Abstract
Seasonal or permanent water scarcity in off-grid communities can be alleviated by recycling water in decentralized wastewater treatment systems. Nature-based solutions, such as constructed wetlands (CWs), have become popular solutions for sanitation in remote locations. Although typical CWs can efficiently remove solids and organics to meet water reuse standards, polishing remains necessary for other parameters, such as pathogens, nutrients, and recalcitrant pollutants. Different CW designs and CWs coupled with electrochemical technologies have been proposed to improve treatment efficiency. Electrochemical systems (ECs) have been either implemented within the CW bed (ECin-CW) or as a stage in a sequential treatment (CW + EC). A large body of literature has focused on ECin-CW, and multiple scaled-up systems have recently been successfully implemented, primarily to remove recalcitrant organics. Conversely, only a few reports have explored the opportunity to polish CW effluents in a downstream electrochemical module for the electro-oxidation of micropollutants or electro-disinfection of pathogens to meet more stringent water reuse standards. This paper aims to critically review the opportunities, challenges, and future research directions of the different couplings of CW with EC as a decentralized technology for water treatment and recovery.
Keywords: Sanitation and reuse, Disinfection, Advanced oxidation, Decentralized systems, Electrification
Graphical abstract
Highlights
-
•
Overview of electrochemical technologies coupled to constructed wetlands.
-
•
Full-scale systems integrating conductive beds for wastewater treatment.
-
•
Challenges and prospects to remove pathogens and recalcitrant compounds.
-
•
CAPEX, energy consumption, and stability to be tackled for electrolysis-based systems.
-
•
Low-income countries' approach to decentralized water reclamation.
Abbreviations
- BDD
boron-doped diamond
- BDL
below detection limits
- BOD
biological oxygen demand
- BOD5
5-day biological oxygen demand
- CW(s)
constructed wetland(s)
- CW + EC
CW followed by EC as two technologies coupled in a treatment train
- CW-MFC
microbial fuel cells integrated into CWs
- COD
chemical oxygen demand
- DO
dissolved oxygen
- ECin-CW
EC features integrated into the CW bed
- EC(s)
electrochemical system(s)
- EC + CW
EC followed by CW as two technologies coupled in a treatment train
- EC-PCW
pyrite-filled CW integrated with EC
- GW
green walls
- HRT
hydraulic retention time
- HSSF-CW
horizontal sub-surface flow constructed wetland
- MFC
microbial fuel cell
- MMO
mixed metal oxides
- SEC
specific energy consumption
- SHE
standard hydrogen electrode
- SF-CW
surface flow constructed wetlands
- SSF-CW
sub-surface flow constructed wetlands
- sMFC
sediment microbial fuel cell
- SMX
sulfamethoxazole
- TC
total coliforms
- TN
total nitrogen
- TSS
total suspended solids
- VSSF-CW
vertical sub-surface flow constructed wetlands
1. Introduction
Recent predictions about water availability indicate that half of the world's population will live in water-stressed areas by 2025 [1,2]. Therefore, there is an urgent need for wiser water management to cope with the ongoing water crisis. Desalination technologies, rainwater harvesting, and water reclamation are proposed solutions placed at the forefront of this effort [3,4].
Typically, water provision and sanitation in urban sectors are served by centralized systems demanding a high capital investment, primarily due to the required extensive pipe networks [5]. However, in rural or peri-urban areas, water service coverage is usually limited as sparse settlements render centralized water treatment financially unsustainable [6]. The pressure to cover the increasing water demand and to remediate untreated or insufficiently treated wastewater can be alleviated by implementing local decentralized systems to enable onsite water treatment and reuse [7].
Constructed wetlands (CWs), a nature-based solution, enable decentralized wastewater treatment and water reclamation [[7], [8], [9]]. CWs are cost-effective and additionally provide co-benefits, such as ecosystem services (e.g., wildlife habitats and recreational environments) [10] and valuable, marketable products (e.g., biomass as fuel) [11]. Decentralized CWs have been implemented for wastewater treatment within the premises of university campus facilities, small industries, and small communities or to serve temporary events [[12], [13], [14]]. However, several studies report that CW effluents consistently fail to comply with the required standards for water reuse in terms of pathogen and nutrient content [9,[15], [16], [17]]. Moreover, the conventional, stand-alone CW cannot eliminate recalcitrant compounds, meaning that water reuse right after CW treatment will be hindered by the presence of emerging contaminants [9,15].
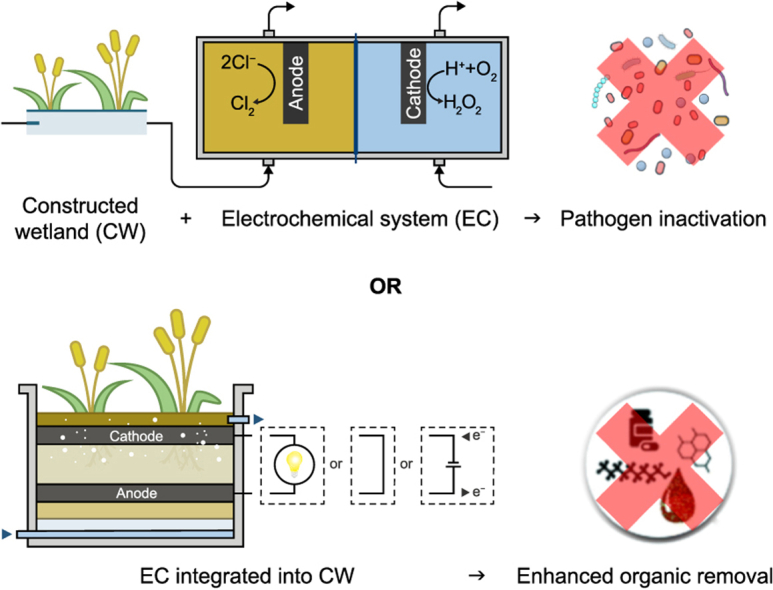
The need to polish CW effluents and upgrade CW operation has been the subject of multiple studies which have investigated CWs combined with other advanced technologies [9,17,18], such as ultraviolet (UV) irradiation [19,20], membrane filtration [14,21], advanced oxidation [[22], [23], [24], [25]], and electrochemical systems (ECs) [[26], [27], [28], [29], [30], [31], [32]]. Among those, EC-based strategies have substantially decreased recalcitrant pollutants and pathogen levels in the treated water. Two main approaches have been proposed for CW and ECs couplings. The first one integrates (bio)electrochemical features within the CW (ECin-CW) by incorporating either a “conventional” electrochemical cell (with wired anode and cathode) or by using bed materials that conduct electrons from the bottom of the bed (low redox potentials) to the top (high redox potentials). The latter ECin-CW variation has been reported to substantially enhance organic removal, and multiple scaled-up systems have recently been implemented successfully [[33], [34], [35]]. The second strategy consists of using ECs to produce oxidizing agents sequentially placed after (CW + EC) or before (EC + CW) the CWs with a primary focus on pathogens removal and biodegradability enhancement [28,30,32]. Such coupled systems could be implemented in decentralized settings to avoid the transportation and storage of concentrated reagents for the treating process in resource-limiting conditions [32,36,37], yet several challenges remain to be tackled before any viable implementation.
In this review, we first introduce the concept of CWs, their design variations, and the need for effluent polishing for either water reuse or discharge (Section 2). Section 3 focuses on the ECs integrated into the CW bed, ECin-CW. We subdivide them into systems that generate electrical energy (microbial fuel cells, (MFCs)), systems that operate under short circuit (microbial electrochemical “snorkels”), and systems that consume electric power (electrolysis cells). Section 4 reviews a treatment train concept that entails a sequential coupling of CWs and ECs, CW + EC. First, the different oxidizing agents that can be electrogenerated for disinfection and/or pollutant removal are introduced and the different treatment sequences proposed so far. Next, the operational performance of the CW + EC reported thus far in the literature is discussed. The applicability of all systems presented in Sections 3, 4 is critically debated regarding water treatment performance, energy consumption, and operational stability. Section 5 reflects upon the possibility and relevance of implementing these systems in less-developed countries and discusses future research directions.
2. Constructed wetlands: design variations and effluent characteristics
Constructed wetlands (CWs) or treatment wetlands imitate natural wetlands with a human-made engineered system that treats water through a combination of biological and physicochemical processes. This eco-technology is considered robust and requires minimum maintenance [38,39]. The usual CW design includes three main components: (i) the granular substrate that corresponds to a filtration bed or, alternatively, to the bottom of a pond, (ii) the macrophytes that aid pollutant removal and provide aeration via the root system, and (iii) the microorganisms that enable pollutant degradation [15,40].
Depending on their flow regime, CWs can be divided into surface flow (SF) and subsurface flow (SSF) systems [38]. SF systems can be categorized further, according to the rooting location of the dominant macrophytes, into rooted emergent, submerged, and free-floating [41]. The most used variant is the rooted emergent macrophytes, where plants like reeds or bulrushes are rooted into the bottom substrate, and water flows on top of the substrate between the plant stems. Another increasingly investigated option is the floating wetland, where vegetation is grown on a supporting material that provides buoyancy. The plant root system develops in the water column beneath the floating structure, and biofilms growing on the roots remediate the water (Fig. 1a) [42,43].
Fig. 1.
Variations in constructed wetland configurations applied for water treatment and reclamation. a, A floating constructed wetland as an example of surface flow (SF) systems. b–c, The sub-surface flow constructed wetlands (SSF) in their vertical (b) and horizontal (c) feeding patterns. d, An example of intensified CWs: the aerated horizontal sub-surface flow CW (HSSF-CW).
On the other hand, sub-surface flow constructed wetlands (SSF-CWs) consist of a granular (gravel, lava rocks, or sand) bed over an impervious liner, and macrophytes are commonly planted at the top. Since the wastewater mainly flows 15–20 cm below the bed surface, the emission of foul odors is diminished compared to the surface flow constructed wetlands (SF-CW). The SSF-CWs are most commonly used to treat domestic wastewater, and they are classified into vertical (VSSF-CW) and horizontal (HSSF-CW) subsurface flow CWs depending on the water route during the treatment [41,44] (Fig. 1b and c). A VSSF-CW usually has a bed depth between 0.6 and 1 m and can treat raw wastewater in the so-called French-type CW [45,46]. The French-type CW is ideal for small communities (<5000 people equivalent) [47] and usually works as a two-stage system, where the first stage retains the sludge and degrades organic matter, and the second one allows nitrification and further organic degradation. Other types of VSSF-CWs require pre-treatment of the wastewater, usually by septic tanks, sedimentation tanks, and, less frequently, by other kinds of anaerobic bioreactors to avoid clogging the granular media [48]. Commonly for VSSF-CW, intermittent feeding with organic loading rates between 20 and 27 g COD m−2 d−1 is usually applied [38]. The feeding process allows the formation of aerobic (top part) and anoxic zones (bottom layer) in the VSSF-CW. Even though the different redox zones can promote nitrification and denitrification reactions, the low denitrification rate usually limits the total nitrogen removal to ∼20% [9,45]. On the other hand, HSSF-CWs exhibit bed depths between 0.3 and 0.7 m and function as a secondary or tertiary treatment with organic loading rates ranging 6–20 g COD m−2 d−1 [38]. Gravel is usually applied at the inlet and outlet zones of the system (Fig. 1c) to enhance water transport horizontally. Coarse materials, other than gravel, can also be implemented in the CW bed [38,39,41]. Anaerobic processes dominate the water treatment in HSSF-CWs with a water-saturated bed assembled with coarse materials [46]. Hence, as nitrification is limited in the anaerobic conditions developed, HSSF-CWs are limited in nitrogen removal when used as a single stage.
Green walls and green roofs are two very recent adaptations of SSF-CWs, where urban wastewater is treated by the same mechanisms employed in a CW. In this variation, the CW is mounted vertically or horizontally on a supportive urban structure. These nature-based solutions fit land-limited urban environments and have been explored and reported in detail in other studies [8,30].
The typical removal efficiencies of the CW designs listed so far are 80–90% for organics, reported as COD, and 80–90% removal of solids, reported as total suspended solids (TSS), demonstrating an acceptable performance level as biological systems. However, other water quality parameters, such as nutrients and pathogens (Table S1), require further attention when water reuse is considered [7,17,26]. To achieve higher performances, different types of CWs are usually combined in series, forming hybrid CWs [46,49]. For instance, VSSF-CWs (with aerobic and anoxic zones) are placed before HSSF-CWs (mostly anoxic) to promote sequential nitrification and denitrification, ultimately enhancing total nitrogen removal [49].
Moreover, some operational strategies, such as substrate variations, recirculation, aeration (Fig. 1d), tidal operation, and implementation of electrodes, enhance the performance in the so-called intensified CWs [[50], [51], [52], [53], [54]]. Intensified CWs can reduce the land area by increasing pollutant removal rates. Researchers increasingly investigate reactive materials to replace or complement the typically inert materials forming CW beds. For example, zeolites promote sorption of NH4+ [55] and antibiotics [56], lightweight expanded clay aggregates favor the removal of anions (e.g., phosphates, nitrites, or chromates) [57], and granular activated carbon enhances the removal of nutrients and micropollutants when added to the CW bed [58]. Mechanical aeration is implemented to accelerate the organic degradation of common and emerging contaminants [50,51]. In the tidal flow CWs, wastewater is pumped back and forth between two wetland beds, increasing the oxygen content in the wetland bed without needing costly artificial aeration [52,59]. Another example is the integration of electrochemically based strategies within the CW bed (ECin-CW), a concept further described in Section 3. Almost a quarter of the new research in CWs is dedicated to intensified CWs, demonstrating the importance of more sophisticated engineering approaches for optimizing CWs [60].
While the primary target of CWs is to decrease the level of organics, all planted CWs can also decrease the concentrations of pathogens to some extent. This can occur because of sedimentation, mechanical filtration, biocides excreted by plants or root exudates, natural die-off, sunlight irradiation, microbial predation (carried out by protozoa and metazoa), and competition [16,61]. Conventional CWs have shown highly varying log10 removal values of pathogens (LRV), ranging from one to four logs. Temporal fluctuations in organics, pathogens load, and weather conditions usually result in that variable CW performance. Occasionally, bacterial regrowth can occur within the CW basin, and animal feces inhabiting the wetland can increase the pathogen loads [16]. As such, it is often challenging to guarantee a CW effluent (e.g., 103–105 CFU per 100 mL as total coliforms, Table S1) that complies with water reuse standards (e.g., 10–103 CFU per 100 mL as total coliforms or Escherichia coli) [9,16]. Thus, it appears crucial to implement further polishing steps to improve the removal of pathogens and guarantee the safe reuse of the treated effluent (Table S1).
3. Electrochemical systems integrated into constructed wetlands
Although CWs is a versatile and cost-effective technology with low operation and maintenance efforts, their surface footprint remains an important drawback, being much larger than conventional intensive wastewater treatment technologies. To minimize surface requirements, efforts to intensify the CW treatment have been undertaken. This transition to intensified systems is supported by the ECin-CW to establish one single hybrid technology, the so-called electroactive wetlands [34].
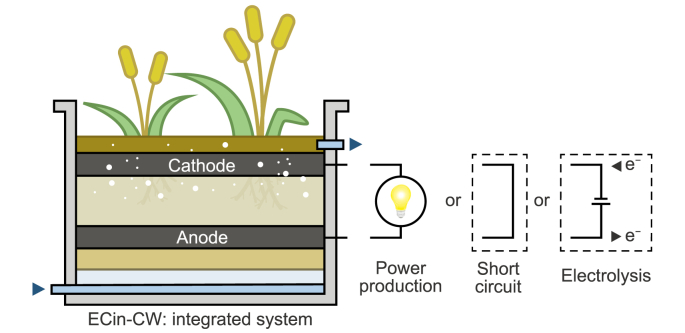
In ECin-CW, fully or partially conductive materials are integrated into the CW bed to allow for electron flow and thus to locally enhance oxidation and reduction reactions. We here identify three kinds of configurations (Fig. 2): (i) those where two separated zones of conductive materials are connected electrically and perform as anode and cathode, respectively, generating electric power as a microbial fuel cell (MFC), called microbial fuel cells integrated into CWs (CW-MFC); (ii) those where conductive materials constitute the entire CW bed, allowing an operation in short-circuit between zones of different redox potentials, the so-called METland®; and (iii) those where separated conductive materials (i.e., anode and cathode) are connected to a power supply to perform electrolysis (basics concepts of electrolysis and relevant parameters can be found in Text S1 or in the literature [62,63]).
