Abstract
Background
Widespread vaccination and emergence of less aggressive SARS-CoV2 variants may have blunted the unfavourable outcomes of COVID-19 in nursing home (NH) residents. We analysed the course of COVID-19 epidemic in NHs of Florence, Italy, during the “Omicron era” and investigated the independent effect of SARS-CoV2 infection on death and hospitalization risk.
Methods
Weekly SARS-CoV2 infection rates between November 2021 and March 2022 were calculated. Detailed clinical data were collected in a sample of NHs.
Results
Among 2044 residents, 667 SARS-CoV2 cases were confirmed. SARS-CoV2 incidence sharply increased during the Omicron era. Mortality rates did not differ between SARS-CoV2-positive (6.9%) and SARS-CoV2-negative residents (7.3%, p = 0.71). Chronic obstructive pulmonary disease and poor functional status, but not SARS-CoV2 infection independently predicted death and hospitalization.
Conclusions
Despite that SARS-CoV2 incidence increased during the Omicron era, SARS-CoV2 infection was not a significant predictor of hospitalization and death in the NH setting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-023-02415-w.
Keywords: COVID-19, Vaccination, Immunization, Functional status, Older adults
Introduction
The COVID-19 pandemic had dramatic effects in older adults, particularly those residing in nursing homes (NHs). Indeed, over 20% lethality and similar rates of hospital admission among SARS-CoV2 positive were observed in NH residents [1, 2]. The introduction of SARS-CoV2 vaccination dramatically changed the course of epidemic, followed by lower infection rates and reduced risk of severe COVID-19 disease, with significantly lower lethality and hospitalization rates also in the NH setting [3, 4]. The Omicron variant identified in November 2021 became the dominant variant worldwide. Of note, in the general population, the Omicron variant has shown greater infectivity, but a lower lethality rate in comparison with the Delta variant, although the difference in death risk seems less evident among subjects aged 70 or older [5]. These findings have been recently confirmed in a large sample of NHs in the UK, showing a two-third decrease of hospital admissions and mortality among infected residents during the “Omicron period”, although part of the protective effect is explained by the higher rate of booster vaccination and previous infection [6, 7].
It is still largely unknown to what extent SARS-CoV2 infection in the present conditions affects the prognosis of older NH residents over and beyond their current pathology and functional impairment. Less aggressive SARS-CoV2 variants—together with widespread vaccination—may have blunted the unfavourable outcomes of COVID-19 in NH residents. To test this hypothesis, we recorded SARS-CoV2 infection incidence, mortality and hospitalization rate of all NH residents in Florence Health District, Tuscany, Italy during the first months of the “Omicron era”. Moreover, we gathered individual data from a sub-sample of NH residents to compare the short-term prognosis (death and hospitalization risk) of residents with and without incident infection, including both SARS-CoV2 infection status and other coexistent clinical features in a multivariate model.
Methods
We conducted a retrospective longitudinal study that involved all residents living in the NHs of Florence, Tuscany, Italy, as of November 1st, 2021. We calculated weekly SARS-CoV2 infection rates since November 1st, 2021, until March 31st, 2022. Hospital admissions and deaths were recorded from local administrative and clinical sources during the period between December 24th, 2021 and March 31st, 2022 (hereinafter referred to as “Omicron era”), soon after the Omicron variant was first detected in the study area. [8]
In the Florence Health District, two BNT162b2 (Pfizer-BioNTech) mRNA vaccine doses were administered during the December 27th, 2020–January 17th, 2021 period (first dose) and January 21st–February 15th, 2021 (second dose) period, and a third dose was administered at the beginning of November 2021. Since the beginning of the vaccine campaign, SARS-CoV2 vaccination is a necessary pre-requisite for new NH admission. Moreover, according to Florence Health District prevention protocols, transmission control strategies—including use of personal protective equipment and regular SARS-CoV2 screening—were maintained throughout the study period. SARS-CoV2-positive residents were isolated in dedicated areas and received standardized on-site care by a hospital-at-nursing home multidisciplinary team, as detailed elsewhere [1].
