Abstract
Yersinia enterocolitica is occasionally detected in kimchi, a traditional food prepared from fermented vegetables. Changes in growth properties of Y. enterocolitica during kimchi fermentation are largely unknown. We investigated the viability of Y. enterocolitica during the fermentation of vegan and non-vegan kimchi at different temperatures. Changes in Y. enterocolitica population, pH, and titratable acidity were measured for 24 days. In a suspension test with kimchi juice, populations of three Y. enterocolitica strains were above 3.30 log10 CFU/mL at pH > 5 for 7 days. Yersinia enterocolitica counts in vegan kimchi were considerably reduced at 0 °C and 6 °C. During fermentation at 6 °C, Y. enterocolitica populations in non-vegan kimchi and vegan kimchi were not detected starting from days 14 and 10, respectively. In kimchi samples stored at 0 °C and 6 °C, Y. enterocolitica survival correlated with pH changes during fermentation; in samples stored for up to 24 days, Y. enterocolitica was not detected. According to the kmax values from the “log-linear with shoulder and tail” model, Y. enterocolitica was more sensitive to vegan kimchi fermentation than to non-vegan kimchi fermentation. Our findings provide an important basis for ensuring the safe production of kimchi without Y. enterocolitica contamination. Further research is necessary to elucidate the mechanism of Y. enterocolitica inactivation and the major bacterial and physicochemical factors involved in kimchi fermentation.
Keywords: Fermented kimchi, Foodborne pathogen, Survival kinetics, Antimicrobial effect
1. Introduction
Kimchi is a traditional food in Korea, comprising various vegetables, such as kimchi cabbage, garlic, red pepper powder, fermented seafood (jeotgal), and radish, fermented at a low temperature. In recent years, kimchi has been recognized worldwide as a fermented vegetable probiotic food that improves health [1,2]. Spontaneous fermentation, without the use of starter cultures or sterilization, leads to the growth of various microorganisms during the ripening of kimchi [3]. Kimchi is prepared using various ingredients harvested from soil and is therefore susceptible to microbial contamination. Moreover, farm products are often mixed with contaminants, and raw ingredients are ingested without thermal processing, which can negatively affect human health [4].
The Korean Food and Drug Administration mandates that kimchi products be “negative” for Yersinia enterocolitica (YE), Salmonella, Vibrio parahaemolyticus, Listeria monocytogenes, enterohemorrhagic Escherichia coli, and Campylobacter jejuni/coli. YE is a gram-negative, rod-shaped, psychrotrophic pathogen that can grow in cold chain environments in food markets [5]. Yersinia may contaminate kimchi during cold storage. The occurrence of YE in ready-to-eat vegetables, water dropwort, Chinese cabbage, lettuce, and frozen fruits [[6], [7], [8]], as well as in water used for salting and washing kimchi cabbages during the manufacturing of kimchi has been reported [9]. Therefore, research on food pathogens, such as YE, in kimchi is important from the perspective of the kimchi industry in South Korea.
The antibacterial activities of organic acids such as lactic acid, acetic acid, and oxalic acid produced by lactic acid bacteria (LAB) in kimchi are well known. The organic compounds produced by lactic acid fermentation potentially lower the risk of foodborne diseases [10,11] and, therefore, consumers can consume cabbage kimchi without anxiety regarding contamination, even though it is a non-thermally processed food [11]. However, the composition of these organic compounds could be influenced by changes in microbial communities owing to the nutritive components and various raw ingredients present at the start of fermentation [1]. The raw ingredients have an important role in establishing the microbial community responsible for fermenting kimchi. In particular, as kimchi attracts global attention as a vegetable fermented food, interest in vegan kimchi (VK) is also increasing. The non-usage of animal ingredients, such as fish sauce, in vegan kimchi can result in differences in microbiological and physicochemical characteristics compared with those of generally used kimchi. However, basic research data related to the differences are limited. Therefore, several studies have profiled and compared the effect of vegan and non-vegan ingredients and assessed the changes in the microbial community of kimchi based on the differences [3,12]. However, little is known about the changes in the growth properties of YE during kimchi fermentation, depending on whether the ingredients are vegan or non-vegan. Therefore, in the present study, we aimed to evaluate the changes in the growth properties of YE during kimchi fermentation at 0 °C and 6 °C. Furthermore, we compared the changes in the physicochemical and microbial properties of YE in VK and non-vegan (NVK) kimchi.
2. Materials and methods
2.1. Sample preparation
Commercial NVK and VK samples (initial pH > 5.5), which were prepared as described below, were purchased from the Kimchi Town market in Gwangju, Korea. For NVK samples, salted cabbage (Brassica rapa subsp. pekinensis) was mixed with kimchi paste composed of 20.8% red pepper powder, 5.0% salt-fermented anchovy, 5.0% salt-fermented shrimp, 5.0% radish, 5.0% green onion, 16.0% glutinous rice paste, 5.0% onion, 1.0% seaweed extract, 1.0% ground garlic, and 0.1% ground ginger. On the contrary, in the preparation of vegan kimchi, 5.0% anchovy and 5.0% salted shrimp were excluded from the above ingredients. The NVK and VK samples were separately packed in approximately 200 g units and stored in sterile plastic kimchi containers (LOCK&LOCK 8 L, Seoul, Korea). They were fermented at 0 °C or 6 °C for 24 days, and measurements were taken at 0, 3, 6, 10, 14, 18, and 24 days. The microbial count of YE (colony-forming units [CFUs]), pH, and titratable acidity (TA) of kimchi samples were estimated. During fermentation, biochemical changes in the samples were monitored for 24 days. For spiking experiments, each kimchi packaging pouch was inoculated with a 10-mL aliquot of YE (6.05 ± 0.25 log10 CFU/g) and stored at 0 °C or 6 °C for up to 24 days. Non-inoculated kimchi served as the negative control.