Fig. 2.
A wetland can integrate an electrochemical cell (ECin-CW) functioning either as a power source (MFC), in short-circuit where electrons flow across a conductive bed (METland®), or in electrolysis mode for wastewater treatment.
3.1. Microbial fuel cells integrated into constructed wetlands
Sediment microbial fuel cells (sMFC) [64] harvest energy directly from a natural environment by submerging an anode in a flooded anoxic sediment and a cathode in an oxidative environment, in most cases an aerated upper layer that is in contact with atmospheric oxygen. In such MFC configurations, electroactive bacteria oxidize organic compounds in the sediment and transfer the corresponding electrons to the anode, ultimately generating electric power [65]. A similar concept can be applied to a CW considering the presence of zones with different redox environments: more negative redox potentials in the oxygen-depleted CW bottom layers and more positive potentials at the CW upper layers. A CW-MFC allows for electron flow along an external circuit from the anode to the cathode, where they reduce available electron acceptors, including oxygen or nitrate [66]. The first published paper on CW-MFC was a laboratory experience with synthetic wastewater to facilitate the removal of methylene blue dye with concomitant electricity generation [67]. Since then, the focus has been expanded from conventional pollutants in real wastewater (e.g., nutrients and easily biodegradable pollutants) to refractory pollutants, including antibiotics [[68], [69], [70], [71], [72], [73]]. The topic has been extensively reviewed during the last decade [74,75] and numerous studies have already explored the impact of stimulating microbial communities via electrode placement in CWs treating wastewater. However, the power densities generated remain marginal (few mW m−2) because of the limited current densities produced by electroactive organisms and the large internal resistance of such systems [70,76]. Since the current generated by the MFC is highly dependent on the organic load, an interesting application of the CW-MFC is to use it as a biosensor for real-time monitoring of the biological oxygen demand (BOD) [77,78].
3.2. Constructed wetlands with an electrically conductive bed: the METland® system
Electro-microbiology concepts can also be integrated into CWs through an alternative strategy to CW-MFC, the METland® configuration (Fig. 3). In such systems, a 0.8–0.1 m bed of typically carbon-rich conductive particles is employed to electrochemically augment the CW performance [34,70]. Particles of 7–10 mm size distribution are connected to each other, allowing the electrons to flow across the whole conductive bed, in a process so-called “microbial electrochemical snorkel” (i.e., under short-circuit) [79]. Electroactive organisms oxidize organics in the anaerobic, low-potential bottom zones, generating electrons that can be transferred to the conductive bed. The electrons flow spontaneously toward the aerated, high-potential upper layers. Thus, the bottom layers of the conductive bed act as the anodic zone, and the upper layers as the cathodic one. The electron flow along METland® beds was demonstrated by measuring the electric potential profile along vertical distances larger than 40 cm [33,34]. The METland® maximizes the availability of electron acceptors for organics removal and stimulates electro-syntrophies between microbial communities, which greatly enhance pollutant removal rates.
Fig. 3.
Scheme of a typical METland® illustrating the electron flow within the conductive bed, enhancing the removal rate of organics and nitrogen compounds. Reprinted (adapted) with permission from Ref. [33]. Copyright 2020 Elsevier.
Several conductive materials have been used in the METland® bed, mainly conductive coke [34,70] or more sustainable materials such as conductive biochar obtained after high-temperature pyrolysis of biomass such as wood [33,53,80,81]. Biochar additionally exhibits substantial electron storage capacities conferred by their redox-active moieties [82]. This allows biochar to act as a redox buffer when local limitations of electron donors or acceptors occur [83]. Recent studies also introduced supplementary, high-potential, electron acceptors (e.g., hypochlorite or oxygenated water) that act as electron sinks. Those so-called “e-sinks” are embedded in the conductive bed and can somewhat favor the organics degradation rate. However, such a system would require frequent maintenance to replace those chemicals once depleted [33].
According to reported studies from community-level physiological profiling [84] or sequencing analysis [33,70], the type of wastewater, the conductive material, and the operational mode applied will determine the specific microbial communities obtained in the process. Diverse designs and granulometry of conductive particles have been tested to optimize the degradation rates of pollutants. A recent study has demonstrated that the water-saturated METland® was able to remove more than 90% of pharmaceutical compounds from wastewater with a hydraulic retention time (HRT) of 0.5 days [85]. Different studies concluded that highly conductive materials, like coke, promote electron transfer mainly due to the percolation of conduction across the particles forming the bed. Biochars are less conductive yet host redox functional groups, such as quinones, that provide them an electron exchange capacity, acting like a rechargeable geobattery [33,53].
The METland® systems can operate similarly to existing CW configurations: flooded, like HSSF-CWs, and non-flooded, like VSSF-CWs (Fig. 4). The METland® was initially designed to operate under flooded conditions (either HSSF or upflow) (Fig. 4a), favoring anoxic metabolism and nitrate removal [70]. The METland® was also effective under non-flooded mode (down-flow operation, Fig. 4b). This configuration takes advantage of passive aeration, with no energy cost, where dissolved oxygen also diffuses to the bottom bed layers and acts as both an electrochemical and a microbial electron acceptor [86]. Although most “anodic” electroactive bacteria are generally accepted as strict anaerobes, still, Geobacter sp. dominated the microbial communities in the inner biofilm layers, suggesting that O2 was sufficiently depleted in the outer biofilm layers [86]. Furthermore, in this mode of operation, nitrification was favored.
Fig. 4.
The two main operational modes of METland® systems: a, flooded upflow; b, non-flooded downflow. Reprinted with permission from Ref. [87]. Copyrights 2022 Creative Commons.
In the last decade, the METland® has been applied under different environmental and operating conditions in diverse geographic regions (Fig. 5) while achieving COD removal efficiencies of about 90% [35]. A life cycle assessment (LCA) study suggested that they are an environmentally sustainable wastewater treatment technology [88]. Their optimal locations for implementation have been investigated using geospatial tools like multi-criteria evaluation and sensitivity analysis [89]. Two configurations can be distinguished: constructed and modular. Constructed METlands® follow similar design methods to CWs. In contrast, modular METlands® are systems that can be implemented without civil engineering but as a “plug-and-play” solution. Modular METlands® can host conductive beds of higher height (2–3 m) than the one applied in conventional CWs (∼0.8 m), upgrading the wastewater load treatment capacity of the system. For instance, such systems have achieved outstanding organics removal rates ranging from 2 kg COD m−2 d−1 when treating wastewater from the oil and gas sector to 10 kg COD m−2 d−1 for winery wastewater (Esteve-Núñez 2022, personal communication), while conventional CWs typically remove 0.01–0.03 kg COD m−2 d−1. Searching for new materials to reduce the cost or target the enrichment of specific bacterial communities is now a matter of research. To accelerate widespread application, the construction of more demo sites is required, as well as technology uptake by early adopters.
Fig. 5.
Different implementations of METland® treating real urban wastewater. a, Modular METland® treating 25 m3 d−1 at Otos municipality (Spain). b, Modular single-house system treating 1 m3 d−1c, Constructed METland® treating 20 m3 d−1 at Orby (Denmark). d, Constructed METland® treating 5 m3 d−1 at Carrion de los Céspedes (Spain). e, Containerized modular METland® operating in series for treating municipal wastewater at Seville (Spain).
3.3. Electrolysis-integrated constructed wetland
In certain CW strategies and when required to treat high nutrient load, the CWs present inadequate nutrient removal. Oxygen depletion in the system limits nitrification, which later impacts denitrification and overall nutrient removal. Additionally, the finite sorption capacity of typical bed materials limits phosphorus removal in the long run [52]. Thus, electrolysis-integrated CWs emerged as a solution to enhance nutrient removal [52,90,91]. Electrolysis in the CW systems can remove phosphorous via electro-coagulation processes [52,90,91]. It can also provide electron acceptors and donors involved in microbial metabolisms, such as O2 for nitrification at the anodic side or H2 for autotrophic denitrification at the cathodic side, which also steers the microbial community in the bed [52,91]. Electrolysis-integrated systems have also been reported as efficient in controlling sulfide-related malodor [52]. Considering that low temperature impairs microbial and plant activity toward nutrient removal [52,90], the electrolysis-integrated CWs have been developed as a system incorporating physical and chemical treatments, which are more reliable to perform effectively in cold climates [40,59].
The effort to integrate electrolysis into a wetland bed started with the study of Ju et al. [52] aiming to improve nutrient removal in tidal flow CWs. The electrolysis-integrated tidal flow CW was built with iron and graphite electrodes in a bio-ceramic bed of a cylindrical reactor. Ju et al. operated the system at 10 V with periods of 4 h where three distinct operational time shifts were applied: (i) the iron plates were operated as cathodes, and the H2 electrogenerated was used as an electron donor to promote autotrophic denitrification; (ii) the iron plates were used as anodes which released Fe3+ particles that removed phosphate by precipitation, adsorption, and flocculation; and (iii) an open circuit mode was applied to allow the phosphate-rich particles to settle. The electrolysis-integrated system successfully removed ≥85% of PO43−, while different controls only achieved 20–65% removal. The removal of NH4+ was around 80%, whether the electrolysis was applied or not. As the iron electrode is periodically employed as a sacrificial anode, it must be regularly replaced, adding to the maintenance and operational costs of the overall system.
Similar efforts to improve nutrient removal were subsequently taken up, combining various materials for the electrodes and for the CW bed, including iron plates in HSSF-CW [90], Mg–Al alloy as the anode, and graphite as the cathode in an ecological floating bed [91], and carbon fiber electrodes in a CW bed formed of granular pyrite [92]. In two studies employing sacrificial electrodes, iron [90] and Mg–Al alloy [91], phosphorus removal was improved by 50% and 66%, respectively, compared to the control beds without electrodes. Similarly, the electrolysis improved nitrate removal by 71% in the electrolysis-integrated HSSF-CW [90], and the total nitrogen (TN) removal by 26% in the electrolysis-integrated ecological floating bed compared to the similar wetland configuration without applying electrolysis [91]. Another study used pyrite (FeS2) as the substrate of the CW to boost both phosphorus and nitrogen removal [92]. The Fe(II) in FeS2 can be oxidized by O2, which releases Fe3+ to precipitate PO43−. While FeS2 is known to act as an electron donor for autotrophic denitrification, cathodically produced H2 can further boost denitrification. Three systems were compared: (i) a CW filled with a FeS2 bed integrating carbon felt electrodes, so-called electrolysis-integrated pyrite constructed wetland (EC-PCW); (ii) a control with a bed made of gravel instead of pyrite, also integrating the carbon felt electrodes; and (iii) another control with pyrite but devoid of electrodes. The total phosphorus removal efficiency was remarkably similar for both systems using pyrite (∼75%), regardless of the presence of electrodes and electrolysis operation, while it was only ∼15% for the control without pyrite. This indicates that pyrite as bed material probably improves removal due to adsorption. The electrolysis contribution on nitrogen removal was more evident, as the EC-PCW achieved the highest TN removal efficiency (68%) compared to the controls (56% for the PCW and 54% for the gravel bed with integrated electrodes, most likely because H2 produced at the cathode complemented pyrite-based denitrification.
Other research focused on removing micropollutants, for example, the model sulfonamide antibiotic sulfamethoxazole (SMX) [59]. Interestingly, this is the first study that investigates the performance of electrolysis-integrated CWs at temperatures below 12 °C. Iron electrodes were implemented in the wetland bed made of zeolite and gravel, and the electrolysis was performed at 10 V. In the electrolysis-integrated tidal flow CW, the SMX removal efficiencies were 22% at 4 °C and 29% at 12 °C, higher than the controls without electrolysis (4% and 7%, respectively). The authors reported that the higher SMX removal under electrolysis was probably a result of combined electrochemical and microbial processes. Oxygen co-generated on the anode surface or hydroxyl radicals produced by the ionization of iron ions could have aided the oxidation of SMX. Electrolysis can trigger antioxidant enzyme activities in the leaves and roots of the planted macrophyte, which is expected to enhance pollutant removal; however, electrolysis may also hinder the photosynthetic ability of the plant, affecting the overall removal of the pollutant [59].
3.4. Challenges and opportunities
CW-MFC systems are relevant to explore the behavior of electroactive communities in a real scenario, favor organics removal, and produce electric energy to some extent. However, they generate particularly low power per surface or volume (≤30 W m−3) [93]. An interesting feature is that the bioelectrochemical current generated by MFCs strongly depends on the level of organics, which provides an opportunity for sensing BOD in real-time [94]. Therefore, implementing the MFC-based sensors can provide a way of monitoring BOD in different wetland zones, including in the discharged effluent. Such a system has been used for assessing COD removal in a pilot CW-MFC treating domestic wastewater [78]. Microbial electrodes have also been proposed to monitor the occurrence of toxicity in real time when the current generated by the microbes decreases upon the release of toxic compounds [95,96]. Implementing affordable sensors that could provide remote monitoring for organics and toxicity could be particularly relevant considering the application in remote locations far away from lab facilities.
METland® systems are already a validated technology for treating urban (e.g., from single housing to around 1000 people) and industrial wastewater (e.g., from food industries or oil and gas sectors). Their vast reduction in the surface requirement by increasing up to 1000 times the organic load of common CWs opens an attractive scenario for decentralized treatments. Like any biofilter-based system, METland® may suffer clogging if not properly operated. To minimize such events, under high organic loading rates, resting periods (i.e., without feeding) or backwash operations are currently being validated under real scenarios.
Although it is not always evident that integrating electrolysis into CWs can boost nitrogen conversions, using sacrificial anodes in CWs demonstrated consistent phosphorus removal via the precipitation of PO43− [52,90,91]. Since the process is abiotic, it is not as severely affected by changes in environmental conditions as biological processes. Therefore, electrolysis can be used to sustain consistent high phosphorus removal, regardless of the climate or both nutrient and organics availability. Certainly, a challenge is the periodic replacement of the sacrificial anodes, which implies an increasing maintenance cost. Another challenge is the possible release of iron as a contaminant in the effluent. For instance, maximum concentrations of 3 mg L−1 Fe2+ and 14 mg L−1 Fe3+ were measured in the effluent of a CW using iron sacrificial anodes [90]. Indicatively, the World Health Organization recommends 0.3 mg L−1 Fe3+ upper limit concentration to ensure drinking water with an acceptable taste and color. The release rate of iron ions, controlled by the current, should be optimized to fit the phosphate loading rate and avoid an excessive release of Fe3+ in the effluent.
One report showed that the electrolysis-integrated system might produce a more alkaline stream (pH 8.2–10) than the one obtained from the sole HSSF-CW (pH 7.1–7.7) [90]. However, other reports presented excess acidification of the CW effluents in electrolysis-integrated systems when high current densities or voltages were applied, leading to excessive H+ production [92] and OH− consumption during phosphorus removal or Fe(OH)3 precipitation [52]. The selection of operational parameters, such as HRT, electrolysis time, and the current or applied voltage, should determine whether the electrolysis-integrated system will present the reported downsides. However, only few studies explored the electrolysis-integrated system with variable configurations and operational settings, in most cases not discussing the reasons behind selecting such parameters. It will be key to assess those impacts rationally and more standardized to optimize the setup performances and assess their applicability.
