Abstract
Abstract
Hepatic fibrosis is the common pathological change that occurs due to increased synthesis and accumulation of extracellular matrix components. Chronic insult from hepatotoxicants leads to liver cirrhosis, which if not reversed timely using appropriate therapeutics, liver transplantation remains the only effective therapy. Often the disease further progresses into hepatic carcinoma. Although there is an increased advancement in understanding the pathological phenotypes of the disease, additional knowledge of the novel molecular signaling mechanisms involved in the disease progression would enable the development of efficacious therapeutics. Ephrin–Eph molecules belong to the largest family of receptor tyrosine kinases (RTKs) which are identified to play a crucial role in cellular migratory functions, during morphological and developmental stages. Additionally, they contribute to the growth of a multicellular organism as well as in pathological conditions like cancer, and diabetes. A wide spectrum of mechanistic studies has been performed on ephrin–Eph RTKs in various hepatic tissues under both normal and diseased conditions revealing their diverse roles in hepatic pathology. This systematic review summarizes the liver-specific ephrin–Eph RTK signaling mechanisms and recognizes them as druggable targets for mitigating hepatic pathology.
Graphical abstract
Keywords: Ephrins, Eph RTKs, Hepatic fibrosis, Hepatocellular carcinoma, Cholangiocarcinoma, NAFLD
Introduction
Eph receptors are the largest known receptor tyrosine kinases (RTKs) discovered in 1987 by Hirai et al. (1987), during a search for the identification of tyrosine kinases that are involved in cancer. The name ‘Eph’ comes from the ‘erythropoietin-producing human hepatocellular carcinoma cell line, a cancer type where it was first identified (Hirai et al. 1987). Initially, due to the lack of known ligands, these Eph receptors were considered orphan receptors. Later, ephrins were discovered independently by Bartley et al. (1994); Beckmann et al. (1994); and Cheng and Flanagan (1994) as their ligands. These discoveries have led to a revolution in the field of signal transduction of RTKs. In recent years, these ephrins and Eph RTKs have gained a lot of importance with several unique physiological functions identified with specific ligand-receptor interactions. To date, 9 isoforms of ephrin ligands and 16 isoforms of Eph receptors have been discovered and classified into different classes (Xu et al. 2013).
Classification
The Eph receptors have been classified based on sequence similarity and binding specificities. They are grouped into EphA (A1–A10) and EphB (B1–B6) isoforms which interact with ephrin-A (A1–A6) and ephrin-B (B1–B3) ligands, respectively (Manning et al. 2002). Unique cross-interactions between these ligands and receptors have been identified between ephrin-B3–EphA4, and ephrin-A5–EphB2 (Himanen and Nikolov 2003). Among the different isoforms identified in the vertebrates, the human genome includes 14 receptors and 8 ligands, excluding EphA9, EphB5, and ephrin-A6. The molecular organization of these isoforms has been reported to occur either due to gene duplication (Himanen et al. 2004) or through an alternate gene splicing (Kullander and Klein 2002) leading to the generation of both ligands and receptors differing in structures from a prototypical domain.
Structure and signaling mechanism
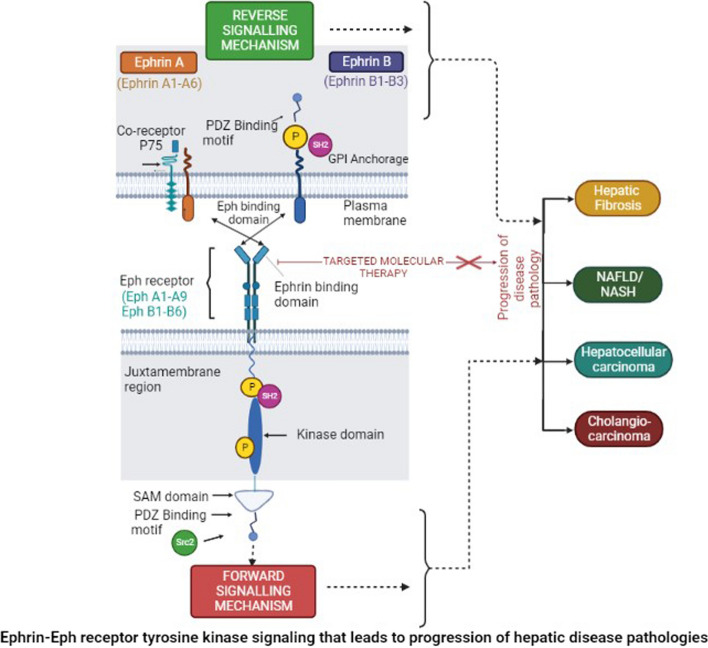
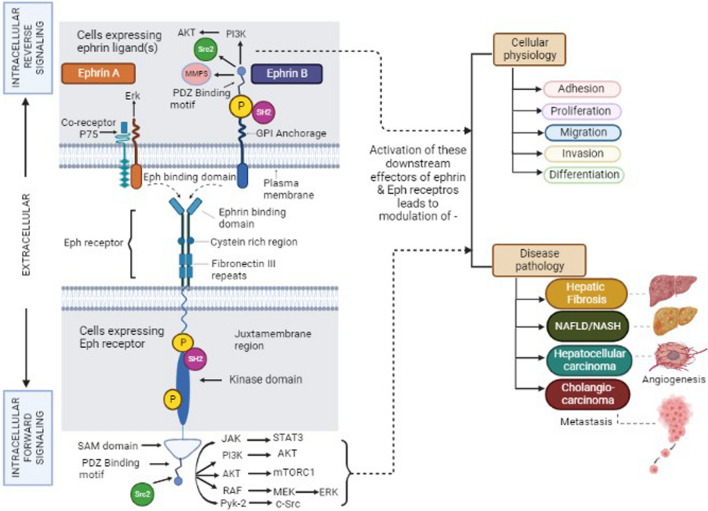
Eph receptor transmembrane proteins consist of an extracellular N-terminal domain that includes the ligand-binding domain (LBD), sequentially followed by a cysteine-rich linker region, Epidermal growth factor-like motif, and two fibronectin type III repeats (Fig. 1). Functionally, the LBD domain possesses a high-affinity ligand binding site that is necessary for the recognition and binding of ephrins. In contrast to LBD, the cysteine-rich region and fibronectin type III repeats possess low-affinity ephrin binding sites that are essential for multimerization/clustering or binding to other proteins like N-methyl-D-aspartate receptor (Dalva et al. 2000). The intracellular C-terminal domain after the transmembrane segment includes the juxtamembrane region followed by a conserved kinase domain involved in the phosphorylation of tyrosine moieties of other proteins as well as the Eph receptor itself. The kinase domain is sequentially followed by the presence of a conserved sterile alpha motif (SAM) domain and a PDZ (post-synaptic 95 density zona occludens) binding motif. SAM domain plays a critical role in receptor dimerization, oligomerization, or binding with adaptor proteins that participate in signaling (Wang et al. 2016a, b). In contrast to the Eph receptors, the ephrin ligands differ in their structure. Ephrin-A ligands consist of the receptor-binding domain (RBD) with glycophosphatidyl inositol anchorage while ephrin-B ligands are transmembrane proteins with RBD and a short conserved cytoplasmic tail consisting of PDZ binding motif that helps in interaction with other proteins (Fig. 1).
Fig. 1.
