ABSTRACT
Klebsiella pneumoniae and Pseudomonas aeruginosa are two leading causes of burn and wound infections, pneumonia, urinary tract infections, and more severe invasive diseases, which are often multidrug resistant (MDR) or extensively drug resistant. Due to this, it is critical to discover alternative antimicrobials, such as bacteriophage lysins, against these pathogens. Unfortunately, most lysins that target Gram-negative bacteria require additional modifications or outer membrane permeabilizing agents to be bactericidal. We identified four putative lysins through bioinformatic analysis of Pseudomonas and Klebsiella phage genomes in the NCBI database and then expressed and tested their intrinsic lytic activity in vitro. The most active lysin, PlyKp104, exhibited >5-log killing against K. pneumoniae, P. aeruginosa, and other Gram-negative representatives of the multidrug-resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, K. pneumonia, Acinetobacter baumannii, P. aeruginosa, and Enterobacter species) without further modification. PlyKp104 displayed rapid killing and high activity over a wide pH range and in high concentrations of salt and urea. Additionally, pulmonary surfactants and low concentrations of human serum did not inhibit PlyKp104 activity in vitro. PlyKp104 also significantly reduced drug-resistant K. pneumoniae >2 logs in a murine skin infection model after one treatment of the wound, suggesting that this lysin could be used as a topical antimicrobial against K. pneumoniae and other MDR Gram-negative infections.
KEYWORDS: bacteriophage, lysin, endolysin, antibiotic resistance, Klebsiella, Pseudomonas, ESKAPE, Gram negative, skin infection
INTRODUCTION
Klebsiella pneumoniae organisms are nonmotile, encapsulated, Gram-negative bacilli that belong to the family Enterobacteriaceae. Pseudomonas aeruginosa organisms are motile, encapsulated Gram-negative bacilli that belong to the family Pseudomonadaceae. Both species have similar epidemiology, are ubiquitous in the environment, and can be found on the skin and in the gastrointestinal and respiratory tracts of healthy people (1). These bacteria are opportunistic pathogens, particularly in immunocompromised individuals, diabetics, alcoholics, neonates, the elderly, and burn patients (2–4). Additionally, cystic fibrosis patients are at severe risk for colonization and chronic infections with P. aeruginosa, the leading cause of mortality for these individuals (5).
K. pneumoniae and P. aeruginosa infections range from mild topical skin, mucosal, and burn wound infections to urinary tract infections and more severe invasive diseases, such as pneumonia, sepsis, and meningitis (4, 6). Importantly, they have become two of the world’s leading causes of Gram-negative nosocomial infections and are readily isolated from patients with hospital-acquired pneumonia (HAP), health care-associated pneumonia (HCAP), and ventilator-associated pneumonia (VAP) (7–9). These infections are associated with high mortality rates, >40% in patients with K. pneumoniae pneumonia or septicemia (2, 10, 11) and about 30% and 50% for P. aeruginosa VAP and bacteremia, respectively (7).
As prominent members of the multidrug-resistant ESKAPE pathogen group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species), the high rate of antibiotic resistance of K. pneumoniae and P. aeruginosa makes treating these infections an even greater challenge (12, 13). Both organisms are inherently resistant to many antimicrobial classes, due to the limited permeability of their outer membrane and to their capacity for biofilm formation, and they can acquire further drug resistance through mutations or the acquisition of new genetic material (14, 15). About 80% of K. pneumoniae isolates and an increasing number of P. aeruginosa strains produce different types of extended-spectrum β-lactamases (ESBLs), making them resistant to penicillins and cephalosporins (16–18). Moreover, due to the increased use of carbapenems, multidrug-resistant (MDR) or even extensively drug-resistant (XDR) K. pneumoniae and P. aeruginosa strains have evolved, resulting in increased morbidity and mortality, extended hospital stays, and high health care expenses (2, 19–23).
There have also been increased incidences of P. aeruginosa and K. pneumoniae community-acquired pneumonia (CAP) and infections (24–26). While P. aeruginosa CAP is often associated with certain underlying health conditions, severe community-acquired K. pneumoniae infections are usually caused by hypervirulent strains with increased mucoid or hypermucoviscous phenotypes (9, 27, 28). Even more concerning, K. pneumoniae isolates with both hypermucoviscosity and antibiotic resistance are emerging, leading to potentially hypervirulent antibiotic-resistant infections that may become untreatable (29). In accordance, the World Health Organization (WHO) listed carbapenem-resistant P. aeruginosa and carbapenem-resistant and third-generation-cephalosporin-resistant Enterobacteriaceae (K. pneumoniae) as the most critical priority for the development of new antibiotics to treat these infections (30).
Bacteriophage lysins have emerged as a potential treatment for respiratory and other drug-resistant bacterial infections (31–33). Phage lysins are enzymes that cleave specific peptidoglycan bonds to disrupt the bacterial cell wall, resulting in hypotonic lysis and the release of phage progeny at the end of their replication cycle (34). The catalytic domains of lysins can be categorized into different classes based on their peptidoglycan cleavage sites, including transglycosylases, glucosaminidases, muramidase, amidases, and endopeptidases (34). Exogenously added lysins have antibacterial activity in both in vitro and in vivo preclinical infection models (34). They have also demonstrated their therapeutic potential against important Gram-positive pathogens in human clinical trials (35–39).
However, due to the protective outer membranes of Gram-negative bacteria, it is more difficult for exogenously added lysins from Gram-negative phage to reach the cell wall peptidoglycan to lyse these bacteria (33). Different approaches have been employed to increase the membrane permeability to lysins, such as the simultaneous addition of membrane-destabilizing agents like poly-l-lysine, EDTA disodium salt (EDTA), or chloroform (40–43). Weak acids, such as citric acid, have also been used to aid in lysin penetration of the outer membrane (44). Additionally, cationic peptide antibiotics like colistin or polymyxin B can destabilize the lipopolysaccharides and phospholipids in the outer membrane and give lysins access to the peptidoglycan layer, thus increasing the bactericidal activity of both compounds (45, 46).
Investigators have also modified recombinant lysins to better penetrate the outer membrane through genetic and protein engineering strategies, including the fusion of the lysins with highly charged amino acids, amphipathic helices, hydrophobic N- or C-terminal amino acid extensions, or membrane-translocating domains (35, 47–49). These modifications allowed the lysins to kill many genera of Gram-negative pathogens in vitro and in vivo without further additives (34).
In contrast, a small number of natural lysins have antimicrobial activity against Gram-negative bacteria without the presence of exogenously added membrane destabilizers or further genetic modifications, including the lysins PlyF307, PlyPa03, PlyPa91, LysAB2, PlyE146, and LysS from bacteriophage targeting A. baumannii, P. aeruginosa, and Escherichia coli (50–55). This activity has been attributed to the presence of one or more amphipathic helices in the structure of the lysins or a positively charged C-terminal tail (50–52, 54–56).
Some of these natural lysins from other Gram-negative bacteriophages have also shown additional activity against K. pneumoniae in preliminary in vitro tests (57). However, no publications have yet described natural K. pneumoniae phage lysins as intrinsically effective against intact K. pneumoniae without the coaddition of other factors (58). In this report, we identified, cloned, and expressed four lysins from the genomic sequences of the lytic and lysogenic phage of Pseudomonas and Klebsiella clinical isolates. The in vitro activity of these lysins was tested against K. pneumoniae and P. aeruginosa isolates without additives or modifications. PlyKp104 was chosen as the best therapeutic candidate, and its activity was further characterized in vitro against other Gram-negative bacterial isolates. Additionally, we validated the in vivo antibacterial efficacy of PlyKp104 against drug-resistant K. pneumoniae in a skin wound infection model.
RESULTS
Lysin identification, sequence analysis, and lysin expression.
To discover phage lysins with potential bacteriolytic activity against K. pneumoniae and P. aeruginosa, we first searched the published genomic sequences of two P. aeruginosa lytic phages (NP1 and NP3) (59) in the NCBI database for annotations of putative lytic proteins. Two lysins, PlyPa101 (accession no. YP_009285812) and PlyPa102 (accession no. AMQ76165), were identified in NP1 (accession no. NC_031058.1) and NP3 (accession no. KU198331.1), respectively. Next, a BLAST search was performed against the available Pseudomonas and Klebsiella genomes in the NCBI database to detect lysin genes with homology to PlyPa101. PlyPa103 (accession no. AQZ96894) and PlyKp104 (accession no. MBC5100607) were identified in lysogenic phage from Pseudomonas phragmitis strain S-6-2 (accession no. GCA_002056295.1) and Klebsiella pneumoniae strain NR-41923/BIDMC 7B (accession no. GCA_000567425.1), respectively (60).