4. Electrochemical systems coupled to constructed wetlands
4.1. Electrolysis to produce chemical agents
Electrochemical systems (ECs) performing electrolysis allow for in situ generation of chemicals for several applications from the industry to the water treatment sector [63,97,98]. Electrolysis has been routinely used, among other applications, for chlorine and sodium hydroxide production in the chlor-alkali industry, for aluminum production, and H2 production via water splitting [99,100]. In the context of water treatment and reclamation, the in situ generation of oxidizing agents to be used for pathogens inactivation and organics oxidation is an evolving technology that has attracted interest in the last two decades [[101], [102], [103]].
To comprehend basic concepts and relevant parameters in electrochemical systems, such as the applied current and current density, the faradic efficiency, the charge density, and the internal resistances, see Text S1 in the supplementary information. Importantly, in the water treatment framework, the specific energy consumption (SEC) (equation (1)) needs to be quantified, defined as the energy investment required per volume of water treated. The SEC is generally expressed in kWh m−3, which is more practical than the SI unit (J m−3) for engineering purposes.
| (1) |
Where U is the operating voltage (in Volt), i the current (in Ampere), Q is the flow rate of the water being treated across the EC (in m3 s−1), and 3.6 × 106 is a factor converting (J m−3) into (kWh m−3). The ratio of current by flow rate corresponds to the charge density, i.e., the number of electric charges used per volume of water treated, expressed in C m−3 in equation (1) or in Ah m−3. The SEC can also be expressed as the energy needed to produce oxidative chemicals (kWh per kg oxidant) or to degrade pollutants (kWh per kg pollutant). Another figure often used is the electrical energy per order, corresponding to the energy necessary per m3 to decrease a pollutant concentration by one order of magnitude [101,102,104].
4.1.1. A portfolio of chemicals
The electrode material and the geometry, the operating current or voltage, and the nature of the electrolyte influence the type of electrochemical product(s) that can be generated and employed for water treatment. The mixed metal oxide (MMO) and the boron-doped diamond (BDD) electrodes are primarily used as anodes in the electrochemical production of oxidant agents [[105], [106], [107]]. Other electrode materials include Pt, graphite, stainless-steel, MnO2, doped SnO2, doped PbO2, and doped Sb2O5 on titanium or platinum base, as well as sub-stoichiometric Ti4O7, have also been used in the lab- and pilot-scale tests for oxidation processes [102,108,109]. To complete the electrical circuit, cheaper materials such as stainless steel are usually selected as the cathode to reduce water when the main targeted oxidant is produced at the anode (Fig. 6a) [28,30,32,110]. Despite the high capital cost, BDD or MMO electrodes can also be implemented symmetrically, as both anode and cathodes, a configuration allowing to perform polarity reversal (i.e., switching between cathodic and anodic operation). Polarity reversal is frequently used to remove inorganic scaling formed near the cathode because of the alkaline conditions obtained during water reduction [111,112]. Various oxidants can be electrogenerated in this way, including Cl2 [32,[113], [114], [115]], HO• [[116], [117], [118]], SO4•− [119], O3 [120,121], and H2O2 [31,122]. Chlorine (Cl2) is the most reported oxidant in ECs used for disinfection and indirect oxidation of organics [32,36,37,105,123]. Cl2 is produced by the oxidation of chloride ions (Cl−) that are, in most cases, already present in the water to be treated (equation (2)):
| (2) |
Fig. 6.
Various electrochemical systems (EC) configurations are applied for the disinfection and enhanced organic degradation of secondary treated wastewater. a, The EC can be designed to operate in a single compartment (membrane-less system) or two-compartment by implementing a separator such as ion exchange membranes (e.g., cation exchange membrane, CEM). b, Bioelectrochemical systems oxidize biodegradable organics at the anode and can reduce oxygen to hydrogen peroxide at the cathode. c–d, The batch (c) and continuous (d) operation of a two-chamber system.
MMO electrodes present a higher affinity towards Cl2 generation [100,105,117] and, in some reports, they have shown a higher disinfection capacity and a lower production of disinfection by-products than BDD electrodes [105,124,125]. Chlorine generated at the anode is transported via diffusion and convection within the electrolyte, where most reactions for pathogen inactivation [115,126,127] and indirect organic oxidation occur [102]. Chlorine is hydrolyzed, forming hypochlorous acid and hypochlorite, whose relative abundance depends on the pH of the solution (equation (3) and (4)) [128]:
| (3) |
| (4) |
If ammonium is present in the water, it can react with Cl2 to form mono-, di-, and tri-chloramines in subsequent reactions (equation (5)–(7)):
| (5) |
| (6) |
| (7) |
All those reactive chlorine species in solution can provide residual protection when pathogen inactivation is targeted, conversely to disinfection using UV or ozonation, since the disinfection action is stopped after passing the UV-lamp and ozone molecules decompose particularly quickly.
In typical domestic wastewater containing urine and feces [129,130], the chloride ions are sufficiently concentrated (30–650 mg L−1) to generate in situ the reactive chlorine species for disinfection purposes [32,126]. However, some researchers have amended the (waste)water by adding exogenous salts containing Cl− to enhance the electrochemical treatment, as the efficiency of chlorine electrogeneration is dependent on chloride concentration [131,132].
The second most common chemical produced for disinfection and the advanced oxidation process is the hydroxyl radical (HO•) [132,133]. It requires no specific chemical precursor (e.g., Cl− ions) but water. The HO• can be generated as an intermediate during oxygen evolution with MMO electrodes and remains chemisorbed in the oxide lattice of the metal for direct oxidation of pollutants adsorbed at the catalytic site of the anode (equation (8)). BDD anodes operating at higher cell voltages can produce physisorbed HO• at a high oxidation potential (2.8 V vs. SHE), resulting in most cases in higher energy consumption (0.6–12 kWh m−3) than chlorine-producing systems (10−4–10 kWh m−3) [103]. MMO anodes are classified as “active” for their lower overpotential for oxygen evolution, whereas BDD anodes are “non-active” with a higher overpotential for oxygen evolution and weakly HO• physisorbed at the anode allowing for pollutant oxidation [102,108,134]. The highly reactive HO• has been acknowledged to have a disinfection action ten thousand times more effective than chlorine [135]. However, in the presence of Cl− during electrolysis in the EC, the active chlorine species dissolved in the electrolyte are more prominent in the disinfection than the HO• adsorbed at the anode [30,113,133].
| (8) |
Where M refers to the catalytic site of the electrode material, and M() is the radical adsorbed on that site [102,134].
Water oxidation can produce ozone at a BDD anode (equation (9)), which brings synergistic effects for disinfection purposes when produced together with chlorine [121]. In general, the combination of oxidants, such as chlorine and ozone or chlorine and hydrogen peroxide, has also been reported to enhance the oxidation processes of organics [30,121,136].
| (9) |
The generation of sulfate radicals has been recently explored as an electrochemical advanced oxidation process. It can be electrogenerated on BDD anodes with a slightly lower energy requirement than for OH•, providing that the electrolyte contains SO42− [119]. A practical limitation can be a low concentration of the precursor (SO42−) in the water to be treated (equation (10)).
| (10) |
Hydrogen peroxide (H2O2) is a common oxidant and disinfectant. H2O2 can be obtained by combining two hydroxyl radicals (HO•) produced as intermediates during the oxidation process on BDD anodes [134]. In contrast to the previously listed compounds that are formed only at the anode, H2O2 can also be cathodically produced via oxygen reduction on carbon-based cathodes (equation (11)) [31,122,137,138] and subsequently used for treating wastewaters [31,139,140]:
| (11) |
For systems electrogenerating H2O2, a catalyzer (e.g., ferrous ions or scrap iron) can be introduced to obtain HO• free in the electrolyte (equation (12)) by performing the so-called electro-Fenton process [108,141].
| (12) |
This process has caught increasing attention for disinfection and various pollutants removal (COD, NH3, total phosphorus, and micropollutants) as the HO• is in the electrolyte [[142], [143], [144]].
4.1.2. (Bio-)electrochemical cell configurations for water treatment
Electrochemical reactors can be built as a single-chamber system (membrane-less), or, in some cases, the cathodic and anodic compartments can be separated by one or several ion exchange membranes (Fig. 6a) [63,108]. Cation-exchange-membranes (CEM) or anion-exchange-membranes (AEM) are employed to avoid the redox cycling of anodically or cathodically generated products or to allow for selective migration of ions to enhance oxidant production and resource recovery (Fig. 6a) [29,32,108,145]. For instance, an AEM-divided EC allows the migration of Cl− from catholyte to anolyte, concentrating Cl− close to the anode and therefore enhancing Cl2 production [32]. Conversely, a CEM-divided EC allows possible NH4+ recovery through electromigration of those cations from the anodic to the cathodic compartment [29,145].
In two-chamber systems, different electrolytes can be used in the two compartments, which increases the number of options and versatility of the EC. For instance, microbial electrochemical systems can be designed to oxidize biodegradable organics by electroactive bacteria attached to an anode (Fig. 6b) [[146], [147], [148]]. The latter system typically uses affordable carbon-based anodes and can save energy, as the low potential electrons harvested from the microbial anode can be used for H2O2 production at the cathode at low electrolysis voltage or even under short circuits. Concentrations sufficient for in situ disinfection (1 g H2O2 L−1) have been reported without substantial impairment of the electroactive biofilm in the adjacent chamber [147,148]. Some microbial electrochemical systems producing H2O2 have also been applied for electro-Fenton strategies, referred to as bio-electro-Fenton processes [149,150]. The usual drawback of such a system is the low current density that can generate those microbial anodes, inducing a low removal rate of organics and a low production rate of H2O2.
EC can work as separated chemical production units with subsequent dosing of oxidants to the water treatment line or operate as an in-line electrochemical reactor as part of the water pipeline [128,151]. The former strategy has been proven useful for high throughput drinking water production in centralized plants [128]. Conversely, most reports using ECs in decentralized water systems operate as in-line configurations in batch or continuous operation (Fig. 6c and d). Key performance factors are disinfection efficiency or pollutant removal, HRT, SEC, and stability. Most electrochemical production of oxidants for water treatment reported in the literature operates in batch mode [28,126,133,149,152,153], while a continuous-flow system is desirable [32,154] to couple the EC to technologies already running in continuous modes, such as CWs. The pattern of the water flux across continuous-flow EC is also a crucial operational parameter that can impact pollutant removal and the system's stability [29,32,108,145]. Water can only cross the anodic compartment or flow sequentially through the anodic and then the cathodic compartment (Fig. 6d). On large-scale systems, the mass transfer of the electrogenerated oxidants from the electrode to the bulk of the flowing water can require further design considerations. Mixing can be improved with turbulence promoters, optimization of the geometrical aspect of the electrode and compartments, and properly positioning the water inlet and outlet points in flow-by EC [155]. Another increasingly studied reactor design is the flow-through mode, where the water circulates through porous electrodes, which mitigates mass transfer limitations [101,104]. Considering the importance of hydraulic behavior, especially for disinfection purposes, simulations based on computational fluid dynamics (CFD) can help optimize water treatment designs [155,156]. More extensive reviews related to the different configurations of ECs for water treatment can be found elsewhere [101,112].
4.2. Electrochemical disinfection of constructed wetlands effluents
The strategy consists of coupling the two different technologies (CW + EC) in subsequent steps of a treatment train (Fig. 7). This coupling of CW + EC provides a synergistic effect since: (i) downstream EC provides supplementary disinfection and pollutants removal to reach regulations, which in turn decreases the land footprint of the CW [9,28], and (ii) CW-pretreatment substantially removes organics, solids, and ammonium, which otherwise would consume a larger fraction of the electrogenerated oxidants and accelerate fouling in an EC [29,37,114,157]. Pre-treating wastewater in CW, therefore, decreases both energy consumption and maintenance requirements for an EC [29,32,136]. For instance, Talekar and Mutnuri (2020) reported that the SEC of the electrochemical system treating septage decreased from 48 to 21 kWh m−3 when the septage was first treated in a CW [29].
Fig. 7.
Electrochemical systems produce oxidants to polish constructed wetland effluents. Naturally present chloride is typically oxidized to chlorine on an anode. Oxygen can be reduced to hydrogen peroxide on carbon-based cathodes. Reprinted and adapted according to Copyright 2021 Royal Society of Chemistry [32].
Pathogen levels in CW effluents usually exceed discharge or water reuse limits [9,16]. Contamination from pathogens is usually assessed by fecal indicators such as total coliforms (TC), fecal coliforms, and Escherichia coli concentrations [158,159]. TC is a more conservative measurement that measures the presence of E. coli and other lactose-fermenter bacteria. For reuse purposes, unrestricted non-potable reuse guidelines focus the attention on a high removal of E. coli (e.g., >5 or 6 LRV) or a low concentration of that indicator on the water to be reclaimed (e.g., <1 or 10 CFU per 100 mL) [17,160,161]. Legislation for water reclamation in irrigation can be less strict when the reclaimed water is not in direct contact with the edible product, e.g., >3 LRV of TC or concentrations of E. coli <100–1000 CFU per 100 mL [159,162]. Electrochemical disinfection has been proven to highly decrease the level of pathogens to reach water discharge and reuse standards by the action of the electrogenerated oxidants that disrupt microbial cell integrity (i.e., oxidative stress in cell walls and membranes) and provoke cell lysis [116,163].
The continuous electrochemical disinfection of CW effluents (CW + EC) was first carried out in India in 2017 by using full-scale HSSF-CWs and a commercial EC (SuMeWa-Autarcon, Germany) with MMO anodes [27]. The coupling allowed overall disinfection (4.9–6.9 LRV of TC) via chlorine generation, and the effluent was reused in agricultural activities. However, limited data were provided about the electrochemical configuration and the real operation or maintenance of the coupling, which makes it difficult to draw more insights from this work. Another study showed that BOD5 could be removed to acceptable levels for water reuse (≤10 mg L−1) by an HSSF-CW [26]. However, pathogens removal (TC and E. coli) was limited to a maximum of 0.5 LRV [26]. Implementing an EC chlorine after the HSSF-CW produced water without detectable pathogens [26]. Similarly, Talekar et al. (2018) reported that most COD (80%), TKN (44%), TAN (84%), and orto-phosphate (93%) were removed by a VSSF-CW, but its disinfection was limited to only 1–2 LRVs of TC. Implementing a downstream chlorine-generating EC completely inactivated the pathogens below the detection limit, achieving an overall LRV of TC higher than 5 [28].
Bakheet et al. (2020) coupled green walls with EC to treat greywater. While green walls alone only removed 1 log of TC and E. coli, the CW + EC coupling accomplished a decrease of E. coli from 4 logs (before the green wall) to <0.3 logs, thanks mostly to the EC (3.5 LRV) generating chlorine, ozone, and OH• on a BDD anode. The generated effluent fitted several reuse guidelines [30,164]. It also exhibited 21% lower turbidity and 29% lower color than when treated only by the green walls. The electrochemical coupling has been shown to improve other water quality parameters, for example, by decreasing TSS and hardness [27].
Mosquera-Romero et al. (2022) investigated the proper chlorine production in a continuous EC for CW effluent disinfection. To do so, they studied the impact of current and water flux across the EC with either AEM or CEM separating the two compartments of the EC. There, it was demonstrated that a current density of 50 A m−2 was sufficient to disinfect CW effluents to typical regulations levels at a high flux (HRT: 15–30 s) and low SEC (∼0.1 kWh m−3) regardless of the type of membrane separating the chambers.