Schematic representation of the structural domains of Ephrin A and B ligands and Eph receptor Formation of the heterocomplex facilitates bi-directional signaling in which numerous signaling pathways play a role in cell function and disease pathology and can be activated through both ephrins—“reverse” and Eph—“forward” signaling. SAM domain plays a regulatory role for target-specific binding and the Src family plays a role in reverse signaling of the Eph and ephrins. These signaling events include activation of PI3K, Src family kinases, JAK-STAT, and RAF GTPases that are downstream of Ephrins and the Eph receptor. (Abbreviation: SAM, Sterile Alpha Motif; PDZ, Postsynaptic 95 Density Zona occludens; GPI, Glycosyl Phosphatidyl Inositol. P; representative of tyrosine phosphorylation sites—JAK, Janus kinase; STAT, signal transducer and activator of transcription)
Unlike other RTKs, these ephrin–Eph interaction generates a unique bi-directional signaling mechanism in both, the receptor-bearing cell—‘forward signaling’ and the ligand-bearing cell—‘retrograde/reverse signaling’ (Wang et al. 2016a, b). Some cells are known to co-express both receptor and ligand wherein cis (between receptor and ligand on the same cell) and/or trans (between receptor and ligand on two opposing cells) type of interactions take place leading to downstream signaling. The cis type of interaction leads to the inhibition of forward signaling while trans interactions are activating downstream effectors (Kao and Kania 2011).
The bidirectional signaling initiates through receptor activation involving the phosphorylation of two tyrosine residues (conserved) on the juxtamembrane region. Other tyrosine phosphorylation sites are present in the kinase domain and SAM domain (Lim et al. 2008). The binding of molecules like SH2-containing proteins and/or Src 2 homology proteins on the Eph receptor activates it through tyrosine phosphorylation and mediates the downstream signaling (Fig. 1). Ephrin-B ligands are also activated through their respective tyrosine residues mediating signals on the PDZ binding motif (Fig. 1) while Ephrin-A ligands signal through the transmembrane proteins and co-receptor like P75, a nerve growth factor receptor (Bochenek et al. 2010). The interaction between the structural motifs on these ephrin–Eph RTKs initiates various cellular physiological and pathological processes.
Role of ephrin–Eph RTKs in cellular pathophysiology
Initially, the ephrin–Eph RTKs were known to be expressed only in the nervous system, but later discoveries identified its expression in almost all the tissues of developing embryos (Arcas et al. 2020). The Eph-mediated molecular signal transduction was observed in various physiological processes–adhesion (Mukai et al. 2017), migration (Yoon et al. 2018), skeletal myogenesis, angiogenesis (Srivastava et al. 2018), osteogenesis (Arthur et al. 2018), osteoclastic activity (Ge et al. 2018), neuronal differentiation (Chen et al. 2020a, b), axonal regeneration (Teng et al. 2019), and bone remodeling (Tazaki et al. 2018). The Eph-mediated molecular signaling has also been identified in severe disease pathologies like colorectal cancer (Mateo-Lozano et al. 2017), pancreatic cancer (Liu et al. 2014), diabetes mellitus (Jain et al. 2013), and Waldenstrom macroglobulinemia (Azab et al. 2013). In diabetes, it was observed that ephrin-A/EphA signaling mediates the glucose-stimulated insulin secretion wherein forward signaling through receptor suppresses and reverse signaling through ligand promotes insulin secretion. Thus, forward signaling favors the development of the disease, and reverse signaling favors the suppression of the disease. Eph receptors—EphA1, EphA2, EphA4, EphA5, and EphA7 regulate different stages of pancreatic ductal adenocarcinoma (Giaginis et al. 2010). Similarly, EphA2, and EphA7 along with EphB2, EphB3, and EphB4 act in different stages of colorectal tumorigenesis by forward and reverse signaling (Batlle et al. 2005). Although the role of Eph signaling has been explored in diverse physiological and pathological states, its expression and function in liver health and diseases are varied. Hence, understanding the functional role of ephrin–Eph receptors interaction in hepatic biology is essential.
We have performed a systematic review of the published literature through electronic databases; Pubmed and ScienceDirect by two independent investigators. The search criteria included all publications from the year 2000 through March 2023 in the English language including both preclinical and clinical studies. The following keywords or terms were included as a search criterion: “Ephrin”, “Eph Receptor”, “Hepatic fibrosis”, “Hepatocellular carcinoma (HCC)”, “Cholangiocarcinoma”, “Non-alcoholic Fatty-Liver Disease (NAFLD)”, and “Non-alcoholic steatohepatitis (NASH)”.
Hepatic diseases
The liver is one of the vital organs playing an important role in glucose metabolism, detoxification, storage of vitamins, etc. Hence, its failure causes a severe effect on the whole body thereby developing fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Some of the common etiologies of liver diseases include hepatitis B or C virus (HBV and HCV) infection, alcohol consumption, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and hemochromatosis.
Hepatic fibrosis
Hepatic fibrosis is a unique injury-recuperating process characterized by the accumulation of fibrous scars. It is formed due to the increased production of extracellular matrix (ECM) components by the activated hepatic stellate cells (HSC) emerging from different persistent liver pathologies (Liu et al. 2016). HSCs are liver-specific pericytes that are vitamin A-storing cells. Due to hepatic insults, HSCs get activated by the various cytokines, chemokines, and growth factors released by hepatocytes, Kupffer cells, and sinusoidal endothelial cells (SEC). Activated HSCs are transdifferentiated into myofibroblast-like cells that promote the formation and secretion of ECM components which further aggravates the commencement and progression of fibrosis into hepatic pathologies (Liu et al. 2016). Chronic fibrosis results in hepatocyte necrosis followed by inflammatory cell infiltration, and myofibroblast activation that often leads to a stage called hepatic cirrhosis. Although fibrosis and cirrhosis are reversible, the liver becomes non-functional over the years with decreased ability to synthesize proteins and process drugs and toxins. Often the condition of hepatic cirrhosis is vulnerable to the development of HCC. The underlying mechanisms of these complications are complex with limited benefits from existing therapeutics. Hence, novel molecular targets for anti-fibrotic therapeutics are warranted.
The CCN family proteins comprising of Cyr61 (Cellular communication network factor 1 - CCN1), CTGF (CCN2), and NOV (CCN3) are matricellular proteins that are present in between the cells in the extracellular matrix (Yeger and Perbal 2016). The connective tissue growth factor (CTGF/CCN2) is one of the major proteins that is produced by the activated HSCs in fibrotic livers. CCN2, a profibrotic protein regulates multiple processes including cell proliferation, migration, adhesion, survival, and ECM production by being both a downstream and cooperative mediator of TGFβ signaling that contribute to fibrogenesis (Tong et al. 2009, Huang et al. 2012). CCN1 also has multiple roles inducing efferocytosis to remove the apoptotic neutrophils thereby leading to polarization of macrophages, TGFβ1 expression in activated HSCs, and ECM production to initiate the tissue repair process (Kim et al. 2022). CCN1 and CCN2 both lead to fibrosis by activating downstream TGFβ signaling. Interestingly, CCN3 counteracts the effect of CCN2 which may be a molecular target for developing anti-fibrotic therapy (El Kader et al. 2013). Similarly, CCN1 has a negative correlation while CCN2 and CCN3 have a positive correlation in the development of HCC (Jia et al. 2016). CCN proteins are potential therapeutic targets for liver fibrosis and HCC like the ephrin–Eph RTKs but to date, there have been no studies that suggest a direct correlation between them in hepatic pathologies.