2.2. Strains and culture conditions
YE strains (ATCC 9610, ATCC 23715, and ATCC 27729) were purchased from the American Type Culture Collection (Manassas, VA, USA). All strains were cultured at 30 °C in tryptic soy broth (Difco Laboratories, Detroit, MI, USA) for 24 h to prepare a final inoculum adjusted to ∼106 log10 CFU/mL before use in the subsequent experiments.
2.3. Suspension test
YE strain suspensions (800 μL; 5.54 ± 0.35 log10 CFU/mL) were mixed with 8 mL of one of the test kimchi juices and adjusted to different pH levels with lactic acid solution (Sigma-Aldrich, St. Louis, MO, USA). The following five groups with different pH levels were created: A, above 5.5; B, 5.0–5.5; C, 4.5–5.0; D, 4.0–4.5; E, below 4.0. The mixtures were vortexed and allowed to stand for 0, 1, 4, and 7 days at 6 °C with agitation. Immediately after the treatment, the mixture was 10-fold serially diluted, and aliquots were analyzed for colony formation (CFU/mL) on cefsulodin irgasan novobiocin (CIN; Difco, Sparks, MD, USA) agar plates incubated for 24 ± 2 h at 30 °C.
2.4. Determination of YE bacterial count during kimchi fermentation
YE ATCC 27729 suspensions were used to determine YE bacterial count during kimchi fermentation. Enumeration of total viable YE was performed using the modified Food Code (Ministry of Food and Drug Safety, Republic of Korea). Kimchi samples were homogenized for 60 s with a stomacher (BagMixer® 400; Interscience, St Nom, France) after putting 25 g of the kimchi sample in a sterile filter bag (3 M, Saint Paul, MN, USA) and adding 225 mL phosphate buffered saline (PBS; Sigma-Aldrich) diluent. The CIN agar (Difco) plates were incubated at 30 °C for 24 ± 2 h, and the YE microbial counts in the kimchi samples were determined as the number of CFUs per gram. We identified a YE colony when a characteristic deep red center with a transparent margin and a diameter of 2–4 mm was observed on the CIN agar. Colonies on plates inoculated with PBS-diluted samples that yielded between 15 and 300 colonies were counted, and the viable counting method was repeated three times. The survival curves of YE during the fermentation of kimchi at 0 and 6 °C were generated.
2.5. Measurement of pH and acidity
Samples (5 g cabbage and 5 g juice) were homogenized in a blender (HR1372; Philips, Amsterdam, The Netherlands). The solid material was removed by filtering through sterilized gauze, and the filtrate was used for analyses. The TA and pH were measured using a pH meter (TitroLine 5000; SI Analytics, Mainz, Germany). TA was determined according to the Association of Official Analytical Chemists (AOAC, 2005) method by titrating the sample to pH 8.3 with 0.1 N NaOH (DaeJung Chemicals and Metals, Busan, Korea) [13]. TA was calculated using the following formula [13]:
| (1) |
2.6. Log-linear mode
Survival regression curves were constructed for the reduction values of the YE counts during kimchi fermentation, using the Geeraerd and Van Impe inactivation model fitting tool (GInaFiT), where log10 CFU/g was plotted against time for each experiment. Predicted curves were fitted according to the Log-linear with shoulder and tail model (Eq. (2)), which is a three-parameter non-linear model and can be written as described by Geeraerd et al. [13]:
| (2) |
where, t is exposure time (day); N is the number of observed colonies (CFU/g) at time t; N0 is the initial number of observed colonies (CFU/g) at time 0; Nres is the residual population (CFU g_1) density (tail region); and kmax is the maximum specific inactivation rate constant (day−1).
2.7. Model validation
The root mean square error (RMSE; Eq. (3)) and standard correlation coefficient (R2; Eq. (4)) were analyzed in Microsoft Excel to verify the best model for each experimental set [15]. Lower RMSE and higher R2 values indicated more acceptable model fits.
| (3) |
where, y is the mean predicted value, yi is the predicted value, and ei is the error in the predicted value.
| (4) |
where, p denotes the number of parameters and n denotes the number of experimental observations; observed and predicted values indicate the observed and predicted bacterial populations, respectively. For inactivation kinetics validation by comparing observations with model fits/predictions, additional experimental data from 2 intermediary days (2 and 12 days) were used for testing model prediction. Accuracy factor (Af; Eq. (5)) and bias factor (Bf; E1. 6) were used to determine the predictive efficacy [15]:
| (5) |
| (6) |
where, y is observed YE population value and n is the number of experimental observations.