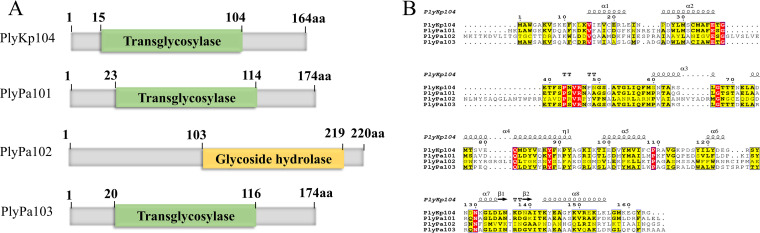
The four lysins were aligned, and domains and structural analysis were predicted in silico (Fig. 1). Three lysins, PlyKp104, PlyPa101, and PlyPa103, possessed a putative transglycosylase domain, while PlyPa102 had a potential glycoside hydrolase domain (Fig. 1A). Amino acid sequence alignments and in silico structural analysis of the lysins showed that most of the residues and secondary structures in the three transglycosylase lysins were highly conserved (Fig. 1B). Further, the C-terminal region of these lysins contained a putative amphipathic alpha helix (amino acids [aa] 143 to 155) (Fig. 1B) surrounded by and containing positively charged or basic amino acid sequences. All four lysin genes were synthesized, cloned into plasmid expression vectors and transformed into E. coli. Lysin expression and purifications were performed according to our previous publication (55), Fig. S1, and the methods described below.
FIG 1.
(A) Schematic representation of the four lysins identified and expressed. Domains and protein families were predicted by using Pfam (https://www.ebi.ac.uk/interpro/entry/pfam/#table). (B) Amino acid sequence alignment of the four lysins compared to PlyKp104. All alignments were generated by Clustal W (https://www.genome.jp/tools-bin/clustalw). The figure was generated by ESPript (https://espript.ibcp.fr/ESPript/ESPript/index.php). Strictly conserved residues are presented in red, highly conserved residues are in yellow, and dots represent gaps in the alignment. A schematic representation of the predicted secondary structure of PlyKp104 is shown above the sequences.
Evaluation of lysin activity against P. aeruginosa, K. pneumoniae, and other Gram-negative organisms.
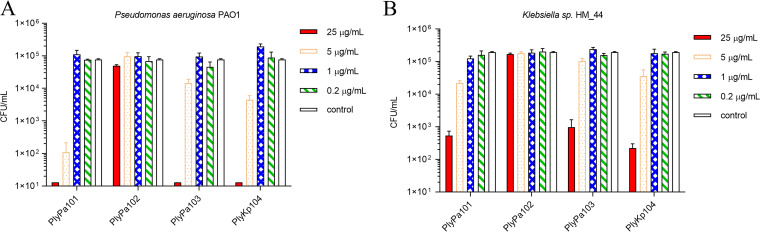
In bactericidal in vitro killing assays, PlyPa101, PlyPa103, and PlyKp104 (25 μg/mL of each lysin) reduced the viability of P. aeruginosa strain PAO1 by more than 4 logs in 1 h (Fig. 2A). Likewise, PlyPa101, PlyPa103, and PlyKp104 were also able to reduce the number of CFU of Klebsiella sp. strain HM_44 by 2 to 3 logs over 1 h (Fig. 2B). However, PlyPa102 had no in vitro bactericidal activity against P. aeruginosa or Klebsiella, so it was not characterized further (Fig. 2).
FIG 2.
Bactericidal activity of lysins against P. aeruginosa PAO1 and Klebsiella sp. strain HM_4. Purified lysins were diluted to various concentrations and incubated with log-phase P. aeruginosa PAO1 or Klebsiella sp. strain HM_4 for 1 h at 37°C in 30 mM HEPES (pH 7.4). Values were established by serial dilution in 30 mM HEPES (pH 7.4) and plating to LB agar. (A) Bactericidal activity of lysins against P. aeruginosa PAO1. (B) Bactericidal activity of lysins against Klebsiella sp. strain HM_4. Experiments were conducted in triplicate. Error bars represent standard deviations.
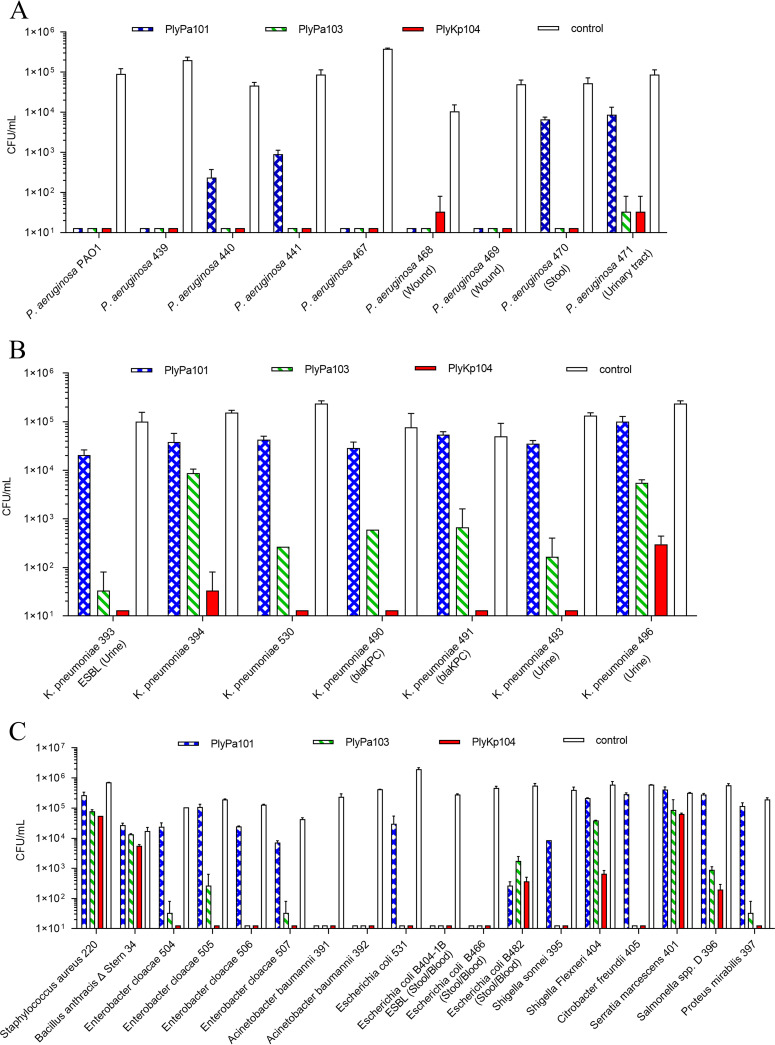
Next, the killing activity of PlyPa101, PlyPa103, and PlyKp104 (100 μg/mL of each lysin) was tested against a panel of clinical isolates and antibiotic-resistant strains of P. aeruginosa and K. pneumoniae (Fig. 3). PlyPa101 reduced most strains of P. aeruginosa by 2 to 4 logs but had less activity against P. aeruginosa isolates 470 and 471 (Fig. 3A). Additionally, PlyPa101 was ineffective against the clinical isolates of K. pneumoniae (<1-log reduction in CFU/mL) (Fig. 3B). PlyPa103 and PlyKp104 reduced all P. aeruginosa strains by 3 to 4 logs (Fig. 3A). PlyPa103 showed moderate killing activity against most K. pneumoniae strains (1- to 4-log reduction, depending on the isolate) (Fig. 3B). PlyKp104 had the best bactericidal activity and reduced most K. pneumoniae strains >3 to 4 logs, to below the limit of detection (66 CFU/mL), including the ESBL- and carbapenemase-producing antibiotic-resistant K. pneumoniae isolates (Fig. 3B). The lysins were also tested against a panel of Gram-positive and other Gram-negative bacteria (Table 1). PlyKp104 had significant bactericidal killing activity (>3-log reduction) against most of the Gram-negative ESKAPE strains tested, including K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter cloacae, and other Enterobacteriaceae (i.e., E. coli, Shigella sonnei, Citrobacter freundii and Proteus mirabilis) (Fig. 3C), demonstrating a broader range of activity than PlyPa101 and PlyPa103. No activity against the Gram-positive organisms tested was seen with any of the four lysins (Fig. 3C). Based on the results above, PlyKp104 was chosen for further in vitro and in vivo analyses.
