of minutes) as well as the penetration (approximately 200 μm). Therefore, studies can only be accomplished in small areas on the surface of the spinal cord and of morphological alterations within finite times after the injury.
Recently, another imaging modality, ultrafast US imaging, has been developed and applied to visualize blood flow dynamics within the spinal cord in rats with high temporal (10 000) frames per second and spatial resolution (>100 μm) (120–122). Moreover, the use of a contrast agent, microbubbles, in combination with ultrafast US imaging (termed contrast-enhanced US [CEUS] imaging) and data processing methods have been developed. This method separates slow flow signals within the MV from faster flow signals from larger (>100 μm) vasculature, resulting in perfusion as well as vessel imaging and flow velocity data (122). Contrast-enhanced US (CEUS) imaging has been examined in both brain (121) and spinal cord and during acute and chronic time points after injury (123) in animal models. Importantly, CEUS imaging can be performed intravitally (121,123), and methods for transcutaneous CEUS imaging for the spinal cord have been developed (124). These advances suggest that ultrafast CEUS imaging has the potential to be used to examine blood flow changes within the MV of the spinal cord in ways not previously examined. In the future, it will be important to determine whether ultrafast CEUS imaging can be used to detect microvascular function and reactivity within the aged human spinal cord.
Summary
The brain MV in normal aging demonstrates decreases in capillary density, increases in vessel tortuosity, and a reduced ability to recover from ischemia and other insults. Endothelial cells are maintained except in areas of rarefaction, but pericytes are lost and glial cells such as astrocytes show increased activation. It is also generally accepted that there are increases in BM at the same time there are decreases in glycocalyx thickness. There is an increase in oxidative stress and inflammatory mediators. Functionally, there is slowed response to perturbations and less ability to return to a steady state once a stress response is initiated. Confidence about the degree of these MV alterations is limited due to inherent difficulties in imaging the human brain MV, the presence of brain atrophy, variability in the aged models used for study, the concurrent presence of age-related diseases, and regional differences in brain structure and function.
Less is known about the microvascular changes in the spinal cord during normal aging. Although reductions in motor neurons within the spinal cord are noted in both human and rodent models, only one preclinical study to date has directly examined aged-related MV changes in the spinal cord. Nonetheless, work from our group noted no significant reduction in MV density during normal aging in the spinal cord, underscoring the importance of utilizing multiple models to define true age-related changes. Ongoing and future studies using state-of-the-art imaging, including the latest advances in MRI/MRA and US, will advance understanding of the anatomy and physiology of the brain and spinal cord MV during normal aging.
Contributor Information
Zin Z Khaing, Department of Neurological Surgery, University of Washington, Seattle, Washington, USA.
Abarajithan Chandrasekaran, Department of Neurological Surgery, University of Washington, Seattle, Washington, USA.
Anjali Katta, Department of Neurological Surgery, University of Washington, Seattle, Washington, USA.
May J Reed, Department of Medicine, Division of Gerontology and Geriatric Medicine, University of Washington, Seattle, Washington, USA.
Funding
We would like to thank the following funding sources: National Institutes of Health R01-NS121191 (Z.Z.K.), AG073929 (Z.Z.K.), R03AG051071 (M.J.R.), R21AG073676 (M.J.R.).
Conflict of Interest
None declared.