2.8. Statistical analysis
All experiments were performed in triplicate with three samples per trial. The results were evaluated for statistical significance, and the results are presented as mean ± standard deviation. Data were analyzed using SPSS v.8.2 for Windows (SPSS Inc., Chicago, IL, USA) to evaluate the significance of the results. Differences between groups were evaluated using Duncan's multiple range test, and results with P < 0.05 were considered statistically significant. The results are expressed as log10 CFU/g, and graphs were plotted using SigmaPlot 14.0 software.
3. Results and discussion
3.1. YE count changes in kimchi juice
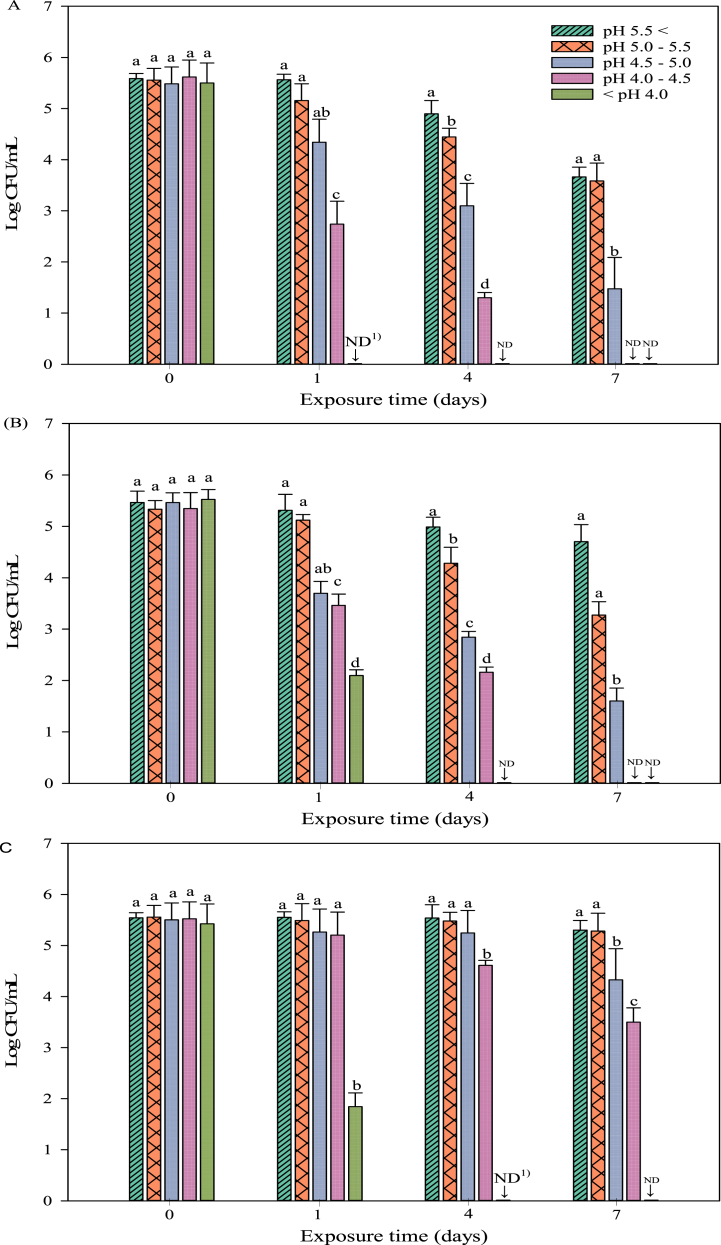
The survival of YE counts in kimchi juice in the five pH groups was evaluated at 0, 1, 4, and 7 days. The inoculation population of YE strains ATCC 9610 (Fig. 1A), ATCC 23715 (Fig. 1B), and ATCC 27729 (Fig. 1C) was 5.54 ± 0.06, 5.35 ± 0.16, and 5.44 ± 0.07 log10 CFU/mL, respectively. The YE strains showed differences in acid resistance; YE ATCC 27729 showed the strongest acid resistance (P < 0.05). All YE strains treated for 7 days in the groups with pH above pH 4.5 survived. In the groups with pH below 4.5, YE ATCC 9610 and ATCC 23715 could not survive for 7 days, whereas YE ATCC 27729 was present at 3.50 ± 0.28 log10 CFU/mL. After 4 days of treatment, YE cells were not detected at pH 4.0. To date, there have been only a few studies on changes in the growth of YE during kimchi fermentation [14]. Therefore, it is difficult to directly compare the cell viability patterns in kimchi juice. Regarding the viability of YE cells in acidic solutions, the kinetics of reduction in YE growth at various concentrations of organic acids, such as lactic and citric acid, at different temperatures has been reported [[15], [16], [17]]. In particular, Virto et al. [17] reported that lactic acid (pH 4.0–5.0) reduces the population of YE strain STCC 4315 up to 4 log levels within 20 min at 4 °C. These results are similar to our findings regarding changes in the viability of YE cells under different pH conditions. Based on these results, we confirmed that there is a possibility that YE can survive in kimchi if it is not sufficiently fermented.
Fig. 1.
Survival properties of Yersinia enterocolitica in kimchi juice at different pH levels. AY. enterocolitica ATCC 9610; BY. enterocolitica ATCC 23715; CY. enterocolitica ATCC 27729. Not Detected (ND) indicates that the concentration of the analyte may have been below the detection limit.