Role of ephrin–Eph RTKs in hepatic fibrosis
Hepatic fibrosis is a well-studied and unusual wound repair response to chronic liver injuries, however, the role of ephrin–Eph RTKs in it has been identified recently (Table 1). HSCs, the major cell type in fibrosis, are in the space of Disse which interacts with SEC in maintaining vascular homeostasis, thereby leading to microvasculature formation (Semela et al. 2008). This interaction is in part due to the platelet-derived growth factor (PDGF) released by the SEC, which binds to its cognate receptor, PDGFRβ present on the HSC. Interestingly, the co-culture of HSC and SEC labeled independently with different colored dyes resulted in the formation of vascular tube-like structures in an in-vitro set-up wherein the SECs were present on the cellular extensions of HSCs (Semela et al. 2008). Furthermore, HSC monocultures when induced with PDGF-BB depicted tube formation that was inhibited by the inhibitor, imatinib mesylate. This suggests the activation of HSCs leads to vascular tubulogenesis (Semela et al. 2008). Similarly, increased tubulogenesis was confirmed in primary HSCs isolated from bile duct ligated as compared to sham-operated rats (Semela et al. 2008). Microarray analysis of tube-forming HSC, when treated with PDGF-BB, has shown increased expression of ephrin-B2, and its cognate receptor EphB4, which was inhibited with imatinib mesylate. Thus, signifying ephrin-B2 and EphB4 RTKs as the downstream effectors of PDGF-BB/PDGFRβ signaling in HSCs (Semela et al. 2008). This study pioneered the role of Eph RTKs during HSC activation leading to hepatic fibrosis. Furthermore, the molecular mechanisms between HSC and SEC interaction also involve ephrin-B2-EphB4 signaling in HSC resulting in the expression and secretion of vascular endothelial growth factor (VEGF) through an extracellular signal-regulated kinase (ERK)-dependent pathway that leads to SEC recruitment (Das et al. 2010) and finally liver fibrosis.
Table 1.
Ephrins and Eph RTKs signaling
| S. No | Hepatic diseases | Ephrin ligand | Eph receptor | Signaling mechanism | Process involved | Tissue/Cell type | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Hepatic fibrosis |
Ephrin-B2 Ephrin-B2 |
EphB4 EphB4 EphB1 |
PDGFB/PDGFRβ ERK-KLF2-VEGF Mechanism unk |
HSC-recruitment SEC-recruitment Prolif, Mig |
HSCs HSCs HSCs |
Semela et al. (2008) Das et al. (2010) Li et al. (2023) |
| 2 |
NAFLD NASH |
– Ephrin-A1 – |
EphA3 EphA2 EphA3 EphB2 |
Mechanism unk Mechanism unk Mechanism unk Notch mechanism |
Undetermined Undetermined Undetermined Migration, Prolif |
Liv tis Liv tis Liv tis Hepatocytes |
Moylan et al. (2014) Alisi et al. (2011) Gerhard et al. (2018) Xiao et al. (2023) |
| 3 | Hepatocellul-ar carcinoma |
– – – Ephrin A1 Ephrin-B1 – – – Ephrin-A3 |
EphA1 EphA2 EphA3 EphA1 EphB1 EphA5 EphA1 EphA2 EphA2 EphB2 |
Mechanism unk Mechanism unk Missense mut p21 upregulation Mechanism unk AKT-ERK-p38 PI3K/AKT-mTOR JAK1/STAT3-AKT SREBP1-ACLY EPHB2/β-catenin/TCF1 |
Prolif, Mig, Inv, Tumorigenesis Inv, Metastasis Tumor-growth Prolif, Angio Tumor-Angio Tumor-growth Tumor-Angio Tumor-growth Tumor-initiation Undetermined |
Huh-7 cells Liv tis Liv tis Liv tis HCC tumor HCC cells HLE, Huh7 Huh-7 MHCC97L, PLC/PRF/5, HepG2 HCC cells and liv tis |
Chen et al. (2010) Yang et al. (2009) Bae et al. (2009) Iida et al. (2005) Sawai et al. (2003) Wang et al. (2019) Wang et al. (2016b) Wang et al. (2021) Husain et al. (2022) Leung et al. 2021 |
| 4 | Cholangioca-rcinoma |
– Ephrin-B1 Ephrin-B2 – |
EphA2 EphB2 & EphB4 EphA2 EphB3 |
AKT/mTORc1, & Pyk-2/c-Src PI3K/AKT, & Wnt NOTCH1 Mechanism unk |
Prolif, Mig, Inv Mig, Metastasis Metastasis Tumorigenesis, Metastasis |
Choi-ck, Cho-ck CCA tissue CCLP1, HUCCT1 CCA tissue |
Cui et al. (2012) Khansaard et al. (2014) Sheng et al. (2019) Wu et al. (2020) |
Specific ephrin ligand and Eph receptor interactions activate downstream signaling-mediated physiological processes in various hepatic disease pathologies. Also, an increased expression of Eph RTKs in tumor cells upregulated the molecular signaling as well as pathophysiological processes
Prolif, Proliferation; Mig, Migration; Inv, Invasion; Tumor-Angio, Tumor angiogenesis; Mut, mutation; unk, unknown; Liv tis, Liver tissue; HLE, Human hepatoma cell line; Huh7, Human hepatoma Huh-7 cells; MHCC97L, hepatocellular carcinoma (HCC) cell line with highly metastatic potential; PLC/PRF/5, Alexander hepatoma cell line; HepG2, human liver cancer cell line; Choi-ck, Cho-ck; CCLP1, Cholangiocarcinoma cell lines; HUCCT1, Cholangiocarcinoma cell line
Malarial pathogenesis leads to the sequestration of infected red blood cells onto the liver endothelium resulting in hepatic fibrosis (Mimche et al. 2015). An increased surface expression of EphB2 on macrophages led to analyze its role in disease generation using an EphB2 knockout (EphB2−/−) mouse model. Protection from hepatic fibrosis with altered hepatocyte function was observed along with decreased infiltration of leukocytes in the EphB2−/− mouse although it had a similar parasite burden as compared with malaria-infected control mice (Mimche et al. 2015). Similarly, EPHB2 was upregulated in several types of infection or inflammation-induced liver fibrosis in mice, and thus it serves as a potential molecular target for novel anti-fibrotic therapeutics. Activation of HSCs was associated with the downregulation of miR-451/miR-185 accompanied by the upregulation of EphB2 and other fibrosis markers. Overexpression of miR-451 led to the repression of the nuclear export receptor, XPO-1 (also known as chromosome region maintenance 1, CRM1) expression which in turn upregulated miR-185 expression at the post-transcriptional level. The synergistic antifibrotic actions between miR-451 and miR-185 were further confirmed in HSCs and CCl4-induced hepatic fibrosis in mice (Chen et al. 2020a, b).
In the CCl4-induced liver fibrosis, EphB2 deficiency also drastically reduced hepatic fibrosis. A dual effect of EphB2 was observed on the pro-inflammatory and pro-fibrotic state in the mouse inflamed liver chronically exposed to CCl4 (Mimche et al. 2018). Interestingly, EPHB1 expression in activated HSCs has been accompanied by a post-translational modification, neddylation that was also observed to be significantly increased in the CCl4-induced mouse model of liver fibrosis (Table 1). Mechanistically, neddylation not only prevents EPHB1 from degradation but also enhances the kinase activity thereby promoting the activation phenotypes of the HSCs—proliferation, and migration (Li et al. 2023). This study provides new insight into the Eph receptor signaling that has a potential role in therapeutic targeting for the treatment of liver fibrosis. Since preclinical studies supported by clinical observations suggest the involvement of ephrins and Eph RTKs in hepatic fibrosis, developing targeted therapeutics against these will be effectively halting the further development of cirrhosis and subsequent HCC pathologies.
Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)
NAFLD is a broad term that characterizes progressive hepatic steatosis which is associated with hepatitis, fibrosis, cirrhosis, and in some cases HCC. The development of NAFLD in patients is associated with obesity, diabetes, dyslipidemias, insulin resistance, and metabolic syndromes. In NAFLD with simple steatosis alone, steatosis occurs with no evidence of hepatic injury and is reversible, whereas NASH is an aggressive form of NAFLD, which causes necro-inflammatory injury with or without fibrosis that progresses to cirrhosis and occasionally leads to HCC (Benedict et al. 2017). Ballooned hepatocytes are a prominent feature of lipotoxic liver injury. The presence of hepatocellular ballooning is used for histological grading and staging of NAFLD and NASH diagnosis. Sonic hedgehog (Shh), a ligand of the developmental hedgehog signaling is produced by the ballooned hepatocytes. Along with hepatocyte injury and death, inflammation is another major histological hallmark of NASH. Kupffer cells (liver resident macrophages) play a critical role in the development of inflammation and fibrosis by initiating the recruitment of other immune cells. Hepatic inflammation is characterized by the accumulation of recruited monocytes/monocyte-derived macrophages and increased numbers of neutrophils and Natural Killer (NK) cells. Kupffer cells are proinflammatory and secrete inflammatory cytokine-tumor necrosis factor, and chemokines including C–C motif chemokine ligand (CCL)2 and IL-8, thereby recruiting monocytes and neutrophils to the liver injury microenvironment. Furthermore, circulating blood monocytes infiltrate the liver and give rise to monocyte-derived macrophages. Like the Kupffer cells, monocyte-derived macrophages also play a vital role in perpetuating inflammation and tissue remodeling. In NASH, monocytes infiltrate the liver via mechanisms largely dependent on chemokine receptors expressed by monocytes, such as C–C chemokine receptor (CCR)2 and CXCR3. Another feature of NASH is hepatic neutrophil infiltration. Even though the extent of their accumulation appears to be less than in alcoholic steatohepatitis, they play important role in NASH progression (Ibrahim et al. 2018). The prevalence and incidences of NAFLD have been observed to be 20–30% in Western countries (Sayiner et al. 2016) whereas a recent meta-analysis showed 31.6% in Asian countries (Riazi et al. 2022).
Role of ephrin–Eph RTKs in NAFLD/NASH
Interestingly, clinical samples from severe NAFLD patients have confirmed the upregulation of the EPHA3 gene (Moylan et al. 2014). Microarray data analysis determined the inverse correlation between rat-specific miRNA expression and proteins in a diet-induced model of NAFLD. There was a significant upregulation of miR-200a, miR-200b, and miR-429 along with downregulation of the protein expression of ephrin-A1 and EPHA2 with a hypercaloric diet (Allisi et al. 2011). Further, gene expression profiling following microarray analysis of human NASH fibrosis samples revealed a differential expression of EPHA3 (Gerhard et al. 2018). At the non-alcoholic fatty liver stage, hepatocytes exhibited metabolic adaptation, whereas, at the NASH stage, a subset of hepatocytes was enriched with the EphB2 signature on cell adhesion and migration. EPHB2, a downstream effector of Notch signaling in hepatocytes, induces cell-autonomous inflammation. Knockdown of Ephb2 in hepatocytes ameliorated inflammation and fibrosis in a mouse model of NASH (Table 1). Thus, EphB2-expressing hepatocytes contribute to NASH progression that may serve as a potential therapeutic target (Xiao et al. 2023).
Hepatocellular carcinoma (HCC)
HCC contributes to the third leading cause of cancer-related deaths worldwide (Sung et al. 2021). HCC incidence occurs mostly in men than in women and is highly prevalent in parts of south-eastern Asia and western and northern Africa due to HBV and HCV infections while the HCC incidence in the American subcontinent has been reported due to higher alcohol consumption (Sung et al. 2021). Although HCC was considered to be the primary malignancy of the liver due to cirrhosis and/or HBV, HCV infection, recent trends suggest that the prevalence has been rapidly taken over due to NAFLD (Younossi et al. 2016). The diseased liver becomes nodular along with symptoms like yellow skin, abdominal pain, and nausea. Surgical removal of the tumor results in 10–20% success. However, if the tumor is not resected completely, 70% of patients develop recurrent HCC resulting in the mortality of patients within 3–6 months of surgery (Sung et al. 2021).
Role of ephrin–Eph RTKs in HCC
Protein array technology revealed an upregulation of 42 phospho-RTKs in HCC (Liu et al. 2011). This discovery led to identifying the probable functional role of Eph RTKs in the disease thereby deciphering the molecular signaling mechanisms in HCC (Table 1). An upregulated EPHA1 in Huh-7, an HCC cell line, increased cellular proliferation, migration, and invasion. Gene silencing of EPHA1 in these cells decreased tumor-inducing ability along with the downregulation of the angiogenic and invasive markers—VEGF, MMP-2, and MMP-9. Further, these cells when transplanted into nude mice resulted in reduced tumor growth and microvessel density (MVD) (Cheng et al. 2010), thus confirming the association of EPHA1 with HCC tumorigenesis. Next, higher expression of MMP-9, EphA2, and CD34 (a marker of MVD) in HCC tissues correlates with the tumor nodules, tumor size, vascularity, and capsule formation that regulates the invasive and metastatic potential of HCC. Clinically, EPHA2+MMP-9+MVDhi HCC patients showed a decreased mean median survival rate (Yang et al. 2009). Thus, EPHA2 serves as the prognostic marker of HCC, suggesting a rationale for drug designing against this molecular target to treat HCC for increased patient survival. Similarly, EPHA3 was also observed to be overexpressed in HCC. The genetic alterations of the gene identified using ‘single-strand conformational polymorphism and sequencing’ in HCC patients revealed D219V missense mutations in the extracellular domain and SAM domain of the EPHA3 receptor leading to the aggressive growth of HCC (Bae et al. 2009). The presence of EPHB1 polymorphisms in the Korean population has been diagnosed with HCC. Among the various single nucleotide polymorphisms of the EPHB1 gene, the genotypes A/A at rs11926992, T/T at rs7644369, A/A at rs6776570, T/T at rs3821502, and G/G at rs6766459 were identified to be associated with the HCC. Additionally, an increased mRNA expression level of EphA7 (26–fold) and EphB2 (9-fold) was observed in HCC tissue as compared with healthy liver tissue. However, at the protein level, immunohistochemical analysis revealed a high EPHB2 but weakly expressed EPHA7 (Hafner et al. 2004). This differential regulation of Eph’s at mRNA and protein levels suggests a post-transcriptional regulation in the parenchymal population of HCC.
Ephrins, the cognate ligand of Eph receptors are also associated with HCC. An increased mRNA expression of the ephrin-A1 ligand that specifically binds to the EPHA1 receptor has been observed in Alpha-fetoprotein (AFP) expressing HCC cell lines and tumor samples (Iida et al. 2005). The functionality of ephrin-A1 was characterized using anti-sense oligonucleotide against ephrin-A1, leading to decreased proliferation of Huh7 cells. On the contrary, overexpression of ephrin-A1 using ephrin-A1 Fc chimera resulted in increased proliferation of HLE cells, an HCC cell line that constitutively expresses low ephrin-A1 (a rescuing back phenomenon). Mechanistically, the expression of p21 (a cell cycle inhibitor gene) was upregulated during ephrin-A1 silencing and downregulated during overexpression thereby modulating the proliferation of cells (Iida et al. 2005). The microarray analysis of cells treated with ephrin-A1 Fc led to an increased expression of AFP, EphA1, TGF-β, BMP, FGF, MMP-2, MMP-7, Rho family members, and decreased tumor angiogenesis inhibitor TSP-1, and p21. Out of these, ephrin-A1 correlated with AFP, EphA1, EphA2, p21, MMP-2, and TSP-1 both at mRNA and protein levels in HCC cells and patient samples (Iida et al. 2005). Thus, ephrin-A1 acts as a tumor promoter by increasing the proliferative capacity of HCC.