FIG 3.
Spectrum of lysin activity against various bacterial isolates. (A) P. aeruginosa; (B) K. pneumoniae; (C) other Gram-positive and Gram-negative species. Bacteria were incubated with 100 μg/mL of lysin in 30 mM HEPES buffer (pH 7.4) for 1 h at 37°C. Viable bacteria were enumerated by serial dilution in 30 mM HEPES buffer (pH 7.4) and plating to LB or BHI agar. Experiments were performed in triplicate; error bars represent standard deviations. Known drug resistance and/or clinical isolation site is given after the isolate name. ESBL, extended-spectrum β-lactamase; blaKPC, carbapenemase (carbapenem-hydrolyzing β-lactamase). See Table 1 for further information on bacterial strains.
TABLE 1.
Bacterial strains used in this study
| Isolate | Descriptiona | Source or reference |
|---|---|---|
| Gram-negative bacteria | ||
| Acinetobacter baumannii 391 | ATCC 17978 | ATCC |
| Acinetobacter baumannii 392 | WCMC Hospital; MDR clinical isolate 1791 | 54 |
| Citrobacter freundii 405 | ATCC 8090 | ATCC |
| Enterobacter cloacae 504 | NR-50391 | BEI Resources |
| Enterobacter cloacae 505 | NR-50392 | BEI Resources |
| Enterobacter cloacae 506 | NR-50393 | BEI Resources |
| Enterobacter cloacae 507 | NR-548555 | BEI Resources |
| Escherichia coli BL21 | Invitrogen | |
| Escherichia coli DH5α | Invitrogen | |
| Escherichia coli 531 | UTI; NYU Hospital | 55 |
| Escherichia coli B404-1B | Stool/blood; cephalosporin resistant (ESBL) | 69 |
| Escherichia coli B466 | Stool/blood; fluoroquinolone resistant | 69 |
| Escherichia coli B482 | Stool/blood; fluoroquinolone resistant | 69 |
| Klebsiella pneumoniae 393 | Urine; ESBL | 55 |
| Klebsiella pneumoniae 394 | ATCC 10031 | ATCC |
| Klebsiella pneumoniae 490 | NR-15410 (blaKPC+) | BEI Resources |
| Klebsiella pneumoniae 491 | NR-15411 (blaKPC+) | BEI Resources |
| Klebsiella pneumoniae 493 | Urine; NR-41923 | BEI Resources |
| Klebsiella pneumoniae 496 | Urine, blood/sepsis; NR-44349 | BEI Resources |
| Klebsiella pneumoniae ATCC 700603 | Urine; ESBL | ATCC |
| Klebsiella sp. strain HM_44 | Strain 1_1_55; BEI Resources | 60 |
| Proteus mirabilis 397 | Clinical isolate; Hunter College Collection | 55 |
| Pseudomonas aeruginosa 439 | Clinical isolate 1108; WCMC Hospital | 55 |
| Pseudomonas aeruginosa 440 | Clinical isolate 1113; WCMC Hospital | 55 |
| Pseudomonas aeruginosa 441 | Clinical isolate 1140; WCMC Hospital | 55 |
| Pseudomonas aeruginosa 467 | LRT; NYU Hospital | 55 |
| Pseudomonas aeruginosa 468 | Wound; NYU Hospital | 55 |
| Pseudomonas aeruginosa 469 | Wound; NYU Hospital | 55 |
| Pseudomonas aeruginosa 470 | Stool; NYU Hospital | 55 |
| Pseudomonas aeruginosa 471 | UTI; NYU Hospital | 55 |
| Pseudomonas aeruginosa PAO1 | Reference strain; ATCC | 55 |
| Salmonella serogroup D 396 | Clinical isolate; Hunter College Collection | 55 |
| Serratia marcescens 401 | Clinical isolate; Hunter College Collection | 55 |
| Shigella flexneri 404 | ATCC 12022 | 55 |
| Shigella sonnei 395 | ATCC 25931 | 55 |
| Gram-positive bacteria | ||
| Bacillus anthracis Δ Stern 34 | Δ Stern | 79 |
| Staphylococcus aureus 220 | Newman | 80 |
WCMC, Weill Cornell Medical Center; Sp. 8.
Biochemical characterization of PlyKp104 antibacterial activity under various conditions.
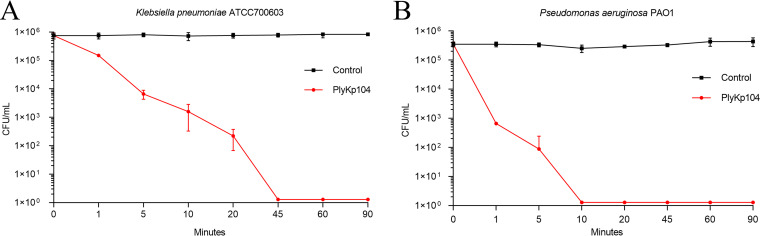
To evaluate the relative rate of killing by PlyKp104, 100 μg/mL of lysin was incubated with bacteria in 30 mM HEPES buffer for 1 to 90 min. The suspension was plated at different time points to analyze CFU reduction. PlyKp104 rapidly killed the ESBL-producing K. pneumoniae strain ATCC 700603, resulting in a 2-log decrease after 5 min and a continued reduction to below the level after detection in 45 min (Fig. 4A). Interestingly, even though PlyKp104 was isolated from the genome of a K. pneumoniae phage, it was more active against P. aeruginosa PAO1. PlyKp104 reduced viable cells by more than 2 logs in 1 min and killed all detectable bacteria in 10 min (Fig. 4B).
FIG 4.
Time-kill curve. Log-phase cells of (A) the ESBL-producing K. pneumoniae strain ATCC 700603 or (B) P. aeruginosa PAO1 were incubated for various times at 37°C with shaking in the presence of 100 μg/mL PlyKp104 in 30 mM HEPES buffer. Surviving bacteria were enumerated by serial dilution in 30 mM HEPES buffer and plating to LB agar. Experiments were performed in triplicate; error bars represent standard deviations.
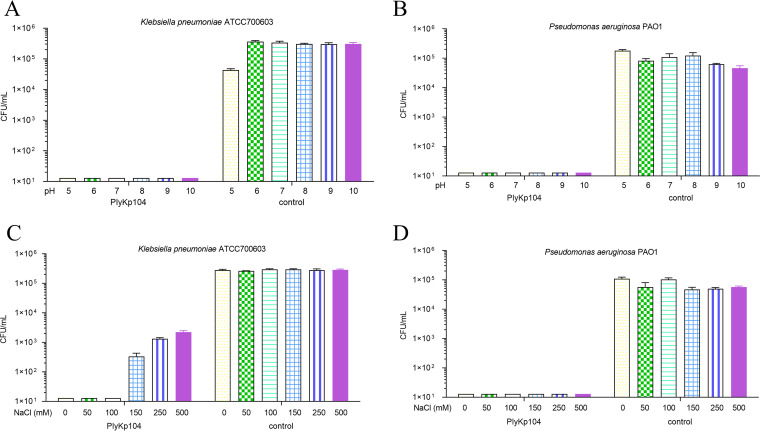
Next, the effect of pH on the activity of PlyKp104 was evaluated in vitro. K. pneumoniae (Fig. 5A) and P. aeruginosa (Fig. 5B) were incubated for 1 h with PlyKp104 under various buffer conditions ranging from pH 5.0 to 10.0. PlyKp104 was highly active in all pH conditions tested, reducing the amount of K. pneumoniae ATCC 700603 and P. aeruginosa PAO1 more than 5 logs, to below the limit of detection under all conditions. While the lysin’s activity was stable at multiple pH values, further experiments were performed at pH 7.4 to mimic physiological and in vivo conditions.
FIG 5.
(A and B) Effect of pH on the activity of PlyKp104. Log-phase cells of (A) the ESBL-producing K. pneumoniae strain ATCC 700603 or (B) P. aeruginosa PAO1 were incubated for 1 h at 37°C with 100 μg/mL lysin or buffer alone (control) in a 25 mM concentration of acetate buffer (pH 5.0), MES buffer (pH 6.0), HEPES buffer (pH 7.0 and 8.0), CHES buffer (pH 9.0), or CAPS buffer (pH 10.0). (C and D) Effect of NaCl on the activity of PlyKp104. Log-phase cells of (C) ESBL-producing K. pneumoniae strain ATCC 700603 or (D) P. aeruginosa PAO1 were incubated with 100 μg/mL PlyKp104 or buffer alone (control) for 1 h at 37°C in 30 mM HEPES and various concentrations of NaCl. Surviving bacteria were enumerated by serial dilution in 30 mM HEPES buffer and plating to LB agar. Experiments were performed in triplicate; error bars represent standard deviations.