3.2. Physicochemical changes in kimchi during fermentation
During kimchi fermentation, LAB degrade various ingredients (e.g., kimchi cabbage, red pepper powder, and garlic) through enzymatic reactions [18]. Among the physicochemical properties, pH and TA are considered significant indicators of kimchi fermentation. You et al. [19] reported that these two factors are important quality indices. Moreover, these factors are determinants of the sour taste developed during fermentation [20]. The changes in pH and TA during fermentation of YE-contaminated kimchi for 24 days at 0 °C and 6 °C are presented in Table 1. Moreover, negative control results pertaining to pH and TA during the fermentation of kimchi uncontaminated with YE are demonstrated in Table S1. The initial pH and TA of NVK and VK ranged between 0.30 and 0.35% and 5.79–5.81, respectively. The TA gradually increased, whereas the pH decreased during kimchi fermentation. The pH values were significantly (P < 0.05) different between NVK and VK from the day when fermentation started. The pH and TA showed significantly larger changes in VK than in NVK, during kimchi fermentation at both temperatures.
Table 1.
Changes in pH and titratable acidity (TA) during the fermentation of non-vegan and vegan kimchi at 0 °C and 6 °C.
| Temperature/days | Non-vegan kimchi |
Vegan kimchi |
|||
|---|---|---|---|---|---|
| pH | TA (%) | pH | TA (%) | ||
| 0 °C | 0 | 5.81 ± 0.02a | 0.35 ± 0.01a | 5.79 ± 0.02a | 0.30 ± 0.01a |
| 3 | 5.65 ± 0.00b | 0.38 ± 0.01a | 5.41 ± 0.01b | 0.34 ± 0.02 ab | |
| 6 | 5.63 ± 0.03b | 0.43 ± 0.01b | 5.23 ± 0.01c | 0.55 ± 0.01b | |
| 10 | 4.95 ± 0.00c | 0.52 ± 0.02bc | 4.79 ± 0.01d | 0.61 ± 0.01bc | |
| 14 | 4.76 ± 0.03c | 0.80 ± 0.02c | 4.57 ± 0.01de | 0.67 ± 0.01c | |
| 18 | 4.55 ± 0.01cd | 0.85 ± 0.01c | 4.44 ± 0.04e | 0.79 ± 0.03d | |
| 24 | 4.31 ± 0.03d | 1.12 ± 0.06d | 4.30 ± 0.01e | 1.03 ± 0.01e | |
| 6 °C | 0 | 5.81 ± 0.02a | 0.35 ± 0.01a | 5.79 ± 0.02a | 0.30 ± 0.01a |
| 3 | 5.76 ± 0.02b | 0.42 ± 0.03a | 5.38 ± 0.01b | 0.37 ± 0.02a | |
| 6 | 4.75 ± 0.03b | 0.68 ± 0.03b | 4.54 ± 0.02c | 0.76 ± 0.01b | |
| 10 | 4.42 ± 0.01c | 0.89 ± 0.02c | 4.11 ± 0.01d | 1.08 ± 0.01c | |
| 14 | 4.12 ± 0.01d | 1.26 ± 0.01d | 4.05 ± 0.06d | 1.25 ± 0.06d | |
| 18 | 4.11 ± 0.01d | 1.34 ± 0.02e | 4.03 ± 0.02d | 1.26 ± 0.01d | |
| 24 | 4.02 ± 0.04e | 1.37 ± 0.04e | 3.97 ± 0.02e | 1.39 ± 0.01e | |
Kimchi samples were contaminated with Yersinia enterocolitica (6.05 log10 ± 0.25 CFU/g).
Different letters in the same column are significantly different at P < 0.05.
The reason for the significant differences in the trends of pH and TA change in NVK and VK could be related to the raw materials in kimchi. The distinguishing characteristic of NVK and VK with regard to the main raw materials is the use of fermented fish sauce as an animal ingredient. Among the components of fermented fish sauce, free amino acids, such as arginine, methionine, isoleucine, leucine, aspartic acid, glutamic acid, alanine, glycine, and proline, add flavor to kimchi [21,22]. It is considered that some of these free amino acids such as arginine, methionine, and alanine reduce the changes in pH and TA of kimchi by acting as buffers, delaying the acidification and change in the taste of kimchi [[21], [22], [23]]. In our study, the changes in pH and TA in the NVK group using fermented fish sauce were larger than those in the VK group. NVK fermentation progressed gradually at 6 °C and attained optimal fermenting conditions after 10 days with 0.89% ± 0.02% TA, whereas the cut-off condition for optimal fermentation of VK was exceeded.
In previous studies, the cut-off conditions for optimal fermentation to ensure the quality of kimchi were as follows: pH > 4.0 and TA < 1.0% [24]. Generally, kimchi exhibits optimal taste, flavor, and texture when suitably fermented at approximately 0.9–1.0% TA or at pH of approximately 4.0–4.3 [18]. According to Mheen and Kwon [25], fermentation at pH in the range of 4.2–4.5 is considered optimal for kimchi. In particular, our results regarding pH changes were similar to those in several previous studies [4,18,26]. The pH of NVK stored at 6 °C steadily decreased from 5.81 on day 0–4.02 on day 7, whereas the pH of NVK stored at 0 °C remained optimal for fermentation until the end of the fermentation period. The TA of NVK and VK increased from 0.35% to 1.12% and from 0.30% to 1.03%, respectively, at 0 °C after 24 days (P < 0.05; Table 1). The TA of VK at 6 °C, which was initially 0.30%, increased to moderate values of 0.7–1.0%, and reached 1.39% at the end of fermentation.