We also collected on-site detailed clinical data of the sub-cohort including the residents of seven NHs, selected among those who were strictly adherent to the cited prevention protocols, including both small (< 60 residents, N = 5) and large (> 60 residents, N = 2) facilities. Information recorded included immunization status, disability, physical performance, comorbidities and COVID-19-related symptoms. Immunization was evaluated as a composite of infection history and vaccination, considering previous infection the same as a vaccine dose. Residents were thus considered as fully immunized if three vaccine doses or two vaccine doses and a previous SARS-CoV2 infection were reported. Mortality and hospitalization rate were recorded over a 3-month follow-up. A composite of death and hospital admission represented the primary study outcome.
Ethical approval for this research was obtained from the local ethic committee (protocol number 2157).
Statistical analysis
Data are presented as means with standard deviations or as medians and interquartile ranges, as appropriate. The independent samples t test (parametric) or the Mann–Whitney U test (non-parametric) was used for comparisons of continuous variables.
A multivariable logistic regression model was used to identify clinical variables independently associated with the risk of the composite outcome including mortality and/or hospitalization, adjusting for age and other variables showing an association with the outcome in univariate analysis (p < 0.05). Statistical analyses were performed using SPSS software version 26 (SPSS, Inc., Chicago, IL).
Results
Global cohort
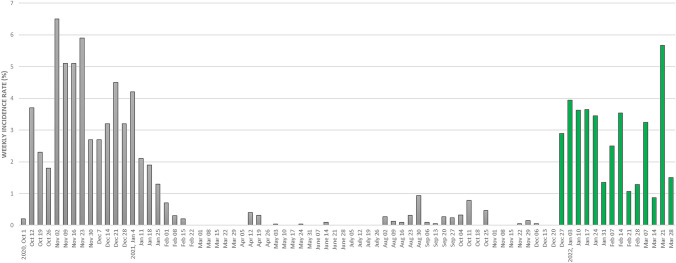
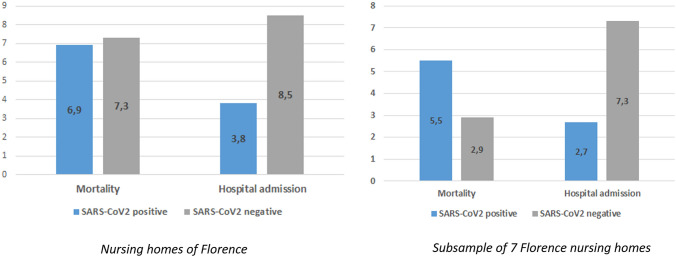
Among 2044 residents living in Florence NHs as of November 1st, 2021 (mean age 86.5 years, 77% female), 667 SARS-CoV2 cases were confirmed during the period November 2021–March 2022. Only sporadic cases were observed until the end of December 2021. SARS-CoV2 cases sharply increased soon afterwards, in parallel with the detection of the Omicron variant in the study area. Indeed, over the following weeks, 7-day incidence rates varied from 1 to 4%, peaking at 5.7% at the end of March 2022 (Fig. 1). Throughout the Omicron era, SARS-CoV2 weekly incidence rates were similar or slightly lower compared to those reported during the first wave of the pandemic (Fig. 1). However, during the Omicron era, mortality rates among NH residents were not significantly different between SARS-CoV2-positive (6.9%) and SARS-CoV2-negative residents (7.3%, p = 0.706), despite a significantly lower hospitalization rate in SARS-CoV2-positive individuals (3.8% vs 8.5% in SARS-CoV2-negative residents, p < 0.001) (Fig. 2, left panel, and Supplementary Table 1).
Fig. 1.