Another ligand known to be associated with HCC is ephrin-A5. This ligand exists in two isoforms, canonical larger ‘L’ and shorter ‘S’ form lacking exon 4. The expression of both isoforms is decreased in HCC tumor tissue (Wang et al. 2012). Additionally, in HCC cell lines, the ephrin-A5 ‘S’ isoform was observed to be more potent than the ephrin-A5 ‘L’ isoform in decreasing cell proliferation, and migration (Wang et al. 2012). Furthermore, a disease-free, as well as overall survival of HCC patients, was efficiently correlated with only the higher expression of the ephrin-A5 ‘S’ isoform (Wang et al. 2012) Thus, the ‘S’ isoform of ephrin-A5 is another prognostic marker that reduces the overall effects of HCC.
Ephrin-B1/EphB1 interaction plays a key role in HCC tumor angiogenesis. Ephrin-B1 mRNA and protein levels are highly expressed in HCC tumor samples as well as in HCC cell lines (Sawai et al. 2003). An overexpression of ephrin-B1 in BalbC mice also resulted in increased tumor growth. Neovascularization as assessed by counting the number of CD31+ stained blood vessels depicted an increased size and number of hematoceles in ephrin-B1 overexpressing tumors as compared with control (Sawai et al. 2003) suggesting the role of ephrin-B1 in HCC vascularization. In the liver microcirculation of adult mice, Notch regulates vascular differentiation and size through ephrin-B2/EPHB4 signaling resulting in angiosarcoma (Dill et al. 2012).
The essential kinases of HCC cells like Anaplastic lymphoma kinase, Fibroblast growth factor receptor 2, and EphA5 together function to maintain the viability of HCC cells through their downstream effectors—protein kinase B (AKT), ERK, and p38-dependent signaling pathways (Table 1). The co-activation of these kinases leads to the growth of HCC cells further resulting in poor clinical prognosis and overall decreased survival of HCC patients (Wang et al. 2019). The preliminary role of tyrosine kinases in HCC cells revealed the phosphorylation of 71 tyrosine kinases that were profiled across 8 HCC cell lines utilizing the human RTK phosphorylation arrays. This has eventually led to the recognition of the plausible role of Eph RTKs in HCC (Wang et al. 2019).
Interestingly, endothelial progenitor cells (EPCs) transplantation in HCC xenograft-bearing nude mice through tail veins facilitated angiogenesis-mediated tumor development. An upregulated EphA1 expression in HCC cells promoted the chemotaxis of EPCs and endothelial cells towards the tumor cells through a paracrine mechanism. Furthermore, in-vitro and in-vivo experiments confirmed the pivotal role of EPHA1 along with the SDF-1/CXCR4 axis-mediated activation of PI3K/AKT and mTOR signaling in the growth of HCC xenografts. Thus, targeting the EPHA1/SDF-1 signaling suggests a plausible anti-angiogenesis-based therapeutic approach to mitigate HCC (Wang et al. 2016a, b). Similarly, targeting EPHA2 using a small molecule, ALW-II-41-27 downregulated the activation of JAK1/STAT3 and AKT signaling in HCC that in turn suppressed tumor growth (Wang et al. 2021).
The intra-tumoral hypoxic microenvironment of these highly proliferative HCCs was evaluated for transcriptomic and genomic profiling (Husain et al. 2022). At a clinical level, hypoxia-inducible monitoring identified the ephrin-A3/EphA2 axis as clinically relevant prognostic markers. Ephrin-A3 upregulation has been correlated with the metastatic potential of HCC. The ephrin-A3/EphA2 forward signaling axis induced the maturation of sterol regulatory element-binding protein 1, and the expression of its transcriptional target, ATP citrate lyase (ACLY) which resulted in the self-renewal capacity of HCC for tumor initiation. Metabolic profiling of EPHA2 in clinical cohorts and stable knockdown of ACLY in HCC cells illustrated critical cross-over in unsaturated fats, cholesterol, and Tricarboxylic acid cycle metabolites. Thus, ACLY the downstream effector of the ephrin-A3/EphA2 axis modulates the self-renewal capacity of HCC (Husain et al. 2022). Poor diagnosis of clinicopathological features and a high tumor recurrence rate were associated with higher expression of EPHA2 and N-cadherin, and reduced expression of E-cadherin, resulting in an E–N cadherin switch (Fan et al. 2013). Additionally, weighted gene co-expression network analysis (WGCNA) employed to detect co-expressed modules and hub genes in differential stages of HCC depicted ephrin signaling to be deregulated at the very early stage and upregulated increasingly with the progression of HCC (Yin et al. 2018). EPHB2 kinase activity was significantly upregulated in the sorafenib-resistant, patient-derived tumor xenografts (Leung et al. 2021). A sequential increase in EPHB2 expression correlated with stages of hepatic pathology from normal liver tissue through fibrotic to HCC tissue. Mechanistically, EPHB2 regulated the stemness and chemoresistance through SRC/AKT/GSK3β/β-catenin signaling while its expression was regulated by TCF1-induced promoter activation, forming a positive Wnt/β-catenin feedback loop (Table 1). Intravenous administration of rAAV-8-shEPHB2 suppressed HCC tumor growth and significantly sensitized HCC cells to sorafenib (Leung et al. 2021) thereby suggesting this as a therapeutically targetable axis to combat acquired drug resistance in HCC.
Cholangiocarcinomas
Cholangiocarcinoma (CCA) is a malignant tumor of epithelial cells arising from the cholangiocytes on the biliary tree (Tabrizian et al. 2015). It is the second leading liver malignancy after HCC. CCA being an aggressive malignancy has a poor prognosis with a 10% 5-year survival rate. Several risk factors associated with CCA tumorigenesis, are congenital hepatic fibrosis, primary sclerosing cholangitis, biliary stone disease, Caroli disease, choledochal cysts, and chronic infection with liver flukes. Depending on the origin of the tumor in the biliary tree, CCA can be classified as intrahepatic (IH-CCA), extra-hepatic (EH-CCA), and distal-hepatic. The EH-CCA is the most common type that accounts for approximately 90% of CCA. Currently, treatment strategies for EH-CCA include systemic chemotherapy, targeted radiation, and surgery. However, the recurrence of the disease often leads to death (Tabrizian et al. 2015).
Role of ephrin–Eph RTKs in CCA
The functional role of Eph RTKs in pathological complications in cholangiocytes suggests the significance of Eph molecular signaling in CCA cells (Table 1). Interestingly, the EPHA2 gene has been identified both as a tumor promoter and suppressor in CCA (Tabrizian et al. 2015). Furthermore, poorly differentiated CCA tumors also correlated with high EphA2 expression (Singh et al. 2017). The differentially high EphA2 expression was induced by epidermal growth factor (EGF) while inhibited by its ligand, ephrin-A1. Thus, ligand-independent EphA2 signaling has been observed to promote CCA. EGF treatment to the cells also upregulated EPHA2 site-specific phosphorylation at a unique serine, S897 but not the usual tyrosine, Y954 moiety. Functionally, EphA2 imparts high migratory and invasive capacities to the CCA cells in-vitro and in-vivo, respectively. The invasiveness of the stably transfected CCA cells with EphA2 revealed higher metastatic nodules in the lungs. Mechanistically, the AKT/mTORC1 and Pyk-2/c-Src signaling were identified as the downstream effector of EphA2 for the proliferative and invasive potential of CCA cells (Cui et al. 2012).