3.3. Microbial changes during kimchi fermentation
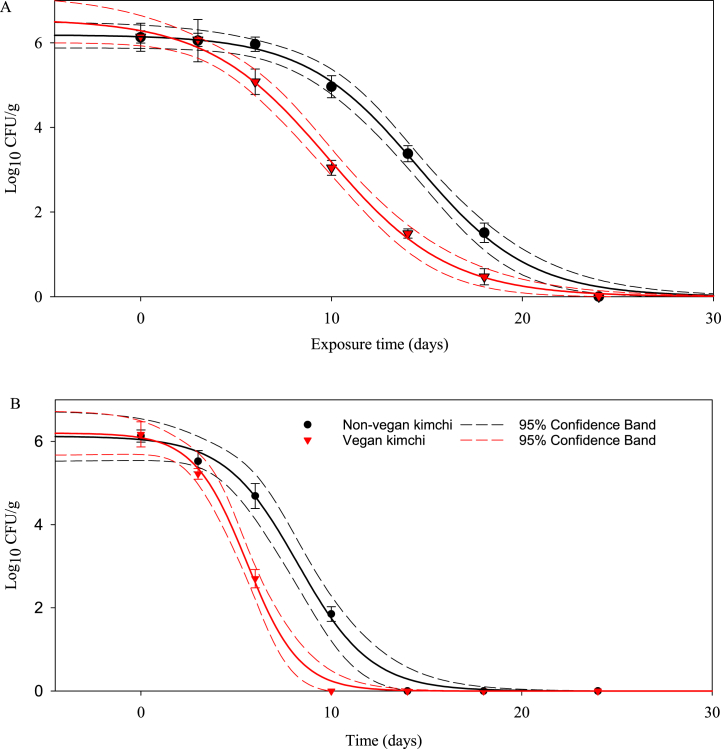
The changes in YE during the fermentation of NVK and VK stored at 0 °C and 6 °C are presented in Fig. 2A and B. The initial counts of YE in NVK and VK were 6.05 ± 0.25 log10 CFU/g. The YE populations in both NVK and VK steadily declined during fermentation at 6 °C and were not detected (with a detection limit of 0.69 log10edecreased to a greater extent in VK than in NVK during fermentation at both temperatures. The YE populations in NVK and VK were stable for 3 days and decreased slowly but steadily thereafter; the populations in NVK (6.05 log10 CFU/g) and VK (6.07 log10 CFU/g) on day 3 were not detected after 24 days. Interestingly, the YE population in the VK sample group was inactivated more rapidly than that in the NVK sample group at both temperatures. Overall, all graphs (Fig. 2) indicate a rapid decrease in YE population as storage temperatures increase. LAB and various organic acids are produced during kimchi fermentation [2]. Changes in the number of LAB during the fermentation process of kimchi markedly affect the microbiological characteristics of kimchi [2]. This scientific proof can be attributed to the direct effect of LAB dominance and the indirect effect of secondary LAB metabolites. Virto et al. [17] evaluated YE population numbers in lactic (0.3–4.0% v/v) and citric (1–20% w/v) acids at different temperatures (4, 20, 40, and 80 °C) and reported that the viability and survivability of YE were dependent on temperature, acid concentration, and duration of exposure to the acid. According to a previous study, at pH 3.0, YE cells do not survive, and the influence of lactic acid concentration on microbial inactivation is dependent on temperature. Similar changes in pH and YE counts were observed in previous studies [17,27]. Although the characterization of LAB population has not been conducted in our study, the inactivation effect of various organic acids produced by LAB has been demonstrated in various studies. Particularly, organic acids and LAB metabolites have been reported to affect YE viability [28]. Moreover, several studies have evaluated the effect of acidic conditions [29] on the growth and survival of YE. The antimicrobial activity of organic acids is thought to arise from the presence of their anions and their ability to acidify the cytoplasm [30]. These results demonstrate that there are a few safety issues associated with fermented kimchi because fermentation inhibits foodborne pathogens such as YE. All in all, the main factors influencing the microbiological safety of kimchi cannot be determined based on a single indicator. Therefore, the broad interpretation of some of the study's findings is not sufficient to guarantee the safety of kimchi consumption. However, we are confident that our findings contribute to maintaining the safety of kimchi. We found that YE in NVK and VK stored at 0 °C or 6 °C for up to 24 days did not survive during fermentation. Our findings provide a considerable basic understanding regarding the safety of kimchi, which is a non-thermally and minimally processed fresh food.
Fig. 2.
Survival curve of Yersinia enterocolitica strain ATCC 27729 during fermentation in non-vegan and vegan kimchi at 0 °C and 6 °C. A Kimchi group stored at 0 °C; B Kimchi group stored at 6 °C.