Implementing EC inactivates not only the regulated pathogens, such as TC and E. coli, but also other microorganisms of concern, such as Enterococcus, Salmonella, and Clostridium [26]. Similarly, efficient removal of E. coli (up to 5 LRV), Klebsiella pneumoniae (up to 7 LRV), and Staphylococcus aureus (up to 8.8 LRV) were reported at high charge densities ranging 620–1105 Ah m−3 [28]. The downstream EC substantially decreased the viability of helminth eggs because of chlorine generation and acidification, further illustrating the ability of such a design to perform efficient disinfection [28]. Another recent study showed the electrochemical inactivation of bacteria (E. coli, Legionella pneumophila, and Campylobacter jejuni), bacterial spores (C. perfringens and Bacillus cereus), bacteriophages (PhiX174 and MS2), protozoa (Acanthamoeba castellanii), and viruses (Coxsackie and Tulane) spiked in CW effluents, aiming for demonstrating safe water reclamation for irrigation purposes [165]. Fore et al. (2023) reported that the inactivation kinetics notably varied per each pathogen type, and hence, suggested evaluating the inactivation profiles of bacteria, virus, and protozoa that are more oxidant resistant to select the correct charge density (42−1000 Ah m−3) required to assure safe water reclamation [165].
At least one organic matter parameter (COD, BOD5, or TOC) and one pathogen indicator (TC, fecal coliforms, or E. coli) are generally reported in studies of CW + EC couplings for disinfection purposes (Table 1 and S2). Other parameters such as TN, TSS, and turbidity are not always reported (Table S2), while they are crucial to evaluate if the treated water meets water reuse standards. The water quality should be assessed at each stage of the treatment train to properly evaluate the respective contributions of each step [9]. For instance, one study reported that a CW + EC coupling efficiently removed pathogens and organics, whereas nitrogen concentrations remained above the permissible limits for discharge [28]. After analyzing the system separately, the VSSF-CW only removed 23% of the nitrogen content, while the EC did not contribute significantly (0–1%). This showed that CWs operation had to be optimized for nitrogen removal, as usually up to 50% of TN can be removed in common VSSF-CW [38], while strategies like intermittent aeration in CWs can enhance TN removal up to ∼80% [166] (Section 2).
Table 1.
Reported parameters and performances for couplings of electrochemical systems (EC) with constructed wetlands (CW) or green walls (GW) focusing on inactivating pathogens for water reuse. Values for organics and pathogens concentrations and removals only correspond to the EC stage.
| Water matrix | System (scale) | Flow rate (m3 d−1) (HRT) | Current density(A m−2) | Disinfectant agents (residual concentration in the EC effluent)(mg L−1) | Conductivity(mS cm−1) | Organic removal COD, BOD5 (initial and final)(mg L−1) | LRVTotal coliforms (TC) or E. coli(initial and final concentrations, log10(CFU per 100 mL)) | SECa (kWh m−3) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Domestic sewage | UASB + VSSF-CW + HSSF-CW + membrane-less EC(pilot) | 10 | NR, commercial EC:SuMeWa-Autarcon | Chlorine(NR) | 1.3 | 37.8% BOD5(from 4.5 to 2.8 mg L−1) | 2 TC(3.8to 1.8) | NR | [27] |
| Domestic sewage | HSSF-CW + zeolite + membrane-less EC(pilot) | 10 | NR, commercial EC:SuMeWa-Autarcon | Chlorine(0.5 ± 0.3 mg Cl2 L−1) | 1.3 | 37.8% BOD5 (from 4.5 to 2.8 mg L−1) | >6.5 TC(6.5 to BDL) | NR | [27] |
| Domestic sewage primarily or secondarily treated | HSSF-CW + zeolite + membrane-less EC(pilot) | 10 | NR, commercial EC:SuMeWa-Autarcon | Chlorine(0–1.1 mg Cl2 L−1) | 1.3 | 44% BOD5 (from 18 to 10 mg L−1) | >3.2 TC(4 to BDL) | NR | [26] |
| >2.2 E. coli (2.2 to BDL) | |||||||||
| Blackwater | VSSF-CW + CEM divided EC(household scale) | 0.18 | 103 | Chlorine(NR) | 1.2 | 28% COD (from 190 to 136 mg L−1) | >5.0 TC(5.0 to BDL) | 16 | [28] |
| Blackwater | VSSF-CW + CEM divided EC(community scale) | 1.3 | 116 | Chlorine(0.9 ± 0.1 mg Cl2 L−1) | 2 | 51% COD(from 269 to 133 mg L−1) | 2.3 TC(3.0 to 0.7) | 16 | [28] |
| Blackwater | VSSF-CW AEM divided EC(bench-scale) | 0.007 (22 min) | 62.5 | Chlorine(2–3.7 mg Cl2 L−1) | NR | 74% COD(from 104 to 27 mg L−1) | >3.5 TC(3.5 to BDL) | 21 | [29] |
| Synthetic greywater | GW + CEM divided EC(bench-scale) | 2 L Batch (10 min) | 250 | Chlorine, HO•, O3(0.3 mg Cl2 L−1) | 0.1 (solid electrolyte) | 0% COD(initial 14 mg L−1) | 3.5 TC(3.5 to BDL) | 0.6 | [30] |
| Domestic wastewater | CW + CEM or AEM divided EC(bench-scale) | 1.2(15 s) | 50 | Chlorine(0.5 mg Cl2 L−1) | 4 | 0% COD(initial 6.5 mg L−1) | >5.4 TC(5.4 to BDL) | 0.1 | [32] |
| Domestic sewage | Lagoons + SF-CW + membrane-less EC (bench scale) | 0.25 L Batch (30 min) | 150 | Chlorine(1.1 mg Cl2 L−1) | 1 | NR | 6 E. coli(NR) | 0.1–1 | [165] |
BDL: below detection limits; HSSF-CW: horizontal subsurface flow constructed wetland; NR: not reported; UASB: upflow anaerobic sludge blanket; VSSF-CW: vertical subsurface flow constructed wetland.
The specific energy consumption only refers to the electrochemical system (EC) implemented.
Talekar et al. (2018) electrochemically treated CW effluent with a medium concentration of organics (269 mg COD L−1) aiming for various contaminants removal, including pathogens, by implementing an average charge density of 1017 Ah m−3. That energy invested was still more than ten times higher compared with other works (<83 Ah m−3) focusing mostly on disinfection [32] or treating CW effluents at lower organic loads (13.6 mg COD L−1) [30]. The charge density directly influences energy consumption; thus, the system of Talekar et al. (2018) was particularly energy intensive (SEC of 16–27 kWh m−3) when compared with other studies (0.05–0.2 kWh m−3) disinfecting various secondarily-treated wastewater [113,133]. There is a substantial variation in the energy consumed per case study, which can be due to the variations in the hydraulic and organic load, the configuration of both CW and EC, the implementation scale, and the quality of the wastewater [9,153]. As the CWs energy consumption is minimal or null [41] compared to the energy input from electrolysis, reports generally only report the latter. The SEC of studies using EC to disinfect CW effluents are reported in Table 1, and it highly varies from 0.1 to 21 kWh m−3.
Implementing an EC after the CW allowed substantial pathogen inactivation in all studies. CW treatment alone removed pathogens with LRV ranging 1–2. The additional LRV induced by the EC was consistently above 2. Some systems produced water following the stricter requirements for water reuse [30,32]. Moreover, most water reuse standards suggest a certain residual chlorine level (e.g., 0.5–1.0 mg L−1) in the treated water to avoid recontamination [158,164]. In that scenario, EC represents the ideal alternative to achieve the desired residual chlorine concentration in the water without introducing exogenous chemical reagents.
4.3. Electro-oxidation to remove organics and emerging contaminants
The persistence of recalcitrant organic compounds and other contaminants, including microbial contamination, in the CW effluents raises environmental concerns [9,28,167]. The coupling of CW + EC has been proposed to alleviate this issue, as the oxidants generated for disinfection (Cl2 and HO•) are also effective in oxidizing organics and nitrogen compounds. In studies specifically designed to remove organics from agro-industrial effluents, the SEC are 1–2 orders of magnitude higher (1–500 kWh m−3) than for systems focusing solely on disinfection (0.05–27 kWh m−3) [30,32]. The main reason is that more charge density is needed to remove the typical level of organics from CW effluents than for generating sufficient disinfecting chemicals. This can also be partially attributed to the 2–10 times higher organic load of the agro-industrial effluents compared to the domestic ones and to the recalcitrant nature of the organic molecules in those specific wastewaters. For instance, researchers focusing on the disinfection of CW effluents also noticed a decrease in COD, TN, color, and turbidity when treating the CW effluents electrochemically [28,30]. However, the relatively low charge densities applied in EC for disinfection, lower than 100 Ah m−3 [30,32,165] are generally not sufficient for the complete removal of organics or nitrogen compounds [28,30]. Studies on electrochemical polishing of agro-industrial effluents that were treated first in CWs, focused mainly on removing low to medium recalcitrant compounds [[167], [168], [169]], nitrogen compounds [170], total suspended solids (TSS), and color [168]. In those studies, high charge densities, usually above a few hundred Ah m−3 (Table 2), mostly in batch operation, were required (Table 2) to comply with discharge permit limits or reuse requirements. Additionally, the coupling of CW and EC was investigated, as in which process should be upstream or downstream, meaning whether EC + CW coupling or EC + CW coupling would be overall more efficient.
Table 2.
Reported parameters and performances for electrochemical systems (EC) couplings with constructed wetlands (CW) targeting the removal of organic compounds, nitrogen compounds, solids, and color. Concentrations reported correspond to the EC influent, and the removal corresponds to the EC stage.
| Water matrix | System | Anode material(size) | Initial COD concentration (mg COD L−1) | Initial nitrogen concentration (mg TN L−1) | % COD removal | % TN removal | Charge density (Ah m−3) | SEC (kWh m−3) | SECCOD (kWh per kg COD) | SECTN (kW per kg TN) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CW + EC |
Table olive washing wastewater | Trickling filter + HSSF-CW + membrane-less EC (pilot) | BDD(16 cm2) | 5000–15 000 | NR | 72 | N/A | 3750 | 105 | 322 | N/A | [167] |
| Olive pomace leachate | VSSF-CW + membrane-less | BDD(70 cm2) | 9740 | 25 | 61 | 9 | 12000 | 462 | 943 | 210000b | [168] | |
| EC, DiaCell®(pilot) | ||||||||||||
| Cooling tower blowdown | VSSF-CW + membrane-less EC | BDD(22 cm2) | 107 | NR | 95 | N/A | 973 | 6 | 106a | N/A | [169] | |
| Cooling tower blowdown |
VSSF-CW + membrane-less EC |
Ru MMO(22 cm2) |
107 |
NR |
55 |
N/A |
1556 |
11 |
347a |
N/A |
[169] |
|
| EC + CW | Olive pomace leachate | VSSF-CW + membrane-less EC | BDD | 9740 | 35 | 39 | 33 | 12000 | N/A | N/A | N/A | [168] |
| EC, DiaCell® (pilot) | ||||||||||||
| Cooling tower blowdown | VSSF-CW + membrane-less EC(bench-scale) | BDD(22 cm2) | 107 | NR | 81 | N/A | 973 | 6 | 66a | N/A | [169] | |
| Cooling tower blowdown | VSSF-CW + membrane-less EC(bench-scale) | Ru MMO(22 cm2) | 107 | NR | 47 | N/A | 1503 | 10 | 208a | N/A | [169] | |
| Polluted surface waterc | HSSF-CW + membrane-less EC, commercial at 6 V(pilot) | TiO2/RuO2/IrO2 (3600 cm2) | 61 | 34 | 36 | 38 | 140 | 1 | 38 | 57 | [170] |
NR: not reported; N/A: not applicable.
Electrolyte volume (380 mL) and cell voltage (5.88 V for the BDD electrode and 6.95 for the MMO electrode) were provided through personal communication with the authors. Based on that, SEC was calculated. In the original paper, 19 and 64 kWh per kg COD are reported for the CW + EC treatment with BDD and MMO anodes, respectively, and 23 and 79 kWh per kg COD for the EC + CW treatment with BDD and MMO anodes, respectively.
Calculated based on the charge density and the TN removal in the electrooxidation unit treating CW effluent.
The only study where the cell was potentiostatically controlled.
The CW + EC treatment has been tested so far with agro-industrial effluents from the olive oil processing industry [167,168] or industrial cooling tower effluents. Both studies that investigated the CW + EC treatment of wastewater from the olive mill and olive-oil processing industry achieved 95% COD removal, 94–100% color removal [167,168], and complete phenols removal [167]. The CW + EC treatment displayed consistently higher removal efficiencies than the control biological treatment (trickling filter + HSSF-CW [167] or VSS-CW [168]), owing to the contribution of EC using a BDD electrode and charge densities that ranged 4–12 kAh m−3. In the biological treatment, the pollutant removal rates were lower, with substantial fluctuations in the removal efficiencies (e.g., 50–86% of COD removal, 0–77% decolorization, and 60–78% of phenols removal) (Table 2). When the CW + EC configuration was used to treat cooling tower water with a BDD electrode, the coupled system removed a maximum of 97% of COD, and the EC unit contributed to 52% of the total COD removed, as it oxidized 95% of the COD of the CW effluent [169].
Surface water and agro-industrial effluents have also been treated by placing the EC before the CW treatment (EC + CW) (Table 2). The EC + CW strategy was applied in studies to oxidize recalcitrant organics and thus increase the wastewater biodegradability to allow for subsequent, uninhibited CW treatment [[168], [169], [170]]. This coupling strategy was used in the surface water treatment aiming to enhance nitrogen removal in the first treatment stage through indirect electrochemical ammonium oxidation [170]. The coupled system achieved a 70% removal efficiency for both COD and TN, and the upstream EC was responsible for 52% of the COD and 54% of the TN removed. The SEC of the system was 1 kWh m−3, 38 kWh per kg COD, and 57 kWh per kg TN, which were considered acceptable when compared with other systems using EC with BDD electrodes [170].
Only two reports treating agro-industrial effluents assessed the impact of positioning the electrochemical treatment before the CW (EC + CW). By placing the EC before the CW to remove recalcitrant organics, the authors initially expected to increase the biodegradability of the residual organics in the downstream CW treatment [168,169]. Conversely to expectation, the electrochemical treatment increased the toxicity of the wastewater in both studies. One of the studies attributed this phenomenon to the production of chlorinated organic and inorganic compounds, ClO3− and ClO4− [169], which can inhibit microbial activity and increase phytotoxicity [168,169]. As a result, the CW + EC system removed more organics than the EC + CW system for treating either olive pomace leachate effluents (COD removal of 95% vs. 81%) or cooling water effluents (97% vs. 76% with a BDD electrode) [168,169]. Additionally, the authors of both studies stated that a lower organic content in the effluent of the CW improved the COD removal efficiency of the EC, as in these cases, the COD decreased exponentially. Indeed, the CW + EC system treating olive pomace leachate removed 61% of the receiving COD, while the EC in the EC + CW system removed only 39% of the receiving COD, and a similar performance was observed in the study treating cooling tower water, either with a BDD or an MMO electrode (Table 2).
4.4. Challenges and opportunities
4.4.1. Oxidant demand and the generation of by-products
Moving from established applications of electrochemistry with solids-free, highly conductive, and well-defined electrolytes to applications where wastewater acts as the electrolyte challenges the efficiency and sustainability of the electrochemical production of oxidants. The presence of reduced components such as ammonium or certain organics can hinder pathways for oxidant production at the anode or consume electrogenerated oxidants in the electrolyte. The cellular material released during cell lysis of the inactivated microorganisms can also consume some of the oxidants [30,121].
The type and required dose of the oxidizing agent can differ depending on the specific treatment goal [134,171,172]. While specified chlorine doses (1.8 mg min L−1) and ozone doses (0.03 mg min L−1) are required to reach the 3 LRV of TC inactivation [128,173], the HO• radicals dosing has not yet been established by any guidelines. The review of Hand and Cusick (2021) reported three times lower contact times (in mg min L−1) to disinfect centralized wastewater with HO• than with Cl2, illustrating the greater inactivation potential of HO• [103,135]. However, the electrogenerated OH• needs to be quantified correctly, as indirect analytical detection can lead to underestimation, and furthermore, the disinfection performance should be thoroughly investigated in the presence of other oxidants (e.g., active chlorine species) in the electrolyte [134,174].