In a screen for identifying the activated protein kinases involved in CCA, a phosphokinase array identified the Eph RTKs along with PI3K/AKT, and Wnt canonical signaling. Among the various subtypes of Eph RTKs, EphB2, and EphB4, along with ligands, ephrin-B1, and ephrin-B2 were associated with CCA (Khansaard et al. 2014). Immunohistochemically the localization of these receptors EphB2, EphB4, and the ligands ephrin-B1, and ephrin-B2 were confirmed on the cell membrane and in the cytoplasm, respectively of the CCA, as well as in the hepatocytes, and blood vessels. Physiologically, EphB2 silencing decreased cell migration that was associated with decreased phosphorylation of focal adhesion kinase and paxillin, the cell migration-related kinases (Khansaard et al. 2014). Furthermore, higher expression of EphB2 in CCA tissues has been associated with the metastasis of the disease. CCA-related angiogenesis revealed the functional role of EphB4, EphB2, and ephrin-B1 to be associated with MVD. Thus, targeting EphA2 and EphB2 signaling might be a potential therapeutic strategy to mitigate CCA-mediated metastasis.
Clinically the whole-transcriptome sequencing and exome sequencing of IH-CCA including lymph node metastases from patients revealed a mutation in EPHA2. Correlation analysis indicated that EPHA2 mutations were closely associated with lymph node metastasis of IH-CCA. This mutation resulted in the ligand-independent phosphorylation of Ser897, thereby modulating the NOTCH1 signaling and promoting lymphatic metastasis of IH-CCA. As observed in HCC, ALW-II-41-27, an inhibitor of Ser897 phosphorylation effectively suppressed the metastasis of IH-CCA with mutated EPHA2 in both in-vitro assays and patient-derived xenografts (Sheng et al. 2019). Thus, designed small molecule inhibitors targeting the EPHA2 activation might be an attractive therapeutic strategy to combat lymphatic metastasis of IH-CCA. Similarly, clinicopathological analysis in peritumoral tissues and adenoma tissues of EH-CCA revealed an upregulated dysadherin with concomitant downregulated EPHB3 expression which correlated with the malignancy (Table 1). The patient survival rates were significantly higher with positive expression of EPHB3 and negative expression of dysadherin than vice versa (Wu et al. 2020). Hence, these molecular targets may serve in mitigating the tumorigenesis and progression of EH-CCA.
Ephrin–Eph RTKs as druggable targets—current and future perspectives
Over the years enormous advances have been made in the therapeutic strategies against various liver pathologies that have improved patient survivability. Molecular profiling of the hepatic tissues from preclinical hepatic disease models as well as clinical samples revealed the role of Ephrin–Eph RTKs that qualifies for the development of targeted therapeutics. So far, the EphA2-derived peptide vaccine with amphiphilic poly nanoparticles (Eph-NPs) exhibited an anti-tumor effect against HCC in mice (Yamaguchi et al. 2010). Similarly, small molecules as pharmacological inhibitors of Eph RTKs (ALW-II-41-27), small peptides as neutralizing antibodies of ephrin (l-Phenylalanine (Phe)-conjugated poly(γ-glutamic acid), monoclonal antibodies against ephrin–Eph RTKs (ephrin-A1 Fc chimera), miRNAs (miR-451 and miR-185), siRNAs (EphB4 siRNA, etc.), and Adeno-associated viral (AAV)-constructs to deliver shRNA against Eph RTKs (rAAV-8-shEphB2) has been used effectively to mitigate the hepatic pathologies in preclinical disease models. Interestingly, a few of the discoveries have also been tested at the clinical trials stage. A phase 1 clinical trial of sEphB4-HSA, a recombinant albumin fusion protein with EPHB4 to target Ephrin-B2 in HCC reported 1 out of 8 HCC patients had a partial beneficial response (El-Khoueiry et al. 2016).
More efforts are needed to explore the mechanistic insights of various other ephrin–Eph and Eph-Eph interactions, receptor activation, downstream signaling, and the function-altering sites in the Eph receptors. This enables into rationale designing of multi-targeted broad-spectrum molecules not only to advance our understanding of the basic molecular mechanisms underlying ephrin–Eph signaling but also the translational outcome. Studies should be conducted in the future using molecular modeling to identify and design small molecules against these targets that can effectively modulate the downstream signaling of ephrin and Eph RTKs to mitigate the hepatic pathologies. Further, these small molecules should be synthesized using green chemistry to maximize the therapeutic efficacy, sustained/controlled release, reduced drug toxicity, and liver-targeted delivery to minimize off-target side effects. Similarly, designed peptides with high affinity and potency targeting selective ephrin–Eph RTKs should be undertaken. Additionally, modifications of these peptides would further increase the half-life to prevent their degradation. Furthermore, CRISPR-Cas9-based gene-editing technology should be employed for gene modification of these identified ephrin–Eph RTKs to overcome the barriers to drug delivery, minimize drug toxicities, and improve the efficacy and safety of drugs targeting the ephrin–Eph RTKs.
Conclusion
Ephrins-Eph RTKs are the largest class of RTKs involved in fundamental cellular and pathological functions. This review highlighted the potential isoforms of ephrin and Eph RTKs which get upregulated during the disease and can serve as key molecular targets for effective therapeutics. A precise role of Eph like EphA1, EphA2, EphA3, EphA5, EphB1, EphB2, EphB4, and ephrins like ephrin-A1, ephrin-A3, ephrin-B1, ephrin-B2 has been observed in liver fibrosis, NAFLD/NASH, HCC, and CCA.
Acknowledgements
Manuscript Communication number: IICT/Pubs./2022/171.
Author contributions
AD—Study concept and design; drafting of the manuscript; critical revision of the manuscript; obtained funding; study supervision. SM & PD—Literature search through databases; figures and tables; drafting of the manuscript; critical revision of the manuscript.
Funding
AD acknowledges the funding provided by the Council of Scientific and Industrial Research (CSIR), Ministry of Science & Technology, Government of India for Niche Creating High Science Project MLP-0052 and Focused Basic Research MLP-0277 under the Health Care Theme. The fellowship provided by UGC-JRF/SRF to SM is gratefully acknowledged.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El Kader T, Kubota S, Janune D, Nishida T, Hattori T, Aoyama E, Perbal B, Kuboki T, Takigawa M. Anti-fibrotic effect of CCN3 accompanied by altered gene expression profile of the CCN family. J Cell Commun Signal. 2013;7:11–18. doi: 10.1007/s12079-012-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, Leoni S, Bottazzo GF, Masotti A, Nobili V. Mirnome analysis reveals novel molecular determinants in the pathogenesis of the diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;9:283–293. doi: 10.1038/labinvest.2010.166. [DOI] [PubMed] [Google Scholar]
- Arcas A, Wilkinson DG, Nieto MA. The evolutionary history of Ephs and Ephrins: toward multicellular organisms. Mol Biol Evol. 2020;37:379–394. doi: 10.1093/molbev/msz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A, Nguyen TM, Paton S, Klisuric A, Zannettino ACW, Gronthos S. The osteoprogenitor-specific loss of ephrinB1 results in an osteoporotic phenotype affecting the balance between bone formation and resorption. Sci Rep. 2018;8:12756. doi: 10.1038/s41598-018-31190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab F, Azab AK, Maiso P, Calimeri T, Flores L, Liu Y, Quang P, Roccaro AM, Sacco A, Ngo HT, Zhang Y. Eph-B2/Ephrin-B2 interaction plays a major role in the adhesion and proliferation of Waldenstrom’s macroglobulinemia. Clin Cancer Res. 2013;18:91–105. doi: 10.1158/1078-0432.CCR-11-0111. [DOI] [PubMed] [Google Scholar]