3.4. Inactivation kinetics and efficiency
The analyzed experimental data of the changes in YE during the fermentation of NVK and VK are shown in Table 2. In the initial stage, shoulder-shape (lag phase) and subsequent log-linear trends (exponential decay) were observed in YE inactivation in both NVK and VK. Based on the shape of the inactivation curves for the four experimental sets, the “log-linear with shoulder and tail” model was applied to analyze the experimental data. The lag phase under environmental conditions is because the planktonic bacterial cells have a specific level of resistance to oxidative stress, as indicated by the shoulder length (SL) [31]. The kinetic parameters for YE ATCC 27729 inactivation are detailed in Table 2.
Table 2.
Estimated parameters of the log-linear with shoulder and tail model and respective RMSE values for Yersinia enterocolitica strain ATCC 27729 inactivation.
| Model | Sample | SL (day) | kmax (day−1) | R2 | RMSE | |
|---|---|---|---|---|---|---|
| Log-linear with shoulder and tail | 0 °C | NVK | 5.7 | 0.41 | 0.9987 | 0.0371 |
| VK | 1.5 | 0.56 | 0.9972 | 0.0401 | ||
| 6 °C | NVK | 2.1 | 0.78 | 0.9967 | 0.0397 | |
| VK | 1.2 | 0.91 | 0.9974 | 0.0411 | ||
NVK, non-vegan kimchi; RMSE, root mean square error; SL, shoulder length; VK, vegan kimchi.
At 0 °C, the mean RMSE value in NVK and VK was 0.0371 and 0.0401, respectively, and the R2 value of NVK and VK was 0.9987 and 0.9972, respectively. The RMSE values close to zero and R2 values close to 1 indicated that the observed experimental data well fit the YE ATCC 27729 inactivation models. As shown in Table 2, the SL value of YE ATCC 27729 in NVK (5.7 days) was higher than that in VK (1.5 days) stored at 0 °C (P < 0.05), and the kmax value of YE ATCC 27729 in NVK (0.41 day−1) was lower than that in VK (0.56 day−1) stored at 0 °C (P < 0.05). The YE ATCC 27729 inactivation pattern observed in NVK and VK stored at 6 °C showed a trend similar to that observed at 0 °C. The mean RMSE value in NVK and VK was 0.0397 and 0.0411, respectively, and the R2 value of NVK and VK was 0.9967 and 0.9974, respectively. The SL value of YE ATCC 27729 in NVK and VK stored at 6 °C was 2.1 and 1.2 days, respectively. According to the kmax values from the inactivation curves (Table 2), YE ATCC 27729 was found to be more sensitive to VK fermentation than to NVK fermentation. Numerous studies have reported that the sensitivity of general microorganisms under particular environmental conditions showed sigmoidal inactivation curves that could be described using the “log-linear with shoulder and tail” model [13,32]. Furthermore, the differences in sensitivity between NVK and VK fermentation may be attributed to various reasons. In particular, the difference in the main components between the NVK and VK samples used in this experiment is the presence or absence of animal ingredients, salt-fermented anchovy, and salt-fermented shrimp. We suggest that the difference in the fermentation patterns of NVK and VK is the primary reason, as the composition of kimchi is different in each case. The faster the fermentation of kimchi proceeds, the higher the inactivation efficiency due to various antibacterial mechanisms, such as the proliferation of LAB and generation of secondary LAB metabolites, which inhibit YE growth or activate control mechanisms. Importantly, this study is the first to directly assess the growth inhibition of YE while comparing the fermentation rates of NVK and VK.
3.5. Regression model fits and predictions
Table 3 presents the predicted YE ATCC 27729 inactivation values during kimchi fermentation, based on the regression models, on varying days of fermentation. For simulations at 0 °C, the regression models confirmed an acceptable model fit at two sampling days (8 and 12 days). For simulations at 6 °C, no scattering was observed in the experimental observation values, and the fit/prediction of the regression model matched the mean values of the experimental data (Table 3). Overall, these results demonstrate that our regression models are well-suited for the 0 °C and 6 °C conditions. Af and Bf values close to 1 indicate that the experimental data were not scattered and that the predictions/fits of the regression models acceptably match the mean values of the experimental data [33]. Bf values less than or greater than 1 indicate that the predicted values of the regression model are overestimates or underestimates. Generally, the Bf value for an acceptable predictive regression model should be between 0.75 and 1.25 [34]. Furthermore, Ross [35] reported that Bf values between 0.7 and 0.9 or 1.06–1.15 denote acceptable model fits; Bf values between 0.9 and 1.05 denote good fits; and Bf values > 1.15 or < 0.7 indicate unacceptable fits.
Table 3.
Accuracy (Af) and bias factor (Bf) for the log-linear with shoulder and tail model, based on the correlation between Yersinia enterocolitica strain ATCC 27729 inactivation and fermentation under simulated kimchi fermentation.
| Simulation sample | Af | Bf | RMSE | ||
|---|---|---|---|---|---|
| 0 °C | 8 day | NVK | 1.027 | 0.979 | 0.048 |
| VK | 1.088 | 1.071 | 0.036 | ||
| 12 day | NVK | 1.038 | 1.089 | 0.066 | |
| VK | 1.071 | 1.107 | 0.081 | ||
| 6 °C | 8 day | NVK | 1.175 | 0.876 | 0.069 |
| VK | 1.134 | 0.893 | 0.077 | ||
| 12 day | NVK | 1.117 | 0.931 | 0.045 | |
| VK | 1.124 | 0.918 | 0.063 | ||
Af, accuracy; Bf, bias factor; NVK, non-vegan kimchi; RMSE, root mean square error; VK, vegan kimchi.