The effect of salt (NaCl) concentration on the activity of PlyKp104 against K. pneumoniae and P. aeruginosa was also tested in vitro. PlyKp104 remained highly active against K. pneumoniae at NaCl concentrations as high as 100 mM, resulting in a 5-log decrease in CFU per milliliter, equal to that seen with the no-salt control (Fig. 5C). The lysin was also active in 150 to 500 mM NaCl against K. pneumoniae, achieving a 2- to 3-log reduction of CFU in 1 h (Fig. 5C). Alternatively, PlyKp104 maintained full lytic activity against P. aeruginosa at all NaCl concentrations tested (0 to 500 mM) (Fig. 5D).
Antibacterial activity of PlyKp104 in lung surfactant, urea, and human serum.
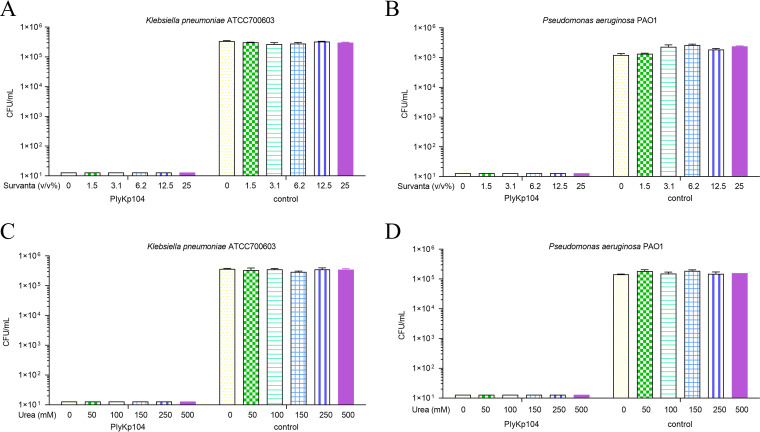
Considering that K. pneumoniae and P. aeruginosa are important pathogens of nosocomial pneumonia (61), we evaluated the effect of Survanta lung surfactants on the lytic activity of PlyKp104. The lysin maintained high activity (>5-log killing) against both K. pneumoniae and P. aeruginosa in lung surfactant, with no effect on PlyKp104 activity at the maximal concentrations tested (0 to 25% [vol/vol]) (Fig. 6A and B).
FIG 6.
Effects of Survanta and urea on the activity of PlyKp104. Log-phase cells of (A) the ESBL-producing K. pneumoniae strain ATCC 700603 or (B) P. aeruginosa PAO1 were incubated for 1 h at 37°C with 100 μg/mL of PlyKp104, or 30 mM HEPES buffer control, in the presence of the indicated concentrations of Survanta. Log-phase cells of (C) the ESBL-producing K. pneumoniae strain ATCC 700603 or (D) P. aeruginosa PAO1 were incubated with 100 μg/mL PlyKp104 for 1 h at 37°C in 30 mM HEPES and various concentrations of urea. Numbers of viable bacterial CFU were determined by serial dilution and plating. Experiments were performed in triplicate; error bars represent standard deviations.
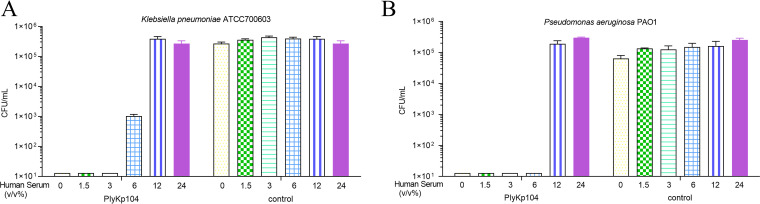
Since PlyKp104 was active in lung surfactants and at high salt concentrations, we also wanted to test it in other components of bodily fluids, such as those in urine (urea) and blood (human serum). PlyKp104 maintained high lytic activity, with >5-log killing, against K. pneumoniae and P. aeruginosa at all urea concentrations tested (0 to 500 mM) (Fig. 6C and D). While serum at a concentration of 12% or more inhibited the activity of PlyKp104, the lysin reduced viable K. pneumoniae and P. aeruginosa by >2.5 logs and >5 logs at 6% serum, respectively, and had potent activity, equal to that in the nonserum control, against both pathogens at less than 6% serum (Fig. 7A and B).
FIG 7.
The activity of PlyKp104 in the presence of human serum. Cells of (A) the ESBL-producing K. pneumoniae strain ATCC 700603 or (B) P. aeruginosa PAO1 were incubated for 1 h at 37°C with 100 μg/mL of the lysins in the presence of the indicated concentration of human serum in 30 mM HEPES buffer. Numbers of viable bacterial CFU are presented. Experiments were performed in triplicate; error bars represent standard deviations.
In vivo antibacterial efficacy of PlyKp104 in a murine skin infection model.
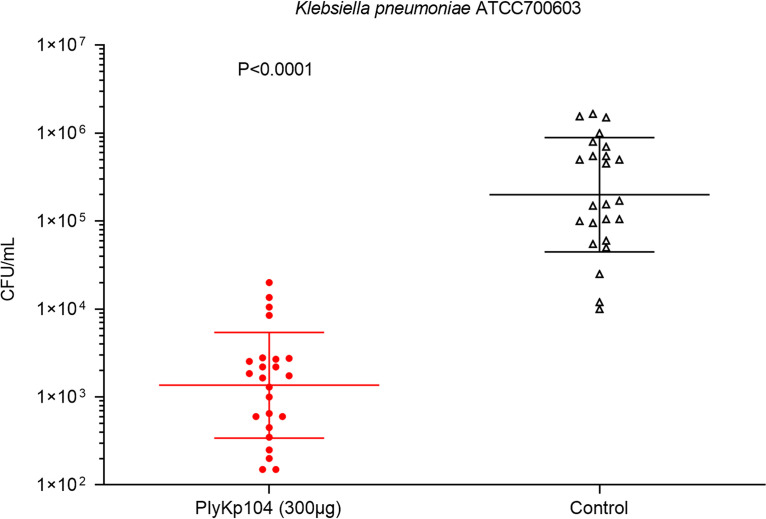
To evaluate the in vivo efficacy of PlyKp104, the lysin was tested in a topical wound infection model (62, 63). The mice were shaved and depilated, and skin abrasions were created on their backs by removing the top layers of the epidermis by tape stripping. The ESBL-producing K. pneumoniae strain ATCC 700603 (104 CFU) was applied to two separate areas of the wounds to establish colonization for 3 h. Then the infected areas were treated with a 300-μg dose of PlyKp104 or the buffer control. After 3 h, the mice were euthanized, the infected skin was excised and homogenized, and the bacterial burden was evaluated by serial dilution and plating. Treatment of the K. pneumoniae-infected mice with a single dose of PlyKp104 significantly reduced the bacterial concentration in the skin by >2 logs compared to buffer controls (P < 0.0001) (Fig. 8).
FIG 8.
In vivo activity of PlyKp104 in a murine skin infection model of K. pneumoniae. Depilated CD1 mice were tape stripped and topically infected with 104 CFU of log-phase the ESBL-producing K. pneumoniae strain ATCC 700603. After 3 h, the infected areas were topically treated with 300 μg PlyKp104 in 50 μL CAPS-buffered saline, pH 6.0 (n = 12; 2 wounds per mouse) or 50 μL buffer control (n = 12; 2 wounds per mouse). Three hours after treatment, the animals were euthanized, and the infected skin was excised and homogenized in PBS. Samples were serially diluted in PBS and plated to LB agar for CFU enumeration. Horizontal lines indicate the geometric mean values of each group, error bars represent geometric standard deviations, and statistical significance was evaluated by Student’s t test.
DISCUSSION
Gram-negative clinical isolates such as K. pneumoniae and P. aeruginosa are becoming more virulent and drug resistant, and there is great concern that they will become resistant to the last line of antibiotics (8, 18). Moreover, Klebsiella spp. are considered a significant source of disseminating extended-spectrum-β-lactamase- and carbapenemase-encoding plasmids to other Gram-negative species (18). Likewise, P. aeruginosa isolates contain numerous mobile elements that are critical in spreading antibiotic resistance between strains (14, 64). For these reasons, novel antibiotics must be discovered to control these pathogens and other MDR Gram-negative infections of the ESKAPE group.