Chlorine is also preferred due to the longer lifetime in solution and due to the residual disinfection effect. Conversely, OH• radicals have a very short lifetime (<1 μs) and cannot provide residual protection against pathogens’ regrowth [124]. In the context of water reuse, preserving a residual Cl2 concentration of 0.5–1 mg L−1 is recommended [158,164]. Remaining NH4+ in the CW effluent is not expected to negatively impact the residual disinfection, as combined Cl2 (e.g., monochloramines and dichloramines) can be formed (equation (7)–(10)) that exerts disinfection for more extended periods. However, that will depend on the respective concentrations of NH4+ and Cl2 in the disinfected effluent.
When electrochemical chlorination is applied in wastewater with high organic content, toxic, organochlorinated compounds can be formed, such as trihalomethanes and haloacetic acids. Furthermore, toxic inorganic compounds, such as ClO3−, ClO4−, and BrO3−, have also been reported during electro-oxidation with BDD and MMO electrodes [136]. Different amounts of disinfection by-products were reported to be produced by BDD and MMO electrodes under similar conditions of electrolyte composition and applied current density [105,124,125,165]. It is important to limit the electrolysis extent to the necessary charge density required for a specific target (e.g., disinfection or threshold COD level) since it optimizes the energy consumption and restricts the formation of by-products as their concentration tends to rise with increasing charge densities [125].
4.4.2. Tuning of operational parameters to target specific water quality
The EC is a versatile, highly controllable, and modular operational unit that can be coupled to CWs for effluent polishing in decentralized settings. However, the operational parameters (flow rate or HRT and applied current or charge density) should be carefully tuned to ensure producing water quality for specific purposes, for example, discharge or reuse. The electrolysis time in a batch mode at a relatively low charge density (83 Ah m−3) can be selected to a minimum (e.g., 20 min) to accomplish the desired treatment when treated only in the anodic compartment at a quasi-neutral pH (pH 6.6), as higher residence time can lead to developing acidic water (pH < 5.6) [30]. In another case, with a two-compartment cell operating in batch operation, an alkaline solution was obtained in the cathodic compartment (pH > 9) and an acidic one in the anodic compartment (pH < 3) by applying a charge density of 800 Ah m−3 while degrading pollutants and inactivating total coliforms and helminths ova [28]. As the circumneutral pH is required for either discharge or reuse purposes, running the system in continuous mode with the wastewater flowing from the anodic to the cathodic compartment (Fig. 6d) has been proposed for effluent neutralization [28]. Moreover, a large proportion of studies focusing on disinfection fail to report the impact of EC treatment on the whole spectrum of water quality indicators. Thus, reporting all indicators listed in the safe water reclamation guidelines is suggested.
4.4.3. Energy consumption
For high-organics content on CW effluent (100–10000 mg L−1), organic mineralization via electrolysis of CW effluents requires 10–100 times higher charge densities than disinfection [167,168]. Therefore, it is important to optimize the upstream CW operation to remove most of the organics originally contained in wastewater at a low cost, as their removal in the downstream electrochemical process is much costlier. CWs can be optimized for biodegradable contaminant removal by increasing HRT or applying intensification strategies, such as aeration or bed material modification (see Section 2).
The smallest SEC during the electrochemical oxidation of recalcitrant (6–11 kWh m−3) are of a similar or lower order of magnitude than other more mature advanced oxidation processes, such as ozonation, UV/H2O2, or wet oxidation [102,104,108]. However, those values are still higher than the lowest SECs required (0.1–0.6 kWh m−3) when disinfection was targeted via the electrochemical treatment of CW effluents [32,107]. It is not surprising that higher SEC is considered acceptable depending on the higher quality of water obtained per application. For example, reverse osmosis membranes for water desalination for drinking water can exhibit energy consumption of up to 10 kWh m−3 [175], compared to the suggested 0.5 kWh m−3 for wastewater disinfection [176].
The conductivity of the wastewater to treat widely varies, from ∼0.5 mS cm−1 (i.e., freshwater level) to ∼50 mS cm−1 in ballast wastewater. Especially for low conductivities, the ohmic drop can become the main contributor to the voltage of the EC. Since the ohmic drop is proportional to the current, it has been proposed that current densities should be kept below 50 A m−2 to maintain a sufficiently low SEC [32,113]. This limit in current, it turns, restrains the water flux that can be treated per volume of the electrochemical cell. Therefore, it is key to minimize the internal resistance of such systems. In some studies, wastewater was amended by dissolving salts to reduce energy consumption [177,178], yet their continuous supply is not a sustainable solution. The internal resistance of the electrochemical cell can also be diminished by minimizing the distance between the anode and cathode. However, shortening that distance can impede convection and increase clogging by naturally present particles or inorganic precipitates generated by the higher pH gradient. A suggested alternative is to use solid polymer electrolytes [30,179]. In such a system, two electrodes are pressed against a membrane made of polymer electrolyte that increases conductivity and further decreases the interelectrode distance.
4.4.4. Stability of the electrochemical cell
Long-term operation at maximum removal rates has been proven arduous for coupled or integrated CW systems. A study reported six months of operation for a CW + EC [27], the longest to date to the extent of our knowledge. Yet, truly little information on that system was provided (e.g., on the operational mode, the charge applied, the water flux or materials used), and it is, therefore, challenging to assess its operational time (duty cycle) or if any maintenance was applied. Another study reported that EC systems treating CW effluent for two months required mechanical cleaning of the electrode membrane assembly every nine days, as the voltage increased to a point where the power source could not maintain the current applied [28].
Fouling is commonly reported in EC treating wastewater. Fouling can occur superficially on electrodes and membranes or by the accumulation and settlement of solids in the compartment(s) of the EC, often owing to the alkaline conditions developed when OH− is produced during water reduction at the cathode [111,112]. Carbonates- and hydroxide-based precipitates will typically accumulate on the cathode and between the cathode and the membrane [111,112]. Thus, hard water with substantial concentrations of divalent cations, mainly Ca2+ and Mg2+, and alkalinity formed by carbonates and phosphates will induce prominent precipitations in EC [28,180]. Fouling increases internal resistance in the system, decreases the available electrode surface area, and impedes a good flow pattern in the cathodic compartment. Consequently, weekly cleaning was typically required in previous studies [28,180]. Another strategy is the periodic reversal of electrode polarity, though it requires costly electrodes that can function as both cathodes and anodes without substantial degradation [108,110,111]. Alternatively, a separate precipitation unit at alkaline conditions (pH 10.5–12.0) can be implemented to remove fouling agents upstream of the EC. The latter will also demand additional energy input to neutralize the alkaline stream in the anodic compartment via oxygen evolution and generate reactive chlorine species in the effluent [108,180].
Moreover, the reported literature for EC treatment is primarily based on batch experiments [28,126,133,149,152,153], therefore reporting higher investment per small amount of water to be reclaimed. An analysis of reactor design, hydrodynamics, applied current or potential, and treatment effectiveness shall be performed [104,181]. Thus, the prioritization of research focusing on the continuous treatment of real wastewater at higher fluxes should be further explored to clearly define the limit capacity of EC.
4.4.5. High capital cost
The capital cost for electrochemical cells remains high compared to the typical flux of wastewater they can treat. The most expensive components are the electrodes and the membranes. This can limit the widespread implementation of the technology, especially in low-income countries [104,182]. Commercial MMO and BDD electrodes cost around €1k–10k m−2 [104,107]. It has been stated that using BDD electrodes for wastewater treatment is still beyond economic interest until material costs are substantially lowered [153]. Research also focuses on developing much cheaper electrode materials. For example, electrodes based on Magnéli phase titanium oxides (TinO2n−1) could be produced at much lower costs and have been used to remove various pollutants at the lab scale, yet they exhibited a lower affinity for chlorine evolution than MMO anodes [104,183]. To solve that trade-off, material scientists are called to develop affordable electrodes that can promote oxidant generation and be cathodically and anodically stable. When membrane-divided cells are implemented, developing cost-competitive ion-exchange membranes resistant to oxidants, highly conductive, and with anti-fouling properties is also encouraged [32,184].
5. A niche in low-income countries: challenge and opportunity
In less developed and landlocked developing countries, the financial sustainability of water treatment technologies is of utmost importance [185]. Affordable and low-maintenance decentralized technologies can provide a cost-efficient alternative to the expensive distribution infrastructure needed in centralized approaches. The electrochemical generation of chemicals, in situ and on-demand, can render a water treatment process train location independent, which is particularly relevant for landlocked countries that depend on their neighboring transit countries for most commodities. The countries listed as developing, least developed, and most of the landlocked developing countries are usually located in zones receiving high solar radiation with relatively low seasonal fluctuations, which can further aid energy independence by ideally powering the system with photovoltaic cells. Encouraging an off-the-electricity-grid system when powered by the EC with renewable energy is highly attractive in resource-limited areas [26,27,186]. Furthermore, warm climate countries are ideal for enhancing microbial and plant activity in CWs and further aiding in pollutant removal.
Even though land availability is not a significant concern in rural and peri-urban areas as compared to the situation in densely-populated areas, the potential of decreasing the surface footprint by implementing an ECin-CW is still advantageous for simplicity in controlling one reactor unit. Moreover, sensors installed in the CW bed can allow for online monitoring and data transfer to a central platform to encourage regional management of decentralized systems, which allows municipalities to avoid often reported problems of abandoned decentralized systems in remote locations [26,187].
While coupling CW and EC technologies appears to be an opportunity for regions that endure limited water treatment capacity, several bottlenecks still need to be overcome to enable widespread application. Especially for CW + EC strategies, the first target should be lowering the capital costs, increasing the water fluxes per reactor volume, and maximizing the robustness of the setup as well as its maintainability by low-to middle-skilled personnel. More pilot projects are encouraged to be implemented and studied in the aforementioned types of countries with a proper technological transfer from experts belonging to institutions where the system was developed, as reported in India [27,28] and Ecuador [32]. User feedback will be highly valuable in assessing the performance and the acceptability of such designs. Finally, life-cycle assessment studies should be carefully performed to assess the competitiveness of such systems against other commonly applied technologies, such as granular filters and solar water disinfection [104,153,188].
6. Conclusions
Associating electrochemical treatment with CW offers opportunities for decentralized water treatment. It can improve water quality up to meeting the required criteria for discharge or reuse applications (e.g., irrigation, non-potable household use, industrial use). Using beds made of conductive particles in CWs induces the formation of a bioelectrochemical system operating in short circuit. The ECin-CW systems highly increase the surface-specific rate of organics degradation. Because of their good operational performances, they are already being successfully implemented to treat domestic and agro-industrial effluents. Conversely, the CW + EC couplings that generate oxidants by electrolysis are at an early investigation stage and target different goals. They have been shown to disinfect CW effluents efficiently at relevantly low energy consumption. Conversely, while they can also degrade pollutants, their high energy consumption makes them less attractive for that specific purpose. The performance of electrolysis-based systems is tunable by regulating the current input, which controls the rates at which chemicals are generated for disinfection or pollutant degradation. As such, they provide a more reliable and consistent treatment performance to overcome possible seasonal variations that commonly affect biological systems, including CWs. Before the strategies discussed can be rationally applied, important challenges should be tackled. Among them are the high capital cost of the electrochemical cells, especially of the electrodes, the relatively low flux of water treated per volume of the EC, and the fouling in ECs. More studies evolving from the lab- or pilot-scale systems to full-scale systems are required to overcome the challenges involved in the scale-up and, ultimately, to better assess the perspectives for widespread applications.