In conclusion, fermented kimchi has a few safety issues because fermentation inactivates the foodborne pathogenic bacterium YE. However, during the initial stages of fermentation, kimchi can be exposed to a high proportion of YE, which can potentially lead to food poisoning outbreaks. In this study, we investigated the changes in the growth characteristics of YE during kimchi fermentation. Furthermore, changes in YE counts, pH, and TA were measured for 24 days during kimchi fermentation. We observed YE inactivation during the fermentation of NVK and VK at different temperatures. Thus, with appropriate fermentation, the safety of kimchi against YE contamination can be ensured. Furthermore, we found that YE did not survive in either type of kimchi stored at 0 °C and 6 °C for up to 24 days. We also found that the survival of YE correlated well with the changes in pH during kimchi fermentation. Based on the analysis results, we confirmed that YE viability was more sensitive to VK fermentation than to NVK fermentation. We believe that our findings provide an important basis for ensuring the safe production of kimchi without YE contamination. Nevertheless, further research is necessary to investigate the mechanism of YE inactivation during kimchi fermentation and to determine the major bacterial and physicochemical factors affecting the process. Further studies investigating the correlation between the specificity of LAB individual changes and the inactivation effect of food-poisoning bacteria are required to clarify the mechanism through which kimchi fermentation inactivates food-poisoning bacteria.
Author contributions
Su-Ji Kim: Performed the experiments; Wrote the paper.
Hae-Won Lee: Analyzed and interpreted the data.
Jae Yong Lee, Eun Woo Moon: Performed the experiment.
Hyeyeon Song: Contributed reagents, materials, analysis tools or data.
Ji-Hyoung Ha: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
This research was supported by the World Institute of Kimchi funded by the Ministry of Science and ICT (grant numbers KE2002-2, and KE2202-2).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e15031.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jung J.Y., Lee S.H., Kim J.M., Park M.S., Bae J.W., Hahn Y., Madsen E.L., Jeon C.O. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 2011;77:2264–2274. doi: 10.1128/AEM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H., Yoon H., Ji Y., Kim H., Park H., Lee J., Shin H., Holzapfel W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011;145:155–161. doi: 10.1016/j.ijfoodmicro.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Zabat M.A., Sano W.H., Cabral D.J., Wurster J.I., Belenky P. The impact of vegan production on the kimchi microbiome. Food Microbiol. 2018;74:171–178. doi: 10.1016/j.fm.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.H., Ha J.H., Lee H.W., Lee J.Y., Hwang Y.S., Lee H.M., Kim S.H., Kim S.J. Analysis of microbiological contamination in kimchi and its ingredients. J. Fd Hyg. Safety. 2018;33:94–101. [Google Scholar]
- 5.Ito A., Sato Y., Kudo S., Sato S., Nakajima H., Toba T. The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr. Microbiol. 2003;47:231–236. doi: 10.1007/s00284-002-3993-1. [DOI] [PubMed] [Google Scholar]
- 6.Kapperud G. Yersinia enterocolitica in food hygiene. Int. J. Food Microbiol. 1991;12:53–65. doi: 10.1016/0168-1605(91)90047-s. [DOI] [PubMed] [Google Scholar]
- 7.Lee T.S., Lee S.W., Seok W.S., Yoo M.Y., Yoon J.W., Park B.K., Moon K.D., Oh D.H. Prevalence, antibiotic susceptibility, and virulence factors of Yersinia enterocolitica and related species from ready-to-eat vegetables available in Korea. J. Food Protect. 2004;67:1123–1127. doi: 10.4315/0362-028x-67.6.1123. [DOI] [PubMed] [Google Scholar]
- 8.Verbikova V., Borilova G., Babak V., Moravkova M. Prevalence, characterization and antimicrobial susceptibility of Yersinia enterocolitica and other Yersinia species found in fruits and vegetables from the European Union. Food Control. 2018;85:161–167. [Google Scholar]
- 9.Kim J.H., Lee Y.K., Yang J.Y. Change of harmful microorganisms in pickling process of salted cabbage according to salting and washing conditions. J. Food Hyg. S. Afr. 2011;26:417–423. [Google Scholar]
- 10.Jung S.R., Hwang H.L., Lee J.H. Effect of lactic acid bacteria on phenyllactic acid production in kimchi. Food Control. 2019;106 [Google Scholar]
- 11.Kang S.J., Kim S.J., Kim S.H., Lee J.H., Park S.Y., Ha S.D. Comparison of the murine norovirus-1 inactivation in cabbage Kimchi with two different salinities during storage. Food Res. Int. 2016;84:96–101. [Google Scholar]
- 12.Lee M., Song J.H., Park J.M., Chang J.Y. Bacterial diversity in Korean temple kimchi fermentation. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108592. [DOI] [PubMed] [Google Scholar]