References
- 1. Farkas E, de Vos RA, Donka G, Jansen Steur EN, Mihály A, Luiten PG.. Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol. 2006;111(2):150–157. doi: 10.1007/s00401-005-0007-y [DOI] [PubMed] [Google Scholar]
- 2. Faber JE, Chilian WM, Deindl E, van Royen N, Simons M.. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34(9):1854–1859. doi: 10.1161/atvbaha.114.303929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schager B, Brown CE.. Susceptibility to capillary plugging scan predict brain region specific vessel loss with aging. J Cereb Blood Flow Metab. 2020;40(12):2475–2490. doi: 10.1177/0271678X19895245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA.. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol (1985) 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006 [DOI] [PubMed] [Google Scholar]
- 5. Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S.. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90(10):E89–E93. doi: 10.1161/01.res.0000020861.20064.7e [DOI] [PubMed] [Google Scholar]
- 6. Kratzer I, Ek J, Stolp H.. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr. 2020;1862(11):183430. doi: 10.1016/j.bbamem.2020.183430 [DOI] [PubMed] [Google Scholar]
- 7. Hablitz LM, Nedergaard M.. The glymphatic system: a novel component of fundamental neurobiology. J Neurosci. 2021;41(37):7698–7711. doi: 10.1523/JNEUROSCI.0619-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mann DM, Eaves NR, Marcyniuk B, Yates PO.. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol Aging. 1986;7(5):321–330. doi: 10.1016/0197-4580(86)90158-2 [DOI] [PubMed] [Google Scholar]
- 10. Brown WR, Blair RM, Moody DM, et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257(1-2):67–71. doi: 10.1016/j.jns.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 11. Bullitt E, Zeng D, Mortamet B, et al. The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging. 2010;31(2):290–300. doi: 10.1016/j.neurobiolaging.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damodarasamy M, Vernon RB, Pathan JL, et al. The microvascular extracellular matrix in brains with Alzheimer’s disease neuropathologic change (ADNC) and cerebral amyloid angiopathy (CAA). Fluids Barriers CNS 2020;17(1):60. doi: 10.1186/s12987-020-00219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sonntag WE, Lynch CD, Cooney PT, Hutchins PM.. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138(8):3515–3520. doi: 10.1210/endo.138.8.5330 [DOI] [PubMed] [Google Scholar]
- 15. Nyúl-Tóth A, Tarantini S, DelFavero J, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320(4):H1370–H1392. doi: 10.1152/ajpheart.00709.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ingraham JP, Forbes ME, Riddle DR, Sonntag WE.. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63(1):12–20. doi: 10.1093/gerona/63.1.12 [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Wang B, Ren C, et al. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8(5):590–610. doi: 10.14336/AD.2017.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR.. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66(5):337–345. doi: 10.1097/nen.0b013e3180537147 [DOI] [PubMed] [Google Scholar]
- 19. Akima M, Nonaka H, Kagesawa M, Tanaka K.. A study on the microvasculature of the cerebral cortex. Fundamental architecture and its senile change in the frontal cortex. Lab Invest. 1986;55(4):482–489. [PubMed] [Google Scholar]
- 20. Cheung CY, Chen C, Wong TY.. Ocular fundus photography as a tool to study stroke and dementia. Semin Neurol. 2015;35(5):481–490. doi: 10.1055/s-0035-1563570 [DOI] [PubMed] [Google Scholar]
- 21. Wang Z, Lyons B, Truscott RJ, Schey KL.. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 2014;13(2):226–234. doi: 10.1111/acel.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G.. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17(1):21–30. doi: 10.1152/physiolgenomics.00136.2003 [DOI] [PubMed] [Google Scholar]
- 23. Rzechorzek W, Zhang H, Buckley BK, Hua K, Pomp D, Faber JE.. Aerobic exercise prevents rarefaction of pial collaterals and increased stroke severity that occur with aging. J Cereb Blood Flow Metab. 2017;37(11):3544–3555. doi: 10.1177/0271678X17718966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiss T, Balasubramanian P, Valcarcel-Ares MN, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41(5):619–630. doi: 10.1007/s11357-019-00074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P.. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF.. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37(Pt 2):529–534. doi: 10.1161/01.hyp.37.2.529 [DOI] [PubMed] [Google Scholar]