CRediT authorship contribution statement
SuannyMosquera-Romero: Conceptualization, Data curation, Writing - Original draft preparation. Eleftheria Ntagia: Conceptualization, Data curation, Writing - Original draft preparation. Diederik Rousseau: Supervision, Reviewing and Editing. Abraham Esteve-Núñez: Writing - Original draft preparation. Antonin Prévoteau: Conceptualization, Supervision, Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Tim Lacoere and Sam Eggeling are kindly acknowledged for help with the artwork. Marlee Wasserman is kindly acknowledged for the English editing of the manuscript. This work was supported by the Fund Ernest Solvay, managed by the King Baudouin Foundation, under grant 2020-B7120700-214824. SM is supported by the Bijzonder Onderzoeksfonds (BOF18/DOS/035) scholarship and ESPOL. AP is supported by a Ghent University Bijzonder Onderzoeksfonds GOA grant (BOF19/GOA/026).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100265.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.FAO . 2007. Coping with Water Scarcity, Challenge of the Twenty-First Century. [Google Scholar]
- 2.JMP . WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation; 2015. 2015 Annual Report. [Google Scholar]
- 3.Saquib S., Gupta A., Joshi A. first ed. Elsevier Inc.; 2022. Emerging Water Crisis: Impact of Urbanization on Water Resources and Constructed Wetlands as a Nature-Based Solution (NbS) [DOI] [Google Scholar]
- 4.Yang S.-Q. 2022. World Water Crisis and Possible Solutions. [DOI] [Google Scholar]
- 5.Siegrist R.L. 2017. Decentralized Water Reclamation Engineering. [DOI] [Google Scholar]
- 6.Libralato G., Volpi Ghirardini A., Avezzù F. To centralise or to decentralise: an overview of the most recent trends in wastewater treatment management. J. Environ. Manag. 2012;94:61–68. doi: 10.1016/j.jenvman.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Capodaglio A.G., Bolognesi S., Cecconet D. Sustainable, decentralized sanitation and reuse with hybrid nature-based systems. Water (Switzerland) 2021:13. doi: 10.3390/w13111583. [DOI] [Google Scholar]
- 8.Boano F., Caruso A., Costamagna E., Ridolfi L., Fiore S., Demichelis F., Galvão A., Pisoeiro J., Rizzo A., Masi F. A review of nature-based solutions for greywater treatment: applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.134731. [DOI] [PubMed] [Google Scholar]
- 9.Castellar J.A.C., Torrens A., Buttiglieri G., Monclús H., Arias C.A., Carvalho P.N., Galvao A., Comas J. Nature-based solutions coupled with advanced technologies: an opportunity for decentralized water reuse in cities. J. Clean. Prod. 2022;340 doi: 10.1016/j.jclepro.2022.130660. [DOI] [Google Scholar]
- 10.Liquete C., Udias A., Conte G., Grizzetti B., Masi F. Integrated valuation of a nature-based solution for water pollution control. Highlighting hidden benefits. Ecosyst. Serv. 2016;22:392–401. doi: 10.1016/j.ecoser.2016.09.011. [DOI] [Google Scholar]
- 11.Masi F., Rizzo A., Regelsberger M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2018;216:275–284. doi: 10.1016/j.jenvman.2017.11.086. [DOI] [PubMed] [Google Scholar]
- 12.Corbella C., Puigagut J., Garfí M. Life cycle assessment of constructed wetland systems for wastewater treatment coupled with microbial fuel cells. Sci. Total Environ. 2017;584–585:355–362. doi: 10.1016/j.scitotenv.2016.12.186. [DOI] [PubMed] [Google Scholar]
- 13.Otter P., Hertel S., Ansari J., Lara E., Cano R., Arias C., Gregersen P., Grischek T., Benz F., Goldmaier A., Alvarez J.A. Disinfection for decentralized wastewater reuse in rural areas through wetlands and solar driven onsite chlorination. Sci. Total Environ. 2020;721 doi: 10.1016/j.scitotenv.2020.137595. [DOI] [PubMed] [Google Scholar]
- 14.Lakho F.H., Le H.Q., Van Kerkhove F., Igodt W., Depuydt V., Desloover J., Rousseau D.P.L., Van Hulle S.W.H. Water treatment and re-use at temporary events using a mobile constructed wetland and drinking water production system. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.139630. [DOI] [PubMed] [Google Scholar]
- 15.Arden S., Ma X. Constructed wetlands for greywater recycle and reuse: a review. Sci. Total Environ. 2018;630:587–599. doi: 10.1016/j.scitotenv.2018.02.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S., Carvalho P., Muller J., Manoj V., Dong R. Sanitation in constructed wetlands: a review on the removal of human pathogens and fecal indicators. Sci. Total Environ. 2016;541:8–22. doi: 10.1016/j.scitotenv.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 17.Nan X., Lavrnić S., Toscano A. Potential of constructed wetland treatment systems for agricultural wastewater reuse under the EU framework. J. Environ. Manag. 2020;275 doi: 10.1016/j.jenvman.2020.111219. [DOI] [PubMed] [Google Scholar]
- 18.Liu R., Zhao Y., Doherty L., Hu Y., Hao X. A review of incorporation of constructed wetland with other treatment processes. Chem. Eng. J. 2015;279:220–230. doi: 10.1016/j.cej.2015.05.023. [DOI] [Google Scholar]
- 19.Azaizeh H., Linden K.G., Barstow C., Kalbouneh S., Tellawi A., Albalawneh A., Gerchman Y. Constructed wetlands combined with UV disinfection systems for removal of enteric pathogens and wastewater contaminants. Water Sci. Technol. 2013;67:651–657. doi: 10.2166/wst.2012.615. [DOI] [PubMed] [Google Scholar]
- 20.Jaén-Gil A., Buttiglieri G., Benito A., Mir-Tutusaus J.A., Gonzalez-Olmos R., Caminal G., Barceló D., Sarrà M., Rodriguez-Mozaz S. Combining biological processes with UV/H2O2 for metoprolol and metoprolol acid removal in hospital wastewater. Chem. Eng. J. 2021;404 doi: 10.1016/j.cej.2020.126482. [DOI] [Google Scholar]
- 21.Lakho F.H., Le H.Q., Mattheeuws F., Igodt W., Depuydt V., Desloover J., Rousseau D.P.L., Van Hulle S.W.H. Decentralized grey and black water reuse by combining a vertical flow constructed wetland and membrane based potable water system: full scale demonstration. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2020.104688. [DOI] [Google Scholar]
- 22.Horn T.B., Zerwes F.V., Kist L.T., Machado Ê.L. Constructed wetland and photocatalytic ozonation for university sewage treatment. Ecol. Eng. 2014;63:134–141. doi: 10.1016/j.ecoleng.2013.12.012. [DOI] [Google Scholar]
- 23.Lancheros J.C., Madera-Parra C.A., Caselles-Osorio A., Torres-López W.A., Vargas-Ramírez X.M. Ibuprofen and naproxen removal from domestic wastewater using a horizontal subsurface flow constructed wetland coupled to ozonation. Ecol. Eng. 2019;135:89–97. doi: 10.1016/j.ecoleng.2019.05.007. [DOI] [Google Scholar]
- 24.Colares G.S., da Silva F.P., de Souza Celente G., de Loreto A.C., Lutterbeck C.A., Machado Ê.L., Kist L.T. Combined system for the treatment and reuse of urban wastewater: the efficiency of anaerobic reactors + hybrid constructed wetlands + ozonation. Water Sci. Technol. 2019;80:254–264. doi: 10.2166/wst.2019.270. [DOI] [PubMed] [Google Scholar]
- 25.Gomes A.C., Silva L., Albuquerque A., Simões R., Stefanakis A.I. Treatment of cork boiling wastewater using a horizontal subsurface flow constructed wetland combined with ozonation. Chemosphere. 2020;260:1–12. doi: 10.1016/j.chemosphere.2020.127598. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V., Otter P., Shukla R., Goldmaier A., Alvarez J.A., Khalil N., Avila C., Arias C., Ameršek I. Application of horizontal flow constructed wetland and solar driven disinfection technologies for wastewater treatment in India, Water Pract. Technol. 2018;13:469–480. doi: 10.2166/wpt.2018.029. [DOI] [Google Scholar]
- 27.Álvarez J.A., Ávila C., Otter P., Kilian R., Istenič D., Rolletschek M., Molle P., Khalil N., Ameršek I., Mishra V.K., Jorgensen C., Garfi A., Carvalho P., Brix H., Arias C.A. Constructed wetlands and solar-driven disinfection technologies for sustainable wastewater treatment and reclamation in rural India: SWINGS project. Water Sci. Technol. 2017;76:1474–1489. doi: 10.2166/wst.2017.329. [DOI] [PubMed] [Google Scholar]
- 28.Talekar G.V., Sharma P., Yadav A., Clauwaert P., Rabaey K., Mutnuri S. Sanitation of blackwater via sequential wetland and electrochemical treatment. Npj Clean Water. 2018;1:1–9. doi: 10.1038/s41545-018-0014-x. [DOI] [Google Scholar]
- 29.Talekar G.V., Mutnuri S. Membrane selection for electrochemical treatment of septage. Front. Energy Res. 2020;8:1–12. doi: 10.3389/fenrg.2020.00020. [DOI] [Google Scholar]
- 30.Bakheet B., Prodanovic V., Deletic A., McCarthy D. Effective treatment of greywater via green wall biofiltration and electrochemical disinfection. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116228. [DOI] [PubMed] [Google Scholar]
- 31.Arends J.B.A., Van Denhouwe S., Verstraete W., Boon N., Rabaey K. Enhanced disinfection of wastewater by combining wetland treatment with bioelectrochemical H2O2 production. Bioresour. Technol. 2014;155:352–358. doi: 10.1016/j.biortech.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 32.Mosquera-Romero S., Prévoteau A., Arends J.B.A., Rousseau D.P.L., Dominguez-Granda L., Rabaey K. Disinfection of constructed wetland effluent by: in situ electrochemical chlorine production for water reuse. Environ. Sci. Water Res. Technol. 2022;8:98–107. doi: 10.1039/d1ew00708d. [DOI] [Google Scholar]
- 33.Prado A., Ramírez-Vargas C.A., Arias C.A., Esteve-Núñez A. Novel bioelectrochemical strategies for domesticating the electron flow in constructed wetlands. Sci. Total Environ. 2020;735 doi: 10.1016/j.scitotenv.2020.139522. [DOI] [PubMed] [Google Scholar]
- 34.Ramírez-Vargas C.A., Arias C.A., Carvalho P., Zhang L., Esteve-Núñez A., Brix H. Electroactive biofilm-based constructed wetland (EABB-CW): a mesocosm-scale test of an innovative setup for wastewater treatment. Sci. Total Environ. 2019;659:796–806. doi: 10.1016/j.scitotenv.2018.12.432. [DOI] [PubMed] [Google Scholar]
- 35.Peñacoba-Antona L., Ramirez-Vargas C.A., Wardman C., Carmona-Martinez A.A., Esteve-Núñez A., Paredes D., Brix H., Arias C.A. Microbial electrochemically assisted treatment wetlands: current flow density as a performance indicator in real-scale systems in mediterranean and northern European locations. Front. Microbiol. 2022;13:1–12. doi: 10.3389/fmicb.2022.843135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H., Vecitis C.D., Hoffmann M.R. Electrochemical water splitting coupled with organic compound oxidation: the role of active chlorine species. J. Phys. Chem. C. 2009;113:7935–7945. doi: 10.1021/jp810331w. [DOI] [Google Scholar]
- 37.Huang X., Qu Y., Cid C.A., Finke C., Hoffmann M.R., Lim K., Jiang S.C. Electrochemical disinfection of toilet wastewater using wastewater electrolysis cell. Water Res. 2016;92:164–172. doi: 10.1016/j.watres.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dotro G., Molle P., Nivala J., Puigagut J., Stein O. IWA publishing; London: 2017. Treatment Wetlands - Biological Wastewater Treatment Series. [Google Scholar]
- 39.Vymazal J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009;35:1–17. doi: 10.1016/j.ecoleng.2008.08.016. [DOI] [Google Scholar]
- 40.Kataki S., Chatterjee S., Vairale M.G., Sharma S., Dwivedi S.K., Gupta D.K. Constructed wetland, an eco-technology for wastewater treatment: a review on various aspects of microbial fuel cell integration, low temperature strategies and life cycle impact of the technology. Renew. Sustain. Energy Rev. 2021;148 doi: 10.1016/j.rser.2021.111261. [DOI] [Google Scholar]
- 41.Vymazal J., Zhao Y., Mander Ü. Recent research challenges in constructed wetlands for wastewater treatment: a review. Ecol. Eng. 2021;169 doi: 10.1016/j.ecoleng.2021.106318. [DOI] [Google Scholar]
- 42.Stefanakis A. John Wiley & Sons, Ltd; West Sussex: 2018. Constructed Wetlands for Industrial Wastewater Treatment. [Google Scholar]
- 43.Pavlidis G., Zotou I., Karasali H., Marousopoulou A., Bariamis G., Tsihrintzis V.A., Nalbantis I. Performance of pilot-scale constructed floating wetlands in the removal of nutrients and pesticides, water resour. OR Manag. 2022;36:399–416. doi: 10.1007/s11269-021-03033-9. [DOI] [Google Scholar]
- 44.Brix H., Arias C.A. The use of vertical flow constructed wetlands for on-site treatment of domestic wastewater: new Danish guidelines. Ecol. Eng. 2005;25:491–500. doi: 10.1016/j.ecoleng.2005.07.009. [DOI] [Google Scholar]
- 45.Molle P., Liénard A., Boutin C., Merlin G., Iwema A. How to treat raw sewage with constructed wetlands: an overview of the French systems. Water Sci. Technol. 2005;51:11–21. doi: 10.2166/wst.2005.0277. [DOI] [PubMed] [Google Scholar]
- 46.Torrens A., de la Varga D., Ndiaye A.K., Folch M., Coly A. Innovative multistage constructed wetland for municipal wastewater treatment and reuse for agriculture in Senegal. Water (Switzerland) 2020;12:1–12. doi: 10.3390/w12113139. [DOI] [Google Scholar]
- 47.Morvannou A., Forquet N., Michel S., Troesch S., Molle P. Treatment performances of French constructed wetlands: results from a database collected over the last 30 years. Water Sci. Technol. 2015;71:1333–1339. doi: 10.2166/wst.2015.089. [DOI] [PubMed] [Google Scholar]
- 48.Fernández del Castillo A., Garibay M.V., Senés-Guerrero C., Orozco-Nunnelly D.A., de Anda J., Gradilla-Hernández M.S. A review of the sustainability of anaerobic reactors combined with constructed wetlands for decentralized wastewater treatment. J. Clean. Prod. 2022;371 doi: 10.1016/j.jclepro.2022.133428. [DOI] [Google Scholar]
- 49.Vymazal J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: a review of a recent development. Water Res. 2013:4795–4811. doi: 10.1016/j.watres.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Auvinen H., Havran I., Hubau L., Vanseveren L., Gebhardt W., Linnemann V., Van Oirschot D., Du Laing G., Rousseau D.P.L. Removal of pharmaceuticals by a pilot aerated sub-surface flow constructed wetland treating municipal and hospital wastewater. Ecol. Eng. 2017;100:157–164. doi: 10.1016/j.ecoleng.2016.12.031. [DOI] [Google Scholar]
- 51.Ilyas H., Masih I. Intensification of constructed wetlands for land area reduction: a review. Environ. Sci. Pollut. Res. 2017;24:12081–12091. doi: 10.1007/s11356-017-8740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ju X., Wu S., Huang X., Zhang Y., Dong R. How the novel integration of electrolysis in tidal flow constructed wetlands intensifies nutrient removal and odor control. Bioresour. Technol. 2014;169:605–613. doi: 10.1016/j.biortech.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Prado A., Berenguer R., Esteve-Núñez A. Evaluating bioelectrochemically-assisted constructed wetland (METland®) for treating wastewater: analysis of materials, performance and electroactive communities. Chem. Eng. J. 2022;440 doi: 10.1016/j.cej.2022.135748. [DOI] [Google Scholar]
- 54.Wu S., Kuschk P., Birx H., Vymazal J., Dong R. Development of constructed wetlands in perfomance intensification for wastewater treatment: a nitrogen and organic matter targeted review. Water Res. 2014:40–55. doi: 10.1016/j.watres.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Bruch I., Fritsche J., Bänninger D., Alewell U., Sendelov M., Hürlimann H., Hasselbach R., Alewell C. Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour. Technol. 2011;102:937–941. doi: 10.1016/j.biortech.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 56.Du L., Zhao Y., Wang C., Zhang H., Chen Q., Zhang X., Zhang L., Wu J., Wu Z., Zhou Q. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020;721:1–10. doi: 10.1016/j.scitotenv.2020.137765. [DOI] [PubMed] [Google Scholar]
- 57.Mlih R., Bydalek F., Klumpp E., Yaghi N., Bol R., Wenk J. Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands – a review. Ecol. Eng. 2020;148 doi: 10.1016/j.ecoleng.2020.105783. [DOI] [Google Scholar]
- 58.Deng S., Chen J., Chang J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: performance and ecological benefits. J. Clean. Prod. 2021;293 doi: 10.1016/j.jclepro.2021.126156. [DOI] [Google Scholar]