Weekly SARS-CoV2 incidence rates in the nursing homes of Florence during the COVID-19 pandemic (October 1st, 2020–March 31st, 2022). The study period (i.e. “Omicron era” in the text) is indicated in green (December 24th, 2021–March 31st, 2022) The date on the x axis indicates the first day of the monitoring week. Vertical lines indicate administration of the first (dotted line), second (dashed line) and third (solid line) vaccine dose.
Fig. 2.
Mortality and hospitalization rates by SARS-CoV2 status in residents of the nursing homes of Florence (left panel) and in a sample of seven randomly selected nursing homes (right panel) during the Omicron era (December 27th, 2021–March 31st, 2022)
Clinical sub-cohort
Table 1 illustrates the clinical characteristics of 457 residents from a selected sample of seven Florence NHs, by SARS-CoV2 status. Residents testing SARS-CoV2 positive during the study period were older and showed a higher prevalence of severe dementia. Moreover, a previous history of SARS-CoV2 infection was significantly less common in residents testing SARS-CoV2 positive.
Table 1.
Baseline characteristics of residents from seven selected Florence nursing homes, by SARS-CoV2 status
| Overall sample (n = 457) | SARS-CoV2-positive residents (n = 182) | SARS-CoV2-negative residents (n = 275) | p | |
|---|---|---|---|---|
| Age, mean (SD) | 81.8 (12.2) | 83.6 (11.9) | 80.6 (12.2) | 0.011* |
| Female, n (%) | 329 (72.0) | 138 (75.8) | 191 (69.5) | 0.138 |
| BADL lost, median (IQR) | 4 (2,5) | 4 (2,5) | 4 (2,5.5) | 0.281 |
| Walking capacity | 0.442 | |||
| Preserved, n (%) | 235 (51,4) | 87 (47.8) | 148 (53.8) | |
| Wheelchair/bedridden | 222 (48.6) | 95 (52.2) | 127 (46.2) | |
| Dementia, n (%) | 0.030* | |||
| No dementia | 122 (26.7) | 45 (24.7) | 77 (28.0) | |
| Mild/moderate dementia | 172 (37.6) | 59 (32.4) | 113 (41.1) | |
| Severe dementia | 163 (35.7) | 78 (42.9) | 85 (30.9) | |
| BPSD, n (%) | 177 (38,7) | 68 (37.4) | 109 (39.6) | 0.625 |
| Cardiac disease, n (%) | 142 (31,1) | 53 (29.1) | 89 (32.4) | 0.463 |
| COPD, n (%) | 65 (14,2) | 28 (15.4) | 37 (13.5) | 0.563 |
| Hypertension, n (%) | 260 (56,9) | 99 (54.4) | 161 (58.5) | 0.381 |
| Renal impairment, n (%) | 80 (17,5) | 31 (17.0) | 49 (17.8) | 0.829 |
| Atrial fibrillation, n (%) | 82 (17,9) | 31 (17.0) | 51 (18.5) | 0.680 |
| Previous stroke, n (%) | 67 (14,7) | 28 (15.4) | 39 (14.2) | 0.722 |
| Diabetes, n (%) | 96 (21) | 35 (19.2) | 61 (22.2) | 0.448 |
| Disease count, median (IQR) | 2 (1,3) | 1 (1,3) | 2 (1,3) | 0.429 |
| Previous SARS-CoV2 infection, n (%) | 138 (30.2) | 45 (24.7) | 93 (33.8) | 0.038* |
| Full immunization, n (%) | 422 (92.3) | 163 (89.6) | 259 (94.2) | 0.069 |
SD, standard deviation; BADL, basic activities of daily living; IQR, interquartile range; BPSD, behavioural and psychological symptoms of dementia; COPD, chronic obstructive pulmonary disease
*Statistical significance p < 0.05
Among residents testing SARS-CoV2 positive, two residents (10%) were admitted to hospital and five (25%) died. Clinical manifestations of SARS-CoV2 infection are detailed in Supplementary Table 2.