- 27. Guebel DV, Torres NV, Acebes A.. Mapping the transcriptomic changes of endothelial compartment in human hippocampus across aging and mild cognitive impairment. Biol Open. 2021;10(5). doi: 10.1242/bio.057950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heringa SM, van den Berg E, Reijmer YD, et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–118. doi: 10.1016/j.psyneuen.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 29. Santoro A, Bientinesi E, Monti D.. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. doi: 10.1016/j.arr.2021.101422 [DOI] [PubMed] [Google Scholar]
- 30. Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA.. Healthy aging and the blood-brain barrier. Nat Aging. 2021;1(3):243–254. doi: 10.1038/s43587-021-00043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stamatovic SM, Martinez-Revollar G, Hu A, Choi J, Keep RF, Andjelkovic AV.. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis. 2019;126:105–116. doi: 10.1016/j.nbd.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen MB, Yang AC, Yousef H, et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 2020;30(13):4418–4432.e4. doi: 10.1016/j.celrep.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rojas-Vázquez S, Blasco-Chamarro L, López-Fabuel I, Martínez-Máñez R, Fariñas I.. Vascular senescence: a potential bridge between physiological aging and neurogenic decline. Front Neurosci. 2021;15:666881. doi: 10.3389/fnins.2021.666881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ungvari Z, Tucsek Z, Sosnowska D, et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68(8):877–891. doi: 10.1093/gerona/gls242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bennett HC, Kim Y.. Pericytes across the lifetime in the central nervous system. Front Cell Neurosci. 2021;15:627291. doi: 10.3389/fncel.2021.627291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berthiaume AA, Schmid F, Stamenkovic S, et al. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat Commun. 2022;13(1):5912. doi: 10.1038/s41467-022-33464-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fjorder AS, Rasmussen MB, Mehrjouy MM, et al. Haploinsufficiency of ARHGAP42 is associated with hypertension. Eur J Hum Genet. 2019;27(8):1296–1303. doi: 10.1038/s41431-019-0382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owasil R, O’Neill R, Keable A, et al. The pattern of AQP4 expression in the ageing human brain and in cerebral amyloid angiopathy. Int J Mol Sci. 2020;21(4). doi: 10.3390/ijms21041225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding F, Liang S, Li R, et al. Astrocytes exhibit diverse Ca(2+) changes at subcellular domains during brain aging. Front Aging Neurosci. 2022;14:1029533. doi: 10.3389/fnagi.2022.1029533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mills WA, 3rd, Woo AM, Jiang S, et al. Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat Commun. 2022;13(1):1794. doi: 10.1038/s41467-022-29475-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Habib N, McCabe C, Medina S, et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci. 2020;23(6):701–706. doi: 10.1038/s41593-020-0624-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Popov A, Brazhe A, Denisov P, et al. Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell. 2021;20(3):e13334. doi: 10.1111/acel.13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA.. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA. 2018;115(8):E1896–E1905. doi: 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM.. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153(5):933–946. doi: 10.1083/jcb.153.5.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stratman AN, Davis GE.. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18(1):68–80. doi: 10.1017/S1431927611012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wallow IH, Bindley CD, Reboussin DM, Gange SJ, Fisher MR.. Systemic hypertension produces pericyte changes in retinal capillaries. Invest Ophthalmol Vis Sci. 1993;34(2):420–430. [PubMed] [Google Scholar]
- 48. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 49. Hawkes CA, Gatherer M, Sharp MM, et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell. 2013;12(2):224–236. doi: 10.1111/acel.12045 [DOI] [PubMed] [Google Scholar]
- 50. Hawkes CA, Härtig W, Kacza J, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121(4):431–443. doi: 10.1007/s00401-011-0801-7 [DOI] [PubMed] [Google Scholar]
- 51. Uspenskaia O, Liebetrau M, Herms J, Danek A, Hamann GF.. Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci. 2004;5:37. doi: 10.1186/1471-2202-5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Candiello J, Cole GJ, Halfter W.. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29(5):402–410. doi: 10.1016/j.matbio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 53. Keable A, Fenna K, Yuen HM, et al. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta. 2016;1862(5):1037–1046. doi: 10.1016/j.bbadis.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ceafalan LC, Fertig TE, Gheorghe TC, et al. Age-related ultrastructural changes of the basement membrane in the mouse blood-brain barrier. J Cell Mol Med. 2019;23(2):819–827. doi: 10.1111/jcmm.13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iozzo RV, Schaefer L.. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Logsdon AF, Rhea EM, Reed M, Banks WA, Erickson MA.. The neurovascular extracellular matrix in health and disease. Exp Biol Med (Maywood). 2021;246(7):835–844. doi: 10.1177/1535370220977195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al-Ahmad AJ, Patel R, Palecek SP, Shusta EV.. Hyaluronan impairs the barrier integrity of brain microvascular endothelial cells through a CD44-dependent pathway. J Cereb Blood Flow Metab. 2019;39(9):1759–1775. doi: 10.1177/0271678X18767748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ando Y, Okada H, Takemura G, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8(1):17523. doi: 10.1038/s41598-018-35976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reed MJ, Damodarasamy M, Banks WA.. The extracellular matrix of the blood-brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers. 2019;7(4):1651157. doi: 10.1080/21688370.2019.1651157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mulivor AW, Lipowsky HH.. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283(4):H1282–H1291. doi: 10.1152/ajpheart.00117.2002 [DOI] [PubMed] [Google Scholar]
- 61. Kutuzov N, Flyvbjerg H, Lauritzen M.. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci USA. 2018;115(40):E9429–E9E38. doi: 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Machin DR, Bloom SI, Campbell RA, et al. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2018;315(3):H531–H539. doi: 10.1152/ajpheart.00104.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Erickson MA, Banks WA.. Age-associated changes in the immune system and blood-brain barrier functions. Int J Mol Sci. 2019;20(7):1632. doi: 10.3390/ijms20071632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elahy M. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immu Aging. 2015;12:2. doi: 10.1186/s12979-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Verheggen ICM, de Jong JJA, van Boxtel MPJ, et al. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience. 2020;42(6):1751–1764. doi: 10.1007/s11357-020-00282-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Montagne A, Barnes SR, Nation DA, Kisler K, Toga AW, Zlokovic BV.. Imaging subtle leaks in the blood-brain barrier in the aging human brain: potential pitfalls, challenges, and possible solutions. Geroscience. 2022;44(3):1339–1351. doi: 10.1007/s11357-022-00571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montagne A, Huuskonen MT, Rajagopal G, et al. Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimers Dement. 2019;15(12):1568–1575. doi: 10.1016/j.jalz.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang AC, Stevens MY, Chen MB, et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583(7816):425–430. doi: 10.1038/s41586-020-2453-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G.. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ungvari Z, Podlutsky A, Sosnowska D, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68(12):1443–1457. doi: 10.1093/gerona/glt057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamazaki Y, Baker DJ, Tachibana M, et al. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke. 2016;47(4):1068–1077. doi: 10.1161/STROKEAHA.115.010835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T.. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-β by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging. 2013;34(7):1902–1912. doi: 10.1016/j.neurobiolaging.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 75. Hussain M, Bork K, Gnanapragassam VS, et al. Novel insights in the dysfunction of human blood-brain barrier after glycation. Mech Ageing Dev. 2016;155:48–54. doi: 10.1016/j.mad.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 76. Rom S, Heldt NA, Gajghate S, Seliga A, Reichenbach NL, Persidsky Y.. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep. 2020;10(1):7274. doi: 10.1038/s41598-020-64349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A.. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 78. Dinenno FA, Seals DR, DeSouza CA, Tanaka H.. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol. 2001;531(Pt 2):573–579. doi: 10.1111/j.1469-7793.2001.0573i.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dinenno FA, Tanaka H, Stauffer BL, Seals DR.. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001;536(Pt 3):977–983. doi: 10.1111/j.1469-7793.2001.00977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donato AJ, Gano LB, Eskurza I, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–H432. doi: 10.1152/ajpheart.00689.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lourenço CF, Ledo A, Barbosa RM, Laranjinha J.. Neurovascular-neuroenergetic coupling axis in the brain: master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic Biol Med. 2017;108:668–682. doi: 10.1016/j.freeradbiomed.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 82. Venturelli M, Pedrinolla A, Boscolo Galazzo I, et al. Impact of nitric oxide bioavailability on the progressive cerebral and peripheral circulatory impairments during aging and Alzheimer’s disease. Front Physiol. 2018;9:169. doi: 10.3389/fphys.2018.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81(11):984–991. doi: 10.1212/WNL.0b013e3182a43e1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hansen TW, Li Y, Staessen JA, et al. Independent prognostic value of the ambulatory arterial stiffness index and aortic pulse wave velocity in a general population. J Hum Hypertens. 2008;22(3):214–216. doi: 10.1038/sj.jhh.1002295 [DOI] [PubMed] [Google Scholar]