- Bae HJ, Song JH, Noh JH, Kim JK, Jung KH, Eun JW, Xie HJ, Ryu JC, Ahn YM, Kim SY, Lee SH. Low-frequency mutation of the Ephrin receptor A3 gene in hepatocellular carcinoma. Neoplasma. 2009;56:331–334. doi: 10.4149/neo_2009_04_331. [DOI] [PubMed] [Google Scholar]
- Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, Lu HS, Colombero AM, Elliott RL, Guthrie BA, Holst PL. B61 is a ligand for the Eck receptor protein-tyrosine kinase. Nature. 1994;368:558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- Beckmann MP, Cerretti DP, Baum P, Vanden Bos T, James L, Farrah T, Kozlovsky C, Hollingsworth T, Shilling H, Maraskovsky E. Molecular characterization of a family of ligands for Eph-related tyrosine kinase receptors. EMBO J. 1994;13:3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9:715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci. 2010;123:1235–1246. doi: 10.1242/jcs.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang Y, Zhou M, Shi H, Yu Z, Zhu Y, Yu F. EphA1 receptor silencing by small interfering RNA has antiangiogenic and antitumor efficacy in hepatocellular carcinoma. Oncol Rep. 2010;23:563–570. doi: 10.3892/or_00000670. [DOI] [PubMed] [Google Scholar]
- Chen Q, Song H, Liu C, Xu J, Wei C, Wang W, Han F. The interaction of EphA4 With PDGFR β regulates proliferation and neuronal differentiation of neural progenitor cells in vitro and promotes neurogenesis in vivo. Front Aging Neurosci. 2020;12:1–14. doi: 10.3389/fnagi.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang D, Wang Y, Chen K, Zhao L, Xu Y, Jiang H, Wang S. Synergistic antifibrotic effects of miR-451 with miR-185 partly by co-targeting EphB2 on hepatic stellate cells. Cell Death Dis. 2020;11:1–3. doi: 10.1038/s41419-020-2613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Cui XD, Lee MJ, Kim JH, Hao PP, Liu L, Yu GR, Kim DG. Activation of mammalian target of rapamycin complex 1 (mTORC1) and Raf/Pyk2 by growth factor-mediated Eph receptor 2 (EphA2) is required for cholangiocarcinoma growth and metastasis. Hepatol. 2012;1:2248–2260. doi: 10.1002/hep.26253. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Das A, Shergill U, Thakur L, Sinha S, Urrutia R, Mukhopadhyay D, Shah VH. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk-dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol. 2010;298:908–915. doi: 10.1152/ajpgi.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill MT, Rothweiler S, Djonov V, Hlushchuk R, Tornillo L, Terracciano L, Meili-Butz S, Radtke F, Heim MH, Semela D. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterol. 2012;142(4):967–977.e2. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- El-Khoueiry A, Gitlitz B, Cole S, Tsao-Wei D, Goldkorn A, Quinn D, Lenz HJ, Nieva J, Dorff T, Oswald M, Berg J. A first-in-human phase I study of sEphB4-HSA in patients with advanced solid tumors with expansion at the maximum tolerated dose (MTD) or recommended phase II dose (RP2D) Eur J Cancer. 2016;1(69):S11. doi: 10.1016/s0959-8049(16)32623-5. [DOI] [Google Scholar]
- Fan M, Liu Y, Xia F, Wang Z, Huang Y, Li J, Wang Z, Li X. Increased expression of EphA2 and E–N cadherin switch in primary hepatocellular carcinoma. Tumori. 2013;99:689–696. doi: 10.1177/030089161309900608. [DOI] [PubMed] [Google Scholar]
- Ge YW, Liu ZQ, Sun ZY, Yu DG, Feng K, Zhu ZA, Mao YQ. Titanium particle-mediated osteoclastogenesis may be attenuated via bidirectional ephrin-B2/Eph-B4 signaling in vitro. Int J Mol Med. 2018;42:2031–2041. doi: 10.3892/ijmm.2018.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard GS, Legendre C, Still CD, Chu X, Petrick A, DiStefano JK. Transcriptomic profiling of obesity-related nonalcoholic steatohepatitis reveals a core set of fibrosis-specific genes. J Endocr Soc. 2018;5:710–726. doi: 10.1210/js.2018-00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaginis C, Tsourouflis G, Zizi-Serbetzoglou A, Kouraklis G, Chatzopoulou E, Dimakopoulou K, Theocharis SE. Clinical significance of ephrin (Eph)-A1, -A2, -a4, -a5 and -a7 receptors in pancreatic ductal adenocarcinoma. Pathol Oncol Res. 2010;16:267–276. doi: 10.1007/s12253-009-9221-6. [DOI] [PubMed] [Google Scholar]
- Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46–51. doi: 10.1016/s0166-2236(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Himanen J, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the Eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (landmark Ed) 2012;17:2495–2507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- Husain A, Chiu YT, Sze KM, Ho DW, Tsui YM, Suarez EM, Zhang VX, Chan LK, Lee E, Lee JM, Cheung TT. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J Hepatol. 2022;8278:125–128. doi: 10.1016/j.jhep.2022.02.018. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Hirsova P, Gores GJ. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut. 2018;67:963–972. doi: 10.1136/gutjnl-2017-315691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, Honda M, Kawai HF, Yamashita T, Shirota Y, Wang BC, Miao H, Kaneko S. Ephrin-A1 expression contributes to the malignant characteristics of α-fetoprotein producing hepatocellular carcinoma. Gut. 2005;54:843–851. doi: 10.1136/gut.2004.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Jain D, Liu Q, Bartosinska B, Wang J, Schumann D, Kauschke SG, Eickelmann P, Piemonte L, Gray NS, Lammert E. Pharmacological inhibition of Eph receptors enhances glucose-stimulated insulin secretion from mouse and human pancreatic islets. Diabetologia. 2013;56:1350–1355. doi: 10.1007/s00125-013-2877-1. [DOI] [PubMed] [Google Scholar]
- Jia Q, Dong Q, Qin L. CCN: core regulatory proteins in the microenvironment that affect the metastasis of hepatocellular carcinoma. Oncotarget. 2016;7:1203–1214. doi: 10.18632/oncotarget.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Kania A. Ephrin-mediated cis -attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Khansaard W, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, Puapairoj A, Loilome W. Increased EphB2 expression predicts cholangiocarcinoma metastasis. Tumor Biol. 2014;35:10031–10041. doi: 10.1007/s13277-014-2295-0. [DOI] [PubMed] [Google Scholar]
- Kim KH, Cheng N, Lau LF. Cellular communication network factor 1-stimulated liver macrophage efferocytosis drives hepatic stellate cell activation and liver fibrosis. Hepatol Commun. 2022;6:2798–2811. doi: 10.1002/hep4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signaling. Nat Commun Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Leung HW, Leung CON, Lau EY, et al. EPHB2 activates β-catenin to enhance cancer stem cell properties and drive sorafenib resistance in hepatocellular carcinoma. Cancer Res. 2021;81:3229–3240. doi: 10.1158/0008-5472.can-21-0184. [DOI] [PubMed] [Google Scholar]