No significant differences were observed in mortality rate when comparing SARS-CoV2-positive (5.5%) and -negative residents (2.9%, p = 0.164). By contrast, similar to the whole population, hospital admission was significantly less frequent among SARS-CoV2-positive residents (2.7% vs 7.3%, p = 0.037; Fig. 2, right panel, and Supplementary Table 1).
A comparison of the clinical characteristics of residents based on the composite outcome of mortality and hospitalization is reported in Table 2. The composite outcome subgroup included residents with lower functional status, worse physical performance and a higher prevalence of chronic obstructive pulmonary disease (COPD), while SARS-CoV2 infection had a similar prevalence in the two groups. A past history of SARS-CoV2 infection was less common in residents who died and/or were admitted to hospital during the study period, although no differences were reported as regards full immunization (Table 3)
Table 2.
Bivariate analysis for the composite endpoint of death and hospital admission
| Death/hospital admission (n = 36) | Others (n = 421) | p | |
|---|---|---|---|
| Age, mean (SD) | 83.6 (12.2) | 81.6 (12.2) | 0.348 |
| Female, n (%) | 29 (80.6) | 300 (71.3) | 0.233 |
| BADL lost, median (IQR) | 5 (4,6) | 4 (2,5) | 0.008* |
| Walking capacity | 0.020* | ||
| Preserved, n (%) | 13 (36.1) | 222 (52.7) | |
| Wheelchair/bedridden | 23 (63.9) | 199 (47.3) | |
| Dementia, n (%) | 0.441 | ||
| No dementia | 7 (19.4) | 115 (27.3) | |
| Mild/moderate dementia | 13 (36.1) | 159 (37.8) | |
| Severe dementia | 16 (44.4) | 147 (34.9) | |
| Behavioural/psychological symptoms of dementia, n (%) | 10 (27.8) | 167 (39.7) | 0.160 |
| Cardiac disease, n (%) | 14 (38.9) | 128 (30.4) | 0.291 |
| Chronic obstructive pulmonary disease, n (%) | 13 (36.1) | 52 (12.4) | 0.001* |
| Hypertension, n (%) | 22 (61.1) | 238 (56.5) | 0.594 |
| Renal impairment, n (%) | 8 (22.2) | 72 (17.1) | 0.438 |
| Atrial fibrillation, n (%) | 9 (25.0) | 73 (17.3) | 0.250 |
| Diabetes, n (%) | 7 (19.4) | 89 (21.1) | 0.811 |
| Previous stroke, n (%) | 3 (8.3) | 64 (15.2) | 0.263 |
| Disease count, median (IQR) | 2 (1,3) | 2 (1,3) | 0.149 |
| SARS-CoV2 infection during the study period, n (%) | 13 (36.1) | 169 (40.1) | 0.635 |
| Previous SARS-CoV2 infection, n (%) | 5 (13.9) | 133 (31.6) | 0.026* |
| Full immunization, n (%) | 32 (88.9) | 390 (92.6) | 0.417 |
SD, standard deviation; BADL, basic activities of daily living; IQR, interquartile range
*Statistical significance p < 0.05
Table 3.
Multivariate logistic regression for the composite outcome of death and hospital admission
| OR | 95% Confidence Interval | p | |
|---|---|---|---|
| Age | 1.005 | 0.975–1.035 | 0.769 |
| Nr. BADL lost | 1.433 | 1.141–1.801 | 0.002 |
| SARS-CoV2 status (positive vs negative) | 0.790 | 0.374–1.672 | 0.538 |
| Chronic obstructive pulmonary disease | 4.290 | 1.975–9.320 | < 0.001 |
| Previous SARS-CoV2 infection | 0.302 | 0.112–0.813 | 0.018 |
In multivariable logistic regression analysis, a SARS-CoV2-positive test was not associated with significantly higher risk of death and hospitalization. By contrast, poor functional status according to the basic activities of daily living and COPD were associated with an increased risk of the composite outcome. Past history of SARS-CoV2 infection, but not full immunization, was associated with a reduced risk of death and hospital admission. Results were similar when analysis was repeated using mortality as outcome (Supplementary Tables 3 and 4).