Lysins are one of the necessary components that phages use to kill their host quickly and spread their viral progeny at the end of the lytic cycle (65). They target the cell wall’s highly conserved and essential peptidoglycan motifs, making bacterial resistance to these enzymes less likely to develop. These characteristics were first used to create antibacterial therapeutics against Gram-positive bacteria (24, 55). Unlike Gram-positive bacteria, the outer membrane of Gram-negative bacteria reduces lytic damage by limiting access to the peptidoglycan target. More recently, some lysins (natural or engineered) that kill Gram-negative bacteria were identified (49, 54, 55, 66). Unfortunately, only a few lysins have been identified in Klebsiella bacteriophages that efficiently kill K. pneumoniae in both in vitro and in vivo infection models, without the coaddition of other membrane-permeabilizing factors. For example, lysins initially identified from Klebsiella phages vB_KpnS_MK54, K11, KP32, and KP27 possess cell wall hydrolase activity. However, the antibacterial action of these lysins against K. pneumoniae is effective only in the presence of outer membrane permeabilizers, such as EDTA or chloroform (41–43).
In the present study, two lysins, PlyPa101 and PlyPa102, were identified from the DNA sequences of the P. aeruginosa lytic phages NP1 and NP3, respectively, and were cloned, expressed, and purified. When their lytic activity was tested, PlyPa102 had no bactericidal effect, but PlyPa101 killed P. aeruginosa and K. pneumoniae during initial in vitro studies. To identify more lysins against K. pneumoniae and P. aeruginosa, a BLAST search for genes with homology to Pseudomonas phage lysin PlyPa101 was performed against the Pseudomonas and Klebsiella genomes available in the NCBI database. PlyPa103 and PlyKp104 were identified in lysogenic bacteriophages from Pseudomonas and Klebsiella, respectively. These new lysins were expressed and purified, and their lytic activity was compared to that of PlyPa101. Our results showed that PlyPa103 and PlyKp104 exhibited significant killing activity against clinical and drug-resistant isolates of P. aeruginosa and K. pneumoniae isolates without additional outer membrane permeabilizers. This attribute may be due to the predicted amphipathic alpha helix region and the positively charged C-terminal region of these lysins, which could interact with and permeabilize the Gram-negative outer membrane. These structures have also been identified and characterized in other intrinsically active lysins targeting different Gram-negative bacterial species (49, 55, 56, 67).
Moreover, PlyKp104 had significant bactericidal activity (>5-log reduction) over a broader range of Gram-negative organisms, including clinical isolates and drug-resistant strains of P. aeruginosa, K. pneumoniae, E. cloacae, E. coli, and A. baumannii. Although our lysins had a diverse range of lytic activity, some species specificity existed, as they did not kill all Gram-negative bacteria to the same extent. Likewise, none of the lysins in our study had substantial activity against the Gram-positive bacteria tested (S. aureus and Bacillus anthracis). This lytic spectrum may be due to differences in the composition of the Gram-positive and Gram-negative bacteria cell walls, preventing PlyKp104 from binding to or cleaving the peptidoglycan layer (33, 35, 66).
Our results are in concert with previous publications describing lysins with intrinsic activity from other phages of Gram-negative bacteria, such as Salmonella enterica serovar Typhi, E. coli, A. baumannii, and P. aeruginosa, which show that a broader spectrum of bactericidal activity occurs more than expected with these lysins (31, 53, 55, 68, 69). As a result, some also have activity against K. pneumoniae and P. aeruginosa but require higher enzyme concentrations and display less killing. Therefore, most of these lysins have not been further characterized in vitro against multiple K. pneumoniae and P. aeruginosa clinical isolates or tested in in vivo infection models (31, 53, 55, 70). In another recent study, an A. baumannii phage lysin, LysAB54, reduced viable A. baumannii, K. pneumoniae, and P. aeruginosa by 0.5 to 3 logs at 100 μg/mL (68). Notably, PlyKp104 had higher bactericidal activity (>5-log reduction) against K. pneumoniae, P. aeruginosa, A. baumannii, and most of the other Gram-negative clinical isolates in our panel at the same concentration (100 μg/mL).
PlyKp104 also displayed rapid killing against P. aeruginosa PAO1, leading to roughly a 3-log reduction within 1 min and a 5-log reduction (and complete sterilization of the suspension) within 10 min. This high activity against P. aeruginosa was also confirmed by the significant killing of multiple strains of P. aeruginosa in our bactericidal assays. When targeting K. pneumoniae, PlyKp104 took 10 min and 45 min to achieve comparable levels of reduction.
PlyKp104 remained fully active against K. pneumoniae and P. aeruginosa throughout a broad pH range and at high concentrations of urea and lung surfactants tested in vitro. However, NaCl concentrations above 100 mM reduced the bactericidal activity of PlyKp104 against K. pneumoniae. However, it still killed K. pneumoniae by roughly 3 logs at a physiological concentration (150 mM NaCl) and 2 logs at 500 mM NaCl. The activities of many Gram-positive and Gram-negative lysins decrease with increasing concentrations of NaCl, as seen with LysK, PlyEc2, and other lysins (57, 69, 71). Higher salt concentrations may reduce the activity of lysins by increasing protein aggregation, interacting with the charged residues on both the lysin and the bacterial surface to prevent the lysin from permeabilizing the outer membrane or cleaving its substrate or reducing bacterial turgor pressure and thus preventing lysis (61, 72). Interestingly, PlyKp104 maintained full lytic activity against P. aeruginosa at all NaCl concentrations tested (0 to 500 mM), indicating that interactions and influence of salt on lysin activity may also depend on the bacterial target.
Human serum at a concentration of 12% or higher also inhibited the activity of PlyKp104, suggesting that blood components other than the physiological NaCl concentration of 150 mM may also reduce lysin activity. In contrast to the synergistic effect of lysin CF-301 with human serum albumin and lysozyme to enhance the bactericidal activity of lysin (35), many Gram-negative lysins are inhibited by human serum (34). This result suggests that PlyKp104 may not be suitable for systemic therapeutic intervention in bacteremia or other internal infections. Instead, it might be more appropriate for topical treatments involving burn or wound infections. For these reasons, we chose to explore the in vivo activity of PlyKp104 against a murine model of topical K. pneumoniae infection.
P. aeruginosa and K. pneumoniae are the most common Gram-negative pathogens associated with hospital-acquired infections. They can lead to severe skin and soft tissue infections of wounds from injury, catheters, and surgical sites in healthy and immunocompromised individuals (73, 74). They are also responsible for a majority of infections in burn patients, and K. pneumoniae accounts for 15% of the microorganisms isolated from these infections (4, 61, 75). As a proof of principle, we tested the in vivo ability of PlyKp104 to treat K. pneumoniae wound infections using a modified mouse tape strip skin infection model (70, 76). After 3 h of infection with an ESBL-producing K. pneumoniae strain (ATCC 700603), mice were topically treated with a single dose of 300 μg of PlyKp104. Three hours later, the skin tissues were examined for viable Klebsiella organisms. PlyKp104 significantly reduced the bacterial load in the skin wound by >2 logs compared to the buffer control. While the time between infection and treatment was not long, these results show that a topically applied PlyKp104 can work in vivo to reduce the bacterial burden in skin wounds and possibly prevent, decolonize, or treat topical K. pneumoniae infections. Higher concentrations or multiple doses of lysin delivered in formulations that allow better and longer tissue penetration could lead to even better treatment efficacy.
In conclusion, this is the first report to describe a native K. pneumoniae phage lysin that is intrinsically bactericidal, in both in vitro and in vivo assays, without the addition of membrane permeabilizers or further protein modifications. While these treatments are still in the preclinical development stage, and further testing is needed to test PlyKp104 against additional bacterial isolates and biofilms, in combination with other antibiotics, and in further in vivo models, our study emphasizes the potential therapeutic role of this and other phage lysins for the treatment of infections caused by K. pneumoniae, P. aeruginosa, and other MDR Gram-negative organisms.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Table 1 describes bacterial strains used in this study and gives their sources. Gram-negative bacteria were cultured in Luria broth (LB; EMD Millipore), and Gram-positive bacteria were grown in Mueller-Hinton broth or brain heart infusion (BHI) media (Difco) at 37°C, with shaking at 200 rpm.