- Li R, Zhang D, Han Y, Chen K, Guo W, Chen Y, Wang S. Neddylation of EphB1 regulates its activity and associates with liver fibrosis. Int J Mol Sci. 2023;24:3415. doi: 10.3390/ijms24043415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75NTR mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gong J, Morishita A, Nomura T, Miyoshi H, Tani J, Kato K, Yoneyama H, Deguchi A, Mori H, Mimura S. Use of protein array technology to investigate receptor tyrosine kinases activated in hepatocellular carcinoma. Exp Therap Med. 2011;2:399–403. doi: 10.3892/etm.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Huang H, Wang C, Kong Y, Zhang H. Involvement of ephrin receptor A4 in pancreatic cancer cell motility and invasion. Oncol Lett. 2014;7:2165–2169. doi: 10.3892/ol.2014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ji Y, Ai H, Ning B, Zhao J, Zhang Y, Dun G. Liver shear-wave velocity and serum fibrosis markers to diagnose hepatic fibrosis in patients with chronic viral hepatitis B. Kor J Radiol. 2016;17:396–404. doi: 10.3348/kjr.2016.17.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- Mateo-Lozano S, Bazzocco S, Rodrigues P, Mazzolini R, Andretta E, Dopeso H, Fernández Y, Del Llano E, Bilic J, Suárez-López L, Macaya I. Loss of the EPH receptor B6 contributes to colorectal cancer metastasis. Sci Rep. 2017;7:43702. doi: 10.1038/srep43702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimche PN, Brady LM, Bray CF, Lee CM, Thapa M, King TP, Quicke K, McDermott CD, Mimche SM, Grakoui A, Morgan ET. The receptor tyrosine kinase EphB2 promotes hepatic fibrosis in mice. Hepatol. 2015;62:900–914. doi: 10.1002/hep.27792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimche PN, Lee CM, Mimche SM, Thapa M, Grakoui A, Henkemeyer M, Lamb TJ. EphB2 receptor tyrosine kinase promotes hepatic fibrogenesis in mice via activation of hepatic stellate cells. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-20926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan CA, Pang H, Dellinger A, Garrett ME, Guy CD, Murphy SK, Ashley-Loch AE, Choi SS, Michelotti GA, Hampton DD. Hepatic gene expression profiles differentiate pre-symptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatol. 2014;59:471–482. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Suruga N, Saeki N, Ogawa K. EphA receptors and ephrin-A ligands are upregulated by monocytic differentiation/maturation and promote cell adhesion and protrusion formation in HL60 monocytes. BMC Cell Biol. 2017;18:1–20. doi: 10.1186/s12860-017-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- Sawai Y, Tamura S, Fukui K, Ito N, Imanaka K, Saeki A, Sauda S, Kiso S, Matsuzawa Y. Expression of ephrin-B1 in hepatocellular carcinoma: possible involvement in neovascularization. J Hepatol. 2003;39:991–996. doi: 10.1016/S0168-8278(03)00498-7. [DOI] [PubMed] [Google Scholar]
- Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the United States and rest of the world. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterol. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Wei J, Zhang Y, Gao X, Wang Z, Yang J, Yan S, Zhu Y, Zhang Z, Xu D, Wang C. Mutated EPHA2 is a target for combating lymphatic metastasis in intrahepatic cholangiocarcinoma. Int J Can. 2019;144:2440–2452. doi: 10.1002/ijc.31979. [DOI] [PubMed] [Google Scholar]
- Singh DR, Ahmed F, Paul MD, Gedam M, Pasquale EB, Hristova K. The SAM domain inhibits EphA2 interactions in the plasma membrane. Biochimica Et Biophysica Acta (BBA)-Mol Cell Res. 2017;1864:31–38. doi: 10.1016/j.bbamcr.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Shukla V, Tiwari D, Gupta J, Kumar S, Kumar A. Targeted therapy of chronic liver diseases with the inhibitors of angiogenesis. Biomed Pharmacother. 2018;105:256–266. doi: 10.1016/j.biopha.2018.05.102. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tabrizian P, Jibara G, Hechtman JF, Franssen B, Labow DM, Schwartz ME, Thung SN, Sarpel U. Outcomes following resection of intrahepatic cholangiocarcinoma. HPB. 2015;17:344–351. doi: 10.1111/hpb.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazaki Y, Sugitani K, Ogai K, Kobayashi I, Kawasaki H, Aoyama T, Suzuki N, Tabuchi Y, Hattori A, Kitamura KI. RANKL, Ephrin–Eph, and Wnt10b are key intercellular communication molecules regulating bone remodeling in autologous transplanted goldfish scales. Comp Biochem Physiol Part A Mol Integrative Physiol. 2018;225:46–58. doi: 10.1016/j.cbpa.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Teng S, Palmieri A, Maita I, Zheng C, Das G, Park J, Zhou R, Alder J, Thakker-Varia S. Inhibition of EphA/Ephrin-A signaling using genetic and pharmacologic approaches improve recovery following traumatic brain injury in mice. Brain Inj. 2019;33:1385–41401. doi: 10.1080/02699052.2019.1641622. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50(3):939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Ng KF, Sen YT, Wang YL, Liang KH, Yeh CT, Chen TC. Peritumoral small EphrinA5 isoform level predicts the postoperative survival in hepatocellular carcinoma. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Q, Zheng Y, Li G, Liu W. Systematic biochemical characterization of the SAM domains in Eph receptor family from Mus Musculus. Biochem Biophys Res Comm. 2016;473:1281–1287. doi: 10.1016/j.bbrc.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu H, Shan Y, Tao C, Wu F, Yu Z, Guo P, Huang J, Li J, Zhu Q, Yu F. EphA1 activation promotes the homing of endothelial progenitor cells to hepatocellular carcinoma for tumor neovascularization through the SDF-1/CXCR4 signaling pathway. J Exp Clin Cancer Res. 2016;35:1–15. doi: 10.1186/s13046-016-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang M, Ping F, Liu H, Sun J, Wang Y, Shen A, Ding J, Geng M. Identification and therapeutic intervention of coactivated anaplastic lymphoma kinase, fibroblast growth factor receptor 2, and ephrin type-A receptor 5 kinases in hepatocellular carcinoma. Hepatol. 2019;69:573–586. doi: 10.1002/hep.29792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hou W, Perera A, Bettler C, Beach JR, Ding X, Li J, Denning MF, Dhanarajan A, Cotler SJ, Joyce C. Targeting EphA2 suppresses hepatocellular carcinoma initiation and progression by dual inhibition of JAK1/STAT3 and AKT signaling. Cell Rep. 2021;34:108765. doi: 10.1016/j.celrep.2021.108765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu R, Xiong L, Miao X, Li D, Zou Q, Yuan Y, Yang Z. Prognostic and clinicopathological significance of EphB3 and dysadherin expression in extrahepatic cholangiocarcinoma. Cancer Manag Res. 2020;12:221–232. doi: 10.2147/CMAR.S232278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Batmanov K, Hu W, Zhu K, Tom AY, Guan D, Jiang C, Cheng L, McCright SJ, Yang EC, Lanza MR, Liu Y, Hill DA, Lazar MA. Hepatocytes demarcated by EphB2 contribute to the progression of nonalcoholic steatohepatitis. Sci Transl Med. 2023;15:9653. doi: 10.1126/scitranslmed.adc9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Tzvetkova-Robev D, Xu Y, Goldgur Y, Chan YP, Himanen JP, Nikolov DB. Insights into Eph receptor tyrosine kinase activation from crystal structures of the EphA4 ectodomain and its complex with ephrin-A5. Proc Nat Acad Sci. 2013;110:14634–14639. doi: 10.1073/pnas.1311000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Tatsumi T, Takehara T, Sasakawa A, Yamamoto M, Kohga K, Miyagi T, Kanto T, Hiramastu N, Akagi T, Akashi M, Hayashi N. EphA2-derived peptide vaccine with amphiphilic poly(gamma-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol Immunother. 2010;59:759–767. doi: 10.1007/s00262-009-0796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Yuan W, He J, Wang J, Yu L, Jin X, Hu Y, Liao M, Chen Z, Zhang Y. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: implications for tumor progression and prognosis. Hepatol Res. 2009;39:1169–1177. doi: 10.1111/j.1872-034X.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- Yeger H, Perbal B. CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal. 2016;10:229–240. doi: 10.1007/s12079-016-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Cai Z, Zhu B, Xu C. Identification of key pathways and genes in the dynamic progression of HCC based on WGCNA. Genes (basel) 2018;9:92. doi: 10.3390/genes9020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Hwang YS, Lee M, Sun J, Cho HJ, Knapik L, Daar IO. TBC1d24-ephrinB2 interaction regulates contact inhibition of locomotion in neural crest cell migration. Nat Comm. 2018;9:3491. doi: 10.1038/s41467-018-05924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]