Discussion
This study describes a sharp increase of SARS-CoV2 incidence in the NH setting in parallel with the Omicron outbreak, with weekly rates approximating those observed during the first pandemic wave. Yet, SARS-CoV2 infection was not associated with an increased risk of death and hospital admission. Functional status and COPD were the strongest predictors of the combined outcome including hospitalization and all-cause mortality. Results were similar when mortality was used as sole outcome.
Our study shows a higher probability of SARS-CoV2 infection in older individuals and in those with no past history of infection. The association between past history of infection and lower risk of reinfection is consistent with available literature showing an approximately 50% risk reduction in individuals with immunity from previous infection [9]. Moreover, clinical outcome of SARS-CoV2-positive subjects was better among subjects with a history of previous infection. This is consistent with the mechanism of hybrid immunity, i.e. the combination of infection-induced immunity and vaccine-induced immunity that has been associated with a more robust and persistent immune response, in comparison with either of the two mechanism [10].
The present study confirmed, as expected, that NH residents with worse physical performance and greater disability in activities of daily living, have higher mortality and hospitalization risk. Moreover, COPD was associated with a worse clinical outcome. Of notice, in our sample these associations were independent of SARS-CoV2 infection, suggesting that concomitant conditions such as disability and comorbidities may have greater influence on individuals’ prognosis in this highly vulnerable population.
Although we reported an increase in SARS-CoV2 incidence rates during the Omicron era, this was not associated with an increase in adverse outcomes among NH residents. The overall mortality rate over a five-month period was 7.1%, which is similar than that reported in Italian NH facilities during the pre-COVID-19 period [11]. Moreover, mortality rate among SARS-CoV2-positive residents (6.8%) remained low and comparable to that observed in the early post-vaccination period (6%) [3] and substantially lower in comparison with the 20% lethality observed at the end of 2020 [1]. We might suppose that high levels of prior infection and vaccination coverage and involvement of the Omicron variant may have contributed to avoid an increase in both hospitalization and mortality in our study sample. Indeed, booster vaccination was found to significantly increase Omicron-specific neutralizing activity in older adults and NH residents [12], providing strong protection against Omicron infection and COVID-19–related hospitalization and death [7]. Another possible explanation for the limited increase in hospital admissions and deaths is a decrease in virulence of the Omicron variant [13].
Since the beginning of the COVID-19 pandemic, on-site acute care of SARS-CoV2-positive residents was implemented in the NHs of Florence, through the development of the GIROT (Gruppo di Intervento Rapido Ospedale-Territorio, Hospital-to-Community Rapid Intervention Team) model [1]. This is a “hospital-at-nursing home” patient-centred care model based on comprehensive geriatric assessment, providing acute care to SARS-CoV2-positive residents on site in the NHs and pursuing a reduction of hospital admissions. This innovative care model is the most likely explanation for the lower hospitalization rate reported in SARS-CoV2-positive versus -negative residents in the present study [1].
Strict infection prevention and transmission control measures have demonstrated to be effective in managing SARS-CoV2 outbreaks in the setting of long-term care facilities [14]. However, it is well known that social isolation and loneliness may lead to patients’ distress, depression, functional and cognitive decline, with a consequent negative impact on well-being of both NH residents and their families. Moreover, it should be considered that principles of good geriatric care have been underutilized throughout the pandemic, leading to reduced quality of care and possibly contributing to the disproportionate impact of COVID-19 on older adults [15]. The present data question whether stringent surveillance and isolation measures should be maintained in the NH setting. As COVID-19 seems to be evolving from a pandemic to an endemic disease, potential harms deriving from isolation and infection control measures should be balanced against infection risks.