Gene synthesis and lysin cloning.
PlyPa101 was amplified from the genome of Pseudomonas phage NP1 (NC_031058.1) using primers 645_5_NP1_SalI (5′-CGCGTCGACATGAAACTTGCCTGGGGCAAAAAGGTC) and 646_3_NP1_NotI (5′-GCGGCGGCCGCCTACAGCTCAAGCGCGAACCGATCC). PlyPa102 was amplified from the genome of Pseudomonas phage NP3 (KU198331.1) using primers 647_5_NP3_SalI (5′-CGCGTCGACATGAAAATCACGAAGGATGTTCTG) and 648_3_NP3_NotI (5′-GCGGCGGCCGCTCAGGAGCCTTGATTGATCGC) (70). These PCR products were inserted into the SalI and NotI sites of a modified pET21 vector, with a hexahistidine tag separated from the protein by a 3C cleavage site, resulting in pAR602-PlyPa101(NP1) and pAR603-PlyPa102(NP3), respectively. DNA sequences for the lysins PlyPa103 and PlyKp104 were synthesized by Genewiz and designed to contain an upstream SalI and downstream NotI restriction site. The two DNA fragments were inserted into the SalI and NotI sites of a modified pET21a plasmid as described above, resulting in pAR737-PlyPa103 and pAR738-PlyKp104.
In silico protein analysis.
Amino acid sequence alignment of the four lysins compared to PlyKp104 was generated by ClustalW (https://www.genome.jp/tools-bin/clustalw). Protein domains and families were predicted by using Pfam (https://www.ebi.ac.uk/interpro/entry/pfam/#table) (77, 78). The figures were generated by ESPript (https://espript.ibcp.fr/ESPript/ESPript/index.php).
Purification of phage lysins.
Overnight cultures of E. coli BL21, containing the expression plasmids described above, were diluted 1:100 into 1 L of LB medium containing 100 μg/mL ampicillin and were incubated at 37°C with shaking at 200 rpm until the culture reached an optical density at 600 nm (OD600) of 0.5. Lysin expression was then induced with 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h at 37°C with shaking, and then the cultures were switched to 4°C at 200 rpm for 18 h. Cells were harvested, resuspended in 40 mL MCAC buffer (30 mM Tris [pH 7.4], 0.5 M NaCl, 10% glycerol, 1 mM dithiothreitol [DTT]), and homogenized using an Emulsiflex-C5 homogenizer (Avestin, Ottawa, ON, Canada). Cell debris was removed by ultracentrifugation, and the supernatant was filtered through a 0.22-μm filter (Millipore). The cleared lysates were loaded onto a nickel-nitrilotriacetic acid (Ni-NTA) column attached to an AKTA Prime fast protein liquid chromatography (FPLC) machine (GE), followed by 6 column washes with MCAC buffer containing 20 mM imidazole, and eluted across a gradient with MCAC buffer containing 150 mM imidazole. The eluted fractions were pooled and supplemented with 10× 3C buffer for a final concentration of 150 mM NaCl, 50 mM Tris (pH 7.6), 10 mM EDTA, and 1 mM DTT. Fifty microliters of 3C protease was added per 1 mg of purified protein and incubated overnight at 4°C to remove the hexahistidine tag from the lysins. Reactions were dialyzed in a 3-kDa-cutoff membrane for 24 h with 3 buffer changes of phosphate-buffered saline (PBS). The protein was then concentrated using an Amicon ultrafiltration device with a 3-kDa molecular weight cutoff. The final concentration was determined using an ND-1000 spectrophotometer (Nanodrop), according to absorbance at 280 nm.
Bactericidal assays.
Overnight bacterial cultures of K. pneumoniae, P. aeruginosa and other Gram-negative bacteria were diluted 1:50 into fresh LB medium. Gram-positive bacteria were diluted 1:50 into fresh BHI. All cultures were grown at 37°C with shaking at 200 rpm to an absorbance of 0.5 at 600 nm (A600). The cells were harvested, washed, and resuspended in 30 mM HEPES buffer, pH 7.4, to achieve a final concentration of 106 CFU/mL (unless otherwise noted). In a U-bottomed 96-well plate, each lysin was diluted in the same buffer to the desired final concentration (0.2 to 100 μg/mL), and then 50 μL of the test bacteria was added to each well. The plate was incubated for 1 h at 37°C with shaking at 200 rpm. The content of each well was then serially diluted 10-fold in 30 mM HEPES buffer, pH 7.4, and plated to LB agar, Mueller-Hinton agar, or BHI agar to quantify viable bacteria by CFU enumeration.
PlyKp104 activity under different biochemical conditions.
Bacteria were grown to exponential phase (A600 ≈ 0.5) as described above, washed, and resuspended to approximately 106 CFU/mL in different buffers, including those with different pH values (25 mM acetate buffer [pH 5.0], MES [morpholineethanesulfonic acid] buffer [pH 6.0], HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer [pH 7.0 and 8.0], CHES [N-Cyclohexyltaurine] buffer [pH 9.0], and CAPS [N-cyclohexyl-3-aminopropanesulfonic acid] buffer [pH 10.0]), NaCl (0 to 500 mM in 30 mM HEPES buffer), urea (0 to 500 mM in 30 mM HEPES buffer), Survanta lung surfactants (0 to 25% [vol/vol] in 30 mM HEPES buffer), and human serum (0 to 24% [vol/vol] in 30 mM HEPES buffer). The resuspended bacteria were then incubated with 100 μg/mL PlyKp104 for 1 h at 37°C with shaking at 200 rpm, followed by serial 10-fold dilutions in 30 mM HEPES buffer at pH 7.4, and plated to LB agar to quantify viable bacteria by CFU enumeration.
Mouse skin infection model.
All experiments were performed at Rockefeller University’s animal housing facility, an AAALAC-accredited research organization, and were approved by The Rockefeller University’s Institutional Animal Care and Use Committee.
The skin wound infection model was established according to previous reports (55, 70, 76). A total of 24 female CD1 mice, 6 to 8 weeks old, were obtained from Charles River and housed 5 per cage with standard chow and tap water. All mice were maintained in a pathogen-free controlled environment of 22°C ± 2°C and 30% to 70% relative humidity with a 12-h light/dark cycle. Mice were anesthetized by an intraperitoneal injection of ketamine (1.2 mg/animal) and xylazine (0.25 mg/animal). The backs of the mice were shaved with an electric razor and treated with depilatory cream to remove the remaining hair. Then, an area of 2 cm2 was tape-stripped 20 times using autoclave tape and sanitized using alcohol wipes, and two 1-cm2 regions were infected with 10 μL of 1 × 106 CFU/mL log-phase ESBL-producing K. pneumoniae organisms (ATCC 700603) each. Infection was established for 3 h, and then the mice were divided by simple randomization into 2 groups: (i) wounds topically treated with 300 μg of PlyKp104 in 50 μL CAPS-buffered saline, pH 6.0 (n = 12; two wounds per mouse), and (ii) wounds topically treated with 50 μL of carrier buffer control (n = 12; two wounds per mouse). The mice were euthanized at 3 h following treatment. The skin regions were excised and homogenized in 500 μL of PBS, using a Stomacher 80 Biomaster for 1 min at the highest setting. The homogenate was then serially diluted in PBS and plated on LB plates to calculate the number of K. pneumoniae CFU per milliliter in the skin sample.
Statistical analysis.
All experiments were performed in triplicate. We used a two-tailed Student’s t test to evaluate the significance of the bactericidal assays and murine skin models. Data were also calculated and statistically analyzed for standard error, 95% confidence intervals, and significance levels (log-rank/Mantel-Cox test), using the Prism (version 7) computer program (GraphPad Software, La Jolla, CA).
ACKNOWLEDGMENTS
This work was supported by The National Key Research and Development Program of China (2022YFD1800903), The Rockefeller University, Hunter College, CUNY, The National Natural Science Foundation of China (32072323 and 31772083), the Special Fund for Technology Innovation of Hubei Province (2019AHB074), and the Natural Science Foundation of Hubei Province (2022CFB659).
We thank Tricia Alston and Ryan Heselpoth for their contributions to this paper. We thank Bruce Levin for providing us with the NP1 and NP3 phages. We thank Lars Westblade of Weill Cornell Medical Center, New York, NY, and Eugene E. Gobern of NYU Hospital for providing us with contemporary clinical isolates and advice regarding the clinical aspects of the study.