- 59.Liu Y., Liu X., Wang H., Fang Y., Li Z., Lu S., Wang A. Performance and mechanism of SMX removal in an electrolysis-integrated tidal flow constructed wetland at low temperature. Chem. Eng. J. 2022;434 doi: 10.1016/j.cej.2022.134494. [DOI] [Google Scholar]
- 60.Wu S., Lyu T., Zhao Y., Vymazal J., Arias C.A., Brix H. Rethinking intensification of constructed wetlands as a green eco-technology for wastewater treatment. Environ. Sci. Technol. 2018;52:1693–1694. doi: 10.1021/acs.est.8b00010. [DOI] [PubMed] [Google Scholar]
- 61.Jasper J.T., Nguyen M.T., Jones Z.L., Ismail N.S., Sedlak D.L., Sharp J.O., Luthy R.G., Horne A.J., Nelson K.L. Unit process wetlands for removal of trace organic contaminants and pathogens from municipal wastewater effluents. Environ. Eng. Sci. 2013;30:421–436. doi: 10.1089/ees.2012.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bard A.J., Faulkner L. second ed. John Wiley & Sons, Inc; 2001. Electrochemical Methods. Fundamentals and Applications.http://www.annualreviews.org/doi/10.1146/annurev.matsci.30.1.117 [Google Scholar]
- 63.Newman J., Thomas-Alyea K.E. Wiley; 2004. Electrochemical Systems.https://books.google.com.ec/books?id=vArZu0HM-xYC [Google Scholar]
- 64.Bond D.R., Holmes D.E., Tender L.M., Lovley D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- 65.Domínguez-Garay A., Berná A., Ortiz-Bernad I., Esteve-Núñez A. Silica colloid formation enhances performance of sediment microbial fuel cells in a low conductivity soil. Environ. Sci. Technol. 2013;47:2117–2122. doi: 10.1021/es303436x. [DOI] [PubMed] [Google Scholar]
- 66.Corbella C., Guivernau M., Viñas M., Puigagut J. Operational, design and microbial aspects related to power production with microbial fuel cells implemented in constructed wetlands. Water Res. 2015;84:232–242. doi: 10.1016/j.watres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Yadav A.K., Dash P., Mohanty A., Abbassi R., Mishra B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012;47:126–131. doi: 10.1016/j.ecoleng.2012.06.029. [DOI] [Google Scholar]
- 68.Fang Z., Song H.L., Cang N., Li X.N. Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour. Technol. 2013;144:165–171. doi: 10.1016/j.biortech.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y., Collum S., Phelan M., Goodbody T., Doherty L., Hu Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: batch and continuous flow trials. Chem. Eng. J. 2013;229:364–370. doi: 10.1016/j.cej.2013.06.023. [DOI] [Google Scholar]
- 70.Aguirre-Sierra A., Bacchetti-De Gregoris T., Berná A., Salas J.J., Aragón C., Esteve-Núñez A. Microbial electrochemical systems outperform fixed-bed biofilters in cleaning up urban wastewater. Environ. Sci. Water Res. Technol. 2016;2:984–993. doi: 10.1039/C6EW00172F. [DOI] [Google Scholar]
- 71.Doherty L., Zhao Y., Zhao X., Wang W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015;266:74–81. doi: 10.1016/j.cej.2014.12.063. [DOI] [Google Scholar]
- 72.Guadarrama-Pérez O., Gutiérrez-Macías T., García-Sánchez L., Guadarrama-Pérez V.H., Estrada-Arriaga E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: a review. Int. J. Energy Res. 2019;43:5106–5127. doi: 10.1002/er.4496. [DOI] [Google Scholar]
- 73.Tang C., Zhao Y., Kang C., Yang Y., Morgan D., Xu L. Towards concurrent pollutants removal and high energy harvesting in a pilot-scale CW-MFC: insight into the cathode conditions and electrodes connection. Chem. Eng. J. 2019;373:150–160. doi: 10.1016/j.cej.2019.05.035. [DOI] [Google Scholar]
- 74.Corbella C., Puigagut J. Microbial fuel cells implemented in constructed wetlands: fundamentals, current research and future perspectives the fundamentals: microbial fuel cells technology. Contrib. Sci. (Los Angel.) 2015;120:113–120. doi: 10.2436/20.7010.01.220. [DOI] [Google Scholar]
- 75.Ji B., Zhao Y., Vymazal J., Mander Ü., Lust R., Tang C. Mapping the field of constructed wetland-microbial fuel cell: a review and bibliometric analysis. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.128366. [DOI] [PubMed] [Google Scholar]
- 76.Doherty L., Zhao Y., Zhao X., Hu Y., Hao X., Xu L., Liu R. A review of a recently emerged technology: constructed wetland - microbial fuel cells. Water Res. 2015;85:38–45. doi: 10.1016/j.watres.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Xu L., Zhao Y., Fan C., Fan Z., Zhao F. First study to explore the feasibility of applying microbial fuel cells into constructed wetlands for COD monitoring. Bioresour. Technol. 2017;243:846–854. doi: 10.1016/j.biortech.2017.06.179. [DOI] [PubMed] [Google Scholar]
- 78.Corbella C., Hartl M., Fernandez-gatell M., Puigagut J. MFC-based biosensor for domestic wastewater COD assessment in constructed wetlands. Sci. Total Environ. 2019;660:218–226. doi: 10.1016/j.scitotenv.2018.12.347. [DOI] [PubMed] [Google Scholar]
- 79.Hoareau M., Erable B., Bergel A. Microbial electrochemical snorkels (MESs): a budding technology for multiple applications. A mini review. Electrochem. Commun. 2019;104 doi: 10.1016/j.elecom.2019.05.022. [DOI] [Google Scholar]
- 80.Schievano A., Berenguer R., Goglio A., Bocchi S., Marzorati S., Rago L., Louro R.O., Paquete C.M., Esteve-Núñez A. Electroactive biochar for large-scale environmental applications of microbial electrochemistry. ACS Sustain. Chem. Eng. 2019;7:18198–18212. doi: 10.1021/acssuschemeng.9b04229. [DOI] [Google Scholar]
- 81.Prado A., Berenguer R., Esteve-Núñez A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon N. Y. 2019;146:597–609. doi: 10.1016/j.carbon.2019.02.038. [DOI] [Google Scholar]
- 82.Prévoteau A., Ronsse F., Cid I., Boeckx P., Rabaey K. The electron donating capacity of biochar is dramatically underestimated. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep32870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan Y., Bolan N., Prévoteau A., Vithanage M., Biswas J.K., Ok Y.S., Wang H. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017;246:271–281. doi: 10.1016/j.biortech.2017.06.154. [DOI] [PubMed] [Google Scholar]
- 84.Ramírez-Vargas C.A., Arias C.A., Zhang L., Paredes D., Brix H. Community level physiological profiling of microbial electrochemical-based constructed wetlands. Sci. Total Environ. 2020;721 doi: 10.1016/j.scitotenv.2020.137761. [DOI] [PubMed] [Google Scholar]
- 85.Pun Á., Boltes K., Letón P., Esteve-Nuñez A. Detoxification of wastewater containing pharmaceuticals using horizontal flow bioelectrochemical filter. Bioresour. Technol. Reports. 2019;7 doi: 10.1016/j.biteb.2019.100296. [DOI] [Google Scholar]
- 86.Aguirre-Sierra A., Bacchetti-De Gregoris T., Salas J.J., de Deus A., Esteve-Núñez A. A new concept in constructed wetlands: assessment of aerobic electroconductive biofilters. Environ. Sci. Water Res. Technol. 2020;6:1312–1323. doi: 10.1039/C9EW00696F. [DOI] [Google Scholar]
- 87.Peñacoba-Antona Lorena. University of Alcalá; 2021. Validating Full Scale METland Solutions for Decentralized Sustaninable Wastewater Treatment: Techno-Environmental and Geospatial Analysis. [Google Scholar]
- 88.Peñacoba-Antona L., Senán-Salinas J., Aguirre-Sierra A., Letón P., Salas J.J., García-Calvo E., Esteve-Núñez A. Assessing METland® design and performance through LCA: techno-environmental study with multifunctional unit perspective. Front. Microbiol. 2021;12:1–14. doi: 10.3389/fmicb.2021.652173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peñacoba-Antona L., Gómez-Delgado M., Esteve-Núñez A. Multi-criteria evaluation and sensitivity analysis for the optimal location of constructed wetlands (METland) at oceanic and mediterranean areas. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph18105415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y., Xie Y.W., Zhang Q., Wang A.L., Yu Y.X., Yang L.Y. Intensified nitrate and phosphorus removal in an electrolysis-integrated horizontal subsurface-flow constructed wetland. Water Res. 2017;108:39–45. doi: 10.1016/j.watres.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 91.Yan C., Wang M., Ma T., Yang S., Kong M., Shen J., Yang L., Gao Y. Study on the experimental performance by electrolysis-integrated ecological floating bed for nitrogen and phosphorus removal in eutrophic water. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-64499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu J., Wang M., Wei J., Kong L., Yang B., Wu G., Lei L., Li Z. Electrolysis-integrated constructed wetland with pyrite filler for simultaneous enhanced phosphorus and nitrogen removal. Chem. Eng. J. 2023;451 doi: 10.1016/j.cej.2022.138542. [DOI] [Google Scholar]
- 93.Ebrahimi A., Sivakumar M., McLauchlan C., Ansari A., Vishwanathan A.S. A critical review of the symbiotic relationship between constructed wetland and microbial fuel cell for enhancing pollutant removal and energy generation. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2020.105011. [DOI] [Google Scholar]
- 94.Prévoteau A., Rabaey K. Electroactive biofilms for sensing: reflections and perspectives. ACS Sens. 2017;2:1072–1085. doi: 10.1021/acssensors.7b00418. [DOI] [PubMed] [Google Scholar]
- 95.Zhai J., Dong S. Recent advances in microbial fuel cell-based toxicity biosensors: strategies for enhanced toxicity response. Curr. Opin. Electrochem. 2022;34 doi: 10.1016/j.coelec.2022.100975. [DOI] [Google Scholar]
- 96.Prévoteau A., Clauwaert P., Kerckhof F.M., Rabaey K. Oxygen-reducing microbial cathodes monitoring toxic shocks in tap water. Biosens. Bioelectron. 2019;132:115–121. doi: 10.1016/j.bios.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 97.Wang X., Aulenta F., Puig S., Esteve-Núñez A., He Y., Mu Y., Rabaey K. Microbial electrochemistry for bioremediation. Environ. Sci. Ecotechnology. 2020;1 doi: 10.1016/j.ese.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martínez-Huitle C., Rodrigo M.A., Scialdone O., editors. Electrochemical Water and Wastewater Treatment. Matthew Deans; Oxford: 2018. [DOI] [Google Scholar]
- 99.Mah D.T. Electrochemical manufacturing in the 21st century. Electrochem. Soc. Interface. 2014;23:47. doi: 10.1149/2.F03143if. [DOI] [Google Scholar]
- 100.Karlsson R.K.B., Cornell A. Selectivity between oxygen and chlorine evolution in the chlor-alkali and chlorate processes. Chem. Rev. 2016;116:2982–3028. doi: 10.1021/acs.chemrev.5b00389. [DOI] [PubMed] [Google Scholar]
- 101.Radjenovic J., Sedlak D.L. 2015. Challenges and Opportunities for Electrochemical Processes as Next- Generation Technologies for the Treatment of Contaminated Water. [DOI] [PubMed] [Google Scholar]
- 102.Martínez-Huitle C.A., Ferro S. Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem. Soc. Rev. 2006;35:1324–1340. doi: 10.1039/b517632h. [DOI] [PubMed] [Google Scholar]
- 103.Hand S., Cusick R.D. Electrochemical disinfection in water and wastewater treatment: identifying impacts of water quality and operating conditions on performance. Environ. Sci. Technol. 2021;55:3470–3482. doi: 10.1021/acs.est.0c06254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaplin B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019;52:596–604. doi: 10.1021/acs.accounts.8b00611. [DOI] [PubMed] [Google Scholar]
- 105.Lacasa E., Llanos J., Cañizares P., Rodrigo M.A. Electrochemical denitrificacion with chlorides using DSA and BDD anodes. Chem. Eng. J. 2012;184:66–71. doi: 10.1016/j.cej.2011.12.090. [DOI] [Google Scholar]
- 106.Mier A.A., Olvera-Vargas H., Mejía-López M., Longoria A., Verea L., Sebastian P.J., Arias D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: from biofilm-bearing anodes to specialized cathodes. Chemosphere. 2021;283 doi: 10.1016/j.chemosphere.2021.131138. [DOI] [PubMed] [Google Scholar]
- 107.Bergmann H. Electrochemical disinfection – state of the art and tendencies. Curr. Opin. Electrochem. 2021;28 doi: 10.1016/j.coelec.2021.100694. [DOI] [Google Scholar]
- 108.Gao R., Mosquera-Romero S., Ntagia E., Wang X., Rabaey K., Bonin L. Review—electrochemical separation of organic and inorganic contaminants in wastewater. J. Electrochem. Soc. 2022;169 doi: 10.1149/1945-7111/ac51f9. [DOI] [Google Scholar]
- 109.Moreira F.C., Boaventura R.A.R., Brillas E., Vilar V.J.P. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017;202:217–261. doi: 10.1016/j.apcatb.2016.08.037. [DOI] [Google Scholar]
- 110.Schaefer C.E., Lavorgna G.M., Webster T.S., Deshusses M.A., Andaya C., Urtiaga A. Pilot-scale electrochemical disinfection of surface water: assessing disinfection by-product and free chlorine formation. Water Sci. Technol. Water Supply. 2017;17:526–536. doi: 10.2166/ws.2016.165. [DOI] [Google Scholar]
- 111.Chow H., Pham A.L.T. Mitigating electrode fouling in electrocoagulation by means of polarity reversal: the effects of electrode type, current density, and polarity reversal frequency. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117074. [DOI] [PubMed] [Google Scholar]
- 112.Muddemann T., Haupt D., Sievers M., Kunz U. Electrochemical reactors for wastewater treatment. ChemBioEng Rev. 2019;6:142–156. doi: 10.1002/cben.201900021. [DOI] [Google Scholar]
- 113.Cano A., Cañizares P., Barrera-Díaz C., Sáez C., Rodrigo M.A. Use of conductive-diamond electrochemical-oxidation for the disinfection of several actual treated wastewaters. Chem. Eng. J. 2012;211–212:463–469. doi: 10.1016/j.cej.2012.09.071. [DOI] [Google Scholar]
- 114.Jasper J.T., Shafaat O.S., Hoffmann M.R. Electrochemical transformation of trace organic contaminants in latrine wastewater. Environ. Sci. Technol. 2016;50:10198–10208. doi: 10.1021/acs.est.6b02912. [DOI] [PubMed] [Google Scholar]
- 115.Kerwick M.I., Reddy S.M., Chamberlain A.H.L., Holt D.M. Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim. Acta. 2005;50:5270–5277. doi: 10.1016/j.electacta.2005.02.074. [DOI] [Google Scholar]
- 116.Jeong J., Kim J.Y., Yoon J. The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ. Sci. Technol. 2006;40:6117–6122. doi: 10.1021/es0604313. [DOI] [PubMed] [Google Scholar]
- 117.Jeong J., Kim J.Y., Cho M., Choi W., Yoon J. Inactivation of Escherichia coli in the electrochemical disinfection process using a Pt anode. Chemosphere. 2007;67:652–659. doi: 10.1016/j.chemosphere.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 118.Li H., Zhu X., Ni J. Inactivation of Escherichia coli in Na2SO4 electrolyte using boron-doped diamond anode. Electrochim. Acta. 2010;56:448–453. doi: 10.1016/j.electacta.2010.08.055. [DOI] [Google Scholar]
- 119.Chen L., Lei C., Li Z., Yang B., Zhang X., Lei L. Electrochemical activation of sulfate by BDD anode in basic medium for efficient removal of organic pollutants. Chemosphere. 2018;210:516–523. doi: 10.1016/j.chemosphere.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 120.Nadeeshani Nanayakkara K.G., Khorshed Alam A.K.M., Zheng Y.M., Paul Chen J. A low-energy intensive electrochemical system for the eradication of Escherichia coli from ballast water: process development, disinfection chemistry, and kinetics modeling. Mar. Pollut. Bull. 2012;64:1238–1245. doi: 10.1016/j.marpolbul.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 121.Rajab M., Heim C., Letzel T., Drewes J.E., Helmreich B. Electrochemical disinfection using boron-doped diamond electrode - the synergetic effects of in situ ozone and free chlorine generation. Chemosphere. 2015;121:47–53. doi: 10.1016/j.chemosphere.2014.10.075. [DOI] [PubMed] [Google Scholar]
- 122.Zhou W., Meng X., Gao J., Alshawabkeh A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: a state-of-the-art review. Chemosphere. 2019;225:588–607. doi: 10.1016/j.chemosphere.2019.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho K., Qu Y., Kwon D., Zhang H., Cid C.A., Aryanfar A., Hoffmann M.R. Effects of anodic potential and chloride ion on overall reactivity in electrochemical reactors designed for solar-powered wastewater treatment. Environ. Sci. Technol. 2014;48:2377–2384. doi: 10.1021/es404137u. [DOI] [PubMed] [Google Scholar]