- 85. Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878. doi: 10.1038/s41467-018-07318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB.. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27(2):260–268. doi: 10.1038/sj.ijo.802225 [DOI] [PubMed] [Google Scholar]
- 87. Bálint AR, Puskás T, Menyhárt A, et al. Aging impairs cerebrovascular reactivity at preserved resting cerebral arteriolar tone and vascular density in the laboratory rat. Front Aging Neurosci. 2019;11:301. doi: 10.3389/fnagi.2019.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thomas BP, Liu P, Park DC, van Osch MJ, Lu H.. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab. 2014;34(2):242–247. doi: 10.1038/jcbfm.2013.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reich T, Rusinek H.. Cerebral cortical and white matter reactivity to carbon dioxide. Stroke. 1989;20(4):453–457. doi: 10.1161/01.str.20.4.453 [DOI] [PubMed] [Google Scholar]
- 90. Vestergaard MB, Jensen ML, Arngrim N, Lindberg U, Larsson HB.. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab. 2020;40(2):341–353. doi: 10.1177/0271678X18818291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mitschelen M, Garteiser P, Carnes BA, et al. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neuroscience. 2009;164(3):918–928. doi: 10.1016/j.neuroscience.2009.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Postert T, Federlein J, Przuntek H, Büttner T.. Insufficient and absent acoustic temporal bone window: potential and limitations of transcranial contrast-enhanced color-coded sonography and contrast-enhanced power-based sonography. Ultrasound Med Biol. 1997;23(6):857–862. doi: 10.1016/s0301-5629(97)00047-1 [DOI] [PubMed] [Google Scholar]
- 93. Boccalini S, Si-Mohamed S, Dessouky R, Sigovan M, Boussel L, Douek P.. Feasibility of human vascular imaging of the neck with a large field-of-view spectral photon-counting CT system. Diagn Interv Imaging. 2021;102(5):329–332. doi: 10.1016/j.diii.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 94. Symons R, Reich DS, Bagheri M, et al. Photon-counting computed tomography for vascular imaging of the head and neck: first in vivo human results. Invest Radiol. 2018;53(3):135–142. doi: 10.1097/RLI.0000000000000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Callewaert B, Jones EAV, Himmelreich U, Gsell W.. Non-invasive evaluation of cerebral microvasculature using pre-clinical mri: principles, advantages and limitations. Diagnostics (Basel). 2021;11(6). doi: 10.3390/diagnostics11060926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao F, Zhao W, Zheng Y, et al. Intravoxel incoherent motion magnetic resonance imaging used in preoperative screening of high-risk patients with Moyamoya disease who may develop postoperative cerebral hyperperfusion syndrome. Front Neurosci. 2022;16:826021. doi: 10.3389/fnins.2022.826021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang Y, Hashizume Y.. Pathological study of age-related vascular changes in the spinal cord. Nihon Ronen Igakkai Zasshi. 1996;33(8):563–568. doi: 10.3143/geriatrics.33.563 [DOI] [PubMed] [Google Scholar]
- 98. SCI-INFO-PAGES. Spinal Cord Injury Facts & Statistics. 2022. [Google Scholar]
- 99. Barbiellini Amidei C, Salmaso L, Bellio S, Saia M.. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60(9):812–819. doi: 10.1038/s41393-022-00795-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mautes AE, Weinzierl MR, Donovan F, Noble LJ.. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):673–687. [PubMed] [Google Scholar]
- 101. Qiu MG, Zhu XH.. Aging changes of the angioarchitecture and arterial morphology of the spinal cord in rats. Gerontology. 2004;50(6):360–365. doi: 10.1159/000080173 [DOI] [PubMed] [Google Scholar]
- 102. Tomlinson BE, Irving D.. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34(2):213–219. doi: 10.1016/0022-510x(77)90069-7 [DOI] [PubMed] [Google Scholar]