The findings of this study are subjected to some limitations. First, SARS-CoV2 variant strains were not routinely investigated in the study area. Second, humoral response to vaccination was not assessed in the present study. Third, SARS-CoV2-positive NH residents might have received a higher amount of medical care in comparison with negative ones as a consequence of the implementation of the GIROT model. This might have contributed to limit the rates of adverse outcomes in SARS-CoV2-positive residents. Fifth, data on medical therapy were not available and the retrospective nature of the study required reliance on review of medical records for data extraction. Finally, the limited sample size of the clinical sample does not allow to identify the prognostic impact of different pathophysiological factors, such as lower strain pathogenicity, previous infection, vaccination, and care organization.
Conclusions
During the Omicron era, SARS-CoV2 incidence substantially increased among NH residents, but SARS-CoV2 infection was not associated with an increased risk of death and hospital admission, in a setting where full immunization and adequate on-site care was provided. Functional status and COPD emerged as predictors of hospitalization and mortality in NH residents during the Omicron era, while a history of previous SARS-CoV2 infection represented an independent protective factor.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge Professor Luigi Ferrucci and Dr. Stefania Bandinelli for their valuable time and efforts in revising the manuscript.
Funding
This study was supported by Azienda USL Toscana Centro and Azienda Ospedaliero-Universitaria Careggi.
Data availability
Data are available from the authors upon request.
Declarations
Conflict of interests
The authors have no conflicts of interests to declare.
Ethical approval
Ethical approval for this research was obtained from the Local Ethic Committee (protocol number 2157).
Informed consent
Patient consent was waived due to retrospective analysis in a frail population whose consensus often could not be collected.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Enrico Mossello and Enrico Benvenuti equally contributed to this paper.
References
- 1.Benvenuti E, Rivasi G, Bulgaresi M, et al. Caring for nursing home residents with COVID-19: a “ hospital—at—nursing home ” intermediate care intervention. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta HB, Li S, Goodwin JS. Risk factors associated with SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw Open. 2021;4:1–14. doi: 10.1001/jamanetworkopen.2021.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivasi G, Bulgaresi M, Mossello E, et al. Course and Lethality of SARS-CoV-2 epidemic in nursing homes after vaccination in Florence, Italy. Vaccines. 2021;9:1174. doi: 10.3390/vaccines9101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mor V, Gutman R, Yang X, et al. Short-term impact of nursing home SARS-CoV-2 vaccinations on new infections, hospitalizations, and deaths. J Am Geriatr Soc. 2021 doi: 10.1111/jgs.17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022 doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krutikov M, Stirrup O, Nacer-Laidi H, et al. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Heal Longev. 2022;3:e347–e355. doi: 10.1016/S2666-7568(22)00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad N, Derado G, Nanduri SA, et al. Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the omicron variant—United States, February 14–March 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:633–637. doi: 10.15585/mmwr.mm7118a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Istituto Superiore di Sanità (2022) Stima della prevalenza delle varianti VOC (Variant Of Concern) e di altre varianti di SARS-CoV-2 in Italia. https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-7-marzo-2022.pdf. Accessed 31 Aug 2022
- 9.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022;310:27–46. doi: 10.1111/imr.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cangiano B, Fatti LM, Danesi L, et al. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging (Albany NY) 2020;12:24522–24534. doi: 10.18632/aging.202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canaday DH, Oyebanji OA, White E, et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. EBioMedicine. 2022;80:104066. doi: 10.1016/j.ebiom.2022.104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratil JM, Biallas RL, Burns J, et al. Non-pharmacological measures implemented in the setting of long-term care facilities to prevent SARS-CoV-2 infections and their consequences: a rapid review. Cochrane database Syst Rev. 2021;9:CD015085. doi: 10.1002/14651858.CD015085.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hanlon S, Dhesi J, Aronson L, Inouye SK. Covid-19: a call for mobilizing geriatric expertise. Eur Geriatr Med. 2021;12:597–600. doi: 10.1007/s41999-021-00500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon request.