- 13.AOAC . eighteenth ed. Association of Official Analytical Chemists; Washington, DC: 2005. Official Method of Analysis. [Google Scholar]
- 14.Lee J.H., Ha J.H., Lee H.W., Lee J.Y., Hwang Y.S., Lee H.M., Kim S.H., Kim S.J. Analysis of microbiological contamination in kimchi and its ingredients. J. Food Hyg. Saf. 2018;33:94. [Google Scholar]
- 15.Geeraerd A.H., Valdramidis V.P., Van Impe J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005;102:95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Gouvea F.S., Padilla-Zakour O.I., Worobo R.W., Xavier B.M., Walter E.H.M., Rosenthal A. Effect of high-pressure processing on bacterial inactivation in açaí juices with varying pH and soluble solids content. Innovat. Food Sci. Emerg. Technol. 2020;66 [Google Scholar]
- 17.Virto R., Sanz D., Alvarez I., Condón R.J., Raso J. Inactivation kinetics of Yersinia enterocolitica by citric and lactic acid at different temperatures. Int. J. Food Microbiol. 2005;103:251–257. doi: 10.1016/j.ijfoodmicro.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Baek S., Maruthupandy M., Lee K., Kim D., Seo J. Freshness indicator for monitoring changes in quality of packaged kimchi during storage. Food Packag. Shelf Life. 2020;25 [Google Scholar]
- 19.You S.Y., Yang J.S., Kim S.H., Hwang I.M. Changes in the physicochemical quality characteristics of cabbage kimchi with respect to storage conditions. J. Food Qual. 2017:1–7. [Google Scholar]
- 20.Kim S.J., Lee J.Y., Yoon S.R., Lee H.W., Ha J.H. Regression analysis for predicting the fermentation state of packaged Kimchi using a colorimetric indicator. J. Food Eng. 2019;240:65–72. [Google Scholar]
- 21.Anggo A.D., Ma’ruf W.F., Swastawati F., Rianingsih L. Changes of amino and fatty acids in anchovy (Stolephorus sp.) fermented fish paste with different fermentation periods. Procedia Environ. Sci. 2015;23:58–63. [Google Scholar]
- 22.Koesoemawardani D., Hidayati S., Subeki Amino acid and fatty acid compositions of Rusip from fermented anchovy fish (Stolephorus sp.) IOP Conf. Ser. Mater. Sci. Eng. 2018;344 [Google Scholar]
- 23.Lee J.H., Hwang H.L. Characterization of arginine catabolism by lactic acid bacteria isolated from kimchi. Molecules. 2018;23:3049. doi: 10.3390/molecules23113049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon E.W., Yang J.S., Yoon S.R., Ha J.H. Application of colorimetric indicators to predict the fermentation stage of kimchi. J. Food Sci. 2020;85 doi: 10.1111/1750-3841.15532. [DOI] [PubMed] [Google Scholar]
- 25.Mheen T.I., Kwon T.W. Effect of temperature and salt concentration on kimchi fermentation. Korean J. Food Sci. Technol. 1984;16:443–450. [Google Scholar]
- 26.Lee H.M., Kim S.J., Lee J.Y., Park B., Yang J.S., Ha S.D., Choi C., Ha J.H. Capsaicinoids reduce the viability of a Norovirus surrogate during kimchi fermentation. LWT. 2019;115 [Google Scholar]
- 27.Bhaduri S. Effect of salt and acidic pH on the stability of virulence plasmid (pYV) in Yersinia enterocolitica and expression of virulence-associated characteristics. Food Microbiol. 2011;28:171–173. doi: 10.1016/j.fm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Brocklehurst T.F., Lund B.M. The influence of pH, temperature and organic acids on the initiation of growth of Yersinia enterocolitica. J. Appl. Bacteriol. 1990;69:390–397. doi: 10.1111/j.1365-2672.1990.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 29.Brackett R.E. Effects of various acids on growth and survival of Yersinia enterocolitica. J. Food Protect. 1987;50:598–601. doi: 10.4315/0362-028X-50.7.598. [DOI] [PubMed] [Google Scholar]
- 30.Lambert R.J., Stratford M. Weak-acid preservatives: modelling microbial inhibition and response. J. Appl. Microbiol. 1999;86:157–164. doi: 10.1046/j.1365-2672.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Serna-Galvis E.A., Troyon J.A., Giannakis S., Torres-Palma R.A., Carena L., Vione D., Pulgarin C. Kinetic modeling of lag times during photo-induced inactivation of E. coli in sunlit surface waters: unraveling the pathways of exogenous action. Water Res. 2019;163 doi: 10.1016/j.watres.2019.114894. [DOI] [PubMed] [Google Scholar]
- 32.Berney M., Weilenmann H.U., Ihssen J., Bassin C., Egli T. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl. Environ. Microbiol. 2006;72:2586–2593. doi: 10.1128/AEM.72.4.2586-2593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996;81:501–508. doi: 10.1111/j.1365-2672.1996.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Rodríguez F., Valero A. Springer; New York: 2013. Predictive Microbiology in Foods; pp. 1–10. [Google Scholar]
- 35.Ross T. Meat and Livestock Australia; Sydney, Australia: 1999. Predictive Food Microbiology Models in the Meat Industry. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.