- 103. Piekarz KM, Bhaskaran S, Sataranatarajan K, et al. Molecular changes associated with spinal cord aging. Geroscience. 2020;42(2):765–784. doi: 10.1007/s11357-020-00172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maxwell N, Castro RW, Sutherland NM, et al. alpha-Motor neurons are spared from aging while their synaptic inputs degenerate in monkeys and mice. Aging Cell. 2018;17(2):e12726. doi: 10.1111/acel.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Toedebusch CM, Garcia VB, Snyder JC, et al. Lumbar spinal cord microglia exhibited increased activation in aging dogs compared with young adult dogs. Geroscience. 2020;42(1):169–182. doi: 10.1007/s11357-019-00133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Buchman AS, Leurgans SE, Nag S, et al. Spinal arteriolosclerosis is common in older adults and associated with parkinsonism. Stroke. 2017;48(10):2792–2798. doi: 10.1161/STROKEAHA.117.017643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kumar H, Ropper AE, Lee SH, Han I.. Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol. 2017;54(5):3578–3590. doi: 10.1007/s12035-016-9910-6 [DOI] [PubMed] [Google Scholar]
- 108. Yamadera M, Fujimura H, Inoue K, et al. Microvascular disturbance with decreased pericyte coverage is prominent in the ventral horn of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5-6):393–401. doi: 10.3109/21678421.2015.1011663 [DOI] [PubMed] [Google Scholar]
- 109. Zhu S, Chen M, Ying Y, et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022;10(1):30. doi: 10.1038/s41413-022-00203-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Montague-Cardoso K, Malcangio M.. Changes in blood-spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. Pain Rep. 2021;6(1):e879. doi: 10.1097/PR9.0000000000000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. NSCISC. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. Secondary Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance 2020. [Google Scholar]
- 112. Gaudet AD, Fonken LK, Ayala MT, Maier SF, Watkins LR.. Aging and miR-155 in mice influence survival and neuropathic pain after spinal cord injury. Brain Behav Immun. 2021;97:365–370. doi: 10.1016/j.bbi.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hooshmand MJ, Galvan MD, Partida E, Anderson AJ.. Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: is age a limitation? Immun Ageing. 2014;11:15. doi: 10.1186/1742-4933-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shanbhag S. Ultra-high-resolution coronary CT angiography: the “final frontier”—are we there yet? Radiology: Cardiothoracic Imaging 2021;3(4):e210196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nael K. High-spatial-resolution contrast-enhanced MR angiography of abdominal arteries with parallel acquisition at 3.0 T: initial experience in 32 patients. AJR Am J Roentgenol. 2006;187(1):W77–W85. doi: 10.2214/AJR.05.1440 [DOI] [PubMed] [Google Scholar]
- 116. Cao Y, Wu T, Yuan Z, et al. Three-dimensional imaging of microvasculature in the rat spinal cord following injury. Sci Rep. 2015;5:12643. doi: 10.1038/srep12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Davalos D, Akassoglou K.. In vivo imaging of the mouse spinal cord using two-photon microscopy. J Vis Exp. 2012;(59):e2760. doi: 10.3791/2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dray C, Rougon G, Debarbieux F.. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci USA. 2009;106(23):9459–9464. doi: 10.1073/pnas.0900222106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Haghayegh Jahromi N, Tardent H, Enzmann G, et al. A novel cervical spinal cord window preparation allows for two-photon imaging of T-cell interactions with the cervical spinal cord microvasculature during experimental autoimmune encephalomyelitis. Front Immunol. 2017;8:406. doi: 10.3389/fimmu.2017.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Khaing ZZ, Cates LN, DeWees DM, et al. Contrast-enhanced ultrasound to visualize hemodynamic changes after rodent spinal cord injury. J Neurosurg Spine. 2018;29(3):306–313. doi: 10.3171/2018.1.SPINE171202 [DOI] [PubMed] [Google Scholar]
- 121. Bruce M, DeWees D, Harmon J, Cates L, Khaing ZZ, Hofstetter CP.. Blood flow changes associated with spinal cord injury assessed by non-linear Doppler contrast-enhanced ultrasound. Ultrasound Med Biol. 2022;48(8):1410–1419. doi: 10.1016/j.ultrasmedbio.2022.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Khaing ZZ, Cates LN, Hyde J, et al. Contrast-enhanced ultrasound for assessment of local hemodynamic changes following a rodent contusion spinal cord injury. Mil Med. 2020;185(Suppl 1):470–475. doi: 10.1093/milmed/usz296 [DOI] [PubMed] [Google Scholar]
- 123. Beliard B, Ahmanna C, Tiran E, et al. Ultrafast Doppler imaging and ultrasound localization microscopy reveal the complexity of vascular rearrangement in chronic spinal lesion. Sci Rep. 2022;12(1):6574. doi: 10.1038/s41598-022-10250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Khaing ZZ, Cates LN, Hyde JE, Hammond R, Bruce M, Hofstetter CP.. Transcutaneous contrast-enhanced ultrasound imaging of the posttraumatic spinal cord. Spinal Cord. 2020;58(6):695–704. doi: 10.1038/s41393-020-0415-9 [DOI] [PubMed] [Google Scholar]