Footnotes
Supplemental material is available online only.
[This article was published on 26 April 2023 with an error in the author affiliations. The affiliations were updated in the current version, posted on 4 May 2023.]
REFERENCES
- 1.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 2.Shon AS, Bajwa RP, Russo TA. 2013. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer RC. 1996. Predominant pathogens found in the European prevalence of infection in intensive care study. Eur J Clin Microbiol Infect Dis 15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 4.Lyczak JB, Cannon CL, Pier GB. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 5.Winstanley C, O’Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams BJ, Dehnbostel J, Blackwell TS. 2010. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 9.Caffrey AR, Appaneal HJ, Liao JX, Piehl EC, Lopes V, Puzniak LA. 2022. Treatment heterogeneity in Pseudomonas aeruginosa pneumonia. Antibiotics (Basel) 11:1033. doi: 10.3390/antibiotics11081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maciejewska B, Roszniowski B, Espaillat A, Kęsik-Szeloch A, Majkowska-Skrobek G, Kropinski AM, Briers Y, Cava F, Lavigne R, Drulis-Kawa Z. 2017. Klebsiella phages representing a novel clade of viruses with an unknown DNA modification and biotechnologically interesting enzymes. Appl Microbiol Biotechnol 101:673–684. doi: 10.1007/s00253-016-7928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Sun X, Ma X. 2017. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 16:18. doi: 10.1186/s12941-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassetti M, Garau J. 2021. Current and future perspectives in the treatment of multidrug-resistant gram-negative infections. J Antimicrob Chemother 76:iv23–iv37. doi: 10.1093/jac/dkab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. 2019. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Hancock RE. 1998. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis 27:S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 16.Potron A, Poirel L, Nordmann P. 2015. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Laudy AE, Róg P, Smolińska-Król K, Ćmiel M, Słoczyńska A, Patzer J, Dzierżanowska D, Wolinowska R, Starościak B, Tyski S. 2017. Prevalence of ESBL-producing Pseudomonas aeruginosa isolates in Warsaw, Poland, detected by various phenotypic and genotypic methods. PLoS One 12:e0180121. doi: 10.1371/journal.pone.0180121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effah CY, Sun T, Liu S, Wu Y. 2020. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob 19:1. doi: 10.1186/s12941-019-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suay-García B, Pérez-Gracia MT. 2019. Present and future of carbapenem-resistant Enterobacteriaceae (CRE) infections. Antibiotics (Basel) 8:122. doi: 10.3390/antibiotics8030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. 2014. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009–11 in 14 European and Mediterranean countries. J Antimicrob Chemother 69:1804–1814. doi: 10.1093/jac/dku048. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo-Troyano A, Sibila O. 2017. The respiratory threat posed by multidrug resistant gram-negative bacteria. Respirology 22:1288–1299. doi: 10.1111/resp.13115. [DOI] [PubMed] [Google Scholar]
- 22.Peña C, Gómez-Zorrilla S, Oriol I, Tubau F, Dominguez MA, Pujol M, Ariza J. 2013. Impact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: predictors of early and crude mortality. Eur J Clin Microbiol Infect Dis 32:413–420. doi: 10.1007/s10096-012-1758-8. [DOI] [PubMed] [Google Scholar]
- 23.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. 2011. Tackling antibiotic resistance. Nat Rev Microbiol 9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sader HS, Flamm RK, Mendes RE, Farrell DJ, Jones RN. 2016. Antimicrobial activities of ceftaroline and comparator agents against bacterial organisms causing bacteremia in patients with skin and skin structure infections in U.S. medical centers, 2008 to 2014. Antimicrob Agents Chemother 60:2558–2563. doi: 10.1128/AAC.02794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. 2019. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong WH, Saha BK, Ramani A, Chopra A. 2021. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 49:591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cillóniz C, Gabarrús A, Ferrer M, Puig de la Bellacasa J, Rinaudo M, Mensa J, Niederman MS, Torres A. 2016. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest 150:415–425. doi: 10.1016/j.chest.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Herridge WP, Shibu P, O’Shea J, Brook TC, Hoyles L. 2020. Bacteriophages of Klebsiella spp., their diversity and potential therapeutic uses. J Med Microbiol 69:176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seetoh T, Lye D, Archuleta S, Fisher D. 2013. Klebsiella pneumoniae liver abscesses. Lancet Infect Dis 13:391–392. doi: 10.1016/S1473-3099(13)70067-7. [DOI] [PubMed] [Google Scholar]
- 30.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 31.Vázquez R, García E, García P. 2018. Phage lysins for fighting bacterial respiratory infections: a new generation of antimicrobials. Front Immunol 9:2252. doi: 10.3389/fimmu.2018.02252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondil VS, Harjai K, Chhibber S. 2020. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int J Antimicrob Agents 55:105844. doi: 10.1016/j.ijantimicag.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, Jeong BC, Lee SH. 2017. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol 7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira H, Melo LD, Santos SB, Nóbrega FL, Ferreira EC, Cerca N, Azeredo J, Kluskens LD. 2013. Molecular aspects and comparative genomics of bacteriophage endolysins. J Virol 87:4558–4570. doi: 10.1128/JVI.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuch R, Cassino C, Vila-Farres X. 2022. Direct lytic agents: novel, rapidly acting potential antimicrobial treatment modalities for systemic use in the Era of rising antibiotic resistance. Front Microbiol 13:841905. doi: 10.3389/fmicb.2022.841905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau M, Seité S, Aguilar L, Da Cruz O, Puech J, Frieling J, Demessant A. 2021. Topical S. aureus-targeting endolysin significantly improves symptoms and QoL in individuals with atopic dermatitis. J Drugs Dermatol 20:1323–1328. doi: 10.36849/jdd.6363. [DOI] [PubMed] [Google Scholar]
- 37.Wire MB, Jun SY, Jang IJ, Lee SH, Hwang JG, Huang DB. 2022. A phase 1 study to evaluate safety and pharmacokinetics following administration of single and multiple doses of the antistaphylococcal lysin LSVT-1701 in healthy adult subjects. Antimicrob Agents Chemother 66:e01842-21. doi: 10.1128/aac.01842-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghose C, Euler CW. 2020. Gram-negative bacterial lysins. Antibiotics (Basel) 9:74. doi: 10.3390/antibiotics9020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler VG, Jr, Das AF, Lipka-Diamond J, Schuch R, Pomerantz R, Jáuregui-Peredo L, Bressler A, Evans D, Moran GJ, Rupp ME, Wise R, Corey GR, Zervos M, Douglas PS, Cassino C. 2020. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest 130:3750–3760. doi: 10.1172/JCI136577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briers Y, Walmagh M, Lavigne R. 2011. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J Appl Microbiol 110:778–785. doi: 10.1111/j.1365-2672.2010.04931.x. [DOI] [PubMed] [Google Scholar]
- 41.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Lavigne R, Neves-Petersen MT, Kluskens LD, Azeredo J. 2014. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS One 9:e108376. doi: 10.1371/journal.pone.0108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu B, Yao X, Han G, Luo Z, Zhang J, Yong K, Wang Y, Luo Y, Yang Z, Ren M, Cao S. 2022. Isolation of Klebsiella pneumoniae phage vB_KpnS_MK54 and pathological assessment of endolysin in the treatment of pneumonia mice model. Front Microbiol 13:854908. doi: 10.3389/fmicb.2022.854908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walmagh M, Boczkowska B, Grymonprez B, Briers Y, Drulis-Kawa Z, Lavigne R. 2013. Characterization of five novel endolysins from gram-negative infecting bacteriophages. Appl Microbiol Biotechnol 97:4369–4375. doi: 10.1007/s00253-012-4294-7. [DOI] [PubMed] [Google Scholar]