- 124.Bruguera-Casamada C., Sirés I., Brillas E., Araujo R.M. Effect of electrogenerated hydroxyl radicals, active chlorine and organic matter on the electrochemical inactivation of Pseudomonas aeruginosa using BDD and dimensionally stable anodes. Sep. Purif. Technol. 2017;178:224–231. doi: 10.1016/j.seppur.2017.01.042. [DOI] [Google Scholar]
- 125.Jasper J.T., Yang Y., Hoffmann M.R. Toxic byproduct formation during electrochemical treatment of latrine wastewater. Environ. Sci. Technol. 2017;51:7111–7119. doi: 10.1021/acs.est.7b01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mezule L., Reimanis M., Krumplevska V., Ozolins J., Juhna T. Comparing electrochemical disinfection with chlorination for inactivation of bacterial spores in drinking water. Water Sci. Technol. Water Supply. 2014;14:158–164. doi: 10.2166/ws.2013.182. [DOI] [Google Scholar]
- 127.Drees K.P., Abbaszadegan M., Maier R.M. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 2003;37:2291–2300. doi: 10.1016/S0043-1354(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 128.Burton F.L., Tchobanoglous G., Tsuchihashi R., Stensel H.D., Metcalf I., Eddy . McGraw-Hill Education; 2013. Wastewater Engineering: Treatment and Resource Recovery.https://books.google.be/books?id=6KVKMAEACAAJ [Google Scholar]
- 129.Kim J., Choi W.J.K., Choi J., Hoffmann M.R., Park H. Electrolysis of urea and urine for solar hydrogen. Catal. Today. 2013;199:2–7. doi: 10.1016/j.cattod.2012.02.009. [DOI] [Google Scholar]
- 130.Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bergmann H., Koparal S. The formation of chlorine dioxide in the electrochemical treatment of drinking water for disinfection. Electrochim. Acta. 2005;50:5218–5228. doi: 10.1016/j.electacta.2005.01.061. [DOI] [Google Scholar]
- 132.Jeong J., Kim C., Yoon J. The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res. 2009;43:895–901. doi: 10.1016/j.watres.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 133.Schmalz V., Dittmar T., Haaken D., Worch E. Electrochemical disinfection of biologically treated wastewater from small treatment systems by using boron-doped diamond (BDD) electrodes - contribution for direct reuse of domestic wastewater. Water Res. 2009;43:5260–5266. doi: 10.1016/j.watres.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 134.Marselli B., Garcia-Gomez J., Michaud P.-A., Rodrigo M.A., Comninellis C. Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J. Electrochem. Soc. 2003;150:D79. doi: 10.1149/1.1553790. [DOI] [Google Scholar]
- 135.Cho M., Chung H., Choi W., Yoon J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004;38:1069–1077. doi: 10.1016/j.watres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 136.Gassie L.W., Englehardt J.D. Advanced oxidation and disinfection processes for onsite net-zero greywater reuse: a review. Water Res. 2017;125:384–399. doi: 10.1016/j.watres.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 137.Li Y., Zhang Y., Xia G., Zhan J., Yu G., Wang Y. Evaluation of the technoeconomic feasibility of electrochemical hydrogen peroxide production for decentralized water treatment. Front. Environ. Sci. Eng. 2021;15:1–15. doi: 10.1007/s11783-020-1293-2. [DOI] [Google Scholar]
- 138.Lu Y., Liu G., Luo H., Zhang R. Efficient in-situ production of hydrogen peroxide using a novel stacked electrosynthesis reactor. Electrochim. Acta. 2017;248:29–36. doi: 10.1016/j.electacta.2017.07.085. [DOI] [Google Scholar]
- 139.Modin O., Kensuke F. Production of high concentrations of H2O2 in a bioelectrochemical reactor fed with real municipal wastewater. Environ. Technol. 2008;111:696–708. doi: 10.1080/09593330.2013.788041. [DOI] [PubMed] [Google Scholar]
- 140.Jiang B., Wang Y., Wang D., Yao M., Fan C., Dai J. Modifying graphite felt cathode by HNO3 or KOH to improve the degradation efficiency of electro-Fenton for landfill leachate. Water Sci. Technol. 2019;80:2412–2421. doi: 10.2166/wst.2020.072. [DOI] [PubMed] [Google Scholar]
- 141.Zhou M., Oturan M.A., Sirés I. Springer; Singapore: 2018. Electro- Fenton: New Trends and Scale-Up. [DOI] [Google Scholar]
- 142.Ren G., Zhou M., Zhang Q., Xu X., Li Y., Su P. A novel stacked flow-through electro-Fenton reactor as decentralized system for the simultaneous removal of pollutants (COD, NH3-N and TP) and disinfection from domestic sewage containing chloride ions. Chem. Eng. J. 2020;387 doi: 10.1016/j.cej.2020.124037. [DOI] [Google Scholar]
- 143.Bruguera-Casamada C., Araujo R.M., Brillas E., Sirés I. Advantages of electro-Fenton over electrocoagulation for disinfection of dairy wastewater. Chem. Eng. J. 2019;376 doi: 10.1016/j.cej.2018.09.136. [DOI] [Google Scholar]
- 144.Aziz H.A., Othman O.M., Abu Amr S.S. The performance of Electro-Fenton oxidation in the removal of coliform bacteria from landfill leachate. Waste Manag. 2013;33:396–400. doi: 10.1016/j.wasman.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 145.Yang Y., Lin L., Tse L.K., Dong H., Yu S., Hoffmann M.R. Membrane-separated electrochemical latrine wastewater treatment. Environ. Sci. Water Res. Technol. 2019;5:51–59. doi: 10.1039/c8ew00698a. [DOI] [Google Scholar]
- 146.Logan B.E., Rabaey K. Conversion of waste into bioelectricity and chemical by using microbial electrochemical technologies. Science. 2013;337(80):686–690. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- 147.Chung T.H., Meshref M.N.A., Hai F.I., Al-Mamun A., Dhar B.R. Microbial electrochemical systems for hydrogen peroxide synthesis: critical review of process optimization, prospective environmental applications, and challenges. Bioresour. Technol. 2020 doi: 10.1016/j.biortech.2020.123727. [DOI] [PubMed] [Google Scholar]
- 148.Mosquera-Romero S., Prévoteau A., Vanwonterghem I., Arends J.B.A., Dominguez L., Rousseau D.P.L., Rabaey K. Hydrogen peroxide in bioelectrochemical systems negatively affects microbial current generation. J. Appl. Electrochem. 2021 doi: 10.1007/s10800-021-01586-6. [DOI] [Google Scholar]
- 149.Hassan M., Pous N., Xie B., Colprim J., Balaguer M.D., Puig S. Employing Microbial Electrochemical Technology-driven electro-Fenton oxidation for the removal of recalcitrant organics from sanitary landfill leachate. Bioresour. Technol. 2017;243:949–956. doi: 10.1016/j.biortech.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 150.Li X., Jin X., Zhao N., Angelidaki I., Zhang Y. Efficient treatment of aniline containing wastewater in bipolar membrane microbial electrolysis cell-Fenton system. Water Res. 2017;119:67–72. doi: 10.1016/j.watres.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 151.Bergmann H. Disinfection of water. Electrochemical. 2014 doi: 10.1007/978-1-4419-6996-5. [DOI] [Google Scholar]
- 152.Sandoval M.A., Calzadilla W., Salazar R. Influence of reactor design on the electrochemical oxidation and disinfection of wastewaters using boron-doped diamond electrodes. Curr. Opin. Electrochem. 2022;33 doi: 10.1016/j.coelec.2022.100939. [DOI] [Google Scholar]
- 153.Stirling R., Walker W.S., Westerhoff P., Garcia-Segura S. Techno-economic analysis to identify key innovations required for electrochemical oxidation as point-of-use treatment systems. Electrochim. Acta. 2020;338 doi: 10.1016/j.electacta.2020.135874. [DOI] [Google Scholar]
- 154.Raut A.S., Parker C.B., Klem E.J.D., Stoner B.R., Deshusses M.A., Glass J.T. Reduction in energy for electrochemical disinfection of E. coli in urine simulant. J. Appl. Electrochem. 2019;49:443–453. doi: 10.1007/s10800-019-01292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cornejo O.M., Murrieta M.F., Castañeda L.F., Nava J.L. Electrochemical reactors equipped with BDD electrodes: geometrical aspects and applications in water treatment. Curr. Opin. Solid State Mater. Sci. 2021;25 doi: 10.1016/j.cossms.2021.100935. [DOI] [Google Scholar]
- 156.Kizilaslan M.A., Demirel E., Aral M.M. Efficiency enhancement of chlorine contact tanks in water treatment plants: a full-scale application. Processes. 2019;7 doi: 10.3390/pr7090551. [DOI] [Google Scholar]
- 157.Kapałka A., Katsaounis A., Michels N.L., Leonidova A., Souentie S., Comninellis C., Udert K.M. Ammonia oxidation to nitrogen mediated by electrogenerated active chlorine on Ti/PtOx-IrO2. Electrochem. Commun. 2010;12:1203–1205. doi: 10.1016/j.elecom.2010.06.019. [DOI] [Google Scholar]
- 158.EPA . 2012. Guidlines for Water Reuse. Washington DC. [Google Scholar]
- 159.The European parliament and the council, regulation (EU) 2020/741, minimum requirements for water reuse, off. Off. J. Eur. Union. 2020;177/33:32–55. [Google Scholar]
- 160.International Organization for Standardization . 2018. Iso 30500- 2018. [Google Scholar]
- 161.Asociación Española de Normalización . 2019. Norma Española UNE-EN 16941. Sistemas in situ de agua no potable - Parte 1: Sistemas para la utilización de aguas lluvias. [Google Scholar]
- 162.Texto unificado de Legislación secundaria de Medio ambiente, registro oficial. Ecuador. 2003;320 https://www.ambiente.gob.ec/wp-content/uploads/downloads/2018/05/TULSMA.pdf [Google Scholar]
- 163.Labas M.D., Zalazar C.S., Brandi R.J., Cassano A.E. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochem. Eng. J. 2008;38:78–87. doi: 10.1016/j.bej.2007.06.008. [DOI] [Google Scholar]
- 164.Leong J.Y.C., Oh K.S., Poh P.E., Chong M.N. Prospects of hybrid rainwater-greywater decentralised system for water recycling and reuse: a review. J. Clean. Prod. 2017;142:3014–3027. doi: 10.1016/j.jclepro.2016.10.167. [DOI] [Google Scholar]
- 165.Forés E., Mejías-Molina C., Ramos A., Itarte M., Hundesa A., Rusiñol M., Martínez-Puchol S., Esteve-Bricullé P., Espejo-Valverde A., Sirés I., Calvo M., Araujo R.M., Girones R. Evaluation of pathogen disinfection efficiency of electrochemical advanced oxidation to become a sustainable technology for water reuse. Chemosphere. 2023;313 doi: 10.2139/ssrn.4229520. [DOI] [PubMed] [Google Scholar]
- 166.Uggetti E., Hughes-Riley T., Morris R.H., Newton M.I., Trabi C.L., Hawes P., Puigagut J., García J. Intermittent aeration to improve wastewater treatment efficiency in pilot-scale constructed wetland. Sci. Total Environ. 2016;559:212–217. doi: 10.1016/j.scitotenv.2016.03.195. [DOI] [PubMed] [Google Scholar]
- 167.Tatoulis T., Stefanakis A., Frontistis Z., Akratos C.S., Tekerlekopoulou A.G., Mantzavinos D., Vayenas D.V. Treatment of table olive washing water using trickling filters, constructed wetlands and electrooxidation. Environ. Sci. Pollut. Res. 2017;24:1085–1092. doi: 10.1007/s11356-016-7058-6. [DOI] [PubMed] [Google Scholar]
- 168.Grafias P., Xekoukoulotakis N.P., Mantzavinos D., Diamadopoulos E. Pilot treatment of olive pomace leachate by vertical-flow constructed wetland and electrochemical oxidation: an efficient hybrid process. Water Res. 2010;44:2773–2780. doi: 10.1016/j.watres.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 169.Saha P., Wagner T.V., Ni J., Langenhoff A.A.M., Bruning H., Rijnaarts H.H.M. Cooling tower water treatment using a combination of electrochemical oxidation and constructed wetlands. Process Saf. Environ. Protect. 2020;144:42–51. doi: 10.1016/j.psep.2020.07.019. [DOI] [Google Scholar]
- 170.Wang C., Zhang M., Ye M., Wang J., Li G. Pilot-scale electrochemical oxidation combined with constructed wetland system for unconventional surface water treatment. J. Chem. Technol. Biotechnol. 2014;89:1599–1606. doi: 10.1002/jctb.4464. [DOI] [Google Scholar]
- 171.Chen J., Shi H., Lu J. Electrochemical treatment of ammonia in wastewater by RuO 2-IrO2-TiO2/Ti electrodes. J. Appl. Electrochem. 2007;37:1137–1144. doi: 10.1007/s10800-007-9373-6. [DOI] [Google Scholar]
- 172.Wang C., He X., Chang F., Li D. The coupling system of electrochemical oxidation and constructed wetland for polluted surface water treatment. 5th Int. Conf. Bioinforma. Biomed. Eng. ICBBE. 2011;(2011):3–6. doi: 10.1109/icbbe.2011.5780726. [DOI] [Google Scholar]
- 173.EPA . 2003. Wastewater Technology Fact Sheet: Disinfection for Small Systems. Washington DC. [Google Scholar]
- 174.Wang X., Tian Y., Liu H., Zhao X., Peng S. Optimizing the performance of organics and nutrient removal in constructed wetland–microbial fuel cell systems. Sci. Total Environ. 2019;653:860–871. doi: 10.1016/j.scitotenv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 175.Dashtpour R., Al-Zubaidy S.N. Energy efficient reverse osmosis desalination process. International Journal of Environmental Science and Development, Int. J. Environ. Sci. Dev. 2012;3:339–345. [Google Scholar]
- 176.Bergmann M.E.H., Koparal A.S. Studies on electrochemical disinfectant production using anodes containing RuO2. J. Appl. Electrochem. 2005;35:1321–1329. doi: 10.1007/s10800-005-9064-0. [DOI] [Google Scholar]
- 177.Barazesh J.M., Hennebel T., Jasper J.T., Sedlak D.L. Modular advanced oxidation process enabled by cathodic hydrogen peroxide production. Environ. Sci. Technol. 2015;49:7391–7399. doi: 10.1021/acs.est.5b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ma P., Ma H., Sabatino S., Galia A., Scialdone O. Electrochemical treatment of real wastewater. Part 1: effluents with low conductivity. Chem. Eng. J. 2018;336:133–140. doi: 10.1016/j.cej.2017.11.046. [DOI] [Google Scholar]
- 179.Clematis D., Panizza M. Electrochemical oxidation of organic pollutants in low conductive solutions. Curr. Opin. Electrochem. 2021;26 doi: 10.1016/j.coelec.2020.100665. [DOI] [Google Scholar]
- 180.Mosquera-Romero S., Prévoteau A., Louage F., Dominguez-Granda L., Rabaey K., Rousseau D.P.L. 2022. Practical factors impeding electrochemical disinfection of constructed wetland effluents: fouling and energy consumption. In Preparation. [Google Scholar]
- 181.Lin C.J., Zhang R., Waisner S.A., Nawaz T., Center L., Gent D.B., Johnson J.L., Holland S. Effects of process factors on the performance of electrochemical disinfection for wastewater in a continuous-flow cell reactor. Environ. Sci. Pollut. Res. 2021;28:36573–36584. doi: 10.1007/s11356-021-13193-1. [DOI] [PubMed] [Google Scholar]
- 182.Ntagia E., Fiset E., Truong Cong Hong L., Vaiopoulou E., Rabaey K. Electrochemical treatment of industrial sulfidic spent caustic streams for sulfide removal and caustic recovery. J. Hazard Mater. 2020;388 doi: 10.1016/j.jhazmat.2019.121770. [DOI] [PubMed] [Google Scholar]
- 183.Kearney D., Bejan D., Bunce N.J. The use of Ebonex electrodes for the electrochemical removal of nitrate ion from water. Can. J. Chem. 2012;90:666–674. doi: 10.1139/v2012-048. [DOI] [Google Scholar]
- 184.Butylskii D.Y., Troitskiy V.A., Ponomar M.A., Moroz I.A., Sabbatovskiy K.G., Sharafan M.V. Efficient anion-exchange membranes with anti-scaling properties obtained by surface modification of commercial membranes using a polyquaternium-22. Membranes. 2022;12:1065. doi: 10.3390/membranes12111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Silver B.J. 2010. Country Classifications, Forces of Labor. 204–204. [DOI] [Google Scholar]
- 186.Mook W.T., Aroua M.K., Issabayeva G. Prospective applications of renewable energy based electrochemical systems in wastewater treatment: a review. Renew. Sustain. Energy Rev. 2014;38:36–46. doi: 10.1016/j.rser.2014.05.042. [DOI] [Google Scholar]
- 187.Alvarado A., Larriva J., Sánchez E., Idrovo D., Cisneros J.F. Assessment of decentralized wastewater treatment systems in the rural area of Cuenca, Ecuador, Water Pract. Technol. 2017;12:240–249. doi: 10.2166/wpt.2017.027. [DOI] [Google Scholar]
- 188.Kalt P., Birzer C., Evans H., Liew A., Padovan M., Watchman M. A solar disinfection water treatment system for remote communities. Procedia Eng. 2014;78:250–258. doi: 10.1016/j.proeng.2014.07.064. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.