- 45.Blasco L, Ambroa A, Trastoy R, Bleriot I, Moscoso M, Fernández-Garcia L, Perez-Nadales E, Fernández-Cuenca F, Torre-Cisneros J, Oteo-Iglesias J, Oliver A, Canton R, Kidd T, Navarro F, Miró E, Pascual A, Bou G, Martínez-Martínez L, Tomas M. 2020. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci Rep 10:7163. doi: 10.1038/s41598-020-64145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thummeepak R, Kitti T, Kunthalert D, Sitthisak S. 2016. Enhanced antibacterial activity of Acinetobacter baumannii bacteriophage ØABP-01 endolysin (LysABP-01) in combination with colistin. Front Microbiol 7:1402. doi: 10.3389/fmicb.2016.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walmagh M, Briers Y, dos Santos SB, Azeredo J, Lavigne R. 2012. Characterization of modular bacteriophage endolysins from Myoviridae phages OBP, 201φ2-1 and PVP-SE1. PLoS One 7:e36991. doi: 10.1371/journal.pone.0036991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, Oliveira H, Azeredo J, Verween G, Pirnay JP, Miller S, Volckaert G, Lavigne R. 2014. Engineered endolysin-based “artilysins” to combat multidrug-resistant Gram-negative pathogens. mBio 5:e01379-14. doi: 10.1128/mBio.01379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukacik P, Barnard TJ, Buchanan SK. 2012. Using a bacteriocin structure to engineer a phage lysin that targets Yersinia pestis. Biochem Soc Trans 40:1503–1506. doi: 10.1042/BST20120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai MJ, Lin NT, Hu A, Soo PC, Chen LK, Chen LH, Chang KC. 2011. Antibacterial activity of Acinetobacter baumannii phage phiAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Appl Microbiol Biotechnol 90:529–539. doi: 10.1007/s00253-011-3104-y. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, Lee DW, Jin JS, Kim J. 2020. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J Glob Antimicrob Resist 22:32–39. doi: 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Larpin Y, Oechslin F, Moreillon P, Resch G, Entenza JM, Mancini S. 2018. In vitro characterization of PlyE146, a novel phage lysin that targets gram-negative bacteria. PLoS One 13:e0192507. doi: 10.1371/journal.pone.0192507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lukacik P, Barnard TJ, Keller PW, Chaturvedi KS, Seddiki N, Fairman JW, Noinaj N, Kirby TL, Henderson JP, Steven AC, Hinnebusch BJ, Buchanan SK. 2012. Structural engineering of a phage lysin that targets gram-negative pathogens. Proc Natl Acad Sci USA 109:9857–9862. doi: 10.1073/pnas.1203472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Schuch R, Fischetti VA. 2015. Novel phage lysin capable of killing the multidrug-resistant gram negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991. doi: 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raz A, Serrano A, Hernandez A, Euler CW, Fischetti VA. 2019. Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection. Antimicrob Agents Chemother 63:e00024-19. doi: 10.1128/AAC.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orito Y, Morita M, Hori K, Unno H, Tanji Y. 2004. Bacillus amyloliquefaciens phage endolysin can enhance permeability of Pseudomonas aeruginosa outer membrane and induce cell lysis. Appl Microbiol Biotechnol 65:105–109. doi: 10.1007/s00253-003-1522-1. [DOI] [PubMed] [Google Scholar]
- 57.Antonova NP, Vasina DV, Lendel AM, Usachev EV, Makarov VV, Gintsburg AL, Tkachuk AP, Gushchin VA. 2019. Broad bactericidal activity of the Myoviridae bacteriophage lysins LysAm24, LysECD7, and LysSi3 against gram-negative ESKAPE pathogens. Viruses 11:284. doi: 10.3390/v11030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu JJK, Poh WH, Hasnuddin NTB, Hew EY, Dam LC, Sahili AE, Rice SA, Goh BC. 2022. Novel phage lysin Abp013 against Acinetobacter baumannii. Antibiotics (Basel) 11:169. doi: 10.3390/antibiotics11020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S, Jin JS, Choi YJ, Kim J. 2020. LysSAP26, a new recombinant phage endolysin with a broad spectrum antibacterial activity. Viruses 12:1340. doi: 10.3390/v12111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Wang LH, Xiang FG, Ding WL, Xi LJ, Wang MQ, Xiao ZJ, Liu JG. 2020. Pseudomonas phragmitis sp. nov., isolated from petroleum polluted river sediment. Int J Syst Evol Microbiol 70:364–372. doi: 10.1099/ijsem.0.003763. [DOI] [PubMed] [Google Scholar]
- 61.Abdelkader K, Gutiérrez D, Grimon D, Ruas-Madiedo P, Lood C, Lavigne R, Safaan A, Khairalla AS, Gaber Y, Dishisha T, Briers Y. 2020. Lysin LysMK34 of Acinetobacter baumannii bacteriophage PMK34 has a turgor pressure-dependent intrinsic antibacterial activity and reverts colistin resistance. Appl Environ Microbiol 86:e01311-20. doi: 10.1128/AEM.01311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clements JA. 1997. Lung surfactant: a personal perspective. Annu Rev Physiol 59:1–21. doi: 10.1146/annurev.physiol.59.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Ramirez-Blanco CE, Ramirez-Rivero CE, Diaz-Martinez LA, Sosa-Avila LM. 2017. Infection in burn patients in a referral center in Colombia. Burns 43:642–653. doi: 10.1016/j.burns.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Yoon EJ, Jeong SH. 2021. Mobile carbapenemase genes in Pseudomonas aeruginosa. Front Microbiol 12:614058. doi: 10.3389/fmicb.2021.614058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sader HS, Castanheira M, Arends SJR, Goossens H, Flamm RK. 2019. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997-2016). J Antimicrob Chemother 74:1595–1606. doi: 10.1093/jac/dkz074. [DOI] [PubMed] [Google Scholar]
- 66.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. 2011. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thandar M, Lood R, Winer BY, Deutsch DR, Euler CW, Fischetti VA. 2016. Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60:2671–2679. doi: 10.1128/AAC.02972-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu S, Campisi E, Li J, Fischetti VA. 2021. Decontamination of Escherichia coli O157:H7 on fresh Romaine lettuce using a novel bacteriophage lysin. Int J Food Microbiol 341:109068. doi: 10.1016/j.ijfoodmicro.2021.109068. [DOI] [PubMed] [Google Scholar]
- 70.Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. 2017. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One 12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fenton M, Ross RP, McAuliffe O, O’Mahony J, Coffey A. 2011. Characterization of the staphylococcal bacteriophage lysin CHAP(K). J Appl Microbiol 111:1025–1035. doi: 10.1111/j.1365-2672.2011.05119.x. [DOI] [PubMed] [Google Scholar]
- 72.Filatova LY, Donovan DM, Foster-Frey J, Pugachev VG, Dmitrieva NF, Chubar TA, Klyachko NL, Kabanov AV. 2015. Bacteriophage phi11 lysin: physicochemical characterization and comparison with phage phi80α lysin. Enzyme Microb Technol 73-74:51–58. doi: 10.1016/j.enzmictec.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Vasina DV, Antonova NP, Grigoriev IV, Yakimakha VS, Lendel AM, Nikiforova MA, Pochtovyi AA, Remizov TA, Usachev EV, Shevlyagina NV, Zhukhovitsky VG, Fursov MV, Potapov VD, Vorobev AM, Aleshkin AV, Laishevtsev AI, Makarov VV, Yudin SM, Tkachuk AP, Gushchin VA. 2021. Discovering the potentials of four phage endolysins to combat gram-negative infections. Front Microbiol 12:748718. doi: 10.3389/fmicb.2021.748718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan FM, Gondil VS, Li C, Jiang M, Li J, Yu J, Wei H, Yang H. 2021. A novel Acinetobacter baumannii bacteriophage endolysin LysAB54 with high antibacterial activity against multiple gram-negative microbes. Front Cell Infect Microbiol 11:637313. doi: 10.3389/fcimb.2021.637313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng NC, Tai HC, Chang SC, Chang CH, Lai HS. 2015. Necrotizing fasciitis in patients with diabetes mellitus: clinical characteristics and risk factors for mortality. BMC Infect Dis 15:417. doi: 10.1186/s12879-015-1144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang F, Ji X, Li Q, Zhang G, Peng J, Hai J, Zhang Y, Ci B, Li H, Xiong Y, Deng X, Lin L. 2020. TSPphg lysin from the extremophilic thermus bacteriophage TSP4 as a potential antimicrobial agent against both gram-negative and gram-positive pathogenic bacteria. Viruses 12:192. doi: 10.3390/v12020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gu J, Feng Y, Feng X, Sun C, Lei L, Ding W, Niu F, Jiao L, Yang M, Li Y, Liu X, Song J, Cui Z, Han D, Du C, Yang Y, Ouyang S, Liu ZJ, Han W. 2014. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-hand-like” calcium-binding phage lysin. PLoS Pathog 10:e1004109. doi: 10.1371/journal.ppat.1004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuch R, Nelson X, Fischetti V. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 80.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti V. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01519-22-s0001.docx, DOCX file, 0.2 MB (227.5KB, docx)